Abstract
Both acute and chronic graft-versus-host disease (GVHD) are major causes of morbidity and mortality in patients undergoing allogeneic hematopoietic stem cell transplantation (AHSCT). The optimal pharmacological regimen for GVHD prophylaxis is unclear, but combinations of a calcineurin inhibitor (cyclosporin or tacrolimus [Tac]) and an antimetabolite (methotrexate or mycophenolate mofetil [MMF]) are typically used. We retrospectively evaluated the clinical outcomes of 414 consecutive patients who underwent AHSCT from sibling (SD) or unrelated donors (UD) with Tac/MMF combination, between January 2005 and August 2010. The median follow-up was 60 months. Less than one third of the patients received a reduced-intensity chemoregimen. The incidence of grades III and IV acute GVHD was 22.3% and 36.5% in SD and UD groups, respectively (P = .0007). The incidence of chronic GVHD was 47.1% and 52.7% in the SD and UD groups, respectively. Nonrelapse mortality (NRM) at 60 months was 33.3% and 46.5% in the SD and UD groups, respectively (P = .0016). The incidence of relapse was 22.4% for UD and 28.8% for SD. Five-year overall survival was 43% and 34% in the SD and UD groups, respectively (P = .0183). GVHD was the leading cause of death for the entire cohort. Multivariable analysis showed that 8/8 HLA match, patient’s age < 60, and low-risk disease were associated with better survival. The use of Tac/MMF for GVHD prophylaxis was associated with a relatively high incidence of severe acute GVHD and NRM in AHSCT from sibling and unrelated donors.
Keywords: Graft-versus-host disease, Hematopoietic stem cell, transplantation, Tacrolimus, Mycophenolate mofetil
INTRODUCTION
Graft-versus-host disease (GVHD) continues to be amajor cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (AHSCT) [1–4]. Methods to prevent GVHD in most centers consist of a combination of methotrexate (MTX) and a calcineurin inhibitor, either cyclosporine A (CSA) or tacrolimus (Tac). Despite the use of pharmacological GVHD prophylaxis, the rate of grade II to IV acute GVHD (aGVHD) ranges from 35% to 50% in transplantations from HLA-matched sibling donors (SD) and up to 70% in transplantations from unrelated donors (UD) [3,5,6]. Therefore, there is a need for safer and more effective GVHD preventive regimens, aimed at improving overall transplantation outcomes.
Mycophenolate mofetil (MMF) is a prodrug of the immune suppressive agent mycophenolic acid. MMF inhibits inosine monophosphate dehydrogenase, an enzyme critical for the guanine monophosphate pathway needed for T and B lymphocyte proliferation [7]. Storb et al. initially, showed evidence of synergy between CSA and MMF in preventing GVHD when tested in dog models [8]. Clinical trials with a CSA/MMF combination resulted in an incidence of aGVHD grade II to IV of 42% to 63% in the context of various intensity conditioning regimens and donor types [9–15]. In a small retrospective cohort of nonmyeloablative transplantations, Le Blanc et al. first reported increased rates of aGVHD when MMF was used instead MTX in combination with CSA for aGVHD prophylaxis [16].
Three prospective trials studied the combination of MMF with Tac after reduced-intensity conditioning and documented rates of grade II to IV aGVHD up to 15% in SD and 54% in UD [17–19]. However, a randomized phase II trial found that the Tac/MMF combination resulted in a high incidence of grades III to IV aGVHD when compared with Tac/MTX (19% versus 4%, P = .03) after a full-intensity preparative regimen [20]. Multiple centers adopted Tac/MMF as the standard GVHD prophylactic regimen, given the advantages of earlier engraftment and less mucositis. Given the heterogeneity of the literature presented above, we aimed to retrospectively evaluate the efficacy of Tac/MMF regimen in a relatively large cohort of patients with a long follow-up period. Our objectives were to evaluate the cumulative incidence and severity of aGHVD, chronic GVHD (cGVDH), nonrelapse mortality (NRM), relapse, and overall survival (OS) in these patients. Additionally we aimed to test, in a multivariable model, donor, recipient, and regimen-related factors for association with aGVHD, cGVHD, and survival. We found that Tac/MMF was associated with a high incidence of grades III to IV aGVHD and high NRM.
METHODS
We retrospectively evaluated all consecutive patients who underwent AHSCT at Karmanos Cancer Center between Jan 2005 and August 2010 and received Tac/MMF for GVHD prophylaxis. This study was approved by the Wayne State University Institutional Review Board. We accessed our transplantation center database (including patient characteristics, GVHD grading, and transplantation outcomes), which was prospectively collected for the Center for International Blood and Marrow Transplant Research database. Two transplantation physicians reviewed every patient’s medical record to check and validate GVHD grading and cause of death. The distinction between acute and chronic GVHD was based on clinical manifestations rather than time of onset after transplantation [21]. The primary endpoint of this study was to evaluate the cumulative incidence (incidence) and severity of aGVHD based on the consensus grading scale [22]. Secondary endpoints included incidence and severity of cGVHD using National Institutes of Health consensus [21], incidence of bronchiolitis obliterans, incidence of cytomegalovirus (CMV) infection, incidence of NRM, incidence of relapse, OS, progression-free survival (PFS), causes of death, and multivariable analysis of possible predictors of aGVHD, cGVHD, and survival.
Inclusion criteria were the following: all adult patients with a diagnosis of hematologic malignancies including myelodysplastic syndromes and myeloproliferative disorders were included in the study. Patients had a suitable HLA 8/8 (A, B, C, and DR) or 7/8 matched SD or UD based on high-resolution molecular typing. Based on our institutional guidelines, patients were required to have adequate organ function for AHSCT, including creatinine clearance of ≥ 50 mL/minute, left ventricular ejection fraction ≥ 50%, and pulmonary function values (forced vital capacity, forced expiratory volume in 1 second, and diffusion capacity of the lung for carbon monoxide) more than ≥ 50% predicted.
Excluded from the analysis were patients who underwent AHSCT for aplastic anemia, patients who had stem cells from cord blood or a haploidentical donor, and patients who received thymoglobulin as part of the preparative regimen or for GVHD prophylaxis. For patients who underwent more than 1 AHSCT, we used data from the first transplantation only.
Disease Diagnosis and Risk Definitions
Diseases at high risk for relapse and death after transplantation were defined primarily based on the Center for International Blood and Marrow Transplant Research criteria, with a few additions based on published literature as detailed and published before [23].
