Abstract
Bioengineering provides unique opportunities to better understand and manage atherosclerotic disease. The field is entering a new era that merges the latest biological insights into inflammatory disease processes with targeted imaging and nanomedicine. Preclinical cardiovascular molecular imaging allows the in vivo study of targeted nanotherapeutics specifically directed toward immune system components that drive atherosclerotic plaque development and complication. The first multicenter trials highlight the potential contribution of multimodality imaging to more efficient drug development. This review describes how the integration of engineering, nanotechnology, and cardiovascular immunology may yield precision diagnostics and efficient therapeutics for atherosclerosis and its ischemic complications.
INTRODUCTION
Atherosclerosis is a chronic progressive disease, affecting the medium and large arteries, in which lipid-triggered inflammation plays a pivotal role (1–3). The major clinical manifestations of atherosclerosis are coronary artery disease (CAD), leading to acute myocardial infarction (MI) and sudden cardiac death; cerebrovascular disease, leading to stroke; and peripheral arterial disease, leading to ischemic limbs and viscera (4). These complications of atherosclerosis are leading causes of death worldwide (5). Despite progress in medical and revascularization therapies for atherothrombotic disease, the incidence of MI and stroke remain high under the current standard of care (6–8), and the past decade has generated few new medical therapies to prevent atherosclerosis-induced events. Similarly, current diagnostic approaches to atherosclerosis do not accurately identify those individuals who will suffer an ischemic complication (9, 10). The field of atherosclerosis is therefore ripe for reengineering in both the therapeutic and diagnostic arenas (9, 11, 12).
Research into the process of atheroma lesion development and maturation has implicated many immune cells including lymphocytes, dendritic cells, and neutrophils (3). The most numerous cells in atherosclerotic plaque are macrophages, which are leukocytes that are central to the innate immunity [see Perspective by Schulz and Massberg (13)]. Because they play a major role in instigating plaque development and complication—both of which are inflammation-related disease processes—leukocytes are promising targets for more effective atherosclerosis treatments (1, 3, 11, 14). However, the complexity of the immune system and its role as a defensive force against infection require novel tools to very precisely identify and treat the inflammatory cells that promote atherosclerosis. Biomedical engineering offers unique possibilities for diagnosing and treating atherosclerotic plaque inflammation. Thus, interfacing engineering with immunology will be essential to meaningful advances in disease management.
This review discusses how recent discoveries in atherosclerosis immunology can provide opportunities for diagnostic imaging of atherosclerotic plaques and cardiovascular complications of atherosclerosis, including translatable molecular imaging techniques. Integrated diagnostic modalities have uncovered new pathways that can serve as potential diagnostic and therapeutic targets, and we predict that these pathways can be specifically modulated by nanomedicine-based interventions.
IMAGING PLAQUE INFLAMMATION
Noninvasive computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound have traditionally been used to image large arteries anatomically, whereas the nuclear imaging techniques positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have been used to image exogenously administered radiotracers (9). In diagnosing atherosclerotic disease, X-ray, MR, or CT angiography can reveal the narrowing of the vessel lumen, known as stenosis (9); CT angiography can even be used to visualize narrowing in smaller, more distal coronary artery segments. Unfortunately, lumen imaging does not necessarily identify which atherosclerotic plaques are at phenotype-dependent risk of rupture or progression, nor can it show outward remodeling of the vessel wall.
An imaging method with superior spatial resolution, intravascular ultrasound (IVUS), uses a catheter that is distally functionalized with a miniaturized ultrasound transducer. IVUS can measure vessel wall thickening and potentially compositional information (“virtual histology”) in atherosclerosis and may be able to predict whether individual plaques will cause ischemic events (14). Because IVUS is an invasive procedure, however, alternative ways to measure vessel wall thickness are under development. MRI, by comparison, can noninvasively visualize the vessel wall (thickness) and characterize plaque composition using multiparametric imaging protocols that typically use different image sequences to generate contrast between plaque structures, such as the fibrous cap and necrotic core (15, 16).
The shift from diagnosing artery lumen stenosis to visualizing vessel wall components paralleled new vascular biology insights into key features of the vulnerable atherosclerotic plaque (Fig. 1). Leukocytes and their associated inflammatory actions are hallmarks of atherosclerotic plaques prone to ruptures that cause catastrophic complications, such as MI and stroke (4). MRI contrast agents can be applied to image such rupture-prone plaques (17). Typically, T1-shortening agents are based on gadolinium chelates (18), whereas T2(*)-shortening agents are based on iron oxide nanoparticles (19). The former agent class brightens T1-weighted MR images; the latter gives rise to T2*-weighted hypointensity at sites of iron oxide accumulation. Iron oxide nanoparticles can be derivatized with coatings and targeting moieties that cause specific interactions with inflammatory plaque components, such as macrophages residing in the plaque or adhesion molecules expressed by endothelial cells, thereby allowing visualization by MRI (Fig. 1) (20). Subsequent refinement of iron oxide contrast agents included additional labeling to enable validation of new targeting components with optical techniques, such as near-infrared fluorescence (NIRF) imaging, fluorescence histology, and flow cytometry (20), which are primarily used in preclinical settings. After preclinical studies that used iron oxide nanoparticles to delineate plaque macrophages (21), the first atherosclerosis imaging studies in patients were conducted more than a decade ago (22, 23).
Fig. 1. Imaging cellular processes and inflammation in atherosclerotic plaques.
Inflammatory atherosclerosis can be diagnosed and monitored by clinical and preclinical imaging methods, some of which require the administration of targeted agents. Preclinically, key inflammation imaging techniques include macrophage imaging in the vessel wall with nanoparticles, adhesion molecule imaging, or protease-specific agents, whereas splenic monocytes that migrate to the plaque can be monitored by intravital microscopy. In a clinical setting, MRI can visualize macrophage burden in human plaques, whereas dynamic contrast-enhanced MRI (DCE-MRI) and FDG-PET/CT allow the quantification of plaque neovessel permeability and inflammation, respectively. Lowercase roman numerals match imaging approach to location in the atherosclerotic vessel. Image in (iii) obtained from (35) with permission; in (iv), from (90) with permission; in (v), from (53) with permission. Image in (vi) is an original image from Z.A.F.
The specific imaging of cellular and molecular biomarkers, inherently the domain of PET and SPECT, has been designated “molecular imaging” (24). As a research field, molecular imaging intersected with cardiovascular disease at the beginning of the 21st century (25, 26). Most PET systems also integrate CT, which provides anatomic detail and enables absolute radiotracer quantification through attenuation correction. Combined PET-MRI systems are now available thanks to a new generation of PET detectors, in which the photomultiplier technology is replaced by magnetic field–insensitive avalanche photodiodes (27). The PET agent 18F-fluorodeoxyglucose (FDG), which is approved for use in humans, is the most widely applied radiotracer for detecting cancer and metastases and has recently been adopted to quantitatively image plaque inflammation (Fig. 1) (28).
