Abstract
A method for quantification of fludarabine (FDB) and clofarabine (CFB) in human plasma was developed with an API5000 LC-MS/MS system. FDB and CFB were extracted from EDTA plasma samples by protein precipitation with trichloroacetic acid. Briefly, 50 µL plasma sample was mixed with 25 µL internal standard (50 ng/mL aqueous 2-Cl-adensosine) and 25 µL 20% trichloroacetic acid, centrifuged at 25,000 g (20,000 rpm) for 3 min, and then transfered to an autosampler vial. The extracted sample was injected onto an Eclipse extend C18 column (2.1 × 150 mm, 5 µm) and eluted with 1 mM NH4OH (pH 9.6) - acetonitrile in a gradient mode. Electrospray ionization in positive mode (ESI+) and multiple reaction monitoring (MRM) were used, and ion pairs 286/134 for FDB, 304/170 for CFB and 302/134 for the internal standard were selected for quantification. The retention times were typically 3.72 min for FDB, 4.34 min for the internal standard, 4.79min for CFB. Total run time was 10 min per sample. Calibration range was 0.5–80ng/mL for CFB and 2–800ng/mL for FDB. The method was applied to a clinical pharmacokinetic study in pediatric patients.
Keywords: Fludarabine, Clofarabine, LC-MS/MS, plasma
1. Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) has become the standard-of-care treatment for a variety of pediatric diseases, including leukemias, immunodeficiencies, and hemoglobinopathies. Although major advancements have been made in recent years through improvements in supportive care, for children with non-malignant disorders and certain myeloid malignancies high rates of engraftment failure and disease relapse remain prominent clinical problems. One of the most common conditioning regimens used in these children prior to alloHCT consists of fludarabine (FDB) combined with the alkylating agent, busulfan[1, 2]. The addition of low-dose clofarabine (CFB) added to standard FDB and busulfan is being evaluated for safety and efficacy in a phase II trial (NCT01596699). CFB, like FDB, is a nucleoside analogue with potent antitumor and immunosuppressive properties[3–5]. At low concentrations, the combination of CFB, FDB, and busulfan showed a higher degree synergistic cytotoxicity when compared with either nucleoside alone in combination with busulfan[6]. Given that both drugs share a similar metabolic pathway, drug-drug interactions may impact pharmacokinetics (PK) and drug disposition through several mechanisms, including altered drug clearance via renal elimination. Currently, no PK data are available for a combination nucleoside analogue regimen containing both CFB and FDB to help inform optimal combination therapy. Such studies are limited by blood volume restrictions in children and dependent on a more sensitive, specific assay of CFB in plasma than those previously published[7].
A number of methods have been reported for the determination of FDB[8–10] and CFB [11–13]. However, methods for the simultaneous determination of these two nucleoside analogues have not yet been reported. Here we report an LC-MS/MS method for the simultaneous determination of FDB and CFB in human plasma with only a 50 µL sample, using 2-chloroadenosine as the internal standard (IS). Based on the regimen of the intended clinical study (40mg/m2 infusion of FDB over 1hr followed by 10mg/m2 infusion of CFB over 2hrs) and published PK studies [7, 14], we expect the minimal concentration in plasma for FDB and CFB will be >1ng/mL and <1ng/mL, respectively. Additionally, the anticipated maximum concentration will be around 1000ng/mL for FDB and 100ng/mL for CFB. Therefore we aim to develop a method with the calibration range at 2–800 ng/mL for FDB and 0.2–80 ng/mL for CFB.
2. Experimental
2.1. Chemicals and reagents
FDB and CFB Figure 1 were purchased from A.K Scientific Inc. (Mountain View, CA, USA); 2-chloroadenosine was from Sigma-Aldrich (St Louis, MO, USA). Acetonitrile (Optima™ LC/MS), water (Optima™ LC/MS), 21% ammonium hydroxide (Optima™), and trichloroacetic acid (Certified ACS) were obtained from Thermo-Fisher Sci., (Fair Lawn, NJ, USA). Mobile phase A was prepared by dissolving 91 µL 21% ammonium hydroxide (NH4OH) in 1L water; 20% trichloroacetic acid (TCA) was prepared by dissolving 2g TCA in 10mL water.
Figure 1.
Chemical structures of fludarabine, clofarabine, and 2-chloro-adenosine (IS).
