Abstract
Contingency management (CM) is an empirically supported intervention for substance dependence, but it has not been evaluated systematically in non maintained opioid-dependent patients. This retrospective analysis examined whether CM was effective in opioid-dependent patients initiating intensive outpatient psychosocial treatment. In the primary trial (Petry, N. M., Weinstock, J., & Alessi, S. M. [2011]. A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology, 79, 686–696), substance-abusing patients (n = 239) at two community-based clinics were randomized to standard care (SC) or SC with CM for 12 weeks; in the CM condition, patients earned opportunities to win prizes for attending treatment and submitting drug-negative samples. For this analysis, patients were further classified as non-opioid-dependent (n = 159), opioid-dependent and not receiving maintenance therapy (n = 33), or opioid-dependent and on methadone or Suboxone maintenance therapy (n = 47). Main effects of opioid dependence/maintenance status, treatment condition, and their interaction were evaluated with respect to attendance and abstinence outcomes. Opioid-dependent patients receiving maintenance pharmacotherapy attended treatment on fewer days and achieved less abstinence than their opioid-dependent counterparts who were not on opioid agonist therapy, with Cohen's d effect sizes of 0.63 and 0.61 for attendance and abstinence outcomes, respectively. Nonmaintained opioid-dependent patients evidenced similar outcomes as substance abusing patients who were not opioid-dependent. CM also improved retention and abstinence (d = .26 and .40, respectively), with no interaction effects with opioid dependence/maintenance status noted. These data suggest that CM may be an effective psychosocial intervention potentially suitable for the growing population of opioid-dependent patients, including those not receiving maintenance pharmacotherapy.
Keywords: contingency management, substance abuse treatment, opioid dependence
Opioid abuse—particularly of prescription opioids—is a growing problem. Opioid use and dependence has almost tripled since 2000 (Office of Applied Studies, 2002; Substance Abuse and Mental Health Services Association [SAMHSA], 2011). With increases in opioid dependence, more patients are seeking treatment, and treatment admission rates for patients with primary opioid problems increased 271% between 1995 and 2005 (SAMHSA, 2010).
Historically, opioid-dependent patients have fared poorly when receiving entirely psychosocial interventions, with extraordinarily high rates of attrition and relapse (Mattick, Breen, Kimber, & Davoli, 2009). Maintenance pharmacotherapy with methadone or buprenorphine/naloxone (Suboxone) is the recommended standard of care for opioid dependence (National Consensus Development Panel on Effective Treatment of Opiate Addiction, 1998), but not all opioid-dependent patients desire or receive maintenance pharmacotherapy.
Contingency management (CM) is efficacious across a range of drug-abusing populations and settings (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). In CM, patients receive tangible reinforcers for objective evidence of behavior change (Petry, 2012). A meta-analysis (Dutra et al., 2008) of psychosocial treatments for substance use disorders found that CM is the intervention with the greatest effect size. It is efficacious for opioid-dependent patients receiving maintenance pharmacotherapies, including methadone (Silverman, Chutuape, Bigelow, & Stitzer, 1999; Peirce et al., 2006; Petry & Martin, 2002; Petry, Martin & Simcic, 2005; Petry, Alessi, Hanson, & Sierra, 2007) and buprenorphine (Kosten et al., 2003; Schottenfeld et al., 2005). CM is also efficacious for improving compliance with naltexone, an opioid antagonsist, to prevent relapse in detoxified patients (Carroll et al., 2001; Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002; Preston et al., 1999).
Little research has examined CM in nonmaintained opioid-dependent patients, and most of it pertains to postdetoxification status. Katz, Chatuape, Jones, and Stitzer (2002) found no benefit of CM in recently detoxified patients who received CM versus those who did not, whereas three others studies, each providing abstinent-contingent housing after residential detoxification, found some benefits of CM (Gruber, Chutuape, Stitzer, 2000; Jones, Wong, Tuten, & Stitzer, 2005; Tuten, Defulio, Jones, & Stitzer, 2012). However, not all opioid-dependent patients are in need of housing or receive residential detoxifications before accessing outpatient treatment.
Opioid-dependent patients are presenting at increasingly rates to clinic that do not offer pharmacotherapies (Treatment Episode Data Set (TEDS); SAMHSA, 2010). Some maintained patients initiate additional treatment to assist with other drug use, whereas other opioid-dependent patients are not receiving maintenance. Opioid-dependent patients who do not receive pharmacotherapy differ from their counterparts who do. In particular, patients with an opioid prescription drug use disorder may be less likely to receive maintenance medications than those who are dependent on heroin (Mendelson, Flower, Pletcher, & Galloway, 2008). Individuals with prescription opioid and heroin use disorders also differ in terms of demographics such as age, race, and years and severity of substance use problems (Brands, Blake, Sproule, Gourlay, & Busto, 2004; Moore et al., 2007; Sigmon, 2006), which may impact not only the types of treatment they seek but also their response to it. However, recent data suggest high rates of prescription opioid, as well as heroin, use in maintenance patients (Moore et al., 2007; Rosenblum et al., 2007). Weiss et al. (2011) found strong benefits of pharmacotherapy in patients with prescription opioid substance use disorders, with high rates of relapse when not maintained on an agonist.