EMERGING MOLECULAR IMAGING METHODS FOR ATHEROSCLEROSIS
Intravital microscopy
Intravital microscopy (epifluorescence, confocal, multiphoton) detects fluorescent agents, proteins, and cells in live organisms. Remarkable advances now allow the study of single-cell behavior in murine hematopoietic tissues (29), atherosclerotic plaques (30–32), and even the mouse heart (33). This modality helped to reveal that splenic leukocyte production increases in atherosclerosis (34), and that inflammatory atherosclerosis progression is further accelerated by positive forward feedback loops after MI (35), leading to frequent secondary ischemia. Major hurdles for applying intravital microscopy in cardiovascular studies include the vigorous and rapid motion of the heart and arteries. Advanced image stabilizing setups and the use of electrocardiography and respiratory gating overcome these, and allow studying leukocyte migration and interaction in cardiovascular tissues of mice that express cell-specific fluorescent reporters (29–35). Going forward, intravital microscopy will play a major role in the preclinical identification of new therapeutic targets and in following therapeutic efficacy with single-cell resolution.
Nanoparticle-enhanced molecular MRI
Inflammatory processes related to atherosclerosis that can be followed with nanoparticle-enhanced molecular MRI include neovascularization, the expression of adhesion molecules on the endothelium, plaque macrophage inflammation, and the action of enzymes produced by inflammatory leukocytes (Fig. 1). In mouse models of atherosclerosis, small-animal MRI scanners can achieve an in-plane resolution below 100 μm at field strengths of generally 7 T or higher (36). Typical atherosclerotic plaque regions that are imaged in mice include the abdominal aorta, the carotids, the aortic arch, and the aortic root. For the aortic root, triggering and gating methods overcome rodents’ rapid respiratory and cardiac motion. In rabbit models of atherosclerosis, the abdominal aorta is typically imaged with clinical MRI scanners at a 250-μm in-plane resolution (37).
Molecular MRI using dextran-coated iron oxide is the most extensively studied method of imaging plaque macrophages in both mouse and rabbit models of atherosclerosis. Dextran-coated iron oxide combines excellent biocompatibility with an inherent affinity for plaque macrophages (20) and is the only nanoparticle agent that has translated to the clinic owing to a favorable toxicity profile and ease of production. Emerging probes for nanoparticle-based MRI include high-density lipoprotein (HDL) nanoparticles, labeled with either iron oxide or gadolinium (Fig. 2) (38), and synthetic nanoparticles functionalized with, for example, ligands that interact with the macrophage scavenger receptor (39). Nevertheless, nanoparticle production, costs, and potential toxicity pose limits to translatability. Oxidative stress resulting from macrophage inflammation has been imaged using the myeloperoxidase sensor bis-5-hydroxytryptamide-diethylenetriami-nepentaacetic acid(Gd) [bis-5HT-DTPA (Gd)] (Fig. 2), which is retained and undergoes increased relaxivity as a result of polymerization in inflamed atherosclerotic lesions (40). Further probe and imaging sequence development will make MRI even more useful in research involving animal models of atherosclerosis.
Fig. 2. Nanoparticle imaging and corresponding diagnostic modalities.
Nanoparticles can be labeled with a variety of imaging agents to enable their detection in plaque cells with CT, MRI, optical methods, or nuclear imaging, such as PET and SPECT. The relative costs, sensitivity, scan time range, and resolution range are indicated. CT image obtained from (47) with permission; MRI image from (52) with permission; optical image from (35) with permission; PET image from (55) with permission. DTPA, diethylenetriamine pentaacetic acid; DOTA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid.
Neovascularization and adhesion molecule expression at the activated endothelium are inherently linked to plaque inflammation (4) and are therefore interesting imaging targets. Extensive work in mice conducted with, αvβ3 integrin–targeted nanoparticles shows the value of angiogenesis molecular imaging for both staging atherosclerosis (41) and monitoring therapeutic response (42). Alternatively, nanoparticle MRI of adhesion molecules—which are proteins that attract and recruit monocytes—is useful for noninvasively assessing statin therapy (43), which allows for direct assessment of vessel wall inflammation.
Nanoparticles are primarily used in preclinical studies of inflammatory pathways and may be readily labeled with fluorophores (Fig. 2) for noninvasive and intravascular NIRF imaging, confocal microscopy, and flow cytometry (20). For example, dye-labeled nanoparticles have shown that atherosclerosis enhances infarct inflammation and post-MI remodeling through oversupply of inflammatory monocytes to the ischemic myocardium (44). Additionally, combining nanoparticle imaging with protease sensors in a spectrally resolved channel has enabled simultaneous assessment of several wound-healing biomarkers in the same mouse (44). These nanoparticles inform on new atherosclerosis biology, which could translate to improved therapeutics and a better understanding of pathogenesis of the human disease.
Multicolor spectral CT
As a molecular imaging modality, conventional CT is limited by inherently low sensitivity for exogenous agents and the consequent need to repeat scans before and after administering the agent, increasing radiation exposure. Recently developed spectral CT (45) allows so-called hot-spot imaging (46), which eliminates the baseline scan. A dedicated detector exploits the x-ray spectrum’s polychromatism and differentiates distinct materials, including tissue-borne calcium, iodine, and gold, which are difficult to differentiate using conventional CT scans. Cormode et al. (47) used this technique to image atherosclerotic plaque macrophages in a mouse model of atherosclerosis with a gold-labeled HDL nanoparticle. In both research and the clinic, CT continues to be an effective imaging tool for atherosclerosis, particularly with the currently available fast and sensitive scanners.
Fluorescence imaging
NIRF imaging is an attractive alternative to nuclear imaging owing to good sensitivity, high spatial resolution, ability to distinguish multiple fluorescent dyes simultaneously, and lack of radiation (48). Additionally, NIRF imaging is fast (a typical study takes <5 min) and is relatively inexpensive because optical imaging devices can be up to an order of magnitude less costly than MRI or PET/CT (Fig. 2). However, the penetration depth of light is limited to about 5 cm, which restricts the use of NIRF imaging to mice and, in larger subjects, superficial structures, such as hands or breasts, particularly if fluorescence detection occurs in reflectance mode.
Fluorescence molecular tomography (FMT) (49) is a form of three-dimensional imaging using fluorescent probes emitting in the near-infrared window. It is often combined with an anatomical diagnostic method like MRI or CT (50). FMT of atherosclerotic plaque inflammation usually uses either NIRF-tagged macrophage probes, such as Cy5.5-labeled nanoparticles, which can visualize plaque macrophages (51), or “activatable” or “smart” probes (Fig. 2). Smart probes are quenched at injection; that is, they are not initially fluorescent, but instead become fluorescent when they interact with certain proteases (for example, cathepsins and matrix metalloproteinases) and can then be quantified with FMT. Combining FMT with smart probes can monitor the progression of atherosclerotic plaque inflammation, and a supplemental high-resolution CT angiography protocol can accurately localize fluorescence originating from the aortic root. For instance, a recent study used smart probes and FMT to visualize protease activity in apolipoprotein E (apoE)–knockout (KO) mice (Fig. 1) (52). By monitoring protease activity, the authors could assess the efficacy of an anti-inflammatory nanotherapeutic. This apoE-KO mouse model is widely used in translational research because it develops an inflammatory atherosclerotic plaque phenotype with high macrophage content and protease activity that are akin to vulnerable lesions in human patients. Atherosclerosis research using FMT is still sparse, but the ability to monitor molecular changes makes it an attractive in vivo molecular readout.