2.2. Instrumental
An AB Sciex API5000 was coupled with Shimadzu Prominence 20ADXR UFLC pumps and SIL-20ACXR autosampler and managed with the software Analyst® 1.5.1. The gases for the MS system were supplied by an LC-MS gas generator (Source 5000™, Parker Balston Inc., Haverhill, MA, USA). LC conditions were as follows: Separation was achieved on a Zorbax Extend C18 (2.1×150 mm, 5µm, Agilent Tech. Inc., Santa Clara, CA, USA) equipped with a guard column (12.5 × 2.1 mm, 5 µm) from the same source. Mobile phase A was 1mM NH4OH and B was acetonitrile (MeCN). One microliter sample was injected onto the column eluted at a flow rate of 0.4 mL/min in a gradient program consisting of 4% solvent B (0–1min), from 4 to 30% B (2–5 min), from 30 to 90% B (5–5.1 min), 90% B (5.1–6 min), 90%–4% B (6.0–6.1 min), and 4% B (6.1–10min). Retention times for CFB, FDB and the IS were 4.7 min, 3.6 and 4.2 min, respectively. Needle wash solvent was 50% MeCN. The divert valve was set to direct LC eluent to mass spectrometer (MS) source at 2 min and to waste line at 5.9min. The MS conditions for FDB, CFB, and the IS were optimized by separate infusion of 50ng/mL corresponding drugs into the MS at a flow rate of 10 µL/min constantly while adjusting MS parameters with auto-tune followed by manual adjustment to achieve maximal signal. The ion pairs 286/134 for FDB, 304/170 for CFB, and 302/134 for the IS were used for quantification in multiple reaction monitoring (MRM) mode. The optimized compound-dependent MS parameters were 56 v (DP), 55 v (CE), 12 v (CXP) for FDB ion pair 286/134, 101 v (DP), 28 v (CE), 24 v (CXP) for CFB ion pair 304/170, and 86 v (DP), 55 v (CE), 10 v (CXP) for the IS ion pair 302/134, respectively. DP was declustering potential, CE was collision energy, and CXP was collision cell exit potential; entrance potential was 10 v for all ion pairs. The MS parameters were optimized to maximize signal for CFB because of higher sensitivity requirement. The optimized MS parameters were as follows: MS source was TurboIon Spray ionization in positive mode (ESI+) with turbo heater set at 600 °C; curtain gas was nitrogen at 40psi, nebulizer gas (gas1) and auxiliary (Turbo) gas (gas 2) were zero air both at 60psi, and collision-deactivated association gas was nitrogen at 9psi; ionspray voltage was 2000v. Data was processed with Analyst 1.5.1. (AB Sciex, Foster City, CA, USA).
2.3. Preparation of calibrators and QC samples
FDB and CFB primary stock solutions were prepared in 25% MeCN separately and diluted to prepare combined working solutions, which were spiked into EDTA human plasma to make calibrators of FDB/CFB at 2/0.2, 5/0.5, 10/1, 50/5, 100/10, 400/40, and 800/80 ng/mL and QC samples at 6/0.6, 60/6, and 700/70 ng/mL. Stock solution of 2-chloroadenosine (the IS) was prepared in 50% MeCN, which was serially diluted in water to make 50ng/mL IS working solution. The prepared solutions and plasma samples were stored at −70 °C until use.
2.4. Sample preparation
Plasma samples (50 µL) were pipetted into 1.5 mL polypropylene eppendorf tubes, to which were added 25 µL IS (50 ng/mL aqueous 2-chloro-adenosine) and 25 µL 20% TCA. After vortexing, the samples were centrifuged at 25,000g for 3 min and 60 µL of the supernatant was transferred to an autosampler vial. The injection volume was 1 µL.
2.5. Validation
The method was validated according to the guidelines of NIH-sponsored AIDS Clinical Trial Group Network[15], which was based on FDA guidelines[16]. One set of calibrators were processed for each run and injected at the beginning and end. Calibration curves were constructed by linear regression of the peak area ratio of analyte to internal standard (Y-axis) versus the nominal analyte concentrations (X-axis) with a weighting factor of 1/x. Precision was reported as relative standard deviation (RSD) and accuracy as percent deviation of the nominal concentration (% dev). The lower limit of quantification (LLOQ) was established with precision <20% and accuracy ±20%. Intra-day precision and accuracy were determined by analysis of at least five replicates of each QC sample at low (0.6/6 ng/mL), medium (6/60 ng/mL), and high (70/700 ng/mL) concentration levels extracted with a set of calibrators in one batch. The same procedure was repeated on at least 2 different days with new samples to determine inter-day precision and accuracy (total: n ≥ 15 per concentration level).
