Abstract
The important role of monocytes and macrophages in diseases like cancer and atherosclerosis has started to get uncovered in the last decade. In addition, subsets of these cell types are believed to participate in the initiation and aggravation of several diseases including cancer and cardiovascular disease. For this reason, monocytes and macrophages have recently been identified as interesting targets for both diagnosis and treatment of the aforementioned pathologies. Compared with free therapeutic or imaging agents, nanoparticle formulations provide several advantages that improve the pharmacokinetics and bioavailability of these agents. In addition, the possibility of surface functionalization creates numerous ways to optimize nanoparticle delivery. Recent advances in nanomedicine have led to the development of multifunctional nanoparticles that allow simultaneous diagnosis and treatment of monocytes and macrophages with high specificity. Relying on the inherent ability of monocytes and macrophages to easily take up foreign particles, the use of nanoparticles provides a precious opportunity for the management of several inflammatory diseases.
Keywords: anti-inflammatory, diagnosis, macrophage, monocyte, nanomedicine, nanoparticles, treatment
The innate immune system regulates the initial defenses toward infection and disease and includes a widely distributed network of cells that ‘patrol’ the body. As we enter the second decade of the 21st century, the authors find that the understanding of the innate immune system has grown remarkably over the last 50 years. While the cooperative functions of the two major components in immunity (cellular and humoral) were initially recognized in 1908, it was not until the late 1960s that the field of innate immunity took a strong foothold, with the discovery that the lack of a well-developed phagocytic function of innate immune cells could cause a severe immunodeficiency disease with fatal outcomes [1]. Since then, the field of innate immunology, facilitated by the development of modern biochemical techniques, has matured to an enormously broad and intriguing web of knowledge that is based on three simple principles, namely recognition of pathogens, killing these pathogens and self-tolerance. The key players in all of these processes are the myeloid cells, among which the monocyte-derived macrophages are probably the most versatile phagocytes [2]. Macrophages cannot be described as a uniform population of cells since they are found throughout the body and their forms and functions are most likely characterized by the location in which they reside. Their numbers are constantly replenished by a pool of circulating blood monocytes that originate either directly from the bone marrow or, as suggested in a very recent study, from a reservoir within the spleen [3]. Moreover, the authors and others have shown that major tissue-resident macrophages may derive from local progenitors and are maintained by local proliferation as was recently confirmed [4]. While most macrophages are unmistakably related to the host’s health maintenance, ‘supervising’ their environment and reacting to pathogen invasion or damage, others are just as well involved in the initiation, progression and aggravation of many major diseases such as cancer [5], cardiovascular disease (CVD) [6], diabetes [7] and tuberculosis [8].
Since macrophages are constantly involved in ‘screening’ for infection or disease, they are naturally widely distributed within the body. Macrophages express an impressive amount of different receptors (e.g., Fc, complement, scavenger and lectin receptors) that allow them to actively recognize a wide array of ligands and molecules. While their ability to engulf pathogens, apoptotic cells and other debris makes them an important player in any type of inflammation, they are also known to be involved in the pathogenesis of many diseases. Because of this bilateral role, macrophages have rapidly become an attractive target for both diagnosis and treatment of diseases. Therefore, the development of new strategies to specifically target macrophages (and monocytes) has become the focus of many research efforts in the last decade [9–12].
The specific targeting of monocytes and macrophages with nanoparticles may hold great promise for both the diagnosis and treatment of the major diseases (cancer, CVD, diabetes and tuberculosis). The application of nanotechnology in (bio)medicine is referred to as nanomedicine, and involves drug delivery [13] or, in case of diagnostics, the delivery of contrast-generating materials [14] to specific targets using so-called nanocarriers. The main advantages of these nanoparticle systems are the possibility to incorporate high payloads of the aforementioned drugs and/or contrast agents, the improved pharmacokinetics and bioavailability of these payloads and the possibility of surface functionalization [15]. The latter includes coating nanoparticles with polymers that improve the circulation half-life as well as derivatization with targeting ligands [16]. In the case of targeting monocytes and macrophages, their ability to easily take up foreign particles provides a precious opportunity for nanomedical intervention. In this review, the authors will present the current state of nanoparticle-based drug delivery and diagnostics in the context of specifically targeting monocytes and macrophages for both treatment and diagnosis of related diseases.
General roles of monocyte & macrophage subsets
Monocytes
Until the discovery of different surface markers, such as CD14 and CD16 on human monocytes, monocytes were considered to be rather homogenous groups of immune cells with similar physiological functions. The identification of different antigenic markers leads to the discovery of at least two different subsets of monocytes in both humans and mice. However, most of the studies on monocyte subsets have been performed in mice, which have a different distribution of mononuclear cells [17]. While the characteristics of both mouse and human monocytes were found to be surprisingly similar [18], one has to be cautious and not forget that the differences in function have not been fully explored.
In the blood circulation, ‘classical’ monocytes, or Ly6Chi in mice and CD14hiCD16− in humans, account for approximately 50% of the monocytes in mice and 90% of the monocytes in humans, respectively. ‘Nonclassical’ monocytes, or Ly6Clow in mice and CD14+CD16+ in humans, most likely account for the rest [19]. Notably, the exact number of subsets of monocytes, their specific role and mutual connections remain unclear. However, the discovery of these subsets has led many to believe in a predisposition of monocytes subsets and fueled new and interesting theories for targeted diagnosis and treatment concerning these cells. An overview of the generally acknowledged subsets and their functions is described below.
The identification of monocyte subsets does not only depend on the expression of main surface markers, but also by the monocytes’ ability to respond to different signaling molecules. In 2003, Geissmann et al. showed that the distinct migratory properties of the two main monocyte subsets were due to the presence of receptors for inflammatory chemokines, like CX3CR1, CXCR2 and CCR2, on Ly6Chi/CD14hiCD16− monocytes, which were absent on Ly6Clow/CD14+CD16+ monocytes (Figure 1). This observation not only confirmed the heterogeneity of monocytes but also put forward a clear functional difference between the two subsets. This functional difference, the ability to respond to inflammation, was further explored in numerous studies, which all showed that the presence of CCR2 on monocytes is a necessity for the proper migration of monocytes to sites of inflammation [20–23]. Thus, while it was already known that tissue macrophages are mainly renewed by local proliferation independently of monocyte influx [24], most inflammatory macrophages still rely on their circulating predecessors in case of acute inflammation. A study by Swirski et al. confirmed this by showing the trafficking of monocytes from the reservoir in the spleen to injured tissue shortly after ischemic myocardial injury [3]. By contrast, Zigmond et al. recently reported on the uniqueness of the gut, as it is believed to be the only organ with a continuous infiltration of Ly6Chi monocytes that participate in normal maintenance [25]. It was only after local inflammatory stimuli that these monocytes were observed to change into proinflammatory effector cells. These studies underlined the importance of Ly6Chi/CD14hiCD16− monocytes in inflammation as well as maintenance in the gut, but also suggest that these monocytes most likely play an active role in fueling certain diseases [26].
Figure 1. Monocyte and macrophage subsets.
