Abstract
Introduction:
Analysis of novel smokeless tobacco products purchased in Round I of the New Product Watch (NPW)—a national tobacco monitoring network—demonstrated that some tobacco constituents vary not only across various brands but also regionally and over time within the same product. In this study, we analyzed snus and dissolvable tobacco products that were purchased in Round II of the NPW.
Methods:
We analyzed tobacco-specific N-nitrosamines (TSNA) and nicotine in snus and dissolvable tobacco products that were purchased in various regions of the country during the spring and summer of 2011. The results were compared against the Round I data, across different U.S. regions, and among products.
Results:
A total of 216 samples were received from different states representing 6 regions of the country. Compared with the previous analyses, TSNA levels increased significantly in Marlboro and Camel Snus and some dissolvable Camel products. The levels of unprotonated nicotine in Marlboro Snus and Camel Snus in this study were not different from Round I but varied significantly by regions; the differences between the highest and the lowest average regional levels were ~3.2-fold in Marlboro Snus ~1.7-fold in Camel Snus.
Conclusions:
Our results indicate that some novel smokeless tobacco products contain TSNA at the levels found in the conventional moist snuff. Observation of regional variations in unprotonated nicotine content in both Round I and Round II of NPW suggest that manufacturers may tailor the levels of this constituent consistently to different regions.
INTRODUCTION
Over the last decades, smokeless tobacco products have been subject of increasing advertisement, promotion, and consumption both globally and in the United States (Nelson et al., 2006). The assortment of smokeless products marketed in the United States includes traditional forms, such as chewing tobacco, nasal and oral snuff, as well as some relatively new varieties, such as Swedish-type snus and dissolvable tobacco products. The latter two types are specifically promoted to smokers, as a substitute in situations when smoking is not possible (Rogers, Biener, & Clark, 2010). Camel Snus and Marlboro Snus, currently available nationwide, first appeared in test markets in 2006 and 2007, respectively. Various dissolvable tobacco products carrying the same brand names were introduced more recently. The information on the use of these products is relatively limited. Surveys show that 5.1%–12.6% of U.S. adults—predominantly young males—try snus, and 14.4% of those who ever tried this product are current users (Biener, McCausland, Curry, & Cullen, 2011; McMillen, Maduka & Winickoff, 2012; Regan, Dube & Arrazola, 2012). Among adults who had heard of dissolvable tobacco, 3.5% had tried it and 0.4% used it at the time of survey (Regan, Dube, & Arrazola, 2012). National surveys have shown high awareness and interest in novel tobacco products as an alternative to conventional cigarettes among some smokers (Parascandola, Augustson, O’Connell, & Marcus, 2009; Parascandola, Augustson, & Rose, 2009). Taking into account the observed recent growth of the tobacco industry’s investments into the advertisement of smokeless tobacco products (Morrison, Krugman, & Park, 2008), accompanied by the rise in smokeless tobacco use in high school students (Centers for Disease Control and Prevention, 2012), it is plausible to expect that the use of these novel products will also continue to grow in the future. Indeed, according to a recent report, market share of snus grew in the United States from 0.1% of all smokeless tobacco products in 2007 to 3.7% of all smokeless tobacco products in 2011(Delnevo et al., 2012).
The potential public health impact of these new smokeless products remains a subject of controversy. On one hand, limited initial reports indicate that these products contain relatively low amounts of tobacco-specific nitrosamines (TSNA) and other carcinogens that are abundant in cigarette smoke and in conventional smokeless tobacco products (Stepanov, Jensen, Hatsukami, & Hecht, 2008; Stepanov et al., 2012a, 2012b), and the existing epidemiological evidence indicates that people who exclusively use low-TSNA Swedish snus experience lower overall cancer risks than regular cigarette smokers (Foulds, Ramstrom, Burke, & Fagerström, 2003; Greer, 2011; Luo et al., 2007). In addition, use of snus in Sweden has been associated in some studies with the reduction in smoking and has been used for smoking cessation (Ramström & Foulds, 2006; Stegmayr, Eliasson, & Rodu, 2005; Stenbeck, Hagquist, & Rosen, 2009). Therefore, encouraging smokers to switch to these novel smokeless products is seen by some as a potential harm-reduction strategy (Bates et al., 2003; Levy et al., 2004; Savitz, Meyer, Tanzer, Mirvish, & Lewin, 2006). On the other hand, potential negative consequences include dual use of smokeless products and cigarettes, recruitment of new smokeless tobacco users, and maintenance of tobacco use in current smokers (Accortt, Waterbor, Beall, & Howard, 2002; Hatsukami, Lemmonds, & Tomar, 2004; Severson, Forrester, & Biglan, 2007; Teo et al., 2006; Tomar, 2003). Additionally, historical patterns of cigarette smoking and snus use in Sweden are influenced by many factors, including strong tobacco control measures, and may not be replicated in the United States (Tomar, Connolly, Wilkenfeld, & Henningfield, 2003; Zhu et al., 2009). Thus, despite the potential benefits to some individuals, the use of these tobacco products may lead to prolonged or increased exposure to tobacco toxicants on a population level, overall negatively affecting public health.
Measurement of toxicant and carcinogen levels, as well as monitoring product availability and use, marketing strategies, and consumer perceptions, can provide valuable information for the evaluation of potential public health impact of these novel smokeless products. The New Product Watch (NPW) is a web-based national monitoring network that provides tools for monitors to periodically report local observations of new oral tobacco products. The monitors are mainly state tobacco control program staff and their community partners who, in addition to reporting observations, collect product samples for analysis of chemical constituents and product packaging (Rogers, Biener, Nyman, & Crow, 2010; Stepanov et al., 2012a). Analysis of TSNA and nicotine in Marlboro Snus, Camel Snus, and dissolvable Camel products Orbs, Sticks, and Strips, that were purchased during the first phase of the NPW in the summer of 2010, demonstrated that the levels of these constituents not only varied across different products but also regionally and over time within the same brand of product (Stepanov et al., 2012a, 2012b).