Preparative Regimens
Choice of preparative regimen was assigned according to disease diagnosis, disease status, age, and comorbidities, and at the discretion of the treating physician. The description of various high- and reduced-intensity conditioning regimens were detailed in a previous publications [23–25].
GVHD Prophylaxis
Tac was started at day −3 intravenously (.03 mg/kg/day) and was converted to oral form (approximately 3 to 4 times the intravenous dose) after the patient demonstrated adequate oral intake after engraftment. Tac dose was adjusted thrice weekly until day +30 to achieve trough blood levels of 10 to 15 nmol/L; tapering started at day +60 to be discontinued by day +180 in the absence of GVHD. MMF was initiated at 10 mg/kg (based on adjusted weight) orally every 8 hours starting day −3, and then on day +1, it was changed to intravenous MMF at 10 mg/kg (based on adjusted weight), infused every 8 hours. After engraftment, MMF was switched to oral (same schedule) whenever patients demonstrated adequate oral intake. Each MMF dose was rounded to 250 mg, 500 mg, 750 mg, or 1000 mg and discontinued without tapering on day +30.
Supportive Care
All peripheral blood stem cell donors weremobilized as per the National Marrow Donor Program standards. All recipients received granulocyte colony–stimulating factor 5 µg/kg starting at day +6 until engraftment. The details of supportive care are mentioned elsewhere [23].
Statistical Analysis
Descriptive analyses for baseline characteristics were performed. The continuous variables were tested with Wilcoxon rank-sum test between the 2 cohorts. The categorical variables were tested with the chi-square test. When the frequency count was small, the Fisher exact test was used. Incidence rates of infections were estimated using proportions and Wilson’s 95% confidence interval. We calculated the cumulative incidence (incidence) for acute and chronic GVHD, with disease relapse or death without GVHD as competing risks. Competing risk for relapse incidence was NRM, for CMV incidence was death, and for NRM incidence was relapse. The Gray’s test P values are not significant unless otherwise mentioned. The incidence of aGVHD or cGVHD was calculated with disease relapse or death without GVHD as competing risks. For calculating the incidence of grade III or IV aGVHD, disease relapse or death without aGVHD were counted as competing risks, and all lower grade aGVHD events were ignored. The Kaplan-Meier estimator was used to calculate PFS (defined as the time from transplantation to relapse or progression or death from any cause) and OS (defined as the time from transplantation to death from any cause). Patients alive without relapse were censored at the date of last follow-up, which was July 31, 2012. Cox regression models adjusted for various prognostic factors were used for GVHD (both acute and chronic) and OS. We evaluated the association between pretransplantation factors (donor type, age, sex, HLA mismatch, preparative regimen) and GVHD (both acute and chronic). Furthermore, we evaluated the association between pretransplantation factors (donor type, HLA mismatch, CMV status, disease risk, patient age) and survival. All P values are 2-sided and not adjusted for multiple testing due to the nature of this exploratory study.
RESULTS
Patient Characteristics
Table 1 summarizes the baseline characteristics of patients who received Tac/MMF for GVHD prophylaxis. Two hundred and eleven patients underwent transplantation from SD, whereas 203 underwent transplantation from UD. The median age of patients was 48 years (range, 23 to 71) in the SD group and 44.5 years (range, 18 to 69) in the UD group (P = .023). The median age of donors was 46.5 years (range, 18 to 75) for the SD and 36.5 years (range,18 to 60) for the UD (P < .0001). There were more HLA-mismatched transplantations in the UD group compared with in the SD group; 33% versus 8% (P <.0001). There were more female donors in the SD group (49%) compared with the UD group (33%) (P = .0016). The median number of CD34+ stem cells infused was 5.78 × 106/kg (range, 2.12 to 14.05) and 7.28 × 106/kg (range, 1.49 to 19.12) of recipient weight for the SD and UD groups, respectively (P <.0001). All patients engrafted at a median of day 11. Three patients in UD group died of graft failure.
Table 1.
Patient Characteristics
| Unrelated Transplantations |
Matched Related Transplantations |
P Value | |
|---|---|---|---|
| No. of patients | 203 | 211 | |
| Patient age, median (range) | 44.5 (18–69) | 48 (23–71) | .0233 |
| Disease | .2132 | ||
| AML | 80 (39%) | 75 (36%) | |
| MDS | 21 (10%) | 26 (12%) | |
| ALL | 30 (15%) | 19 (9%) | |
| NHL | 32 (16%) | 46 (22%) | |
| Other* | 40 (20%) | 45 (21%) | |
| Conditioning | .4541 | ||
| Bu/flu | 70 (34%) | 61 (29%) | |
| Bu/flu/TBI+ | 38+(2) (20%) | 43+(1) (21%) | |
| Flu/mel/TBI+ | 11+(3) (7%) | 13+(5) (9%) | |
| R+/− BEAM | 19 (9%) | 30 (14%) | |
| Other† | 60 (30%) | 58 (27%) | |
| Donor/recipient sex | .0016 | ||
| F/f | 33 (16%) | 48 (23%) | |
| F/m | 35 (17%) | 55 (26%) | |
| M/f | 58 (29%) | 42 (20%) | |
| M/m | 77 (38%) | 66 (31%) | |
| Donor/recipient CMV status | .1952 | ||
| Neg/neg | 62 (31%) | 69 (33%) | |
| Neg/pos | 65 (32%) | 48 (23%) | |
| Pos/neg | 22 (11%) | 28 (13%) | |
| Pos/pos | 54 (27%) | 66 (31%) | |
| Donor age, median (range) | 36.5 (18–60) | 46.5 (18–75) | <.0001 |
| CD 34+median (range), 106/kg | 7.28 (1.49–19.12) | 5.78 (2.12–14.05) | <.0001 |
| HLA match | <.0001 | ||
| 8/8 | 136 (67%) | 194 (92%) | |
| 7/8 | 67 (33%) | 17 (8%) | |
| Disease risk | .3217 | ||
| Low | 75 (37%) | 88 (42%) | |
| High | 128 (63%) | 123 (58%) | |
| Stem cell source | .4960‡ | ||
| PBSC | 198 (98%) | 208 (99%) | |
| BM | 5 (2%) | 3 (1%) | |
| No. of prior transplantations | .0521 | ||
| 0 | 193 (95%) | 190 (90%) | |
| 1 or more (auto) | 10 (5%) | 21 (10%) |
AML indicates acute myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; ALL, acute lymphoblastic leukemia; M, male; F, female; Bu, busulfan; mel, melphalan; BEAM, carmustine, etoposide, cytarabine and melphalan; TBI, total body irradiation; PBSC, peripheral blood stem cells; BM, bone marrow.
