Abstract
Assessment of drug–liver interactions is an integral part of predicting the safety profile of new drugs. Existing model systems range from in vitro cell culture models to FDA-mandated animal tests. Data from these models often fail, however, to predict human liver toxicity, resulting in costly failures of clinical trials. In vitro screens based on cultured hepatocytes are now commonly used in early stages of development, but many toxic responses in vivo seem to be mediated by a complex interplay among several different cell types. We discuss some of the evolving trends in liver cell culture systems applied to drug safety assessment and describe an experimental model that captures complex liver physiology through incorporation of heterotypic cell–cell interactions, 3D architecture and perfused flow. We demonstrate how heterotypic interactions in this system can be manipulated to recreate an inflammatory environment and apply the model to test compounds that potentially exhibit idiosyncratic drug toxicity. Finally, we provide a perspective on how the range of existing and emerging in vitro liver culture approaches, from simple to complex, might serve needs across the range of stages in drug discovery and development, including applications in molecular therapeutics.
Keywords: 3D, clearance, co-culture, flow, hepatocyte, idiosyncratic, in vitro, inflammation, Kupffer, lipopolysaccharide, liver, metabolism, model, ranitidine, safety, tissue engineering, toxicity
1. Introduction
Liver tissue engineering is driven by disparate forces on two separate fronts: large (organ)-scale models aimed at replacement of function in patients of liver failure and small scale laboratory systems for the purpose of studying drug metabolism, toxicity, hepatocellular function and liver disease [1]. The success of both types of models hinges on maintenance of liver function in vitro. This paper focuses on emerging in vitro liver models used for testing drug safety, an application that despite our considerable understanding of fundamental liver biology remains challenging in the development of safe, therapeutic drug candidates. In this context, the criteria for defining ‘maintenance of liver function’ in vitro are arguably growing, from criteria classically focused on preservation of hepatocyte Phase I and II enzyme functions to include now a broader range of tissue responses essential for parsing human liver toxicity.
Incentive to create new in vitro models arises from the massive cost of candidate drug failures due to liver toxicity [2], the low correlation between animal and human liver toxicities [3] and increasing challenges faced by the use of existing whole animal studies including animal welfare, legal/ethical issues, high costs, time, limited throughput, dose extrapolation issues and the other limitations of existing in vitro systems [4–7].
In vitro liver systems that are currently used in pharmaceutical drug discovery and development laboratories for evaluating drug metabolism, clearance and toxicity include freshly isolated primary hepatocytes, plated hepatocytes and immortalized cell lines, microsomal preparations, precision-cut liver slices, isolated perfused livers and complex organotypic reconstructed tissue [8–10]. Cryopreserved primary human hepatocytes remain the preferred method for studying in vitro drug metabolism and hepatic drug uptake. The activity of Phase I and II enzymes in these cellular systems generally remains stable enough over time scales required to gain both quantitative and qualitative information on metabolic conversion (typically on the order of several hours) [11–15], although analysis of enzyme induction, which requires more culture time, can be impaired [9]. Additionally, cyropreserved hepatocytes in suspension are a model system for assessment of hepatic drug uptake mediated by sinusoidal transporters responsible for influx of xenobiotics into the liver [16,17]. When the cells are plated in static cultures, they can be used to capture a significant fraction (~ 50%) of known toxicity events in small molecule drugs [18]. Thus, such culture systems retain an important place in drug development.
There remain many areas in which improved test systems can provide further value, motivating development of more organotypic culture schemes. For example, the utility of hepatocyte cultures to quantify the toxicity of molecular therapeutics such as gene therapy agents is limited due to the complex interplay between the hepatocytes and non parenchymal cells, especially immune cells [19].
Often, a significant challenge for studying and understanding mechanisms of toxicity is the need to administer several doses in vivo before observing a toxicological outcome, as exemplified by the drug troglitizone [20]. Because acute dosing does not often identify the true toxic potential of a molecule, more sophisticated secondary in vitro screens for assessing drug toxicity that maintain liver function over a time course that permits long-term multiple dosing of a new drugs are desirable.
Toxicity mechanisms for molecular therapeutics are also often complex [21,22]. Drugs that become toxic following metabolism may seem benign in culture systems in which the activity of CYP450s and other drug metabolizing enzymes are greatly reduced. Drug toxicity can arise from a wide range of mechanisms beyond those that can be discerned in acute dosing assays; hence, a second tier of analysis is needed to further probe candidate compounds that operate by mechanisms such as those involving immune or other cell interactions that manifest themselves over longer-term exposure to drug [2,23–27]. In an attempt to retain liver phenotype and function, various approaches have been used with the common aim of preserving or creating an in vivo-like environment. In this paper, we cover some of the approaches and design considerations in building a liver tissue model and describe at length a 3D liver model built in our laboratory that has been applied in the study of drug clearance and safety.
2. Evolving trends in liver tissue engineering in vitro
Hepatocyte metabolism plays such a central role in mediating liver responses to drugs that most liver tissue engineering efforts focus on preservation or enhancement of hepatocyte function, with other cell types often viewed as playing supporting roles. However, hepatocytes in vivo are complex metabolic cells whose function is dependant on their microenvironment, which not only consists of direct cell–cell and cell–matrix interactions but also myriad diffusible factors secreted by nearby non-parenchymal cells (NPCs) [28] as well as chemical gradients caused by the unique hepatic circulatory pattern [1,29,30]. The complex interplay of signals within and along the sinusoid result in gradients of hepatocellular function along the sinusoid in both homeostasis and pathological response [29,31,32]. Approaches to preserve hepatocyte function in culture have ranged from simple interventions such as adding medium components and growth factors resembling serum or changing the substrate to provide signaling akin to extracellular matrix to more complex attempts to preserve or create 3D cell–cell architecture (liver slices, spheroids etc.) or even perfusing the systems to recapitulate the cues provided by the flow of blood [33–41]. Described below are three general trends in the field of liver tissue engineering.
2.1 Towards heterotypic co-cultures
Historically, in vitro studies for drug metabolism and toxicity have used purified hepatocyte populations. While these systems have shown great utility in the drug discovery process (and will continue to play an important role), there are many areas in which enhanced models can add tremendous value. For example, it is well documented that NPCs in the liver, including Kupffer cells and endothelial cells, can contribute directly or indirectly to drug induced hepatotoxicity [42–48]. Identifying these toxicities in vitro will require a further level of testing using systems that contain more than hepatocytes alone. Indeed, short-term hepatocyte co-cultures with Kupffer cells have demonstrated synergistic interactions between xenobiotics and lipopolysachcharide (LPS) [49].
Another major driver for including NPC types is the enhancement of hepatocellular phenotype. An abundance of literature over the past few decades has shown the beneficial effects of each of the NPCs and other cell types in not only extending the survival of hepatocytes, but also in maintaining its differentiated state [50–61,63]. The positive effect is attributed to the heterotypic direct cell–cell interactions as well as matrix and diffusible growth factors and cytokines secreted by the NPC types. For example, Kupffer cells release both proproliferative (e.g., TNF-α, IL-6) and anti-proliferative cytokines and signals (IL-1, TGF-β) [62–65]. TNF-α released from the Kupffer cells has been shown to downregulate the enzyme CYP2B1 in hepatocytes in co-cultures and might play an important role in the modulation of xenobiotic metabolism in liver [66]. Hepatocyte growth factor is a mitogen for hepatocytes and is synthesized by quiescent hepatic stellate cells [67] and co-culture with stellate cells improves hepatic function [68]. Studies using co-cultures of hepatocytes with sinusoidal endothelial cells [50,51] demonstrated that these cell types support long-term hepatocyte functional activity. The interaction has been shown to be reciprocal in enhancing the survival and retention of phenotype of the NPCs as well [69,70].
Just as cell density plays a prominent role in governing function of hepatocyte culture – cells must generally be plated at densities < 40,000 cells/cm2 to show robust DNA synthesis with mitogen simulation and densities above ~ 50,000 cells/cm2 to retain high levels of differentiated function [71,72] – the geometry of interactions between cells in co-cultures can influence outcome. Co-cultures initiated by stochastic seeding often evolve islands of two cell types; micropatterning approaches allow the degree of interactions between two cell types to be controlled more precisely on the culture substrate, with some improvement in function [61,63,73]. Whether 2D micropatterning approaches can capture all facets of physiology relevant for predicting toxicology remains an open question. Hence, development of 3D systems remains an attractive path forward for toxicology.
2.2 Towards 3D architecture
The idea of direct cell–cell and cell–matrix interactions promoting survival was suggested and subsequently demonstrated by reports that maintenance of differentiated phenotype in hepatocytes was enhanced by sandwiching mono-layers of cells between layers of extracellular matrix [37,38,74–78]. Studies of various tumor models also long since demonstrated that maintenance of differentiated phenotype was enhanced when cells form spheroids or multi-layer cell aggregates [79,80]. Formation of these structures requires cell–cell interactions mediated by E-cadherin, an intercellular adhesion molecule known to regulate hepatocyte cell functions [81,82]. The spatial constraints imposed by a 3D environment on cells determine how they perceive and interpret biochemical cues from the surrounding microenvironment [83–87]. Additionally, 3D architecture provides another dimension for external mechanical inputs and for cell adhesion, dramatically affecting integrin ligation, cell contraction and associated intracellular signaling [88,89]. In a 3D environment, the surrounding extracellular matrix both controls solute diffusion and binds many effector proteins, such as growth factors and enzymes, thereby establishing tissue-scale solute concentration gradients, as well as local pericellular gradients [1]. For liver, the earliest versions of fully 3D systems consisted of spheroids either of hepatocytes alone or co-cultured with NPCs [68,90–94]. These demonstrated a better retention of hepatocyte phenotype and function. Again, challenges in the stochastic nature of seeding cells in standard culture formats have driven micropatterning approaches to control size and geometric interactions between spheroids [95].
Another early 3D system was the liver slice. Many of the initial hurdles with liver slices (i.e., reproducibility) have been addressed with newer precision slicing systems and advanced incubation methods [96,97]. When slices are too thick, there are diffusion limitations into the tissue at the center of the slice and when slices are too thin, there is too much necrotic tissue from the cut relative to normal tissue inside the slice. Currently, liver slices can survive and function for 24 – 72 h making them useful for some toxicology applications [98–100]. Liver slices are also widely used to study liver fibrosis [101–103].
Major challenges in 3D cultures include the relatively short penetration depth of oxygen and macromolecules in 3D liver culture (~ 0.1 mm), and provision of appropriate mechanical stimuli in the absence of perfusion through the local tissue microenvironment. These challenges have driven development of more complex 3D set ups across both applications areas of liver tissue culture, bioartificial liver devices and microscale laboratory/testing models [104–106].