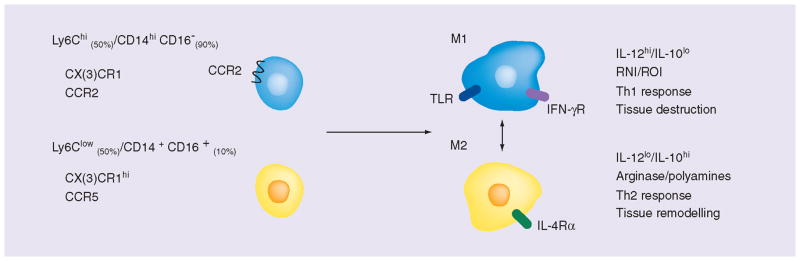
Monocytes and macrophages are generally divided into two subsets that represent different expression patterns of surface markers and functions. Monocytes are divided into Ly6Chi/CD14hiCD16– monocytes (50% in mice and 90% in humans, respectively) and Ly6Clow/CD14+CD16+ monocytes (50% in mice and 10% in humans, respectively). Ly6Chi/CD14hiCD16− monocytes are characterized by expression of CCR2, which allows for trafficking of the monocytes towards inflamed tissues. Ly6Clow/CD14+CD16+ monocytes lack CCR2 but express CCR5 instead. It is not known if one of these subsets specifically matures into one of the two major macrophage subsets. M1 macrophages are known as inflammatory macrophages. They produce inflammatory cytokines and reactive oxygen species. They primarily stimulate the Th1 response and are involved in tissue destruction in many inflammatory diseases. M2 macrophages are described as anti-inflammatory or regulatory macrophages. They produce IL-10 to dampen local inflammation and are mainly involved in the Th2 response and chronic diseases. Their ability to produce tissue remodeling factors makes them valuable in the remodeling and regeneration of tissue. However, their functions are also a cause of unwanted tolerance in cancer.
The role of Ly6Clow/CD14+CD16+ or nonclassical monocytes is less well understood and their exact function remains subjected to divergent research results. As numbers of human CD14+CD16+ monocytes in the blood were found to increase in relation to several pathologies, including cancer [27] and atherosclerosis [28], they were at first described as ‘inflammatory’ monocytes. However, this view is probably not precise as their role seems far more complex and situational. Indeed, it was found that the migratory ability of Ly6Clow/CD14+CD16+ monocytes to inflamed sites is far less than that of the Ly6Chi/CD14hiCD16− counterparts [18, 29]. The nonclassical monocytes express CCR5 instead of CCR2 (Figure 1) and the current belief is that these monocytes express an anti-inflammatory phenotype, mostly being involved in later stages of inflammation and wound healing [3]. Interestingly, a recent publication by Carlin et al. revealed a detrimental role for Ly6Clow monocytes in the survey of endothelium lining the capillaries [30]. Ly6Clow monocytes were found to respond to TLR-7-dependent danger signals from the endothelium by increasing their intravascular retention, attracting neutrophils and orchestrating the elimination of endangered endothelial cells. Thus, while Ly6Clow monocytes are unlikely to participate in inflammation dependent on CCR2, their role in the maintenance of the vascular system could help explain why Ly6Clow monocytes have previously been linked to atherosclerosis and cancer [26].
Macrophages
Macrophages are mononuclear cells with a strong phagocytic function. They reside within practically all tissues, ready to engulf a wide variety of pathogens, apoptotic cells and other debris, but also participate in resolving inflammation and tissue remodeling, making them indispensible in both immunity and maintenance [31]. They have a close relation to dendritic cells as they originate from the same common myeloid progenitor, share many surface markers and react to the same growth factors [32], although dendritic cells are primarily known for activation of lymphocytes [33]. Macrophages are a heterogeneous group of cells that cannot easily be divided into subsets. There is a wide array of macrophage subsets that perform different functions depending on their anatomical location, for example, osteoclasts (bone) [34], Kupffer cells (liver) [35] and alveolar macrophages (lung) [36]. Within a single organ, such as the spleen, many subsets of macrophages with divergent functions coexist. For example, red pulp macrophages engulf old red blood cells while different subsets of white pulp macrophages are involved in the immune response [37]. However, in the case of inflammation, two general roles of macrophages can be distinguished, namely inflammatory and anti-inflammatory (or regulatory).
Inflammatory macrophages, also called M1 macrophages, are often referred to as being classically activated, as opposed to the alternatively activated regulatory macrophages, or M2 macrophages. The M1 macrophages are known to react strongly to invading pathogens (LPS and lipoteichoic acid) or other danger signals (IFN-γ and tumor necrosis factors) by producing proinflammatory cytokines, inducible nitric oxide synthases and reactive oxygen species (Figure 1) [38]. They are the type of macrophages that are predominantly seen in the early phase of acute inflammation or in the later stages of chronic inflammation [39]. While they exert most of their functions locally, the secretion of proinflammatory cytokines, like CXCL9 and CXCL10, attracts T cells and natural killer cells to aid in the inflammatory process [5]. However, in some cases, the M1 macrophages are unable to cease their production of inflammatory mediators, leading to an uncontrolled inflammation and finally tissue destruction as a result of reactive oxygen species and the recruitment of other immune cells. Many autoimmune diseases are believed to originate from this process. In atherosclerosis, activated M1 macrophages turn into so-called foam cells after accumulating lipids. These foam cells are believed to contribute substantially to atherosclerotic plaque formation and will be discussed in more detail below.
The anti-inflammatory macrophages or M2 macrophages are known as regulatory macrophages with tolerogenic and restorative properties. The M2 macrophage, which is induced by IL-4 [5], is mainly active in the later stages of acute inflammation, in Th2 skewed inflammation and many chronic diseases (Figure 1) [31]. As stated before, at the point where the immune system has resolved the infection, it becomes crucial to control the inflammation. In these later stages, M2 macrophages produce anti-inflammatory mediators, such as IL-10 and IL1-receptor antagonist, and arginase 1, to dampen the inflammation. In addition, they produce cytokines, like CCL-22, to attract supporting cells (Th2 cells and Tregs) [40] that help to maintain homeostasis. They are also actively involved in wound healing and fibrosis by phagocytosis of cellular debris and the secretion of several factors with tissue remodeling properties, like matrix metalloproteases, growth factors and angiogenic factors [41]. However, the anti-inflammatory activities of M2 macrophages can also cause unwanted tolerance in cancer [5]. The suppressive role of tumor-associated macrophages (TAMs) results in growth and metastasis of the tumor and is discussed in more detail below.
While the general subsets of M1 and M2 macrophages are acknowledged, the classical view of predestined inflammatory or anti-inflammatory macrophages has become untenable. It now seems that subsets of macrophages exist only because they are programmed by their environment to become inflammatory or anti-inflammatory. Switching between different phenotypes along the course of an inflammation, from a M1 to a M2, seems to be the rule rather than the exception [42,43]. The fact that macrophages are constantly submerged in high levels of anti-inflammatory IL-10 in many tissues also points toward a scenario of interchangeable phenotype. It is only when the initial regulatory state is broken by infection or disease that macrophages tend to turn toward a more inflammatory phenotype. Adding to this, many inflammatory (chronic) disorders, like inflammatory bowel disease (IBD) [44], rheumatoid arthritis (RA) [45] and diabetes [46], are caused by a change in the environment, which leads to a broken regulatory/tolerant state of the macrophages involved.
