Abstract
Impairment of dendritic cells (DC), the most effective activators of anticancer immune responses, is one mechanism for defective antitumor immunity, but the causes of DC impairment are incompletely understood. We evaluated the association of impaired DC differentiation with angiogenesis-associated molecules D-dimer, vascular endothelial growth factor (VEGF), urokinase plasminogen activator (uPA), and plasminogen activator inhibitor (PAI-1) in peripheral blood from 41 patients with lung, breast, and colorectal carcinoma. Subsequently, we studied the effect of administration of the anti-VEGF antibody (bevacizumab) on DC maturation and function in vivo. Compared with healthy volunteers, cancer patients had a bias toward the immunoregulatory DC2, had deficits in DC maturation after overnight in vitro culture, and had a significant increase in immature myeloid cell progenitors of DC (0.50 ± 0.31% vs. 0.32 ± 0.16% of peripheral blood mononuclear cells, respectively, P = 0.011). A positive correlation was found between the percentage of DC2 and PAI-1 (R = 0.50) and between immature myeloid cells and VEGF (R = 0.52). Bevacizumab administration to cancer patients was associated with a decrease in the accumulation of immature progenitor cells (0.39 ± 0.30% vs. 0.27 ± 0.24%, P = 0.012) and induced a modest increase in the DC population in peripheral blood (0.47 ± 0.23% vs. 0.53 ± 0.30%). Moreover, anti-VEGF antibody treatment enhanced allo-stimulatory capacity of DC and T cell proliferation against recall antigens. These data suggest that DC differentiation is negatively associated with VEGF levels and may be one explanation for impaired anticancer immunity, especially in patients with advanced malignancies.
Keywords: Dendritic cell, Immature myeloid cell, VEGF, Cancer, Immunity
Introduction
The role of the immune system in controlling tumors has been suggested by numerous animal models and anecdotal reports of spontaneous regressions of tumors in humans following septic episodes or following various attempts at immune stimulation. Central to pathways for activating tumor antigen-specific T cell and antibody responses is the uptake, processing, and presentation of the tumor antigen by dendritic cells (DC), professional antigen-presenting cells [1, 2]. The importance of endogenous DC has been highlighted by better clinical outcomes for patients with various tumor types when DC are found to be infiltrating the tumor [3, 4].
A major obstacle in the effort to develop effective cancer vaccines has been the capacity of tumors to evade immune surveillance. One mechanism for doing so is the inhibition of DC function [5]. DC isolated from tumor-bearing mice [6] and humans [7] have a diminished ability to stimulate T cells. Furthermore, the generation of “functionally mature” HLA-DR + DC from CD34+ precursors is diminished [8, 9]. Such inhibition leads to an imbalance between mature and immature myeloid cells (iMCs), and there is increasing evidence that progressive tumor growth is associated with an increase in iMCs, monocytes/macrophages, and a decrease in mature, functional DC [10–13].
Several soluble factors have been implicated in defective DC maturation including vascular endothelial growth factor (VEGF). VEGF is critical for tumor-induced angiogenesis by promoting endothelial cell proliferation [14, 15]. Furthermore, high VEGF levels appear to block DC differentiation from immature precursors [16]. Specifically, VEGF binds to hematopoietic progenitor CD34+ cells through the VEGF-receptor-1 (VEGFR1/Flt-1) and inhibits the activation of the transcription factor NF-kB [17, 18], a component of which, RelB, has been implicated in the generation of mature DC. Conversely, anti-VEGF antibodies block the inhibitory effects of tumor cell supernatants on DC in vivo [16]. When anti-VEGF antibodies are used alongside DC immunotherapy, the antitumor effect is much more prolonged and pronounced [19]. These studies all suggest that blocking VEGF signaling may be one approach to improving DC response and hence improve host immune system function and the efficacy of antitumor immunotherapy in cancer patients.
We performed this study to determine the impact of pro-angiogenic molecules on DC and their precursors and to study the immunologic impact of eliminating VEGF with bevacizumab (Avastin, Genentech) in patients with advanced malignancies. We demonstrate that VEGF is associated with defective DC maturation, which is restored by therapy with bevacizumab and that markers of immune function improve following bevacizumab treatment.
Methods
Patients
This study protocol was approved by the Institutional Review Board of the Duke University Medical Center and the Protocol Review Group of GlaxoSmithKline. Patients with colorectal (n = 31), lung (n = 5), breast (n = 4), and unknown primary cancers (n = 1) at the Duke University Medical Center Comprehensive Cancer Center and two groups of healthy volunteers (n = 30 total) were enrolled into the portion of the study. The majority (n = 30) had metastatic disease and the remainder had high risk localized disease that had been resected. The majority (n = 27) was not receiving active chemotherapy at the time of enrollment onto the study. After obtaining informed consent, blood was drawn into heparin tubes for dendritic cell evaluation and into EDTA tubes for circulating endothelial cell (CEC), circulating endothelial progenitor (CEP), and plasma biomarker analysis.