In this study, we present the results of TSNA and nicotine analyses in Marlboro, Camel, and Skoal Snus and dissolvable tobacco products that were purchased between April and July of 2011as part of the second phase of the NPW project.
MATERIALS AND METHODS
Tobacco Samples
A total of 13 monitors from 11 states representing 6 regions of the country purchased products in retail stores between April and July of 2011. The regions (and the corresponding states) were as follows: West (CO and UT), Midwest (IN and KS), Pacific Northwest (AK), Northeast (NH, NY, and MA), Mid-Atlantic/Appalachia (West VA and NC), and South (GA). As in the first round of purchases, we sought to obtain representative averages for constituent levels by acquiring a sample of all flavors of each product from three different locations within each region. Samples were purchased, labeled, and handled according to the protocol described for the first phase of this project (Stepanov et al., 2012a). In the laboratory, samples were sealed in plastic sleeves and stored at 4 °C until analysis.
Tobacco Analysis
The analyses were initiated after all the samples were obtained in the laboratory. Due to the large number of samples and the laborious sample purification procedures, the samples were divided into four subsets, and each subset was analyzed separately. For each sample, the new package was open and four portions were weighed for the measurement of moisture content (100mg), pH (200mg), nicotine (50mg), and TSNA (200mg) into corresponding pre-labeled tubes. During sample preparation for corresponding analyses, each set of samples was supplemented with one negative control (corresponding extraction solvent) and one positive control sample (Copenhagen Snuff with known nicotine and TSNA content, the same product that was used in our previous round of analyses; Stepanov et al., 2012a) to monitor for potential contamination and day-to-day analytic variation. To minimize the potential day-to-day variation in instrument performance, analyses of the prepared samples for nicotine were performed in a single run. The same approach was used for the analysis of TSNA.
Tobacco-Specific N-Nitrosamines
Four commonly measured TSNA were analyzed: 4-(meth ylnitrosamino)-1-(3-pyridyl)-butanone (NNK); Nʹ-nitroso nornicotine (NNN); Nʹ-nitrosoanatabine (NAT); and Nʹ-nitrosoanabasine. Analysis of these TSNA was performed by gas chromatography interfaced with a thermal energy analyzer, identically to the method described for the first round of purchases (Stepanov et al., 2012a).
Nicotine and Unprotonated Nicotine
Nicotine and unprotonated nicotine were measured by using the same methods as in the first phase of the NPW project (Stepanov et al., 2012a). Briefly, nicotine was analyzed by gas chromatography-mass spectrometry-selected ion monitoring, and the amount of unprotonated nicotine was calculated using the Henderson–Hasselbalch equation (Richter & Spierto, 2003).
Moisture content and pH were measured as previously described (Stepanov et al., 2008, 2012a).
Statistical Analysis
The statistical analysis of differences among regions and between Round I and Round II of the NPW was restricted to products for which three samples were obtained from different vendors in multiple locations around the country. A descriptive analysis indicated high variability in some flavors of Camel Snus within region; the decision was made to include all data and analyze it on the log scale. Thus, analysis of variance was conducted to compare the geometric means of the products. When the overall F test was significant, t tests were constructed to make the comparisons of interest. All p-values were adjusted using the method of Bonferroni. Te level of significance was set to 0.05.
RESULTS
The range of products purchased in this phase of the NPW Project was slightly different from our first round of purchases (Stepanov et al., 2012a). For instance, Marlboro Snus in this phase was available in two forms—slim six-pouch packs inserted in paper sleeves (same as in the previous phase) and new metal round tins. In some locations, the four flavors of the “slim pack” Marlboro Snus included Marlboro Snus Mild, whereas in others, the same orange label was called “amber.” In this study, “mild” and “amber” varieties of Marlboro Snus were treated as the same flavor. The new round tins of Marlboro Snus were available in two flavors, original and mint. All flavors and all samples of Camel Snus purchased in this round were packed in the new design tins. In contrast, only two samples (robust and winterchill flavors) of this new version were found by our purchasers in the first round. Dissolvable Camel products in this round were found only in mint flavor. However, two new dissolvable product varieties were purchased this time—Marlboro Sticks and Skoal Sticks—each available in four different flavors.
A total of 216 samples were received: 110 samples of Marlboro Snus (rich, mild/amber, spearmint, and peppermint in slim packs with paper sleeves, and original and mint in new round tins); 12 samples of dissolvable Marlboro Sticks (original, rich, mint, and cool mint); 68 samples of Camel Snus (mellow, frost, robust, and winterchill); 12 samples of Camel dissolvable products (Orbs, Sticks, and Strips; all mint flavor); 2 Skoal Snus samples (mint and smooth mint); and 12 samples of dissolvable Skoal Sticks (original, rich, mint, and smooth mint). Marlboro Snus (both slim packs and round tins) and Camel Snus products were obtained from all six regions. Although we received three samples of each variety and flavor of Marlboro and Camel Snus from Mid-Atlantic/Appalachia, two out of three samples were purchased in the same store. Therefore, a mean value for each constituent was calculated for each pair of samples purchased in the same store, and this value was used as a single entry for statistical analyses. Products purchased in the South region were available from only one state (GA); however, these samples were purchased in three different cities to maintain the “three locations per region” approach. Camel dissolvable products were found only by observers in NC and four samples of each type (Orbs, Sticks, and Strips) were purchased in different stores. Marlboro and Skoal dissolvable sticks were found only in KS, and for both brands, three samples of each flavor were purchased in the same store. Skoal Snus was found only by observers in Newton, MA, where two samples (mint and smooth mint flavors) were purchased.
