Abstract
The effectiveness of antiretroviral therapy to control HIV infection has led to the emergence of an older HIV population who are at risk of chronic diseases. Through a comprehensive search of major databases, this Review summarises information about the associations between chronic obstructive pulmonary disease (COPD), asthma, and HIV infection. Asthma and COPD are more prevalent in HIV-infected populations; 16–20% of individuals with HIV infection have asthma or COPD, and poorly controlled HIV infection worsens spirometric and diffusing capacity measurements, and accelerates lung function decline by about 55–75 mL/year. Up to 21% of HIV-infected individuals have obstructive ventilatory defects and reduced diffusing capacity is seen in more than 50% of HIV-infected populations. Specific pharmacotherapy considerations are needed to care for HIV-infected populations with asthma or COPD–protease inhibitor regimens to treat HIV (such as ritonavir) can result in systemic accumulation of inhaled corticosteroids and might increase pneumonia risk, exacerbating the toxicity of this therapy. Therefore, it is essential for clinicians to have a heightened awareness of the increased risk and manifestations of obstructive lung diseases in HIV-infected patients and specific therapeutic considerations to care for this population. Screening spirometry and tests of diffusing capacity might be beneficial in HIV-infected people with a history of smoking or respiratory symptoms.
Introduction
With the introduction of antiretroviral therapy (ART), the HIV epidemic has undergone a tremendous shift in life expectancy and age distribution. ART has substantially improved survival with HIV1 and, by 2015, 50% of people living with HIV in the USA will be aged 50 years and older.2 Additionally, the age standardised death rate attributable to HIV/AIDS has reduced by 68% in the past 20 years.3 Since the introduction of ART, deaths attributable to classic AIDS-defining opportunistic infections have decreased whereas causes related to lifestyle and ageing have increased.4 Subsequent to the rise in life expectancy, the risk of age-associated chronic diseases (eg, cardio vascular, metabolic, renal, neurological) and non-AIDS defining malignancies is increasing in HIV-infected individuals.2,4–7 The prevalence of multiple morbidities in HIV-infected individuals is 65%,8 with lower nadir CD4 cell count and higher viral load associated with greater multimorbidity.9 Data suggest an increased prevalence of obstructive lung diseases in HIV-infected individuals, including both asthma and chronic obstructive pulmonary disease (COPD).10–13 The mechanisms underlying this association are unclear. This Review summarises the present epidemiological data for associations between COPD, asthma, and HIV infection. To help to inform the clinician caring for HIV-infected patients who are at-risk of obstructive lung diseases, we present data for pulmonary function testing and lung cancer screening in HIV-infected individuals, and specific pharmacotherapy considerations for patients on ART. We conclude with a discussion of the current gaps in information about the management of HIV-associated obstructive lung diseases.
HIV-associated obstructive lung diseases before the introduction of antiretroviral therapy
Before the introduction of effective ART regimens in the mid-1990s, the predominant pulmonary complications of HIV related to infectious causes, with scarce attention focused on chronic, non-infectious, lung diseases. However, several case reports and case-control studies described accelerated radiographic emphysema, air trapping, and diffusing capacity impairments in patients with HIV infection.12,14–16 Early in the HIV epidemic, a reduction in diffusing capacity of the lung for carbon monoxide (DLCO) of less than 80% predicted was associated with more rapid development of an AIDS-defining diagnosis, but spirometric measures were not temporally associated with disease progression.15 In other studies, pulmonary function and radiographic abnormalities seemed to be independent of opportunistic infections; for example, one report described a 15% prevalence of radiographic emphysema in HIV-infected people without a history of opportunistic infection, compared with a 2% prevalence in age-matched and smoking-matched HIV-uninfected individuals.14 One of the largest studies before the ART era to document this association was from the Pulmonary Complications of HIV Infection Study Group.17 This multicentre study measured symptoms, spirometry, and DLCO in 1127 HIV-infected individuals without AIDS and 167 HIV-uninfected controls from similar risk groups. Although spirometric measures were not different between HIV-infected and HIV-uninfected participants, spirometric measurements (eg, forced expiratory volume in 1 s [FEV1], and forced vital capacity [FVC]) were 10–15% lower than healthy reference populations. Additionally, HIV-infected participants had a lower absolute and percentage of predicted mean DLCO, and this association was driven predominantly by reduced DLCO in patients with lower CD4 cell counts.
The method of HIV acquisition seems to affect pulmonary function test measurements. Injection drug users had greater reductions in spirometry and DLCO measurements compared with homosexual men and female sexual partners of HIV-infected men, but differential distribution of smoking habits and ethnic origins among these risk groups might have confounded some of these findings. Even with the challenges of accounting for risk behaviour and confounding characteristics, these studies12, 14–16 (largely from the era before ART) emphasise the early recognition of raised susceptibility to obstructive lung diseases in HIV-infected individuals. At the time of these publications, whether optimum HIV control with ART could ultimately change the risk for development of obstructive lung diseases was unclear.