Nevertheless, given the growing rate of opioid-dependent patients seeking psychosocial treatments (TEDS; SAMHSA, 2010), there is need to evaluate alternate interventions. The purpose of this retrospective analysis was to evaluate outcomes of patients receiving outpatient psychosocial treatment, with and without CM, based on opioid dependence status. Data were derived from a study of CM (Petry et al., 2011) conducted in clinics that did not provide pharmacotherapy. The clinics treated patients with heterogeneous substance use disorders, and the trial likewise included patients with alcohol, stimulant, and opioid use disorders. In the primary report (Petry et al., 2011), CM was efficacious in enhancing attendance and durations of abstinence.
For these analyses, patients were classified by drug use diagnoses, and opioid-dependent patients were further divided into those receiving maintenance pharmacotherapy and those not. Both groups of opioid-dependent patients were compared with one another, as well as with patients who were dependent upon substances other than opioids. One hypothesis was that opioid-dependent patients not receiving pharmacotherapy would evidence high rates of relapse and poor retention, given past research on this population (Mattick et al., 2009; Weiss et al., 2011). CM was expected to improve outcomes regardless of opioid-dependence status, as data find beneficial effects of this intervention across populations (Lussier et al., 2006; Prendergast et al., 2006).
Method
Participants (n = 239) were initiating intensive outpatient treatment for substance use disorders between 2005 and 2009 at one of two community-based clinics that did not provide agonist (or antagonist) medications. The clinics were located in urban areas, that were served by several independent methadone maintenance clinics, as well as private doctor's offices that provided Suboxone treatment. Patients were eligible for the CM study (Petry et al., 2011) if they met past-year Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnosis of cocaine, alcohol, or opioid abuse or dependence (American Psychiatric Association, 2000) and were 18 years or older. Non-English speaking, inability to understand the study, uncontrolled psychotic symptoms, or in recovery for pathological gambling (because prize CM has an element of chance, but see Petry & Alessi, 2010; Petry et al., 2006a) were exclusionary criteria. University and hospital Institutional Review Boards approved study procedures.
Procedures
After obtaining informed consent, research assistants (RAs) administered demographic questionnaires, modules adapted from the Structured Clinical Interview for DSM-IV for assessing substance use diagnoses (First, Spitzer, Gibbon, & Williams, 1996), the Addiction Severity Index (ASI) (McLellan et al., 1985), and the Service Utilization Form (SU) (Rosenheck, Fontanam, & Cottrol, 1995). The ASI is a well-established instrument (Bovasso, Alterman, Cacciloa, & Cook, 2001; Leonhard, Mulvey, Gastfriend, & Schwartz, 2000) that evaluates severity of psychosocial problems related to substance use in seven domains. Composite scores are derived in each domain and range from 0 to 1, with higher scores reflecting greater problems. The SU collects information about types of medical, substance use and psychological treatments received, including methadone and Suboxone. It contains similar items as the Treatment Services Review (McLellan, Alterman, Cacciola, Metzger, & O'Brien, 1992) but is more extensive.
In the main study (Petry et al., 2011), follow-up evaluations were scheduled for 1, 3, 6, 9, and 12 months after randomization (see below). At follow-ups, patients submitted urine and breath samples and completed the ASI and SU. Participants were compensated $40 for each evaluation, and >87% of follow-ups were completed at each time point, with no differences (ps > .05) noted between treatment conditions or drug dependence status.
Assignment to Treatment Conditions
RAs randomly assigned patients to conditions using a computerized urn randomization program. Treatment conditions were balanced (Stout, Wirtz, Carbonari, & Del Boca, 1994) on past-year dependence on opioids, cocaine, and alcohol. Separate programs were used at each clinic, so conditions were balanced by clinic as well.
Standard care (SC) at both clinics was group counseling that included relapse prevention, HIV education, life skills training, and 12-step oriented therapy. Clinic counselors, ranging from no degree to Masters level, conducted group sessions. Patients were involved with intensive care (four hours per day) for four to six weeks. Although intensive care was available up to five days per week, most patients were scheduled to attend only three days per week for four weeks. After completion of intensive care, care decreased in intensity, down to a minimum of one group session per week for up to 12 months, with patients typically attending only once a week after about four or six weeks. A retrospective review of 105 patients who remained in treatment for 12 weeks revealed the average expected days at treatment over this time frame was 21.8 + 8.1 days.
As part of study participation, patients submitted up to 24 urine and breath samples (two per week) for the 12-week study period. Urine specimens were tested for opioids, cocaine, and methamphetamine using OnTrak TesTstiks (Varian, Inc., Walnut Creek, CA), and breath samples for alcohol via an Intoximeter Breathalyzer (Intoximeters, Inc., St. Louis, Mo). RAs screened the samples and congratulated patients when they tested negative; they encouraged patients testing positive to discuss use in group counseling sessions.