For the pilot study with anti-VEGF antibody (bevacizumab), 16 patients with colorectal cancer were originally enrolled, and 3 patients were additionally enrolled for functional analysis. Blood was drawn from these patients before bevacizumab administration, and post-treatment blood was also drawn approximately 14 days after administration for immune analysis.
Dendritic cell analysis
For dendritic cell analysis, antibodies were added to whole blood and stained for 30 min, and then a red blood cell lysis buffer (FACS lysis buffer, Becton-Dickinson, San Jose, CA) was added. To gate-out cells of other lineages as well as hematopoietic precursors, a lineage antibody cocktail was made with FITC-labeled anti-CD3, CD14, CD15, CD19, and CD57, or with FITC-labeled Lineage Cocktail 1 (Becton Dickinson), anti-CD15, and CD34. Cells were also stained with anti-HLA-DR-PerCP, anti-CD45-APC, and PE-labeled CD80, CD86, and CD83. Using flow cytometry, the percentage of CD45+ lineage negative (Lin−), HLA-DR+ (DC), and HLA-DR− (iMC) was determined, and the maturation status of DC (expression of CD80, CD86, and CD83) was evaluated. Cells were also stained with anti-CD11c and anti-CD123 to examine the proportion of DC subsets, DC1 and DC2. In selected experiments, to better characterize the iMCs (CD45+ lineage− HLA-DR−), cells were stained with anti-CD13 or CD33.
To examine if defects in DC maturation persist ex vivo, 1 ml of whole blood was mixed with 9 ml of AIM-V medium (GIBCO, Grand Island, NY) and incubated overnight at 37°C [20]. Then, the cells were harvested and stained for maturational markers as described earlier.
Biomarker analysis
Circulating endothelial cell (CEC) and circulating endothelial progenitor (CEP) analysis was performed on CD34+-enriched cells using four-color flow cytometry with antibodies against Flk-1-perCP, CD15-FITC, CD133-PE, and CD45-APC. CECs were defined as CD45−/Flk+/CD15− and CEPs as CD45−/Flk+/CD133+ [21, 22]. D-dimer, VEGF, urokinase plasminogen activator (uPA), and plasminogen activator inhibitor-I (PAI-1) analyses were performed on platelet-depleted plasma using commercially available ELISA kits [23–26].
Mixed leukocyte reaction
The effect of anti-VEGF antibody on allo-stimulatory capacity of antigen-presenting cells was tested in mixed leukocyte reactions (MLR). Blood was drawn from the patients prior to and 2 weeks after anti-VEGF-treatment, and PBMCs were isolated by density gradient centrifugation over ficol. PBMCs were frozen down and kept at −80°C until use. For the assay, PBMCs were washed, counted, irradiated (50 Gy), and used as stimulators. Allogeneic peripheral blood lymphocytes (PBLs) of normal donors were isolated from PBMCs by negative isolation technique using CD14 and CD19 DynaBeads (Dynal Biotech, Oslo, Norway). PBMCs were mixed with allogeneic PBLs (100,000 cells/well) in 96-well U-bottomed plates to make 1:1, 2:1, and 4:1 stimulator:responder ratios, and incubated for 4 days. One microcurie of [3H]-thymidine was added to each well 18 h prior to harvesting the cells. [3H]-thymidine uptake was counted in a liquid scintillation counter (TRILUX, Wallac, Turku, Finland).
Antigen-specific T cell response/proliferation
The effect of anti-VEGF antibody on antigen-presenting capacity of peripheral blood mononuclear cells was tested by measuring antigen-specific T cell proliferation. Briefly, PBMCs from blood samples obtained prior to and after bevacizumab treatment were isolated as described earlier. PBMCs (100,000 cells/well) were incubated in each well of 96-well U bottom plate, with antigens, including Candida albicans (10 μg/ml, Greer Lab, Lenior, NC), tetanus toxoid (0.5 μg/ml, Calbiochem, Darmstadt, Germany), phytoheamagglutinin (5 μg/ml, Sigma, St Louis, MO), and pokeweed mitogen (1 μg/ml, Sigma). After 4 days of culture, 1 μCi of [3H]-thymidine was added to each well, and 18 h later, uptake was counted by liquid scintillation counter. Stimulation Index was calculated by dividing the [3H]-thymidine uptake values with the value of control (without antigen).
IL-12 enzyme-linked immunosorbent assay
PBMCs from blood samples obtained prior to and after bevacizumab treatment were isolated as described earlier, and 800,000 cells were incubated in each well of 96-well U bottom plate in the medium with CD40-ligand trimer (1 μg/ml; Immunex, Seattle, WA), GM-CSF (Leukine; 800 U/ml; Berlex, Seattle, WA), and Interferon-γ (ActimmuneTM; 1,000 U/ml; InterMune, Palo Alto, CA) [27]. For the detection of IL-12 production by PBMCs, ELISA was performed on 48 h cultured supernatants using the QuantikineTM IL-12 HS ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions.