TSNA and nicotine were not detected in negative control samples. Analysis of nicotine, NNN, and NNK in Copenhagen Snuff produced inter-set relative standard deviations (RSD) of 1.5%, 5.6%, and 5.9%, respectively. We also determined the RSD for Marlboro and Skoal Sticks, for which three samples of all flavors were purchased in the same store. The RSD values were 3.7% for total TSNA, 4.1% for nicotine, 0.5% for pH, and 6.2% for moisture.
Constituent levels in all products analyzed in this study are summarized in Tables 1 and 2.
Table 1.
Portion Weights, Moisture Content, and Tobacco-Specific N-Nitrosamine Levels in Smokeless Products Analyzed in This Studya
| Product | Flavor (n) | Portion weight, g | Moisture, % | µg/g dry weight | ||||
|---|---|---|---|---|---|---|---|---|
| NNN | NNK | NAT | NAB | Total TSNA | ||||
| Snus | ||||||||
| Marlboro Snus (slim pack) | Rich (19) | 0.443±0.02 | 19.5±1.7 | 0.781±0.14 | 0.185±0.03 | 0.665±0.10 | 0.048±0.06 | 1.68±0.25 |
| Mild/amber (20) | 0.387±0.01 | 12.1±1.2 | 0.700±0.10 | 0.220±0.03 | 0.505±0.06 | 0.050±0.06 | 1.48±0.15 | |
| Spearmint (19) | 0.383±0.02 | 13.4±1.0 | 0.688±0.09 | 0.242±0.04 | 0.542±0.07 | 0.034±0.03 | 1.51±0.15 | |
| Peppermint (20) | 0.404±0.02 | 12.8±0.7 | 0.717±0.09 | 0.220±0.03 | 0.579±0.11 | 0.066±0.13 | 1.58±0.21 | |
| Marlboro Snus (round tin) | Original (16) | 0.922±0.03 | 29.5±1.2 | 0.746±0.12 | 0.274±0.03 | 0.710±0.14 | 0.044±0.03 | 1.78±0.22 |
| Mint (16) | 0.852±0.06 | 29.9±1.1 | 0.682±0.10 | 0.232±0.03 | 0.629±0.20 | 0.060±0.06 | 1.60±0.23 | |
| Camel Snus | Robust (17) | 0.931±0.06 | 33.5±1.6 | 1.84±0.56 | 0.730±0.20 | 0.915±0.39 | 0.142±0.06 | 3.63±0.82 |
| Mellow (17) | 0.543±0.05 | 33.2±1.3 | 1.93±1.11 | 0.685±0.19 | 0.907±0.49 | 0.169±0.20 | 3.69±1.55 | |
| Frost (17) | 0.542±0.04 | 32.8±1.5 | 1.98±0.84 | 0.696±0.16 | 0.927±0.38 | 0.133±0.07 | 3.73±1.30 | |
| Winterchill (17) | 0.904±0.07 | 32.9±1.0 | 1.81±0.74 | 0.659±0.14 | 0.950±0.39 | 0.131±0.06 | 3.55±1.13 | |
| Skoal Snus | Mint (1) | 0.980 | 30.9 | 2.04 | 0.356 | 2.19 | 0.165 | 4.75 |
| Smooth mint (1) | 0.924 | 29.3 | 2.41 | 0.534 | 2.24 | 0.073 | 5.26 | |
| Dissolvable products | ||||||||
| Marlboro Sticks | Original (3) | 0.184±0.01 | 10.1±0.3 | 2.09±0.05 | 0.770±0.02 | 1.74±0.06 | 0.083±0.01 | 4.68±0.13 |
| Rich (3) | 0.191±0.01 | 9.2±0.8 | 2.28±0.04 | 0.806±0.01 | 1.68±0.05 | 0.079±0.00 | 4.85±0.09 | |
| Smooth mint (3) | 0.196±0.02 | 9.7±0.9 | 2.15±0.11 | 0.860±0.01 | 1.58±0.06 | 0.071±0.01 | 4.67±0.17 | |
| Cool mint (3) | 0.186±0.01 | 9.2±0.4 | 2.24±0.14 | 0.884±0.05 | 1.66±0.11 | 0.076±0.01 | 4.87±0.30 | |
| Camel Orbs | Mint (4) | 0.235±0.00 | 6.1±1.9 | 0.300±0.01 | 1.13±0.06 | 0.285±0.02 | 0.018±0.00 | 1.73±0.07 |
| Camel Sticks | Mint (4) | 0.463±0.01 | 6.1±0.8 | 0.267±0.01 | 0.251±0.02 | 0.185±0.01 | 0.018±0.00 | 0.720±0.03 |
| Camel Strips | Mint (4) | 0.207±0.01 | 10.4±0.8 | 0.378±0.02 | 0.871±0.07 | 0.305±0.01 | 0.025±0.01 | 1.58±0.09 |
| Skoal Sticks | Original (3) | 0.223±0.00 | 9.3±0.8 | 2.30±0.15 | 0.870±0.07 | 2.24±0.16 | 0.102±0.00 | 5.15±0.39 |
| Rich (3) | 0.220±0.01 | 8.9±1.0 | 2.66±0.06 | 0.913±0.03 | 2.30±0.08 | 0.111±0.01 | 5.98±0.14 | |
| Mint (3) | 0.217±0.02 | 8.7±0.2 | 2.44±0.05 | 0.839±0.04 | 2.20±0.05 | 0.128±0.01 | 5.60±0.14 | |
| Smooth mint (3) | 0.220±0.01 | 10.4±0.3 | 2.51±0.08 | 0.815±0.04 | 2.14±0.08 | 0.032±0.02 | 5.50±0.19 | |
Note. NAB = Nʹ-nitrosoanabasine; NAT = Nʹ-nitrosoanatabine; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-butanone; NNN = Nʹ-nitrosonornicotine; TSNA = tobacco-specific N-nitrosamines.
aArithmetic M ± SD are shown for products/flavors for which three or more samples were available. Otherwise, results of single sample analyses are shown.