HIV-associated obstructive lung diseases after the introduction of antiretroviral therapy
HIV infection and asthma
In the general population, asthma and COPD are associated with tremendous morbidity and mortality. Asthma prevalence has been increasing since 2001,18 and HIV infection is associated with an increase of all-cause mortality in adults with asthma of more than three times (odds ratio [OR] 3·64, 95% CI 1·34–9·87).19 Results from prevalence studies before the ART era show variable associations between HIV infection and asthma, which range from 20% to 54%, depending on the criteria used.20,21 Several studies after the introduction of ART show a rise in asthma prevalence in children with vertically-acquired HIV infection.22–25 In a study of 451 children infected perinatally with HIV compared with 227 HIV-exposed but uninfected children,22 children with HIV had a higher prevalence of asthma diagnoses (25% vs 20%) and use of asthma drugs (31% vs 22%). Results from a study of 83 HIV-infected children,23 of whom 75% were receiving ART, showed a 34% prevalence of a diagnosis of asthma as defined by the International Classification of Diseases, 9th revision (ICD-9). This prevalence was much higher than the 5·9% noted in general paediatric patients in the same hospital and the 5·0% in the surrounding communities.23 No differences in HIV viral load or CD4 cell count were seen between HIV-infected patients with or without asthma, although a trend towards lower CD8 cell counts was seen in the asthma group. The Women and Infants Transmission Study,24 a prospective observational study of HIV-infected women and their children, showed that HIV-infected children given ART used more asthma drugs compared with HIV-infected children not receiving ART.24 The cumulative incidence of asthma medicine use at age 13·5 years was 33·5% compared with 11·5% in HIV-infected adolescents not on ART. In this cohort, the risk of asthma was heightened after HIV-infected children were given ART.
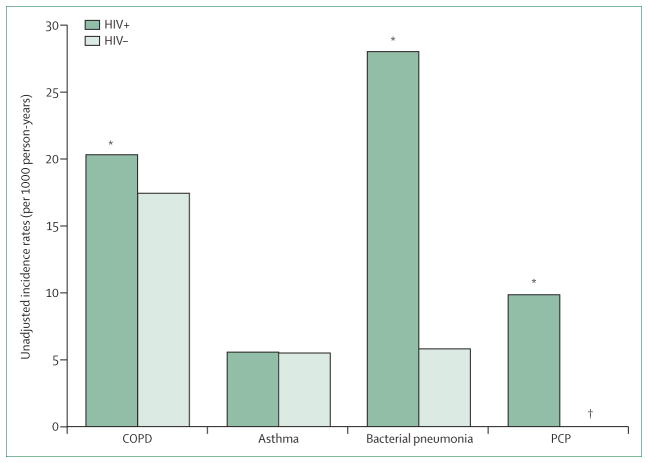
Data for the risk of asthma in HIV-infected adults in the era after the introduction of ART are scarce. Data were obtained from a virtual cohort, a data analysis cohort generated by the combination of standardised electronic medical record data from several sites, derived from the Veterans Ageing Cohort study.26 These data were analysed to define the prevalence of ICD-9 diagnosis of incident pulmonary disease in 33 4203 HIV-infected veterans compared with 66 840 HIV-uninfected veterans matched for age, race, sex, ethnic origin, and study site. Asthma diagnosis in this cohort was low, although HIV-uninfected individuals had a greater baseline prevalence of asthma than those infected with HIV (2·4% vs 2·0%; p<0·001). Asthma was the second most common non-infectious pulmonary disease in the HIV-infected individuals, although the incidence rate did not differ by HIV serostatus (5·6 per 1000 person-years in both groups; figure 1).26 In a single-site study in an urban HIV clinic, Gingo and colleagues27 noted a substantially higher self-reported prevalence of physician-diagnosed asthma in 223 HIV-infected individuals than was reported in the Veterans’ virtual cohort. 21% of participants self-reported a physician’s diagnosis of asthma, and an even higher incidence (30%) of inhaler use in the previous year. Asthma diagnosis was independently associated with female sex, higher body-mass index (BMI), history of respiratory infection (bacterial or pneumocystis) and no current use of ART. No differences in CD4 cell count or viral load were seen between HIV-infected people with or without asthma. Although this study did not have an HIV-uninfected cohort to provide comparative prevalence estimates, the 21% prevalence noted by Gingo and colleagues27 exceeds the 8% prevalence of self-reported doctor diagnosis of asthma described in the general population of the USA.18
Figure 1. Unadjusted incidence rates (per 1000 person-years) of obstructive lung diseases for HIV-infected patients and HIV-uninfected patients.
COPD=chronic obstructive pulmonary disease. PCP=Pneumocystis pneumonia. HIV+=patients infected with HIV. HIV−=patients uninfected with HIV. *p<0·05 for HIV-infected versus HIV-uninfected patients. †incidence in PCP group for HIV-uninfected patients was 0·02. Reproduced from Crothers and colleagues26 by permission of the American Thoracic Society.
Whether HIV infection can be directly linked to an increased risk of asthma therefore remains unclear. Although some data suggest that asthma prevalence might increase with ART, these epidemiological studies have limitations including an absence of HIV-negative comparator groups, confounding behaviours, pulmonary infections in HIV-infected individuals, and methodological limitations in the ascertainment of asthma diagnosis and ART use. These limitations preclude a definitive conclusion on the association between asthma, HIV infection, and ART.
