Abstract
Objective
To investigate the longitudinal performance of a surgically implanted neuroprosthesis for lower extremity exercise, standing, and transfers after spinal cord injury.
Design
Case series.
Setting
Research or outpatient physical therapy departments of four academic hospitals.
Participants
15 subjects with thoracic or low-cervical level spinal cord injuries who had received the 8-channel neuroprosthesis for exercise and standing.
Interventions
After completing rehabilitation with the device, the subjects were discharged to unrestricted home use of the system. A series of assessments were performed before discharge and at a follow-up appointment approximately one year later.
Main Outcome Measure(s)
Neuroprosthesis usage, maximum standing time, body weight support, knee strength, knee fatigue index, electrode stability, and component survivability.
Results
Levels of maximum standing time, body weight support, knee strength, and knee fatigue index were not statistically different from discharge to follow-up (p > 0.05). Additionally, neuroprosthesis usage was consistent with subjects choosing to use the system on approximately half of the days during each monitoring period. Although the number of hours using the neuroprosthesis remained constant, subjects shifted their usage to more functional standing versus more maintenance exercise, suggesting that the subjects incorporated the neuroprosthesis into their lives. Safety and reliability of the system were demonstrated by electrode stability and a high component survivability rate (>90%).
Conclusions
This group of 15 subjects is the largest cohort of implanted lower extremity neurorprosthetic exercise and standing system users. The safety and efficiency data from this group, and acceptance of the neuroprosthesis as demonstrated by continued usage, indicate that future efforts towards commercialization of a similar device may be warranted.
Keywords: Electrical Stimulation, Exercise, Neural Prostheses, Spinal Cord Injuries, Weight-Bearing
There are between 227,000 and 301,000 individuals with spinal cord injuries (SCI) in the United States (US) today1. Focus group studies of individuals with SCI show that being able to stand and walk are important priorities2, 3, along with being more independent and not relying on caregivers and attendants for assistance4. While wheelchairs offer a means of efficient transportation over unobstructed level surfaces, individuals with SCI still need options for negotiating architectural barriers, completing essential transfers, and gaining access to high cabinets, cupboards or shelves that are difficult or impossible to reach from a seated position.
Neuroprostheses employing functional electrical stimulation (FES) provide a means to facilitate these activities. If the neurological damage from SCI is confined to upper motor neurons, then intact peripheral nerves can be excited with small electric currents, causing contractions of the muscle fibers they innervate. By coordinating the actions of a number of muscles to produce useful movements from the otherwise paralyzed limb, FES technology can provide individuals paralyzed by thoracic or low cervical spinal cord injuries with the ability to exercise, stand, and transfer5, 6, 7. Neuroprostheses can allow their users to circumvent environmental barriers and increase the ability to participate in meaningful activities8, 9, 10, 11, facilitate tasks that were previously difficult or impossible from the wheelchair, and improve the health, self-image, and sense of well-being of persons with paralysis 12, 13, 14.
FES applications have been developed to provide standing to individuals with SCI by delivering stimulation with a variety of electrode types. Surface stimulation can provide standing times greater than seven minutes, but systems are typically limited to use only within the laboratory15. A commercially available walking system has been used for at-home standing with four out of eight subjects continuing to use the system occasionally at a mean follow-up interval of 15 months16. To stimulate a larger set of muscles, implanted systems using epimysial, epineural, and epidural electrodes have been developed, although these studies generally have no more than three subjects 17, 18, 19, 20, 21. After 80 sessions of tonic epineural spinal cord stimulation, one subject could start and maintain full weight-bearing standing without manual facilitation, but assistance provided for balance, for a maximum of 4.25 minutes17. Three individuals who received the Praxis multifunctional implantable FES system (Neopraxis Pty. Ltd, Lane Cove, NSW, Australia) achieved maximum standing times of 2, 16, and 34 minutes and could also use the system to perform upright mobility tasks such as a bathroom transfer18.
Implanted systems have shown repeatable and consistent stimulated responses, including less than 10% variation of neural electrode capacitance at five years post-op in one subject19 and 15 out of 20 epineural electrodes producing observable threshold and maximal muscle contractions with joint movements in one subject at 23 months post-op20. Similarly, stable functional results and stimulated muscle strength were found over three years in one pediatric recipient of an implanted lower extremity neuroprosthesis21.
Our research group previously reported9 a snap-shot of the acute performance of an implanted 8-channel neuroprosthesis utilizing epimysial and intramuscular electrodes for standing and exercise after SCI. At the time of that preliminary report, eight subjects had completed the exercise portion of the rehabilitation protocol and could generate an average of 35 Nm of knee extension moment, which is necessary for a person of average stature to stand22. Seven of these subjects had advanced to the standing training and rehabilitation portion of the protocol, and five were able to stand with enough body weight through their legs to release one hand to grab an object in the environment. Prior studies have not addressed how the subjects used their systems at home or how the systems performed across time.
The purpose of this study was to determine the longitudinal performance of the implanted lower extremity neuroprosthesis for standing after SCI through repeated assessments of 15 subjects at two points in the study protocol: when subjects were discharged from rehabilitative training after learning to use the system and when they returned to the lab for their first follow-up evaluation after approximately one year of use at home. This summary documents the long-term performance, safety, stability, and usage of the implanted standing system and represents the largest study of such a system to date with a minimum follow-up of one year of home use.
Methods
System components
A schematic and photos of the internal and external components of the neuroprosthesis are shown in the Figure 1. Intramuscular (IM) electrodes were implanted bilaterally at the L1-L2 spinal roots to activate erector spinae for trunk extension23. Epimysial (EP) electrodes were sutured near the motor points on the surfaces of bilateral vastus lateralis for knee extension and gluteus maximus and semimembranosus for hip extension24, 25. These electrodes were connected to a surgically implanted pulse generator (eight-channel implantable receiver-stimulator, or IRS-8), which was sutured to the abdominal wall26. The IRS-8 delivered constant current, charge balanced biphasic pulses with independently controlled amplitude (2, 8, 14, 20 mA), pulse duration (0-200 μs), and frequency (1-50 Hz) set on a channel-by-channel basis.
Figure 1.