Role of monocytes & macrophages in cancer & CVD
Cancer
Over the last few decades, a vast amount of evidence has been collected that highlighted the importance of monocytes and macrophages in cancer. Inflammatory (M1) macrophages are involved in eliminating unwanted tumor cells, but M2 TAMs represent a more pronounced subset that can inhibit the antitumor response, enhance tumor growth and promote metastasis [47]. The presence and number of these TAMs are in many cases correlated with a poor prognosis for patient survival [48]. Likewise, splenectomized mice showed a great reduction of relocating monocytes and subsequent TAM numbers, which resulted in reduced tumor growth and metastasis [49]. The exact origin of these TAMs is currently unknown, although several mechanisms have been proposed. The tumor environment is characterized by hypoxia, dying cells and chronic inflammation [50], and these features attract and activate monocytes and macrophages alike. Within this environment, TAMs are strongly influenced by the secretion of signal molecules by other immune cells and tumor cells. It is molecules such as CCL-2 [51], CSF-1, IL-10 and TGF-β that are of key importance to the monocyte recruitment and polarization toward an M2 phenotype of TAMs. It must be noted, however, that different tumors can induce differential skewing of macrophages, depending on the origin of the tumor [52].
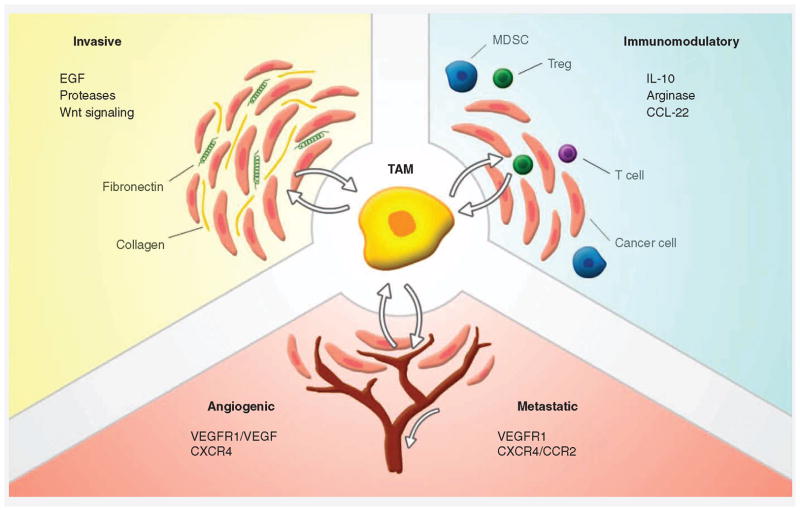
While most TAMs are generally characterized by impaired antigen presentation and inflammatory capabilities [5], several subsets of TAMs can be distinguished. Transcriptome analysis shows the different roles that TAMs can adopt, depending on the tumor in which they reside (Figure 2) [47]. Invasive TAMs produce growth factors such as EGF that promote tumor growth. Furthermore, they produce proteases that can remodel the extracellular matrix, providing easy passage for tumor cells. Immunomodulatory TAMs participate in the production of anti-inflammatory cytokines, like IL-10, and can recruit Tregs by the release of CCL-22. They are thought to be related to myeloid-derived suppressor cells, which can suppress inflammatory responses by recruiting Tregs. Angiogenic and metastatic TAMs produce factors like VEGF that promote blood vessel growth and promote metastasis. Furthermore, they express the VEGFR1, which is linked to premetastatic niche forming (Figure 2) [47].
Figure 2. Subsets of tumor-associated macrophages can influence the tumor environment.
Tumor-associated macrophages (TAMs) are polarized toward phenotypes favoring tumor growth. These ‘educated’ macrophages have been shown to interact with the tumor cells and provide them with several benefits. By producing anti-inflammatory mediators, TAMs create a tolerant environment for the tumor cells to thrive. Expression of growth factors and proteases by TAMs allows for further growth and invasiveness of the tumor. TAMs also promote angiogenesis by secreting several growth factors, allowing further progression of the tumor. Metastasis is promoted by angiogenesis but also by the formation of metastatic niches by TAMs at distant locations.
CVD & atherosclerosis
Atherosclerosis, the major contributor to CVD, is characterized by the development of lipid-rich lesions within the major arteries. As the lesions progress into atherosclerotic plaques, they can narrow the artery lumen and reduce blood flow, or more importantly, can rupture into the lumen. A ruptured plaque causes the formation of a thrombus (Figure 3C) [6,53], which can break free and cause obstructions, frequently leading to a myocardial infarction or stroke.
Figure 3. Monocytes and macrophages in the development of an atherosclerotic lesion.
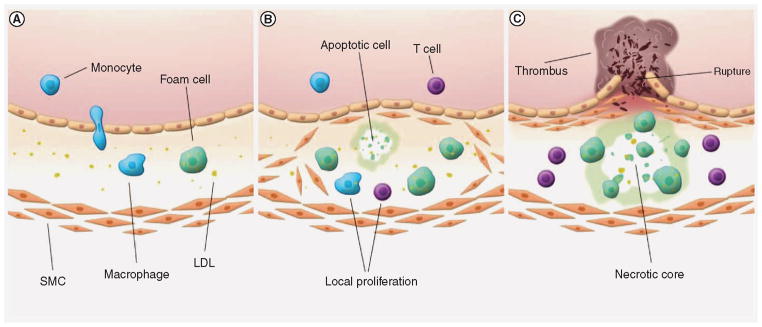
(A) The initial processes of atherosclerotic plaque initiation are unknown. However, early inflammation in the arterial wall results in the infiltration of monocytes that differentiate into activated macrophages. Macrophages will take up oxidized LDL and free lipids, transforming into lipid-rich foam cells. (B) A deregulation of the inflammatory process causes increased expression of adhesion molecules on endothelial cells above, resulting in enhanced influx of immune cells. At the same time, SMCs are thought to grow outward and establish themselves under the endothelial layer, producing extracellular matrix proteins that eventually form a fibrous cap. Local proliferation of macrophages and lymphocytes increases atherosclerotic plaque growth. (C) As more immune cells promote the inflammation, foam cells and SMCs go into apoptosis and form a necrotic core. Proteases produced by macrophages further destabilize the lesion, which is believed to increase the risk of plaque rupture. A ruptured lesion exposes its tissue factors to coagulation factors in the blood, resulting in the formation of a thrombus. LDL: Low-density lipoprotein; SMC: Smooth muscle cell.
The processes that are involved in the initiation and progression of the disease have recently been described in great detail by others [53,54], and the importance of monocytes and macrophages in these processes has become undisputed. As low-density lipoprotein (LDL) in the blood starts to accumulate at subendothelial locations within the artery walls, monocytes and other leukocytes will be attracted to these sites (Figure 3A). Initial inflammatory processes result in oxidized LDL that causes the expression of more adhesion molecules by the endothelial cell layer above. Especially proinflammatory CCR2-expressing monocytes accumulate within the developing plaque [55], eventually maturing into inflammatory macrophages. Within the lesion, macrophages engulf lipids, including oxidized LDL and cholesterol crystals, and transform into so-called ‘foam cells’ [56]. Interestingly, Kadl et al. recently identified a possible new macrophage subset that develops in response to atherogenic (oxidized) phospholipids [57]. At the same time, smooth muscle cells are believed to grow outward from the underlying media, producing extracellular matrix molecules that make up most of the lesion’s fibrous cap. The amount of immune cells in the lesions increases over time, either by infiltration or local proliferation [58], stimulating atherosclerotic plaque growth (Figure 3B). As more macrophages, T cells and mast cells promote the ongoing inflammatory process [59,60], many smooth muscle cells and foam cells undergo apoptosis, promoting the formation of a necrotic core at the center of the lesion. Macrophages produce proteases that break down extracellular matrix, such as collagen fibers, and destabilize the plaque. Eventually, these necrotic and unstable plaques can rupture, resulting in the exposure of plaque components to the blood and subsequent thrombus formation within the arterial lumen by platelet adhesion (Figure 3C).