Quantification of vessel wall inflammation by PET
In atherosclerotic rabbits, FDG-PET signal correlated with plaque macrophage burden (53). FDG affinity for plaque macrophages relies on the high metabolic activity of leukocytes, which consequently limits the specificity of the approach. Adjacent tissues with high glucose uptake, such as the myocardium (heart tissue) next to coronary arteries, may show a high FDG signal. Coronary studies in people are already occurring with FDG-PET, but they are not easy owing to high glucose—and, hence, FDG uptake—in healthy heart tissue.
One alternative to FDG in PET imaging uses nanoparticles coated with dextran, a polysaccharide composed of many glucose molecules. Radiolabeled dextran nanoparticles are readily taken up by macrophages (54), but not by myocardium. For example, a 64Cu-labeled dextran–coated iron oxide nanoparticle enabled hybrid PET-CT imaging of macrophages in inflammatory atherosclerosis in apoE-KO mice (51). More recently, a next-generation version of this probe, which uses a core-free 13-nm dextran nanoparticle radiolabeled with 89Zr (Fig. 2), was used for PET-MRI of macrophages in atherosclerotic mice (55). This approach effectively monitored plaque response to a small interfering RNA (siRNA) anti-inflammatory treatment. Ongoing development of novel radiolabeled nanoparticles will further improve the PET’s ability to quantitatively image atherosclerosis-related processes.
ATHEROSCLEROSIS IMAGING IN PATIENTS
The latest imaging techniques described above are not routinely used to diagnose and monitor inflammation in atherosclerosis in clinical practice, although they have been explored in several clinical studies and clinical trials. Traditionally, novel anti-atherosclerotic compounds are evaluated in large patient cohorts, using morbidity, mortality, and secondary event occurrence rates as endpoints (56). Such trials often follow 5000 to 10,000 patients for several years and are thus very costly and lengthy; moreover, they do not necessarily inform on direct drug action. Imaging trials could precede these large and costly programs, serving as faster, more cost-effective preliminary screenings by directly measuring drug action in plaque after several months of treatment, rather than patient mortality after several years, in cohorts two orders of magnitude smaller (n = 100 to 200) than traditional studies (Table 1). It may be beneficial to first study, for instance, a new drug’s action on plaque inflammation in a small patient cohort before embarking on a trial that studies outcome by measuring clinical events and mortality.
Table 1. Traditional endpoint trial versus imaging trial.
A traditional endpoint trial typically monitors endpoints that are the consequence of the disease, such as clinical events or death, in a large cohort of patients during an extended period. An imaging trial aims to evaluate the direct effects of anti-atherosclerotic medication by evaluating its effect on the atherosclerotic vessel wall thickness and inflammatory state in a relatively small group of patients, typically in a 2-year time frame. The relative costs and number of participants are indicated.
| Endpoint trial | Time (years) | n participants | Endpoints | Cost (million USD) |
|---|---|---|---|---|
| Traditional | 5 | 5000 | Hospitalization or death | $250 |
| Imaging | 2 | 200 | CT, PET, MRI | $5 |
In patients, the structural composition of the atherosclerotic vessel wall can be visualized and characterized with high-resolution multi-contrast MRI. Multicontrast MRI protocols typically combine bright blood time of flight imaging, to visualize blood vessels, with T1-, T2-, and proton density–weighted black blood images, to delineate the vessel lumen from the vessel wall and identify typical plaque features. These include wall thickness, intraplaque hemorrhage, calcification, the thickness of the fibrous cap, and outward vessel wall remodeling (15). A recent study used multicontrast MRI to longitudinally study carotid artery plaque progression in symptomatic patients (57). In some cases, Gd contrast–enhanced MRI is also used to help analyze atherosclerotic plaques, as will be discussed later.
USPIO-enhanced MRI of plaque macrophages
Several studies have demonstrated that iron oxide nanoparticle–enhanced MRI can stratify plaque inflammation in both animals and humans, which could help differentiate plaques at risk for rupture from stable lesions (58). If proven predictive, this method would enable clinical decision-making and facilitate emerging immunotherapeutics that aggressively alter leukocyte function in high-risk patients. Clinically, ultrasmall superparamagnetic nanoparticles of iron oxide (USPIO), which have long circulation times and can directly penetrate the plaque, have been used to measure inflammatory burden (22, 23) and the effects of lipid-lowering therapy in patients (59). Multicenter human trials are still needed to validate atherosclerotic plaque imaging before universally robust protocols are established and implemented.
Imaging of arterial wall inflammation by FDG-PET/CT
FDG-PET/CT is widely applied in people for the detection and staging of various cancers and to monitor therapeutic response. Compared to USPIO-enhanced MRI, FDG-PET is easier to implement and analyze, more quantitative, faster, and does not require a baseline scan (Fig. 2). Rudd and colleagues pioneered applying this method to image atherosclerotic plaque inflammation (60). Subsequent studies by several independent groups improved and validated the reproducibility and robustness of the technique in atherosclerosis (61, 62). Since then, several clinical trials with anti-atherosclerotic therapies have integrated FDG-PET as an imaging endpoint (for example, NCT00655473 and NCT00695305). Despite its promise, the specificity of FDG-PET remains low because the cellular uptake mechanism is nonspecific, and precise data interpretation, imaging quality, and quantification need improvement.
Using FDG-PET in multicenter clinical drug trials creates additional challenges, including scanner differences among sites and vendors, discrepancies in image acquisition, and variations in data transfer and analysis. The dal-PLAQUE study, a recent placebo-controlled multi-center imaging trial (NCT00655473), investigated FDG-PET imaging of plaque inflammation and MRI of plaque volume (63, 64). This imaging trial was part of a large program designed to evaluate the effects of dalcetrapib, a new cholesteryl ester transfer protein inhibitor, and showed FDG-PET to be a good indicator of the dalcetrapib treatment outcome. However, the drug trial was later halted. In another multi-center clinical trial (NCT00695305), the beneficial effects of intensified statin therapy were validated and differentiated using FDG-PET/CT imaging (65).
The value of FDG-PET was exemplified in human studies, revealing a connection between systemic inflammation severity and vascular FDG uptake. Steady-state monocyte production occurs in the hematopoietic niche of the bone marrow. Hematopoietic activity regulates the supply of blood monocytes, which increases in mice and patients with atherosclerosis. In mice, ischemic events such as MI or other inflammatory stimuli may accelerate splenic leukocyte production through modulation of the microenvironment in hematopoietic niches in the bone marrow and the spleen (Fig. 1) (35). FDG PET imaging in patients with acute coronary syndrome documented that bone marrow and spleen PET signal increases after myocardial injury and correlates with vascular inflammation (66). A different study revealed that patients with rheumatoid arthritis have increased aortic FDG uptake compared to patients who have stable cardiovascular disease (67). Anti–tumor necrosis factor–α therapy, which reduces inflammation in diseased joints, also led to reduced aortic inflammation in patients with arthritis. Further FDG-PET imaging research is needed to elucidate if and how PET signal reflects such immune system dynamics in disease and in response to therapy.