HIV infection and COPD
Death rates attributable to COPD ranked third worldwide in 2010 and COPD is a major contributor to global years of life lost.28 Key risk factors for the development of COPD, including high smoking prevalence, low socioeconomic status, and injection drug use, are more common in HIV-infected populations than in the general population.29–31 Several clinic-based studies have examined the association between HIV infection and COPD.32–34 In an Italian study of 111 HIV-infected and 65 HIV-uninfected matched controls,32 COPD prevalence was higher in HIV-infected patients than in HIV-uninfected controls, but ART use was not associated with COPD diagnosis. In an urban Canadian clinic of HIV-infected patients with more than 90% ART use, chronic airflow limitation defined by spirometry was present in 35% of the cohort, and a lower nadir CD4 cell count was independently associated with airflow limitation.33 In the UK, results from a stable HIV outpatient population, in which 96% were receiving ART, showed that 16% had spirometry-defined airflow obstruction.34 In this analysis, older age and longer duration of HIV infection were the factors with the greatest association with airflow obstruction. These studies, although limited by study size, heterogeneity of selection criteria, and method of COPD diagnosis, emphasise the substantial prevalence and raised awareness by clinicians and researchers of COPD in HIV populations.
Several large epidemiological cohorts from the USA have been powered to more rigorously assess the association between HIV infection and diagnosis of COPD.27,35–37 Crothers and colleagues35 examined the prevalence of COPD defined by self-report and diagnosis as defined by ICD-9 in a cohort of 1014 HIV-positive and 713 HIV-negative men from the Veterans Ageing Cohort Study, an elderly population with heavy tobacco use. Nearly 80% of the HIV-infected cohort were receiving antiretroviral therapy. After adjustment for age, race, smoking intensity, injection drug use, and alcohol misuse, the investigators showed that HIV infection raised the odds of COPD by 50–60% (by ICD-9 codes: OR 1·47, 95% CI 1·01–2·13; by patient self-report: OR 1·58, 95% CI 1·14–2·18). Comparison of HIV-infected individuals with and without COPD showed that median CD4 cell count was substantially lower in those with an ICD-9 diagnosis of COPD. However, no differences in HIV viral load or use of ART were seen between groups with and without COPD. An analysis26 of a larger sample from the Veterans cohort extended these findings by comparing the incidence of COPD in HIV-infected veterans with those who did not have HIV. Data for more than 33000 HIV-infected individuals and more than 66 000 HIV-uninfected individuals matched for clinical and demographic characteristics showed that the incidence of COPD was significantly higher in HIV-infected individuals (20·3 per 1000 person-years) compared with individuals who were not HIV-infected (17·5 per 1000 person-years; p<0·001) (figure 1). COPD was the most common non-infectious pulmonary disease in HIV-infected individuals, with a prevalence of 16%. Similar to trends reported in the general population, the incidence of COPD tended to increase in both HIV-infected and HIV-uninfected groups with age. However, in adjusted analyses, HIV infection remained independently associated with a higher risk of COPD—the incidence of COPD was significantly higher in patients with higher HIV viral load (Incidence Rate Ratio [IRR] 1·08, 95% CI 1·03–1·13, p<0·05) and lower in those on ART at baseline (IRR 0·90, 95% CI 0·82–0·99, p<0·05).
Injection drug users are another population at risk for obstructive lung diseases, including COPD. Analyses of a cohort of urban injection drug users with nearly ubiquitous tobacco use showed that, similar to the Veterans Aging Study population, poor HIV control was associated with greater prevalence of airflow obstruction.36 Pre-bronchodilator spirometry to define COPD showed that HIV-infected individuals with very high HIV RNA levels had a more than three times rise in the odds of airflow obstruction (OR 3·41, 95% CI 1·24–9·39, p=0·02). The association between raised viral load and airflow obstruction was not attenuated by (self-reported) ART use. Notably, 50% of this cohort with spirometrically confirmed airflow obstruction had never received a physician diagnosis of obstructive lung disease; current ART use was independently associated with greater prevalence of unrecognised obstructive lung disease.38 A combined analysis of data from two large observational cohorts of HIV-infected men and women obtained before and after widespread ART use provided insight into the potential role of ART in mitigating longitudinal COPD risk.37 Compared with HIV-uninfected men in the Multicentre AIDS Cohort Study, HIV-infected men had a nearly three times greater risk of self-reported COPD diagnosis in the era before ART (hazard ratio [HR] 2·9, 95% CI 1·02–8·4). However, after ART became available, when most HIV-infected men received effective ART, this risk was attenuated and COPD diagnoses by HIV status were no longer significant (HR 1·61, 95% CI 0·36–7·19).
These studies emphasise that HIV infection seems to be independently associated with a risk of COPD, particularly if HIV infection is not well controlled. The consistent findings for this association across several unique clinic-based and epidemiological cohorts further strengthen these conclusions. Unlike asthma, for which immune reconstitution mediated by ART might heighten the risk, data suggest that HIV infection is associated with increased COPD risk and HIV disease control might be associated with a reduction in COPD risk. Whether the increased risk represents a biological mechanism mediated by the HIV virus or shows confounding by behaviours associated with poor HIV treatment outcomes that lead to COPD development is unclear. In view of the increased risk of COPD associated with HIV infection, clinicians should consider screening HIV-infected individuals with spirometry, especially in the presence of additional risk factors (ie, smoking) or unaccounted respiratory symptoms. Present guidelines for the general population suggest screening spirometry to assess for obstructive lung disease in smokers aged older than 40 years with any respiratory symptoms such as cough or phlegm production.39,40 The high smoking prevalence and ageing of the HIV-infected population mean that a large proportion of HIV-infected individuals would merit such screening. Although the effect of ART on asthma risk has not been clearly assessed, evidence suggests that HIV-infected individuals, especially those with suboptimum treatment for their HIV, are at accelerated risk of COPD.