Statistical analysis
Spearman rank-order correlation was used as a nonparametric measure of association between the biomarkers. Differences between population subgroups for the biomarkers were assessed using the Kruskal-Wallis (Wilcoxon) nonparametric test. Differences of DC and immature myeloid cells between pre- and post-treatment samples were analyzed by Students’ t test. Differences at P < 0.05 were considered statistically significant.
Results
Deficits in DC maturation in cancer patients
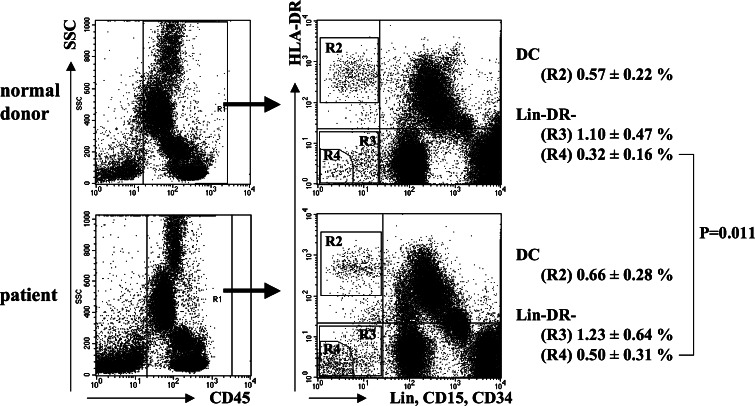
Two strategies were utilized to enumerate blood DC in cancer patients. First, we stained whole white blood cells with a cocktail of lineage antibodies against CD3, 14, 15, 19, 34, and 57, and then gated on the lineage negative population to identify the HLA-DR + cells as blood DC. This seemed to overestimate the percentage of blood DC (2.06 ± 1.57% of the mononuclear cells by this method) compared with the percentage generally reported in the literature (data not shown). To improve upon the method of DC quantification, we next used a different antibody cocktail consisting of antibodies against CD3, 14, 15, 16, 19, 20, 34, and 56, and gated on the lineage negative, HLA-DR + cells (see Fig. 1, region R2). Because this method was reproducible (coefficient of variance of 3.8% for three replicate analyses), it was used throughout the remainder of this report. There was no difference in the percentage of DC between the cancer patients (0.66 ± 0.28%) and healthy volunteers (0.57 ± 0.22%, P = 0.28) (Fig. 1). The percentage of freshly analyzed DC that expressed CD86 was greater amongst healthy volunteers (71.54 ± 8.63%, Fig. 2a) compared with cancer patients (61.80 ± 11.29%, Fig. 2b) (P = 0.043), but there were no other differences in phenotypic markers between the cancer patients and healthy volunteers DC. We evaluated the subtypes of DC and found that the percentage of DC1 (CD11c + CD123−) tended to be lower in cancer patients (57.1 ± 15.8%) than in normal volunteers (66.6 ± 9.9%) (P = 0.058) [2], and the percentage of DC2 (CD123+) was nonsignificantly higher in cancer patients (29.3 ± 12.4%) than in normal volunteers (26.2 ± 10.7%) (P = 0.29).
Fig. 1.
Schema for enumerating dendritic cells and immature myeloid cells. CD45+ cells were gated and analyzed for lineage marker and HLA-DR expression. R2 shows DC population. R3 shows CD45+ lineage− HLA-DR− cell population. R4 shows the cell population with the least fluorescence for lineage markers and HLA-DR
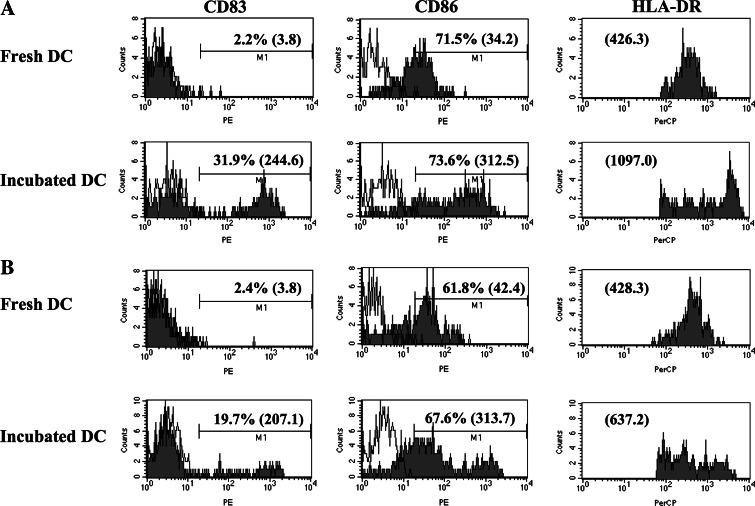
Fig. 2.