Dynamic contrast-enhanced MRI
The dal-PLAQUE study incorporated DCE-MRI of plaque permeability (Fig. 1), which is related to plaque neovascularity (68), as a secondary endpoint. In DCE-MRI—a technique routinely applied in oncological studies—T1-weighted MR images are acquired before and continuously after the administration of a paramagnetic contrast agent, typically Gd-DTPA or any of the other available Gd-based agents (Fig. 2). The cumulative signal enhancement that results from contrast agent injection can be used to create area under the curve (AUC) graphs or to build mathematical models that extract parameters like k-trans, a measure of vascular permeability.
DCE-MRI of the atherosclerotic vessel wall is challenging owing to blood signal from the vessel lumen and the limited wall thickness. Improving DCE-MRI acquisition speed will advance spatial resolution and allow full three-dimensional assessment of a vessel bed. Studies in both rabbits (69, 70) and humans (71) have shown good reproducibility of the correlation between DCE-MRI–derived parameters, such as AUC and k-trans, with plaque microvasculature. Because the latter parameter is a hallmark of plaque vulnerability, this technique may potentially identify atherosclerosis patients at risk of rupture.
Inflammation imaging in coronary arteries
Most clinical methods of imaging plaque inflammation target the larger vessels, such as the aorta and carotid arteries. Nevertheless, there is great interest in directly imaging plaque features in the coronary arteries because these plaques cause acute MI. Imaging the coronaries is far more challenging than the larger vessels because the smaller plaque volume requires high-resolution imaging methods, and the challenge of cardio-respiratory motion often necessitates gating strategies to deblur images. Accordingly, insight into human coronary atheroma has mostly been obtained through invasive intravascular imaging methods.
FDG-PET and 18F-sodium fluoride PET have preliminarily enabled coronary plaque imaging of inflammation and osteogenesis, respectively (72). In Fig. 1, a PET/CT image of the neck region shows uptake of FDG in the coronary arteries of a patient. Although carotid FDG-PET/CT imaging has been shown to be reproducible and reliable, its application for the coronary arteries is challenging because of glucose consumption by the heart muscle itself. Alternatively, 18F-sodium fluoride PET demonstrated less myocardial background signal than FDG-PET owing to the lack of uptake of 18F-sodium fluoride by the myocardial wall (73).
Intravascular optical imaging using optical coherence tomography (OCT) can physically detect plaque macrophages in patients. Macrophages are relatively large cells with varying refractive indices that produce elevated signal during OCT imaging (74, 75). However, areas of calcification may limit the assessment of plaque macrophages by OCT.
Intravascular NIRF molecular imaging is an emerging catheter-based approach for imaging fluorescent molecular probes in coronary-sized arteries. NIRF catheters can detect inflammatory targets (protease activity and macrophages) through blood because NIR light travels efficiently (76). In a recent advance, combining NIRF with OCT enabled a dual-modal molecular-structural imaging approach based on clinical OCT (77). With the continued development of fluorescence imaging agents (Fig. 2) (78, 79), intravascular NIRF imaging is becoming relevant for clinical intracoronary imaging of plaque inflammation.
NANOTHERAPY IN ATHEROSCLEROSIS
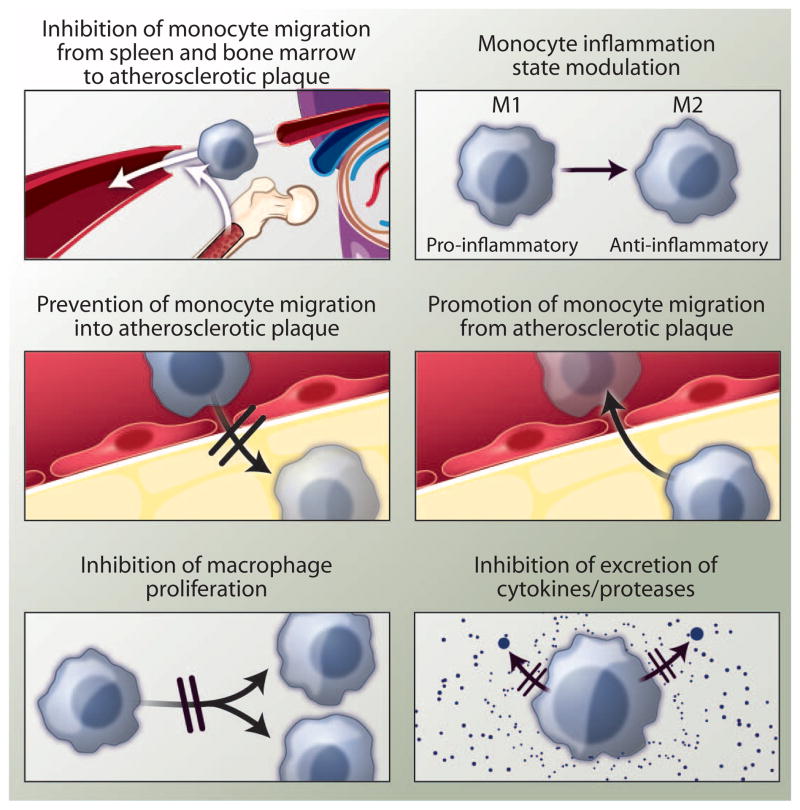
The dynamics of monocyte and macrophage migration determine the overall number of cells in the plaque. For instance, if oversupply and recruitment of monocytes outpaces macrophage death and departure, the number of plaque-resident cells swells and atherosclerosis progresses. In growing plaques, macrophages become dysfunctional. Instead of removing the ingested lipoprotein deposits from the artery, the cells differentiate to foam cells and die, contributing to necrotic cores. The process of macrophage death and necrotic core development can destroy local tissue architecture by secreting proteases and inflicting oxidative stress on the vessel wall. In particular, matrix-degrading enzymes such as metalloproteases may wreak havoc on the arterial wall structure while digesting the fibrous cap, thereby initiating plaque rupture. These insights make macrophages, which readily ingest nanomaterials, a prime target for novel therapeutics [see Perspective by Fredman et al. (80)]. Therapeutically reducing their numbers can be achieved by targeting several processes (Fig. 3), including monocyte phenotype and migration (81), as well as by reducing the burden (82) and proliferation (83) of macrophages.
Fig. 3. Therapeutic targets in inflammatory atherosclerosis.
A cascade of processes that contribute to macrophage activity and inflammation in atherosclerotic plaques can be specifically targeted to halt the progression of the disease. The migration of monocytes from the bone marrow or spleen to atherosclerotic plaque can be inhibited; the phenotype of monocytes can be modulated; monocyte migration into the plaque can be halted, or monocytes/macrophages departing the plaque can be stimulated. Moreover, macrophage proliferation and the excretion of harmful proteases or cytokines are compelling therapeutic targets.