Recovery and matrix effect was evaluated according to the approach published by Matuszewski, et al.[17]. Three sets of validation samples at low, medium, and high concentration were prepared. Set 1 samples were prepared by spiking both drugs (FDB/CFB) in 5%TCA in water at 3/0.3, 30/3, 350/35 ng/mL, respectively. The IS concentration was 12.5 ng/mL. One sample at each concentration was prepared and injected for 6 times. Six different lots of plasma were used to prepare set 2 and set 3 validation samples. Set 2 samples were prepared by extracting blank plasma then spiking FDB/CFB and IS into the extracted matrix at the same concentration as set 1. Set 3 were prepared by spiking FDB/CFB at 6/0.6, 60/6, and 700/70 ng/mL in 6 different lots of plasma and extracting the samples as described in sample preparation section. Samples were injected in the order of set 1, 2, and 3 for each of the 6 lots of matrices for low concentration, followed by medium and high concentrations in the same injection order. The data from set 1 and set 2 were used to define overall system and detector performance, absolute and relative matrix effects, results from set 3 defined recovery and overall process efficiency.
The stability of FDB and CFB was evaluated in the following conditions: 3-freeze-thaw cycles, freezer (−70 °C), room temperature (22–25 °C) in plasma for 72hr and injection solvent (in autosampler vial) for 24hr and 96hr. Stock solution of FDB was tested for 2 months at −70 °C. IS working solution was tested at room temperature (22–25 °C) for 8 days. The treated samples were measured in triplicate at low and high concentrations and compared to the corresponding untreated samples. Stability was expressed as % remained and calculated as follows: % remained =100×Ctreated/Cuntreated, where C represents the measured mean concentration.
2.6. Application
The assay has been used to quantify FDB and CFB in an ongoing clinical trial in pediatric HCT recipients. The University of California San Francisco Committee on Human Subjects’ Research approved this study and all patients and/or guardians provided written informed consent to participate PK sampling. Per protocol, FDB (40mg/m2) was administered intravenously over 60 minutes, followed immediately by CFB (10mg/m2) infusion over 2 hours. An optimal sampling strategy using D-optimality methods was used to determine the PK collections times. For convenience, all collection times corresponded to the start of the FDB infusion. Following the first dose of analogue therapy, blood samples were collected 2 hours, 4 hours, 8 hours and 24 hours post start of FDB infusion. To assess inter-occasion variability, PK sample was repeated following a subsequent dose of analogue therapy (dose 2, 3, 4) at 2 hours and 24 hours post start of FDB infusion. In total, 6 blood samples per patient were collected over the entire course of nucleoside analogue treatment to gather preliminary data on FDB and CFB drug exposure. Blood samples for each plasma PK time point were collected in a 2mL spray-dried K2EDTA tube. Samples were immediately placed on wet ice and sent to the pediatric clinical research center for processing. Samples were spun at 3000–4000 rpm for 10 minutes in a refrigerated centrifuge at 4 °C. All specimens were centrifuged, aliquoted and frozen at −70 °C within 30 minutes of drawing and before analysis.
3. Results and discussion
3.1. LC-MS/MS optimization
The method was optimized based on a previous assay for FDB developed in our laboratory. During assay development of the prior method, we found ammonium hydroxide increased signal intensity by ~5-fold compared to neutral or acidic mobile phase solvents. This effect has been reported by others[18, 19], but the mechanism remains unclear. It may be that ammonium gas facilitates evaporation of ionized analytes in the ion source. The MS parameters were optimized to maximize CFB signal. The optimal Gas 2 was 40psi for FDB, however, the signal was 35% higher at 60psi for CFB, therefore, Gas 2 was set at 60psi. The optimal ionspray voltage was 5500v for FDB and 2000v for CFB. The assay utilized 2000v.
3.2. Sample preparation
FDB and CFB are both hydrophilic. A published method for FDB utilized MeCN to precipitate plasma protein, which required reconstitution to match the low initial organic mobile phase[10]. To simplify the procedure, here we used aqueous TCA to precipitate plasma protein. The supernatant could be directly injected onto LC-MS/MS system without compromising the peak shape. In addition, sample dilution caused by TCA protein precipitation could be minimized by using equal or less volume of TCA, while protein precipitation with organic solvents typically need 3-fold or more volume of solvent[10, 20]. The following procedure was used in this assay: 25 µL 20%TCA and 25 µL IS solutions were added into a 50uL plasma sample. After vortex-mixing and centrifugation, 70 µL of the supernatant was transferred into an autosampler vial and 1 µL was injected onto the LC-MS/MS system.
