Abstract
Background
While elevated pulmonary artery systolic pressure (PASP) is associated with heart failure (HF), whether PASP measurement can help predict future HF admissions is not known, especially in African-Americans, who are at increased risk for HF. We hypothesized that elevated PASP is associated with increased risk of HF admission and improves HF prediction in African-American (AA) population.
Methods and Results
We conducted a longitudinal analysis using the JHS cohort (n=3125, 32.2% men) with baseline echo-derived PASP and follow-up for HF admissions. Hazard ratio for HF admission was estimated using Cox-proportional hazard model adjusted for variables in the Atherosclerosis Risk in Community (ARIC) HF prediction model. Over median follow up of 3.46 years, 3.42% of the cohort was admitted for HF. Subjects with HF had a higher PASP (35.6 ± 11.4 mm Hg vs. 27.6 ± 6.9 mm Hg, p<0.001). The hazard of HF admission increased with higher baseline PASP (adjusted HR/10 mmHg increase in PASP: 2.03, 95% CI: 1.67-2.48; adjusted HR for highest (≥33 mmHg) versus lowest quartile (<24 mmHg) of PASP: 2.69, 95% CI: 1.43-5.06) and remained significant irrespective of history of HF, or preserved/reduced ejection fraction. Addition of PASP to the ARIC model resulted in a significant improvement in model discrimination (AUC = 0.82 before vs. 0.84 after, p = 0.03), and improved net reclassification index (11-15%), using PASP as a continuous or dichotomous (cutoff: 33 mm Hg) variable.
Conclusions
Elevated PASP predict HF admissions in African Americans and may aid in early identification of at risk subjects for aggressive risk factor modification.
Keywords: pulmonary artery systolic pressure, heart failure, African-American
Heart failure (HF) is associated with substantial morbidity, mortality and cost 1. It is common in the African-American (AA) population with a prevalence of 4.5% in males and 3.8% in females1. Moreover, the age-adjusted incidence rate of HF is highest in AA compared to other ethnicities1-3 and is associated with higher case fatality rates 3. Hence, identifying novel markers for predicting HF admissions would be clinically important for early identification of these at-risk subjects.
Elevated pulmonary artery systolic pressure (PASP) is associated with increased mortality and morbidity in the general population and in patients with HF4-8. In the AA population, elevated PASP is independently associated with co-morbidities that increase the risk of HF such as obesity, diabetes and hypertension 9. Furthermore, left atrial hypertension due to cardiac dysfunction commonly results in elevation of PASP. However, despite the epidemiological and pathophysiological link, PASP estimates are not part of major HF risk prediction models 10-12. In this study, we used the Jackson Heart Study (JHS) data to test the hypothesis that elevated PASP is associated with increased risk of HF admission and significantly improves HF prediction in a community-based AA population when added to a traditional HF prediction model, ARIC 10, which was derived from a cohort with substantial AA representation.
Methods
We conducted a longitudinal analysis using the JHS cohort. The conduct of the JHS was approved by the University of Mississippi Medical center Institutional Review Board. The participants gave written informed consent to participate in the research study. The current analysis of the JHS data was approved by the Providence VA Medical Center Institutional Review Board. The Providence VAMC Institutional Review Board waived the requirement for informed consent for this analysis, as the data available to the authors did not contain identifiable information.
Population
The JHS is a longitudinal population-based cohort study that recruited 5,301 AA participants between 2000-2004 residing in Jackson, MS 13, 14. Participants were enrolled from each of 4 recruitment pools: random, 17%; volunteer, 22%; currently enrolled in the Atherosclerosis Risk in Communities (ARIC) Study, 30%; and secondary family members, 31%. The participants answered predefined questionnaires, and underwent echocardiographic evaluation and spirometry at the time of first exam (2000-2004). The participants were followed up at regular intervals. The cohort used for the current study included participants that had echocardiography data available (n= 5,076), measurable tricuspid regurgitant (TR) velocity (n=3,282) and follow up contact after 12/31/2004 (n=3,125).
Outcome
The main outcome is time to probable or definite heart failure admission after adjudication based on available data on history, physical exam, laboratory analysis and medication use similar to those used in the ARIC study 15, 16. The adjudication of heart failure outcomes began on 01/01/2005 and heart failure admission data was available for a median of 3.46 years (4-1,461 days) after that date. Our secondary outcome was time to death starting from the time of the index echocardiographic exam.