The association between HIV infection and obstructive lung diseases represents the ultimate sequelae of as-yet undefined damage to the lung, which had probably happened over many years. Although these data are informative, the clinician also needs to understand the association of HIV with different measures to test pulmonary function that are independent of established lung diagnoses in the era after the introduction of ART. Here we present insights on the effect of HIV infection on spirometry, lung volumes, bronchodilator response, diffusion impairment, and bronchial hyper-reactivity, independent of disease diagnoses (panel 1).
Panel 1. Summary of pulmonary function abnormalities in HIV infection.
Spirometry
7–21% obstructive ventilatory defect (defined as forced expiratory volume in 1 s [FEV1]/ forced vital capacity [FVC]<0·70)
Reduced FEV1
Reduced FVC
Accelerated FEV1 decline in poorly controlled HIV
Lung volumes
No conclusive data
Diffusion impairment
50–64% of individuals have reduced diffusing capacity of the lung for carbon monoxide (DLCO)
Reduction in DLCO greatest with advanced HIV
DLCO abnormalities present in never-smokers
Bronchial hyper-reactivity
Greater prevalence in HIV-infected patients than in controls (26% vs 14%)
Bronchodilator reversibility
9% prevalence
No association with ART or disease control
Abnormalities in pulmonary function tests in patients with HIV
Spirometry
Spirometric measurements, including FEV1 and FVC, are the definitive measurements to ascertain the presence of airflow obstruction such as that seen in asthma and COPD. The prevalence of obstructive ventilatory defects (defined as FEV1/FVC<0·70) in HIV-infected people ranges from 7% to 21% despite the widespread use of ART.11,36,41–44 This increase in prevalence is seen in both children and adults with HIV infection.11,36,41–44 Chronic HIV infection in vertically infected children is associated with abnormal results of spirometric testing.45,46 Adolescent survivors of vertically acquired HIV infection in Africa are susceptible to small airways disease; for example, nearly 50% of 116 adolescents from one study had an FEV1 of less than 80% predicted, despite 69% of the entire study cohort receiving ART.46 In adults, Gingo and colleagues11 reported that 21% of HIV-infected individuals had irreversible airflow obstruction, and this finding was more common in patients who had ever smoked than those who had never smoked. ART (present in 81%) and high numbers of pack-years smoked were independently associated with fixed airflow obstruction.11 In this analysis, no association between nadir CD4 cell count or zenith HIV viral load and airway obstruction was noted. Additional studies have described an association between ART use and reduced FEV1/FVC.42 A study of 275 HIV-infected individuals,47 nearly all on ART with an undetectable viral load, had a 17% prevalence of spirometric airflow limitation. Smoking exposure and previous tuberculosis were the main risk factors for airflow limitation.
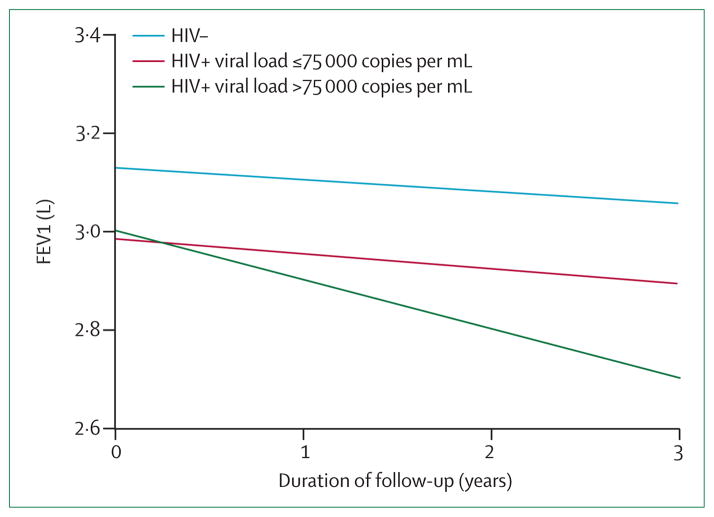
Two studies have extended these cross-sectional results by assessing longitudinal changes in spirometric measures in HIV-infected individuals.48,49 Kristoffersen and colleagues48 followed up 88 HIV-infected patients with measurements for spirometry and DLCO at baseline and again at a median follow-up of 4·5 years. Nearly all the patients in this cohort were receiving ART and 40% were smokers. At the follow-up assessment, the prevalence of reduced FEV1 (less than the 5th percentile of predicted FEV1) increased from 8% to 13% and FEV1/ FVC reduction increased from 10% to 19%. The widespread use of ART in this cohort did not allow for assessment of the effect of HIV disease control on spirometric changes over time. In a US study from Baltimore49—in which 1064 individuals with, or at-risk of, HIV infection underwent longitudinal spirometric measurements every 6 months for almost 3 years—markers of poorly controlled HIV infection were independently associated with more rapid annual decline of FEV1. Specifically, HIV infection with HIV viral load higher than 75 000 copies per mL was associated with a 76 mL/year greater rate of decline in FEV1 compared with HIV-uninfected individuals (p<0·01) (figure 2). Similarly, HIV-infected patients with a CD4 cell count of less than 100 cells per mL had a 57 mL/year greater decline in FEV1 than HIV-uninfected controls. Participants with markers indicating well controlled HIV disease had lung function changes similar to HIV uninfected individuals. It is difficult to determine the causal association between HIV disease control and lung function from these heterogeneous observational studies. ART use might be a marker of previous opportunistic infections that contribute to lung function decline. Conversely, uncontrolled HIV infection could represent a direct biological response to the HIV virus or merely show the consequences of factors related to poor HIV treatment (eg, increased smoking and drug use, more frequent respiratory infections, and less access to health care).