Maturation of DC in vitro. Expression of maturation markers by peripheral blood DC of normal donors and cancer patients was analyzed by flow cytometry. Leukocytes in the whole blood were stained with antibody cocktails soon after blood drawing or after overnight incubation in the medium. DC expression of maturation markers, CD80, CD83, and CD86, was analyzed and compared between fresh and overnight-incubated cells. Percentages of positive cells and mean fluorescence intensity (in the parenthesis) are shown in each histogram. Representative cases are shown for normal donors (a) and cancer patients (b)
We next examined the ability of DC to mature to CD83+ cells after an overnight incubation without additional cytokines added to culture conditions (Fig. 2). A portion of the DC obtained from cancer patients (Fig. 2b) was able to mature spontaneously as determined by CD83 upregulation (19.7 ± 14.7%), although DC from healthy volunteers (Fig. 2a) showed higher CD83 upregulation (31.9 ± 12.7%, P = 0.054). Mean fluorescent intensity of HLA-DR expression on DC was significantly higher for DC from normal donors than those from cancer patients after overnight incubation (1,097 ± 469 vs. 637 ± 377, P = 0.047). In summary, these data show that the cancer patients, as a group, had deficits in DC maturation and a bias toward the immunoregulatory DC2 compared to DC from normal volunteers.
Accumulation of immature myeloid cells in cancer patients
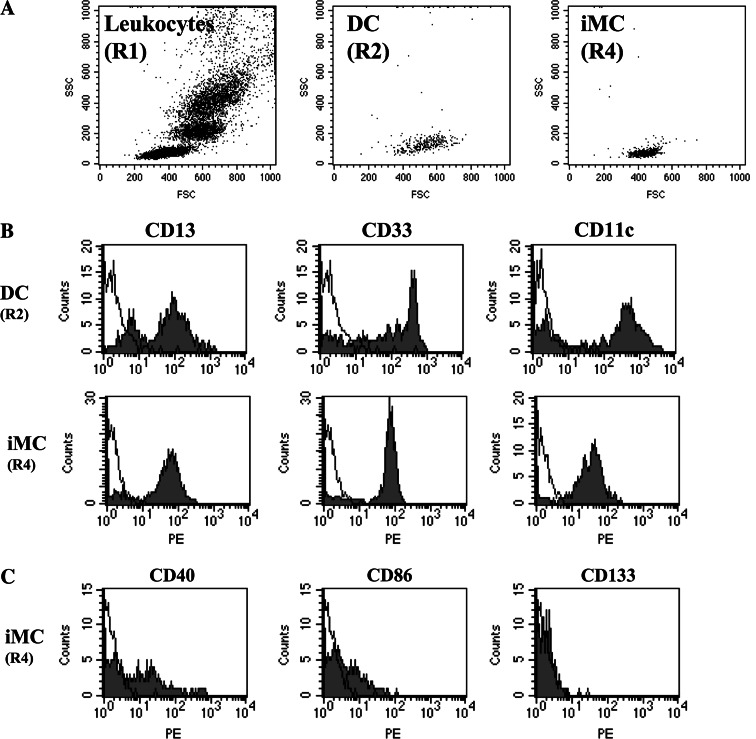
We next sought to determine whether there were defects in differentiation of cells toward the DC lineage, resulting in an increase in cells without lineage or differentiation markers (iMCs) in cancer patients. We first enumerated the entire population of cells that was CD45+ Lin− HLA-DR− (Fig. 1, region R3) and found no significant differences between cancer patients and healthy volunteers (1.23 ± 0.64% compared with 1.10 ± 0.47% of the CD45+ cells, P = 0.25). Because it is possible that NK and other cells with dim expression of the lineage markers might have been included in this gate (R3), we next focused on the population of cells with the least fluorescence for lineage markers and HLA-DR (see Fig. 1, region R4). There was a significantly higher percentage of these iMC in the cancer patients (0.50 ± 0.31%) compared with 0.32 ± 0.16% of the CD45+ cells in healthy volunteers (P = 0.011). A forward scatter/side scatter gram of iMC showed their distribution in the overlapping area of the lymphocyte and DC populations, suggesting that these might be the precursor of DC, but they are not red blood cell contamination or cell debris (Fig. 3a). These iMCs expressed myeloid markers, CD13, CD33, and CD11c (Fig. 3b), although with less intensity than DC, and only a minority was weakly positive for CD40 and CD86 expression. They were negative for CD80 and CD83, indicating their lack of maturation. They also lacked CD133 expression, indicating they were not stem cells (Fig. 3c).
Fig. 3.