CM treatment was provided in addition to SC and sample monitoring outlined above. CM patients were separated from SC patients for the first session of the day; in this session, all CM patients put their name into a hat at least once. On Tuesdays through Fridays, patients who had their urine and breath samples tested since the last group session put their name in the hat one extra time (i.e., twice) if samples were negative for all substances tested: opioids, cocaine, methamphetamine, and alcohol. On Mondays (or the first group session of a week if Monday was a holiday), names went into the hat once for attendance plus a bonus number of times. The bonus related to the number of weeks in a row the patient attended all scheduled group sessions and submitted all negative breath and urine samples. Thus, a patient who attended all group sessions six weeks in a row and submitted all negative samples for six weeks would put his name in the hat seven times (once for attendance that Monday plus six bonus times). The bonuses were arranged on Mondays because of the traditionally low rates of attendance on Mondays.
Bonus name slips were forfeited and reset if patients provided a sample positive for any substance (cocaine, methamphetamine, opioids, or alcohol), refused to provide a sample, or had an unexcused absence from one or more scheduled group sessions. Excused absences were rare (M = 1.3 in both treatment conditions) and include court appearance, family emergencies, and commitments cleared 24 hours in advance by the primary therapist. After a reset, the next week of consecutive attendance and negative samples would result in a patient's name going into the hat twice on Monday (once for attendance that day, plus once more for one week of continuous attendance/abstinence). Being late to the group session resulted in a forfeit of one's name going in the hat that day but did not reset name slips the next Monday.
On Tuesdays through Fridays, three name slips were drawn from the hat at the start of the first counseling session. The individuals whose names were drawn drew once from a standard prize bowl. This bowl comprised 200 cards: 174 were small prizes (choice of $1 McDonald's coupons, food items, bus tokens, etc.), 25 were large prizes (choice of $20 movie tickets, CDs, watches, dish sets, etc.), and one was a jumbo prize (choice of stereo, DVD player, or TV). Sample prizes were available for selection immediately in group, or patients could choose from the full range of prizes after the session. Cards were returned to the prize bowl after drawings, so probabilities were constant, but name slips were not replaced into the hat, and after each counseling session, all name slips were discarded. Nevertheless, patients' names could be selected more than once on a day if they had recently provided a substance negative sample.
On Mondays, six names were randomly chosen from the hat. The first five people whose names were selected drew for one prize each, and the sixth person drew for five prizes. Drawings on Mondays were from an Enhanced Prize Bowl with 30 cards; 25 were for small prizes, four for larges, and one for a jumbo. Thus, on Mondays, probabilities of winning jumbos were high and more patients overall won prizes. As during other days, patients' names could be selected more than once, and in such cases they could earn more than one prize; similarly to other days, all name slips were discarded after the drawing session.
Data Analyses
Patients were classified into one of three groups based upon DSM-IV criteria for opioid dependence and whether they were receiving opioid maintenance therapy. (Only three patients met criteria for opioid abuse alone, without dependence; these patients also had other substance use disorders, and they were classified in the nonopioid dependent category.) One group comprised patients not meeting criteria for opioid dependence and not receiving maintenance pharmacotherapy throughout study participation (e.g., alcohol and/or cocaine abusing/dependent patients, n = 159). Another group comprised opioid-dependent individuals receiving agonist therapy with methadone or Suboxone (n = 47) at the baseline, Month 1 or Month 3 evaluations, and the third group comprised opioid-dependent individuals receiving no opioid medications (n = 33) at any of these time points. (Twelve opioid-dependent patients not reporting maintenance therapy at time of study initiation began maintenance therapy, and six patients who were on maintenance therapy at study initiation did not report receiving maintenance treatment throughout the study period. Because these subgroups were small, we did not further subdivide those who moved in or out of maintenance therapy, and for the purposes of these analyses, these 18 individuals were classified as maintained.) No opioid-dependent patients reported receiving naltrexone. Chi-square and analysis of variance evaluated differences across these three groups with respect to demographics and baseline characteristics.
In evaluating outcomes, we used an intent-to-treat approach, including all 239 randomized patients. Two primary outcome measures, most often reported in CM treatments, were evaluated to minimize Type I error. These outcomes were available for 100% of patients: number of days attended counseling sessions, and longest duration of abstinence. A week of abstinence was counted for two consecutively scheduled samples that tested negative for all substances from which abstinence was reinforced (opioids, cocaine, methamphetamine, and alcohol). If patients refused or missed a sample because of an unexcused absence, the string of abstinence was broken. These two outcomes were correlated at 0.51, p < .001.