3.3. Validation
3.3.1. Calibration range
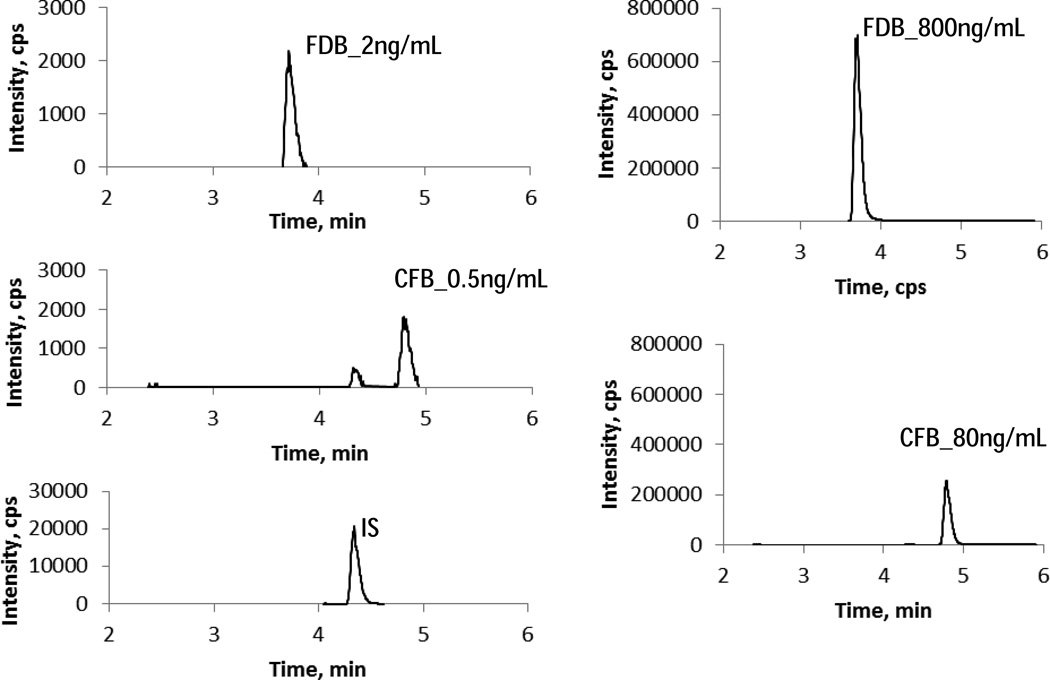
The LLOQ in this assay was initially set at 0.2ng/mL for CFB and 2ng/mL for FDB. The ULOQ was set at 80 and 800ng/mL for CFB and FDB, respectively. Since there was no baseline signal, LLOQ could not be determined by signal noise ratio. For CFB, the signal intensity was 1000cps (peak area, 6070) at 0.2ng/ml, but the signal from blank sample was present at significant level (>20%). We found mobile phase solvent was a source of contamination, and LC-MS grade solvents gave the least signal from blank sample injection. We raised LLOQ for CFB to 0.5ng/mL. The signal intensity was 1800 cps (peak area, 1.05×104) for 0.5ng/mL CFB and 2170cps (peak area, 1.20×104) for 2ng/mL FDB. The calibration curve was constructed with least square linear regression weighted by 1/x. The inter-day back-calculated concentrations of calibrators over 3 days were listed in table 1. Representative MRM ion chromatograms of FDB and CFB at LLOQ and ULOQ levels were shown in Figure 2.
Table.1.
Inter-day average back-calculated standard concentrations (n = 3)
| Fludarabine | Clofarabine | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Norminal conc, ng/mL | 2 | 5 | 10 | 50 | 100 | 400 | 800 | R | 0.5 | 1 | 5 | 10 | 40 | 80 | R |
| Mean, ng/mL | 1.87 | 4.79 | 10 | 52.3 | 103 | 403 | 791 | 0.9993 | 0.469 | 1.01 | 5.17 | 10 | 39 | 81 | 0.9997 |
| Precision (RSD,%) | 9.67 | 2.53 | 3.12 | 1.77 | 4.07 | 1.43 | 1.35 | 0.060 | 5.56 | 3.14 | 1.51 | 1.12 | 2.19 | 1.15 | 0.017 |
| Accuracy (% dev) | −6.50 | −4.27 | 3.23 | 4.67 | 3.30 | 0.83 | −1.08 | −6.13 | 1.17 | 3.40 | 3.33 | −2.50 | 0.67 | ||
| n | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||
Figure 2.
Representative chromatograms of FDB and CFB at LLOQ and ULOQ levels.
3.3.2. Precision and accuracy
The intra-day precision (n = 6) was within 10% at low, medium, and high concentrations for both FDB and CFB. The inter-day precision, calculated with the individual mean concentration from 3 days, was also within 10% at the three concentration levels for both FDB and CFB. The intraday accuracy and inter-day accuracy were all within ±10% for both FDB and CFB. At the LLOQ levels, the precision and accuracy met the criteria (20%) (Table 2).
Table 2.