Exposure
The main exposure is PA systolic pressure (PASP). The PA systolic pressure was calculated by addition of 5 mm Hg right atrial pressure to the trans-tricuspid gradient measured on echocardiogram 4.
Clinical Variables
Coronary heart disease was considered as present when the subject reported a history of myocardial infarction, abnormal stress test, prior coronary artery bypass graft surgery or prior coronary angioplasty. Presence of diabetes was defined as a history of diabetes, use of diabetes medications, HgbA1c ≥ 6.5, or a fasting blood glucose ≥ 126 mg/dL. Presence of systemic hypertension was defined as subject having a systolic BP ≥ 140 mm Hg, or a diastolic BP ≥ 90 mm Hg, or taking anti-hypertensive medications. Body mass index (BMI) was categorized as normal (<25 kg/m2), overweight (25-<30 kg/m2) and obese (≥30kg/m2). Severe mitral or aortic valve disease was considered as present if the qualitative assessment by echocardiography showed presence of severe mitral regurgitation, mitral stenosis, aortic regurgitation, or aortic stenosis. Heart rate was measured on a baseline EKG. History of heart failure was considered present if the patients responded to the question “Has a doctor ever said you had heart failure or congestive heart failure?” in affirmative at the time of first annual telephone follow up.
Chronic lung disease was considered present if the subjects responded to the question “Has your doctor or health professional ever said you have chronic lung disease, such as bronchitis or emphysema?” in the affirmative. Cigarette smoking status was derived from interview and categorized as never smoker (one who reported having smoked less than 400 cigarettes in one's life), former smoker (smoked >400 cigarettes but not currently smoking), and current smoker.
Spirometry Measurements
Based on the spirometry measurements, subjects were considered to have an obstructive pattern if the FEV1/FVC ratio was < 0.7, or a restrictive pattern if the FEV1/FVC ratio was ≥ 0.7 and the FVC was <80% predicted. Subjects that had neither an obstructive nor a restrictive pattern were considered as having normal spirometry.
Echocardiographic parameters
Detailed echocardiography procedures are available online17. Briefly, echocardiograms were recorded by trained sonographers and interpreted by experienced cardiologists in the Echocardiography Reading Center located at the University of Mississippi Medical Center. Standard echocardiographic views were obtained and measurements performed by the interpreting physician who was blinded to the participants' clinical data 18. No tissue Doppler measurements were performed. The echocardiography data used for this current study included PA systolic pressure (calculated from TR jet peak gradient + 5 mm Hg), semi-quantitative ejection fraction (to nearest 5%), and left atrial (LA) diameter (measured on 2D images at endsystole). Left atrial diameter was indexed to height to adjust for body habitus. All measurements used were performed in 2D images. Valvular disease was qualitatively graded.
Statistical Analysis
We first compared the baseline clinical characteristics, pulmonary function and echocardiographic parameters using Student's test and Chi-square analysis, for continuous and categorical variables, respectively.
We then used a Cox-proportional hazard model to estimate the hazard ratio of HF admission using either PASP (every 10 mm Hg increment) or quartiles of PASP as main exposure. Subsequently, we adjusted our model with variables used in the ARIC HF prediction model (age, sex, coronary heart disease, diabetes, systolic blood pressure, blood pressure medication use, heart rate, smoking status, and body mass index). Race was a part of the ARIC model but was dropped as a covariate since our population is exclusively African-American. Subjects that died prior to a HF event were censored. In order to confirm that censoring subjects that died (competing event) did not significantly alter the hazards of HF admission (main event), we repeated our analysis by estimating the sub-hazard ratios using the competing-risks regression model, according to the method of Fine and Gray 19.
Subgroup analyses was then performed to estimate the hazard ratios using Cox-proportional hazard model with either PASP as a continuous variable or elevated PASP (≥ 33 mm Hg, the cut-off of 4th quartile of PASP) as a categorical variable adjusted for the ARIC model in subjects with or without prior HF history, in subjects with reduced or preserved EF, and in subjects with different spirometry profiles. We also performed sensitivity analyses by excluding the subjects with (a) reduced EF, prior HF history or severe left sided vavular disease and (b) obstructive spirometry profile, and then estimating the hazard ratios for HF admission for the resulting subgroup by PASP (as a continuous or categorical variable), after adjustment for the ARIC model.
We performed receiver operating characteristic (ROC) curve analyses to estimate the area under the curve (AUC) using the ARIC model, ARIC model + PASP as continuous variable, and ARIC model + elevated PASP (≥33 mm Hg) as a categorical variable to assess if addition of PASP variables improved the discriminatory power of the ARIC model. The ROC curves were then compared for significant difference in the AUC.