Univariate analysis of variance examined the impact of opioid dependence/maintenance therapy status, treatment condition, and the interaction between treatment condition and opioid dependence/maintenance status on the primary outcomes. Analyses controlled for clinic, baseline urine toxicology result, race, whether the admission was prompted by the legal system, DSM–IV alcohol dependence criteria, and age. The latter two variables were entered as continuous variables and others as categorical. Cohen's d was also calculated as an estimate of effect size.
Logistic regressions evaluated predictors of abstinence, as assessed by a substance negative toxicology screen, at month 12. Baseline variables (opioid dependence/maintenance status, clinic, baseline urine toxicology result, race, whether the admission was prompted by the legal system, DSM–IV alcohol dependence criteria, and age) were included in step one of the regression, and in step two, treatment condition and longest duration of abstinence achieved (a potent predictor of long-term outcomes; Higgins, Badger, & Budney, 2000; Petry et al., 2005, 2006b, 2007b, 2011) were added. Step three included a treatment condition by opioid-dependence/maintenance status interaction term. These analyses were conducted twice—first only using patients who submitted a sample at the Month 12 follow-up, and second using all randomized patients coding those without a sample at Month 12 as positive. Analyses were performed on SPSS for Windows (v 15). Two-tailed alphas <0.05 were considered significant.
Results
Demographic and Baseline Characteristics
Table 1 shows baseline characteristics of patients classified into the three groups based on opioid dependence and maintenance status. The three groups differed with respect to age and race, as well as drug use characteristics. The non opioid-dependent group was older, they endorsed more alcohol dependence criteria than the opioid-dependent groups, and few reported regular use of heroin, opioids, or benzodiazepines; they also had the lowest ASI-drug scores and highest ASI-alcohol scores. The non-maintained opioid-dependent group was most likely to be European American and least likely to be African American. They also endorsed the greatest number of DSM–IV opioid dependence criteria, and intermediary numbers of alcohol dependence criteria relative to the other two groups; every patient in this group reported regular marijuana use. At time of outpatient treatment initiation, the non-maintained opioid-dependent patients were least likely to test positive for cocaine, and the maintained opioid-dependent patients were more likely to test positive for opioids.
Table 1. Demographic and Baseline Characteristics.
| Variable | Not opioid dependent | Opioid dependent non maintained | Opioid dependent on maintenance | Significance test value (df), p |
|---|---|---|---|---|
| N | 159 | 33 | 47 | |
| Treatment condition, % (n) | χ2(2) = 2.80, .25 | |||
| Standard care | 49.1 (78) | 45.5 (15) | 61.7 (29) | |
| Contingency management | 50.9 (81) | 54.5 (18) | 38.3 (18) | |
| Age | 40.4a ± 10.3 | 30.6b ± 9.6 | 33.3b ± 11.6 | F(2, 236) = 17.27, .000 |
| Male, % (n) | 57.9 (92) | 57.6 (19) | 53.2 (25) | χ2(1) = 0.33, .84 |
| Years of education | 12.5 ± 2.2 | 12.8 ± 2.0 | 12.6 ± 1.6 | F(2, 235) = 0.23, .80 |
| Income | $14,630 ± 18,400 | $12,667 ± 20,646 | $16,058 ± 27,623 | F(2, 234) = 0.26, .77 |
| Race/ethnicity, % (n) | χ2(6) = 18.82, .004 | |||
| African American | 37.7 (60)a | 3.0 (1)b | 25.5 (12)a | |
| European American | 48.4 (77) | 84.8 (28) | 63.8 (30) | |
| Hispanic American | 9.4 (15) | 9.1 (3) | 8.5 (4) | |
| Other | 4.4 (7) | 3.0 (1) | 2.1 (1) | |
| Number of DSM-IV criteria endorsed | ||||
| Opioid dependence | 0.6a ± 0.3 | 6.3b ± 1.1 | 5.5c ± 2.6 | F(2, 236) = 566.21, .000 |
| Alcohol dependence | 4.1a ± 2.6 | 2.9b ± 2.6 | 1.8c ± 2.3 | F(2, 236) = 15.66, .000 |
| Cocaine dependence | 3.8 ± 3.1 | 3.7 ± 3.0 | 5.0 ± 2.6 | F(2, 236) = 3.32, .06 |