2.3 Towards flow based systems
One of the major limitations when creating dense 3D tissue structures is the delivery of oxygen to the tissue [1]. Efforts at ensuring adequate oxygen delivery in 3D systems have driven the design of systems that incorporate fluid flow across or through the tissue. Most efforts in this regard arise from the needs of large-scale systems for maintenance of cell viability during extracorporeal liver support, and prominently include membrane-based reactors (in which cells are cultured outside semi-permeable, hollow-fiber membranes), perfusion reactors (in which cells are grown in porous scaffolds and fluid is pumped around them) and stirred suspension-culture reactors (in which aggregates of cells are kept in suspension) [40,41,107–112]. The large scale of these devices makes them impractical to use in basic research applications, although concepts from large-scale systems have been applied to scaled-down versions of these systems [113]. Perfusing culture medium thorough the tissue can overcome oxygen transport limitation by increasing the amount of oxygen available to the tissue [114,115]. Perfusion flow can be achieved in small set ups with the help of microfluidic pumps and valves designed to circulate culture medium through small culture units on the order of a few thousand cells [116–119]. In addition to providing nutrients and oxygen, flow can be used to control the oxygen gradient across the tissue, a feature of particular significance in the liver on account of the heterogeneity in phenotype and metabolic patterns seen in vivo between periportal and pericentral hepatocytes in the hepatic lobule [29,30,120–123]. Additionally, flow of medium through the tissue can potentially recapitulate the mechanical cues provided by blood flow in the sinusoids [1].
The features and incremental benefits of these evolving models over traditional hepatocyte systems are listed in Table 1.
Table 1. Evolution of in vitro liver tissue models.
| Approach | Specific feature | Observed benefit | Ref. |
|---|---|---|---|
| Co-culturing hepatocytes with other cell types | Hepatocytes with endothelial cells | Maintenance of differentiation and function | [50,51] |
| Hepatocytes with stellate cells | Preserve hepatocyte function, hepatocyte proliferation | [52–54] | |
| Hepatocytes with Kupffer cells | Optimize hepatocyte function | [60] | |
| Micropatterned hepatocytes with NPCs | Preserve hepatocyte phenotype | [55,59] | |
| Hepatocytes with other cell lines | Preservation of hepatocyte function and phenotype | [56,58] | |
| 3D co-cultures | Hepatocyte-stellate cell spheroids | Preservation of hepatocyte function | [68,94,164,165] |
| Layered hepatocytes and endothelial cells | Upregulation of metabolism genes | [167] | |
| Hepatocytes with cell lines | Preservation of hepatocyte function | [166] | |
| Flow based systems | Continuous flow around hepatocytes in spinner baskets | Higher levels of DNA synthesis | [168] |
| Perfused scaffolds with 3D hepatocyte aggregates | Better morphology and viability | [118] | |
| Perfused scaffolds with 3D hepatocyte-NPC aggregates | Sinusoidal phenotype maintained long-term | [119] |
NPC: Non-parenchymal cell.
3. Considerations and design principles for perfused 3D liver toxicity models
There are many factors that play a role in creating a predictive model for drug hepatotoxicity. The first is that the test system must represent the population who will ultimately receive the drug. Second, the exposure of the compound to the system must be of sufficient duration and concentration to generate a relevant response. Next, appropriate end points must be measured and either mimic or correlate to clinically observed end points indicative of toxicity. Various assays for toxicology have been discussed expansively in literature [18,124–127]. Finally, and probably most importantly, the data must be used appropriately to make decisions that will guide the development process. It is critical to understand the limitations of any test system and how they influence results. Another difficult challenge is extrapolation of concentrations and results from an in vitro model to in vivo, or between species. Understanding there are many elements that influence the predictive nature, we focus here on the test system. To design an in vitro model of liver that functions like in vivo liver with regard to drug metabolism, immune response and toxicity, the following factors need consideration.
3.1 Cell source, type and quality
Because significant species differences can exist in the metabolism and toxicity of various compounds [128,129], selection of cells to use in the system (be it from human or various animal species) should be done with the understanding of potential limitations in translation to the clinical setting. The most common sources are freshly isolated or cryopreserved enriched fractions of hepatocytes [8,9]. Addressing the need for a reproducible source of highly functional cells, an immortalized human cell line that can be induced to express a high degree of differentiated function has been commercialized [130]. Promising, but still only on the verge of commercial application, are the many approaches to differentiation of tissue-specific (liver) or pluripotent stem cells (including mesenchymal and embryonic) into mature hepatocytes [131–136]. Regardless of the type or species of cells, they need to be reliably sourced with minimal variability from batch to batch for the model to provide reproducible results. In the case of human cells, given the great inter-individual variability of cytochrome P450 patterns between donors, prediction of drug metabolic profiles, drug–drug interactions and toxicity of new drugs should be investigated in hepatocytes from several donors [6,8].
When maintaining heterotypic cultures, the relative numbers and dedifferentiated phenotype of different cell types can change during culture. It is thus important to ensure the functional phenotype of cells in the system is maintained throughout the assay duration. An array of markers can be used for functional activity; for instance, probe compounds can be used to check maintenance of specific drug metabolizing enzymes [8,137], albumin can be a functional marker for hepatocytes or the membrane antigen SE-1 can reflect functionality and differentiated phenotype of rat sinusoidal endothelial cells in co-cultures. Quantitative analysis of these markers is a continuing challenge, making it essential to standardize seeding numbers and operating conditions.
3.2 Determining length scales and flow rates based on predicted tissue requirements
As with the liver in vivo, in a 3D hepatic tissue unit, concentration gradients can exist for soluble and diffusible culture-medium components that are consumed or produced by cells, from oxygen to basic nutrients or secreted factors. Gradients of oxygen tension in vivo give rise to the phenomenon of zonation, driving differences in phenotype across the lobule. At periportal regions where oxygen tension is higher and gluconeogenesis occurs, oxygen consumption is higher with respect to pericentral regions where oxygen tension is lower and glycolysis occurs. In addition to oxygen consumption rates, the distribution of drug metabolizing enzymes and transporters is heterogeneous in the liver. Most CYPs are located in the perivenous region [29,30]. Transporters, such as OATP1A4 are expressed more abundantly in the midzonal perivenous region and not the periportal region [138]. However, some transporters (e.g., MRP2 and MDR1) have been shown to be expressed equally in both periportal and perivenous regions of the liver [139,140]. In the liver, the oxygen tension is typically lower than other organs due to the portal blood supply. The baseline metabolism and functions occur typically in environments ranging from 30 to 90 mmHg O2, a value significantly lower than atmospheric oxygen tension (160 mmHg O2) [141].
In vitro, oxygen is rapidly depleted due to its relatively low solubility in culture medium and the high metabolic activity of the cells. To achieve a physiologically relevant 3D microenvironment, it is important to design length scales based on known or predicted cellular requirements. Experimental values of cellular utilization are available in literature [142,143] and can be used in conjunction with diffusion and flow parameters as the basis for determining the upper bounds for length scales. In cases of floating spheroids where all the surfaces are exposed to oxygen saturated media, oxygen transport by diffusion can support a cellular mass of greater diameter than when oxygen transport only occurs across one plane, such as tissue packed into a well. For example, based on reported oxygen consumption rates [144], a spherical mass of tissue at an elevated oxygen consumption rate would need to be smaller than 380 µm to allow oxygen to diffuse into the center. Likewise, a static culture would need to be < 110 µm deep to allow for enough oxygen to reach the bottom layer of cells. The depth of static medium above a tissue surface also imposes diffusion limitations. At cell densities regularly used in tissue culture, a depth of 2 mm can reduce oxygen availability at the cells to 10 – 50% of the oxygen concentration at the air–liquid interface [145].
To overcome diffusion limitations, flow can be used to transport oxygen to the tissue. In a system that is perfused, or when culture medium flows across the tissue surface, it is important to consider the effects of shear stresses. Higher than physiological shear rates, such as those seen after partial hepatectomy, are known to perturb the different cell types, giving rise to a range of cell signaling events and cell growth seen in hepatic regeneration [146]. It has been shown that while a small amount of shear stress is beneficial to cultures of hepatocytes (below 5 dynes/cm2), larger amounts of shear are shown to be damaging [147–149].
3.3 Scaffold material and geometry
A scaffold is used to provide support for 3D organization of cells. The composition, architecture and mechanical properties of the scaffold work together to dictate tissue morphogenesis and continued evolution of tissue function. A facile means to create scaffolds is by drilling cell-holding features into a thin polymer sheet of materials ranging from polystyrene to polycarbonate, and post-coat the scaffold with extracellular matrix [150]. Finally, it is critical that a scaffold allows access to tissue for measurement of the desired end points. Depending on the application, scaffolding should be conducive to imaging or harvesting the cells for protein or gene expression levels. Although relatively stiff materials such as polystyrene provide mechanical stability to support cells under flow, the growing appreciation that stiff substrates activate stellate cells [151] and influence hepatocellular function [152] is driving development of gel-based scaffolds that provide a closer match to liver extracellular matrix.
4. A 3D perfused liver bioreactor
A liver tissue-engineered perfused bioreactor that shows significant promise for studying drug toxicity and metabolism has been described recently [153,154]. The core of the device is a scaffold (Figure 1A) containing a matrix of 3D liver tissue units that mimic the liver capillary bed. Culture medium continually circulates through the scaffold and bioreactor (Figure 1B). To enhance throughput as well as integration into existing automated cell culture paradigms, the device was designed to mimic the format of a multi-well plate, and houses 12 isolated bioreactors each with an integrated micro pump for controlling flow (Figure 1C). The capillary bed is formed by seeding a suspension of liver cells into a reactor well; the cells adhere to the scaffold contained in the well and form tissue-like structures under continuous perfusion. As culture medium flows through the tissue bed, oxygen is consumed resulting in a gradient across the tissue that is similar to the oxygen gradient in the in vivo liver sinusoid [154]. The system was designed such that this gradient and the actual concentrations can be tuned based on flow rate and other operating parameters [154]. As a result, mechanical parameters in the system can be easily adjusted and coupled with functional output to help optimize the system effectively. Several generations of these 3D perfused bioreactors have been developed and each generation maintains a core scaffold that is continually perfused with culture medium [33,34,117,118]. In the most recent version, several reactor chambers were integrated with their associated pumping systems onto a single plate for increased throughput, reliability and ease of use [154].