In order to calculate the net reclassification improvement (NRI), the 3-year (approximate median follow up time of the cohort) event rate was calculated for each subject using the linear prediction from the fitted model and the predicted baseline survivor function at 3 years with ARIC variables alone and with ARIC variables plus PASP, in both continuous (every 10 mm Hg increment) and dichotomous (≥33 mm Hg vs. <33 mmHg) manner. Category-less net reclassification improvement was also estimated as proposed by Pencina et al. 20. Briefly, the predicted event rate of HF admission using the ARIC model alone was subtracted from the predicted event rate of HF admission using the ARIC model + PASP continuous variable or ARIC model + PASP dichotomous variable for each subject and category-less NRIs were estimated. Next, to estimate NRI based on categories of HF admission risk, we divided the event risk in categories (Yearly risk: <0.5%, 0.5-<1%, 1-<2%, and ≥2%) and performed NRI calculations using these categories.
For our secondary outcome (time to death), we used a Cox-proportional hazard model to estimate the hazard ratio of death using PASP (every 10 mm Hg increment) as main exposure and adjusted for the ARIC HF variables.
Data was 81% complete for all covariates included in the model. Missing data (9% of the study population) ranged from 0.09% (Body Mass Index) to 18.6% (BP medication use). Missing data were imputed based on 5 sets of simulated values generated from non-missing variables using the multiple imputation method in STATA (StataCorp, College Station, Tex).21 Analyses were performed on each of the 5 data sets completed with imputed values, and then combined using Rubin's combination rules to consolidate the individual estimates into a single set of estimates using the MI estimate command in STATA22.
All analysis was performed using STATA/SE version 11.2 software (StataCorp LP, College Station, TX). A 2-sided p- value of < 0.05 was considered significant.
Results
Of the 3,282 subjects that had a measurable trans-tricuspid gradient, we have follow-up information on heart failure admissions starting 01/01/2005 on 3,125 subjects. Before the adjudication of heart failure admissions started, 48 subjects had died and 109 subjects were lost to follow up. The median gap from index echocardiogram to initiation of heart failure adjudication was 898 days (275-1,557 days). The median follow up time starting 01/01/2005 was 3.46 years (4-1,461 days). A total of 301 confirmed heart failure events occurred during the follow-up in 107 subjects (3.42% of cohort). The incidence of heart failure admission was 1.04% per person-year during the adjudication period.
Baseline Characteristics
Table 1 shows the baseline clinical and echocardiographic characteristics of the cohort. The average age of the population was 56.3 ± 12.5 years with 32.2% of subjects male. There was a high prevalence of obesity (51.1%), hypertension (61.1%) and diabetes (24.2%). The mean PA systolic pressure in the cohort was 27.9 ± 7.3 mm Hg.
Table 1. Baseline characteristics.
| Total (Mean ± SD or %), (n= 3,125) | Control group (Mean ± SD or %), (n= 3,018) | HF group (Mean ± SD or %), (n= 107) | P value | |
|---|---|---|---|---|
| Age (years) | 56.3 ± 12.5 | 55.9 ± 12.4 | 65.8 ± 10.5 | <0.001 |
| Male | 32.2% | 32.0% | 36.4% | NS |
| PA Systolic Pressure (mm Hg) | 27.9 ± 7.3 | 27.6 ± 6.9 | 35.6 ± 11.4 | <0.001 |
