Abstract
Measuring inflammation-induced changes in thresholds of hind paw withdrawal from mechanical pressure is a useful technique to assess changes in pain perception in rodents. Withdrawal thresholds can be measured first at baseline and then following drug, venom, injury, allergen, or otherwise evoked inflammation by applying an accurate force on very specific areas of the skin. An electronic von Frey apparatus allows precise assessment of mouse hind paw withdrawal thresholds that are not limited by the available filament sizes in contrast to classical von Frey measurements. The ease and rapidity of measurements allow for incorporation of assessment of tactile sensitivity outcomes in diverse models of rapid-onset inflammatory and neuropathic pain as multiple measurements can be taken within a short time period. Experimental measurements for individual rodent subjects can be internally controlled against individual baseline responses and exclusion criteria easily established to standardize baseline responses within and across experimental groups. Thus, measurements using an electronic von Frey apparatus represent a useful modification of the well-established classical von Frey filament-based assays for rodent mechanical allodynia that may also be applied to other nonhuman mammalian models.
Keywords: Neuroscience, Issue 82, Natural Science Disciplines, Life Sciences (General), Behavioral Sciences, mechanical hyperalgesia, mice, electronic pressure meter, inflammation, snake venom
Introduction
Chronic noncommunicable diseases are responsible for a disproportionate share of the human burdens of mortality and morbidity1. Chronic pain is increasingly recognized as an important component of the global burden of disease2. This has provided great impetus to innovations in the prevention, management, and treatment of pain. At the same time, there is abundant evidence that the underlying biology of pain is complex3. The mechanisms underlying protective as well as maladaptive and chronic pain point to intricate interplay between the immune and nervous systems3,4. The elucidation of these mechanisms demands rigorous quantitative, reproducible experimental approaches from molecular dissection to behavioral analysis.
Rodent models have been invaluable in the characterization of molecular players of inflammatory and neuropathic pain5. Reduction in time and intensity of previously specified responses to noxious stimuli are used to measure pain states in these animals as rodent subjects are not otherwise able to report a sensation of pain6. Altered responses to temperature and pressure are commonly used as metrics of tissue sensitivity in rodent models of inflammatory nociception that can be caused by infection7,8, chemical exposure6, 9,10, surgical incision11, as well as neuropathic pain caused by nerve ligation12.
Mechanical sensitivity, or response to pressure stimuli, is often measured using classical von Frey filaments (CvF) - a series (e.g. 0.4-15 g13) of weighted tips manually applied to test tissue in serial increments until pain behaviors are induced in controls vs. experimental subjects. There are various methods for using these tips or hairs to assess mechanical sensitivity6,13; the most frequently used method is as follows. A tip with a certain pressure (within the series; e.g. 2 g13) is selected as a starting point and a rat or mouse's 50% withdrawal threshold (the stimulus intensity required to produce a response 50% of the times the tip is applied to the tissue) is determined by moving up and down in tip weight based on the display of previously specified response behaviors13. Absolute 50% withdrawal thresholds for treatment groups are then compared between baseline and post-treatment time points. This manual technique is quite time-consuming. In addition to increased time spent by each subject confined in a von Frey chamber, this also increases the number of times that test tips of various weights are applied to inflamed tissue causing greater discomfort to the rodents in the study. Furthermore, 50% withdrawal thresholds are calculated and compared between groups of rodents; the individual responses of experimental subjects are typically not normalized to their individual untreated baseline responses with, to our knowledge, one exception6. As a consequence, rodent subjects are not usually reported as assigned to treatment groups on the basis of consistent baseline responses within a previously determined range and neither are individual subjects evaluated on the basis of a consistently applied set of exclusion criteria that allow for baseline responses to be standardized for any particular set of studies and assays.