Table 2.
pH and Nicotine Levels in Smokeless Productsa
| Product | Flavor (n) | pH | Total nicotine, mg/g dry weight | Unprotonated nicotine, % of total | Unprotonated nicotine, mg/g dry weight |
|---|---|---|---|---|---|
| Snus | |||||
| Marlboro Snus (slim pack) | Rich (19) | 6.72±0.26 | 22.56±2.53 | 5.6±3.5 | 1.23±0.71 |
| Mild/amber (20) | 6.65±0.24 | 17.76±1.88 | 4.7±3.0 | 0.83±0.49 | |
| Spearmint (19) | 6.80±0.19 | 17.95±1.49 | 6.1±2.7 | 1.10±0.48 | |
| Peppermint (20) | 6.77±0.20 | 18.38±1.99 | 5.8±3.0 | 1.05±0.49 | |
| Marlboro Snus (round tin) | Original (16) | 6.72±0.19 | 17.43±1.28 | 5.2±2.6 | 0.91±0.44 |
| Mint (16) | 6.68±0.20 | 16.95±1.00 | 4.8±2.7 | 0.82±0.45 | |
| Camel Snus | Robust (17) | 7.55±0.17 | 13.18±1.34 | 25.9±7.2 | 3.42±1.06 |
| Mellow (17) | 7.57±0.14 | 14.34±1.80 | 26.6±6.1 | 3.83±1.00 | |
| Frost (17) | 7.59±0.18 | 14.34±1.45 | 28.0±7.6 | 4.06±1.34 | |
| Winterchill (17) | 7.61±0.13 | 13.27±1.28 | 28.3±5.6 | 3.78±0.87 | |
| Skoal Snus | Mint (1) | 6.80 | 24.89 | 5.7 | 1.41 |
| Smooth mint (1) | 6.55 | 26.92 | 3.3 | 0.88 | |
| Dissolvable products | |||||
| Marlboro Sticks | Original (3) | 7.91±0.03 | 7.56±0.32 | 43.6±1.8 | 3.30±0.23 |
| Rich (3) | 7.82±0.05 | 7.81±0.26 | 38.8±2.8 | 3.03±0.25 | |
| Smooth mint (3) | 8.04±0.08 | 7.51±0.26 | 50.9±4.3 | 3.82±0.46 | |
| Cool mint (3) | 8.00±0.04 | 7.54±0.48 | 48.9±2.1 | 3.68±0.14 | |
| Camel Orbs | Mint (4) | 7.62±0.27 | 4.39±0.09 | 29.9±10.9 | 1.31±0.48 |
| Camel Sticks | Mint (4) | 7.86±0.05 | 4.97±0.14 | 41.1±2.8 | 2.04±0.12 |
| Camel Strips | Mint (4) | 8.00±0.05 | 4.54±0.20 | 48.8±3.0 | 2.21±0.09 |
| Skoal Sticks | Original (3) | 7.34±0.04 | 5.14±0.27 | 17.1±1.4 | 0.88±0.11 |
| Rich (3) | 7.30±0.04 | 4.94±0.34 | 16.1±1.1 | 0.80±0.10 | |
| Mint (3) | 7.51±0.03 | 4.92±0.17 | 23.5±1.4 | 1.16±0.10 | |
| Smooth mint (3) | 7.51±0.03 | 5.25±0.28 | 23.7±1.1 | 1.25±0.12 | |
aArithmetic M ± SD are shown for products/flavors for which three or more samples were available. Otherwise, results of single sample analyses are shown.
Mean values for single portion weights, moisture content, and TSNA levels per gram dry weight for all products and flavors are provided in Table 1. Among various flavors of Marlboro Snus “slim pack,” rich version had larger pouches and higher moisture content (p < .0001 for both comparisons) than mild/amber, spearmint, and peppermint versions combined (these were not different from each other). The new “round tin” version of Marlboro Snus had larger pouch size and higher moisture content than all flavors of the “slim pack” version (p < .0001 for all comparisons). Pouch weight of the original flavor of Marlboro Snus “round tin” was slightly, but statistically significantly, higher than pouch weight of mint flavor (p = .0094). There were no significant differences in the sum of all four measured TSNA (referred to as total TSNA) levels among various types (slim pack and round tin) and flavors of Marlboro Snus products. Analysis of individual TSNA, however, shows that Marlboro Rich (slim pack) had lower NNK content (p < .0001) and higher NAT levels (p = .0004) than the rest of slim pack flavors. The “round tin” version of Marlboro Snus had higher NNK levels than Marlboro Rich (p = .0069) and other flavors of “slim pack” (p < .0001), and had higher NAT content than nonrich flavors of “slim pack” version (p < .0001). Regional comparisons of total TSNA or the sum of NNN and NNK (carcinogenic TSNA) in Marlboro Snus products revealed no detectable differences (Figure 1A). Among various flavors of Camel Snus, robust and winterchill flavors had larger pouches than mellow and frost (p < .0001). There were no detectable differences among various Camel flavors in moisture or TSNA content. Regional comparisons showed that Camel Snus products purchased in Mid-Atlantic/Appalachia and the Midwest had higher moisture content than those purchased in the Northeast (p = .0068 and p = .0110, respectively) or the Pacific Northwest (p = .0023 and p = .0034, respectively). The sum of carcinogenic NNN and NNK was higher in Camel Snus purchased in the Midwest than in any other region (p < .0001 for the comparisons with Mid-Atlantic/Appalachia, Northeast, Pacific Northwest, and South; and p = .0002 for the comparison with the West; Figure 1B). Temporal comparisons showed that all flavors of Marlboro Snus “slim pack” in this study contained higher levels of the sum of NNN and NNK than the same version of Marlboro Snus purchased in Round I; the sum of these carcinogens was also higher in Camel Snus purchased in Round II than in Round I (p < .0001 for both comparisons).