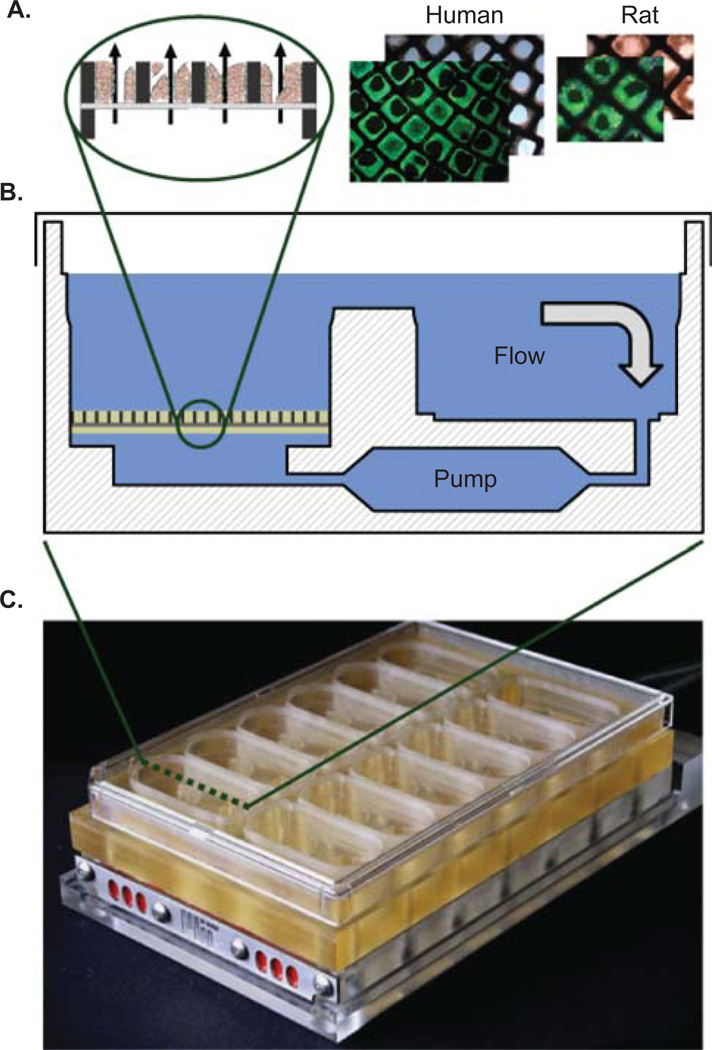
Figure 1. A live-dead stain of human and rat hepatocytes cultured in the scaffold alongside a schematic cross-section.
(A). The scaffold resides within a well of the bioreactor (B) and there are 12 isolated 3D perfused bioreactors on each culture plate (C).
Photograph courtesy of Karel Domansky, MIT 2008.
This perfused liver bioreactor system has been well characterized in the literature and has been shown to enhance the maintenance of liver function in many respects [34,117–119]. Non parenchymal cells when co-cultured with hepatocytes in this system have been shown to retain expression of SE-1, a marker of sinusoidal endothelial cell phenotype for as long as 14 days [119], a period much longer than otherwise reported in literature [155,156]. Kupffer cell presence, as evidenced by staining of the functional marker ED-2, has also been demonstrated after 2 weeks of culture (Dash, Griffith and Tannenbaum, manuscript in progress). Here, we describe how the liver environment is maintained, and how this environment enhances the maintenance of liver function, and finally provide an example of the system’s utility.
4.1 Maintenance of liver function
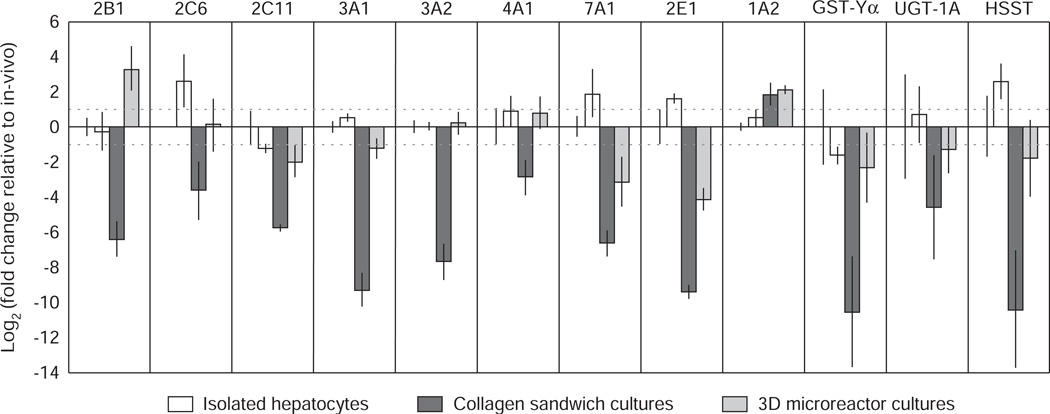
Expression and function of P450 enzymes is rapidly lost in static culture preparations [157–159] whereas it seems to be well maintained in rat hepatocytes cultured in the 3D perfused environment (Figure 2) [117]. In addition to gene expression, the function of selected P450 enzymes has been assessed. Assays were performed by culturing human and rat hepatocytes in the 3D perfused environment for 4 – 6 days, followed by incubating a probe compound in the system for 24 h. The disappearance of probe compounds was measured using LC/MS/MS, and clearance was calculated from these values. Metabolic activity in the perfused bioreactor was compared with historical data from a standardized 4-h suspension assay of freshly thawed rat or human cells (Table 2) [160].
Figure 2. RT-PCR analysis of relative expression of Phase I and II drug metabolizing genes.
Constitutive expression of Phase I and II genes in isolated rat hepatocytes, collagen sandwich culture (day 7) and 3D microreactor (day 7) cultures expressed as log twofold change relative to rat liver in vivo.
Reproduced from Sivaraman et al. 2005 [117] with permission from Bentham Science Publishers Ltd (2009).
RT-PCR: PCR after reverse transcription of RNA.
Table 2. Metabolic clearance of three compounds in rat and human.
Suspension are taken from Pfizer Research Technology Center values, LiverChip values are day 6 for rat (two experiments) and day 4 for human (four experiments).
| Drug | Enzyme | Clearance values (µL per min, per million cells) |
|||
|---|---|---|---|---|---|
| Rat suspension | Rat LiverChip | Human suspension | Human LiverChip | ||
| Midazolam | 3A4 | 35 | 38 | 13 | 13 |
| Naloxone | Phase II | 51 | 39 | 30 | 29 |
| Propranolol | 1A2, 2D6 | 61 | 48 | 19 | 8 |
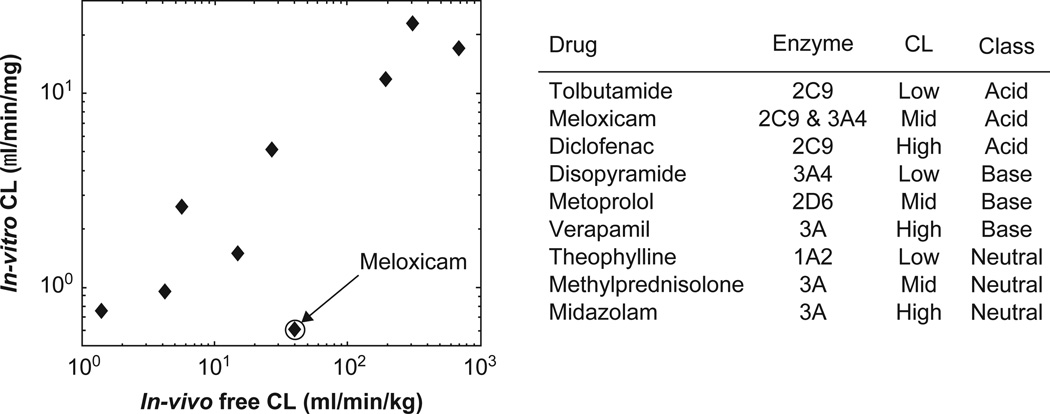
One application for systems that maintain metabolic function is the prediction of in vivo clearance rates. Figure 3 shows the alignment of human in vivo data with in vitro clearance in the perfused 3D liver for a set of nine compounds (Sevidal, Kellet and Obach, manuscript in preparation). Assays were performed using cultures of cryopreserved human hepatocytes, lot Hu4000 (CellzDirect, Inc. Durham, NC, USA). Following 6 days in culture, the probe compound was incubated in the system for 3 days and medium samples were taken periodically. Clearance values were calculated based on disappearance of the parent compound throughout the incubation period. Good alignment between in vitro and in vivo was observed (Figure 3). The exception in this study is the compound meloxicam, in which the perfused microenvironment under-predicts in vivo clearance. This was potentially the result of genetic variation in the specific donor tissue used for the study, as cells from the same donor also under-predict clearance of meloxicam in a 4-h suspension assay (data not shown).
Figure 3. Measured clearance in 3D perfused culture of cryopreserved human hepatocytes compared with in vivo human CL for nine compounds: Sevidal, Kelly and Obach, manuscript in progress.
CL: Clearance.
5. Application of the 3D perfused bioreactor as a model for drug toxicity
Drug induced liver injury (DILI) is the number one cause of death from acute liver failure [161] and the most common cause of drug withdrawal from the market [162]. Broadly, DILI can be categorized into DILI type I (e.g., predictable from animal models, high frequency of occurrence, dose-dependent) and DILI type II (rare occurrence, difficult to predict from preclinical species, little correlation with dose). DILI type II, also referred to as idiosyncratic hepatotoxicity, is a major concern in drug development due to its unpredictable nature and appearance at ordinarily non-toxic doses. One hypothesis for the mechanism responsible for these idiosyncratic responses is due to the presence of inflammatory response elements (e.g., cytokines, chemokines). Numerous in vivo studies have shown that treatment with certain drugs, such as ranitidine, trovafloxacin and diclofenac, in a modest inflammatory setting triggered by bacterial LPS can precipitate idiosyncratic liver injury in rats [23,42,163]. Corresponding in vitro experiments required conditioned medium from neutrophils to elicit toxicity, emphasizing the necessary role of other cell types in the inflammatory response [23]. Other studies that used Kupffer cells and hepatocytes to create an inflammatory response highlight the need of direct cell–cell contact between different cell types in vitro to achieve this effect, and point to a reciprocal interaction between the two cell types [43].