Expression of myeloid markers on immature myeloid cells. a Forward scatter and side scatter gram of DC (R2 in Fig. 1) and immature myeloid cells (R4 in Fig. 1). b The cell population with the least fluorescence for lineage markers and HLA-DR (R4 in Fig. 1) was analyzed for their expression of myeloid cell markers, CD13, CD33, and CD11c. Immature myeloid cells (iMC) express these myeloid markers. c CD40, CD86, and CD133 expression. Immature myeloid cells have weak expression of CD40 and CD86 but do not express CD133
Immature myeloid cells correlate with markers of angiogenesis
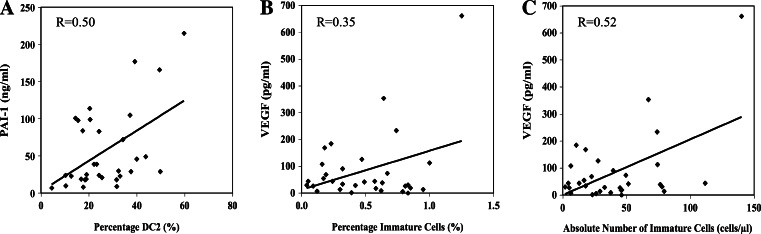
High levels of VEGF have been shown to increase the number of iMC, but other angiogenic markers have not been studied in detail. We therefore analyzed various markers of angiogenesis to determine whether an association might exist between these biomarkers and percentages of mature and immature DC and iMC. We found that D-dimer and VEGF were significantly elevated in platelet-poor plasma of patients compared to controls. There was no correlation between the percentage of DC or DC1 and D-dimer, VEGF, uPA, and PAI-1. However, a moderate positive correlation existed between the percentage of DC2 and PAI-1 (R = 0.50) (Fig. 4a). A weak positive correlation was also found to exist between the percentage of iMC and VEGF levels (R = 0.35), and a stronger positive correlation was found between the absolute number of iMC per microliter of blood and VEGF levels (R = 0.52) (Fig. 4b, c). Other markers such as D-dimer, uPA, and PAI-1 had no correlation. These data suggest that higher VEGF levels may be associated with an increased frequency of iMC. Analysis of CEC by flow cytometry showed a modest (1.3-fold) but not statistically significant increase in cancer patients compared to normal donors; however, CEP (Flk+/CD133+) was similar between patients and controls (data not shown). CEC and CEP levels in cancer patients with metastatic and localized disease were also not significantly different (data not shown). There were no correlations between iMC and CEC or CEP.
Fig. 4.
Correlation between angiogenesis-associated molecules in the serum and cellular components in the peripheral blood of cancer patients. a Correlation between percentage of CD123+ DC2 and plasminogen activator inhibitor-1 (PAI-1). A moderate positive correlation was found (R = 0.50). b and c Correlation between immature myeloid cells and VEGF levels in the serum. A weak positive correlation was found to exist between the percentage of immature myeloid cells and VEGF levels (R = 0.35), and a stronger positive correlation was found between the absolute number of immature myeloid cells/microliter/blood and VEGF levels (R = 0.52)
Anti-VEGF antibody treatment reduces immature myeloid cells in cancer patients
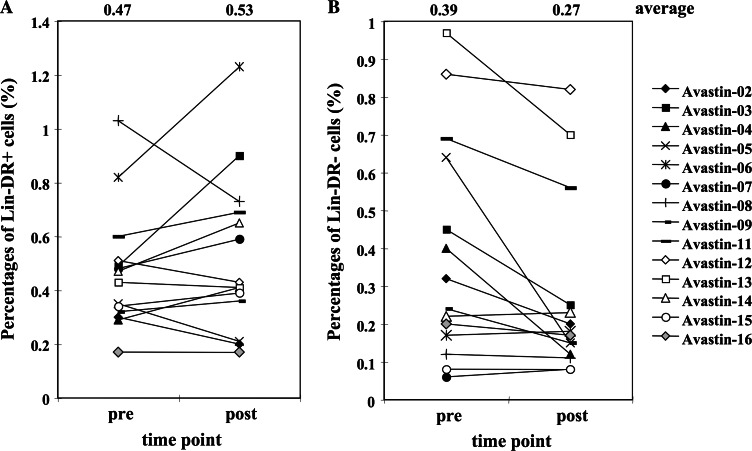
Since VEGF level in the serum showed a moderate correlation with the number of iMC in this study, we performed a pilot study in which cancer patients were treated with the anti-VEGF mAb (bevacizumab), to examine if manipulation of VEGF level can affect iMC or DC or both. Sixteen patients were originally enrolled and both pre- and post-treatment blood were available from 14 patients. Interestingly, administration of anti-VEGF mAb mildly increased DC numbers (pretreatment 0.47 ± 0.23 % vs. 0.53 ± 0.30% following bevacizumab); however, it did not affect DC1/DC2 ratio nor maturation status of DC (Fig. 5a, data not shown). However, anti-VEGF treatment induced a significant decrease in the number of iMC in peripheral blood (0.39 ± 0.30% pretreatment compared with 0.27 ± 0.24% following bevacizumab, P = 0.012) (Fig. 5b). Thus, the decrease in VEGF level may release the block on differentiation of the iMC.
Fig. 5.