| PA Systolic Pressure Quartiles | ||||
| Quartile 1 (10-23 mm Hg) | 27.8% | 28.4% | 12.2% | |
| Quartile 2 (24-27 mm Hg) | 25.9% | 26.4% | 13.1% | |
| Quartile 3 (28-32 mm Hg) | 24.5% | 24.8% | 18.7% | |
| Quartile 4 (33-69 mm Hg) | 21.7% | 20.5% | 56.1% | <0.001 |
| BMI category | ||||
| Normal | 15.2% | 15.3% | 15.0% | NS |
| Overweight | 33.7% | 33.8% | 30.8% | |
| Obese | 51.1% | 51.0% | 54.2% | |
| Systolic blood pressure (mmHg) | 127 ± 18 | 126 ± 18 | 136 ± 25 | <0.001 |
| Diastolic blood pressure (mmHg) | 78 ± 10 | 78 ± 10 | 76 ± 12 | NS |
| Hypertension | 61.1% | 60.2% | 86.9% | <0.001 |
| Diabetes mellitus | 24.2% | 23.0% | 58.5% | <0.001 |
| Coronary artery disease | 8.0% | 7.4% | 24.3% | <0.001 |
| History of heart failure | 2.7% | 2.0% | 21.8% | <0.001 |
| History of lung disease | 6.7% | 6.5% | 10.4% | NS |
| Smoking history | ||||
| Never smoker | 68.5% | 69.0% | 53.8% | 0.001 |
| Former smoker | 20.0% | 19.6% | 33.0% | |
| Current smoker | 11.5% | 11.4% | 13.2% | |
| Spirometry Profile | ||||
| Normal | 70.2% | 71.1% | 42.6% | <0.001 |
| Obstructive | 9.2% | 8.8% | 21.3% | |
| Restrictive | 20.6% | 20.1% | 36.2% | |
| Medications | ||||
| Antihypertensives | 62.1% | 61.2% | 85.7% | <0.001 |
| Diabetes medication | 15.6% | 14.5% | 43.9% | <0.001 |
| Beta blockers | 12.7% | 12.4% | 21.4% | 0.008 |
| Calcium channel blockers | 23.1% | 22.5% | 37.8% | <0.001 |
| Diuretics | 39.5% | 38.5% | 63.3% | <0.001 |
| Statins | 14.1% | 13.8% | 22.4% | 0.016 |
| Echo characteristics | ||||
| LVEF < 50% | 2.5% | 2.0% | 16.0% | <0.001 |
| LA diameter index (mm/m2) | 18.0 ± 2.5 | 17.9 ± 2.4 | 19.8 ± 3.7 | <0.001 |
| Severe left-sided valvular heart disease | 0.2% | 0.1% | 2.8% | <0.001 |
Subjects that were admitted with heart failure were older, had higher systolic blood pressure, and were more likely to have hypertension, diabetes, coronary artery disease, history of prior heart failure, smoking history, and an obstructive or restrictive spirometry profile. As expected, they were more likely to be on anti-hypertensive, anti-diabetic, and statin medications. On echocardiography, subjects admitted for heart failure had a higher PA systolic pressure (35.6 ± 11.4 mm Hg vs. 27.6 ± 6.9 mm Hg, p<0.001), reduced LV ejection fraction, and higher LA diameter index. Fifty six percent of subjects with heart failure event were in the top quartile of PA systolic pressure (33-69 mm Hg) compared to 20.5% of subjects that did not have heart failure admission.
The baseline characteristics of cohort categorized by the PASP quartiles are shown in Supplementary Table 1. Higher PASP were associated with older subjects and a higher prevalence of women, obesity, hypertension, coronary artery disease, diabetes, heart failure, lung disease, smoking, obstructive and restrictive spirometry profile.
Relationship of PA Systolic Pressure with Heart Failure Admissions
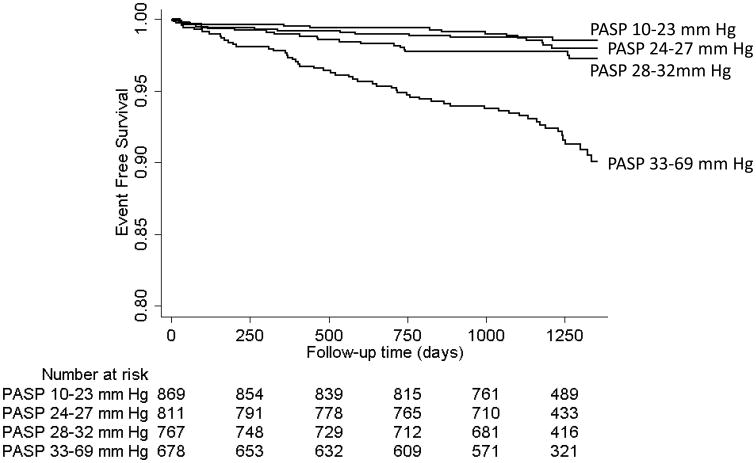
The hazard of HF admission with each 10 mm Hg increment in PASP increased by 2.75 fold (95% CI: 2.31-3.26). Similarly, the hazard of HF admission increased by 6.23 fold (95% CI: 3.42-11.34) in subjects with PASP between 33-69 mm Hg (Quartile 4) compared to subjects in the first quartile with PASP between 10-23 mm Hg (Table 2). The Figure shows the unadjusted Kaplan Meier curves of subjects grouped by the quartiles of PA systolic pressure being free of heart failure admission from the beginning of follow up (time 0 = 12/31/2004).
