Abstract
Harderian gland tumors are extremely rare in female F344 rats. An expansive enlarging lesion of the Harderian gland with compression, distortion and invasion of the surrounding muscle was found in a 110-week-old female F344/DuCrj rat, which was diagnosed as a Harderian gland adenocarcinoma. Epithelial growth patterns such as glandular, lobular, papillary and duct forming patterns were exhibited in most areas of the tumor. The tumor cells were pleomorphic and atypical. In one part of the tumor, poorly differentiated areas were found. This case was observed in the middle dose group of a carcinogenicity study of diphenylamine, which was not carcinogenic, we determine to be this case was a spontaneous tumor.
Keywords: Harderian gland, adenocarcinoma, adenoma, rat, F344, spontaneous tumor
The carcinogenic activities of many chemicals have been tested in 2-year carcinogenicity studies using rats and mice1. Spontaneous Harderian gland tumors in several strains of rats have been described in the literature2,3,4,5, but Harderian gland tumors are extremely rare in F344 rats6,7,8,9,10. According to the historical control data of the National Cancer Institute (NCI) and National Toxicology Program (NTP) of the USA published in 1984, only 1 case of a Harderian gland adenoma and 1 case of a Harderian gland adenocarcinoma occurred in 2320 control F344/N male rats, and no cases were reported in 2370 control females9. In the NCI/NTP data updated in 1998, no cases were reported in 2259 male rats, and 2 cases of Harderian gland adenoma were reported in 2254 female rats10. According to the historical control data on F344/DuCrj rats of the Japan Bioassay Research Center (JBRC), Harderian gland adenomas occurred in 3 of 2748 male rats and 2 of 2547 female rats, and Harderian gland adenocarcinoma occurred in 1 male rat and 0 female rats (data unpublished).
F344/DuCrj rats were obtained from Charles River Laboratories Japan Inc. (Kanagawa, Japan) and used in a 2-year carcinogenicity study. All rats were housed singly in stainless steel wire cages in environment-controlled barrier system rooms maintained at a temperature of 24 ± 2°C with a humidity of 55 ± 10% under a 12 hour light/dark cycle, fed a commercial diet (CRF-1; Oriental Yeast Co., Ltd.) and provided with drinking water sterilized by ultraviolet light ad libitum. Rats were necropsied at the age of 110 weeks or when found dead or in a moribund condition. All necropsies were carried out on anesthetized rats in accordance with the Guide for the Care and Use of Laboratory Animals. All organs and tissues were collected and fixed in 10% buffered formalin. Histological sections for Harderian glands were obtained by longitudinal section through the eyeball, optic nerve and Harderian gland and stained with hematoxylin and eosin (H&E).
A female rat that had a Harderian gland nodule was found in the middle dose (1000 ppm) group on a carcinogenicity study of diphenylamine administered in diet at the age of 110 weeks11. The nodule was stained with H&E, Periodic acid-Schiff (PAS), Berlin blue and Masson’s trichrome stains, and tested immunohistochemically for alpha smooth muscle actin [SMA, monoclonal, DAKO, M0851, diluted 1:200, antigen retrieval: microwave oven, distilled water (DW), detected using DAB], vimentin (monoclonal, Dako, M725, 1:100, microwave oven, DW, DAB) and keratin (wide spectrum, polyclonal, Dako, Z0622, 1:400, DAB).
Exophthalmos was not found in a clinical observation. A nodule in the Harderian gland was observed during necropsy after sacrifice. The nodule expanded into the retro-orbital space with transition to the normal Harderian gland, indicating that it had originated in the Harderian gland. The nodule was white, 5 mm in diameter and spherically-shaped (Fig. 1). It was not adhesion to the orbit of the eye and was easily removed.
Fig. 1.
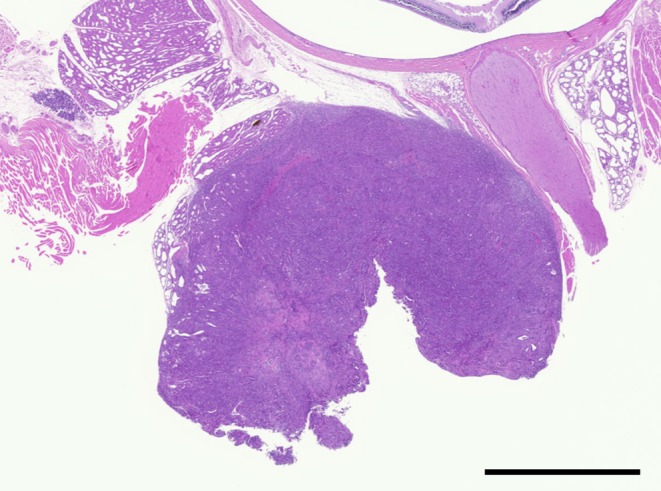
Low magnification of the Harderian gland tumor. The tumor was spherically shaped. H&E, bar = 2 mm.