Intra- and inter-day precision and accuracy.
| FDB | CFB | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FDB | Intra-day | Inter-day | Intra-day | Inter-day | ||||||||||||
| Nominal, ng/mL |
2.00 | 6.00 | 60.0 | 700 | 2.00 | 6.00 | 60.0 | 700 | 0.500 | 0.600 | 6.00 | 70 | 0.500 | 0.600 | 6.00 | 70.0 |
| mean, ng/mL | 1.80 | 6.12 | 65.1 | 713 | 1.95 | 6.29 | 64.5 | 723 | 0.491 | 0.558 | 6.45 | 74.6 | 0.457 | 0.589 | 6.48 | 75 |
| SD | 0.08 | 0.26 | 3.1 | 17 | 0.13 | 0.23 | 0.8 | 9.3 | 0.029 | 0.039 | 0.34 | 2.6 | 0.033 | 0.031 | 0.08 | 1.0 |
| RSD,% | 4.66 | 4.17 | 4.76 | 2.45 | 6.84 | 3.71 | 1.23 | 1.3 | 5.88 | 6.98 | 5.27 | 3.43 | 7.23 | 5.21 | 1.25 | 1.3 |
| %dev | −10.0 | 1.94 | 8.42 | 1.90 | −2.3 | 4.8 | 7.4 | 3.3 | −1.87 | −7.00 | 7.50 | 6.55 | −8.7 | −1.9 | 8.0 | 7.8 |
| n | 6 | 6 | 6 | 6 | 3 | 3 | 3 | 3 | 6 | 6 | 6 | 6 | 3 | 3 | 3 | 3 |
3.3.3. Matrix effect and recovery
Matrix effect was evaluated based on data from set 1, set 2, and set 3 (see supplemental table s1). Absolute matrix effect and recovery are listed in Table 3. The recovery was 95.7–106% for FDB, 81.6–89.2% for CFB, and 105–109% for the IS. Absolute matrix effect was evaluated with mean peak area values from set 1 and 2. A value of 100% means no matrix effect. If >100%, then ion enhancement was observed; if <100%, then ion suppression was observed. Ion suppression was observed for both FDB and IS, but ion enhancement was found for CFB. However, the matrix effect was within 85–115% at all concentration levels for both FDB and CFB (Table 3). These results indicate that matrix effect in the method is not significant. Relative matrix effect was evaluated by comparing the CV% from set 1 and set 2 (Table 4). The differences between CV% of peak areas from set 1 and 2 were −0.5, −0.8, and 1.0 for FDB, and − 2.9, −2.6, and −0.3 for CFB at low, medium, and high concentration levels, respectively; the corresponding values for IS were 0.4, −0.1, and 2.2, respectively. When comparing CV% from the peak area ratios, these values were 3.9, 0.3, and 2.3 for FDB, and −1.6, −1.3, and 0.3 for CFB, respectively. All values were within 5%, suggesting that there was no significant relative matrix effect.
Table 3.
Matrix effect, Recovery, and process efficiency calculated from mean peak areas of 6 replicates of samples (n=6)
| Conc (FDB/CFB) | Matrix Effect, % | Recovery, % | Process Efficiency,% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ng/mL | FDB | CFB | IS | FDB | CFB | IS | FDB | CFB | IS |
| Low (6/0.6) | 90.2 | 107 | 94 | 106 | 89.2 | 109 | 95.9 | 95.4 | 103 |
| Med (60/6) | 86.6 | 105 | 94 | 103 | 85.9 | 106 | 89.4 | 90.6 | 98.9 |
| High (700/70) | 89.9 | 103 | 93 | 95.7 | 81.6 | 105 | 86.0 | 84.2 | 98.5 |
Table 4.
Precision of peak areas and peak area ratio in set 1 and 2 (n=6). RSD represents relative standard deviation calculated from 6 replicates of samples.
| Conc.(FDB/CFB) | FDB peak area RSD, % | CFB peak area RSD, % | IS peak area RSD, % | Ratio (FDB/IS) RSD, % | Ratio (CFB/IS) RSD, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/mL | Set 1 | Set 2 | Set2-Set1 | Set 1 | Set 2 | Set2-Set1 | Set 1 | Set 2 | Set2-Set1 | set1 | set2 | set2-Set1 | set1 | set2 | set2-Set1 |
| Low (6/0.6) | 8.8 | 8.3 | −0.5 | 4.9 | 2.1 | −2.9 | 9.0 | 9.3 | 0.4 | 0.8 | 4.7 | 3.9 | 9.9 | 8.3 | −1.6 |
| Med (60/6) | 3.2 | 2.4 | −0.8 | 5.9 | 3.3 | −2.6 | 2.8 | 2.6 | −0.1 | 3.5 | 3.8 | 0.3 | 4.6 | 3.3 | −1.3 |
| High (700/70) | 3.1 | 4.1 | 1.0 | 5.7 | 5.4 | −0.3 | 3.1 | 5.3 | 2.2 | 2.9 | 5.2 | 2.3 | 4.4 | 4.8 | 0.4 |
Furthermore, slopes of lines connecting low, medium, and high QC samples from each lot of plasma were calculated. The CV% from set 3 was 4.55% for FDB and 4.07 for CFB (< 5%), confirming absence of significant matrix effect on quantification (Table 5).