Other includes chronic myelogenous leukemia, MPD, multiple myeloma, chronic lymphatic leukemia, PLL, and Hodgkin disease.
Other includes VP16/TBI, CY/TBI, BAC, CY/FLU/TBI, CVB+(R), and BU/CY.
Fisher exact test. The others were chi-square test for the categorical variables.
Graft-Versus-Host Disease
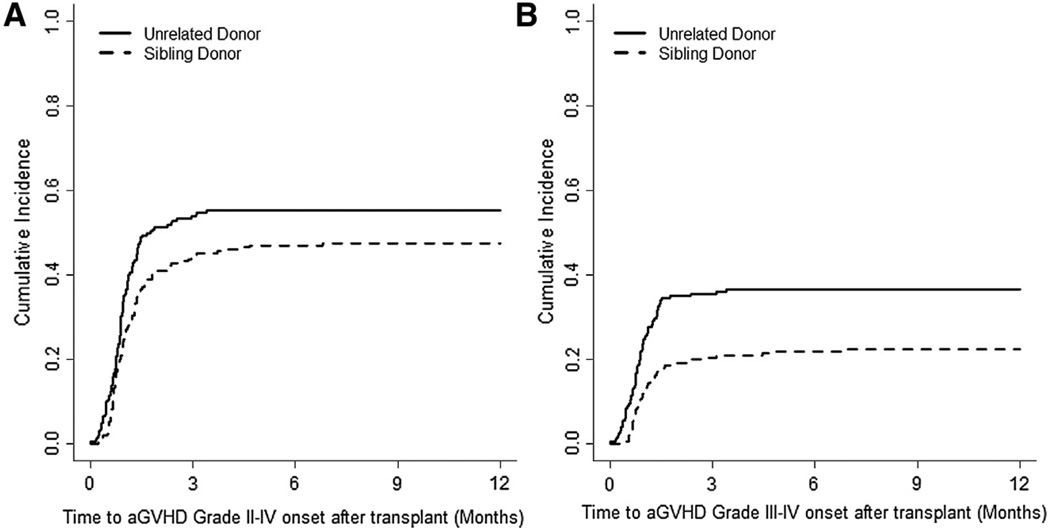
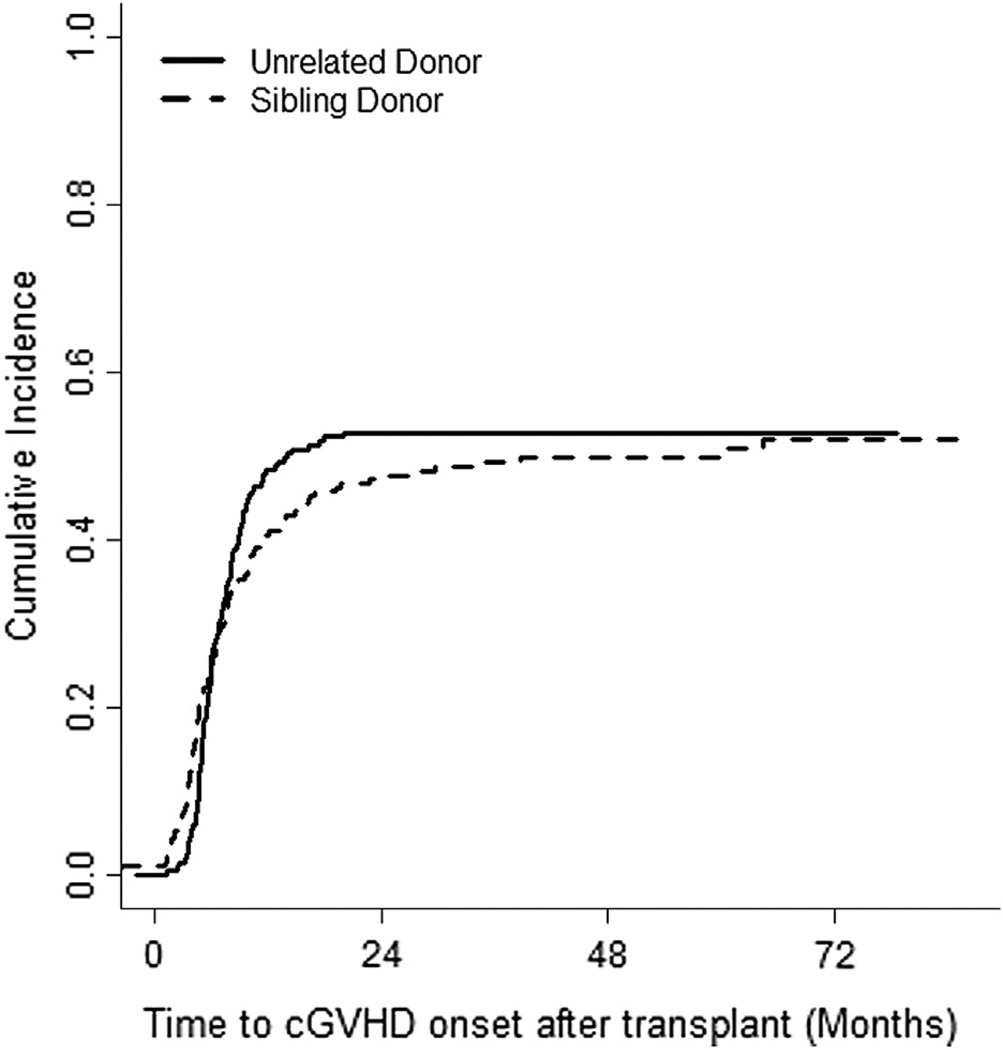
The incidence of grades II to IV aGVHD was 47.4% (95% confidence interval [CI] 40% to 54%) and 55.2% (95% CI, 48% to 61%) in SD and UD groups, respectively (Figure 1A). The incidence of severe aGVHD (grades III to IV) was 22.3% (95% CI, 16% to 28%) in the SD group versus 36.5% (95% CI, 29% to 43%) in the UD group (P = .0007) (Figure 1B). The incidence of cGVHD at 24 months was 47.1% (95% CI, 40% to 53%) and 52.7% (95% CI, 45% to 59%) in SD and UD groups, respectively (Figure 2). The incidence of severe cGVHD (NIH grade 3) at 24 months was 27.6% (95% CI, 22% to 34%) and 26.1% (95% CI, 20% to 32%) in SD and UD groups, respectively. The incidence of bronchiolitis obliterans at 60 monthswas 14.0% (95% CI, 9% to 19%) and 11.6% (95% CI, 7% to 16%) in SD and UD groups, respectively.