Table 2. Unadjusted and Adjusted Hazard Ratios of Heart Failure Admission.
| Unadjusted HR (95% CI) | Adjusted HR for ARIC model‡ (95% CI) | |
|---|---|---|
| Hazard ratio of HF event with 10 mm Hg increase in PA systolic Pressure (continuous) | 2.75 (2.31-3.26)* | 2.03 (1.67-2.48)* |
| PASP Quartile 1 | Referent | Referent |
| PASP Quartile 2 | 1.18 (0.55-2.51) | 0.96 (0.45-2.05) |
| PASP Quartile 3 | 1.76 (0.88-3.54) | 1.03 (0.51-2.10) |
| PASP Quartile 4 | 6.23 (3.42-11.34)* | 2.69 (1.43-5.06) † |
P<0.001;
P=0.002.
Age, sex, CHD, diabetes, systolic blood pressure, blood pressure medication use, heart rate, smoking status, and body mass index.
Figure 1.
Kaplan Meier curves showing heart failure admission free survival in quartiles of pulmonary artery systolic pressure (PASP). The events are heart failure admissions and the follow-up data starts after 12/31/2004, when the adjudication for heart failure admissions began.
After adjustment for the ARIC HF model, the adjusted hazard ratio of HF admission with each 10 mm Hg increase in PASP was 2.03 (95% CI: 1.67-2.48) and the adjusted hazard ratio of subjects with PASP in the 4th quartile was 2.69 (95% CI: 1.43-5.06) compare to those with PASP in the 1st quartile (Table 2). The unadjusted and adjusted hazard ratios did not significantly change using competing risk regression model with death as a competing risk (Supplementary Table 2).
The increased hazard of HF admission with elevated PASP adjusted for the ARIC HF model remained significant in the subgroups of subjects with or without prior history of heart failure, with preserved or reduced ejection fraction, and in subjects with normal and restrictive spirometry profiles (Table 3). Interestingly, an elevated PASP was not associated with a significantly increased hazard of heart failure admission in subjects with airflow obstruction on spirometry. In the subgroup of subjects with preserved EF, no significant left-sided valvular disease and no prior history of heart failure, PASP remained a significant predictor of subsequent HF admissions after adjustment for the ARIC model (adjusted HR for each 10 mm Hg increase in PASP: 1.77, 95% CI: 1.38-2.27; adjusted HR for PASP≥33 mm Hg: 2.31, 95% CI: 1.43-3.71). Similarly, in the subgroup of subjects without obstructive spirometry profile, the adjusted hazards of HF admissions increased with higher PASP (adjusted HR for each 10 mm Hg increase in PASP: 2.13, 95% CI: 1.69-2.67; adjusted HR for PASP≥33mm Hg: 3.12, 95% CI: 1.99-4.91).
Table 3. Subgroup Analysis.
| Subgroups | Adjusted* Hazard Ratio of Heart Failure Admission with PASP≥ 33 mm Hg (95% CI) | Adjusted* Hazard ratio of HF event with 10 mm Hg increase in PASP |
|---|---|---|
| Prior HF History | ||
| Absent | 2.45 (1.55-3.90) | 1.83 (1.43-2.33) |
| Present | 3.29 (1.27-8.56) | 2.18 (1.35-3.53) |
| LV ejection Fraction | ||
| ≥50% | 2.25 (1.45-3.50) | 1.83 (1.46-2.30) |
| <50% | 3.53 (1.05-11.85) | 1.70 (1.01-2.85) |
| Spirometry Profiles | ||
| Normal | 3.34 (1.71-6.53) | 1.98 (1.39-2.84) |
| Obstructive | 0.88 (0.31-2.48) | 1.20 (0.78-1.86) |
| Restrictive | 1.71 (0.84-3.49) | 1.83 (1.28-2.62) |
PASP: PA systolic pressure.
Age, sex, CHD, diabetes, systolic blood pressure, blood pressure medication use, heart rate, smoking status, and body mass index.
Addition of PA systolic pressure to ARIC prediction model of HF admission
The ARIC HF model performed very well in predicting heart failure admission in our population (Area under ROC curve: 0.82, 95% CI: 0.786-0.860). Nonetheless, the addition of PASP variable to the model resulted in a significant improvement in the area under the ROC curve both as a continuous variable (Area under ROC curve: 0.85, 95% CI: 0.81-0.88, p=0.02) and as a dichotomous variable (area under ROC curve: 0.84, 95% CI: 0.81-0.88, p=0.03).