Table 5.
Slopes of standard lines plotted through low, medium, and high QC from each matrix in set 1–3.
| FDB slope | CFB slope | |||||
|---|---|---|---|---|---|---|
| matrix# | set1 | set2 | set3 | set1 | set2 | set3 |
| 1 | 0.064 | 0.059 | 0.054 | 0.217 | 0.225 | 0.185 |
| 2 | 0.060 | 0.059 | 0.056 | 0.207 | 0.244 | 0.188 |
| 3 | 0.060 | 0.062 | 0.053 | 0.211 | 0.256 | 0.185 |
| 4 | 0.062 | 0.062 | 0.056 | 0.232 | 0.248 | 0.198 |
| 5 | 0.060 | 0.061 | 0.055 | 0.211 | 0.24 | 0.182 |
| 6 | 0.063 | 0.054 | 0.049 | 0.226 | 0.227 | 0.175 |
| mean | 0.062 | 0.059 | 0.054 | 0.217 | 0.240 | 0.186 |
| SD | 0.002 | 0.003 | 0.002 | 0.010 | 0.012 | 0.008 |
| CV% | 2.95 | 5.35 | 4.55 | 4.50 | 5.03 | 4.07 |
3.3.4. Stability
FDB and CFB were stable in both plasma and MeCN-water solution. No significant degradation was found under tested condition (Table 6). Further investigation is ongoing to define long term stability in −70 °C freezer.
Table 6.
Stability of FDB and CFB. Percentage of the remained drug (% remained) was calculated by comparing mean concentrations of the treated samples to the untreated samples in triplicate (n=3); RSD represents relative standard deviation for the treated samples.
| Conditions | FDB | CFB | |||
|---|---|---|---|---|---|
| In autosampler vial, 22–25 °C | % remained | RSD, % | % remained | RSD, % | |
| 24hr Low(6/0.6ng/mL) | 98 | 6.1 | 102 | 7.9 | |
| High(700/70ng/mL) | 109 | 1.9 | 104 | 2.6 | |
| 96hr Low(6/0.6ng/mL) | 84.2 | 1.3 | 106 | 4.3 | |
| High(700/70ng/mL) | 95.6 | 3.6 | 87.7 | 1.3 | |
| In plasma, 22–25 °C, 72hr | |||||
| Low(6/0.6ng/mL) | 98.6 | 1.7 | 100 | 4.8 | |
| High(700/70ng/mL) | 107 | 3.1 | 107 | 3.1 | |
| 3 Freeze-thaw cycles | |||||
| Low(6/0.6ng/mL) | 97.8 | 7.2 | 91.7 | 5.2 | |
| High(700/70ng/mL) | 97.1 | 3.7 | 106 | 5.4 | |
| plasma 2 months, −70 °C | |||||
| Low(6/0.6ng/mL) | 103 | 2.5 | 108 | 9.5 | |
| High(700/70ng/mL) | 105 | 0.9 | 106 | 3.2 | |
| Stock in 25% MeCN, 2 months, −70 °C | 99.8 | 4.1 | |||
| IS in water, 8 days, 22–25 °C | 99.7 | 3.3 | |||
3.3.5. Application
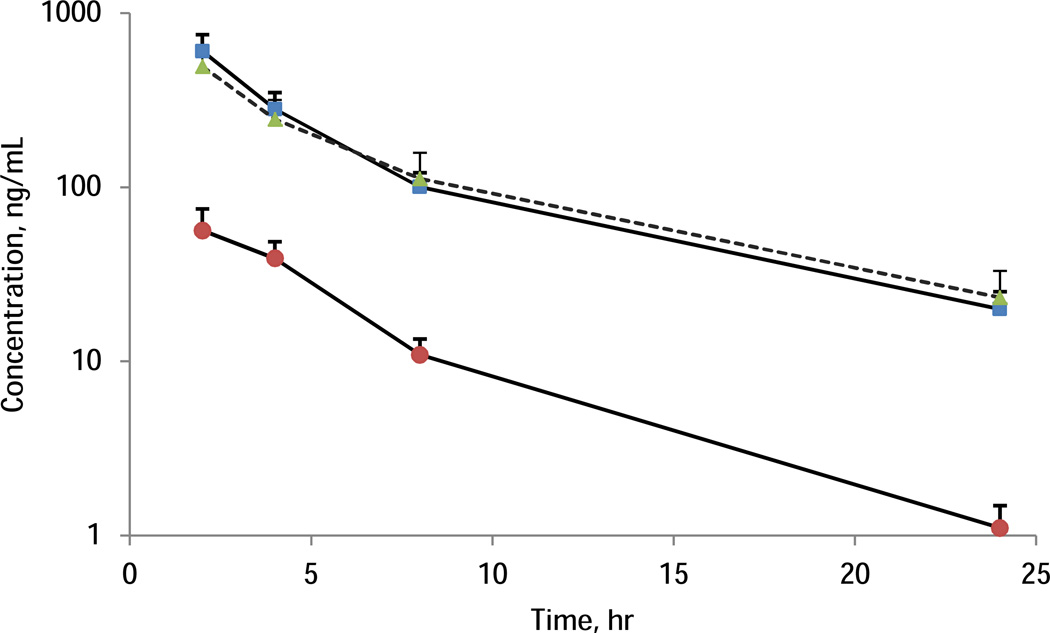