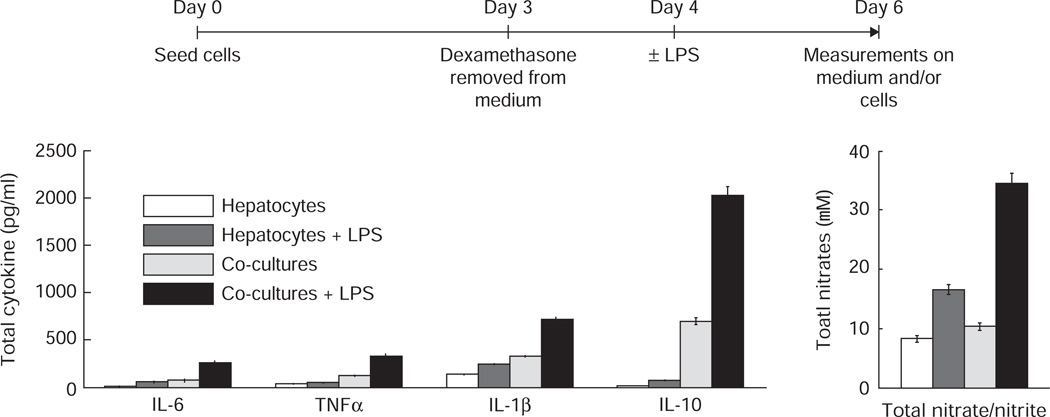
To design a liver model for drug toxicity capable of maintaining an inflammatory component, we used the micro perfused reactor system described in section 4. Monocultures containing an enriched population of hepatocytes (≥ 95%) and co-cultures containing NPCs in near physiological proportions (Table 3) were seeded into 12 wells in the reactors. After 4 days, six of the wells were stimulated with 1 µg/ml LPS. Elevated levels of both pro-inflammatory (IL-1, IL-6, TNF-α) and anti-inflammatory (IL-10) cytokines as well as higher levels of nitrites/nitrates observed in the medium after 48 h demonstrated that the co-cultures containing Kupffer cells exhibited a robust inflammatory response to the LPS compared to the hepatocyte only cultures (Figure 4).
Table 3. Characterization of cellular input into bioreactors: hepatocytes were isolated by a modified two-step collagenase perfusion process described earlier [117] and isolates with viability > 90% were consistently used.
NPC fractions were purified from supernatants based on published methods [119] and stained with different immunostains (SE-1 for sinusoidal endothelial cells, ED-2 for Kupffer cells, GFAP for stellate cells) before quantifying by flow cytometry. NPC isolates were consistently shown to have a viability of > 95%. Based on the proportions, defined numbers of each cell type were added back into reactors at the time of seeding.
| Cell type | Function | Reference in vivo values |
Seeding numbers/reactor well |
|
|---|---|---|---|---|
| Monocultures | Co-cultures | |||
| Hepatocytes | Main metabolic cells | 60% | 800,000 (95%) | 400,000 (40%) |
| Endothelial cells | Filtration, secrete cytokines | 20% | (1 –2%) | 400,000 (40%) |
| Kupffer cells | Macrophages, inflammatory response | 15% | (1 –2%) | 150,000 (15%) |
| Stellate cells | Fibroblasts, store fat, secrete ECM | 5% | (< 1 %) | 50,000 (5%) |
NPC: Non-parenchymal cell.
Figure 4. Monocultures or co-cultures (initial numbers shown in Table 3) were seeded into the reactors.
After allowing the cultures to stabilize for 3 days, dexamethasone was removed from the medium and 24 h later, 50% the wells were stimulated with 1 µg/ml LPS. Measurements of cytokines and total nitrates/nitrites were made from the medium 48 h after treatment. The results show an enhanced inflammatory response in co-cultures. Dash, Griffith and Tannenbaum, publication in progress.
LPS: Lipopolysachcharide.
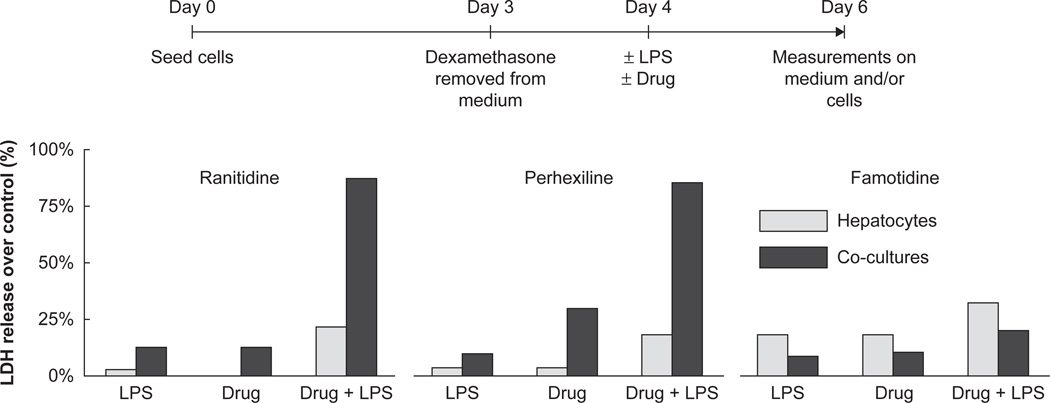
Under these inflammatory conditions, different test drugs known to exhibit idiosyncratic toxicity were then tested using this model along with non-idiosyncratic controls. Cultures were set up as before and the test compounds were added in non-toxic doses to LPS stimulated and DMSO treated control wells. Medium was sampled over 48 h for markers of toxicity and the tissue was stained for cytotoxic response. Over the dose range studied, treatment with the compounds or LPS in isolation caused minimal toxicity in the co-cultures (as evidenced by lactate dehydrogenase release). However, when LPS was administered in combination with known idiosyncratic toxicants (e.g., ranitidine), a marked toxic response was observed (Figure 5) as compared to control drugs from the same therapeutic group (famotidine). Currently, a wider range of idiosyncratic drugs paired with their non-toxic counterparts are under evaluation.
Figure 5. Monocultures containing a mostly hepatocytes (95%) or co-cultures containing NPCs in defined proportions were seeded into the reactors.
After allowing the cultures to stabilize for 3 days, dexamethasone was removed from the medium and 24 h later, 50% the wells were stimulated with 1 µg/ml LPS and/or the test drugs (ranitidine (800 mg/ml), perhexiline (5 mmol), famotidine (175 mg/ml) or DMSO controls). Measurements of LDH release were made from the medium 48 h after treatment. LPS markedly increased toxicity of the known idiosyncratic drug ranitidine but not its corresponding negative control famotidine. Dash, Griffith and Tannenbaum, publication in progress.
LDH: Dehydrogenase: LPS: Lipopolysachcharide: NPC: Non-parenchymal cell.
6. Choosing an appropriate toxicity model
Each of the different approaches listed in Table 1 (or a combination of them) incorporates novel enhancements to current cell culture approaches in an attempt to more closely mimic in vivo liver physiology and function. These features introduce a level of complexity to account for several factors that have a role in influencing cellular phenotype, function and behavior as well as response to a drug or xenobiotic. The key advantages of such complex heterotypic systems is that they clearly result in better maintenance of basic cellular function [55,117,164–168] and that they begin to capture the function of entire organs rather than isolated cells. By virtue of being more like the in vivo setting, these culture systems approximate drug disposition and toxicity in intact preclinical and clinical species in terms of physiological relevance as compared to isolated primary cells plated on a culture dish in a static environment. The applications of these complex models can be specifically tailored to individual needs by manipulating the cell types (as described above) and operating conditions; for instance, varying shear stress or nutrition/oxygen gradients. The increased longevity of viable functional tissue allows for experimental designs such as repeat dosing and long-term evaluation that cannot be achieved in conventional cell cultures with primary cells. Having a system amenable to imaging can provide visualization of cellular level events and morphological changes in a 3D tissue context. Last but not the least, the incorporation of human cells into these systems provides a platform to test drugs in a clinically relevant setting, thereby, bridging the gap between animal and human studies.
While creating a heterotypic model, we have tried to incorporate most of the cell types present in the liver. Kupffer cells, which form almost 80% of the resident macrophage population, when stimulated with LPS in our system elicited an inflammatory background to test the drugs. However, one has to recognize that responses arising due to the recruitment of circulating immune cells such as T lymphocytes and neutrophils are responsible for further mechanisms of toxicity seen in vivo, and these may need to be captured in a different model designed accordingly.
There are also challenges that come with increased complexity that make simpler models preferable in some cases. Cost and capacity are important considerations when selecting an appropriate model at a given stage of drug discovery and development and more simplistic models that address specific research questions will continue to play a crucial role in drug discovery. Rather than replace these models, tissue engineered systems will probably be used in parallel or at later stages to augment data generated in more simplistic systems. Nonetheless, the ultimate choice of model should be driven by the scientific question posed and what physiologic and mechanical components are critical to generate the experimental outcome in the in vitro setting. Given the importance of drug induced liver toxicity in the ultimate development and success of any drug, we recommend having several models available to methodically evaluate compounds at several stages of drug discovery and development. Ultimately, a model that incorporates flow, NPCs and hepatocytes, as well as the ability to evoke an inflammatory response to endotoxins and other immune activators, may be the appropriate tool for understanding mechanisms of toxicity and drug disposition. This model likewise would better enable preclinical study design (including species selection), and better predict the possibility of adverse effects in clinical trials for novel therapeutic agents.
7. Conclusion
Technological strides in the field of liver tissue engineering provide opportunities to transform standard cell culture methods into more complex systems that retain liver function over time. As a direct result, more predictive and reliable tools for understanding drug toxicity and disposition are now emerging. We have discussed some of the evolving approaches, and how they can be combined to create a 3D heterotypic co-culture system that fosters survival of functional hepatocytes and NPCs. Studies in the bioreactor system described above demonstrated its ability to retain gene expression levels for several cytochrome P450s closer to freshly isolated levels as compared to static cultures as well as to maintain metabolic function allowing for prediction of clearance rates similar to in vivo values. Benefits of using this liver bioreactor include the ability to study a metabolically competent system over a long period of time. This provides an important advantage for assessment of the metabolism of low clearance compounds, the formation of secondary/tertiary metabolites and the impact of multiple dosing on drug disposition. It is also crucial for studying the safety of compounds when toxicity is potentially mediated by metabolites. Finally, the ability to compare results between human and animal species is very useful for designing and understanding preclinical pharmacokinetics and safety studies.
To explore this system as a possible model for predicting idiosyncratic toxicity, we utilized the presence of NPC–hepatocyte interaction and characterized a reproducible response to LPS as an inflammatory stimulus. The resulting model was used to demonstrate sensitivity of certain drugs such as ranitidine at non-toxic doses in an inflammatory environment and could serve as a potential model for idiosyncratic hepatotoxicity.