Histologically, the tumor was continuous with the normal Harderian gland. It exhibited expansive growth, resulting in compression of the surrounding tissue. The border between the tumor and the normal Harderian gland was well-demarcated. The tumor manifested an epithelial growth pattern in most regions and poorly differentiated sarcomatous growth in several small regions. The tumor epithelium was stratified with a basal membrane positive for PAS staining. The epithelial regions were comprised of glandular, lobular, papillary and tubular growth patterns, with the papillary growth pattern being the most prominent (Fig. 2). Tumor cells proliferated forming solid sheets, and were arranged in tubular or glandular-like structures. The tumor consisted of cuboidal or columnar epithelial cells and had structural atypia. Tumor cells were pleomorphic with cellular atypia. The tumor cell density was high, and the cells had vacuolated cytoplasm, eccentrically round to oval nuclei or giant nuclei and prominent nucleoli. Numerous mitotic figures were observed. The tumor cells invaded the surrounding muscle (Fig. 3), but metastases were not found in other organs. This lesion was diagnosed as a Harderian gland adenocarcinoma. In addition, the other parts of the Harderian gland were normal, and the rest of rats neither found hyperplastic lesions nor tumor of the Harderian gland in this study.
Fig. 2.
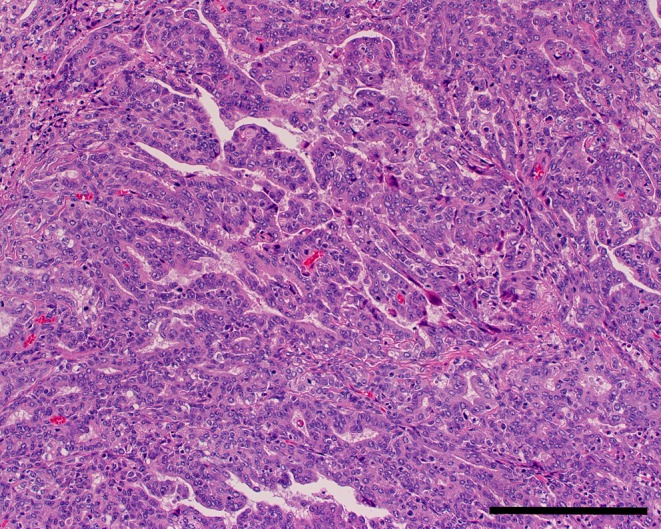
Epithelial growth area (papillary growth). H&E, bar = 200 μm.
Fig. 3.
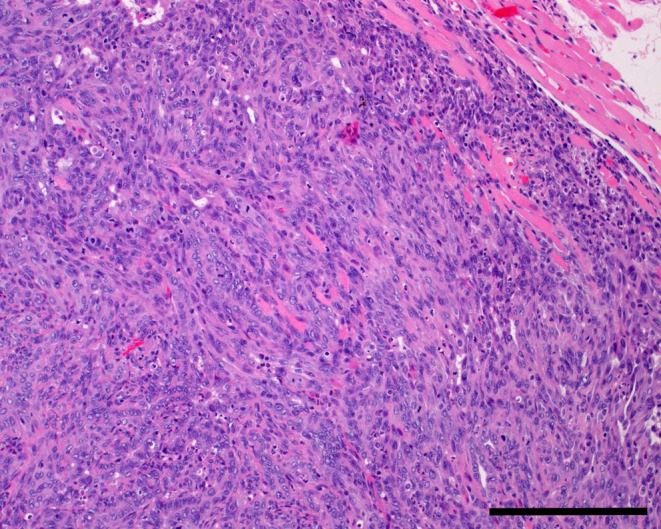
Muscle invasion by the tumor. H&E, bar = 200 μm.
Some glandular cavities in the tumor tissue contained brown pigment. They were negative for Berlin blue stain. Therefore, the pigment was concluded to be porphyrin pigment from the Harderian gland.
In some areas of the tumor, spindle-shaped cells with eosinophilic cytoplasm and spindle-shaped nuclei were present. Typically, the epithelial growth areas of the tumor were surrounded by spindle-shaped cells (Fig. 4). Since spindle-shaped cells were positive with SMA (Fig. 5), they were determined to be myoepithelial cells. This suggests that these cells merely proliferated due to the occurrence of adenocarcinoma, but that their growth pattern was nonneoplastic.
Fig. 4.