In net reclassification analysis using four risk categories (Yearly Risk of HF event: <0.5%, 0.5-<1%, 1-<2%, and ≥2%), the addition of PASP (≥33 mm Hg) to the ARIC model resulted in a NRI of 15.1% (Table 4). Using the same risk categories, the NRI was 11.3% (Supplementary Table 3) when PASP was added as a continuous variable to the ARIC model.
Table 4. Net Reclassification Improvement (with categories) after addition of presence of PASP ≥33 mm Hg to the ARIC model.
| Number of participants that did not have a heart failure admission: | |||||
|---|---|---|---|---|---|
| Risk Catagories (%/year) using ARIC Model | Risk Categories (%/year) with addition of presence of PASP ≥33 mm Hg to the ARIC Model | ||||
| <0.5% | 0.5% to <1% | 1% to <2% | ≥2% | Total | |
| <0.5% | 1,596 | 126† | 3† | 0† | 1,725 |
| 0.5% to <1% | 212* | 234 | 112† | 0† | 558 |
| 1% to <2% | 0* | 189* | 129 | 98† | 416 |
| ≥2% | 0* | 0* | 112* | 207 | 319 |
| Total | 1,808 | 549 | 356 | 305 | 3,018 |
| Non- Event NRI: 5.8% | |||||
| Number of participants that had a heart failure admission: | |||||
| Risk Categories (%/year) with addition of presence of PASP ≥33 mm Hg to the ARIC Model | |||||
| <0.5% | 0.5% to <1% | 1% to <2% | ≥2% | Total | |
| <0.5% | 8 | 4* | 0* | 0* | 12 |
| 0.5% to <1% | 4† | 4 | 9* | 1* | 18 |
| 1% to <2% | 0† | 6† | 4 | 15* | 25 |
| ≥2% | 0† | 0† | 9† | 43 | 52 |
| Total | 12 | 14 | 22 | 59 | 107 |
| Event NRI: 9.3% | |||||
Cells with * reflect appropriate change in risk after addition of presence of PASP ≥33 mm Hg in the model containing ARIC variables (Age, sex, CHD, diabetes, systolic blood pressure, blood pressure medication use, heart rate, smoking status, and body mass index).
Cells with † reflect inappropriate change in risk after addition of presence of PASP ≥33 mm Hg in the model containing ARIC variables.
In category-less net reclassification analysis, we found significant net reclassification improvement to the ARIC HF model with either addition of PASP as a continuous variable (Category-less NRI: 36.8%, Supplementary Table 4), or as a categorical variable (Category-less NRI: 59.5%, Supplementary Table 5). The category-less net reclassification improvement was more among subjects without HF admission (non-event NRI) compared to the subjects with HF admission (event NRI).
Relationship of PA systolic Pressure with Overall Survival
As opposed to HF admission events, survival data was available starting from the date of the index echocardiogram. Over a median follow up of 2,102 days (n=3,240, range 20-3,002 days), 201 subjects died. The unadjusted hazard of death with every 10 mm Hg increase in PASP was 1.87 fold (95% CI: 1.62-2.17, p<0.001). After adjusting for ARIC variables, the hazard ratio for death with every 10 mm Hg increase in PASP remained significantly increased at 1.38 (95% CI: 1.18-1.62, p<0.001).
Discussion
In this study, we show that in an ambulatory, community-based African American cohort with a yearly HF admission risk of ∼ 1%, having an elevated PASP is associated with increased risk of future HF admission and provides incremental value in predicting hospitalization risk over a validated HF model. After adjusting for co-morbidities, participants with a PASP of ≥33 mm Hg had a 2.7 fold increase in the hazard for a heart failure admission compared to those with PASP < 24 mm Hg and, for every 10 mm Hg increase in PASP, there was a doubling of the hazard for HF admission.