Figure 1.
The cumulative incidence of (A) grade II to IV aGVHD and (B) grade III-IV aGVHD.
Figure 2.
The cumulative incidence of cGVHD.
Infections, NRM, and Cause of Death
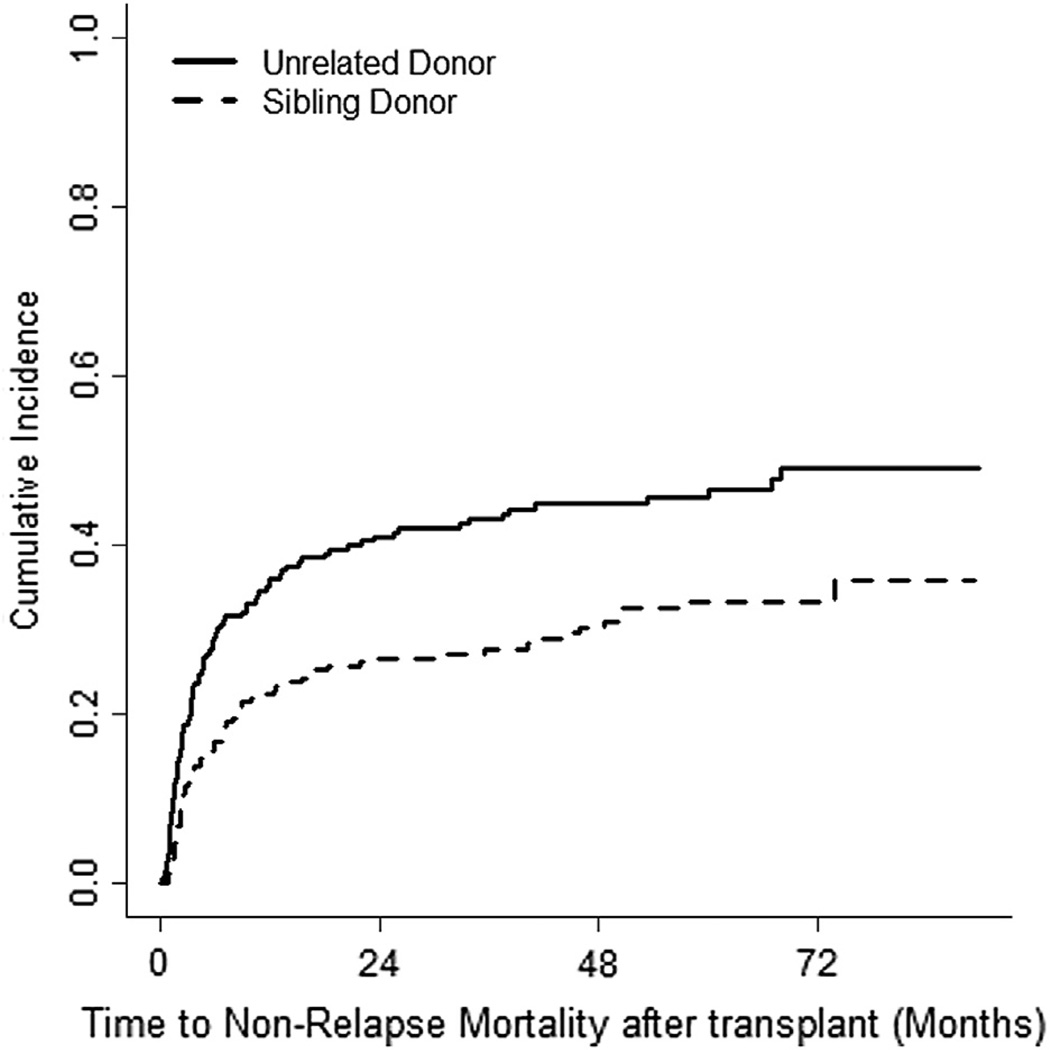
The incidence of CMV reactivation was 27.2% (95% CI, 21% to 33%) and 23.2% (95% CI, 18% to 29%) in SD and UD groups, respectively. The incidence of NRM at 60 months was 33.3% (95% CI, 27% to 40%) versus 46.5% (95% CI, 39% to 53%) for the SD and UD groups, respectively (P = .0016), as shown in Figure 3. Only 15% of patients with a history of grade III to IV aGVHD were alive by the last date of follow-up.
Figure 3.
The cumulative incidence of nonrelapse mortality.