| Lifetime regular use, % (n) | ||||
| Heroin | 3.8 (6)a | 69.7 (23)b | 83.0 (39)b | χ2(2) = 143.79, .000 |
| Prescription opioids | 8.2 (13)a | 72.7 (24)b | 59.6 (28)b | χ(2) = 88.49, .000 |
| Benzodiazepines | 8.2 (13)a | 45.5 (15)b | 25.5 (12)b | χ(2) = 30.50, .000 |
| Cocaine | 72.3 (115) | 75.8 (25) | 83.0 (39) | χ2(2) = 2.46, .29 |
| Marijuana | 61.6 (98)a | 100.0 (33)b | 78.7 (33)a | χ2(2) = 21.92, .000 |
| On probation/parole | 35.2 (56) | 42.4 (14) | 24.5 (12) | χ2(2) = 2.63, .27 |
| Admission prompted by legal system, % (n) | 35.8 (57)a | 48.5 (16)a | 17.0 (8)b | χ2(2) = 9.38, .009 |
| Urinalysis positive at baseline, % (n) | 13.8 (22)a | 3.0 (1)a | 34.0 (16)b | χ2(2) = 15.80, .000 |
| Opioid | 0.6 (1)a | 3.0 (1)a | 25.5 (12)b | χ2(2) = 41.35, .000 |
| Cocaine | 13.8 (22)a | 0.0 (0)b | 17.0 (8)a | χ2(2) = 5.83,.05 |
| Methamphetamine | 1.3 (2) | 0.0 (0) | 2.1 (1) | χ2(2) = 0.71, .70 |
| Addiction Severity Index scores | ||||
| Medical | 0.25 ± 0.35 | 0.27 ± 0.34 | 0.21 ± 0.32 | F(2, 236) = 0.36, .70 |
| Employment | 0.66 ± 0.29 | 0.69 ± 0.30 | 0.61 ± 0.28 | F(2, 236) = 0.78, .46 |
| Alcohol | 0.29a ± 0.26 | 0.10b ± 0.16 | 0.11b ± 0.17 | F(2, 236) = 17.41, .000 |
| Drug | 0.11a ± 0.11 | 0.17b ± 0.09 | 0.20b ± 0.10 | F(2, 236) = 12.64, .000 |
| Legal | 0.13 ± 0.21 | 0.20 ± 0.22 | 0.16 ± 0.24 | F(2, 236) = 1.23, .29 |
| Family/social | 0.24 ± 0.21 | 0.22 ± 0.15 | 0.24 ± 0.20 | F(2, 236) = 0.24, .79 |
| Psychiatric | 0.26 ± 0.24 | 0.27 ± 0.22 | 0.23 ± 0.24 | F(2, 236) = 0.37, .69 |
Note. Values are means and standard deviations unless otherwise noted. Groups with different superscripts differ significantly from one another according to post hoc tests.
During Treatment Outcomes
Figure 1 shows treatment attendance by opioid dependence/maintenance status for patients assigned to SC and CM conditions. Opioid dependence/maintenance status was associated with attendance, F(2, 225) = 4.51, p = .01. Post hoc tests indicated that maintained opioid-dependent patients attended outpatient psychosocial treatment for significantly fewer days than their non-maintained opioid-dependent counterparts (Cohen's d = 0.63), whereas other groups did not differ significantly. Substance abuse treatment admission being prompted by the legal system was positively related to attendance, F(1, 225) = 11.01, p = .001, d = 0.44. Treatment condition was also associated with attendance, F(1, 225) = 16.38, p = .001, d = 0.26, with patients assigned to CM attending more sessions than those assigned to SC. No other independent variables, nor the opioid-dependence/maintenance status by treatment condition interaction, were significant.
Figure 1.
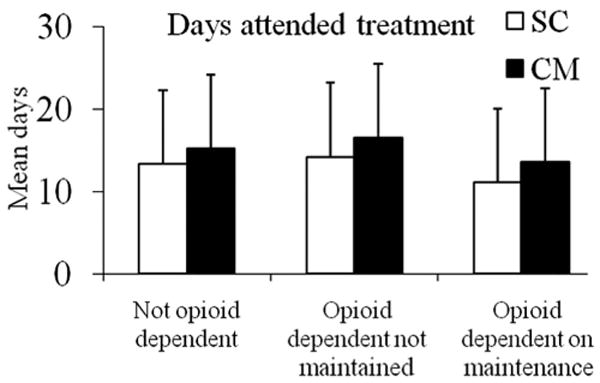
Days attended treatment. Data are shown based on opioid dependence/maintenance status and treatment condition assignment. Patients randomized to standard care (SC) are shown in open bars, and patients randomized to contingency management (CM) are shown in solid bars. The number of participants in the six groups represented from left to right are 78, 81, 15, 18, 29, and 18. Values represent adjusted means and standard deviations. The main effects of opioid dependence/maintenance status and treatment condition were significant (ps < .05). The interaction was not significant. See text for further details.
Figure 2 shows data related to longest duration of abstinence achieved. Opioid dependence/maintenance status was significantly related to this outcome measure as well, F(2, 225) = 8.05, p = .002, with the maintained opioid-dependent patients evidencing significantly shorter durations of abstinence than their non maintained opioid-dependent counterparts (d = 0.61). CM increased duration of abstinence relative to standard care, F(2, 225) = 8.47, p = .01, d = 0.40. Baseline toxicology result was associated with longest duration of abstinence achieved, F(2, 225) = 16.61, p < .001, d = 0.45, but the treatment condition by opioid dependence/maintenance status interaction was not significant, p > .93.
Figure 2.