8. Expert opinion
A decade ago, the utility of hepatocyte cultures in preclinical drug development was just becoming appreciated as standard methods for isolation, shipment and culture of human hepatocytes made it possible to develop in vitro tests that correlated with in vivo toxic events for a subset of compounds known to be toxic. Multi-well plate cultures of hepatocytes have passed an important milestone in that they can be utilized to capture known toxicities with minimal false positive outcomes (i.e., reporting a known safe compound as toxic), although a large fraction of known toxic compounds fail to be identified using these technologies [18,169]. Multi-well plate-type hepatocyte cultures thus have an important role in early screens, but must be augmented by further approaches to improve predictive power. In response to this success with its attendant shortcomings, the field is moving in several directions simultaneously. At one end of the range, technologies are emerging that push versions of accepted assays for hepatocellular metabolism and toxicity into cheaper and more robust high-throughput modes through miniaturization [170]. Miniaturized cell culture formats are moving into the commercial realm at a rapid pace for both general culture and specific applications; hence, success of these formats for toxicology is highly probable. Still, while such technologies have the potential to greatly increase the number of assays and the quantitative information available for each compound and to potentially include some non-liver target cell effects [171], a fundamental question remains whether they will offer a significant advance in predictive power (i.e., whether they will capture a significantly greater fraction of toxic compounds).
Several emerging efforts suggest that standard hepatocyte cultures, and their miniaturized counterparts, will be pushed toward better performance (i.e., greater statistical correlation with known toxic compounds) by judicious combinations of culture conditions, measurement methods and data interpretation that incorporate mechanistic hypotheses. For example, multimode imaging can reveal more subtle effects on cells [18] and combinations of inflammatory cytokines with drugs capture toxicities that are not evident without the co-morbid stress [169]. These approaches, combined with systems biology [172], will improve the predictive power and lower cost in the next 5 – 7 years. The future of these technologies depends on whether they can be validated to improve prediction of toxicants; that is, whether they can capture not only toxicities identified in existing preclinical screens, but also a significant number of toxicities that were missed with current screens, but were identified in clinical trials. If proven through such validation, these tools would establish a platform for better prediction of clinically adverse events and could decrease the post-marketing failures seen with several recent therapeutic agents. We speculate that these technologies may become integral aspects of regulatory submissions to help support safety and disposition information on novel therapeutic agents.
But although we foresee dramatic improvement in these hepatocellular assays, we anticipate that tissue engineering approaches will provide essential further screens for many compounds, and possibly a primary screen for new classes of drugs. As one simple example, we have highlighted the hypothesis that a substantial percent of idiosyncratic toxicities arise from co-morbidity of drug plus inflammation. A preliminary study in hepatocytes treated with inflammatory cytokines shows that predictive power improves, but still falls far short of desirable [169]. Although resolution in this assay might be improved by manipulating protocol variables such as timing of cytokine additions, fed/fasted state of cells and hypoxia, our view is that the complex interactions that lead to toxicity will be difficult to replicate economically in a 2D screen compared to a 3D screen, as many of the variables that must be represented individually in different wells in 2D screens (hypoxia, cytokine combinations) are captured naturally in a single well of a 3D culture due to the presence of nonparenchymal cells and zonation along the path of flow.
We anticipate that the need for tissue-engineered models will be underscored by the needs for preclinical assessment of molecular therapeutics such as siRNA and other gene therapy vectors, which are often taken up by liver even when they are not intended to be. Molecular therapeutics have a highly complex interaction with liver, which in addition to metabolism is the largest immune organ in the body. Although the focus is typically on the Kupffer cell–hepatocyte interactions when clearance and toxicity of molecular therapeutics are discussed, the prominence of sinusoidal endothelium as players in the immune response of liver is rapidly emerging [173]. Sinusoidal endothelial cells are very difficult to culture in a differentiated, functional state [119]; hence, co-cultures that replicate features of the complex interactions among cells in the liver sinusoid will be needed to understand clearance and toxicity of these rapidly-emerging new therapeutics. Finally, tissue engineering will almost surely be needed to assess chronic responses, including those involving trafficking of bone-marrow-derived cells into liver [174].
What is the best path for development? Advances are occurring rapidly in several fields that together impinge on creation of robust systems for tissue engineering. Synthetic biomaterials that are easier to process and store, and have more reproducible lot-to-lot properties, are moving into the mainstream in many areas of cell biology [175]. These are being combined with advances in microfluidics to create integrated devices that can be translated into pharmaceuticals labs [34,115,117,119,154,176,177]. As these tools come on line, their ultimate success in transforming predictive toxicology will rely on using them to address well-posed mechanistic questions and on their integration with systems biology approaches that can parse several types and hierarchies of information.
Acknowledgements
The authors thank K Domansky from MIT for his contributions regarding the 3D perfused bioreactor and R Dunn, J Lawrence and C Afshari from Amgen, Inc. for early discussions on the subject.
Declaration of interest
The authors acknowledge financial support from the NSF ERC, R01ES015241, NIEHS U19ES011399-05S1, DuPont, Inc., Pfizer, Inc. and Amgen, Inc.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1. Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–324. doi: 10.1038/nrm1858. •• This review describes in detail the approach of designing a 3D organotypic model featured in the current review and presents additional data.
- 2.Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: mechanisms and test systems. Hepatology. 2001;33:1009–1113. doi: 10.1053/jhep.2001.23505. [DOI] [PubMed] [Google Scholar]
- 3.Olson H, Betton G, Robinson D, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 4.Li AP. Accurate prediction of human drug toxicity: a major challenge in drug development. Chem Biol Interact. 2004;150:3–7. doi: 10.1016/j.cbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese EJ. Suitability of animal-models for predictive toxicology - theoretical and practical considerations. Drug Metab Rev. 1984;15:505–623. doi: 10.3109/03602538409029971. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor JT, Collins JM, Sugiyama Y, et al. In vitro human tissue models in risk assessment: report of a consensus-building workshop. Toxicol Sci. 2001;59:17–36. doi: 10.1093/toxsci/59.1.17. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien PJ, Chan K, Silber PM. Human and animal hepatocytes in vitro with extrapolation in vivo. Chem Biol Interact. 2004;150:97–114. doi: 10.1016/j.cbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt NJ, Lechon MJG, Houston JB, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- 9.Vermeir M, Annaert P, Mamidi RN, et al. Cell-based models to study hepatic drug metabolism and enzyme induction in humans. Expert Opin Drug Metab Toxicol. 2005;1:75–90. doi: 10.1517/17425255.1.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Li AP. In vitro approaches to evaluate ADMET drug properties. Curr Top Med Chem. 2004;4:701–896. doi: 10.2174/1568026043451050. [DOI] [PubMed] [Google Scholar]
- 11.Ferrini JB, Pichard L, Domergue J, Maurel P. Long-term primary cultures of adult human hepatocytes. Chem Biol Interact. 1997;107:31–45. doi: 10.1016/s0009-2797(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 12.Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect. 1998;106:511–632. doi: 10.1289/ehp.98106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soars MG, McGinnity DF, Grime K, Riley RJ. The pivotal role of hepatocytes in drug discovery. Chem Biol Interact. 2007;168:2–15. doi: 10.1016/j.cbi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Li AP, Gorycki PD, Hengstler JG, et al. Present status of the application of cryopreserved hepatocytes in the evaluation of xenobiotics: consensus of an international expert panel. Chem Biol Interact. 1999;121:117–123. doi: 10.1016/s0009-2797(99)00081-2. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Houston JB. Comparison of the use of liver models for predicting drug clearance using in vitro kinetic data from hepatic microsomes and isolated hepatocytes. Pharm Res. 2004;21:785–992. doi: 10.1023/b:pham.0000026429.12114.7d. [DOI] [PubMed] [Google Scholar]
- 16.Shitara Y, Li AP, Kato Y, et al. Function of uptake transporters for taurocholate and estradiol 17beta-D-glucuronide in cryopreserved human hepatocytes. Drug Metab Pharmacokinet. 2003;18:33–41. doi: 10.2133/dmpk.18.33. [DOI] [PubMed] [Google Scholar]
- 17.Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of Oatp2 (Oatp1B1) and Oatp8 (Oatp1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–246. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- 18.Xu JHJ, Henstock PV, Dunn MC, et al. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol Sci. 2008;105:97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- 19.Descamps D, Benihoud K. Two key challenges for effective adenovirus-mediated liver gene therapy: innate immune responses and hepatocyte-specific transduction. Curr Gene Ther. 2009;9:115–227. doi: 10.2174/156652309787909544. [DOI] [PubMed] [Google Scholar]
- 20.Julie NL, Julie IM, Kende AI, Wilson GL. Mitochondrial dysfunction and delayed hepatotoxicity: another lesson from troglitazone. Diabetologia. 2008;51:2108–3616. doi: 10.1007/s00125-008-1133-6. [DOI] [PubMed] [Google Scholar]