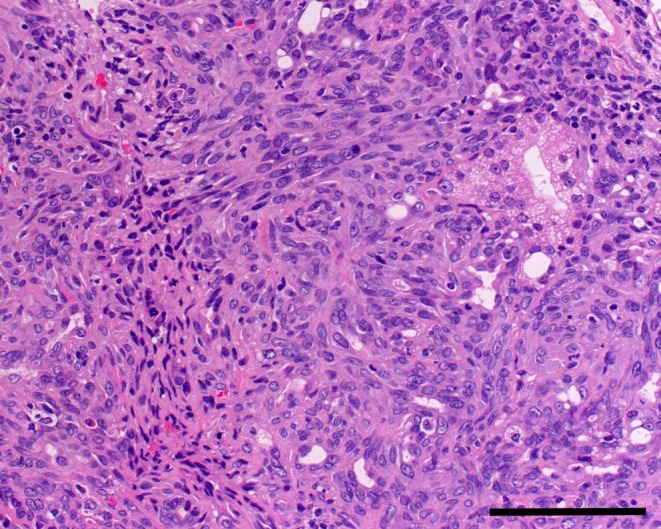
Sarcoma-like growth area (spindle-shaped cells) around a glandular growth area. H&E, bar = 100 μm.
Fig. 5.
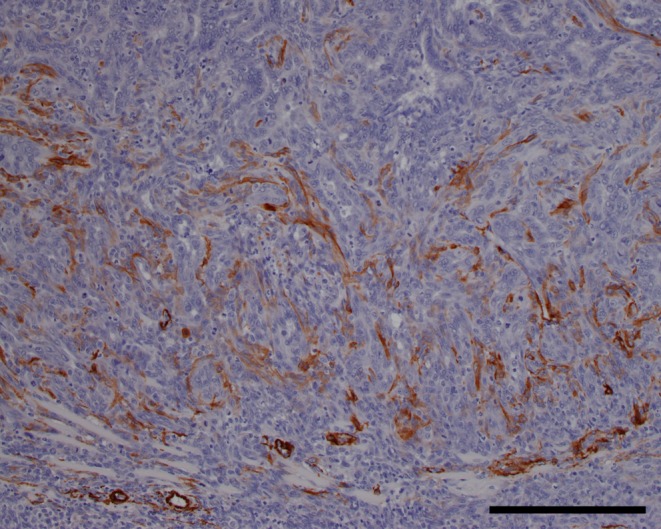
Immunohistochemical stain. Alpha smooth muscle actin, bar = 200 μm.
Solid sarcomatous growth areas were also observed in the tumor tissue. Necroses were observed in these solid sarcomatous growth areas. Cells of the sarcomatous growth areas were pleomorphic and atypical. Moreover, they were positive for both vimentin and keratin (Table 1, Fig. 6). This suggests that the tumor cells transitioned into poorly differentiated cells or mesenchymal-like cells.
Table 1. Immunohistochemistry of the Tumor.
Fig. 6.
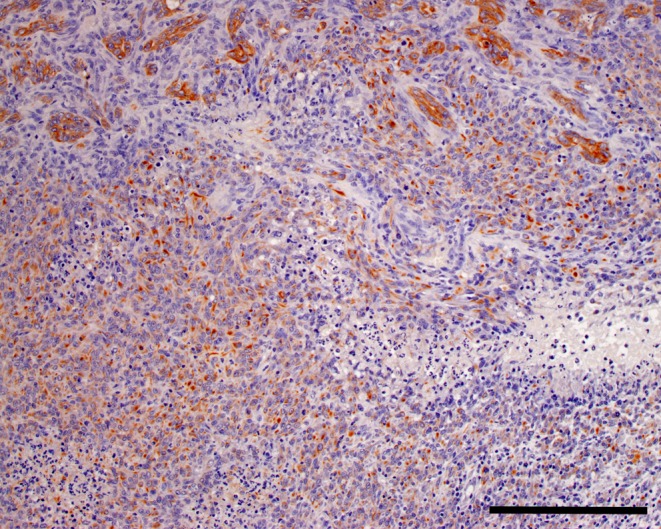
Immunohistochemical stain. Keratin, bar = 200 μm.
Although there is no histological classification of Harderian gland tumors in rats, Harderian gland adenoma in mice can be classified into papillary, cystic papillary, acinar and cystic types12, 13. This case appears to correspond to the papillary type. However, it was an adenocarcinoma, not an adenoma.
In conclusion, this case was diagnosed as a tumor originating from the Harderian gland. The tumor was composed primarily of papillary adenocarcinoma cells that were pleomorphic with cellular atypia. The tumor had a solid sheet growth pattern and stratified epithelium. In addition, it contained sarcomatous cells. Therefore, it was diagnosed as a Harderian gland adenocarcinoma.