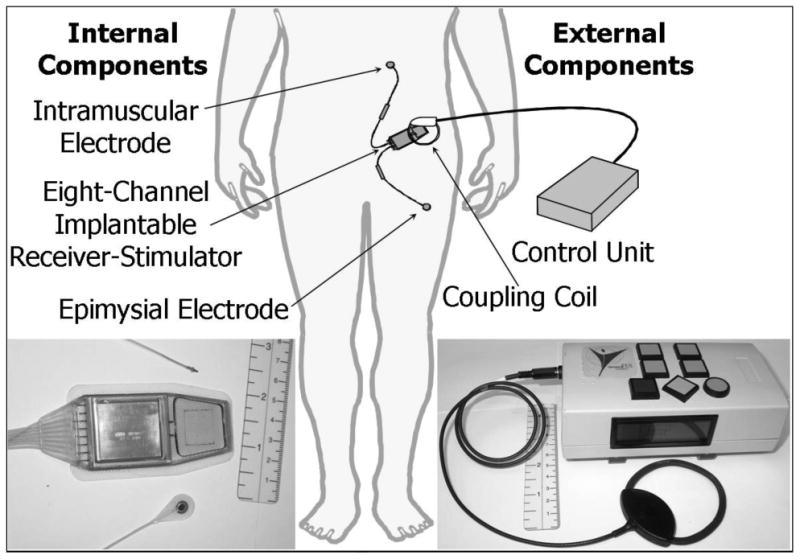
A schematic of the standing neuroprosthesis with photos of both the internal and external components.
Implanted components were produced at Case Western Reserve University (Cleveland OH), provided by NeuroControl Corporation (Cleveland OH), or obtained from Ardiem Medical Inc. (Indiana PA), a contract manufacturer. These implanted components were identical to those utilized by the Freehand System® (NeuroControl Corporation, Cleveland OH) for hand grasp after tetraplegia27. Application to standing after paraplegia represents an off-label use of the technology that was regulated by a separate investigational device exemption from the US Food and Drug Administration (FDA) for this purpose. All study procedures were approved by the institutional review boards of the collaborating centers: Louis Stokes Cleveland Department of Veterans Affairs Medical Center (Cleveland OH), MetroHealth Medical Center (Cleveland OH), Veterans Affairs Ann Arbor Healthcare System (Ann Arbor MI), University of Michigan (Ann Arbor MI), University of Kentucky Medical Center (Lexington KY), and Albany Medical Center (Albany NY).
Patterns of stimulation to produce lower extremity exercise and standing were programmed into an external control unit (ECU) that delivered both power and commands to the IRS-8 through an inductive link with an external coil taped to the skin28. Neuroprosthesis users could scroll between pre-programmed stimulation patterns using the buttons on the ECU enclosure. The stimulation programs included strengthening (30 Hz) and endurance building (16 Hz) exercises along with a pattern for standing. When a user wanted to stand with the neuroprosthesis, he or she first prepared by donning ankle foot orthoses (AFOs) to brace the ankles, as stimulation was not delivered to the muscles supporting those joints. Once the coil was in place, the user would scroll to the “stand” pattern in the ECU. After hitting “go”, the user would hold onto a walker and position his or her body in preparation for the sit-to-stand transition. After a 3 second delay, the ECU would give an audio cue (beep) to signal the start of the ramp of increasing stimulation and the user would push up with his or her arms to assist the body to move into the standing position. Another beep would sound when the stimulation achieved the pre-programmed level for standing. When the user was ready to return to sitting, the same procedure was followed with stimulation ramping down to zero.
Subject pool
Inclusion criteria for the study are summarized in Table 1. Study participants received the implanted standing neuroprosthesis between 1996 and 2007 in studies supported by the US Department of Veterans Affairs and the Office of Orphan Product Development of the US FDA. In a conservative effort to protect the implanted components and allow encapsulation tissue time to form, subjects were instructed during a six-week post-operative recovery period not to engage in strenuous activities, to limit themselves to four transfers per day, and not to bend more than 90 degrees forward in their chairs for personal care or to retrieve items from the floor.
Table 1.
Inclusion criteria for subjects to receive the standing neuroprosthesis.
|
American Spinal Injury Association (ASIA)
Before initiating both the exercise and standing training phases of the protocol, a “profile” of each electrode was performed which consisted of determining the threshold (minimum pulse width that caused a visible muscle contraction) and saturation (pulse width above which muscle strength did not increase or an undesirable movement or sensation occurred) for each muscle. These values were used as the low and high point of the stimulation ramps in the exercise or standing patterns. Sometimes a pulsewidth value different than the profiled saturation value was used during standing to make sure that the responses from the multiple muscle groups were balanced and did not cause any undesired reflexes.
After the recovery period, neuroprosthesis recipients underwent a standardized eight-week reconditioning program of lower extremity strength- and endurance-building exercise with the implanted stimulation system29. Strength training consisted of three sets of 10 repetitions of progressive resistance knee extension while sitting. Individual contractions within each set were held for 11 seconds separated by 16 seconds of rest, and additional rest periods of five minutes between consecutive sets. Resistance provided by adjustable ankle weights was increased every two weeks to the maximum load that could be applied and still complete all 30 repetitions. Endurance training was performed in the supine position and consisted of contractions of all the muscles used for standing. According to subject tolerance the duty cycle of stimulation and the total exercise time were increased every two weeks to a maximum of 26 seconds ON, 10 seconds OFF, and 120 minutes, respectively. Subjects were instructed to perform strength and endurance exercises on at least six days per week to prepare the paralyzed muscles for standing.
After the eight weeks of reconditioning exercise, subjects began rehabilitation and balance/transfer training to learn to safely operate the system and stand with the neuroprosthesis during specialized research physical therapy sessions multiple times per week. Therapy sessions began with standing in a standing frame with stimulation to ensure that muscle contractions were strong enough support body weight, and to build tolerance to the upright position in order to avoid orthostatic hypotension, and progressed to assisted standing in parallel bars and finally to independent standing in a rolling walker. Subjects were instructed on how to recognize the signs of fatigue and monitor their own standing times for safety and to minimize the risk of falls. This training also included safe operation of the ECU, understanding the audio cues, proper sit-to-stand and stand-to-sit maneuvers, balanced quiet standing, and pivot transfers utilizing stimulation. Strength and endurance exercise was continued throughout the rehabilitation and training period.
Rehabilitation and standing balance/transfer training was nominally 12 weeks, but was individualized for each subject according to their progress and personal circumstances such as travel, work schedule, etc. After achieving safe and independent standing using a walker as a support device, as well as independent sit-to-stand and stand-to-sit transitions, subjects were discharged from rehabilitative training to have unrestricted home use of the system. To maintain muscle strength and endurance, a home exercise program that was similar in the number of repetitions and duty cycle timing to the last phase of the reconditioning program was also provided. A series of data collection assessments, described below, were completed at discharge and at follow-up assessment of their status approximately one year later.