The field of oncological nanoparticle therapy has matured well in the preclinical context and is starting to become increasingly established clinically. First-generation nanoparticle therapies (for example, liposomes of daunorubicin or amphotericin) have been approved for clinical use, whereas second-generation targeted and controlled release systems are currently under trial (84). Nanoparticle targeting of tumors has been thoroughly investigated, but there have been few studies on atherosclerosis thus far. Still, several different targeting methods have been proposed (85). Since 2003, several studies have actively targeted atherosclerotic neovessels with nanoparticles that recognize αvβ3 integrins (41). Natural nanoparticle targeting principles based on the inherent affinity of lipoproteins for plaques have also been studied extensively, primarily in the context of atherosclerotic plaque molecular imaging (38).
However, studies on nonspecific accumulation of long-circulating nanoparticles over the permeable endothelium have only recently appeared (53). A biomedical engineering approach that combined advanced microfluidics technology and in vivo studies in atherosclerotic rabbits revealed a direct correlation between the translocation of polymeric nanoparticles and endothelial permeability (86). In a rabbit model, these polymeric nanoparticles enter the plaque via the luminal side and through adventitial neovessels that originate from the vasa vasorum. Integrating a variety of imaging modalities proved essential to unraveling the targeting mechanism.
Neovascularization within the atherosclerotic plaque is closely related to the inflammatory process as it facilitates monocyte recruitment and transmigration. In rabbits, fumagillin-loaded nanoparticles that were targeted to αvβ3-expressing endothelial cells through a peptidomimetic have shown potential, particularly in combination with subsequent oral statin therapy, to reduce plaque neovascularization (42, 87). Also, several strategies to directly inhibit plaque inflammation were recently reported. The first strategy targets atherosclerotic plaque with a nanoparticle containing an anti-inflammatory drug (52, 53), whereas the second strategy focuses on systemically preventing monocytes from migrating to the atherosclerotic plaque (Fig. 3) (81, 88). Additional inquiry into nanoparticle treatment for atherosclerosis, particularly focused on inhibiting proinflammatory processes (89), will validate these strategies and discover others.
Direct inhibition of plaque macrophage inflammation
In atherosclerotic rabbits, Lobatto et al. (53) observed marked and persistent plaque inflammation inhibition using a liposomal nanoparticle containing glucocorticoids. A single dose significantly reduced uptake of FDG as determined by PET imaging, indicating that the plaque was less inflamed after therapy. In these studies, FDG-PET/CT imaging, discussed in an earlier section, was complemented by DCE-MRI, which corroborated the anti-inflammatory effect. The integration of a local delivery strategy with advanced multimodal clinical imaging methods (Fig. 1), including DCE-MRI and PET/CT, shows how advances in biomedical engineering produce novel approaches to managing atherosclerotic disease. This study (53) provided the foundation for two subsequent first-in-man studies of nanotherapy in atherosclerosis patients, in which the same imaging protocols are being used to monitor therapeutic efficacy of the drug-loaded liposomal particle (NCT01601106).
An alternate nanotherapeutic strategy involves statins, a class of lipid-lowering drugs with modest but well-established anti-inflammatory properties, rather than glucocorticoids. Duivenvoorden et al. sought to better exploit and improve statins’ anti-inflammatory properties via an HDL-based nanotherapeutic carrier. The carrier served as a “Trojan horse” to accumulate simvastatin in the atherosclerotic vessel wall, ultimately exerting direct anti-inflammatory action on plaque macrophages in the aortic roots of apoE-KO mice (52). Using preclinical high-field MRI and FMT/CT as well as quantitative histology and mRNA analyses, we observed potent anti-inflammatory effects in both preventive and regressive treatment regimens.
Inhibiting monocyte migration to atherosclerotic lesions
In the above examples, the atherosclerotic plaque macrophages were directly targeted to reduce vessel wall inflammation and subsequently achieve plaque stabilization. An alternative approach used systemic nanotherapy to silence the chemokine receptor CCR2 in monocytes with siRNA (81). Recruitment of inflammatory monocytes to the atherosclerotic plaque and other inflammatory sites depends on the chemokine/chemokine receptor pair MCP-1/CCR2. A lipid-like nanoparticle carrying siRNA targeting CCR2 accumulated in circulating monocytes and in the spleen monocyte reservoir (90). Efficient silencing of CCR2 expression in inflammatory monocytes subsequently inhibited their migration to atherosclerotic plaques in apoE-KO mice, resulting in a lower inflammatory burden in the aortic root. Mice that underwent ischemia reperfusion injury also showed reduced myocardial infarct size after treatment with nanoparticle-enabled RNA interference (RNAi) (83). Recent proof-of-concept studies in non-human primates (91) and humans (92) have confirmed the potential of RNAi therapy, which may be further facilitated through nanoparticle formulations.
Patients who suffer a MI or stroke are at higher risk of recurrent events. In mice, leukocyte overproduction accelerates atherosclerosis and aggravates plaque inflammation (35). Therefore, anti-inflammatory nanotherapies, which directly affect plaque inflammation, may inhibit plaque progression and could reduce recurrent events. To test this hypothesis and thoroughly evaluate the nanotherapy-imaging strategies detailed above, future studies should integrate immunologically focused in vivo imaging with nanotherapeutic interventions that efficiently curb plaque inflammation and aggravation, as outlined in Fig. 3.
TRANSLATIONAL CONSIDERATIONS
The atherosclerosis community has focused on whether lesion-specific imaging can supplement clinical risk prediction models (for example, risk scores, blood biomarkers, and coronary artery calcium scoring) to better define individual risk. Thus far, the landmark PROSPECT trial (14) (NCT00180466) has demonstrated that three-vessel IVUS-virtual histology can identify unique plaque progression predictors, including plaque burden ≥70%, thin-capped fibroatheroma, and mean luminal area ≤4.0 mm2. However, owing to the relatively low positive predictive value of this approach (plaques with all three features still have only an 18% event rate over the 3.4-year median follow-up) and the invasive nature of IVUS, this method is not routinely used in the clinic. Consequently, new diagnostic approaches are still required.
Molecular imaging–based strategies have the potential to add orthogonal biological information about plaque phenotypes. By complementing structural or molecular imaging, these additional plaque diagnostics may improve risk prediction (that is, improve c-statistics and net reclassification indices). For example, a small FDG-PET study demonstrated that carotid plaque inflammation, but neither patient age nor degree of stenosis, was predictive for recurrent stroke (93). In the coronary space, intra-vascular NIRF technology may enable similar inflammation imaging and predictive studies of nonculprit plaques in patients with acute MI. The FDG-PET study (93) and promising NIRF methodology exemplify the clinical roadmap for molecular imaging: to demonstrate utility beyond conventional imaging and clinical biomarkers, such as low-density lipoprotein (LDL) and C-reactive protein.
A second emerging field within biomedical engineering is intelligent drug delivery. Recent insights into the immunology of atherosclerosis offer new therapeutic points of attack (Figs. 1 and 3). This could include interfering with the life cycle of inflammatory leukocytes, including production, migration, and retention of macrophages in plaque. In addition, emerging ideas about modulating adaptive immune responses present bioengineering opportunities (94) in future vaccination strategies for heart disease, once these become feasible. However, it is also clear that we must develop tailored therapies that spare the immune system’s important protective functions. For instance, macrophages are the first line of defense against infections, and that must be preserved. It is imperative to deploy our insight into the phenotypic differences that distinguish plaque-destabilizing inflammatory macrophages from resident macrophages with key sentinel functions for detecting infectious agents. That is, we must pursue interventional strategies that avoid collateral damage. Any viable immunotargeting therapy should be specific to either detrimental immune cells or noncellular immune components. Alternatively, dosing should be individually customized and closely monitored to avoid compromising the host defense system.