Here, we present an alternative method for measuring mouse hind paw tactile sensitivity following exposure to Bothrops jararaca venom using an electronic von Frey apparatus (EvF). Cunha and colleagues6 have pioneered the use of EvF techniques in rodents and systematically compared CvF and EvF efficacy to demonstrate that EvF provides "a more sensitive, objective and quantitative" measure of tactile sensitivity in rodent models of inflammatory pain. Briefly, in EvF measurements, a nonhygroscopic polypropylene von Frey tip of uniform diameter (0.8 mm) of suitable rigidity for the tissue being tested is attached to an electronic probe and readout that displays the force/weight at which the rodent retracts from the tip. There are several important differences between CvF and EvF measurements. First, the process of measuring both baseline and experimental responses using EvF is greatly streamlined as pressure on the hind paw can be applied with increasing force until there is a response and there is no need to change tips as in CvF; animals spend less time confined in the assay chambers and are therefore less stressed and more comfortable during an experiment. Second, EvF allows for relative ease of implementation of rational exclusion criteria for study subjects in the form of ranges of acceptable baseline thresholds. This allows for standardization of baseline responses within and across treatment groups. Third, a subject's response following an experimental treatment is always compared to its own individual baseline response creating important internal controls for measurements. Fourth, the accuracy of the withdrawal thresholds is recorded at a higher level of resolution as pressure can be applied continuously rather than in increments available in the form of weights of manual filaments. As with well-designed CvF assays, EvF-based experiments are also carried out with the same investigator (blind to treatment) applying the force and recording the response behaviors. It is important to note that this technique requires time and effort on the part of investigators to establish internal consistency of measurements and requires experiments to be planned such that appropriate allowances are made for mice that may have to be excluded due to inconsistent baseline responses.
An easy to implement, effective, well-controlled assessment of mechanical sensitivity facilitates the addition of behavioral assessment to models of inflammatory events such as envenomation - the example we have used here to illustrate the capacities and power of EvF measurements in the measurement of changes in tactile sensitivity. We found that male ND4 Swiss mice showed an enhanced mechanical sensitivity following injection of venom derived from the South American pit viper Bothrops jararaca in the hind paw. Behavioral responses were accompanied by edema, influx of neutrophils, and mast cell degranulation in the affected tissue.
Protocol
Macalester College's Institutional Animal Care and Use Committee (IACUC) approved all protocols. 8-12 week-old male ND4 Swiss mice were used for these experiments.
1. Bothrops jararaca Venom Preparation and Injection
CAUTION: B. jararaca venom is toxic. Wear gloves, mask, and eye protection when handling the powder and preparing solutions.
Administer 45 µg/kg B. jararaca venom (regimen determined by earlier dose-response experiments) or 0.9% saline vehicle (10 µl) via intra-plantar injection into both hind paws using 3/10 ml insulin syringes with 29 G x 1/2 in needles. Note: Depending on experimental design, treatment and control solutions can be separately injected into each hind paw respectively to provide internal controls within each subject.
2. Electronic von Frey Apparatus Setup and Operation
Place the electronic von Frey unit onto the probe stand. Ensure that the probe and cable are appropriately connected to the display unit, and turn the instrument on. Set the readout to zero (0.00 g) in the setup mode, and gently place the provided test weight (5.00 g) onto the cone of the von Frey probe.
Record the readout in a laboratory notebook, and repeat this procedure upon finishing the testing session. If there is a discrepancy greater than 0.20 g between your test weight readouts before and after the testing session, or if the readout fluctuates once the test weight is applied, recalibration of the apparatus (by the manufacturer) may be necessary.
To assess withdrawal threshold of the mouse hind paw, use the appropriate polypropylene tip from among those supplied with the probe. Tip choice depends on the tissue being probed. Rigid tips work well for the less sensitive, tougher hind paw tissue while semi-flexible tips may be a better choice for a more tender area such as the ano-genital ridge (Chatterjea, unpublished). Gently mount the rigid tip onto the cone of the probe.
To record the maximum applied pressure, switch the operating mode from setup to operation such that the maximum applied pressure will be recorded on the display. In this mode, the maximum applied pressure readout will be saved on the display screen when the probe is retracted.
3. General Considerations for Hind Paw Withdrawal Threshold Assessment
Ideally, two investigators are needed to measure hind paw withdrawal threshold (mechanical sensitivity) using an EvF apparatus - one to operate the apparatus and carry out the measurements, and another to transcribe the recorded measurements and handle mice.
In order to reduce bias, the investigator operating the apparatus should be blinded to treatments and to the recorded withdrawal threshold values. Furthermore, to ensure consistency between measurements, the same individual should assess all baseline and experimental withdrawal thresholds.