Figure 2. Higher viral load is associated with more rapid annual FEV1 decline.
FEV1=forced expiratory volume in 1 s. Higher viral load in HIV-infected patients (>75 000 copies per mL, green line) is associated with more rapid adjusted annual FEV1 decline compared with HIV-infected patients with viral load ≤75 000 copies per mL (red line) and HIV-uninfected people (blue line). Reproduced from Drummond and colleagues49 by permission of Lippincott Williams and Wilkins/Wolters Kluwer Health: AIDS.
Lung volumes and diffusing capacity
Data for lung volumes in HIV-infected individuals are scarce. One study showed that total lung capacity is reduced after acute pneumocystis infection, but there was no difference in total lung capacity between individuals with no history of pneumocystis and those with one previous episode.50 Studies of pulmonary function in the post-ART era did not report consistent abnormalities in static lung volumes in people infected with HIV.44,47
Similar to results from studies of diffusing capacity in HIV-infected individuals before the introduction of ART,15,17,51 reductions in DLCO are common in HIV-infected individuals in the post-ART era, with prevalence estimates that ranged from 50% to 64%.11,47,52,53 DLCO of less than 80% predicted was present in 64% of HIV-infected patients from one study,11 and was independently associated with greater numbers of pack-years smoked, and use of pneumocystis prophylaxis (an indicator of lower CD4 cell counts), but not ART use.11 In an analysis of 300 HIV-infected men and 289 HIV-uninfected men from the Lung-HIV cohort,54 a moderate to severe reduction in DLCO was noted in 30% of HIV-infected patients compared with 18% of HIV-uninfected people (p<0·001). Despite widespread use of ART in these patients, DLCO was lowest in HIV-infected men with lower CD4 cell counts. In this analysis, HIV was independently associated with reduced DLCO after adjustment for race, pack-years of smoking, and clinical centre. Sampériz and colleagues47 noted that low BMI, being a smoker, and having advanced HIV infection were independent risk factors for reduced DLCO, present in 52% of their HIV-infected population in Spain. Importantly, in HIV-infected patients who had never smoked, reductions in DLCO were still noted and might be associated with raised sputum neutrophils and lymphocytes, which suggest an underlying inflammatory process that is not attributable to smoking.52 Reductions in DLCO are associated with reductions in performance status, as measured by a 6 min walk test in HIV-infected patients.44 With the high prevalence of diffusing capacity abnormalities noted in people with HIV infection, clinicians should think about measuring DLCO in this population, especially in individuals with abnormal spirometry, respiratory symptoms, or evidence of oxygen desaturation at rest or with exertion.
Bronchial hyper-reactivity and bronchodilator reversibility
Bronchial hyper-reactivity to methacholine, which occurs in many pulmonary disorders including asthma, has been assessed in HIV-infected individuals. Early smaller studies did not find an association between HIV infection and bronchial hyper-reactivity to methacholine.21,55 Few studies in the post-ART era have characterised bronchial hyper-responsiveness.22,28 In a study of 248 HIV-infected men,22 26% had hyper-responsiveness to methacholine compared with 14% from a general population control group. In this analysis, current cigarette use was more prevalent in the HIV-infected group, potentially confounding the results. The largest study of bronchodilator reversibility in HIV-infected individuals involved 223 participants, of whom 21% had a physician diagnosis of asthma.28 Bronchodilator reversibility, defined by improvement in FEV1 or FVC of at least 200 mL or 12% after inhaled bronchodilator, was present in 9% of the cohort. No differences in use of antiretroviral drugs, CD4 cell count, or viral load levels were seen between those with and without bronchodilator reversibility. In multivariable analysis, bronchodilator reversibility was independently associated only with sputum eosinophil count.28 These studies represent the available data for airways hyper-reactivity and bronchodilator reversibility in people with HIV-infection in the post-ART era.
Lung cancer screening in smokers infected with HIV
Substantial data lend support to an association between HIV infection and lung cancer risk, independent of smoking.7 In HIV-uninfected smokers, low-dose CT screening reduced lung cancer mortality.56 In view of the high smoking prevalence30,31 and lung cancer risk in HIV-infected patients, this population is expected to have an increased need for screening CT in the near future. Whether HIV infection should be regarded as an additional lung cancer risk factor and prompt screening at younger ages in this population has been debated.57 Data for screening in HIV-infected populations are very sparse and so far, lung cancer screening guidelines have not been adapted specifically for patients with HIV infection. A challenge for clinicians screening asymptomatic HIV-infected smokers is the potential increased risk of false-positive CT findings related to previous infectious or inflammatory events. The additional imaging and diagnostic tests needed in this population might attenuate some of the benefit reported in general population screening trials. Sigel and colleagues57 compared the frequency of incidental CT abnormalities in 160 HIV-infected and 138 HIV-uninfected asymptomatic smokers enrolled in the multicenter Examinations of HIV Associated Lung Emphysema Study. The proportion of CT scans defined as positive by screening guidelines did not differ significantly by HIV status (29% in HIV-infected smokers vs 24% in HIV-uninfected smokers, p=0·3). HIV-infected participants with CD4 cell count less than 200 cells/mm3 had a greater prevalence of abnormalities on CT scan than those with higher CD4 cell count (55% vs 25%, p=0·008). In a multivariable analysis, a CD4 cell count less than 200 cells per mL was the only predictor of false-positive findings. Despite this difference, rates of follow-up procedures (such as imaging, taking a biopsy sample) did not differ by HIV status. Although broader replication and, preferably, clinical trial data are needed, this study suggests that lung cancer screening with CT imaging in HIV-infected populations might not show a notably higher proportion of nodules that need follow-up, and could have similar benefits to those noted in clinical trials in the general population.