Some chemicals are known to induce tumors in multiple organs in rats and mice, and Harderian gland tumors can be caused by the administration of such chemicals as N-ethyl-N-nitrosourea and urethane14,15,16 in mice. However, there are no reports of Harderian gland tumors in rats induced by chemical agents. Since this case was observed in a rat in the middle dose (1000 ppm) feeding group of a two-year carcinogenicity study of diphenylamine11, which was not carcinogenic, we conclude that this case was a spontaneous tumor.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Ward JM. The Two-Year Rodent Carcinogenesis Bioassay? Will It Survive? J Toxicol Pathol. 20: 13–19. 2007. [Google Scholar]
- 2.Yoshitomi K, and Boorman GA. Eye and associated glands. In: Pathology of the Fisher Rat. GA Boorman, SL Eustis, MR Elwell, CA Montgomery, WF MacKenzie (eds). Academic Press, Inc. San Diego, New York, Boston. 239-259. 1990. [Google Scholar]
- 3.Ackerman LJ, Yoshitomi K, Fix AS, and Render JA. Proliferative lesions of the eye in rats. In: Guides for Toxicologic Pathology. STP/ARP/AFIP. Washington, DC. 1998. [Google Scholar]
- 4.Elwell MR, and Boorman GA. Tumours of the harderian gland. In: Pathology of Tumors in Laboratory Animals. Vol. 1, Tumors of the Rat, 2nd ed. VS Turusov, and U Mohr (eds). IARC Scientific Publications No.99, Lyon. 79-88. 1990. [PubMed] [Google Scholar]
- 5.Mohr U. International Classification of Rodent Tumours. Part I, The Rat, 7. Central Nervous System; Heart; Eye; Mesothelium. IARC Publication No.122. Lyon. 34-51. 1994. [PubMed] [Google Scholar]
- 6.Iwata H, Hirouchi Y, Koike Y, Yamakawa Y, Kobayashi K, Yamamoto T, Kobayashi K, Inoue H, and Enomoto M. Historical control data of nonneoplastic and neoplastic lesions in F344/DuCrj rats. J Toxicol Pathol. 4: 1–24. 1991. [Google Scholar]
- 7.Chandra M, and Frith CF. Spontaneous neoplasms in aged control Fischer 344 rats. Cancer Lett. 62: 49–56. 1992. [DOI] [PubMed] [Google Scholar]
- 8.Takaki Y, Kitamura S, Uekusa T, Honma S, Aze Y, Wakabayashi K, Kuwabara N, and Fukuda Y. Spontaneous tumors in F-344/Jcl rats. J Toxicol Sci. 14: 181–195. 1989. [DOI] [PubMed] [Google Scholar]
- 9.Haseman JK, Huff J, and Boorman GA. Use of historical control data in carcinogenicity studies in rodents. Toxicol Pathol. 12: 126–135. 1984. [DOI] [PubMed] [Google Scholar]
- 10.Sheldon WG, Curtis M, Kodell RL, and Weed L. Primary harderian gland neoplasms in mice. J Natl Cancer Inst. 71: 61–68. 1983. [PubMed] [Google Scholar]
- 11.Japan Bioassay Research Center English Summary of Study Report, August 2011. Summary of feed carcinogenicity study of diphenylamine in F344 rats. 2011, from Ministry of Health, Labour and Welfare (MHLW) Website: http://anzeninfo.mhlw.go.jp/user/anzen/kag/pdf/gan/0684_DiphenylAmineRats.pdf.
- 12.Ihara M, Tajima M, Yamate J, and Shibuya K. Morphology of spontaneous Harderian gland tumors in aged B6C3F1 mice. J Vet Med Sci. 56: 775–778. 1994. [DOI] [PubMed] [Google Scholar]
- 13.Vesselinovitch SD, Rao KVN, Mihailovich N, Rice JM, and Lombard LS. Development of broad spectrum of tumors by ethylnitrosourea in mice and the modifying role of age, sex, and strain. Cancer Res. 34: 2530–2538. 1974. [PubMed] [Google Scholar]
- 14.Vesselinovitch SD, and Mihailovich N. The neonatal and infant age periods as biologic factors which modify multicarcinogenesis by urethan. Cancer Res. 27: 1422–1429. 1967. [PubMed] [Google Scholar]
- 15.Vesselinovitch SD, and Mihailovich N. The role of periodic and interrupted treatment of newborn and infant mice with urethan on leukemogenesis. Cancer Res. 27: 350–352. 1967. [PubMed] [Google Scholar]
- 16.Kajimura T, Kashimoto Y, Satoh H, and Furuhama K. Rapid induction of tumors in the harderian gland of mice receiving urethane after initiation with N-ethyl-N-nitrosourea. J Toxicol Pathol. 16: 85–91. 2003. [Google Scholar]