Nanomedicine
The use of nanotechnology in (bio)medicine, referred to as nanomedicine, is comprised of several distinct applications including treatment and diagnosis of disease [61]. Nanomedicine makes use of nanomaterials (usually <100 nm in one dimension) that, due to their size, possess unique features compared with larger particles (>100 nm) [62]. For example, it has been shown that the size of the nanoparticles influences their distribution and uptake in vivo [63,64] and also influences drug loading and release capabilities, as well as the stability of the nanoparticles [65]. In addition, smaller nanoparticles have increased surface to mass ratio, allowing for more efficient incorporation of surface molecules that can optimize their functionality. Compared with free therapeutic or imaging agents, nanoparticle systems provide several advantages that improve the pharmacokinetics and bioavailability of these agents. In addition, the possibility of surface functionalization creates numerous ways to optimize nanoparticle delivery [66]. The development of nanoparticle systems is a comprehensive task that, among others, involves the adjustment of features like size, stability, surface characteristics, targeting, drug loading, drug release, circulation half-life and eventually therapeutic or diagnostic efficacy and toxicity [62]. As research continues, the fabricated systems have become more advanced and more adapted to a specific goal [64]. For example, while many of the first-generation nanoparticles lacked specificity and were cleared from the body at a fast rate, surface modification with, for example, a poly(ethylene) glycol coating increases their stability and elimination half-life dramatically [13]. In addition, nanoparticles can be equipped with specific targeting moieties to increase their accumulation in targeted biological systems, providing a more selective treatment with potentially less adverse effects. Although much progress has been made, several challenges in nanoparticle research still exist, including the integration of controlled release or theranostic properties [67], and will remain the focus of research for many years to come.
Over the course of the past few decades, a wide range of nanoparticle systems, differing in size, composition and function, have been developed and approved for clinical use [68]. The most used drug delivery systems are first-generation liposomal nanoparticles and polymeric nanoparticles. Liposomes were the first class of nanoparticles to be considered for targeted drug delivery. They consist of a bilayered lipid membrane surrounding a variable-sized aqueous core. While the core is used to load therapeutics, derivatization of the membrane with various compounds can improve the stability and efficacy of the liposomes [13]. Because of their biodegradable nature and ability to improve drug bioavailability, several liposomal variants are already in clinical use. Polymeric nanoparticles are comprised of several biodegradable (co)polymers and are often formed through self-assembly [69], a process driven by the hydrophobic, hydrophilic and amphiphilic characteristics of the different (co)polymers. Because of the vast amount of different polymer combinations, the variations in polymeric nanoparticle composition and properties are endless [69]. In addition, many types of drugs can either be attached to or encapsulated in polymeric nanoparticles, while derivatization with targeting ligands can improve their effectiveness [62].
As the use of nanoparticles for the delivery of drugs has been shown to hold great promise, more and improved nanoparticle systems for the treatment and diagnosis of disease are being developed and several of them are now in clinical trials [68]. Besides liposomes and polymeric nanoparticles, other systems include dendrimers [70], inorganic nanocrystals [71] and polymeric micelles [72]. Especially in the field of oncology [73] and to a lesser extent in CVD [74], nanoparticle systems that provide better methods of treatment and diagnostics are currently under development. Since the focus of this review lies in the therapeutic possibilities of targeting monocytes and macrophages specifically, an overview of the most prominent nanoparticle systems targeting these cells will be provided below.
Applications of nanomedicine in targeting monocytes & macrophages
Targeting monocytes & macrophages
As the understanding of the role of monocytes and macrophages in many diseases, including cancer [5], CVD [6], diabetes [7], tuberculosis [8], RA [75], IBD [76] and chronic obstructive pulmonary disease (COPD) [77], grows, a lot of recent research has been dedicated toward targeting these cells. As mentioned before, the specific targeting of monocytes and macrophages represents a unique opportunity that is facilitated by the advantages provided by nanoparticle systems and the inherent ability of monocytes and macrophages to effectively take up foreign materials. While most of the currently approved nanoparticle systems were designed to avoid uptake by the mononuclear phagocyte system (MPS) to increase their half-life, a novel approach consists of applying nanoparticles that include modifications that enhance their uptake by monocytes and macrophages. These modifications include changes in size, surface charge and the use of specific targeting ligands [78]. Since monocytes and macrophages possess a great repertoire of receptors (e.g., scavenger receptors, lectin receptors, Fc receptors and integrins) for the internalization of ligands, a wide variety of lipids, proteins, lectins and antibodies can be used for this purpose. In general, the combination of characteristics of nanoparticles that increase their uptake by the MPS include a >100 nm size [79,62], a negative surface charge [80,81] and derivatization with ligands, targeting one of the characteristic receptors of these cells. In addition, subsets of macrophages can be targeted specifically by decorating nanoparticles with small synthetic molecules. For example, while dextran-coated metal nanoparticles are a popular choice for targeting macrophages in general, additional decoration with glycine increases their uptake by activated macrophages [82]. A selection of nanoparticle systems targeted to monocytes and macrophages is provided in Table 1.
Table 1.
Nanoparticle systems targeting macrophages for diagnostics and treatment.
| Particle type | Ligand | Imaging/therapeutic | Disease | Ref. |
|---|---|---|---|---|
| Diagnostic | ||||
| CLIO | Dextran | MRI | Diabetes | [98] |
| Ferumoxytol (USPIO) | Carboxy-dextran | MRI | Cancer/atherosclerosis | [99,100] |
| P1133 (USPIO) | Folate | MRI | Cancer | [84] |
| 64Cu-DTPA-NP (MION) | Dextran | PET-CT/MRI | Atherosclerosis | [101] |
| Protein cage | LyP-1 | Fluorescence imaging | Atherosclerosis | [102] |
| 99mTc-nanobody | Anti-MMR | SPECT | Arthritis/cancer | [103,104] |
| Quantum dot | Fluorescence imaging | Cancer | [105] | |
| Treatment | ||||
| Liposome | Anionic lipids | Rifampicin | Tuberculosis | [106] |
| Liposome | Muramyl tripeptide | MTP-phosphotidylethanolamine | Cancer | [107] |
| Liposome | Anti-VCAM-1 | Prostaglandins | Atherosclerosis | [108] |
| Liposome | Mann-C4-Chol | Dexamethasone | Inflammatory lung disease | [109] |
| Dendrimer | Folic acid | Methotrexate | Inflammatory arthritis | [110] |
| Chitosan | Chitosan | Anti-TNF-α siRNA | Inflammatory arthritis | [111] |
| Quantum dot | Doxorubicin | Inflammatory lung disease | [112] | |
| Gelatin | Mannan | Didanosine | HIV [113] | |
| Theranostic | ||||
| CLIO-AF750-THPC | Dextran | Fluorescence imaging/phototoxicity | Atherosclerosis | [114] |
CLIO: Crosslinked dextran-coated iron oxide; MION: Monocrystalline iron oxide nanoparticle; THPC: Meso-tetra(hydroxyphenyl)chlorin; USPIO: Ultra-small superparamagnetic iron oxide.