The primary study team in Cleveland OH assembled, equipped, and trained collaborating teams in Lexington KY, Albany NY, and Ann Arbor MI to recruit, implant, and rehabilitate subjects at their own facilities. All sites succeeded in implementing the enrollment, exercise, standing/transfer training, and assessment of basic system performance. One implant surgery was performed outside of Cleveland (Ann Arbor MI), and due to availability of certain specialized laboratory equipment (force plates, dynamometers, instrumented parallel bars, etc.), some centers were not able to perform certain outcome assessments.
Dynamometry
Knee extensor strength and fatigue resistance were measured using a Biodex Pro System 3 dynamometer (Shirley NY) at both the discharge and follow-up intervals. While sitting erect, the neuroprosthesis extended the subject's knee from approximately 90 degrees of flexion to full extension. The stimulus pulse duration and the current amplitude were the same as used for typical standing and exercise. Twelve repetitions per leg were performed for the strength testing at a dynamometer speed of 30 deg/s, stimulation frequency of 30 Hz, and duty cycle of 3 s on and 15 s off. The peaks of each of the twelve trials were averaged to calculate the knee strength for each subject. To determine the resistance to fatigue, 40 minutes of cyclic knee extension contractions were performed with a dynamometer speed of 60 deg/s, stimulation frequency of 16 Hz, and duty cycle of 1 s on and 3 s off. The knee fatigue index was defined as the ratio of the average of the peaks of the last 3 repetitions to the average of the peaks of the first 3 repetitions.
Neuroprosthesis Usage
The ECU recorded the time, date, and duration each standing and exercise pattern were used, which allowed tracking neuroprosthesis usage across time. Subjects were aware that usage was continuously being recorded, but they did not know the time intervals that would be examined. The number of different days the system was used, as well as the total number of hours of usage, was calculated for the 28 day period following discharge to home use with the neuroprosthesis and for the 28 days preceding the follow-up visit. To understand how subjects choose to utilize the neuroprosthesis at home, the number of hours standing and the number of hours exercising were summarized and examined separately.
Maximum Standing Time
As part of both the discharge and follow-up evaluations, subjects stood for as long as they could using the implanted neuroprosthesis to contract knee, hip, and trunk extensors and using the upper extremities only as needed for balance. The maximum standing times were recorded, as were the reasons for ending the test and sitting, such as loss of sufficient knee extension with fatigue, hip extension weakness with fatigue that caused the pelvis and torso to lean forward and exert too much pressure on the arms, or simply the subjective request for a break. The maximum stand test was usually terminated by the subject, as a key part of the training was self-identifying signs of fatigue and the need to sit. The test would only be terminated by the research physical therapist if necessary for subject safety.
Body Weight Distribution
At both the discharge and follow-up intervals, subjects stood on forceplates (Advanced Mechanical Technology, Inc., Watertown MA) with support and balance provided as needed by holding parallel bars instrumented with 3-D load cells (JR3, Inc., Woodland CA). The amount of body weight supported by both the lower and upper extremities was calculated over approximately 30 s of quiet standing. The percentage of body weight supported by the lower extremities was calculated by dividing the weight on the force plates by the sum of the weight on the force plates and the parallel bars.
Electrode Stability
The procedure for profiling and characterizing the contractile properties of each muscle-electrode combination utilized to construct the stimulation patterns for exercise and functional standing was repeated for each electrode in each subject at both the discharge and follow-up intervals. The smallest stimulus pulse duration capable of eliciting a contraction (i.e., the stimulus “threshold”) was determined. Consistency in this “threshold” pulse duration over time was used as an indicator of electrode stability.
Component Survivability
The integrity and functionality of the implanted devices were recorded over time. Failure dates and frequencies were used to summarize and predict the probability of component survival.
Results
Subject pool
Characteristics of the study cohort are listed in Table 2 below. A total of 15 subjects received the implanted standing system and completed the discharge and follow-up assessments. Most of the study cohort (14 subjects) exhibited injuries between C6 and T9: nine were motor and sensory complete (American Spinal Injury Association (ASIA) A), four were motor complete and sensory incomplete (ASIA B), and one was motor and sensory incomplete (ASIA C). The one remaining subject had a mid-cervical complete (C5, ASIA A) injury. The implant recipients were predominantly male (14) and averaged 175.73 ± 8.56 cm in height (160 – 188 cm) and 77.47 ± 16.67 kg in weight (49.9 – 113.4 kg). Time post-injury at implantation ranged from 13 – 202 months (72.60 ± 71.87 months).
Table 2.
Demographics and data collected for the subject pool.
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | F | M | M | M | M | M | M | M | M | M | M |
| Injury Level | C6 | T4 | T9 | T8 | C7 | T6 | C5 | T5 | T8 | T4 | T5/6 | C7 | T6 | T4 | C6 |
| ASIA Class | C | A | A | A | B | A | A | B | A | A | A | B | B | A | A |
| Height (cm) | 183 | 188 | 165 | 163 | 168 | 173 | 175 | 175 | 185 | 175 | 180 | 180 | 183 | 183 | 160 |
| Weight (kg) | 81.6 | 113.4 | 49.9 | 76.2 | 56.7 | 86.2 | 68.0 | 89.8 | 99.8 | 62.6 | 79.4 | 73.5 | 80.3 | 83.9 | 60.7 |
| Months Post Injury at Time of Implant | 83 | 46 | 27 | 33 | 20 | 15 | 106 | 202 | 13 | 200 | 197 | 22 | 18 | 88 | 19 |
| Implant Site * | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | AA |
| Rehab Center * | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Cle | Lex | Lex | Alb | Alb | AA | AA |
| Strength | x | x | x | x | x | x | x | x | |||||||
| Fatigue | x | x | x | x | x | x | x | ||||||||
| Usage | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Standing Time | x | x | x | x | x | x | x | x | x | x | x | ||||
| % Body Weight | x | x | x | x | x | x | x | ||||||||
| Stability | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Survival | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
Cle = Cleveland OH; Lex = Lexington KY; Alb = Albany NY; AA = Ann Arbor MI
The follow-up assessment ranged from 6 – 56 months post discharge (mean = 15.87 ± 11.69 months, median = 14 months). Removing the one subject with the delayed follow-up visit of 56 months, the range was 6 – 19 months (mean = 13.00 ± 3.78 months, median = 13.5 months). The subjects were 16 – 65 months post implant at the follow-up visit (mean = 28.93 ± 12.46 months, median = 26 months). Without the one subject with the delayed follow-up visit of 56 months, the range was 16 – 41 months (mean = 26.36 ± 7.75 months, median = 24.5 months).