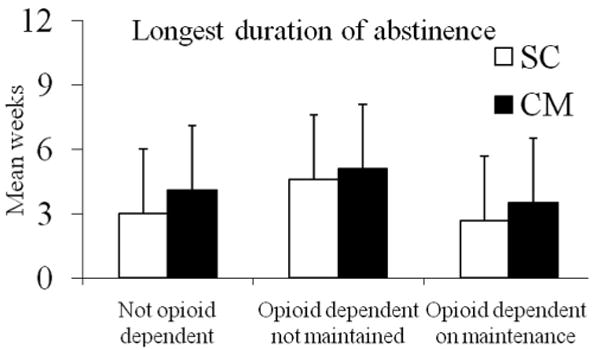
Longest duration of abstinence. Data are shown based on opioid dependence/maintenance status and treatment condition assignment. Patients randomized to standard care (SC) are shown in open bars, and patients randomized to contingency management (CM) are shown in solid bars. The number of participants in the six groups represented from left to right are 78, 81, 15, 18, 29, and 18. Values represent adjusted means and standard deviations. Duration of abstinence refers to toxicology confirmed negatives tests for cocaine, amphetamine, methamphetamine, opioids, and alcohol. The main effects of opioid dependence/maintenance status and treatment condition were significant (ps < .05). The interaction was not significant. See text for further details.
Posttreatment Outcomes
At the Month 12 follow-up, 72.5% (87/120) of non–opioid-dependent patients tested negative for cocaine, methamphetamine, opioids, and alcohol. Among opioid-dependent patients, 71.4% (20/28) of those who did not receive maintenance pharmacotherapy tested negative, and 59.5% (22/37) of those who did receive maintenance pharmacotherapy tested negative. When patients who failed to attend the Month 12 follow-up were considered positive, the respective proportions for the three groups were 54.7% (87/159), 60.6% (20/33), and 46.8% (22/47).
In predicting a substance-negative sample at the 12-month follow-up, Step 1, including baseline characteristics, was not significant, ps > .23. Whether only patients who submitted a urine toxicology screen at Month 12 or the full sample was analyzed (assuming missing samples were positive), Step 2, which included treatment condition and longest duration of abstinence achieved, was significant, ×2(2) = 5.95, p = .05, for the completer sample and for the full sample χ2(2) = 13.12, p < .001. The proportions of correctly classified patients were 73.5% and 63.2%, in the two respective samples. The only variable that was significantly associated with abstinence at Month 12 was the duration of abstinence achieved during treatment. Results from the logistic regressions are shown in Table 2. The significant odds ratios of 1.12 and 1.14 indicate that each additional week of abstinence during treatment was associated with a 12–14% increased probability of abstinence at Month 12. The addition of the opioid dependence/maintenance status by treatment condition interaction in Step 3 was not significant in either the completer or full sample, ps > .84.
Table 2. Logistic Regressions Predicting a Negative Toxicology Screen at Month 12 Follow-Up.
| Variable | B (SE) | Wald | p value | Odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Completer sample (n = 185) | ||||
| Clinic | 0.06 (0.40) | 0.03 | .83 | |
| Baseline toxicology | 0.33 (0.50) | 0.43 | .51 | |
| Race | 5.03 | .17 | ||
| Age | 0.03 (0.02) | 2.69 | .10 | |
| Treatment prompted by legal system | 0.48 (0.40) | 1.49 | .22 | |
| Alcohol dependence criteria | 0.01 (0.07) | 0.02 | .89 | |
| Opioid dependence/maintenance status | 0.47 | .79 | ||
| Treatment condition | 0.19 (0.34) | 0.30 | .58 | |
| Longest duration of abstinence | 0.12 (0.05) | 5.19 | .02 | 1.12 (1.02–1.23) |
| Full sample (n = 239) | ||||
| Clinic | 0.10 (0.33) | 0.09 | .76 | |
| Baseline toxicology | 0.22 (0.41) | 0.28 | .60 | |
| Race | 0.61 | .90 | ||
| Age | 0.02 (0.01) | 1.49 | .22 | |
| Treatment prompted by legal system | 0.08 (0.30) | 0.07 | .80 | |
| Alcohol dependence criteria | −0.02 (0.06) | 0.15 | .70 | |
| Opioid dependence/maintenance status | 0.09 | .96 | ||
| Treatment condition | 0.17 (0.28) | 0.40 | .53 | |
| Longest duration of abstinence | 0.13 (0.04) | 11.31 | <.001 | 1.14 (1.06–1.24) |
Discussion
In this sample of individuals seeking outpatient psychosocial treatment for substance abuse, almost one third were opioid-dependent, and nearly half of them were not maintained on opioid medications. These data are consistent with epidemiological data indicating that up to 50% of opioid-dependent persons are not receiving opioid maintenance therapies (McCarty et al., 2010). Use of Suboxone was low in this sample (n = 8), most likely because of the low-income and uninsured or underinsured population served by these clinics. Given that a large proportion of opioid-dependent individuals do not receive maintenance treatment, it is important to investigate psychosocial treatments for this population.