Nanoparticle-facilitated diagnosis of monocytes & macrophages
Nanoparticle systems are well suited for the diagnosis of disease and can provide prognostic information on patients with established diseases. As mentioned before, diagnostic nanoparticles not only provide an enhanced circulation half-life of the contrast agent, but can also improve the specificity through targeted imaging. There are a multitude of diagnostic imaging techniques that can be combined with a variety of nanoparticle contrast agents for molecular imaging purposes [83]. MRI is often combined with iron oxide nanoparticles or nanoparticles loaded with gadolinium chelates [84,85]; gold nanoparticles are a potent x-ray computed tomography (CT) agent [86,87]; in addition, 98Zr and 111In-labeled nanoparticles can be used for PET [11,88] and single-photon emission computed tomography (SPECT) [89], respectively. In addition to clinical imaging modalities, preclinical fluorescence/optical imaging methods benefit from nanoparticulate luminescent materials such as quantum dots [86]. To render inorganic nanoparticles more biocompatible for in vivo use, a multitude of coatings, including phospholipids, polysaccharides and HDL, have been researched. Each of the different coatings provides unique features that influence the functionality and specificity of the nanoparticle [71]. Moreover, they allow for the incorporation of additional imaging labels that can add optional ways of visualizing them.
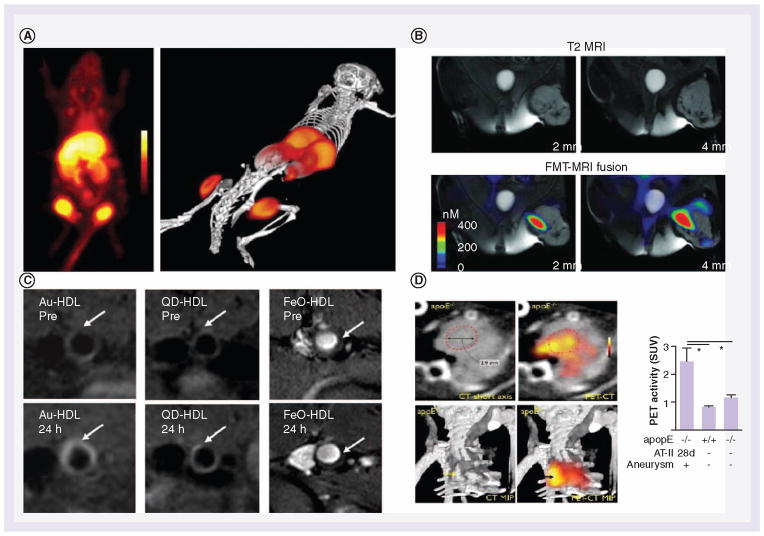
The location, numbers and different subsets of macrophages in tumors and atherosclerotic plaques has been studied in great detail and can be used to predict the prognosis of diseases [48,90]. Therefore, multiple studies on visualizing (subsets of ) macrophages for diagnostic purposes have been performed in both cancer and CVD. Recently, Keliher and colleagues reported a macrophage-specific nanoparticle that allowed in vivo imaging of tumors by PET [10]. To accomplish this, small dextran nanoparticles (13 nm) that had been previously shown to accumulate in macrophages [91], were radiolabeled with 98Zr and applied in a syngeneic colon carcinoma mouse model. Figure 4A shows the localization of the nanoparticles within four mouse tumors visualized by PET. Histological Mac-3 staining showed colocalization of 98Zr dextran nanoparticles with TAMs, suggesting uptake by these cells.
Figure 4. Diagnostics in cancer and cardiovascular disease.
(A) A macrophage-specific nanoparticle that allowed for in vivo imaging of tumors by PET scan. Small dextran nanoparticles were shown to accumulate in macrophages and were radiolabeled with 98Zr. The nanoparticles can be seen to be localized in four different tumors in 2D and a 3D rendered image. (B) MRI (top) in combination with fluorescence molecular tomography (bottom) for tumor-associated macrophage (TAM) visualization in a mouse model of soft tissue sarcoma. A magnetofluorescent nanoparticle with high specificity for activated macrophages was used, revealing distribution of TAMs within the tumor. Both imaging techniques showed TAMs to be located at the same sites. (C) A multimodality contrast agent platform was created to identify macrophages in atherosclerosis. Combination of a HDL shell surrounding a variable inorganic core mimicked HDL particles present in the body. The core consisted of either gold, quantum dots or iron oxide. In vivo MRI shows localization of the nanoparticles within the aortic wall. Accumulation within macrophages was confirmed afterwards. (D) PET-CT imaging for the detection of monocytes and macrophages in aortic aneurysms (AA). Dextran-coated iron oxide nanoparticles were used as a base for subsequent 18F conjugation to the particle. Nanoparticle content in AA was visualized by PET and guided by CT. PET intensity in AA was significantly higher compared with the controls, as displayed in the graph. Up to 90% of the nanoparticles were tracked specifically to monocytes and macrophages in the AA by staining.
(A) Reproduced with permission from [10].
(B) Reproduced with permission from [92].
(C) Reproduced with permission from [86].
(D) Reproduced with permission from [85].
Another recent study used fluorescence molecular tomography (FMT) and MRI to visualize TAMs in a mouse model of soft tissue sarcoma in the left crural muscle [92]. In this study, a magnetofluorescent nanoparticle with high specificity for activated macrophages was used, combining a dextran-coated iron oxide core supplemented with the VT680 fluorochrome. LSL-KrasG12D-p53−/− mice with established tumors were injected with the imaging agent. After 24 h, in vivo imaging by MRI and FMT revealed the distribution of TAM within the tumor (Figure 4B). FMT-MRI fusion images revealed colocalization of both signals. Histological analysis of tumor tissue confirmed uptake of the nanoparticles by tumor-associated macrophages with an M2 phenotype, while no uptake was reported in leukocytes or tumor cells in the region.
Compared with the field of cancer research, the field of CVD is relatively new to the use of nanoparticle systems. However, when specifically targeting monocytes and macrophages, the same diagnostic techniques can be used. A nice example of the use of MRI to identify macrophages in atherosclerosis was reported in a study by Cormode et al. in 2008. In this study, a multimodality contrast agent platform was created by surrounding a variable inorganic core with an HDL shell, mimicking HDL particles present in the body [86]. The core consisted of either gold, quantum dots or iron oxide. ApoA-1 incorporated in the lipoprotein shell provided affinity to macrophages, while the inclusion of rhodamine provided optional (in vitro) visualization possibilities. The nanocrystals core HDL particles were injected into apolipoprotein E−/− mice with advanced atherosclerotic plaques. In vivo MRI showed localization of the nanoparticles within the aortic wall (Figure 4C). Further ex vivo analyses and fluorescence measurements confirmed the nanoparticles to be localized mainly within the present macrophages. In addition, control nanoparticles without ApoA-1 showed almost no accumulation within the aortic wall.
Another study by the group of Nahrendorf used PET-CT imaging for the detection of monocytes and macrophages in aortic aneurysms (AA) [85]. In AA, monocytes and macrophages are linked to inflammation of the arterial wall that in turn can lead to acceleration of complications [93]. Early detection of such inflammation can possibly predict the increased risk. Dextran-coated iron oxide was used as a contrast agent scaffold for subsequent 18F conjugation. Apolipoprotein E−/− mice which had been kept on a high-cholesterol diet for 6 months were subsequently administered with angiotensin-II for 28 days, a model for AA induction. Initial aneurisms were detected by micro-CT while nanoparticle uptake in AA was visualized by PET (Figure 4D) and guided by CT. PET intensity was significantly higher in AA compared with controls, as displayed in the graph of Figure 4D. Up to 90% of the nanoparticles were found to be specifically associated with monocytes and macrophages in AA, as established by immunostaining and flow cytometry. Despite the limited resolution of PET, this technique showed the macrophage burden in AA through the use of nanoparticles, and in the future, may provide better assessment of risk and allow for a more personalized treatment regimen.