- 21.Appledorn DM, McBride A, Seregin S, et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 2008;15:1606–1736. doi: 10.1038/gt.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiedner G, Bloch W, Hertel S, et al. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic kupffer cell-mediated activation of endothelial cells. Hum Gene Ther. 2003;14:1631–1741. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- 23.Luyendyk JP, Maddox JF, Cosma GN, et al. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- 24.Holt MP, Ju C. Mechanisms of drug-induced liver injury. AAPS J. 2006;8:E48–E54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uetrecht J. Idiosyncratic drug reactions: past, present, and future. Chem Res Toxicol. 2008;21:84–92. doi: 10.1021/tx700186p. [DOI] [PubMed] [Google Scholar]
- 26.Jones BE, Czaja MJ. Mechanisms of hepatic toxicity III Intracellular signaling in response to toxic liver injury. Am J Physiol Gastrointest Liver Physiol. 1998;38:G874–G988. doi: 10.1152/ajpgi.1998.275.5.G874. [DOI] [PubMed] [Google Scholar]
- 27.Bourdi M, Reilly TP, Elkahloun AG, et al. Macrophage migration inhibitory factor in drug-induced liver injury: a role in susceptibility and stress responsiveness. Biochem Biophys Res Commun. 2002;294:225–330. doi: 10.1016/S0006-291X(02)00466-7. [DOI] [PubMed] [Google Scholar]
- 28.Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425–531. doi: 10.1016/s1084952102001301. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura T, Kauffman FC, Meren H, Thurman RG. O2 uptake in periportal and pericentral regions of liver lobule in perfused liver. Am J Physiol. 1986;250:G800–G985. doi: 10.1152/ajpgi.1986.250.6.G800. [DOI] [PubMed] [Google Scholar]
- 30.Meren H, Matsumura T, Kauffman FC, Thurman RG. Relationship between oxygen tension and oxygen uptake in the perfused rat liver. Adv Exp Med Biol. 1986;200:467–576. doi: 10.1007/978-1-4684-5188-7_58. [DOI] [PubMed] [Google Scholar]
- 31.Colletti M, Cicchini C, Conigliaro A, et al. Convergence of Wnt signaling on the HNF4a-driven transcription in controlling liver zonation. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.038. [In press] [DOI] [PubMed] [Google Scholar]
- 32.Braeuning A, Ittrich C, Kohle C, et al. Differential gene expression in periportal and perivenous mouse hepatocytes. FEBS J. 2006;273:5051–6261. doi: 10.1111/j.1742-4658.2006.05503.x. [DOI] [PubMed] [Google Scholar]
- 33.Domansky K, Sivaraman A, Griffith LG. Micro and Nanotechnologies for Life Science. Netherlands: Springer; 2005. Micromachined bioreactor for in vitro cell self-assembly and 3D tissue formation. Lab-on-chips for cellomics; pp. 319–546. [Google Scholar]
- 34.Powers MJ, Janigian DM, Wack KE, et al. Functional behavior of primary rat liver cells in a three-dimensional perfused microarray bioreactor. Tissue Eng. 2002;8:499–513. doi: 10.1089/107632702760184745. [DOI] [PubMed] [Google Scholar]
- 35.Lecluyse EL, Bullock PL, Parkinson A. Strategies for restoration and maintenance of normal hepatic structure and function in long-term cultures of rat hepatocytes. Adv Drug Deliv Rev. 1996;22:133–286. [Google Scholar]
- 36.Gomez-Lechon MJ, Jover R, Donato T, et al. Long-term expression of differentiated functions in hepatocytes cultured in three-dimensional collagen matrix. J Cell Physiol. 1998;177:553–662. doi: 10.1002/(SICI)1097-4652(199812)177:4<553::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Gross-Steinmeyer K, Stapleton PL, Tracy JH, et al. Influence of Matrigel-overlay on constitutive and inducible expression of nine genes encoding drug-metabolizing enzymes in primary human hepatocytes. Xenobiotica. 2005;35:419–538. doi: 10.1080/00498250500137427. [DOI] [PubMed] [Google Scholar]
- 38.Page JL, Johnson MC, Olsavsky KM, et al. Gene expression profiling of extracellular matrix as an effector of human hepatocyte phenotype in primary cell culture. Toxicol Sci. 2007;97:384–497. doi: 10.1093/toxsci/kfm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vickers AEM, Fisher RL. Organ slices for the evaluation of human drug toxicity. Chem Biol Interact. 2004;150:87–96. doi: 10.1016/j.cbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Baudoin R, Corlu A, Griscom L, et al. Trends in the development of microfluidic cell biochips for in vitro hepatotoxicity. Toxicol In Vitro. 2007;21:535–544. doi: 10.1016/j.tiv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Gebhardt R, Hengstler JG, Muller D, et al. New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab Rev. 2003;35:145–213. doi: 10.1081/dmr-120023684. [DOI] [PubMed] [Google Scholar]
- 42.Deng XM, Stachlewitz RF, Liguori MJ, et al. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J Pharmacol ExpTher. 2006;319:1191–1259. doi: 10.1124/jpet.106.110247. [DOI] [PubMed] [Google Scholar]
- 43. Hoebe KHN, Witkamp RF, Fink-Gremmels J, et al. Direct cell-to-cell contact between kupffer cells and hepatocytes augments endotoxin-induced hepatic injury. Am J Physio Gastrointest Liver Physiol. 2001;280:G720–G988. doi: 10.1152/ajpgi.2001.280.4.G720. •• This paper demonstrates the need of actual cell-cell contact as opposed to just conditioned medium for some of the heterotypic interactions to occur.
- 44.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of kupffer cells in carbon-tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275–629. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
- 45.Horbach M, Gerber E, Kahl R. Influence of acetaminophen treatment and hydrogen peroxide treatment on the release of a CINC-related protein and TNF-alpha from rat hepatocyte cultures. Toxicology. 1997;121:117–226. doi: 10.1016/s0300-483x(97)00061-9. [DOI] [PubMed] [Google Scholar]
- 46.Nastevska C, Gerber E, Horbach M, et al. impairment of TNF-alpha expression and secretion in primary rat liver cell cultures by acetaminophen treatment. Toxicology. 1999;133:85–92. doi: 10.1016/s0300-483x(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 47.Adachi Y, Bradford BU, Gao WS, et al. Inactivation of kupffer cells prevents early alcohol-induced liver-injury. Hepatology. 1994;20:453–560. [PubMed] [Google Scholar]
- 48.Deleve LD, Ito Y, Bethea NW, et al. Embolization by sinusoidal lining cells obstructs the microcirculation in rat sinusoidal obstruction syndrome. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1045–G1552. doi: 10.1152/ajpgi.00526.2002. [DOI] [PubMed] [Google Scholar]
- 49.Tukov FF, Maddox JF, Amacher DE, et al. Modeling inflammation-drug interactions in vitro: a rat kupffer cell-hepatocyte coculture system. Toxicol In Vitro. 2006;20:1488–1599. doi: 10.1016/j.tiv.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Morin O, Normand C. Long-Term maintenance of hepatocyte functional-activity in coculture - requirements for sinusoidal endothelial-cells and dexamethasone. J Cell Physiol. 1986;129:103–105. doi: 10.1002/jcp.1041290115. [DOI] [PubMed] [Google Scholar]
- 51.Goulet F, Normand C, Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured-hepatocytes. Hepatology. 1988;8:1010–1258. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- 52.Loreal O, Levavasseur F, Fromaget C, et al. Cooperation of ito cells and hepatocytes in the deposition of an extracellular-matrix in-vitro. Am J Pathol. 1993;143:538–644. [PMC free article] [PubMed] [Google Scholar]
- 53.Rojkind M, Novikoff PM, Greenwel P, et al. Characterization and functional-studies on rat-liver fat-storing cell-line and freshly isolated hepatocyte coculture system. Am J Pathol. 1995;146:1508–1620. [PMC free article] [PubMed] [Google Scholar]
- 54.Uyama N, Shimahara Y, Kawada N, et al. Regulation of cultured rat hepatocyte proliferation by stellate cells. J Hepatol. 2002;36:590–699. doi: 10.1016/s0168-8278(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 56.Schrode W, Mecke D, Gebhardt R. Induction of glutamine synthetase in periportal hepatocytes by cocultivation with a liver epithelial cell line. Eur J Cell Biol. 1990;53:35–41. [PubMed] [Google Scholar]
- 57.Zinchenko YS, Culberson CR, Coger RN. Contribution of non-parenchymal cells to the performance of micropatterned hepatocytes. Tissue Eng. 2006;12:2241–2251. doi: 10.1089/ten.2006.12.2241. [DOI] [PubMed] [Google Scholar]
- 58.Bhandari RNB, Riccalton LA, Lewis AL, et al. Liver tissue engineering: a role for co-culture systems in modifying hepatocyte function and viability. Tissue Eng. 2001;7:345–557. doi: 10.1089/10763270152044206. [DOI] [PubMed] [Google Scholar]
- 59.Kidambi S, Sheng LF, Yarmush ML, et al. Patterned co-culture of primary hepatocytes and fibroblasts using polyelectrolyte multilayer templates. Macromol Biosci. 2007;7:344–453. doi: 10.1002/mabi.200600205. [DOI] [PubMed] [Google Scholar]
- 60.Zinchenko YS, Schrum LW, Clemens M, Coger RN. Hepatocyte and kupffer cells co-cultured on micropatterned surfaces to optimize hepatocyte function. Tissue Eng. 2006;12:751–861. doi: 10.1089/ten.2006.12.751. [DOI] [PubMed] [Google Scholar]
- 61.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nature Biotechnology. 2008;26:120–136. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 62.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 63.Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver-regeneration. Hepatology. 1995;21:1443–1559. [PubMed] [Google Scholar]
- 64.Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth-factor-beta in rat-liver - evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–555. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boulton R, Woodman A, Calnan D, et al. Nonparenchymal cells from regenerating rat liver generate interleukin-1 alpha and −1 beta: a mechanism of negative regulation of hepatocyte proliferation. Hepatology. 1997;26:49–58. doi: 10.1053/jhep.1997.v26.pm0009214451. [DOI] [PubMed] [Google Scholar]
- 66.Milosevic N, Schawalder H, Maier P. Kupffer cell-mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes. Eur J Pharmacol. 1999;368:75–87. doi: 10.1016/s0014-2999(98)00988-1. [DOI] [PubMed] [Google Scholar]
- 67.Schirmacher P, Geerts A, Pietrangelo A, et al. Hepatocyte growth-factor hepatopoietin-A is expressed in fat-storing cells from rat-liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology. 1992;15:5–11. doi: 10.1002/hep.1840150103. [DOI] [PubMed] [Google Scholar]
- 68.Riccalton-Banks L, Liew C, Bhandari R, et al. Long-term culture of functional liver tissue: three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003;9:401–510. doi: 10.1089/107632703322066589. [DOI] [PubMed] [Google Scholar]