Perform all measurements in a quiet, temperature-controlled room, and at the same time of day14.
The experimenter recording measurements should place each mouse into individual chambers of multiunit polymethyl methacrylate (PMMA) housing set on a mesh floor stand. Cover the chambers with perforated PMMA lids, and place a heavy object on top, to prevent mice from escaping. Place absorbent material below the stand to absorb/collect excreted waste. Allow mice to acclimate for 15 min in chambers prior to baseline or experimental withdrawal threshold assessment 6,7,10.
The hind paws of the mice have to be easily accessible through the openings of the mesh floor; therefore, place the mesh floor stand at a comfortable height on a cart with brakes, or on a steady benchtop that is accessible from all sides.
During measurements, mice may have to be distracted with a food pellet15 placed just outside the measurement chamber or a very slight noise produced by tapping or running a pen along the wire mesh of the floor of the chamber before, but not during, the measurement. This induces the mouse to hold still enabling the experimenter to begin taking measurements more easily.
4. Baseline Hind Paw Withdrawal Threshold Assessment
The first set of baseline measurements should be performed 48 hr prior to administering treatment. Zero the probe upon mounting the rigid tip. Using two hands, raise the probe slowly to stimulate the middle (footpad area) of either the left or the right hind paw (if both paws received the same treatment).
Increase pressure gradually until defined nociceptive response behaviors such as hind paw retraction, hind paw licking, or four-paw jumping6,10 are observed. Instruct the other investigator to transcribe the pressure that elicited the observed behavior.
Measure the withdrawal threshold of the mouse situated in the adjacent chamber. Continue until you have assessed withdrawal thresholds of each mouse once. Repeat another four times, so that each mouse is measured 5x as previously described10.
For each mouse, calculate the median value of the 5 measurements, and then calculate how much each value deviates from the median. Select 3 values that deviate the least, and average them to obtain a baseline withdrawal threshold as previously described10. In this demonstration, measurement has been standardized by using 3 out of 5 values; while the specific numbers of measurements can vary by experimental design, it is important to ensure that all mice are poked an equal number of times to assess baselines.
Repeat the baseline measurements 24 hr later. Take 5 measurements per mouse, and average the 3 that are closest to the median to obtain a baseline withdrawal threshold.
To calculate the average baseline withdrawal threshold of a mouse, average its baseline withdrawal thresholds taken at 48 and 24 hr prior to treatment. If the average baseline withdrawal threshold is below 3.50 g, or if the two baseline thresholds differ by 2.00 g or more, exclude the mouse from the experiment. These criteria ensure that all mice in the experiment have consistent baseline responses, and sufficiently high baseline withdrawal thresholds that allow observing lower thresholds following treatment.
Note: The exclusion threshold will depend on the tissue being probed, the strain of animals studied, and the type of tip used. For example, we have found 3.5 g to be a useful benchmark for ND4 hind paw tissue but more tender areas such as the ano-genital ridge need a lower exclusion threshold (Chatterjea, unpublished).
Assign mice into treatment groups so that the average withdrawal baseline of the experimental groups is as similar as possible before treatments are administered.
Note: Baseline measurements can be performed prior to 48 hr before an experiment. However, at least 2 sets of baseline measurements at regular intervals before the start of treatments should be completed.
5. Experimental Hind Paw Withdrawal Threshold Assessment
Assess hind paw withdrawal threshold (mechanical sensitivity) following treatment at appropriate time points determined by the design of the experiment. Return mice to their cages with access to food and water promptly after measurements are completed.
Follow steps outlined above; take 3 instead of 5 measurements to decrease the possibility of over-stimulating the (treated) hind paw tissue especially if sensitivity is to be assessed at multiple time points. If any two measurements out of the taken three differ by more than 2.00 g, take a fourth measurement.
Calculate the average of 3 measurements to obtain experimental withdrawal threshold for each mouse. For any mice for which 4 measurements were taken, compute the average of three that deviate least from the median, in order to obtain experimental withdrawal threshold.
To obtain delta withdrawal threshold for each mouse at a particular time point, subtract the baseline from the experimental withdrawal threshold. Repeat for all mice and all experimental time points9.