Changes in dendritic cell and immature myeloid cell population in anti-VEGF-treated patients. Peripheral blood was drawn from patients, who were enrolled in anti-VEGF clinical trials, before and 2 weeks after anti-VEGF antibody administration. The percentages of DC (Lin− HLA-DR+, a) and immature myeloid cells (Lin− HLA-DR−, b) were shown for individual patients. Anti-VEGF antibody administration induced significant decrease in the number of immature myeloid cells (P = 0.012) and mild increase of DC
Anti-VEGF antibody treatment enhances immune function in cancer patients
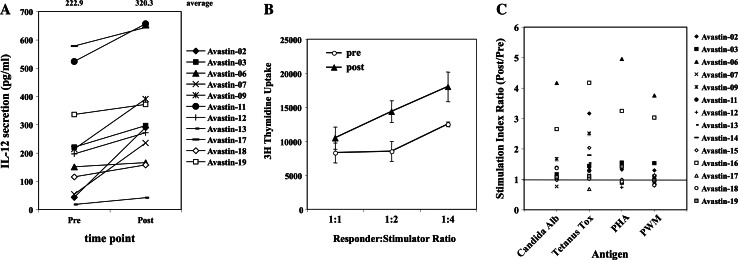
To assess the effect of anti-VEGF antibody treatment on DC function, PBMCs from pre- and post-treatment blood were incubated with CD40 ligand, GM-CSF, and IFN-γ for 48 h, and IL-12 secretion was evaluated. As shown in Fig. 6a, 8 out of 11 available cases showed increase (more than 1.2 times) in IL-12 secretion, and the average for pre- and post-treatment PBMCs were 222.9 and 320.3 pg/ml, respectively (P < 0.01).
Fig. 6.
Changes in IL-12 secretion and in antigen-presenting capacity of peripheral blood in anti-VEGF-treated patients. Peripheral blood was drawn from patients, who were enrolled in anti-VEGF clinical trials, before and 2 weeks after anti-VEGF antibody administration. a PBMCs were stimulated in the medium supplemented with CD40-ligand/GM-CSF/IFN-γ. After 48-h incubation, supernatants were collected and IL-12 production was analyzed by ELISA. Mean values of pre- and post-treatment samples are shown at the top of figure. b PBMCs isolated from patients’ blood, used as stimulator cells, were irradiated (50 Gy), plated in 96-well plate with allogeneic PBMCs (100,000 cells/well) from normal donors to make 1:1, 2:1 and 4:1 ratio. Cells were incubated for 4 days, 1 μCi of [3H]-thymidine was added to each well, and uptake was measured 18 h later. One representative case is shown. c PBMCs (200,000 cells/well) were incubated for 4 days with Candida albicans protein, tetanus toxoid, PHA or pokeweed antigen in the medium, 1 μCi of [3H]-thymidine was added to each well, and uptake was measured 18 h later. Stimulation Index was calculated by dividing the values with the value of control (without antigen). Ratio of stimulation index (post/pre) of individual patients was plotted in the figure
Mixed leukocyte reaction (MLR) and proliferation assays, using Candida Albicans, tetanus toxoid, phytohaemagglutinin antigen, and pokeweed mitogen, were performed on the peripheral blood mononuclear cells from three cancer patients before and approximately 14 days after they received bevacizumab alone. Allostimulatory function was enhanced in post-treatment samples in 9 of 14 patients, unchanged in 3 (Fig. 6b), and was suppressed in 2 patients. Proliferation of PBMCs against these antigens was enhanced in post-treatment samples in majority of the cases, especially for Candida Albicans and tetanus toxoid antigens (Fig. 6c). Significant increase in stimulation index (post/pre ratio > 1.2) was observed in 6, 9, 5, and 4 out of 14 patients for Candida Albicans, tetanus toxoid, PHA, and PWM, respectively. On the other hand, decrease in stimulation index (post/pre ratio < 0.8) was observed in only one case for each antigen. These results indicate that the anti-VEGF therapy is associated with enhanced antigen-presenting capacity of the peripheral blood mononuclear cells, especially DCs. We suspect that the improvement in the immune response is due to the fact that the iMCs have an inhibitory activity in dendritic cell functional assays, because iMC do not possess any ability to present antigen in proliferation assays to autologous T cells nor do they activate an allo-immune response (data not shown).
Discussion
Cancer patients, particularly those with advanced stages of disease, have a diminished ability to activate immune responses against their tumor. Although the mechanisms responsible for this immune “defect” are multifactorial, DC dysfunction plays a key role [6, 7, 16, 27]. In the earlier studies performed by Gabrilovich, Carbone, and colleagues [10, 28, 29], the presence of increased iMC in cancer patients, which is associated with elevated levels of VEGF, was demonstrated to be involved in mediating DC dysfunction. Here, we extend these observations by focusing on an association of VEGF with DC maturation and the accumulation of iMC that correlates with serological biomarkers of angiogenesis. We observed an increase in iMC as defined by the lineage−, HLA-DR− cells in cancer patients compared with healthy volunteers and found a moderate correlation with VEGF levels (Fig. 4), but not the other angiogenic markers (i.e., D-dimer, uPA, and PAI-1). The only other correlation identified was that of DC2 and PAI-1 (R = 0.50). These data implicate VEGF as an inhibitor of DC maturation, in addition to its role in directing neovascularization of tumors. They also suggest a possible association between PAI-1 and immunotolerizing DC, though not a direct cause and effect. Since PAI-1 is associated with poor prognosis of patients with different types of cancers [24, 25, 30], PAI-1 may have indirect correlation with immunoregulatory DC (DC2). Interestingly, Koretz et al. [31] observed that DC within colon cancers strongly expressed u-PA and, at a lower level, also PAI-1.