Heart failure is an emerging crisis in the US with > 1 million hospitalizations annually that are associated with substantial morbidity, mortality and costs. 23. Notably, patients hospitalized for HF are at high risk for all-cause rehospitalization, with a 1-month readmission rate of 25% 24. While screening asymptomatic patients is not recommended, Doppler echocardiography is frequently used in assessment of patients with cardiovascular co-morbidities. Pulmonary artery systolic pressures are easily obtained in about two-thirds of patients undergoing echocardiograms4, 9. Our data suggests that PASP can be used to identify patients that are at risk for subsequent HF admission, especially in patients with preserved EF. Since at this time we do not know if elevated PASP is a modifiable risk factor or a target for treatment in heart failure, the management of these at-risk patients would include aggressive control of their modifiable risk factors, such as hypertension, diabetes, metabolic syndrome and obesity, and guideline-recommended therapy 25. As novel therapies become available especially for HF with preserved ejection fraction, it may be reasonable to study their benefit in these at risk subjects to prevent HF admissions.
The relationship between PASP and HF events was independent of clinical comorbidities and was significant in subgroups with either reduced or preserved EF. Both left ventricular systolic and diastolic dysfunction are associated with elevations in left atrial pressure that result in higher PASP by passive mechanism and/or by increase in vascular remodeling (reactive). However, it is surprising how relatively modest elevations in PASP, to levels not typically considered clinically significant, were related to subsequent HF hospitalizations in our analysis. Similar findings were noted in other studies, where mild to modest elevation on PASP related to mortality 4, and to HF events in patients with stable CAD 7 or AMI 8. We speculate that the underlying mechanism may be related to progression of increased vascular stiffness and/or preclinical left atrial hypertension that ultimately manifests as clinical heart failure. Since elevated PASP independently predicted HF admissions in participants with or without a prior self-reported history of HF, our data suggest that elevated PASP can be used as an additional marker to identify patients in preclinical stages (Stage A or Stage B) of heart failure 25.
It has been shown that heart failure is common in patients with COPD and is frequently unrecognized 26, 27. However, in subgroup analysis, we found that PASP did not predict HF hospitalization in participants with airflow obstruction on spirometry. Given that the obstructive spirometry phenotype may be independently related to elevated PASP in AA due to reasons other than cardiac function (e.g. chronic pulmonary disease and hypoxia associated vascular remodeling),9 it is not surprising that an association between elevated PA pressures and HF hospitalization in this subgroup of subjects was not observed. Hence, alternative risk predictors, such as BNP, may be more relevant in these patients.
We report that addition of PASP, either as a continuous or dichotomous variable, significantly improved the area under the ROC curve of an existing HF prediction model. We used the ARIC model as our baseline model to predict HF events since other models such as ABC and Framingham HF prediction models 11, 12 did not include a significant proportion of AAs in their respective cohorts. Moreover, the ARIC model has been shown to perform better in a cohort with AA compared to the others 10. Indeed, 30% of JHS participants were also part of the ARIC cohort that was used to develop the ARIC model. The baseline ARIC model performed extremely well in our study cohort considering the reported area under the ROC curve used to design the model in the ARIC cohort (c-index of 0.77-0.79) 10. Despite the already good performance of the ARIC model, addition of the PASP significantly improved the discriminatory power in predicting HF events. The NRI analysis confirmed that the addition of PASP for identifying high-risk subjects reclassifies 11-15% of the subjects into more appropriate yearly risk categories. The NRI for PASP is of similar magnitude to the NRI for addition of commonly used biomarkers such as NT-proBNP (NRI: 13.5% when added to the ARIC model)10 or CRP (NRI: 11.8% when added to a multivariable prediction model in the Framingham Offspring cohort in predicting coronary heart disease events)28. Future studies will be needed to determine if PASP and NT-proBNP have overlapping or synergistic effects when added together to the ARIC model.
Our study has some limitations. The adjudication of heart failure events started in 2005. Hence we do not have any event data between the baseline study enrollment dates (2000-2004) and 2005. Thus, we may be underestimating the association between PASP and HF hospitalization assuming that the HF event rates remained constant throughout the study period. Pulmonary artery systolic pressures can be dynamic. The echocardiography was performed at the baseline visit and there were no repeated measures available for PASP in this cohort. We are therefore unable to ascertain if temporal trends in PASP may relate to HF events or mortality. Despite this limitation, our results suggests that even modestly elevated PASP can serve as a significant risk factor for remote HF events and that the underlying pathophysiology may predate the actual event by years similar to other chronic diseases such as DM and HTN. There were no IVC measurements, so we utilized an assumed RAP of 5mm Hg as done by others in a population based cohort 4, which may have led to underestimation of PA pressure in subjects with more elevated RAPs. Also, a right heart catheterization derived PASP is more accurate, which unfortunately is not feasible or practical to perform in population studies. Despite its limitations and inability to accurately assess PA pressures, Doppler echocardiography is frequently used to estimate the PA systolic pressure 4. Hence, it is possible that the lack of precision in measured PASP may have affected the association between estimated PASP and HF admission. History of heart failure is self-reported and as such subject to recall bias. Thus, we are unable to accurately discern between the incident and recurrent HF hospitalizations in the current study.