Ideally, nanotechnology will guide drug delivery to culprit cells and plaque, possibly with specifically engineered drug delivery particle properties, such as size, surface charge, and affinity ligands (Fig. 4) (85). Therapeutic payloads could also be designed to interfere only with harmful immune functions while preserving others deemed essential for tissue homeostasis. Such nanotherapies could perhaps deactivate transcription factors such as interferon regulatory factor 5 (95), a master regulator that promotes an inflammatory macrophage phenotype. To achieve these goals, interdisciplinary teams that combine bioengineering expertise with biological insight into mechanisms of disease progression and regression will have to be formed; this is the mission of the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute’s Program of Excellence in Nanotechnology (www.nhlbi-pen.net), for example.
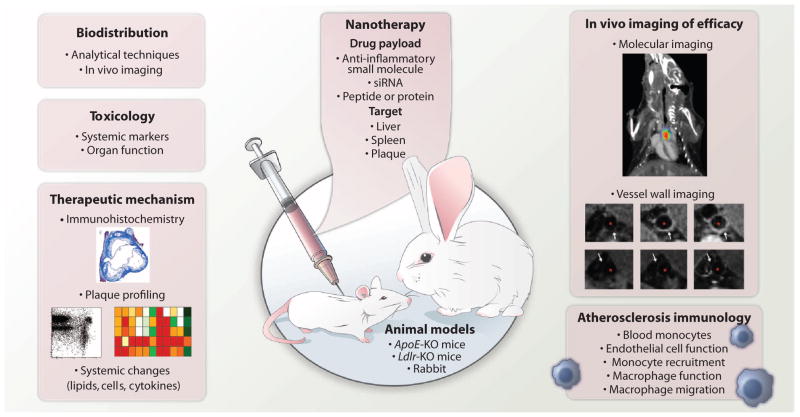
Fig. 4. Considerations for an imaging-, nanotherapy-, and immunology-facilitated atherosclerosis preclinical study.
The early-stage development of an anti-inflammatory nanotherapy for atherosclerosis requires comprehensive evaluation of various pharmacological aspects and effects. These include the determination of the biodistribution (by imaging), toxicology, and therapeutic mechanism. Nanotherapy formulations should consider the target, delivery mechanism, and what type of drug will be included. Preclinically, efficacy of the nanotherapy and the immunological effects can be determined commonly in a mouse or rabbit model of atherosclerosis. Immunohistochemistry image obtained from (35) with permission. Plaque profiling images obtained from (52) with permission. Molecular imaging panel obtained from (96) with permission. Vessel wall images obtained from (52) with permission.
Preclinical studies investigating nanotherapeutic interventions in atherosclerosis remain few in number, although there are substantial efforts in novel nanomaterial design and production. However, for nanotherapies to successfully translate for cardiovascular disease, their functionality and effects need to be thoroughly studied. Traditional histological assessments of plaque phenotype, microvessel, and macrophage burden do not suffice and should be complemented with gene expression and immunological readouts that elucidate the mechanisms of the nanotherapeutic interventions (Fig. 4). Additionally, benefits will be evaluated by integrating noninvasive imaging to longitudinally assess atherosclerotic plaque biology as a function of nanotherapy intervention. It is important to closely and accurately monitor systemic effects, possibly with similar imaging methods. These imaging readouts will report on early efficacy and eventually be complemented by long-term studies of hard endpoints. Functional imaging methods, such as FMT, can monitor plaque biology in preclinical studies (51, 83). FMT of protease activity closely correlates with plaques’ inflammatory states and can indicate changes in plaque macrophage numbers or phenotypes (96). However, FMT is an imaging modality that only works in small animals and is not used on human subjects. FDG-PET/CT may be less specific to the inflammatory process (97) but can be readily integrated into human trials. A small number of trials have used FDG-PET/CT as an inflammation/metabolic imaging biomarker to determine atherosclerosis drug efficacy.
The relative cost and complexity of human nanotherapy trials restrict the number of patients who can be included (Table 1). In atherosclerosis, another major challenge is determining primary endpoints, such as the direct effects that nanotherapy may exert on vessel wall inflammation. Integrating clinically viable imaging endpoints is a prerequisite for effective nanoparticle therapies for atherosclerosis. Recently, two first-in-human nanotherapy trials were conducted in atherosclerosis patients (NCT01647685 and NCT01601106). Engineered nanotherapeutics present an interesting new treatment paradigm for halting plaque progression and rupture. Clinically, new nanotherapeutic strategies will need to demonstrably reduce incidence of MI, stroke, and mortality. Proving endpoint efficacy on top of a standard of care (that is, medical therapy with aspirin and statins) is challenging.
Nonetheless, atherosclerosis carries significant risk of recurrence, even after aggressive medical therapy, especially when considered over a patient’s lifetime. For example, several studies have shown that up to 80% of patients do not reach LDL cholesterol goals on statin therapy. On a regional level, novel treatments, such as prophylactic, macrophage-targeted therapy, or “vascular paint” (insulation of inflammatory tissue from the clotting factors in blood) (98), may benefit patients already undergoing invasive cardiac catheterization. Similarly, lesion-based therapy—initially with biodegradable, drug-eluting, or other specialized stents—is an intriguing option for high-risk plaques in patients undergoing invasive cardiac catheterization (99). In applying regional or local invasive therapies, identifying patients with very high risk of ischemic events will be critical to realizing suitable benefit-to-risk ratios.
OUTLOOK
Twenty-first century innovations in diagnosing and treating atherosclerosis are the result of integrating cardiovascular medicine, immunology, medical imaging, bioengineering, and (bio)chemical engineering. Cross-pollination among these fields will further increase their contributions to atherosclerotic disease management. Medical imaging is poised to noninvasively monitor atherosclerosis beyond vessel stenosis and wall thickness by assessing plaque composition and quantifying inflammatory cells and processes. In the preclinical setting, advanced molecular imaging methods already accomplish these goals with nanoparticle MRI and PET, optical techniques such as FMT, intravascular NIRF imaging and intravital microscopy, as well as the latest-generation CT imaging. Advanced high-resolution imaging that follows culprit immune cell action in inflamed plaque at the single-cell level reveals the underlying immune processes that lead to ischemic complications. Additionally, nanotechnology offers innovative tools for the developing target-specific therapeutics in atherosclerosis. Although most imaging and nanotherapeutic studies for atherosclerosis are in the preclinical realm, early insights in first-in-human trials suggest that the most promising approaches described in this Review will affect patient care.
Acknowledgments
Funding: This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute, as a Program of Excellence in Nanotechnology (PEN) Award, Contracts HHSN268201000045C (Z.A.F.) and HHSN268201000044C; the NIH grants NIBIB R01 EB009638 and NHLBI R01 HL071021 (Z.A.F.), NHLBI R01HL114477 and R01 NHLBI HL108229 (F.A.J.), NHLBI R01HL096576 (M.N.), NCI R01 CA155432 (W.J.M.M.), as well as AHA #13GRNT17060040 (F.A.J.); and NWO ZonMW Vidi 91713324 (W.J.M.M.).