Although our results are largely consistent with those of Gabrilovitch and colleagues, we did not observe an actual decrease in the total number of DC in cancer patients compared with healthy volunteers; however, we did detect fewer of the immunostimulatory DC1 and a trend toward more of the immunotolerizing DC2 amongst the cancer patients. We also found that DCs in cancer patients were less matured based on costimulatory molecule expression (CD86) and did not mature (less upregulation of CD83 and HLA-DR expression) after overnight culture. These findings may explain the defective function of DC in cancer patients, because mature DCs have a more potent immunostimulatory function than immature DC.
We found a significant decrease of iMC in cancer patients after the administration of anti-VEGF mAb (Fig. 5b). Since we observed a moderate correlation between the serum VEGF level and the number of iMC in the peripheral blood of cancer patients (Fig. 4), this finding of anti-VEGF mAb effect on iMC might suggest the direct correlation between VEGF and iMC. Almand et al. reported three metastatic lung cancer patients, who experience a decline in iMC following treatment with chemotherapy and anti-VEGF mAb for several months [32]. However, the respective roles of the chemotherapy and anti-VEGF therapy in the decline in iMC cannot be determined from their study. Our study with anti-VEGF treatment alone can provide more direct evidence of possible association between eliminating VEGF and reducing iMC in humans.
The mechanism for the effect of VEGF on DC maturation and function could either occur at the level of bone marrow precursors or at later stages of development during the transition from immature to mature DC. VEGF could be a growth or survival factor for iMC. It is also possible that VEGF promoted differentiation of iMC to granulocytes/macrophages rather than toward DC. In our study, there was a greater effect of anti-VEGF therapy on reducing iMC than on increasing DC number (Fig. 5), which supports the former scenario; however, we also observed that cancer patients’ DC do not mature as readily after overnight culture as the DC of healthy volunteers (Fig. 2), which supports a further role for VEGF in inhibiting the ability of DC to mature. However, in our in vitro incubation system of human leukocytes, we cannot rule out the possibility that coexisted iMC might have inhibited the maturation of DC during overnight incubation time. By using in vitro culture system of murine cells, Dikov et al. [17] and Laxmanan et al. [33] reported that the direct impairment of DC function by VEGF is mediated primarily by VEGFR1/Flt-1. Dikov et al. reported that VEGF acted on early stages of DC differentiation but not on later phase of maturation. On the other hand, Mimura et al. [34] recently reported, using matured human monocyte-derived DC, that VEGF did not affect DC phenotype, but inhibited allo-stimulatory capacity through VEGFR2 receptor. Thus, more studies are needed to reveal the detailed mechanism of VEGF effect on human DC.
Because Gabrilovich and colleagues [10, 11] showed that the undifferentiated hematopoietic cells from cancer patients inhibited the function of fully differentiated DC, we hypothesized that administration of anti-VEGF Ab would result in enhanced DC functional capacity to activate alloimmune and antigen-specific immune responses. We could confirm the decrease of VEGF level in the sera of patients after anti-VEGF treatment, with some variations in the magnitude (data not shown). As shown in Fig. 6b, c, we observed that anti-VEGF mAb treatment enhanced the MLR (9 out of 14 patients) and antigen-specific T cell response (6, 9, 5, and 4 out of 14 patients for Candida Albicans, tetanus toxoid, PHA, and PWM, respectively), which coincided with a decrease of iMC. Interestingly, the patients with larger decrease in VEGF level by anti-VEGF treatment demonstrated strongly enhanced stimulation index for Candida albicans or PHA (data not shown). Thus, the decrease in VEGF level by anti-VEGF treatment seems to enhance antigen-presenting capacity of DC, probably, at least partly, by reducing iMC, which is reported to be inhibitory for DC function [10–13]. As mentioned earlier, we did not see clear change of DC phenotype or numbers in anti-VEGF-treated patients but still detected enhanced DC functional capacities after anti-VEGF treatment. The elimination of VEGF might not induce differentiation of iMC to DC, but rather induce differentiation to other cell types (i.e., granulocytes, macrophage). Alternatively, the direct effect of VEGF on DC is playing the major role in VEGF-induced suppression of DC function, and phenotype or number of peripheral blood DC might not be affected by short-term reduction of VEGF level in the serum. Larger number of patients with anti-VEGF treatments are needed to elucidate more precise mechanism of VEGF-mediated inhibition of human DC function.