Opioid-dependent patients, regardless of maintenance status, were younger than non–opioid-dependent substance abusing patients, and the group of non-maintained opioid-dependent patients was almost exclusively European American. Although African Americans constituted nearly a third of the full sample, only one African American was opioid-dependent and not on maintenance pharmacotherapy, suggesting that opioid-dependent African Americans are more likely to seek maintenance therapy, at least in New England.
The two opioid-dependent groups did not differ with respect to self-reported regular use of heroin or prescription opioids. These data are consistent with emerging studies of a growing rate of prescription opioid users accessing maintenance pharmacotherapy (Brands et al., 2004; Moore et al., 2007; Weiss et al., 2011), and a relative dearth of “pure” prescription opioid or “pure” heroin using patients among the opioid-dependent population (Brands et al., 2004). Nevertheless, the non-maintained opioid-dependent group was the most likely to report benzodiazepine usage, and all of them reported regular lifetime use of marijuana; they also endorsed more alcohol dependence criteria than their maintained opioid-dependent counterparts, suggesting high rates of polydrug use in non-maintained opioid-dependent individuals who seek psychosocial substance abuse treatment.
Despite their polysubstance abuse, all but one non-maintained opioid-dependent patient presented to treatment with a negative urine sample, a good prognostic indicator of long-term outcomes (Petry et al., 2004; Stitzer et al., 2007). These data suggest that either non-maintained opioid-dependent patients have less severe substance use problems than their maintained opioid-dependent counterparts, or they have made greater changes in their substance use before initiating treatment. The number of DSM–IV dependence criteria endorsed in this group was higher for both opioids and alcohol than in the maintained opioid-dependent group, and ASI drug and alcohol scores were similar, suggesting that low severity of substance use problems was not the reason for low rates of positive samples at treatment initiation in this group.
Non-maintained opioid-dependent patients fared as well in psychosocial treatment as their non–opioid-dependent counterparts, both in terms of attendance and longest duration of abstinence achieved. Opioid-dependent patients, regardless of maintenance status, had significantly improved outcomes when assigned to CM. Further, similarly to other studies (Higgins et al., 2000; Higgins et al., 2003; Petry et al., 2005, 2006b, 2007), longest duration of abstinence during treatment was a significant predictor of long-term outcomes, regardless of treatment condition or opioid dependence status.
Although this study found positive treatment outcomes among non-maintained opioid-dependent patients, these patients may have done better if they concurrently received methadone or Suboxone. Similarly, the relatively poor outcomes achieved by maintained opioid-dependent patients does not imply that maintenance pharmacotherapy is ineffective among this group, as without it they may fare even more poorly. The cohort of maintained opioid-dependent patients who present for additional outpatient substance abuse treatment services may represent a group “mandated” to additional psychosocial treatment to continue receiving maintenance pharmacotherapy. As such, this group may represent a severe and heavily substance-using population, although their DSM criteria and ASI composite scores did not differ significantly from their counterparts not receiving pharmacotherapy for opioid dependence. The relatively poor outcomes in this group may also relate to the burden associated with attending two drug abuse treatment programs concurrently (both for maintenance and psychosocial treatment alone).
Limitations of this study are that the data utilized to classify patients, both in terms of drug dependence status and pharmacotherapies, were based on self report. Further, the reasons for not initiating pharmacotherapy among opioid-dependent patients were not assessed; some patients may have recently detoxified from opioids, others may have been on waiting lists for maintenance therapies, some may have been discharged from maintenance clinics, and still others may have objected to pharmacotherapy. As noted above, the reasons why methadone maintained patients were seeking ancillary outpatient treatment for substance use were also not obtained. Many of these patients may have been told they could not continue with maintenance therapy unless their substance use problems dissipated; soon after these patients satisfied the requirement of their maintenance programs by enrolling in intensive outpatient treatment, they may have withdrawn from it, thereby impacting treatment outcomes.
This study is also limited by small sample sizes when opioid dependence and maintenance status were subdivided by treatment conditions. Moreover, two community-based clinics in New England participated in this study. Characteristics of opioid-dependent patients and access to maintenance pharmacotherapy may differ in other areas of the country. Although rates of follow-up were acceptable, missing data may have impacted the ability to ascertain between group differences, and follow-up results beyond one year would also be informative. Further, the two primary dependent variables were interrelated and highly correlated, so it is not surprising similar effects were noted with respect to both outcome measures.
Despite these limitations, this study found that more than 40% of opioid-dependent patients seeking outpatient psychosocial treatment were not receiving the standard of care for opioid dependence—maintenance medications. Nevertheless, non-maintained opioid-dependent patients responded better to psychosocial treatment than their maintained opioid-dependent counterparts, and as well as their substance abusing counterparts who were not dependent upon opioids. Data from this study suggest that CM appears to be an effective addition to standard outpatient treatment for the growing population of opioid-dependent individuals, and even among those who are not receiving maintenance pharmacotherapies.