Figure 1.
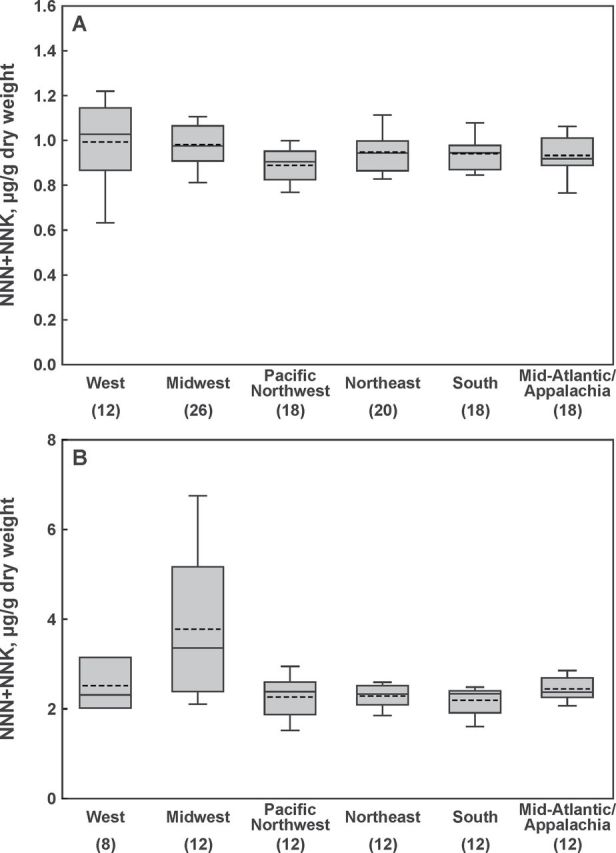
Regional variations in the sum of Nʹ-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-butanone (NNK). (A) Marlboro Snus (all flavors, slim pack and round tin combined); (B) Camel Snus (all flavors combined). Blocks = 95% confidence interval; bars = range; solid lines = median; dashed lines = geometric mean; numbers in parentheses = the number of samples analyzed for a given region.
Mean values for pH and the levels of total and unprotonated nicotine per gram dry weight for all products and flavors analyzed in this study are provided in Table 2. Total nicotine content in Marlboro Snus Rich was higher than in the other flavors of the “slim pack” version and both flavors of the round tin version (p < .0001 for all comparisons). There were no detectable differences in product pH or unprotonated nicotine content among Marlboro Snus products. Regional comparisons of Marlboro Snus products showed that there were no differences in total nicotine content. However, Marlboro Snus purchased in the Midwest had higher pH than Marlboro Snus purchased in any other region (p < .0001 for each comparison), and Marlboro Snus from Pacific Northwest had higher pH than from South (p = .0017). The levels of unprotonated nicotine were also significantly higher in the Midwest than in any other region (p < .0001 for each comparison; Figure 2A). There were no detectable differences in total nicotine, pH, and unprotonated nicotine levels among different flavors of Camel Snus. Total nicotine content in Camel Snus products did not differ by regions. Camel Snus products purchased in the Midwest had lower pH than in Mid-Atlantic/Appalachia (p < .0001), Northeast (p < .0001), or Pacific Northwest (p = .0002), and samples purchased in the West had lower pH than in Mid-Atlantic/Appalachia (p = .0062) and Northeast (p = .0082). The lowest levels of unprotonated nicotine were found in products purchased in the Midwest (p < .0001 for comparisons with Mid-Atlantic/Appalachia and the Northeast, and p = .0005 for comparison with the Pacific Northeast; Figure 2B).
Figure 2.
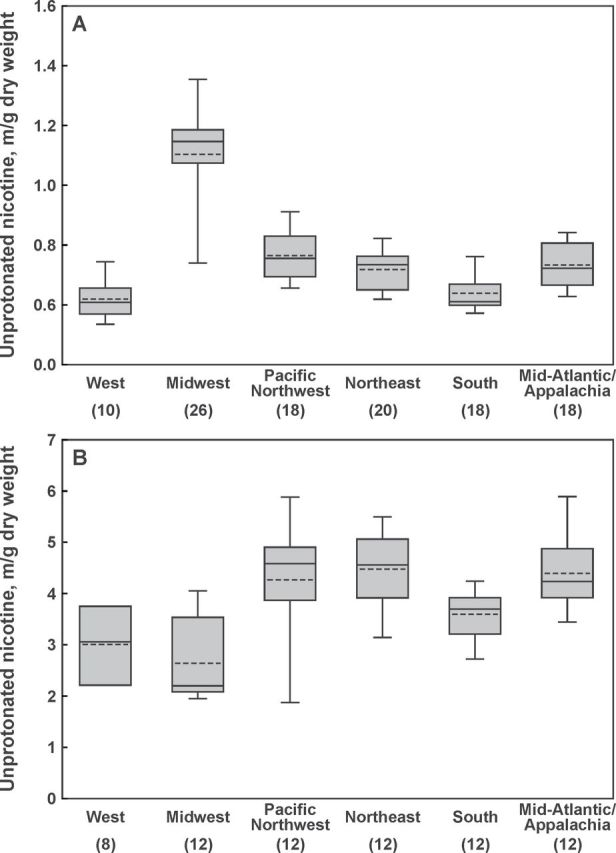
Regional variations in average unprotonated nicotine level. (A) Marlboro Snus (all flavors, slim pack and round tin combined); (B) Camel Snus (all flavors combined). Blocks = 95% confidence interval; bars = range; solid lines = median; dashed lines = geometric mean; numbers in parentheses = the number of samples analyzed for a given region.