All assessments were not completed for all subjects. Some assessments were added after the three initial subjects had already completed the terms of participation in the original study protocol. Some assessments were not collected at some collaborating centers, which affected data for three to five subjects. Some assessments were inappropriate for individuals with upper extremity weakness and therefore could not be performed on the one subject with a higher level (C5) injury.
Dynamometry
At both the discharge and follow-up intervals, strength data were collected from eight subjects and fatigue data from seven subjects. Mean times from discharge to follow-up were 19.13 ± 15.07 months and 19.86 ± 16.12 months for strength and fatigue data, respectively. Strength and endurance established during reconditioning exercise with the implanted system were largely maintained during the follow-up period as illustrated in Figure 2. The mean isokinetic knee extension strength was 35.18 ± 13.75 Nm at discharge and did not significantly change at follow-up to a value of 31.75 ± 11.68 Nm (p = 0.39, Figure 2a). The knee fatigue index was 0.69 ± 0.20 at discharge and showed no significant change at follow-up with a value of 0.63 ± 0.18 (p = 0.25, Figure 2b).
Figure 2.
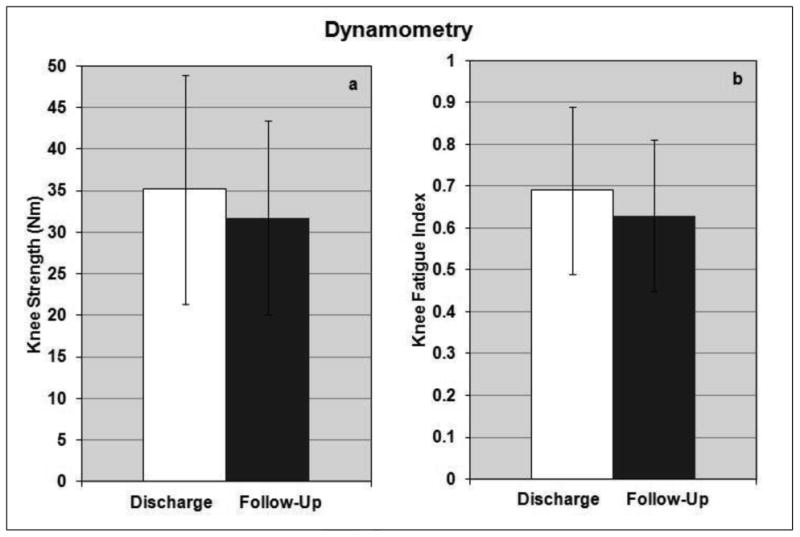
The levels of knee extension strength (a) and fatigue resistance (b) that were established from the reconditioning exercise program and standing training with the neuroprosthesis were maintained at the follow-up visit.
Neuroprosthesis Usage
Usage data were collected from 12 subjects at the two study intervals. Patterns of use changed over the 17.33 ± 12.64 months post-discharge, but total amount of time using the system remained consistent over time. System recipients spent more total time exercising than standing. Recipients used the neuroprosthesis on 12.75 days of the 28 day data monitoring period following discharge for a total time of 12.03 hours, comprised of 0.66 hours standing and 11.37 hours exercising (Table 3). Preceding follow-up examination, the same subjects used the neuroprosthesis for essentially the same amount of time: 12.67 out of 28 days for 11.37 hours. Although the total time using the neuroprosthesis remained nearly constant, the proportion of time standing increased and the proportion of time exercising decreased, reflected by 2.25 hours standing and 9.12 hours exercising (Table 4).
Table 3.
Neuroprosthesis usage for 12 subjects recorded by the ECU over a 28 day period following discharge to home use of the system.
| Neuroprosthesis Usage at Discharge | ||||
|---|---|---|---|---|
| Subject | Total Days | Total Hours | Hours Standing | Hours Exercising |
| 4 | 10 | 1.48 | 1.32 | 0.17 |
| 5 | 21 | 60.05 | 0.00 | 60.05 |
| 6 | 17 | 7.16 | 1.56 | 5.60 |
| 7 | 27 | 6.55 | 0.30 | 6.24 |
| 8 | 1 | 0.10 | 0.04 | 0.06 |
| 9 | 6 | 2.48 | 0.23 | 2.25 |
| 10 | 17 | 10.82 | 1.07 | 9.74 |
| 11 | 4 | 0.31 | 0.31 | 0.00 |
| 12 | 13 | 9.68 | 2.25 | 7.44 |
| 13 | 3 | 0.79 | 0.14 | 0.65 |
| 14 | 24 | 34.43 | 0.75 | 33.68 |
| 15 | 10 | 10.55 | 0.00 | 10.55 |
| Mean ± SD | 12.75 ± 8.55 | 12.03 ± 17.78 | 0.66 ± 0.73 | 11.37 ± 17.90 |
Table 4.
Neuroprosthesis usage for 12 subjects recorded by the ECU over a 28 day period prior to the one year follow-up assessment.
| Neuroprosthesis Usage at Follow-Up | ||||
|---|---|---|---|---|
| Subject | Total Days | Total Hours | Hours Standing | Hours Exercising |
| 4 | 22 | 7.12 | 2.84 | 4.27 |
| 5 | 26 | 48.58 | 18.50 | 30.08 |
| 6 | 11 | 10.74 | 0.13 | 10.61 |
| 7 | 12 | 0.06 | 0.06 | 0.00 |
| 8 | 3 | 1.56 | 0.03 | 1.54 |
| 9 | 0 | 0.00 | 0.00 | 0.00 |
| 10 | 6 | 0.71 | 0.71 | 0.00 |
| 11 | 9 | 2.06 | 0.66 | 1.40 |
| 12 | 9 | 4.72 | 0.89 | 3.82 |
| 13 | 9 | 8.58 | 0.43 | 8.14 |
| 14 | 26 | 28.73 | 2.16 | 26.57 |
| 15 | 19 | 23.56 | 0.61 | 22.95 |
| Mean ± SD | 12.67 ± 8.66 | 11.37 ± 14.95 | 2.25 ± 5.19 | 9.12 ± 11.10 |
Maximum Standing Time
On average, as shown in Figure 3, the maximum standing times for 11 subjects decreased over the 17.18 ± 13.25 months between the discharge and follow-up evaluation intervals from 27.4 ± 38.6 minutes to 15.6 ± 21.8 minutes, but the change was not statistically significant (p = 0.12). Because mean standing times could have been skewed by the three high performing subjects who were able to stand for more than 30 minutes, median values were also computed at discharge and follow-up. The median values showed the same trend as the mean data, decreasing from 4.33 minutes at discharge to 3.33 minutes at follow-up.