Treatment of disease by monocytes & macrophage nanoparticle targeting
Today, many of the available liposomal and polymeric nanoparticle systems are aimed at the treatment of cancer [73]. Most of them were developed with the goal to transport a drug into a targeted (solid) tumor as they provide longer circulation half-life and better bioavailability compared with free drugs. The tumor vasculature is inherently ‘leaky’ and enables the extravasation of nanoparticles into tumor tissue through the enhanced permeability and retention (EPR) effect [64]. In addition, as tumors have a less developed lymphatic system, clearance of nanoparticles from the tumor is impaired. The EPR effect has provided the basis for many effective nanoparticle therapies. Problems that had to be avoided using this tactic were the possible side effects from poor targeting and low half-life due to clearance by the MPS. However, as the pivotal role of monocytes and macrophages in cancer and other diseases like atherosclerosis has become apparent, the specific targeting of the MPS seems to hold great promise for future therapies.
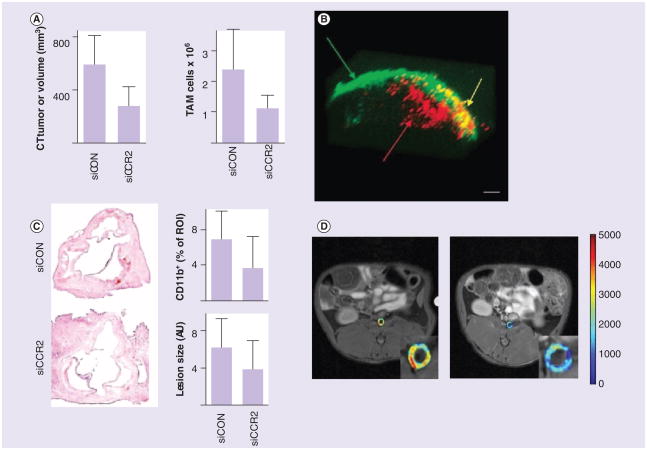
A study by Leuschner et al. exemplified the potential of nanomedicine to target monocytes and macrophages in inflammatory diseases. Their nanoparticle therapy was aimed at reducing the expression of CCR2 receptor on the Ly6Chi monocyte subset by nanoparticle-delivered siRNA [11]. To that end, the authors incorporated fluorescently labeled siRNA into a lipid-based nanoparticle system that was readily taken up by splenic monocytes as determined by in vivo fluorescence measurements. Since CCR2 is monocyte (and even subset) specific and responsible for migration of monocytes from the bone marrow [21] and the spleen to the site of inflammation, the siRNA nanoparticles were found to decrease monocyte recruitment in atherosclerosis, myocardial infarction, diabetes and cancer. For example, the therapy reduced the myocardial infarct size by 34% and increased islet graft survival after pancreatic islet transplantation. In a mouse model of lymphoma, it reduced the number of TAMs by 54% as well as the tumor size (Figure 5A). In a mouse model for atherosclerosis, the treatment reduced the number of monocytes and macrophages in lesions by 82%, as shown by flow cytometry analysis. Immunohistochemical staining for CD11b showed up to 46% reduction in the number of myeloid cells while the lesion size itself was decreased by 38% (Figure 5C). The versatility of this nanomedical method signifies that nanoparticle-based treatments targeted toward monocytes can have a great impact on the treatment for a variety of diseases.
Figure 5. Treament of cancer and cardiovascular disease.
Except for (D), studies used monocyte- and macrophage-specific nanoparticle systems. (A) An effective therapy based on inhibition of the production of the CCR2 receptor on the Ly6Chi monocyte subset by siRNA technology. Tumor size reduction and reduced TAM numbers. siCON represents control values while siCCR2 represents inhibitory siRNA (n = 5–7). Supplementary in vivo fluorescence measurements (not shown) of the labeled siRNA confirmed its location to be mainly in the spleen. The siRNA was found to decrease monocyte recruitment. In a mouse model of lymphoma it reduced the number of TAMs by 54% as well as the tumor size. (B) Monocytes and macrophages in combination with nanoparticles to create a ‘Trojan horse’ therapy for an in vitro model of cancer. Nanoparticles consisting of a silica core surrounded by a gold shell absorb light in a near-infrared spectrum that resonates with the nanoparticle. Light is then converted to heat that can kill the surrounding tissue. Monocytes and macrophages (red) were observed to infiltrate into the spheroids (green), where laser illumination resulted in tissue destruction (yellow). (C) The effective therapy based on inhibition of the production of the CCR2 receptor on the Ly6Chi monocyte subset by siRNA technology. An immunohistochemical analysis of aortic roots is shown on the left. The amount of CD11b-positive cells was decreased as well as lesion size. siCON represents control values while siCCR2 represents inhibitory siRNA (n = 8–14). (D) Dynamic contrast-enhanced MRI shows the potential of a liposomal combination of glucocorticoids to treat atherosclerosis in a rabbit cancer model. Initial MRI (left) showed nanoparticles to be localized in the inflamed vessel wall. The antiangiogenic effect of glucocorticoids reduced the permeability within 2 days after the treatment (right). Nanoparticles were found to be strongly associated with macrophages inside the atherosclerotic aorta.
(A) Adapted with permission from [11].
(B) Reproduced with permission from [9].
(C) Adapted with permission from [11].
(D) Reproduced with permission from [95].
Another study from 2007 also targeted the recruitment of monocytes. However, instead of inhibiting their trafficking, Choi et al. used monocytes in combination with nanoparticles to create a ‘Trojan horse’ therapy for an in vitro model of cancer [9]. These nanoparticles consisted of a silica core surrounded by a gold shell that can absorb light in a near-infrared spectrum that penetrates tissue. While the nanoparticles stay naturally inert within the cell, illumination by a laser with the corresponding wavelength to the resonance of the nanoparticles allowed it to convert the light into heat [94]. This heat increased the local temperature to such levels that it killed the surrounding tissue. While other similar therapies use nanoparticle systems that rely on targeting the tumor cells, in this study monocytes were used to infiltrate the tumor instead. Cultured monocytes were incubated with the nanoparticles and were differentiated into macrophages. Subsequently, macrophages containing the nanoparticles were added to spheroids, an in vitro model for hypoxic tumors. Macrophages were observed to infiltrate into the spheroids, where laser illumination resulted in tissue destruction (Figure 5B). However, near infrared can only pass through a few inches of tissue, which until now only allows for the treatment of subcutaneous tumors. New techniques that allow near-infrared light to be used in deeper tissues will have to be assessed, for example, through the use of intravascular catheters.