Relapse, Survival and Causes of Death
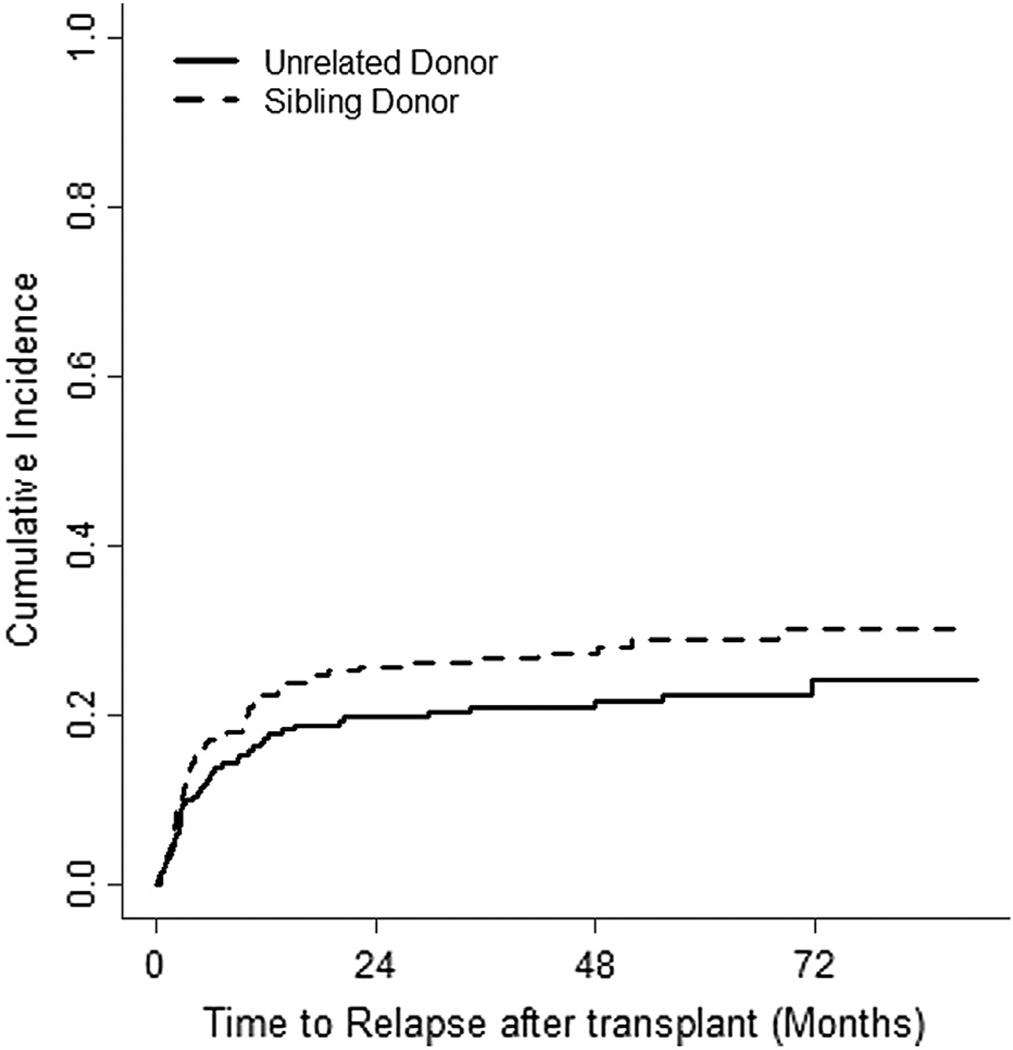
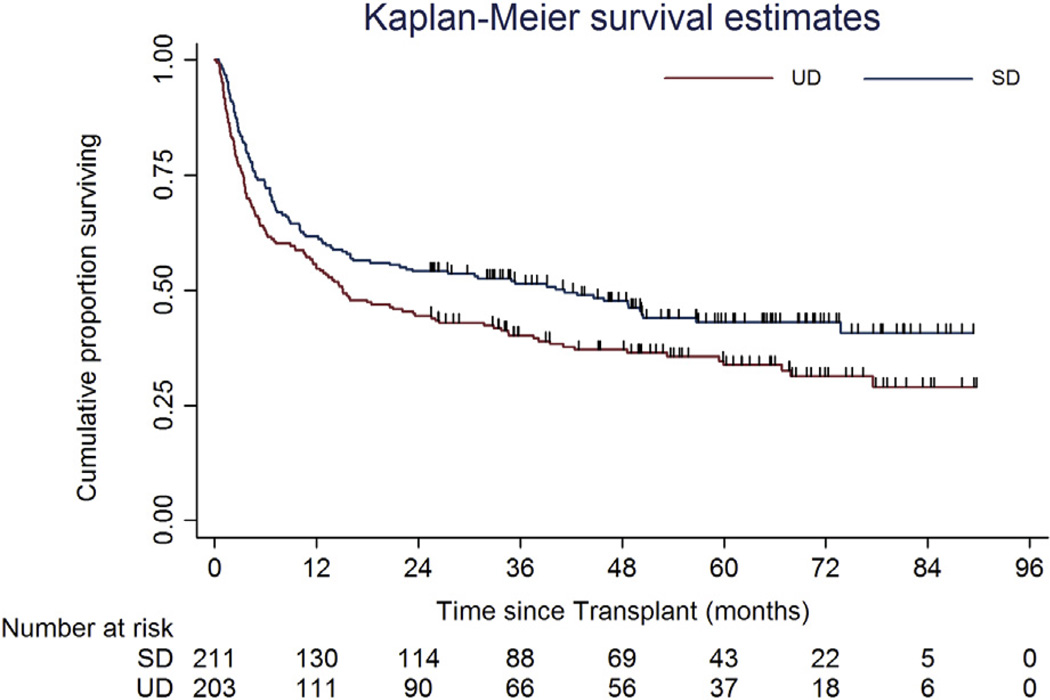
The incidence of relapse was 28.8% (95% CI, 23% to 35%) and 22.4% (95% CI, 17% to 29%) in SD and UD groups, respectively (Figure 4). The causes of death are shown in Table 2. The main cause of death was GVHD, followed by relapse in both groups. With a median follow-up of 60 months (95% CI, 54 to 64), the 5-year OS was 43% (95% CI, 34% to 51%) and 34% (95% CI, 27% to 41%) in SD and UD groups, respectively (log-rank P = .0183) (Figure 5). Median survival was 40.3 months (95% CI, 15 to 73 months) for recipients of allografts from SD and 15.1 months (95% CI, 11 to 26 months) for recipients of allografts from UD.
Figure 4.
The cumulative incidence of relapse.
Table 2.
Causes of Death
| Causes of Death | Related | Unrelated | Total |
|---|---|---|---|
| No. of deaths | 115 (55%) | 133 (66%) | 248 (60%) |
| GVHD | 56 (49%) | 76 (57%) | 132 (53%) |
| Relapse/progression | 48 (42%) | 39 (29%) | 87 (35%) |
| Sepsis | 3 (3%) | 9 (7%) | 12 (5%) |
| MOF | 3 (3%) | 5 (4%) | 8 (3%) |
| Graft failure | 0 | 3 (2%) | 3 (1%) |
| DAH | 2 (2%) | 0 | 2 (1%) |
| Secondary malignancy | 3 (3%) | 1 (1%) | 4 (2%) |
GVHD indicates graft-versus-host disease; MOF, multiorgan failure; DAH, diffuse alveolar hemorrhage.
Data presented are n (%).
Figure 5.
Overall survival.