Using[JL1] this method, 77 samples containing both FDB and CFB and 42 samples containing FDB only were tested. Figure 3 showed a mean plasma concentration-time profile of FDB and CFB with error bar representing standard deviation. There is no significant difference of FDB exposure between administration of FDB alone (dash line with triangle markers, n=24) and in the context of low dose CFB (solid line with square markers, n=13). For FDB, 10 out of 119 samples were >ULOQ and required dilution, no samples were below LLOQ. For CFB, 1 sample was below LLOQ and 5 samples were above ULOQ. During the analysis, 16 QC samples at each QC level were processed. The precision of QC at low, medium, and high levels were 7.84%, 7.00%, and 8.13%, respectively for FDB, and 12.6%, 4.50%, and 8.62%, respectively for CFB. All QCs for FDB were within the accuracy range (85–115%). For CFB, bigger variation were found at low QC and 6 low QCs, one medium, and one high QC were out of the 85–115% range. The results demonstrate the sensitivity of the method met the requirement of the intended study. While 1mL plasma sample was collected in current clinical protocol, capillary sampling method could be used in the future to collect only 50–100 µL plasma. The quantification assay could be accordingly modified to use less than 50 µL sample volume as the injection volume was only 1µL in this reported assay. The assay could be modified to use less sample volume (e.g 25 µL) and more injection volume (e.g 2–10 µL). In that case, a partial validation will be performed.
Figure 3.
Mean plasma concentration-time profile of FDB and CFB. The dash line with triangle markers represents FDB alone from 24 subjects (n=24); solid lines represent combination of FDB (square markers) and CFB (circle markers). The error bar represents one standard deviation.
4. Conclusion
Using a simplified protein precipitation for sample preparation, a sensitive LC-MS/MS method was developed and validated for simultaneous determination of FDB and CFB in human plasma. The sensitivity of the assay met the requirement of the intended clinical study in pediatric patients.
Supplementary Material
Highlights.