Perform appropriate statistical analysis on collected data. Here we used JMP 9.0 (SAS, Cary, NC) to carry out One-way ANOVA and Tukey HSD post hoc analysis.
Representative Results
Local B. jararaca venom administration causes changes in hind paw tactile sensitivity and tissue edema in ND4 Swiss male mice.
1.5 hr after the administration of 45 µg/kg B. jararaca venom, ND4 Swiss male mice withdrew their hind paws at a pressure 3.0 g lower than baseline (Figure 1A). Baseline withdrawal for all groups occurred at ~4.5 g of applied pressure; after venom-treatment, experimental mice withdrew their hind paws at 1-1.5 g of applied pressure (data not shown). Saline-treated control mice showed little to no reduction in response to pressure compared to baseline at all time points tested. Decreased response thresholds in treated mice were clearly maintained at 3 and 6 hr post-envenomation (Figure 1A) and were indistinguishable from controls by 24 hr (data not shown). The hind paws of envenomated mice showed tissue edema characteristic of inflammation. At 1.5 hr post venom administration, treated hind paws, measured as previously described10, were 1.5 mm thicker than at baseline; edema was sustained through 3 and 6 hr (Figure 1B). In contrast, saline treated paws showed no swelling or change in thickness through the experimental period. For the representative experiment shown here, a total of 21 mice were used for baseline measurements and 14 selected for inclusion in the experiment. These were assigned to treatment groups such that average withdrawal responses at baseline were around 4.5 g for each group as a whole.
B. jararaca venom-induced changes in tactile sensitivity are accompanied by mast cell degranulation and neutrophil influx into the hind paws of ND4 Swiss male mice.
In addition to increased tactile sensitivity and tissue edema, we also assessed neutrophil influx (Figures 2A-B) and mast cell degranulation (Figures 2C-D) in the hind paws of envenomated vs. saline-treated mice. Neutrophils are the first inflammatory cells to be recruited into a lesion from the circulation and are known to mediate inflammatory nociceptive responses10,16,17. Mast cells are tissue-resident sentinel cells18 and mast cell degranulation has been associated with nociceptive responses in the hind paw of mice10 and the dura mater of rats19.
Here, we found that neutrophil influx into the envenomated hind paws was increased almost 1,000-fold in comparison to saline-treated hind paws (Figure 3A) as evaluated by counting hematoxylin-eosin stained 4 µm paraffin embedded sagittal plantar tissue sections10 harvested at 1.5 hr post venom administration from euthanized mice.
In saline treated paws, plantar mast cells in the hind paw tissue were largely intact or showed mild degranulation (Figure 3B). In contrast, envenomated hind paws showed 15-20% moderate and extensive degranulation of mast cells (Figure 3B). Tissue sections described above were stained with toluidine blue and numbers of visible granules counted per mast cell observed to ascertain degranulation status as previously described10.
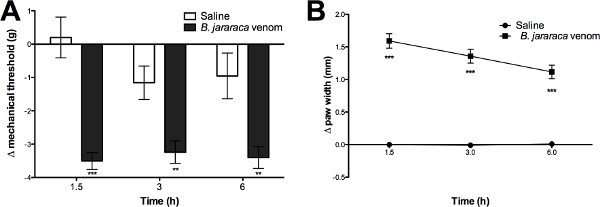 Figure 1. Local B. jararaca venom administration causes changes in hind paw mechanical sensitivity, and tissue edema in ND4 Swiss male mice. Baseline withdrawal thresholds and baseline paw width were assessed at 48 and 24 hr prior to intra-plantar B. jararaca venom (Bjv; 45 µg/kg) or vehicle (saline) treatment (10 µl). Withdrawal thresholds were assessed at 1.5, 3, and 6 hr following treatment (A). Average paw width (mean width of the left and the right paw of a mouse) at the mid-plantar region was assessed using digital calipers at baseline and then 1.5, 3, and 6 hr following treatment to determine tissue edema as previously described10 (B). Significances are compared to Sal-treated mice (* = p < 0.05, ** = p < 0.01, *** = p < 0.001 respectively). n = 10-11 mice per treatment group. Click here to view larger image.