Factors Associated with GVHD and Survival
Cox’s multivariable analysis showed that transplantations from donors HLA-matched at 7/8 antigens and UD were associated with higher risk for aGVHD (P < .0001 and P < .014, respectively), as shown in Table 3. Furthermore, transplantation from UD, older donors (>30 years), and the use of total body irradiation were associated with higher risk for cGVHD (P < .0001, P = .011, and P = .040, respectively) (Table 4). Patient age (<60 years), low risk for relapse/death, and absence of an HLA mismatch were associated with better overall survival (P < .0001, P < .0001, and P = .003, respectively, as shown in Table 5).
Table 3.
Cox’s Multivariable Models for Time to aGVHD*
| Parameter | Category | P Value | HR | 95% CI |
|---|---|---|---|---|
| HLA match | Mismatch | <.0001 | 2.320 | 1.708–3.151 |
| Donor type | UD | .014 | 1.479 | 1.084–2.018 |
| Conditioning regimen | High intensity | .4885 | .905 | .684–1.199 |
| Donor age, yr | >45 | .0661 | 1.420 | .977–2.065 |
| Donor age, yr | 31–45 | .4416 | 1.137 | .820–1.575 |
| Donor sex | Female | .3886 | 1.119 | .867–1.444 |
| TBI | With TBI | .4503 | 1.167 | .781–1.744 |
aGVHD indicates acute graft-versus-host disease; HR, hazard ratio; CI, confidence interval; TBI, total body irradiation; UD, unrelated donor.
All grades of GVHD.
Table 4.
Cox’s Multivariable Models for Time to cGVHD*
| Parameter | Category | P Value | HR | 95% CI |
|---|---|---|---|---|
| Ag mismatch | Mismatch | .4990 | .873 | .588–1.295 |
| Donor type | UD | .0001 | 1.975 | 1.388–2.810 |
| Conditioning regimen | High intensity | .0771 | 1.327 | .970–1.816 |
| Donor age | > 45 | .0654 | 1.492 | .975–2.285 |
| Donor age | 31–45 | .011 | 1.569 | 1.103–2.230 |
| Donor sex | Female | .2287 | 1.188 | .897–1.573 |
| TBI | With TBI | .040 | .623 | .398–.975 |
cGVHD indicates chronic graft-versus-host disease; HR, hazard ratio; CI, confidence interval; ag, antigen; TBI, total body irradiation; UD, unrelated donor.
All grades of GVHD.
Table 5.
Cox’s Multivariable Models for OS
| Parameter | Category | P Value | HR | 95% CI |
|---|---|---|---|---|
| Patient age, yr | >60 | <.0001 | 1.973 | 1.455–2.675 |
| Relapse risk | High | <.0001 | 1.786 | 1.361–2.342 |
| HLA match | Mismatch | .0033 | 1.579 | 1.164–2.143 |
| D/r (−/+) CMV | Yes | .6028 | 1.077 | .815–1.424 |
| Donor type | UD | .2210 | 1.183 | .904–1.550 |
OS indicates overall survival; D/r, donor/recipient; CMV, cytomegalovirus; UD, unrelated donor; HR, hazard ratio; CI, confidence interval.
DISCUSSION
We report on the GVHD and survival outcomes in a cohort of AHSCT patients treated with a uniform GVHD prophylaxis regimen of Tac/MMF. To our knowledge, this is the largest published cohort of patients (N = 414) with the longest follow-up (median of 60 months). We observed a relatively high incidence of severe aGVHD (grade III to IV) in both the SD and UD groups (22.3% and 36.5%, respectively) with high NRM (32.5% and 46.5% at 5 years, respectively). These rates of grade III to IV aGVHD are higher than previously published randomized GVHD prevention trials using calcineurin inhibitors and MTX, as well as single arm phase II trials using Tac/MMF (Tables 6 and 7) [5,6,17–20,26–28]. Large proportion of patients in our cohort received high-intensity preparative regimens as well as transplantations from HLA-mismatched donors, both of which are known to be associated with higher risk of GVHD [4,13,14].
Table 6.
Randomized GVHD Prevention Trials with Calcineurin Inhibitor and Methotrexate
| Study | aGVHD Prophylaxis | Intensity | No. of Patients |
Graft Type |
Graft Source |
HLA Match |
aGVHD III–IV, % | cGVHD at 2 yr, % | NRM at 2 yr, % |
|---|---|---|---|---|---|---|---|---|---|
| Ratanatharathorn 1998 Ph III | CSA/MTX versus Tac/MTX | Full | 329 | BM | SD | 6/6 | 17 versus 13 | 49 versus 55 | 28 versus 35* |
| Nash 2000 Ph III | CSA/MTX versus Tac/MTX | Full | 180 | BM | UD | 6/6† | 25 versus 17 | 70 versus 76 | 33 versus 42 |
| Finke 2009 Ph III | CSA/MTX- −/+ ATGF | Full | 202 | PBSC | UD | 10/10‡ | 24 versus 11 | 58 versus 30 | 28 versus 19 |
| Perkins 2010 Ph II | Tac/MTX versus Tac/MMF | Full | 89 | PBSC | Mixed | 10/10§ | 4 versus 19‖ | 56 versus 58*,# | 30 versus 32* |
| Pidala 2012 Ph II | Tac/MTX versus Tac/Siro | Full | 97 | PBSC | Mixed | 8/8 | 11 versus 14 | 64 versus 24‖ at 30 m | 8 versus 28‖ |
| Cutler 2012 Ph III | Tac/MTX versus Tac/Siro | Full | 304 | PBSC | SD | 6/6 | 15 versus 8‖ | 43 versus 54‖,¶ | NA |
Tac indicates tacrolimus; GVHD, graft-versus-host disease; aGVHD, acute GVHD; BM, bone marrow; PBSC, peripheral blood stem cells; UD, unrelated donor; SD, sibling donor; NA, not available; CSA, cyclosporine A; MTX, methotrexate; MMF, mycophenolate mofetil; Siro, sirolimus; ATGF, ATG fresenius.
Estimated from the curve or from the tables.
About 18% of the patients had at least 1 antigen mismatch. Also HLA C typing was not done.
HLA C mismatch in 14% to 20% of patients.
HLA 9/10 or 10/10 matched donors were included (the author did not specify the percentage of mismatched donors).
Statistically significant.
At unknown duration of follow-up.
Moderate or severe cGVHD.
Table 7.