Figure 3.
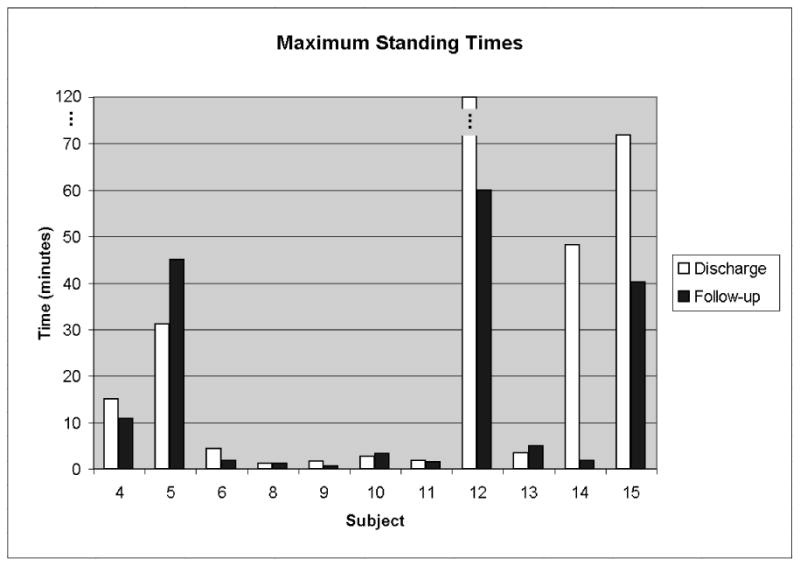
Maximum standing times remained relatively constant or decreased slightly when comparing the follow-up value to that at discharge.
Six subjects were able to stand between one and five minutes, which still allowed sufficient time to perform a standing-pivot transfer, the primary task for which this system was originally designed. Standing time increased (> 120%) between the discharge and follow-up intervals for three subjects, remained the same (80-120%) for two subjects, and decreased (< 80%) for six subjects.
Body Weight Distribution
The percentage of total body weight supported by the lower extremities for each subject is shown for the discharge and follow-up evaluation intervals in Figure 4. Seven subjects had body weight distribution measurements at follow-up (15 ± 2 months post discharge), however only three had paired measurements at discharge. The portion of body weight supported by the lower extremities decreased between the discharge and follow-up evaluation intervals from 90.0 ± 13.1% to 76.7 ± 22.2%, but the change was not statistically significant (p = 0.16). Body weight was sufficiently supported by the lower extremities for all subjects to be able to release one hand from their support device to reach above shoulder height and manipulate objects in the environment.
Figure 4.
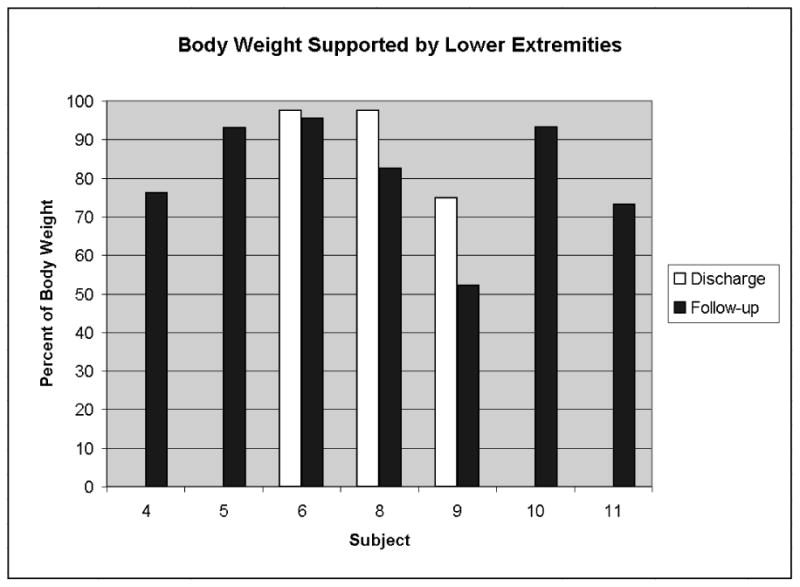
Body weight distribution data was collected for seven subjects at follow-up, but only three subjects had paired data at discharge. A trend of decreased body weight supported by the lower extremities was seen, but was not statistically significant from the discharge values.
Electrode Stability
Electrodes from all 15 subjects were examined for stability. For the 74 epimysial and 35 intramuscular electrodes implanted, the percentages of electrodes that exhibited changes in threshold pulse duration of less than 10 μs, 10-50 μs, and greater than 50 μs were tabulated for the 28.93 ± 12.46 month period between the discharge and follow-up evaluations. As shown in Figure 5, the majority (approximately 90%) of electrodes of both types were stable over time because they had small threshold changes (<10 μs, representing less than 5% of the available pulse width range) between the two intervals studied. The one epimysial electrode that exhibited a threshold change of over 50 μs was likely in the early stages of failing due to mechanical stresses that may have separated the platinum stimulating disk from its surrounding silicone elastomer skirt and leadwire17.
Figure 5.