Figure 1. Local B. jararaca venom administration causes changes in hind paw mechanical sensitivity, and tissue edema in ND4 Swiss male mice. Baseline withdrawal thresholds and baseline paw width were assessed at 48 and 24 hr prior to intra-plantar B. jararaca venom (Bjv; 45 µg/kg) or vehicle (saline) treatment (10 µl). Withdrawal thresholds were assessed at 1.5, 3, and 6 hr following treatment (A). Average paw width (mean width of the left and the right paw of a mouse) at the mid-plantar region was assessed using digital calipers at baseline and then 1.5, 3, and 6 hr following treatment to determine tissue edema as previously described10 (B). Significances are compared to Sal-treated mice (* = p < 0.05, ** = p < 0.01, *** = p < 0.001 respectively). n = 10-11 mice per treatment group. Click here to view larger image.
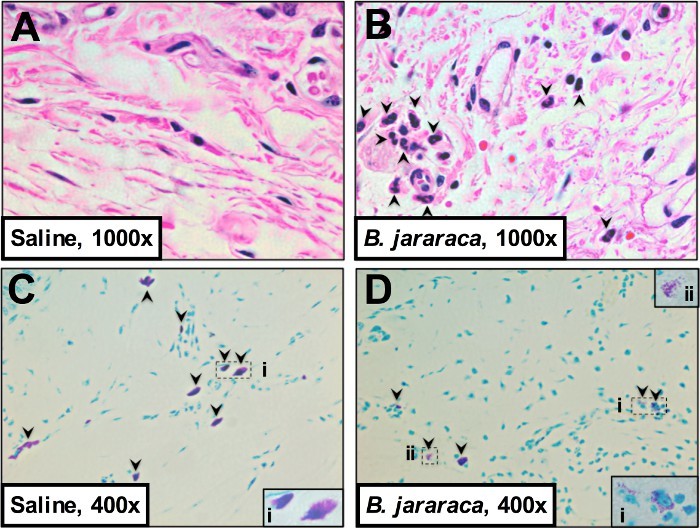 Figure 2. B. jararaca venom-induced mechanical sensitivity is accompanied by mast cell degranulation and neutrophil influx into the hind paws of ND4 Swiss male mice. 3 mice/treatment were euthanized by CO2 at 1.5 hr post treatmentand their hind paws were excised, stored in 10% formalin for 24 hr, and transferred to 70% ethanol, decalcified for 1-2 weeks in 15% EDTA, and embedded in paraffin. 4 µm sagittal sections were stained with hematoxylin-eosin (A-B) and toluidine blue (C-D) for visualizing neutrophils (A-B) and mast cells (C-D), respectively. Images were acquired at 400X and 1,000X as annotated. Click here to view larger image.
Figure 2. B. jararaca venom-induced mechanical sensitivity is accompanied by mast cell degranulation and neutrophil influx into the hind paws of ND4 Swiss male mice. 3 mice/treatment were euthanized by CO2 at 1.5 hr post treatmentand their hind paws were excised, stored in 10% formalin for 24 hr, and transferred to 70% ethanol, decalcified for 1-2 weeks in 15% EDTA, and embedded in paraffin. 4 µm sagittal sections were stained with hematoxylin-eosin (A-B) and toluidine blue (C-D) for visualizing neutrophils (A-B) and mast cells (C-D), respectively. Images were acquired at 400X and 1,000X as annotated. Click here to view larger image.
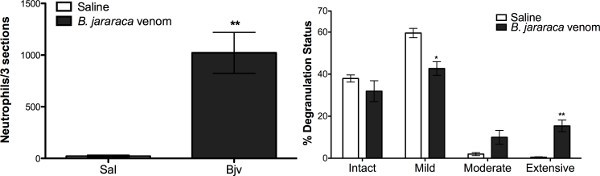 Figure 3. B. jararaca venom-induced mechanical sensitivity is accompanied by significant increases in mast cell degranulation levels and neutrophil influx into the hind paws of ND4 Swiss male mice. Neutrophils and mast cells on stained tissue sections were counted using an Olympus CX21LED microscope. Neutrophils were counted in 3 hind paws per treatment from the heel to the toes in adjacent fields of view at 400X magnification (A). Mast cells were counted in 3 footpads/treatment and their degranulation status was scored based on the number of visible granules outside of the cell boundary: intact (0), mild (0-10), moderate (10-20), extensive (21+), as described previously10 (B). To avoid counting the same cells twice, the investigators assessing neutrophil numbers and mast cell degranulation levels in the hind paw sections used a grid inscribed in the eyepiece reticle. Investigators were blinded to treatments. Significances are compared to Sal-treated mice (* = p < 0.05, ** = p < 0.01, *** = p < 0.001 respectively). n =3 mice per treatment group. Click here to view larger image.