Phase II GVHD Prevention Trials with Tacrolimus and Mycophenolate Mofetil
| Study | aGVHD Prophylaxis, n | Preparative Regimen | Graft Type | Graft Source | HLA Match | aGVHD III–IV | cGVHD | NRM* | Median Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Nieto 2006 | 32 | Flu/TBI 200 | PBSC | SD | 6/6 | 3% | 41%† | 15% | 19 mo |
| Sabry 2009 | 131 | Flu/Cy | PBSC | SD | 6/6 | .2%‡,§ | 76%† | 15% | 32 mo |
| Zohren 2010 | 50 | Flu/TBI 200 | PBSC | UD | 10/10‖ | 16% | 61% | 26% | 37 mo |
GVHD indicates graft-versus-host disease; Flu, fludarabine; TBI, total body irradiation; Cy, cyclophosphamide; PBSC, peripheral blood stem cells; SD, sibling donor; UD, unrelated donor; NRM, nonrelapse mortality.
At the median follow up.
Extensive cGVHD.
Additional 12% developed overlap syndrome with no grade severity mentioned.
Estimated from the curve or from the tables.
Six patients had a donor with one antigen mismatch, while one had a donor with 2 antigen mismatches.
It is not clear if the use of Tac/MMF combination for GVHD prophylaxis has been maximally optimized. Wakahashi et al., in a small retrospective study of AHSCT from UD, showed that patients with measured MMF area under the curve of > 30 µg hour/mL had no grade II to IV aGVHD or extensive cGVHD [29]. Recently, McDermott et al. evaluated the association of MMF pharmacokinetics and transplantation outcomes in a large retrospective report of patients (n = 308) who underwent transplantation from SD and UD with a reduced-intensity conditioning regimen. In patients who underwent transplantation from UD donors, a low mycophenolic acid level (steady state) was associated with increased grade III to IV aGVHD and NRM [30]. Furthermore, in a small retrospective study, Nishikawa et al. showed that extended duration of exposure to MMF was associated with less grade II to IV aGVHD, compared with patients who stopped MMFat day +30 after transplantation [31]. Whether these maneuvers can improve the future efficacy of this GVHD prevention regimen remains to be evaluated.
One potential limitation in our study is its retrospective nature and inherent constraints associated with this type of analysis. However, only 15% of patients who developed grade III to IV aGVHD in this series are long-term survivors, which is similar to the survival rates published before for patients with grade III to IV aGVHD [32,33]. Another unique aspect of our cohort is the use of post-transplantation granulocyte colony–stimulating factor, which has been suspected in some retrospective studies to increase aGVHD rates when used after bone marrow grafts but not after peripheral blood stem cell grafts [34–41].
In addition to the high incidence of severe aGVHD, we observed a parallel high NRM in our cohort of patients compared with previous trials. Considering, the long follow-up for our cohort, we observed progressive NRM with no plateau at 5 years, possibly reflecting the long-term impact of cGVHD. This observation highlights the need for long term follow-up to fully evaluate transplantation outcomes for these patients.
Multivariate analysis showed that transplantation from an HLA-mismatched and UD was associated with higher risk of aGVHD, whereas the use of UD, total body irradiation, and older donors were associated with higher risk of cGVHD, in agreement with previous literature [42–48]. In contrast with previous reports, regimen intensity and donor sex (female donors) were not shown to be predictive of GVHD outcomes in our cohort [43–45,47]. Finally, patient’s age < 60, transplantation from an HLA-matched donor, and low-risk disease were associated with better overall survival [4,43,44,49,50].
In summary, the use of the Tac/MMF combination as pharmacological prophylaxis was associated with high incidence of severe aGVHD and NRM, especially after transplantations from UD. Alternative GVHD prevention regimens are needed to improve AHSCT outcomes. New GVHD prevention agents and regimens are showing some promise in lowering GVHD rates [23,51,52]. Phase III randomized trials (with long-term follow-up) are needed to prove the efficacy of these regimens in preventing GVHD and improving survival.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Chang YJ, Weng CL, Sun LX, Zhao YT. Allogeneic bone marrow transplantation compared to peripheral blood stem cell transplantation for the treatment of hematologic malignancies: a meta-analysis based on time-to-event data from randomized controlled trials. Ann Hematol. 2012;91:427–437. doi: 10.1007/s00277-011-1299-8. [DOI] [PubMed] [Google Scholar]
- 2.Valcarcel D, Sierra J, Wang T, et al. One-antigen mismatched related versus HLA-matched unrelated donor hematopoietic stem cell transplantation in adults with acute leukemia: Center for International Blood and Marrow Transplant Research results in the era of molecular HLA typing. Biol Blood Marrow Transplant. 2011;17:640–648. doi: 10.1016/j.bbmt.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 5.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 6.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 7.Eugui EM, Allison AC. Immunosuppressive activity of mycophenolate mofetil. Ann N Y Acad Sci. 1993;685:309–329. doi: 10.1111/j.1749-6632.1993.tb35881.x. [DOI] [PubMed] [Google Scholar]
- 8.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 9.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 10.Feinstein L, Sandmaier B, Maloney D, et al. Nonmyeloablative hematopoietic cell transplantation. Replacing high-dose cytotoxic therapy by the graft-versus-tumor effect. Ann N Y Acad Sci. 2001;938:328–337. discussion 337–329. [PubMed] [Google Scholar]
- 11.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez R, Parker P, Nademanee A, et al. Cyclosporine and mycophenolate mofetil prophylaxis with fludarabine and melphalan conditioning for unrelated donor transplantation: a prospective study of 22 patients with hematologic malignancies. Bone Marrow Transplant. 2004;33:1123–1129. doi: 10.1038/sj.bmt.1704493. [DOI] [PubMed] [Google Scholar]
- 13.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–625. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 14.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Mohty M, de Lavallade H, Faucher C, et al. Mycophenolate mofetil and cyclosporine for graft-versus-host disease prophylaxis following reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:527–530. doi: 10.1038/sj.bmt.1704640. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc K, Remberger M, Uzunel M, et al. A comparison of nonmyeloablative and reduced-intensity conditioning for allogeneic stem-cell transplantation. Transplantation. 2004;78:1014–1020. doi: 10.1097/01.tp.0000129809.09718.7e. [DOI] [PubMed] [Google Scholar]