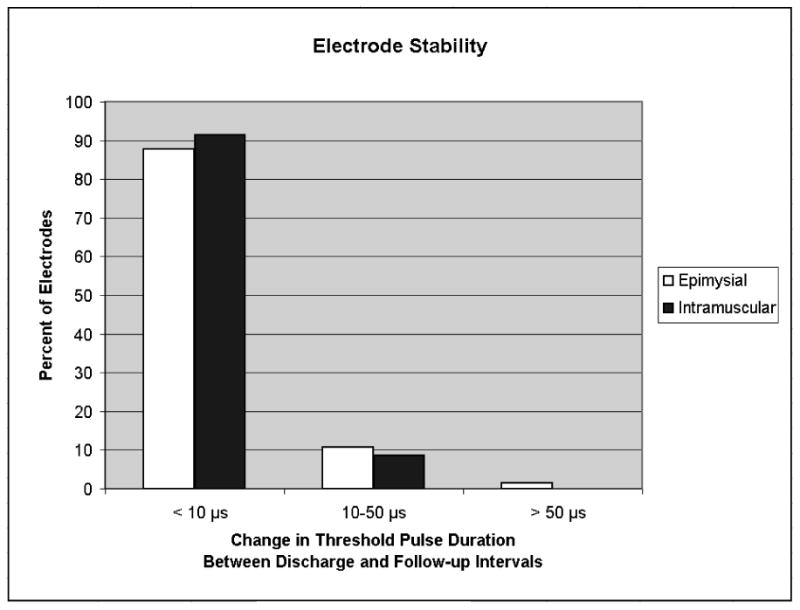
The majority of both epimysial and intramuscular electrodes were stable over time as supported by small threshold changes (<10 μs) between the two intervals studied.
Component Survivability
Table 5 summarizes the survival of the implanted components in all 15 subjects over the 28.93 ± 12.46 month follow-up period and tabulates the number of IRS-8s, IM electrodes, and EP electrodes implanted, the number still operational at each subject's discharge evaluation, and the number still operational at each subject's follow-up evaluation. In general, the implanted components exhibited a high rate of survival from implantation to discharge (93.8%, 97.2%, and 95.1% for IRS-8s, IMs, and EPs, respectively). Survival rates between discharge and follow-up intervals were relatively constant indicating that the devices had stabilized sufficiently to endure unsupervised home and community use and may have been maximally stressed during the rigorous exercise and rehabilitation program. Overall survival rates calculated by comparing initial quantities with those still functioning at follow-up were in excess of 90% for all components.
Table 5.
Number and percentage of devices operational at discharge and follow-up.
| SURVIVABILITY | |||
|---|---|---|---|
| Number of devices | IRS-8s | IMs | EPs |
| At implant | 16 | 36 | 82 |
| Still operational at discharge | 15 (93.8 %) | 35 (97.2%) | 78 (95.1%) |
| Still operational at follow-up | 15 (93.8 %) | 35 (97.2%) | 74 (90.2%) |
IRS-8 = eight-channel implanted receiver-stimulator, IM = intramuscular electrode, EP = epimysial electrode
In addition to considering longevity with respect to study intervals, the probability of survival was calculated with respect to calendar months from the date of implantation using the Kaplan-Meier method, as illustrated in Figure 6. Exponential fits indicate the statistical probability of a device remaining operational as a function of time. According to estimates based on the relatively small sample size available for analysis, the probability of device survival at 30 months post-surgery is 93.0% for IRS-8s, 97.1% for IM electrodes, and 90.4% for EP electrodes. Additional follow-up studies of these devices would allow extending these plots for a longer timespan.
Figure 6.
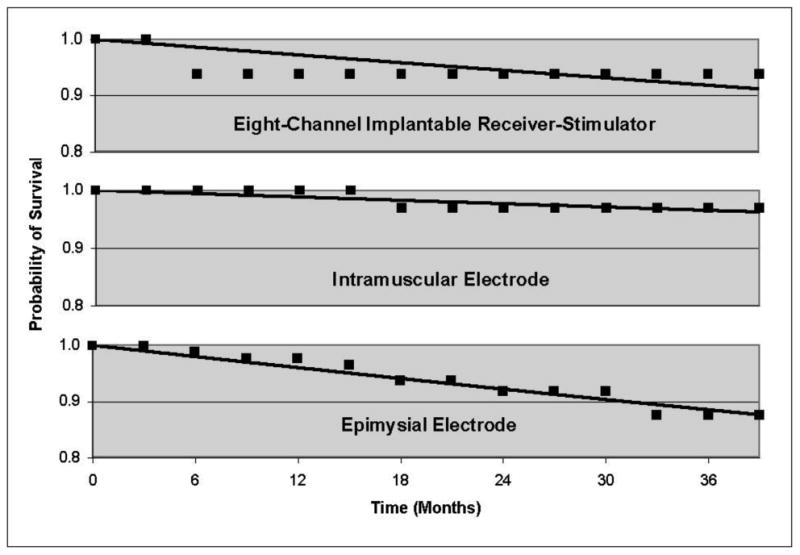
Kaplan-Meyer estimates of probability of device survival. At 30 months post surgery probability of survival was 93.0% for IRS-8s, 97.1% for IM electrodes, and 90.4% for EP electrodes.
Discussion
Maximum standing time, body weight support, knee strength, and knee fatigue index all showed no significant change from discharge to follow-up. This indicated that subjects utilized the neuroprosthesis sufficiently at home to maintain performance levels achieved during the intensive rehabilitative training period that preceded discharge.
The subject with the C5 injury (Subject 7) was a special case and IRB approval was obtained for this exception. He received the standing system to relieve his caregiver of the heavy lifting involved with a maximum assist standing-pivot transfer. The system provided the “lifting” from sitting to standing and the caregiver only had to reposition his body to complete the pivot portion of the transfer. This subject could only use the system for exercise and assisted transfers and not independent standing.
Even though the median maximum standing time decreased from 4.33 minutes at discharge to 3.33 minutes at follow-up, the system still provided ample time for all subjects to perform a standing pivot transfer, which was the primary objective of the study. Since Subject 7 had limited upper extremity function due to his C5 injury, he needed an assistant to accomplish the task. All of the other 14 subjects, even those with the shortest maximum standing time, were independent with the standing pivot transfer using a walker for stability and balance.
A possible explanation for the decreasing trend in maximum standing time between the studied intervals is that the subjects were not as focused on isolated long duration stands on their own as they may have been during training. Many subjects anecdotally reported using the neuroprosthesis at home for shorter duration, more frequent standing to assist with activities of daily living. A specific example can be seen for subject 14, who had a noticeable decrease in maximum standing time at follow-up of 1.78 minutes from 48.25 minutes at discharge even though his time spent using the neuroprosthesis for standing at home actually increased from 0.75 hours at discharge to 2.16 hours at follow-up. It is reasonable to hypothesize that he valued using the neuroprosthesis for short duration stands and was simply no longer focused on maximum standing time without frequent visits to the laboratory and research staff encouragement to do so.