Pharmacotherapy recommendations for HIV-infected patients with obstructive lung disease
Prevalence of asthma and COPD is increasing in the HIV-infected population, and therefore pulmonologists, infectious disease specialists, and general practitioners need to more frequently consider the potential interactions of treatments for these comorbid diseases. The mainstays of inhaled therapy for obstructive lung diseases include inhaled corticosteroids, short-acting and long-acting β-agonists, and long-acting muscarinic antagonists. Inhaled corticosteroid use in the HIV-infected population warrants particular caution, because this population is at increased risk of respiratory infections. Inhaled corticosteroids have been associated with a raised risk of pneumonia58,59 and tuberculosis60,61 in people without HIV infection, and their use in HIV-infected populations might potentially further increase this risk, although this association has not been described in the scientific literature thus far. Additionally, inhaled corticosteroids are implicated in drug–drug interactions with HIV therapy because inhaled corticosteroids are metabolised through the cytochrome P450 CYP3A4 pathway. Ritonavir, a protease inhibitor used at low doses to boost concentrations of other protease inhibitors, is a potent inhibitor of the cytochrome P450 pathway. Concomitant use of ritonavir and inhaled corticosteroids results in symptomatic hypercortisolism; the most well described interactions have occurred with the inhaled corticosteroid fluticasone.62–67 There are a range of hypercortisolism-related symptoms and findings reported in HIV-infected individuals on boosted ritonavir regimens and inhaled corticosteroids (panel 2).62–67 Most case reports describe interactions between fluticasone and protease inhibitors, but the clinician should be aware that other inhaled corticosteroids including budesonide and, to a lesser extent, beclomethasone, can lead to hypercortisolism symptoms if they are given concomitantly with protease inhibitors.68–70 The 2013 update of the antiretroviral treatment guidelines71 advised clinicians to avoid co-administration of ritonavir-based ART with inhaled budesonide or fluticasone, unless the benefits clearly outweighed the risks; however, inhaled beclomethasone was not included in this advice. The adverse side-effects of inhaled budesonide or fluticasone combined with ART seem reversible with cessation of the inhaled corticosteroid. If systemic steroids are needed to treat disease exacerbations in patients on protease inhibitors, clinicians should recognise that, in addition to increased glucocorticoid concentrations and related side-effects, the levels of protease inhibitors might potentially be reduced.
Panel 2. Adverse effects of interactions between protease inhibitors and inhaled corticosteroids.
Implicated inhaled corticosteroids
Fluticasone
Budesonide
Beclomethasone
Hypercortisolism is associated with
Fatigue
Weight gain
Truncal obesity
Hirsutism
Cushing’s syndrome
Osteonecrosis
No interactions between non-corticosteroid inhaler regimens and ART have so far been reported. Additionally, the newer generation integrase inhibitors used to treat HIV infection have not been shown to raise the accumulation of glucocorticoids with inhaled corticosteroid therapy.72 One case report described increased neuropsychiatric symptoms with concurrent use of efavirenz with montelukast, a leukotriene receptor antagonist used to treat asthma.73 Although present pulmonary guidelines for treatment of asthma and COPD do not suggest specific modifications to ART,39,74 it is reasonable for the clinician to carefully consider the role of inhaled corticosteroid as the first-line treatment of asthma and COPD. In asthma, for which inhaled corticosteroid are first-line therapy for disease not controlled with short-acting β-agonist therapy,74 the lowest effective dose of inhaled corticosteroid should be preferentially used and patients should be regularly assessed for hypercortisol symptoms. In COPD, consideration should be given for long-acting muscarinic antagonists as first-line inhaled therapy.
Uncertainties in HIV-associated obstructive lung diseases
Increasing attention is being paid to complications of obstructive lung disease that arise in people with chronic HIV infection, so that clinicians will recognise and improve concurrent management of these comorbidities. Despite the substantial increase in the number of studies contributing data for the prevalence, risk, and sequelae of obstructive lung diseases in the HIV-infected population, many areas of uncertainty still remain. Should the diagnosis of obstructive lung disease in HIV patients be approached differently than in HIV-uninfected individuals? Present guidelines do not recommend screening spirometry to detect COPD in asymptomatic smokers.40 However, since unrecognised abnormalities in spirometric measurements and DLCO are highly prevalent in HIV-infected individuals, widespread screening of all HIV-infected smokers might be of benefit, irrespective of symptoms. Does early treatment with ART modify lung function decline in the HIV-infected? If so, obstructive lung disease could represent an additional indication for ART initiation, irrespective of the extent of immunosuppression, which could have substantial implications in many areas of the world that have intersecting epidemics of HIV and tobacco smoking but scarce resources for providing ART. What are the expected normal versus abnormal CT scan findings in HIV-infected individuals free from acute respiratory symptoms? Use of CT scan imaging for lung cancer screening is increasing, so practitioners will be called on to interpret the clinical significance of asymptomatic abnormalities. Finally, what are the best methods to distinguish exacerbations of obstructive lung disease from acute respiratory infections? In view of the major differences in therapeutic management of exacerbations (inhaled corticosteroids) versus infection (antibiotics), distinguishing these processes is key to the optimisation of clinical care. The table emphasises the present understanding and gaps in knowledge about HIV-associated obstructive lung diseases, and outlines potential future studies and challenges with respect to HIV-associated obstructive lung disease.