Comparison of various products showed that the moisture content and the sum of carcinogenic NNN and NNK were significantly higher in Camel Snus than in Marlboro Snus (p < .0001 for both comparisons). Comparison of dissolvable products with the same brand names, however, revealed that the sum of NNN and NNK in Marlboro Sticks was significantly higher than in Camel Orbs, Sticks, and Strips combined (p < .0001). The levels of NNN and NNK in Skoal Sticks were not different from those found in Marlboro Sticks. Overall, Camel Sticks contained the lowest total TSNA levels among all products analyzed in this study: geometric mean for total TSNA in this product was 0.72 (95% CI = 0.67, 0.77) µg/g dry weight. The highest total TSNA among all products analyzed in this study was found in Marlboro and Skoal dissolvable sticks (p < .0001; Table 1). The levels of TSNA in two available samples of Skoal Snus were also among the highest observed in this study (Table 1). Camel Snus had lower total nicotine content, but higher pH and consequently higher unprotonated nicotine levels than any Marlboro Snus product (p < .0001 for all comparisons). Dissolvable Camel products, however, had significantly lower unprotonated nicotine content than Marlboro Sticks (p < .0001). Skoal Sticks had the lowest pH and the lowest unprotonated nicotine levels among dissolvable products.
DISCUSSION
This study reports the results of chemical analyses performed on a range of novel smokeless tobacco samples purchased in the United States as a part of the Round II of the NPW Project. With this category of tobacco products being continuously modified by the manufacturers, the NPW Project provides a unique opportunity to document the potential temporal and regional variations within and among different brands by periodically collecting samples of novel tobacco products in various regions of the United States and recording their features and chemical composition. The first round of the project demonstrated some variations in TSNA and nicotine content across novel smokeless brands and regionally within the same brand of product, and it also demonstrated some changes in the constituent levels compared with previously reported data for the same products. In this study, we analyzed TSNA and nicotine in snus and dissolvable tobacco products that were purchased in various regions of the country during the spring and summer of 2011.
We observed noticeable shifts in the assortment of novel products available on the market since our first round of purchases. The changes included new flavors and packaging design for existing products, and also appearance of new product brands. For example, in addition to the “slim pack” version of Marlboro Snus that was marketed during our first round of purchases in 2010, a new version of Marlboro Snus was available in this round. The new version comes in two flavors and is packaged in round metal tins, similar to the packaging used for the conventional moist snuff products. The pouches of the “round tin” version are larger and have higher moisture content than those of the “slim pack” version. The reason for this change is not clear. Higher moisture content has been associated with higher pH (Richter, Hodge, Stanfill, Zhang, & Watson, 2008); however, in this study, pH and free nicotine content were not different between the two versions of Marlboro Snus (Table 1). Camel Snus, which underwent several changes in packaging design, with different versions being available for purchase at any given time in the past (Stepanov et al., 2012b), was found in only one latest package version—oval tins containing 15 snus pouches. Moreover, the current packaging includes a statement regarding the pouch size: “15 pouches” for mellow and frost flavors, and “15 large pouches” for robust and winterchill flavors. This is in contrast to the previously reported absence of such information on packages of Camel Snus carrying pouches of different sizes (Stepanov et al., 2012b). It is noteworthy that since their first introduction to the market, the common changes in both Marlboro Snus and Camel Snus include larger pouch size and higher moisture content. These modifications may reflect changes made as a result of the test marketing of earlier versions of these products. It is important to understand to which category of potential customers (current smokers, smokeless tobacco users, or new tobacco users) the latest designs of Marlboro and Camel Snus appeal and how the changes in pouch size and moisture content affect product use and toxicant exposures. The new products purchased in this round included Marlboro Sticks and Skoal Sticks, indicating an expansion of the dissolvable tobacco market.
In addition to the observed changes in the packaging and product inventory, certain changes occurred in the chemical composition of products that were available in both rounds of purchases. For instance, comparison of data obtained for Camel Snus and Marlboro Snus “slim pack” in this study with the analyses of the same products in the previous round of purchases showed that the levels of TSNA increased for both Marlboro Snus and Camel Snus, with increases in both NNN and NNK contributing to these changes (p < .0001 for NNN and NNK increases in both brands). Comparison of dissolvable Camel products, however, shows a different trend: there was a roughly 3-fold increase in NNK levels in Camel Orbs and Camel Strips in the new mint version purchased in 2011 compared with Camel Orbs and Strips purchased in 2010; however, the levels of NNK in Camel Sticks and the levels of other TSNA in all dissolvable Camel products did not change. These variations in TSNA levels within the same products purchased in different years are most likely due to changes in the composition or processing of tobacco used in the manufacturing of these products. The initial reports on TSNA contents in snus and dissolvable tobacco brands marketed in the United States inferred that the manufacturing of these products employs approaches that assure low levels of these carcinogens. However, the observed increases in TSNA levels in the newer versions of most of these products, as well as TSNA levels in the new products such as Marlboro Sticks, Skoal Sticks, and Skoal Snus, which are comparable to the amounts found in conventional moist snuff (Stepanov et al., 2008), caution against automatically classifying snus and dissolvable tobacco as a low-TSNA category of products.
In contrast with the changes in TSNA levels, the levels of unprotonated nicotine were not different within the same product brands purchased in the two rounds, suggesting that the product manufacturers control the amounts of this constituent more carefully than the levels of TSNA. Some other results of this study are also consistent with the results of the previous round. For instance, Camel Snus has higher TSNA, higher pH, and higher unprotonated nicotine levels than Marlboro Snus. Among Marlboro Snus “slim pack” flavors, rich has larger pouches, higher moisture content, lower NNK levels, higher NAT levels, and higher total nicotine content than all other flavors—strikingly consistent with the measurements in the previous round (Stepanov et al., 2012a). Also consistent with the previous observations, pH of dissolvable tobacco products is generally higher than pH of snus products; therefore, despite the relatively low total nicotine content in dissolvable tobacco, the levels of unprotonated nicotine are comparable to those found in snus (Table 2).