- 69.Krause P, Markus PM, Schwartz P, et al. Hepatocyte-supported serum-free culture of rat liver sinusoidal endothelial cells. J Hepatol. 2000;32:718–826. doi: 10.1016/s0168-8278(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 70.Edwards S, Lalor PF, Nash GB, et al. Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology. 2005;41:451–529. doi: 10.1002/hep.20585. [DOI] [PubMed] [Google Scholar]
- 71.Tomomura A, Sawada N, Sattler GL, et al. The control of Dna-synthesis in primary cultures of hepatocytes from adult and young-rats - interactions of extracellular-matrix components, epidermal growth-factor, and the cell-cycle. J Cell Physiol. 1987;130:221–327. doi: 10.1002/jcp.1041300208. [DOI] [PubMed] [Google Scholar]
- 72.Benzeev A, Robinson GS, Bucher NLR, Farmer SR. Cell cell and cell matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci USA. 1988;85:2161–3255. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery-system for cultured-cells using plasma-treated polystyrene dishes grafted with Poly(N-Isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1551. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, LeCluyse EL, Brouwer KR, et al. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999;277:G12–G21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- 75.LeCluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266:C1764–C1874. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- 76.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–645. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 77.Farkas D, Bhat VB, Mandapati S, et al. Characterization of the secreted proteome of rat hepatocytes cultured in collagen sandwiches. Chem Res Toxicol. 2005;18:1132–1259. doi: 10.1021/tx0500225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farkas D, Tannenbaum SR. Characterization of chemically induced hepatotoxicity in collagen sandwiches of rat hepatocytes. Toxicol Sci. 2005;85:927–1034. doi: 10.1093/toxsci/kfi145. [DOI] [PubMed] [Google Scholar]
- 79.Hoffman RM. To do tissue-culture in 2 or 3 dimensions-that is the question. Stem Cells. 1993;11:105–119. doi: 10.1002/stem.5530110205. [DOI] [PubMed] [Google Scholar]
- 80.Schwachofer JHM. Multicellular tumor spheroids in radiotherapy research. Anticancer Res. 1990;10:963–998. [PubMed] [Google Scholar]
- 81.Nagaoka M, Ise H, Akaike T. Immobilized E-cadherin model can enhance cell attachment and differentiation of primary hepatocytes but not proliferation. Biotechnol Lett. 2002;24:1857–1962. [Google Scholar]
- 82.Awata R, Sawai H, Imai K, et al. Morphological comparison and functional reconstitution of rat hepatic parenchymal cells on various matrices. J Gastroenterol Hepatol. 1998;13:S55–S61. doi: 10.1111/jgh.1998.13.s1.55. [DOI] [PubMed] [Google Scholar]
- 83.Bissell MJ, Radisky DC, Rizki A, et al. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–646. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ingber DE. Cancer as a disease of epithelial-mesenchymal interactions and extracellular matrix regulation. Differentiation. 2002;70:547–660. doi: 10.1046/j.1432-0436.2002.700908.x. [DOI] [PubMed] [Google Scholar]
- 85.Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–847. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–585. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 87.Radisky D, Muschler J, Bissell MJ. Order and disorder: the role of extracellular matrix in epithelial cancer. Cancer Invest. 2002;20:139–253. doi: 10.1081/cnv-120000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knight B, Laukaitis C, Akhtar N, et al. Visualizing muscle cell migration in situ. Curr Biol. 2000;10:576–685. doi: 10.1016/s0960-9822(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 89.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–13582. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li AP, Colburn SM, Beck DJ. A simplified method for the culturing of primary adult rat and human hepatocytes as multicellular spheroids. In Vitro Cell Dev Biol. 1992;28A:673–787. doi: 10.1007/BF02631045. [DOI] [PubMed] [Google Scholar]
- 91.Yuasa C, Tomita Y, Shono M, et al. Importance of cell aggregation for expression of liver functions and regeneration demonstrated with primary cultured hepatocytes. J Cell Physiol. 1993;156:522–630. doi: 10.1002/jcp.1041560311. [DOI] [PubMed] [Google Scholar]
- 92.Tong JZ, De Lagausie P, Furlan V, et al. Long-term culture of adult rat hepatocyte spheroids. Exp Cell Res. 1992;200:326–432. doi: 10.1016/0014-4827(92)90179-c. [DOI] [PubMed] [Google Scholar]
- 93.Takezawa T, Yamazaki M, Mori Y, et al. Morphological and immuno-cytochemical characterization of a hetero-spheroid composed of fibroblasts and hepatocytes. J Cell Sci. 1992;101:495–501. doi: 10.1242/jcs.101.3.495. [DOI] [PubMed] [Google Scholar]
- 94.Abu-Absi SF, Hansen LK, Hu WS. Three-dimensional co-culture of hepatocytes and stellate cells. Cytotechnology. 2004;45:125–240. doi: 10.1007/s10616-004-7996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Otsuka H, Hirano A, Nagasaki Y, et al. Two-dimensional multiarray formation of hepatocyte spheroids on a microfabricated PEG-brush surface. Chembiochem. 2004;5:850–985. doi: 10.1002/cbic.200300822. [DOI] [PubMed] [Google Scholar]
- 96.Price RJ, Ball SE, Renwick AB, et al. Use of precision-cut rat liver slices for studies of xenobiotic metabolism and toxicity: comparison of the krumdieck and brendel tissue slicers. Xenobiotica. 1998;28:361–471. doi: 10.1080/004982598239470. [DOI] [PubMed] [Google Scholar]
- 97.Toutain HJ, Moronvalle-Halley V, Sarsat JP, et al. Morphological and functional integrity of precision-cut rat liver slices in rotating organ culture and multiwell plate culture: effects of oxygen tension. Cell Biol Toxicol. 1998;14:175–290. doi: 10.1023/a:1007458408863. [DOI] [PubMed] [Google Scholar]
- 98.Lerche-Langrand C, Toutain HJ. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicology. 2000;153:221–353. doi: 10.1016/s0300-483x(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 99.Moronvalle-Halley V, Sacre-Salem B, Sallez V, et al. Evaluation of cultured, precision-cut rat liver slices as a model to study drug-induced liver apoptosis. Toxicology. 2005;207:203–314. doi: 10.1016/j.tox.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 100.Vickers AEM, Fisher RL. Organ slices for the evaluation of human drug toxicity. Chem Biol Interact. 2004;150:87–96. doi: 10.1016/j.cbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Guyot C, Combe C, Balabaud C, et al. Fibrogenic cell fate during fibrotic tissue remodelling observed in rat and human cultured liver slices. J Hepatol. 2007;46:142–250. doi: 10.1016/j.jhep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 102.de Bovenkamp MV, Groothuis GMM, Meijer DKF, Olinga P. Liver fibrosis in vitro: cell culture models and precision-cut liver slices. Toxicol In Vitro. 2007;21:545–657. doi: 10.1016/j.tiv.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 103.van de Bovenkamp M, Groothuis GMM, Draaisma AL, et al. Precision-cut liver slices as a new model to study toxicity-induced hepatic stellate cell activation in a physiologic milieu. Toxicol Sci. 2005;85:632–738. doi: 10.1093/toxsci/kfi127. [DOI] [PubMed] [Google Scholar]
- 104.Lin KH, Maeda S, Saito T. Long-term maintenance of liver-specific functions in three-dimensional culture of adult rat hepatocytes with a porous gelatin sponge support. Biotechnol Appl Biochem. 1995;21(Pt 1):19–27. [PubMed] [Google Scholar]
- 105.Semino CE, Merok JR, Crane GG, et al. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71:262–370. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- 106.Gerlach J, Encke J, Muller C, Neuhaus P. A model of hepatocyte cultures in bioreactors for hybrid liver support. Zentralbl Chir. 1994;119:334–440. [PubMed] [Google Scholar]
- 107.Martin Y, Vermette P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials. 2005;26:7481–8503. doi: 10.1016/j.biomaterials.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 108.MacDonald JM, Wolfe SP, Roy-Chowdhury I, et al. Effect of flow configuration and membrane characteristics on membrane fouling in a novel multicoaxial hollow-fiber bioartificial liver. Ann NY Acad Sci. 2001;944:334–543. doi: 10.1111/j.1749-6632.2001.tb03845.x. [DOI] [PubMed] [Google Scholar]
- 109.Zeilinger K, Holland G, Sauer IM, et al. Time course of primary liver cell reorganization in three-dimensional high-density bioreactors for extracorporeal liver support: an immunohistochemical and ultrastructural study. Tissue Eng. 2004;10:1113–1224. doi: 10.1089/ten.2004.10.1113. [DOI] [PubMed] [Google Scholar]
- 110.Curcio E, Salerno S, Barbieri G, et al. Mass transfer metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials. 2007;28:5487–6497. doi: 10.1016/j.biomaterials.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 111.Nyberg SL, Shatford RA, Peshwa MV, et al. Evaluation of a hepatocyte-entrapment hollow fiber bioreactor-a potential bioartificial liver. Biotechnol Bioeng. 1993;41:194–203. doi: 10.1002/bit.260410205. [DOI] [PubMed] [Google Scholar]
- 112.De Bartolo L, Morelli S, Lopez LC, et al. Human galactosylated membrane bioreactor for the long-term maintenance of liver specific functions. Desalination. 2006;199:147–259. [Google Scholar]
- 113.De Bartolo L, Salerno S, Curcio E, et al. Human hepatocyte functions in a crossed hollow fiber membrane bioreactor. Biomaterials. 2009;30:2531–3543. doi: 10.1016/j.biomaterials.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 114.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng. 2007;97:1340–1456. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 115.Mehta K, Mehta G, Takayama S, Linderman J. Quantitative inference of cellular parameters from microfluidic cell culture systems. Biotechnol Bioeng. 2009;103(5):966–1074. doi: 10.1002/bit.22334. [DOI] [PubMed] [Google Scholar]
- 116.Song JW, Gu W, Futai N, et al. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal Chem. 2005;77:3993–4989. doi: 10.1021/ac050131o. [DOI] [PubMed] [Google Scholar]
- 117. Sivaraman A, Leach JK, Townsend S, et al. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab. 2005;6:569–691. doi: 10.2174/138920005774832632. •• This paper describes the experiments that provide the gene expression data presented in this review.
- 118. Powers MJ, Domansky K, Kaazempur-Mofrad MR, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78:257–369. doi: 10.1002/bit.10143. •• This paper describes the prototype 3D perfused model on which the system described in this review was based.
- 119. Hwa AJ, Fry RC, Sivaraman A, et al. Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 2007;21:2564–79. doi: 10.1096/fj.06-7473com. • This paper demonstrates the ability of a perfused 3D system to retain functionality on non-parenchymal cells over an extended period of time.