Figure 3. B. jararaca venom-induced mechanical sensitivity is accompanied by significant increases in mast cell degranulation levels and neutrophil influx into the hind paws of ND4 Swiss male mice. Neutrophils and mast cells on stained tissue sections were counted using an Olympus CX21LED microscope. Neutrophils were counted in 3 hind paws per treatment from the heel to the toes in adjacent fields of view at 400X magnification (A). Mast cells were counted in 3 footpads/treatment and their degranulation status was scored based on the number of visible granules outside of the cell boundary: intact (0), mild (0-10), moderate (10-20), extensive (21+), as described previously10 (B). To avoid counting the same cells twice, the investigators assessing neutrophil numbers and mast cell degranulation levels in the hind paw sections used a grid inscribed in the eyepiece reticle. Investigators were blinded to treatments. Significances are compared to Sal-treated mice (* = p < 0.05, ** = p < 0.01, *** = p < 0.001 respectively). n =3 mice per treatment group. Click here to view larger image.
Discussion
The measurement of changes in withdrawal responses is an important tool used to assess changes in tactile sensitivity in rodent models of pain and inflammation. Here we demonstrate the utility of an electronic von Frey apparatus to perform this assessment. There are several advantages to this technique: i) we can rapidly assess withdrawal responses to applied pressure for multiple mice at multiple time points that are fairly close together, ii) we can use a single tip to apply continuous and specific pressure to a small area of tissue, iii) we can accurately measure the precise pressure applied to obtain a withdrawal response, and iv) we can easily implement robust baseline cutoffs, exclusion criteria, and internal controls to ensure that behavioral assessments are as precise and objective as possible.
The critical steps to ensure best outcomes for an EvF assay are prior calibration of the instrument and consistent measurement practices. As detailed in the protocol section, the instrument needs to be handled with care and proper calibration ensured before and during use. Before any measurements are taken, the test weight readings must be confirmed to ensure optimal machine function. If the readings fluctuate significantly or if the test weight readings are not accurate, the manufacturer should be promptly contacted to calibrate the apparatus. With this in mind, having a backup electronic von Frey apparatus is advisable, if possible, so as not to compromise time-sensitive experiments due to an unexpected need for calibration of the instrument. Further, a single, well-trained investigator should take all baseline and experimental measurements for a given study or series of experiments while remaining blinded to treatment groups. Mice should be evaluated in a quiet room, held at a constant temperature, acclimated to the EvF chambers for at least 15 min but not restrained in the chamber any longer than necessary (especially if multiple measurements are taken within a single day) so as to minimize stress and discomfort-induced behavioral variations.
EvF measurements offer significant improvements with respect to the existing CvF techniques. Researchers can apply continuous pressure and do not have to switch between tips of various weights as is done in CvF filament experiments6. This leads to fewer pokes on the tissue, which is especially relevant in the case of inflamed or injured tissue. Precise pressure at which withdrawal occurs can be measured vs. using 50% withdrawal measurements that are also limited by available filament weights used in CvF measurements13. Because rational and uniform exclusion criteria can be implemented (as discussed above), mice can be assigned to experimental groups such that all groups have similar average baselines at the beginning of the experiment. EvF measurements assess the changes in behavioral response to a given stimulus, and not the absolute responses themselves. Therefore each mouse's change in nociceptive response is measured as the difference between its individual experimental and baseline withdrawals thus providing internal controls for every measurement taken.