Table.
Current knowledge, gaps in knowledge, and future research needed into HIV-associated obstructive lung diseases
| Gaps in knowledge | Future research | Research challenges | |
|---|---|---|---|
| HIV infection increases asthma risk in children | Impact of HIV viral control on asthma risk | Longitudinal studies of clinical and physiological outcomes in HIV-infected individuals with asthma and COPD | Selection of representative HIV risk groups and appropriate comparator participants |
| COPD is more prevalent in HIV-infected individuals | Appropriate screening guidelines for COPD in HIV-infected individuals Optimum approaches to distinguish obstructive lung disease exacerbations from respiratory infections | Clinic-based studies of approaches to screen for COPD | Adjustment for confounding behaviours, HIV therapies, and infections |
| Lung cancer risk is increased with HIV infection | Anticipated findings in screening CT scans of HIV-infected smokers | Retrospective analyses of CT databases of HIV-infected smokers | Disentangling the effect of HIV therapy from reduction of confounding behaviours |
| Spirometric and diffusion impairments are common in HIV- infected individuals | Effect of early treatment of HIV on lung function decline | Assessment of lung function outcomes in clinical trials of HIV therapies | Harmonisation of pulmonary-specific outcomes across cohort studies of HIV-infected individuals |
| Inhaled corticosteroids can interact with ritonavir-containing HIV therapies | Risk of respiratory infections in HIV-infected individuals being treated with inhaled corticosteroids | Randomised controlled trials of inhaled corticosteroids versus other therapies for COPD | Identification of eligible HIV-infected individuals with COPD for clinical trials |
COPD=chronic obstructive pulmonary disease.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed, Embase, Cochrane, and Scopus for English-language articles published from Jan 1, 2011, to Jan 24, 2014, containing terms such as: “HIV and lung function”, “HIV” and “HIV treatments and respiratory medicines”, and “HIV and respiratory diseases”. Full details of the search strategy and results can be requested from the authors. MeSH terms and related keywords were included in search strategies to ensure thoroughness. 5199 potentially relevant citations were identified, and after removal of duplicates, the titles and abstracts were screened by MBD for inclusion in the Review; published abstracts presented at meetings were largely excluded. Relevant articles published before January, 2011, were identified through handsearching and cited reference searching.
In summary, the success of ART in improving life expectancy in HIV-infected individuals has led to a population at increased risk of asthma and COPD. Although the mechanisms underlying the development of these lung diseases are unclear, clinicians need to have a heightened awareness of the increasing risk and manifestations of obstructive lung diseases in HIV-infected patients, and that HIV infection seems to negatively affect spirometric and diffusing capacity measurements. Caution should be used if considering inhaled corticosteroids for the management of obstructive lung disease in HIV-infected individuals on protease inhibitors. Ultimately, close collaboration and coordination between pulmonologists, general practitioners, and infectious disease physicians can ensure that the best possible care for lung disease is delivered to HIV-infected individuals in the post-ART era.
Key messages.
Asthma and COPD are more prevalent in HIV-infected populations
Up to 21% of HIV-infected individuals have obstructive ventilatory defects
Reduction in diffusing capacity is seen in more than 50% of HIV-infected populations
Inhaled corticosteroids should be used with caution in HIV-infected patients on regimens containing ritonavir
Screening spirometry and diffusing capacity testing might be needed in HIV-infected people with a history of smoking or respiratory symptoms
Acknowledgments
MBD is funded by a grant from National Institutes of Health, outside the submitted manuscript. (NIH-National Heart, Lung and Blood Institute [K23HL103192]). GDK is funded by grants from the National Institutes of Health outside the submitted manuscript (grants U01-HL-121814; R34-HL-117349; and U01-DA-036297). We thank Carrie Price for her assistance in the literature search for this manuscript.
Footnotes
Contributors
MBD and GDK contributed equally to the concept and design of the Review. MBD undertook the literature search and drafted the manuscript. GDK critically revised the manuscript for important intellectual content. Both authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Declaration of interests
We declare that we have no competing interests.