Additional examination of the total hours of neuroprosthesis usage between discharge and follow-up shows that usage increased (> 120%) for six subjects, remained the same (80-120%) for two subjects, and decreased (< 80%) for three subjects. During the same interval, maximum standing time increased (> 120%) for three subjects, remained the same (80-120%) for two subjects, and decreased (< 80%) for six subjects, demonstrating that there was not a direct correlation between usage and maximum standing time.
The level of neuroprosthesis usage was maintained from discharge to follow-up with subjects choosing to use the system on approximately half of the days during each monitoring period. Although the number of hours using the neuroprosthesis remained constant, subjects shifted their usage to more functional standing versus more maintenance exercise, which could be interpreted to mean that the subjects incorporated the neuroprosthesis into their lives.
The minimal change in electrode thresholds between the study intervals indicates that the components remained in good working order, did not move away from the nerves they were activating and did not develop scar tissue or other obstacles to exciting their target muscles. All components of the system exhibited low failure rates, although additional follow-up studies would strengthen this assertion. The modular design of the implanted components has allowed for successful replacement of failed IRS-8s and both intramuscular and epimysial electrodes. The selection of a particular electrode configuration would therefore depend on many other factors including implant ease, access to the motor point, and anatomical configuration of the muscle/nerve. The slightly higher failure rate of the epimysial electrode, as compared with the intramuscular electrode, especially coupled with the facts that both electrodes were similarly stable and that the surgical implantation of the epimysial is more invasive and lengthy, may suggest that future surgical implementation of such neuroprostheses favor use of the intramuscular design.
Study Limitations
One limitation of this study is the incomplete data set from all 15 volunteers. Due to availability of certain specialized laboratory equipment (force plates, dynamometers, instrumented parallel bars, etc.), certain satellite centers were not able to perform all of the outcome assessments that could be performed in Cleveland. Additionally, some assessments were added after the three initial subjects had already completed the terms of participation in the original study protocol, and some were inappropriate for individuals with upper extremity weakness and therefore could not be performed on the one subject with a higher level (C5) injury. This led to a different number of subjects with paired discharge and follow-up data for each of the different assessments, but with the exception of body weight distribution data, all other assessments had complete data sets for at least seven subjects. Electrode stability and component survivability data were available for all 15 subjects, allowing us to report on the largest cohort of implanted neuroprosthetic systems for exercise and standing to date.
Future studies should continue to investigate how neuroprosthesis users choose to utilize their systems at longer follow-up intervals of three to five years as statistical confidence would improve with additional subjects and complete data sets. However, this longitudinal study demonstrates that the implanted components are robust and function is maintained after at least one year of home use with the system. An implanted standing neuroprosthesis is a viable clinical addition to traditional means of mobility and provides a way to activate paralyzed muscles for exercise and functional tasks to increase independence for persons with SCI.
Conclusion
Implanted neuroprostheses for standing after SCI can be reliable and measures of technical and clinical performance of the systems are consistent over time. Maximum standing time, body weight support, knee strength, and knee fatigue index all demonstrated no significant change from discharge to follow-up (p > 0.05); the technical performance of the neuroprosthesis was constant over the study interval of approximately one year post discharge to home use with the system. Safety and reliability of the system were demonstrated by electrode stability and a high component survivability rate (>90%) for this group of 15 subjects, which is the largest cohort of implanted lower extremity neurorprosthetic exercise and standing systems.
Patterns of system usage changed over time, but neuroprothesis recipients continued regular use of the devices. The level of neuroprosthesis usage was maintained from discharge to follow-up with subjects choosing to use the system on approximately half of the days surveyed during each monitoring period. Although the number of hours using the neuroprosthesis remained constant (> 12 hours over the 28 day monitoring period), subjects shifted their usage to more short duration functional standing versus more prolonged endurance building and strength maintenance exercise, which could be interpreted to mean that the subjects incorporated the neuroprosthesis into their lives and daily routines.
The long-term technical and clinical performance of the systems, and the apparent acceptance of the neuroprosthesis as demonstrated by its continued usage, indicate that future efforts towards commercialization or wider spread distribution of a similar device would be justified.
Acknowledgments
The authors would like to thank the investigators and staff at the satellite centers for their contributions towards the study (affiliations are listed as at the time the study was conducted): Darryl J. DiRisio, Albany Medical Center (Albany NY); Jason P. Gagnon, Albany Medical Center (Albany NY); Julie A. Mannlein, University of Michigan (Ann Arbor MI); Susan McDowell, University of Kentucky (Lexington KY); Nancy E. Quick, University of Kentucky (Lexington KY); JoAnne Riess Resig, University of Kentucky (Lexington KY); Gianna M. Rodriguez, University of Michigan (Ann Arbor MI); Melissa L. Wright, University of Michigan (Ann Arbor MI).
This study was supported by grants from the Rehabilitation Research & Development Service of the US Department of Veterans Affairs (EW66CA, B3155R), the Office of Orphan Product Development of the US Food and Drug Administration (FD-R-001244) and the New York State Department of Health (C-08616). This publication was also made possible in part by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
We (the authors) certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript (see above paragraph).