Acknowledgments
This research and preparation of this report were supported in part by National Institutes of Health grants P30-DA023918, R01-DA027615, R01-DA022739, R01-DA13444, R01-DA018883, P50-DA09241, P60-AA03510, R01-HD075630, and M01-RR06192.
Contributor Information
Nancy M. Petry, Department of Medicine, University of Connecticut School of Medicine
Kathleen M. Carroll, Department of Psychiatry, Yale University School of Medicine
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th text rev. Washington DC: Author; 2000. [Google Scholar]
- Bovasso GB, Alterman AI, Cacciola JS, Cook TG. Predictive validity of the Addiction Severity Index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychology of Addictive Behaviors. 2001;15:171–176. doi: 10.1037/0893-164X.15.3171. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug and Alcohol Dependence. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Archives of General Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10:54–63. doi: 10.1037/1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders. Washington, DC: American Psychiatric Publishing; 1996. [Google Scholar]
- Gruber K, Chutuape MA, Stitzer ML. Reinforcement-based intensive outpatient treatment for inner city opiate abusers: A short-term evaluation. Drug and Alcohol Dependence. 2000;57:211–223. doi: 10.1016/S0376-8716(99)00054-X. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger G, Budney A. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037/1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Jones HE, Wong CJ, Tuten M, Stitzer ML. Reinforcement-based therapy: 12-month evaluation of an outpatient drug-free treatment for heroin abusers. Drug and Alcohol Dependence. 2005;79:119–128. doi: 10.1016/j.drugalcdep.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Katz EC, Chutuape MA, Jones HE, Stitzer ML. Voucher reinforcement for heroin and cocaine abstinence in an outpatient drug-free program. Experimental and Clinical Psychopharmacology. 2002;10:136–143. doi: 10.1037/1064-1297.10.2.136. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug and Alcohol Dependence. 2003;70:315–325. doi: 10.1016/S0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Leonhard C, Mulvey K, Gastfriend DR, Schwartz M. Addiction Severity Index: A field study of internal consistency and validity. Journal of Substance Abuse Treatment. 2000;18:129–135. doi: 10.1016/S0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews. 2009;1 CD002209. [Google Scholar]
- McCarty D, Perrin NA, Green CA, Polen MR, Leo MC, Lynch F. Methadone maintenance and the cost and utilization of health care among individuals dependent on opioids in a commercial health plan. Drug and Alcohol Dependence. 2010;111:235–240. doi: 10.1016/j.drugalcdep.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O'Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. Journal of Mental and Nervous Disease. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciloa J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Mental and Nervous Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Flower K, Pletcher MJ, Galloway GP. Addiction to prescription opioids: Characteristics of the emerging epidemic and treatment with buprenorphine. Experimental and Clinical Psychopharmacology. 2008;16:435–441. doi: 10.1037/a0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O'Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: Comparison of heroin and prescription opioid dependent patients. Journal of General Internal Medicine. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. The Journal of American Medical Association. 1998;280:1936–1943. doi: 10.1001/jama.280.22.1936. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies. (NHSDA Series H-14, DHHS Publication No SMA 02–3640) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2002. National and State Estimates of the Drug Abuse Treatment Gap: 2000 National Household Survey on Drug Abuse. [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York, NY: Routledge; 2012. [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, Sierra S. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006b;74:592–601. doi: 10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75:983–991. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling: Results of the National Drug Abuse Treatment Clinical Trials Network multi-site study. Drug and Alcohol Dependence. 2006a;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037/0022-006X.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology. 2011;79:686–696. doi: 10.1037/a0024813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug and Alcohol Dependence. 1999;54:127–135. doi: 10.1016/S0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug and Alcohol Dependence. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Fontana A, Cottrol C. Effect of clinician-veteran racial pairing in the treatment of posttraumatic stress disorder. The American Journal of Psychiatry. 1995;152:555–563. doi: 10.1176/ajp.152.4.555. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. The American Journal of Psychiatry. 2005;162:340–349. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. Characterizing the emerging population of prescription opioid abusers. The American Journal on Addictions. 2006;15:208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Peirce J, Petry NM, Kirby K, Roll J, Krasnansky J, Li R. Abstinence-based incentives in methadone maintenance: Interaction with intake stimulant test results. Experimental and Clinical Psychopharmacology. 2007;15:344–350. doi: 10.1037/1064-1297.15.4.344. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balance distributions of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11–4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. [Google Scholar]
- Substance Abuse and Mental Health Services Administration-Office of Applied Studies. The TEDS Report: Substance Abuse Treatment Admissions Involving Abuse of Pain Relievers: 1998 and 2008. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010. [Google Scholar]
- Tuten M, Defulio A, Jones HE, Stitzer M. Abstinence-contingent recovery housing and reinforcement-based treatment following opioid detoxification. Addiction. 2012;107:973–982. doi: 10.1111/j.1360-0443.2011.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]