Perhaps the most significant limitation of EvF is that, similar to its manual counterpart, the same researcher must take all measurements for a particular series of baseline and experimental measurements. This makes scaling up of the technique challenging from the standpoints of time, personnel and equipment. We find that ~20 mice at a time (depending on the configuration of assay chambers) can be measured in 30-45 min or less at multiple timepoints provided there are at least 45 min between measurements. A related consideration in this regard is the learning curve of individual experimenters. Internal consistency has to be established before an individual researcher can embark on a full-scale experiment and this requires many hours of dedicated practice involving repeated baseline response measurements until the desired consistency is reached. In addition, using baseline cutoffs implies that not all mice baselined for an experiment can be included; we find that 25-30% of mice baselined per experiment are typically excluded.
In terms of modifications and future applications, EvF behavioral measurements add a powerful dimension to a wide range of models of inflammation to analyze concomitant acute and/or chronic pain that can underlie inflammatory pathologies. In envenomation, we found that pronounced, rapid-onset changes in tactile sensitivity accompanied the expected outcomes of tissue swelling, leukocyte recruitment and mast cell degranulation in the hind paws of ND4 male mice. These findings corroborate previous observations about the contributions of mast cell mediators to thermal sensitivity in rats20 and mechanical sensitivity induced by B. jararaca venom in ND4 Swiss mice measured using CvF21. The EvF protocol can easily be modified for use on tissues other than the hind paw e.g. abdomen7 and ano-genital ridge8 where CvF is currently used. Rodent models of allergies, wound healing, neuropathies and other pathologies can be easily adapted to include measures of mechanical sensitivity in tissues including and beyond the hind paw.
In summary, EvF is a useful and effective technique that updates a classical approach of measurement of nociception in rodents and is an important new tool in probing the complex underlying mechanisms of pain in a wide array of disease models.
Disclosures
The authors have no competing financial interests to disclose.
Acknowledgments
NIH R15 NS067536-01A1 (to DC) and the Macalester College Wallace Research Fund (to DC) supported these studies. Macalester College Library supported the open access publication of this article. In addition, we thank current and former Chatterjea lab members for their support.
References
- Meetoo D. Chronic diseases: the silent global epidemic. Br J Nurs. 2008. pp. 17–21. [DOI] [PubMed]
- Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11 doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. What is this thing called pain. J. Clin. Invest. 2010;120(11):3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009;89(2):707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, et al. An electronic pressure-meter nociception paw test for mice. Braz. J. Med. Biol. Res. 2004;37(3):401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast Cell-Derived Histamine Mediates Cystitis Pain. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MA, et al. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci. Transl. Med. 2011;3(101) doi: 10.1126/scitranslmed.3002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102(4):937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- Chatterjea D, et al. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem. Biophys. Res. Commun. 2012;425:237–243. doi: 10.1016/j.bbrc.2012.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SM, et al. Involvement of mast cells in a mouse model of postoperative pain. Eur. J. Pharmacol. 2011;672(1-3):88–95. doi: 10.1016/j.ejphar.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Wu A, Moalem-Taylor G. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J. Vis. Exp. 2012. [DOI] [PMC free article] [PubMed]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Akunne HC, Soliman KF. Serotonin modulation of pain responsiveness in the aged rat. Pharmacol. Biochem. Behav. 1994;48(2):411–416. doi: 10.1016/0091-3057(94)90545-2. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. J. Vis. Exp. 2012. [DOI] [PMC free article] [PubMed]
- Cunha TM, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc. Biol. 2008;83(4):824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- Lavich TR, et al. Neutrophil infiltration is implicated in the sustained thermal hyperalgesic response evoked by allergen provocation in actively sensitized rats. Pain. 2006. pp. 125–121. [DOI] [PubMed]
- Galli SJ, et al. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1-2):166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavita AG, et al. Contribution of mast cells and snake venom metalloproteinases to the hyperalgesia induced by Bothrops jararaca venom in rats. Toxicon. 2006;147(8):885–893. doi: 10.1016/j.toxicon.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Zychar BC, Dale CS, Demarchi DS, Gonçalves LR. Contribution of metalloproteases, serine proteases and phospholipases A2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon. 2010;55(2-3):227–234. doi: 10.1016/j.toxicon.2009.07.025. [DOI] [PubMed] [Google Scholar]