REFERENCES AND NOTES
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 3.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Mitka M. New basic care goals seek to rein in global rise in cardiovascular disease. JAMA. 2012;308:1725–1726. doi: 10.1001/jama.2012.13721. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. The forgotten majority: Unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Milonas C, Jernberg T, Lindbäck J, Agewall S, Wallentin L, Stenestrand U RIKS-HIA Group. Effect of angiotensin-converting enzyme inhibition on one-year mortality and frequency of repeat acute myocardial infarction in patients with acute myocardial infarction. Am J Cardiol. 2010;105:1229–1234. doi: 10.1016/j.amjcard.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Chen IY, Wu JC. Cardiovascular molecular imaging: Focus on clinical translation. Circulation. 2011;123:425–443. doi: 10.1161/CIRCULATIONAHA.109.916338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 10.Owen DR, Lindsay AC, Choudhury RP, Fayad ZA. Imaging of atherosclerosis. Annu Rev Med. 2011;62:25–40. doi: 10.1146/annurev-med-041709-133809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborn EA, Jaffer FA. The year in molecular imaging. JACC Cardiovasc Imaging. 2012;5:317–328. doi: 10.1016/j.jcmg.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz C, Massberg S. Multiple pathways to lesional macrophages in atherosclerosis. Sci Transl Med. 2014;6:239ps2. doi: 10.1126/scitranslmed.3008922. [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 15.Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, Ohayon J, Pettigrew R, Sabatine MS, Tearney GJ, Waxman S, Domanski MJ, Srinivas PR, Narula J. Detection of high-risk atherosclerotic plaque: Report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5:941–955. doi: 10.1016/j.jcmg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerwin WS, Hatsukami T, Yuan C, Zhao XQ. MRI of carotid atherosclerosis. AJR Am J Roentgenol. 2013;200:W304–W313. doi: 10.2214/AJR.12.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 18.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 19.Strijkers GJ, Mulder WJM, van Tilborg GAF, Nicolay K. MRI contrast agents: Current status and future perspectives. Anticancer Agents Med Chem. 2007;7:291–305. doi: 10.2174/187152007780618135. [DOI] [PubMed] [Google Scholar]
- 20.Heidt T, Nahrendorf M. Multimodal iron oxide nanoparticles for hybrid biomedical imaging. NMR Biomed. 2013;26:756–765. doi: 10.1002/nbm.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 22.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 23.Tang T, Howarth SPS, Miller SR, Trivedi R, Graves MJ, King-Im JU, Li ZY, Brown AP, Kirkpatrick PJ, Gaunt ME, Gillard JH. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke. 2006;37:2266–2270. doi: 10.1161/01.STR.0000236063.47539.99. [DOI] [PubMed] [Google Scholar]
- 24.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 25.Osborn EA, Jaffer FA. The year in molecular imaging. JACC Cardiovasc Imaging. 2010;3:1181–1195. doi: 10.1016/j.jcmg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majmudar MD, Nahrendorf M. Cardiovascular molecular imaging: The road ahead. J Nucl Med. 2012;53:673–676. doi: 10.2967/jnumed.111.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichler BJ, Kolb A, Nägele T, Schlemmer HP. PET/MRI: Paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51:333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 28.Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, Tahara N, Fuster V, Warburton EA, Fayad ZA, Tawakol AA. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: Ready for prime time? J Am Coll Cardiol. 2010;55:2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 29.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson EE. Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation. 2011;124:2129–2138. doi: 10.1161/CIRCULATIONAHA.111.030627. [DOI] [PubMed] [Google Scholar]
- 31.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell–APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chèvre R, González-Granado JM, Megens RTa, Sreeramkumar V, Silvestre-Roig C, Molina-Sánchez P, Weber C, Soehnlein O, Hidalgo A, Andrés V. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circ Res. 2014;114:770–779. doi: 10.1161/CIRCRESAHA.114.302590. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Vinegoni C, Feruglio PF, Fexon L, Gorbatov R, Pivoravov M, Sbarbati A, Nahrendorf M, Weissleder R. Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nat Commun. 2012;3:1054. doi: 10.1038/ncomms2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HWM, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinreb DB, Aguinaldo JGS, Feig JE, Fisher EA, Fayad ZA. Non-invasive MRI of mouse models of atherosclerosis. NMR Biomed. 2007;20:256–264. doi: 10.1002/nbm.1148. [DOI] [PubMed] [Google Scholar]
- 37.Lobatto ME, Calcagno C, Metselaar JM, Storm G, Stroes ESG, Fayad ZA, Mulder WJM. Imaging the efficacy of anti-inflammatory liposomes in a rabbit model of atherosclerosis by non-invasive imaging. Methods Enzymol. 2012;508:211–228. doi: 10.1016/B978-0-12-391860-4.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skajaa T, Cormode DP, Falk E, Mulder WJM, Fisher EA, Fayad ZA. High-density lipoprotein–based contrast agents for multimodal imaging of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:169–176. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulder WJM, Strijkers GJ, Briley-Saboe KC, Frias JC, Aguinaldo JGS, Vucic E, Amirbekian V, Tang C, Chin PTK, Nicolay K, Fayad ZA. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58:1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 40.Ronald JA, Chen JW, Chen Y, Hamilton AM, Rodriguez E, Reynolds F, Hegele RA, Rogers KA, Querol M, Bogdanov A, Weissleder R, Rutt BK. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009;120:592–599. doi: 10.1161/CIRCULATIONAHA.108.813998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin–targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 42.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Endothelial αvβ3 integrin–targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 43.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 44.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roessl E, Proksa R. K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors. Phys Med Biol. 2007;52:4679–4696. doi: 10.1088/0031-9155/52/15/020. [DOI] [PubMed] [Google Scholar]
- 46.Bulte JWM. Hot spot MRI emerges from the background. Nat Biotechnol. 2005;23:945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 47.Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, Fuster V, Fisher EA, Mulder WJM, Proksa R, Fayad ZA. Atherosclerotic plaque composition: Analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256:774–782. doi: 10.1148/radiol.10092473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 49.Ntziachristos V, Tung CH, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8:757–760. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 50.Ale A, Ermolayev V, Herzog E, Cohrs C, de Angelis MH, Ntziachristos V. FMT-XCT: In vivo animal studies with hybrid fluorescence molecular tomography–X-ray computed tomography. Nat Methods. 2012;9:615–620. doi: 10.1038/nmeth.2014. [DOI] [PubMed] [Google Scholar]