A promising translational study by Lobatto et al. showed the potential of liposomal glucocorticoids to treat atherosclerosis in a rabbit model [95]. Glucocorticoids, such as prednisolone, are potent anti-inflammatory hormones that are used to combat several inflammatory diseases [96]. To increase the efficacy and decrease the adverse effect, prednisolone was incorporated in long-circulating liposomal carrier. Additional labeling with paramagnetic GD-DTPA-DSA lipids enabled the visualization of its accumulation in the inflamed vessel wall by clinical MRI. Within 7 days after treatment, 18F-fluorodeoxyglucose PET revealed a reduction in inflammation in arterial wall, which indicated the effectiveness of the treatment. As glucocorticoids are known to have an antiangiogenic effect as well, the permeability of the target region was evaluated by dynamic contrast-enhanced MRI. Two days after the treatment, plaque permeability was found to be decreased, showing the effectiveness of the treatment (Figure 5D). The exact therapeutic and nanoparticle targeting mechanism are still a topic of investigation. Interestingly, while long-circulating liposomes are actually designed to avoid uptake by macrophages, they were found to be strongly associated with macrophages inside the atherosclerotic aorta. As the amount of macrophages in plaques also decreased after several days of treatment, correlating with the decreased permeability, this could indicate a possible role for macrophages in the observed therapeutic effects. As this specific nanoparticle system was found to be rather effective in treating several facets of atherosclerosis, it would be interesting to see the effect of glucocorticoid-loaded nanoparticle systems that specifically target macrophages.
Expert commentary
Advances in nanochemistry in general and novel nanoparticle production methods in particular have paved the way for exciting novel applications. While an impressive number of promising nanoparticle systems are currently available, several aspects need to be addressed. As of today, only a handful of nanoparticle systems have been approved for use in the clinic. While many nanoparticle systems have been shown to possess great potential for treatment and diagnosis of disease, toxicity issues remain a concern as systemic exposure and accumulation in the body is significantly elevated compared with low molecular weight agents. Controlled release second-generation nanoparticle systems are now tested in clinical trials and are believed to have longer lasting effects at lower doses [97]. While these nanoparticle systems use more advanced techniques, like ligand targeting and activated drug release, multifunctionality is usually not exploited. Theranostic nanoparticle systems, which are nanoparticle drug formulations that can also be tracked by means of noninvasive imaging, hold promise for screening patients amenable for such nanotherapy. In case imaging reveals the nanotherapy to not accumulate at the diseased site a patient can be easily excluded from the therapy at a very early stage. However, while theranostic nanoparticles can be very advantageous for clinical use, their production and the involvement of elaborate diagnostic procedures may not be cost effective and complicate clinical exploitation.
Five-year view
Future applications
A lot of time and effort has been spent on the development of so-called stealth nanoparticles. These nanoparticles have improved half-life because their surfaces are coated with hydrophilic polymers that increase stability and avoid clearance by the MPS – that is, uptake by monocytes and macrophages. Conversely, the important role of monocytes and macrophages in cancer and atherosclerosis has been uncovered in the last decade. As described in this review, several research groups have now started to explore the possibilities of monocyte and macrophage targeted nanotherapeutics. For example, nanoparticle systems can specifically inhibit the trafficking of monocytes to inflamed tissues, thereby reducing inflammation in a variety of pathological processes. In addition, diagnostic nanoparticle systems targeting macrophages provide multiple ways of locating and diagnosing atherosclerotic lesions or tumors in vivo.
The role of monocytes and macrophages in many diseases (RA, IBD, diabetes and COPD) is still under investigation, and it is likely that new insights may create new opportunities for nanomedical interventions. It is not unlikely that, with the help of nanoparticle systems, we will soon be able to polarize monocyte and macrophage subsets and use them to help treat disease. The design of novel nanoparticle systems should therefore be tailored to novel insights in immunopathology. The progress the field has witnessed in the past 5 years indicates that 5 years from now, as our understanding of diseases increases and more advanced and safe nanoparticle systems enter clinical trials, nanoparticle treatment will have started to enter the clinic for a variety of inflammation-related conditions.
Key issues.
While the presence of most monocytes and macrophages is related to the host’s health maintenance, some subsets of these cells are involved in the initiation, progression and aggravation of major diseases such as cancer and atherosclerosis.
Nanoparticle systems provide several advantages that improve the pharmacokinetics and bioavailability of therapeutic or imaging agents. In addition, the possibility of surface functionalization allows for direct targeting of monocytes and macrophages.
Monocytes and macrophages have been shown to be promising targets for both diagnosis and treatment of cancer and cardiovascular disease.
Newly developed multifunctional nanoparticles will provide multiple ways of locating and diagnosing atherosclerotic lesions or tumors in vivo.
Footnotes
Financial & competing interests disclosure
This work was financially supported by the National Heart, Lung, and Blood Institute, NIH, as a Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C, the NIH grant R01CA155432 (WJM Mulder) and the American Heart Association Award #13PRE14350020-Founders (J Tang) The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Holmes B, Quie PG, Windhorst DB, Good RA. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966;1(7449):1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- 2.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 6.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in Type 1 and Type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl. 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 8.Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annu Rev Pathol. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 9.Choi MR, Stanton-Maxey KJ, Stanley JK, et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7(12):3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 10.Keliher EJ, Yoo J, Nahrendorf M, et al. 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging. Bioconjug Chem. 2011;22(12):2383–2389. doi: 10.1021/bc200405d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–1010. doi: 10.1038/nbt.1989. Shows the role of inflammatory monocytes in several diseases and provides a monocyte-specific therapeutic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davignon JL, Hayder M, Baron M, et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013;52(4):590–598. doi: 10.1093/rheumatology/kes304. [DOI] [PubMed] [Google Scholar]
- 13.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 14.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29(7):992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012;41(7):2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62(2):90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(10):1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 19.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74(7):2527–2534. [PubMed] [Google Scholar]
- 20.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201(11):1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7(3):311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 22.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. 2010;17(1):53–59. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 24.Randolph GJ. Immunology. No need to coax monocytes. Science. 2011;332(6035):1268–1269. doi: 10.1126/science.1208480. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond E, Varol C, Farache J, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37(6):1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleh MN, Goldman SJ, LoBuglio AF, et al. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85(10):2910–2917. [PubMed] [Google Scholar]
- 28.Schlitt A, Heine GH, Blankenberg S, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92(2):419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 29.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211(6–8):609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. An overview of the different macrophage subsets and their relation to health and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181(9):5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 33.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10(6):453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henriksen K, Bollerslev J, Everts V, Karsdal MA. Osteoclast activity and subtypes as a function of physiology and pathology–implications for future treatments of osteoporosis. Endocr Rev. 2011;32(1):31–63. doi: 10.1210/er.2010-0006. [DOI] [PubMed] [Google Scholar]
- 35.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26(10):1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2(5):403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214(2):161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 41.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30(3):245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175(1):342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 43.Hagemann T, Lawrence T, McNeish I, et al. ‘Re-educating’ tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206(9):1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinne RW, Stuhlmüller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther. 2007;9(6):224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. Detailed description of all the different roles that tumor-associated macrophages can fulfill in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritu- moral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol. 2011;28(4):1447–1452. doi: 10.1007/s12032-010-9638-5. [DOI] [PubMed] [Google Scholar]