- 17.Nieto Y, Patton N, Hawkins T, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12:217–225. doi: 10.1016/j.bbmt.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Sabry W, Le Blanc R, Labbe AC, et al. Graft-versus-host disease prophylaxis with tacrolimus and mycophenolate mofetil in HLA-matched nonmyeloablative transplant recipients is associated with very low incidence of GVHD and nonrelapse mortality. Biol Blood Marrow Transplant. 2009;15:919–929. doi: 10.1016/j.bbmt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Zohren F, Schroeder T, Czibere A, et al. Tacrolimus and mycofenolate mofetil as GvHD prophylaxis following nonmyeloablative conditioning and unrelated hematopoietic SCT for adult patients with advanced hematologic diseases. Bone Marrow Transplant. 2011;46:747–755. doi: 10.1038/bmt.2010.167. [DOI] [PubMed] [Google Scholar]
- 20.Perkins J, Field T, Kim J, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16:937–947. doi: 10.1016/j.bbmt.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. 1994. [PubMed] [Google Scholar]
- 23.Al-Kadhimi Z, Gul Z, Rodriguez R, et al. Anti-thymocyte globulin (thymoglobulin), tacrolimus, and sirolimus as acute graft-versus-host disease prophylaxis for unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1734–1744. doi: 10.1016/j.bbmt.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratanatharathorn V, Karanes C, Lum LG, et al. Allogeneic bone marrow transplantation in high-risk myeloid disorders using busulfan, cytosine arabinoside and cyclophosphamide (BAC) Bone Marrow Transplant. 1992;9:49–55. [PubMed] [Google Scholar]
- 25.Law LY, Horning SJ, Wong RM, et al. High-dose carmustine, etoposide, and cyclophosphamide followed by allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2006;12:703–711. doi: 10.1016/j.bbmt.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 27.Hoda D, Pidala J, Salgado-Vila N, et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010;45:1347–1351. doi: 10.1038/bmt.2009.343. [DOI] [PubMed] [Google Scholar]
- 28.Cutler C, Logan BR, Nakamura R, et al. Tacrolimus/sirolimus vs. tacrolimus/methotrexate for graft-vs.-host disease prophylaxis after HLA-matched, related donor hematopoietic stem cell transplantation: results of Blood and Marrow Transplant Clinical Trials Network Trial 0402. ASH Annual Meeting Abstracts Blood. 2012;120:739. [Google Scholar]
- 29.Wakahashi K, Yamamori M, Minagawa K, et al. Pharmacokinetics-based optimal dose prediction of donor source-dependent response to mycophenolate mofetil in unrelated hematopoietic cell transplantation. Int J Hematol. 2011;94:193–202. doi: 10.1007/s12185-011-0888-6. [DOI] [PubMed] [Google Scholar]
- 30.McDermott CL, Sandmaier BM, Storer B, et al. Nonrelapse mortality and mycophenolic acid exposure in nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1159–1166. doi: 10.1016/j.bbmt.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikawa S, Okamura A, Yamamori M, et al. Extended mycophenolate mofetil administration beyond day 30 in allogeneic hematopoietic stem cell transplantation as preemptive therapy for severe graft-versus-host disease. Transplant Proc. 2009;41:3873–3876. doi: 10.1016/j.transproceed.2009.06.231. [DOI] [PubMed] [Google Scholar]
- 32.Yanada M, Naoe T, Iida H, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: significant roles of total body irradiation and chronic graft-versus-host disease. Bone Marrow Transplant. 2005;36:867–872. doi: 10.1038/sj.bmt.1705148. [DOI] [PubMed] [Google Scholar]
- 33.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 34.Ringden O, Labopin M, Gorin NC, et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 35.Remberger M, Naseh N, Aschan J, et al. G-CSF given after haematopoietic stem cell transplantation using HLA-identical sibling donors is associated to a higher incidence of acute GVHD II–IV. Bone Marrow Transplant. 2003;32:217–223. doi: 10.1038/sj.bmt.1704108. [DOI] [PubMed] [Google Scholar]
- 36.Bishop MR, Tarantolo SR, Geller RB, et al. A randomized, double-blind trial of filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 2000;96:80–85. [PubMed] [Google Scholar]
- 37.Ozcan M, Ustun C, Akcaglayan E, et al. Recombinant human granulocyte colony-stimulating factor (rh-G-CSF) may accelerate hematopoietic recovery after HLA-identical sibling allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;27:499–505. doi: 10.1038/sj.bmt.1702816. [DOI] [PubMed] [Google Scholar]
- 38.Khoury HJ, Loberiza FR, Jr, Ringden O, et al. Impact of posttransplantation G-CSF on outcomes of allogeneic hematopoietic stem cell transplantation. Blood. 2006;107:1712–1716. doi: 10.1182/blood-2005-07-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Przepiorka D, Smith TL, Folloder J, et al. Controlled trial of filgrastim for acceleration of neutrophil recovery after allogeneic blood stem cell transplantation from human leukocyte antigen-matched related donors. Blood. 2001;97:3405–3410. doi: 10.1182/blood.v97.11.3405. [DOI] [PubMed] [Google Scholar]
- 40.Stinson TJ, Adams JR, Bishop MR, et al. Economic analysis of a phase III study of G-CSF vs placebo following allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2000;26:663–666. doi: 10.1038/sj.bmt.1702579. [DOI] [PubMed] [Google Scholar]
- 41.Ho VT, Mirza NQ, Junco Dd D, et al. The effect of hematopoietic growth factors on the risk of graft-vs-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis. Bone Marrow Transplant. 2003;32:771–775. doi: 10.1038/sj.bmt.1704228. [DOI] [PubMed] [Google Scholar]
- 42.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 45.Martires KJ, Baird K, Steinberg SM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118:4250–4257. doi: 10.1182/blood-2011-04-350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passweg JR, Zhang MJ, Rocha V, et al. Donor characteristics affecting graft failure, graft-versus-host disease, and survival after unrelated donor transplantation with reduced-intensity conditioning for hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1869–1873. doi: 10.1016/j.bbmt.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saliba RM, de Lima M, Giralt S, et al. Hyperacute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007;109:2751–2758. doi: 10.1182/blood-2006-07-034348. [DOI] [PubMed] [Google Scholar]
- 48.Wojnar J, Giebel S, Krawczyk-Kulis M, et al. Acute graft-versus-host disease. The incidence and risk factors. Ann Transplant. 2006;11:16–23. [PubMed] [Google Scholar]
- 49.Arora M, Klein JP, Weisdorf DJ, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood. 2011;117:6714–6720. doi: 10.1182/blood-2010-12-323824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi SW, Braun T, Chang L, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15:87–95. doi: 10.1016/S1470-2045(13)70512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]