Regional variations in constituent levels among novel products were previously observed (Stepanov et al., 2012a) and are of particular interest. For instance, we previously found significant variations in unprotonated nicotine content in Camel Snus samples purchased in different locations (Stepanov et al., 2012a), which we hypothesized was indicative of an important role of these alterations in the manufacturer’s test-marketing strategy. In that study, regional differences in unprotonated nicotine levels in Marlboro Snus were not as drastic as those in Camel Snus. In contrast with those findings, regional variation in unprotonated nicotine levels in Marlboro Snus in this study was roughly 3-fold, with samples purchased in the Midwest containing significantly higher levels of unprotonated nicotine than in other regions (Figure 2A). Unprotonated nicotine in Camel Snus varied only1.7-fold (Figure 2B). Interestingly, the levels of NNN and NNK in Camel Snus samples purchased in the Midwest were significantly higher than in samples purchased in other regions (Figure 1A and B); in our previous study, Marlboro Snus purchased in the same region had the highest levels of NNN and NNK (Stepanov et al., 2012a). The reasons for the higher levels of TSNA observed in samples purchased in the Midwest in both rounds of the NPW Project are not clear. However, it should be noted that the higher levels of TSNA in Camel Snus purchased in the Midwest seem to be driven by the relatively high levels of these constituents in the set of samples (all flavors) purchased in one particular store in Indianapolis, IN. The same was true for the Marlboro Snus samples purchased in the previous round of the NPW—samples obtained from a store in Richmond, MN had relatively high TSNA levels compared with the rest of Marlboro Snus samples purchased in that round. This is an important observation as it indicates that differences in product handling conditions may contribute to differences in TSNA levels in this category of products at the point of sale.
An important outcome of this phase of the NPW Project is that we used our established network and our developed standard protocol to collect samples of novel tobacco products for the second consecutive year. The number of samples obtained in this round of purchases was higher than in the first round, mostly due to the larger number of novel product varieties available on the market in 2011 compared with 2010. Therefore, our model of the nationwide tobacco product monitoring network may be a sustainable and unique resource for the collection of information about the marketing of novel tobacco products in various regions of the country and for the chemical characterization of these products at the point of sale.
In summary, we report here the results of TSNA and nicotine analyses in Marlboro, Camel, and Skoal novel oral tobacco products that were purchased in various regions of the United States between April and July of 2011. We found that the levels of TSNA increased in some products that were previously shown to contain relatively low levels of these carcinogens. In addition, new products Marlboro Sticks, Skoal Sticks, and Skoal Snus contain TSNA at the levels found in the conventional moist snuff (Stepanov et al., 2008). These findings caution against referring to snus and dissolvable tobacco as a low-TSNA category of products. We also found less of the regional variation in the unprotonated nicotine content in Camel Snus compared with the first round of purchases. However, analysis of Marlboro Snus showed larger regional variation in unprotonated nicotine levels than in the past. Our overall observation is that TSNA levels in novel products tend to vary over time, whereas unprotonated nicotine levels tend to vary regionally. The reasons for these variations are not clear, however, it is known that both constituents can be controlled by the manufacturers. Therefore, our findings further stress the importance of tobacco product regulation and the need for the continued monitoring of this category of products at the point of sale as the existing products are being modified and new brands are being introduced.
FUNDING
This study was supported by National Cancer Institute contract HHSN261201100513P, by grants R01-CA141631 and R01-CA135884 from the National Institutes of Health, and by the startup funds from the Masonic Cancer Center, University of Minnesota, to IS.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We would like to thank our monitors who volunteered to help us with obtaining product samples from various U.S. regions. We also thank A. Knezevich for help with tobacco analyses and R. Carlson for editorial assistance.
REFERENCES
- Accortt N. A., Waterbor J. W., Beall C., Howard G. (2002). Chronic disease mortality in a cohort of smokeless tobacco users. American Journal of Epidemiology, 156, 730–737. 10.1093/aje/kwf106 [DOI] [PubMed] [Google Scholar]
- Bates C., Fagerström K. -O, Jarvis M. J., Kunze M., McNeill A., Ramström L. (2003). European Union policy on smokeless tobacco: a statement in favour of evidence based regulation for public health. Tobacco Control, 12, 360–367. 10.1136/tc.12.4.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L., McCausland K., Curry L., Cullen J. (2011). Prevalence of trial of snus products among adult smokers. American Journal of Public Health, 101, 1874–1876. 10.2105/AJPH.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2012). Current tobacco use among middle and high school students - United States, 2011. Morbidity and Mortality Weekly Report, 61, 581–585 Retrieved from www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a1.htm [PubMed] [Google Scholar]