- 51.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ESG, Fuster V, Fisher EA, Fayad ZA, Mulder WJM. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, Dickson SD, Nicolay K, Banciu M, Schiffelers RM, Metselaar JM, van Bloois L, Wu HS, Fallon JT, Rudd JH, Fuster V, Fisher EA, Storm G, Mulder WJM. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm. 2010;7:2020–2029. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13:125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 55.Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112:755–761. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 57.Kwee RM, Truijman MTB, van Oostenbrugge RJ, Mess WH, Prins MH, Franke CL, Korten AGGC, Wildberger JE, Kooi ME. Longitudinal MRI study on the natural history of carotid artery plaques in symptomatic patients. PLOS One. 2012;7:e42472. doi: 10.1371/journal.pone.0042472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang TY, Muller KH, Graves MJ, Li ZY, Walsh SR, Young V, Sadat U, Howarth SPS, Gillard JH. Iron oxide particles for atheroma imaging. Arterioscler Thromb Vasc Biol. 2009;29:1001–1008. doi: 10.1161/ATVBAHA.108.165514. [DOI] [PubMed] [Google Scholar]
- 59.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, UKIJM, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 61.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 62.Fifer KM, Qadir S, Subramanian S, Vijayakumar J, Figueroa AL, Truong QA, Hoffmann U, Brady TJ, Tawakol A. Positron emission tomography measurement of periodontal 18F-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J Am Coll Cardiol. 2011;57:971–976. doi: 10.1016/j.jacc.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 63.Fayad ZA, Mani V, Woodward M, Kallend D, Bansilal S, Pozza J, Burgess T, Fuster V, Rudd JH, Tawakol A, Farkouh ME. Rationale and design of dal-PLAQUE: A study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Am Heart J. 2011;162:214–221.e2. doi: 10.1016/j.ahj.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A dal-PLAQUE Investigators. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): A randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JHF, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 66.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18F-fluorodeoxyglucose positron emission tomographic imaging. Circ Cardiovasc Imaging. 2014;7:454–460. doi: 10.1161/CIRCIMAGING.113.001093. [DOI] [PubMed] [Google Scholar]
- 67.Mäki-Petäjä KM, Elkhawad M, Cheriyan J, Joshi FR, Ostör AJK, Hall FC, Rudd JHF, Wilkinson IB. Anti-tumor necrosis factor-a therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126:2473–2480. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 68.Calcagno C, Ramachandran S, Millon A, Robson PM, Mani V, Fayad Z. Gadolinium-based contrast agents for vessel wall magnetic resonance imaging (MRI) of atherosclerosis. Curr Cardiovasc Imaging Rep. 2013;6:11–24. doi: 10.1007/s12410-012-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calcagno C, Cornily JC, Hyafil F, Rudd JH, Briley-Saebo KC, Mani V, Goldschlager G, Machac J, Fuster V, Fayad ZA. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calcagno C, Vucic E, Mani V, Goldschlager G, Fayad ZA. Reproducibility of black blood dynamic contrast-enhanced magnetic resonance imaging in aortic plaques of atherosclerotic rabbits. J Magn Reson Imaging. 2010;32:191–198. doi: 10.1002/jmri.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaens ME, Backes WH, Rozel S, Lipperts M, Sanders SN, Jaspers K, Cleutjens JPM, Sluimer JC, Heeneman S, Daemen MJAP, Welten RJTJ, Daemen JWH, Wildberger JE, Kwee RM, Kooi ME. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: Model selection, reproducibility, and validation. Radiology. 2013;266:271–279. doi: 10.1148/radiol.12120499. [DOI] [PubMed] [Google Scholar]
- 72.Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, Brady TJ, Tawakol A. Feasibility of FDG imaging of the coronary arteries: Comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3:388–397. doi: 10.1016/j.jcmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 73.Dweck MR, Chow MWL, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJR, Boon NA, Rudd JHF, Newby DE. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 74.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 75.MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, Shishkov M, Kauffman CR, Houser SL, Aretz HT, DeJoseph D, Halpern EF, Tearney GJ. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44:972–979. doi: 10.1016/j.jacc.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 76.Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, Weissleder R, Libby P, Ntziachristos V. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoo H, Kim JW, Shishkov M, Namati E, Morse T, Shubochkin R, McCarthy JR, Ntziachristos V, Bouma BE, Jaffer FA, Tearney GJ. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17:1680–1684. doi: 10.1038/nm.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinegoni C, Botnaru I, Aikawa E, Calfon MA, Iwamoto Y, Folco EJ, Ntziachristos V, Weissleder R, Libby P, Jaffer FA. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3:84ra45. doi: 10.1126/scitranslmed.3001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJG, van der Zee AGJ, Bart J, Low PS, Ntziachristos V. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-a targeting: First in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 80.Fredman G, Ozcan L, Tabas I. Common therapeutic targets in cardiometabolic disease. Sci Transl Med. 2014;6:239ps5. doi: 10.1126/scitranslmed.3008908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lobatto ME, Fuster V, Fayad ZA, Mulder WJM. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim Y, Lobatto ME, Kawahara T, Lee Chung B, Mieszawska AJ, Sanchez-Gaytan BL, Fay F, Senders ML, Calcagno C, Becraft J, Tun Saung M, Gordon RE, Stroes ESG, Ma M, Farokhzad OC, Fayad ZA, Mulder WJM, Langer R. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci USA. 2014;111:1078–1083. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kühn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJC. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci USA. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novobrantseva TI, Borodovsky A, Wong J, Klebanov B, Zafari M, Yucius K, Querbes W, Ge P, Ruda VM, Milstein S, Speciner L, Duncan R, Barros S, Basha G, Cullis P, Akinc A, Donahoe JS, Narayanannair Jayaprakash K, Jayaraman M, Bogorad RL, Love K, Whitehead K, Levins C, Manoharan M, Swirski FK, Weissleder R, Langer R, Anderson DG, de Fougerolles A, Nahrendorf M, Koteliansky V. Systemic RNAi-mediated gene silencing in nonhuman primate and rodent myeloid cells. Mol Ther Nucleic Acids. 2012;1:e4. doi: 10.1038/mtna.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DWY, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 93.Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, Dolan E, Moroney J, Murphy S, O’Rourke K, O’Malley K, O’Donohoe M, McDonnell C, Noone I, Barry M, Crowe M, Kavanagh E, O’Connell M, Kelly PJ. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71:709–718. doi: 10.1002/ana.23553. [DOI] [PubMed] [Google Scholar]
- 94.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4:148rv9. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 95.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 96.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, Marinelli B, Aikawa E, Pittet MJ, Swirski FK, Weissleder R. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29:1444–1451. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 98.Kastrup CJ, Nahrendorf M, Figueiredo JL, Lee H, Kambhampati S, Lee T, Cho SW, Gorbatov R, Iwamoto Y, Dang TT, Dutta P, Yeon JH, Cheng H, Pritchard CD, Vegas AJ, Siegel CD, MacDougall S, Okonkwo M, Thai A, Stone JR, Coury AJ, Weissleder R, Langer R, Anderson DG. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc Natl Acad Sci USA. 2012;109:21444–21449. doi: 10.1073/pnas.1217972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramcharitar S, Gonzalo N, van Geuns RJ, Garcia-Garcia HM, Wykrzykowska JJ, Ligthart JMR, Regar E, Serruys PW. First case of stenting of a vulnerable plaque in the SECRITT I trial—The dawn of a new era? Nat Rev Cardiol. 2009;6:374–378. doi: 10.1038/nrcardio.2009.34. [DOI] [PubMed] [Google Scholar]