- 49.Cortez-Retamozo V, Etzrodt M, Newton A, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci USA. 2012;109(7):2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 51.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Bögels M, Braster R, Nijland PG, et al. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology. 2012;1(6):798–809. doi: 10.4161/onci.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 54••.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. Provides an overview of the pathogenesis of atherosclerosis and recent advances in diagnosis and treatment. [DOI] [PubMed] [Google Scholar]
- 55.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. Explains the different roles of macrophages in atherosclerosis in great detail. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107(6):737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol. 1995;147(3):668–677. [PMC free article] [PubMed] [Google Scholar]
- 59.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 61.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 62.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 63.Chono S, Tauchi Y, Morimoto K. Influence of particle size on the distributions of liposomes to atherosclerotic lesions in mice. Drug Dev Ind Pharm. 2006;32(1):125–135. doi: 10.1080/03639040500390645. [DOI] [PubMed] [Google Scholar]
- 64••.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. Overview of the current applications of nanoparticle systems in the diagnosis and treatment of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 67•.Tang J, Lobatto ME, Read JC, Mieszawska AJ, Fayad ZA, Mulder WJ. Nanomedical Theranostics in Cardiovascular Disease. Curr Cardiovasc Imaging Rep. 2012;5(1):19–25. doi: 10.1007/s12410-011-9120-6. Update on the current status of advanced theranostic nanoparticles in the field of cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. Comprehensive overview of most of the nanoparticle systems that are applied in medicine. [DOI] [PubMed] [Google Scholar]
- 69.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Baker JR., Jr Dendrimer-based nanoparticles for cancer therapy. Hematology Am Soc Hematol Educ Program. 2009:708–719. doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]
- 71.Cormode DP, Sanchez-Gaytan BL, Mieszawska AJ, et al. Inorganic nanocrystals as contrast agents in MRI: synthesis, coating and introduction of multifunctionality. NMR in Biomed. 2013;10 doi: 10.1002/nbm.2909. 1002/nbm 2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 73.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 74.Lobatto ME, Calcagno C, Metselaar JM, et al. Imaging the efficacy of anti-inflammatory liposomes in a rabbit model of atherosclerosis by non-invasive imaging. Meth Enzymol. 2012;508:211–228. doi: 10.1016/B978-0-12-391860-4.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19(3):289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 76.Heinsbroek SE, Gordon S. The role of macrophages in inflammatory bowel diseases. Expert Rev Mol Med. 2009;11:e14. doi: 10.1017/S1462399409001069. [DOI] [PubMed] [Google Scholar]
- 77.Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1(1):59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 78.Kelly C, Jefferies C, Cryan SA. Targeted liposomal drug delivery to monocytes and macrophages. J Drug Deliv. 2011;2011:727241. doi: 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Epstein-Barash H, Gutman D, Markovsky E, et al. Physicochemical parameters affecting liposomal bisphosphonates bioactivity for restenosis therapy: internalization, cell inhibition, activation of cytokines and complement, and mechanism of cell death. J Control Release. 2010;146(2):182–195. doi: 10.1016/j.jconrel.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 80.Fidler IJ, Raz A, Fogler WE, Kirsh R, Bugelski P, Poste G. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980;40(12):4460–4466. [PubMed] [Google Scholar]
- 81.Ahsan F, Rivas IP, Khan MA, Torres Suarez AI. Targeting to macrophages: role of physicochemical properties of particulate carriers–liposomes and microspheres–on the phagocytosis by macrophages. J Control Release. 2002;79(1–3):29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 82.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23(11):1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 83.Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov. 2011;10(11):835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daldrup-Link HE, Golovko D, Ruffell B, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17(17):5695–5704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nahrendorf M, Keliher E, Marinelli B, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31(4):750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cormode DP, Skajaa T, van Schooneveld MM, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8(11):3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mieszawska AJ, Mulder WJ, Fayad ZA, Cormode DP. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol Pharm. 2013;10(3):831–847. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen K, Conti PS. Target-specific delivery of peptide-based probes for PET imaging. Adv Drug Deliv Rev. 2010;62(11):1005–1022. doi: 10.1016/j.addr.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 89.Gorantla S, Dou H, Boska M, et al. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J Leukoc Biol. 2006;80(5):1165–1174. doi: 10.1189/jlb.0206110. [DOI] [PubMed] [Google Scholar]
- 90.Swirski FK, Pittet MJ, Kircher MF, et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA. 2006;103(27):10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121(1):442–445. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leimgruber A, Berger C, Cortez-Retamozo V, et al. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia. 2009;11(5):459–468. doi: 10.1593/neo.09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26(5):987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 94.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 95.Lobatto ME, Fayad ZA, Silvera S, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm. 2010;7(6):2020–2029. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 97.Hrkach J, Von Hoff D, Mukkaram Ali M, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 98.Fu W, Wojtkiewicz G, Weissleder R, Benoist C, Mathis D. Early window of diabetes determinism in NOD mice, dependent on the complement receptor CRIg, identified by noninvasive imaging. Nat Immunol. 2012;13(4):361–368. doi: 10.1038/ni.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30(1):15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yancy AD, Olzinski AR, Hu TC, et al. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: critical determinants of atherosclerotic plaque labeling. J Magn Reson Imaging. 2005;21(4):432–442. doi: 10.1002/jmri.20283. [DOI] [PubMed] [Google Scholar]
- 101.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uchida M, Kosuge H, Terashima M, et al. Protein cage nanoparticles bearing the LyP-1 peptide for enhanced imaging of macrophage-rich vascular lesions. ACS Nano. 2011;5(4):2493–2502. doi: 10.1021/nn102863y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Put S, Schoonooghe S, Devoogdt N, et al. SPECT Imaging of Joint Inflammation with Nanobodies Targeting the Macrophage Mannose Receptor in a Mouse Model for Rheumatoid Arthritis. J Nucl Med. 2013 doi: 10.2967/jnumed.112.111781. [DOI] [PubMed] [Google Scholar]
- 104.Movahedi K, Schoonooghe S, Laoui D, et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72(16):4165–4177. doi: 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]
- 105.Jackson H, Muhammad O, Daneshvar H, et al. Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery. 2007;60(3):524–9. doi: 10.1227/01.NEU.0000255334.95532.DD. discussion 529. [DOI] [PubMed] [Google Scholar]
- 106.Vyas SP, Kannan ME, Jain S, Mishra V, Singh P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int J Pharm. 2004;269(1):37–49. doi: 10.1016/j.ijpharm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 107.Nardin A, Lefebvre ML, Labroquère K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6(2):123–133. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- 108.Homem de Bittencourt PI, Jr, Lagranha DJ, Maslinkiewicz A, et al. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis. 2006;193(2):245–258. doi: 10.1016/j.atherosclerosis.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 109.Wijagkanalan W, Higuchi Y, Kawakami S, Teshima M, Sasaki H, Hashida M. Enhanced anti-inflammation of inhaled dexamethasone palmitate using mannosylated liposomes in an endotoxin-induced lung inflammation model. Mol Pharmacol. 2008;74(5):1183–1192. doi: 10.1124/mol.108.050153. [DOI] [PubMed] [Google Scholar]
- 110.Thomas TP, Goonewardena SN, Majoros IJ, et al. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011;63(9):2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, Kjems J. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17(1):162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chakravarthy KV, Davidson BA, Helinski JD, et al. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomedicine. 2011;7(1):88–96. doi: 10.1016/j.nano.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaur A, Jain S, Tiwary AK. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: in vitro and in vivo evaluation. Acta Pharm. 2008;58(1):61–74. doi: 10.2478/v10007-007-0045-1. [DOI] [PubMed] [Google Scholar]
- 114.McCarthy JR, Korngold E, Weissleder R, Jaffer FA. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6(18):2041–2049. doi: 10.1002/smll.201000596. [DOI] [PMC free article] [PubMed] [Google Scholar]