In conclusion, we report that elevated PASP predicts heart failure admissions and mortality in African Americans independent of clinical co-morbidities and provides incremental value as a risk assessment marker for future HF hospitalizations. Identification of these at-risk subjects using PASP measurements may be helpful in targeting aggressive risk factor modification. Future studies are needed to confirm the validity of our findings in other populations.
Acknowledgments
Sources of Funding: This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service (MERIT Review Award to GC, IBX000711A). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities, with additional support from the National Institute on Biomedical Imaging and Bioengineering. None of the authors have any relationship with industry to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Disclosures: None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB Subcommittee AHASCaSS. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: The multi-ethnic study of atherosclerosis. Archives of internal medicine. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Lam CSP, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: A community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ristow B, Ali S, Ren X, Whooley MA, Schiller NB. Elevated pulmonary artery pressure by doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: The heart and soul study. J Am Coll Cardiol. 2007;49:43–49. doi: 10.1016/j.jacc.2006.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutlak D, Lessick J, Carasso S, Kapeliovich M, Dragu R, Hammerman H, Agmon Y, Aronson D. Utility of pulmonary hypertension for the prediction of heart failure following acute myocardial infarction. Am J Cardiol. 2012;109:1254–1259. doi: 10.1016/j.amjcard.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary G, Jankowich M, Wu WC. Prevalence and clinical characteristics associated with pulmonary hypertension in african-americans. PloS one. 2013;8:e84264. doi: 10.1371/journal.pone.0084264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: The atherosclerosis risk in communities (aric) study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Archives of internal medicine. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB, Health ABCS. Incident heart failure prediction in the elderly: The health abc heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in african americans: Design and methods of the jackson heart study. Ethn Dis. 2005;15:S6–4. 17. [PubMed] [Google Scholar]
- 14.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting african-american research participation in the jackson heart study: Methods, response rates, and sample description. Ethn Dis. 2005;15:S6–18. 29. [PubMed] [Google Scholar]
- 15.Jackson Heart Study. Instructions for heart failure final diagnosis form. [Accessed : 01/09/2014]; http://www2.cscc.unc.edu/aric/sites/default/files/public/forms/HDX_QXQ.pdf.
- 16.Jackson Heart Study. Heart failure diagnosis form. [Accessed : 01/09/2014]; http://www2.cscc.unc.edu/aric/sites/default/files/public/forms/HDXB_Form_012313.pdf.
- 17.Jackson Heart Study. Echocardiography manual. [Accessed : 01/09/2014]; http://jhs.jsums.edu/jhsinfo/Portals/0/pdf/manuals1/Echocardiography_manual6.pdf.
- 18.Samdarshi TE, Taylor HA, Edwards DQ, Liebson PR, Sarpong DF, Shreenivas SS, Howard G, Garrison RJ, Fox ER. Distribution and determinants of doppler-derived diastolic flow indices in african americans: The jackson heart study (jhs) Am Heart J. 2009;158:209–216. doi: 10.1016/j.ahj.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 22.Stata Corp. Multiple-imputation analysis using stata's mi command. 2010 http://www.stata.com/meeting/boston10/boston10_marchenko.pdf.03/27/2013.
- 23.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circulation Cardiovascular quality and outcomes. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 26.McCullough PA, Hollander JE, Nowak RM, Storrow AB, Duc P, Omland T, McCord J, Herrmann HC, Steg PG, Westheim A, Knudsen CW, Abraham WT, Lamba S, Wu AH, Perez A, Clopton P, Krishnaswamy P, Kazanegra R, Maisel AS Investigators BNPMS. Uncovering heart failure in patients with a history of pulmonary disease: Rationale for the early use of b-type natriuretic peptide in the emergency department. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2003;10:198–204. doi: 10.1111/j.1553-2712.2003.tb01990.x. [DOI] [PubMed] [Google Scholar]
- 27.Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. European heart journal. 2005;26:1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW, Pencina M, Jacques P, Selhub J, D'Agostino R, Sr, O'Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the framingham heart study. Circulation Cardiovascular quality and outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]