In summary, our data indicates an association between VEGF and defects in DC maturation and function. Eliminating VEGF with anti-VEGF mAb in vivo resulted in a decrease in iMC, and induced a trend to an enhanced antigen-presenting function of DC. Future studies are needed to determine whether eliminating VEGF prior to cancer vaccines will lead to improvements in antitumor immunotherapy efficacy in cancer patients. We are currently evaluating the effect of eliminating VEGF with the anti-VEGF antibody bevacizumab in human clinical trials.
Acknowledgments
We wish to thank Elizabeth Anderson and Emily Privette for their help in obtaining blood samples from the cancer patients and Sharon Peplinski for flow cytometric expertise. This project was supported by a grant from GSK and by 5 P01 CA78673.
References
- 1.Flores-Romo L. In vivo maturation and migration of dendritic cells. Immunology. 2001;102:255–262. doi: 10.1046/j.1365-2567.2001.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse MA, Lyerly HK, Gilboa E, Nair SK. Optimization of the sequence of antigen loading and CD40 ligand-induced maturation of dendritic cells. Cancer Res. 1998;58:2965–2968. [PubMed] [Google Scholar]
- 3.Coventry BJ, Morton J. CD1a-positive infiltrating-dendritic cell density and 5-year survival from human breast cancer. Br J Cancer. 2003;89:533–538. doi: 10.1038/sj.bjc.6601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeid NA, Muller HK. S100 positive dendritic cells in human lung tumors associated with cell differentiation and enhanced survival. Pathology. 1993;25:338–343. doi: 10.3109/00313029309090853. [DOI] [PubMed] [Google Scholar]
- 5.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 8.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 9.Lathers DM, Lubbers E, Beal NM, Wright MA, Young MR. Cultures derived from peripheral blood CD34+ progenitor cells of head and neck cancer patients and from cord blood are functionally different. Hum Immunol. 1999;60:1207–1215. doi: 10.1016/S0198-8859(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 10.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 11.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–1139. doi: 10.1158/0008-5472.CAN-03-1715. [DOI] [PubMed] [Google Scholar]
- 13.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 15.Martiny-Baron G, Marme D. VEGF-mediated tumour angiogenesis: a new target for cancer therapy. Curr Opin Biotechnol. 1995;6:675–680. doi: 10.1016/0958-1669(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 17.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 18.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hematopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 19.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 20.Thomas R, Davis LS, Lipsky PE. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1994;150:821–834. [PubMed] [Google Scholar]
- 21.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 22.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 23.Dirix LY, Salgado R, Weytjens R, Colpaert C, Benoy I, Huget P, van Dam P, Prove A, Lemmens J, Vermeulen P. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86:389–395. doi: 10.1038/sj.bjc.6600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noel A. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker EA, Bergin FG, Leaper DJ. Plasminogen activator system, vascular endothelial growth factor, and colorectal cancer progression. Mol Pathol. 2000;53:307–312. doi: 10.1136/mp.53.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackwell K, Hurwitz H, Lieberman G, Novotny W, Snyder S, Dewhirst M, Greenberg C. Circulating D-dimer levels are better predictors of overall survival and disease progression in patients with metastatic colorectal carcinoma. Cancer. 2004;101:77–82. doi: 10.1002/cncr.20336. [DOI] [PubMed] [Google Scholar]
- 27.Mosca PJ, Hobeika AC, Colling K, Clay TM, Thomas EK, Caron D, Lyerly HK, Morse MA. Multiple signals are required for maturation of human dendritic cells mobilized in vivo with Flt3 ligand. J Leukoc Biol. 2002;72:546–553. [PubMed] [Google Scholar]
- 28.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 29.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 30.Look MP, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Ferno M, Eppenberger-Castori S, Sweep CG, Ulm K, Peyrat JP, Martin PM, Magdelenat H, Brunner N, Duggan C, Lisboa BW, Bendahl PO, Quillien V, Daver A, Ricolleau G, Meijer-van Gelder ME, Manders P, Fiets WE, Blankenstein MA, Broet P, Romain S, Daxenbichler G, Windbichler G, Cufer T, Borstnar S, Kueng W, Beex LV, Klijn JG, O’Higgins N, Eppenberger U, Janicke F, Schmitt M, Foekens JA. Pooled analysis of prognostic impact of urokinase-type plasminogen activators and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst. 2002;94:116–128. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- 31.Koretz K, Moller P, Schwartz-Albiez R. Plasminogen activators and plasminogen activator inhibitors in human colorectal carcinoma tissues are not expressed by the tumour cells. Eur J Cancer. 1993;29A:1184–1189. doi: 10.1016/s0959-8049(05)80312-0. [DOI] [PubMed] [Google Scholar]
- 32.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 33.Laxmanan S, Robertson SW, Wang E, Lau JS, Briscoe DM, Mukhopadhyay D. Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochem Biophys Res Commun. 2005;334:193–198. doi: 10.1016/j.bbrc.2005.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother. 2007;56:761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]