Simultaneous quantification of fludarabine and clofarabine
Sensitive LC-MS/MS method suitable for pediatric studies
The method was applied to a clinical pharmacokinetic study in pediatric patients
Acknowledgments
Funding support: This work was supported by UCSF-GIVI Center for AIDS Research (AI027763) and the Thrasher Research Fund. Additionally, support was also provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health USA, through UCSF-CTSI Grant Number UL1 RR024131 and UCSF-CTSI Grant Number KL2 TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Horn B, Baxter-Lowe LA, Englert L, McMillan A, Quinn M, Desantes K, Cowan M. Reduced intensity conditioning using intravenous busulfan, fludarabine and rabbit ATG for children with nonmalignant disorders and CML. Bone Marrow Transplant. 2006;37:263–269. doi: 10.1038/sj.bmt.1705240. [DOI] [PubMed] [Google Scholar]
- 2.Law J, Cowan MJ, Dvorak CC, Musick L, Long-Boyle JR, Baxter-Lowe LA, Horn B. Busulfan, fludarabine, and alemtuzumab as a reduced toxicity regimen for children with malignant and nonmalignant diseases improves engraftment and graft-versus-host disease without delaying immune reconstitution. Biol. Blood Marrow Transplant. 2012;18:1656–1663. doi: 10.1016/j.bbmt.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Dow LW, Bell DE, Poulakos L, Fridland A. Differences in metabolism and cytotoxicity between 9-beta-D arabinofuranosyladenine and 9-beta-D-arabinofuranosyl-2-fluoroadenine in human leukemic lymphoblasts. Cancer Res. 1980;40:1405–1410. [PubMed] [Google Scholar]
- 4.Brockman RW, Cheng YC, Schabel FM, Jr, Montgomery JA. Metabolism and chemotherapeutic activity of 9-beta-D-arabinofuranosyl-2-fluoroadenine against murine leukemia L1210 and evidence for its phosphorylation by deoxycytidine kinase. Cancer Res. 1980;40:3610–3615. [PubMed] [Google Scholar]
- 5.Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 6.Valdez BC, Li Y, Murray D, Champlin RE, Andersson BS. The synergistic cytotoxicity of clofarabine, fludarabine and busulfan in AML cells involves ATM pathway activation and chromatin remodeling. Biochem Pharmacol. 2011;81:222–232. doi: 10.1016/j.bcp.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long-Boyle J, Huang J, Rydholm N, Smith A, Orchard P, Tolar J, Jacobson P. Pharmacokinetics of clofarabine in patients with high-risk inherited metabolic disorders undergoing brain-sparing hematopoietic cell transplantation. J. 2011;51:679–686. doi: 10.1177/0091270010372519. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra R, Calabro ML, Tommasini S, Villari A, Melardi S, Coppolino S, Semreen M, Ficarra P. Determination of fludarabine in a pharmaceutical formulation by LC. JPharm Biomed Anal. 1999;21:1077–1081. doi: 10.1016/s0731-7085(99)00189-2. [DOI] [PubMed] [Google Scholar]
- 9.Ng ES, Kangarloo SB, Daly A. Improved quantitative method for fludarabine in human plasma by liquid chromatography and tandem mass spectrometry. J Chromatogr B. 2013;931:103–110. doi: 10.1016/j.jchromb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Silvertand LH, Vazvaei F, Weigl P, Rosing H, Hillebrand MJ, van Maanen MJ, Beijnen JH. Simultaneous quantification of fludarabine and cyclophosphamide in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:3673–3680. doi: 10.1002/rcm.2242. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh Y, Duncan CJ, Lee S, Liu M. Comparison of fast liquid chromatography/tandem mass spectrometric methods for simultaneous determination of cladribine and clofarabine in mouse plasma. J Pharm Biomed Anal. 2007;44:492–497. doi: 10.1016/j.jpba.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Zhu B, ZHao L, YAn M, Fang Y, Li Y. Determination of clofarabine in human plasma by LC-MS/MS. Zhongguo Yaoxue Zazhi. 2010;45:1496–1499. [Google Scholar]
- 13.Bonate PL, Cunningham CC, Gaynon P, Jeha S, Kadota R, Lam GN, Razzouk B, Rytting M, Steinherz P, Weitman S. Population pharmacokinetics of clofarabine and its metabolite 6-ketoclofarabine in adult and pediatric patients with cancer. Cancer Chemother Pharmacol. 2011;67:875–890. doi: 10.1007/s00280-010-1376-z. [DOI] [PubMed] [Google Scholar]
- 14.Long-Boyle JR, Green KG, Brunstein CG, Cao Q, Rogosheske J, Weisdorf DJ, Miller JS, Wagner JE, McGlave PB, Jacobson PA. High fludarabine exposure and relationship with treatment-related mortality after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:20–26. doi: 10.1038/bmt.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Pharmacology Quanlity Assurance (CPQA) program. CPQA Guidelines for chromatographic method development and validation based on (and including) FDA guidelines dated May 2001. Version 4.0. 2012:1–52. in, www.fstrf.org. [Google Scholar]
- 16.US Department of Health and Human Services. Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation. [last accessed on January 6, 2014];2001 May; in, http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdfCenter.
- 17.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 18.Mess JN, Lahaie M, Furtado M, Garofolo F. Effect of high pH mobile phase on the sensitivity of multiple drugs by LC positive electrospray ionization MS/MS. Bioanalysis. 2009;1:1419–1430. doi: 10.4155/bio.09.133. [DOI] [PubMed] [Google Scholar]
- 19.Peng L, Farkas T. Analysis of basic compounds by reversed-phase liquid chromatographyelectrospray mass spectrometry in high-pH mobile phases. J Chromatogr A. 2008;1179:131–144. doi: 10.1016/j.chroma.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Marzan F, Jayewardene AL, Lizak PS, Li X, Aweeka FT. Development and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for determination of isoniazid in human plasma. J Chromatogr B. 2009;877:285–290. doi: 10.1016/j.jchromb.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.