Abbreviations
- ASIA
American Spinal Injury Association
- AFO
ankle foot orthosis
- IRS-8
eight-channel implantable receiver-stimulator
- EP
epimysial
- ECU
external control unit
- FDA
Food and Drug Administration
- FES
functional electrical stimulation
- IM
intramuscular
- SCI
spinal cord injury
- US
United States
Footnotes
Preliminary data (without follow-up data) on first seven subjects were reported at the Annual Meeting of the American Spinal Cord Injury Society, Chicago IL, April 2000. John A. Davis, Jr. DDS, MD: presenter and first author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. 2008 Jan; doi: 10.1179/1079026813Z.000000000136. www.spinalcord.uab.edu. [DOI] [PMC free article] [PubMed]
- 2.Brown-Triolo DL, Roach MJ, Nelson K, Triolo RJ. Consumer perspectives on mobility: implications for neuroprosthesis design. JRRD. 2002;39(6):659–670. [PubMed] [Google Scholar]
- 3.Brown-Triolo DL, Triolo RJ, Roach MJ, Nelson K, Peckham PH. Mobility issues in paraplegia. J Spinal Cord Med. 1999;22(1):29–30. [Google Scholar]
- 4.Kilgore KL, Scherer M, Bobblitt R, Dettloff J, Dombrowski DM, Godbold N, Jatich JW, Morris R, Penko JS, Schremp ES, Cash LA. Neuroprosthesis consumers' forum: consumer priorities for research directions. JRRD. 2001;38(6):655–660. [PubMed] [Google Scholar]
- 5.Jaeger RJ. Lower extremity applications of functional neuromuscular stimulation. Assist Tech. 1992;4(1):19–30. doi: 10.1080/10400435.1992.10132189. [DOI] [PubMed] [Google Scholar]
- 6.Marsolais EB, Kobetic R. Functional electrical stimulation for walking in paraplegia. J Bone Joint Surg (Am) 1987;69A:728–733. [PubMed] [Google Scholar]
- 7.Marsolais EB, Kobetic R, Chizeck HJ, Jacobs JL. Orthoses and electrical stimulation for walking in complete paraplegia. J Neuro Rehab. 1991;5(1-2):13–22. [Google Scholar]
- 8.Triolo RJ, Bogie K. Lower extremity applications of functional neuromuscular stimulation after spinal cord injury. Topics in SCI Rehab. 1999;5(1):44–65. [Google Scholar]
- 9.Davis JA, Triolo RJ, Uhlir J, Bieri C, Rohde L, Lissy D, Kukke S. Preliminary performance of a surgically implanted neuroprosthesis for standing and transfers – Where do we stand? JRRD. 2001;38(6):609–617. [PubMed] [Google Scholar]
- 10.Grill WM, Kirsch RF. Neuroprosthetic applications of electrical stimulation. Assist Tech. 2000;12:6–20. doi: 10.1080/10400435.2000.10132006. [DOI] [PubMed] [Google Scholar]
- 11.Quintern J. Application of functional electrical stimulation in paraplegic patients. Neurorehab. 1998;10:205–250. [Google Scholar]
- 12.Agarwal S, Triolo RJ, Kobetic R, Miller M, Bieri C, Kukke S, Rohde L, Davis JA. Long-term user perceptions of an implanted neuroprosthesis for exercise, standing and transfers after spinal cord injury. JRRD. 2003;40(3):241–252. [PubMed] [Google Scholar]
- 13.Guest RS, Klose KJ, Needham-Shropshire BM, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: Part 4. Effect on physical self-concept and depression. Arch Phys Med Rehab. 1997;78:804–807. doi: 10.1016/s0003-9993(97)90191-x. [DOI] [PubMed] [Google Scholar]
- 14.Kralj A, Bajd T. Functional Electrical Stimulation: Standing and Walking after Spinal Cord Injury. Boca Raton, FL: CRC Press Inc.; 1989. pp. 110–122. [Google Scholar]
- 15.Braz GP, Russold M, Smith RM, Davis GM. Efficacy and stability performance of traditional versus motion sensor-assisted strategies for FES standing. J Biomech. 2009;42(9):1332–8. doi: 10.1016/j.jbiomech.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Gallien P, Brissot R, Eyssette M, Tell L, Barat M, Wiart L, Petit H. Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia. 1995;33:660–4. doi: 10.1038/sc.1995.138. [DOI] [PubMed] [Google Scholar]
- 17.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epineural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–47. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston TE, Betz RR, Smith BT, Benda BJ, Mulcahey MJ, Davis R, Houdayer TP, Pontari MA, Barriskill A, Creasey GH. Implantable FES system for upright mobility and bladder and bowl function for individuals with spinal cord injury. Spinal Cord. 2005;43:713–23. doi: 10.1038/sj.sc.3101797. [DOI] [PubMed] [Google Scholar]
- 19.Guiraud D, Stieglitz T, Koch KP, Divoux JL, Rabischong P. An implantable neuroprosthesis for standing and walking in paraplegia: 5-year patient follow-up. J Neural Eng. 2006;3(4):268–75. doi: 10.1088/1741-2560/3/4/003. [DOI] [PubMed] [Google Scholar]
- 20.Davis R, MacFarland WC, Emmons SE. Initial results of the Nucleus FES-22 implanted system for limb movement in paraplegia. Stereotact Funct Neurosurg. 1994;63(1-4):192–7. doi: 10.1159/000100314. [DOI] [PubMed] [Google Scholar]
- 21.Betz RR, Johnston TE, Smith BT, Mulcahey MJ, McCarthy JJ. Three-year follow-up of an implanted functional electrical stimulation system for upright mobility in a child with a thoracic level spinal cord injury. J Spinal Cord Med. 2002;25:345–50. doi: 10.1080/10790268.2002.11754548. [DOI] [PubMed] [Google Scholar]
- 22.Bajd T, Kralj A, Turk R. Standing-up of a healthy subject and a paraplegic patient. J Biomech. 1982;15(1):1–10. doi: 10.1016/0021-9290(82)90029-x. [DOI] [PubMed] [Google Scholar]
- 23.Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Biomed Eng. 1994;2(2):80–91. [Google Scholar]
- 24.Akers JM, Peckham PH, Keith MW, Merritt K. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans Rehabil Eng. 1996;5(2):207–20. doi: 10.1109/86.593301. [DOI] [PubMed] [Google Scholar]
- 25.Uhlir JP, Triolo RJ, Davis JA, Bieri C. Performance of Epimysial Stimulating Electrodes in the Lower Extremities of Individuals with Spinal Cord Injury. IEEE Trans Rehabil Eng. 2004;12(2):279–87. doi: 10.1109/TNSRE.2004.827224. [DOI] [PubMed] [Google Scholar]
- 26.Davis JA, Triolo RJ, Uhlir JP, Bhadra N, Lissy D, Nandurkar S, Marsolais EB. Surgical technique for installing an 8-channel neuroprosthesis for standing. Clin Orthop and Rel Res. 2001;4:237–52. doi: 10.1097/00003086-200104000-00035. [DOI] [PubMed] [Google Scholar]
- 27.Keith MW, Peckham PH, Thrope GB, Buckett JR, Stroh KC, Menger V. Functional neuromuscular stimulation neuroprostheses for the tetraplegic hand. Clin Orthop Relat Res. 1988;233:25–33. [PubMed] [Google Scholar]
- 28.Buckett J, Triolo R, Ferencz D, Katorgi M, Bieri C, Weisgarber J, Vrabec T, Johnson D. A wearable controller for clinical studies involving multi-implant FNS systems. J Spinal Cord Med. 1998;21(2):179. [Google Scholar]
- 29.Bogie KM, Triolo RJ. Effects of regular use of neuromuscular electrical stimulation on tissue health. JRRD. 2003;40(6):469–476. doi: 10.1682/jrrd.2003.11.0469. [DOI] [PubMed] [Google Scholar]