- Delnevo C. D., Waskowski O. A., Giovenco D. P., Bover Manderski M. T., Hrywna M., Ling P. M. (2012). Examining market trends in the United States smokeless tobacco use: 2005–2011. Tobacco Control, 10.1136/tobaccocontrol-2012–050739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Ramstrom L., Burke M., Fagerström K. -O. (2003). Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control, 12, 349–359. 10.1136/tc.12.4.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer R. O., Jr (2011). Oral manifestations of smokeless tobacco use. Otolaryngologic Clinics of North America, 44, 31–56. 10.1016/j.otc.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Hatsukami D. K., Lemmonds C., Tomar S. L. (2004). Smokeless tobacco use: harm reduction or induction approach? Preventive Medicine, 38, 309–317. 10.1016/j.ypmed.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Levy D. T., Mumford E. A., Cummings K. M., Gilpin E. A., Giovino G., Hyland A., . . ., Warner K. E. (2004). The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: estimates of a panel of experts. Cancer Epidemiology, Biomarkers & Prevention, 13, 2035–2042 Retrieved from http://cebp.aacrjournals.org/content/13/12/2035 [PubMed] [Google Scholar]
- Luo J., Ye W., Zendehdel K., Adami J., Adami H. O., Boffetta P., Nyren O. (2007). Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet, 369, 2015–2020. 10.1016/S0140-6736(07)60678-3 [DOI] [PubMed] [Google Scholar]
- McMillen R., Maduka J., Winickoff J. (2012). Use of emerging tobacco products in the United States. Journal of Environmental and Public Health, 2012, 989474. 10.1155/2012/989474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. A., Krugman D. M., Park P. (2008). Under the radar: smokeless tobacco advertising in magazines with substantial youth readership. American Journal of Public Health, 98, 543–548. 10.2105/AJPH.2006.092775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. E., Mowery P., Tomar S., Marcus S., Giovino G., Zhao L. (2006). Trends in smokeless tobacco use among adults and adolescents in the United States. American Journal of Public Health, 96, 897–905. 10.2105/AJPH.2004.061580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascandola M., Augustson E., O’Connell M. E., Marcus S. (2009). Consumer awareness and attitudes related to new potential reduced-exposure tobacco product brands. Nicotine & Tobacco Research, 11, 886–895. 10.1093/ntr/ntp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascandola M., Augustson E., Rose A. (2009). Characteristics of current and recent former smokers associated with the use of new potential reduced-exposure tobacco products. Nicotine & Tobacco Research, 11, 1431–1438. 10.1093/ntr/ntp157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramström L. M., Foulds J. (2006). Role of snus in initiation and cessation of tobacco smoking in Sweden. Tobacco Control, 15, 210–214. 10.1136/tc.2005.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan A. K., Dube S. R., Arrazola R. (2012). Smokeless and flavored tobacco products in the U.S.: 2009 styles survey results. American Journal of Preventive Medicine, 42, 29–36. 10.1016/j.amepre.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Richter P., Hodge K., Stanfill S., Zhang L., Watson C. (2008). Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine & Tobacco Research, 10, 1645–1652. 10.1080/14622200802412937 [DOI] [PubMed] [Google Scholar]
- Richter P., Spierto F. W. (2003). Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine & Tobacco Research, 5, 885–889. 10.1080/14622200310001614647 [DOI] [PubMed] [Google Scholar]
- Rogers J. D., Biener L., Clark P. I. (2010). Test marketing of new smokeless tobacco products in four U.S. cities. Nicotine & Tobacco Research, 12, 69–72. 10.1093/ntr/ntp166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. D., Biener L., Nyman A. L., Crow R. (2010). Building a national surveillance infrastructure for new smokeless tobacco and nicotine products., Baltimore, MD: Society for Research on Nicotine and Tobacco [Google Scholar]
- Savitz D. A., Meyer R. E., Tanzer J. M., Mirvish S. S., Lewin F. (2006). Public health implications of smokeless tobacco use as a harm reduction strategy. American Journal of Public Health, 96, 1934–1939. 10.2105/AJPH.2005.075499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson H. H., Forrester K. K., Biglan A. (2007). Use of smokeless tobacco is a risk factor for cigarette smoking. Nicotine & Tobacco Research, 9, 1331–1337. 10.1080/14622200701705209 [DOI] [PubMed] [Google Scholar]
- Stegmayr B., Eliasson M., Rodu B. (2005). The decline of smoking in northern Sweden. Scandinavian Journal of Public Health, 33, 321–324 [DOI] [PubMed] [Google Scholar]
- Stenbeck M., Hagquist C., Rosén M. (2009). The association of snus and smoking behaviour: a cohort analysis of Swedish males in the 1990s. Addiction, 104, 1579–1585. 10.1111/j.1360-0443.2009.02661.x [DOI] [PubMed] [Google Scholar]
- Stepanov I., Biener L., Knezevich A., Nyman A. L., Bliss R., Jensen J., … Hatsukami D. K. (2012a). Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: findings from Round 1 of the New Product Watch. Nicotine & Tobacco Research, 14, 274–281. 10.1093/ntr/ntr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Jensen J., Biener L., Bliss R. L., Hecht S. S., Hatsukami D. K.(2012b). Increased pouch sizes and resulting changes in the amounts of nicotine and tobacco-specific N-nitrosamines in single pouches of Camel Snus and Marlboro Snus. Nicotine & Tobacco Research, 10.1093/ntr/ntr292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Jensen J., Hatsukami D., Hecht S. S. (2008). New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research, 10, 1773–1782. 10.1080/14622200802443544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo K. K., Ounpuu S., Hawken S., Pandey M. R., Valentin V., Hunt D., … Yusuf S. (2006). Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet, 368, 647–658 [DOI] [PubMed] [Google Scholar]
- Tomar S. L. (2003). Is use of smokeless tobacco a risk factor for cigarette smoking? The U.S. experience. Nicotine & Tobacco Research, 5, 561–569. 10.1080/1462220031000118667 [DOI] [PubMed] [Google Scholar]
- Tomar S. L., Connolly G. N., Wilkenfeld J., Henningfield J. E. (2003). Declining smoking in Sweden: is Swedish Match getting the credit for Swedish tobacco control’s efforts? Tobacco Control, 12, 368–371. 10.1136/tc.12.4.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. H., Wang J. B., Hartman A., Zhuang Y., Gamst A., Gibson J. T., … Galanti M. R. (2009). Quitting cigarettes completely or switching to smokeless tobacco: do US data replicate the Swedish results? Tobacco Control, 18, 82–87. 10.1136/tc.2008.028209 [DOI] [PMC free article] [PubMed] [Google Scholar]