- 120.Buhler R, Lindros KO, Nordling A, et al. Zonation of cytochrome-P450 isozyme expression and induction in rat-liver. Eur J Biochem. 1992;204:407–512. doi: 10.1111/j.1432-1033.1992.tb16650.x. [DOI] [PubMed] [Google Scholar]
- 121.Jungermann K, Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982;2:385–495. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- 122.Gebhardt R. Metabolic zonation of the liver-regulation and implications for liver-function. Pharmacol Ther. 1992;53:275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- 123.Gebhardt R, Gaunitz F. Cell-cell interactions in the regulation of the expression of hepatic enzymes. Cell Biol Toxicol. 1997;13:263–373. doi: 10.1023/a:1007431307300. [DOI] [PubMed] [Google Scholar]
- 124.Xu JJ, Diaz D, O’Brien PJ. Applications of cytotoxicity assays and pre-lethal mechanistic assays for assessment of human hepatotoxicity potential. Chem Biol Interact. 2004;150:115–228. doi: 10.1016/j.cbi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 125.Lautraite S, Bigot-Lasserre D, Bars R, Carmichael N. Optimisation of cell-based assays for medium throughput screening of oxidative stress. Toxicol In Vitro. 2003;17:207–320. doi: 10.1016/s0887-2333(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 126.O’Brien PJ, Irwin W, Diaz D, et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol. 2006;80:580–604. doi: 10.1007/s00204-006-0091-3. [DOI] [PubMed] [Google Scholar]
- 127.Davila JC, Xu JJ, Hoffmaster KA, et al. Current in vitro models to study drug-induced liver injury. In: Sahu SC, editor. Hepatotoxicity:genomics from vitro to in vivo in Models. John Wiley & Sons, Ltd; 2008. pp. 3–55. [Google Scholar]
- 128.Bogaards JJP, Bertrand M, Jackson P, et al. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–1252. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- 129.Mutlib AE, Gerson RJ, Meunier PC, et al. The species-dependent metabolism of efavirenz produces a nephrotoxic glutathione conjugate in rats. Toxicol Appl Pharmacol. 2000;169:102–113. doi: 10.1006/taap.2000.9055. [DOI] [PubMed] [Google Scholar]
- 130.Josse R, Aninat C, Glaise D, Dumont J, et al. Long-term functional stability of human HepaRG hepatocytes and use for chronic toxicity and genotoxicity studies. Drug Metab Dispos. 2008;36:1111–1218. doi: 10.1124/dmd.107.019901. [DOI] [PubMed] [Google Scholar]
- 131.Davila JC, Cezar GG, Thiede M, et al. Use and application of stem cells in toxicology. Toxicol Sci. 2004;79:214–323. doi: 10.1093/toxsci/kfh100. [DOI] [PubMed] [Google Scholar]
- 132.Wauthier E, Schmelzer E, Turner W, et al. Hepatic stem cells and hepatoblasts: identification, isolation, and ex vivo maintenance. Stem Cell Cult. 2008;86:137–225. doi: 10.1016/S0091-679X(08)00008-3. [DOI] [PubMed] [Google Scholar]
- 133.Cheng N, Wauthier E, Reid LM. Mature human hepatocytes from ex vivo differentiation of alginate-encapsulated hepatoblasts. Tissue Eng Part A. 2008;14:1–7. doi: 10.1089/ten.a.2007.0131. [DOI] [PubMed] [Google Scholar]
- 134.Sicklick JK, Li YX, Melhem A, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–G970. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 135.Basma H, Soto-Gutierrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–1099. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412–1589. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- 137.Brown HS, Griffin M, Houston JB. Evaluation of cryopreserved human hepatocytes as an alternative in vitro system to microsomes for the prediction of metabolic clearance. Drug Metab Dispos. 2007;35:293–301. doi: 10.1124/dmd.106.011569. [DOI] [PubMed] [Google Scholar]
- 138.Reichel C, Gao B, Van Montfoort J, et al. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology. 1999;117:688–795. doi: 10.1016/s0016-5085(99)70463-4. [DOI] [PubMed] [Google Scholar]
- 139.Liu LC, Mak E, Tirona RG, et al. Vascular binding, blood flow, transporter, and enzyme interactions on the processing of digoxin in rat liver. J Pharmacol Exp Ther. 2005;315:433–548. doi: 10.1124/jpet.105.088039. [DOI] [PubMed] [Google Scholar]
- 140.Tirona RG, Pang KS. Bimolecular glutathione conjugation kinetics of ethacrynic acid in rat liver: in vitro and perfusion studies. J Pharmacol Exp Ther. 1999;290:1230–1341. [PubMed] [Google Scholar]
- 141.Martinez I, Nedredal G, Oie C, et al. The influence of oxygen tension on the structure and function of isolated liver sinusoidal endothelial cells. Comp Hepatol. 2008;7:4 doi: 10.1186/1476-5926-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kellner K, Liebsch G, Klimant I, et al. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 143.Glicklis R, Merchuk JC, Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol Bioeng. 2004;86:672–880. doi: 10.1002/bit.20086. [DOI] [PubMed] [Google Scholar]
- 144.Matsumura T, Yoshihara H, Jeffs R, et al. Hormones increase oxygen uptake in periportal and pericentral regions of the liver lobule. Am J Physiol. 1992;262:G645–G650. doi: 10.1152/ajpgi.1992.262.4.G645. [DOI] [PubMed] [Google Scholar]
- 145.Guppy M, Leedman P, Zu X, Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J. 2002;364:309–415. doi: 10.1042/bj3640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Braet F, Shleper M, Paizi M, et al. Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp Hepatol. 2004;3:7 doi: 10.1186/1476-5926-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kan P, Miyoshi H, Yanagi K, Ohshima N. Effects of shear stress on metabolic function of the co-culture system of hepatocyte/nonparenchymal cells for a bioartificial liver. ASAIO J. 1998;44:M441–M524. doi: 10.1097/00002480-199809000-00023. [DOI] [PubMed] [Google Scholar]
- 148.Tilles AW, Baskaran H, Roy P, et al. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng. 2001;73:379–489. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]
- 149.Kan P, Miyoshi H, Ohshima N. Perfusion of medium with supplemented growth factors changes metabolic activities and cell morphology of hepatocyte-nonparenchymal cell coculture. Tissue Eng. 2004;10:1297–1307. doi: 10.1089/ten.2004.10.1297. [DOI] [PubMed] [Google Scholar]
- 150.Andersson H, van den BA. Microfabrication and microfluidics for tissue engineering: state of the art and future opportunities. Lab Chip. 2004;4:98–103. doi: 10.1039/b314469k. [DOI] [PubMed] [Google Scholar]
- 151.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:S158–S261. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 152.Lindblad WJ, Schuetz EG, Redford KS, Guzelian PS. Hepatocellular phenotype in vitro is influenced by biophysical features of the collagenous substratum. Hepatology. 1991;13:282–328. [PubMed] [Google Scholar]
- 153.Domansky K, Inman W, Owens B, et al. Perfused multiwell tissue culture plates with integrated microfluidic system. MicroTAS. 2006:951–53. [Google Scholar]
- 154. Domansky K, Inman W, Serdy J, et al. Perfused multiwell plate for 3D liver tissue engineering. Submitted Lab Chip. 2009:966–1074. doi: 10.1039/b913221j. •• This paper describes in detail the multiwell 3-D perfused model on which the clearance and toxicity experiments described in this article were performed.
- 155.Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype n vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301–3210. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 156.Yoshida M, Nishikawa Y, Omori Y, et al. involvement of signaling of VEGF and TGF-beta in differentiation of sinusoidal endothelial cells during culture of fetal rat liver cells. Cell Tissue Res. 2007;329:273–382. doi: 10.1007/s00441-007-0387-5. [DOI] [PubMed] [Google Scholar]
- 157.Rodriguez-Antona C, Donato MT, Boobis A, et al. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: molecular mechanisms that determine lower expression in cultured cells. Xenobiotica. 2002;32:505–620. doi: 10.1080/00498250210128675. [DOI] [PubMed] [Google Scholar]
- 158.Gomez-Lechon MJ, Donato MT, Castell JV, Jover R. Human Hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab. 2004;5:443–562. doi: 10.2174/1389200043335414. [DOI] [PubMed] [Google Scholar]
- 159.LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–468. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 160.Reddy A, Heimbach T, Freiwald S, et al. Validation of a semi-automated human hepatocyte assay for the determination and prediction of intrinsic clearance in discovery. J Pharm Biomed Anal. 2005;37:319–426. doi: 10.1016/j.jpba.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 161.Lazerow SK, Abdi MS, Lewis JH. Drug-induced liver disease 2004. Curr Opin Gastroenterol. 2005;21:283–392. doi: 10.1097/01.mog.0000160043.10804.60. [DOI] [PubMed] [Google Scholar]
- 162.Lee WM. Drug-Induced Hepatotoxicity. New Engl J Med. 2003;349:474–585. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 163.Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci. 2007;100:259–466. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 164.Thomas RJ, Bhandari R, Barrett DA, et al. The effect of three-dimensional co-culture of hepatocytes and hepatic stellate cells on key hepatocyte functions in vitro. Cells Tissues Organs. 2005;181:267–79. doi: 10.1159/000091096. [DOI] [PubMed] [Google Scholar]
- 165.Lu HF, Chua KN, Lim WS, et al. Three-dimensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta Biomater. 2005;1:399–410. doi: 10.1016/j.actbio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 166.Chia SM, Lin PC, Yu H. TGF-beta 1 regulation in hepatocyte-NIH3T3 co-culture is important for the enhanced hepatocyte function n 3D microenvironment. Biotechnol Bioeng. 2005;89:565–673. doi: 10.1002/bit.20372. [DOI] [PubMed] [Google Scholar]
- 167.Ohno M, Motojima K, Okano T, Taniguchi A. Up-regulation of drug-metabolizing enzyme genes in layered co-culture of a human liver cell line and endothelial cells. Tissue Eng Part A. 2008;14:1861–1989. doi: 10.1089/ten.tea.2007.0160. [DOI] [PubMed] [Google Scholar]
- 168.Takeshita K, Bowen WC, Michalopoulos GK. Three-dimensional culture of hepatocytes in a continuously flowing medium. In Vitro Cell Dev Biol Anim. 1998;34:482–595. doi: 10.1007/s11626-998-0082-1. [DOI] [PubMed] [Google Scholar]
- 169.Cosgrove BD, Alexopoulos LG, Hasan MA, et al. Synergistic drug-cytokine induction of hepatocellular death as an n vitro approach for the study of inflammation-associated idiosyncratic drug hepatotoxicity. Toxicol Appl Pharmacol. 2009;237(3):317–530. doi: 10.1016/j.taap.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee MY, Kumar RA, Sukumaran S, et al. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc Natl cad Sci USA. 2008;105:59–63. doi: 10.1073/pnas.0708756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee MY, Park CB, Dordick JS, Clark DS. Metabolizing enzyme toxicology assay chip (MetaChip) for high-throughput microscale toxicity analyses. Proc Natl Acad Sci USA. 2005;102:983–1207. doi: 10.1073/pnas.0406755102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cosgrove BD, Griffith LG, Lauffenburger DA. Fusing tissue engineering and systems biology toward fulfilling their promise. Cell Mol Bioeng. 2008;1:33–41. [Google Scholar]
- 173.Elvevold K, Smedsrod B, Martinez I. The liver sinusoidal endothelial cell: cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol. 2008;294:G391–G400. doi: 10.1152/ajpgi.00167.2007. [DOI] [PubMed] [Google Scholar]
- 174.Li C, Kong Y, Wang H, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174–1283. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 175.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 176.Mehta G, Lee J, Cha W, et al. Hard top soft bottom microfluidic devices for cell culture and chemical analysis. Anal Chem. 2009;81:3714–4522. doi: 10.1021/ac802178u. [DOI] [PubMed] [Google Scholar]
- 177.Sung JH, Shuler ML. A micro cell culture analog (microCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip. 2009;9:1385–1524. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]