References
- 1.Samji H, Cescon A, Hogg RS, et al. and the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Abraham J, Ali MK, et al. the US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewden C, Salmon D, Morlat P, et al. the Mortality 2000 study group. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–30. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 5.Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309:1397–405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk GD, Merlo CA the Lung HIV Study. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8:326–32. doi: 10.1513/pats.201009-061WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert AA, Merlo CA, Kirk GD. Human immunodeficiency virus-associated lung malignancies. Clin Chest Med. 2013;34:255–72. doi: 10.1016/j.ccm.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DJ, Westfall AO, Chamot E, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61:600–05. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011;53:1256–64. doi: 10.1093/cid/cir673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crothers K, Thompson BW, Burkhardt K, et al. the Lung HIV Study. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8:275–81. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–96. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med. 1992;116:124–28. doi: 10.7326/0003-4819-116-2-124. [DOI] [PubMed] [Google Scholar]
- 13.Morris AM, Huang L, Bacchetti P, et al. The Pulmonary Complications of HIV Infection Study Group. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. Am J Respir Crit Care Med. 2000;162:612–16. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 14.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–72. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM. Reduced carbon monoxide transfer factor (TLCO) in human immunodeficiency virus type I (HIV-I) infection as a predictor for faster progression to AIDS. Thorax. 1993;48:481–85. doi: 10.1136/thx.48.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell DM, Fleming J, Pinching AJ, et al. Pulmonary function in human immunodeficiency virus infection. A prospective 18-month study of serial lung function in 474 patients. Am Rev Respir Dis. 1992;146:745–51. doi: 10.1164/ajrccm/146.3.745. [DOI] [PubMed] [Google Scholar]
- 17.Rosen MJ, Lou Y, Kvale PA, et al. the Pulmonary Complications of HIV Infection Study Group. Pulmonary function tests in HIV-infected patients without AIDS. Am J Respir Crit Care Med. 1995;152:738–45. doi: 10.1164/ajrccm.152.2.7633736. [DOI] [PubMed] [Google Scholar]
- 18.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;35:1–67. [PubMed] [Google Scholar]
- 19.Sumino K, O’Brian K, Bartle B, Au DH, Castro M, Lee TA. Coexisting chronic conditions associated with mortality and morbidity in adult patients with asthma. J Asthma. 2014;51:306–14. doi: 10.3109/02770903.2013.879881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace JM, Stone GS, Browdy BL, et al. the Pulmonary Complications of HIV Infection Study Group. Nonspecific airway hyperresponsiveness in HIV disease. Chest. 1997;111:121–27. doi: 10.1378/chest.111.1.121. [DOI] [PubMed] [Google Scholar]
- 21.Poirier CD, Inhaber N, Lalonde RG, Ernst P. Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am J Respir Crit Care Med. 2001;164:542–45. doi: 10.1164/ajrccm.164.4.2010019. [DOI] [PubMed] [Google Scholar]
- 22.Siberry GK, Leister E, Jacobson DL, et al. Increased risk of asthma and atopic dermatitis in perinatally HIV-infected children and adolescents. Clin Immunol. 2012;142:201–08. doi: 10.1016/j.clim.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster SB, Paul ME, Kozinetz CA, Macias CG, Shearer WT. Prevalence of asthma in children and young adults with HIV infection. J Allergy Clin Immunol. 2007;119:750–52. doi: 10.1016/j.jaci.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Foster SB, McIntosh K, Thompson B, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol. 2008;122:159–65. doi: 10.1016/j.jaci.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutin F, Butt A, Alame W, Thomas R, Secord E. Asthma in immune-competent children with human immunodeficiency virus. Ann Allergy Asthma Immunol. 2009;102:438. doi: 10.1016/S1081-1206(10)60518-2. [DOI] [PubMed] [Google Scholar]
- 26.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–95. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingo MR, Wenzel SE, Steele C, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129:708–14. doi: 10.1016/j.jaci.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. [accessed Jan 15, 2014];HIV surveillance report. 2011 http://www.cdc.gov/hiv/topics/surveillance/resources/reports.
- 30.Marshall MM, Kirk GD, Caporaso NE, et al. Tobacco use and nicotine dependence among HIV-infected and uninfected injection drug users. Addict Behav. 2011;36:61–67. doi: 10.1016/j.addbeh.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns DN, Hillman D, Neaton JD, et al. the Terry Beirn Community Programs for Clinical Research on AIDS. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:374–83. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- 32.Madeddu G, Fois AG, Calia GM, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection. 2013;41:347–53. doi: 10.1007/s15010-012-0330-x. [DOI] [PubMed] [Google Scholar]
- 33.Mtambo A, Shaipanich T, Guillemi SA, et al. Demographic and clinical predictors of chronic airflow limitation (CAL) among HIV+ patients attending an urban HIV clinic in Vancouver, BC. 6th International AIDS Society Conference on HIV pathogenesis, treatment and prevention; Rome, Italy. July 17–20, 2011; p. CDB128. [Google Scholar]
- 34.Hollington R, Malbon R, Dickson N, et al. Prevalence of chronic obstructive pulmonary disease in an HIV-infected population. HIV Med. 2013;14 (suppl 2):12–77. (abstr P95) [Google Scholar]
- 35.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC the Veterans Aging Cohort 5 Project Team. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–33. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 36.Drummond MB, Kirk GD, Astemborski J, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67:309–14. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gingo MR, Balasubramani GK, Kingsley L, et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS One. 2013;8:e58812. doi: 10.1371/journal.pone.0058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drummond MB, Kirk GD, Astemborski J, et al. Prevalence and risk factors for unrecognized obstructive lung disease among urban drug users. Int J Chron Obstruct Pulmon Dis. 2011;6:89–95. doi: 10.2147/COPD.S15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.From the Global Strategy for the Diagnosis. [accessed Jan 29, 2014];Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014 http://www.goldcopd.org/
- 40.Qaseem A, Wilt TJ, Weinberger SE, et al. the American College of Physicians, and the American College of Chest Physicians, and the American Thoracic Society, and the European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 41.Hirani A, Cavallazzi R, Vasu T, et al. Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med. 2011;105:1655–61. doi: 10.1016/j.rmed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 42.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS ONE. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, Smieja M. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther. 2010;7:6. doi: 10.1186/1742-6405-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campo M, Oursler KK, Huang L, et al. Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J Acquir Immune Defic Syndr. 2013 Dec 16; doi: 10.1097/QAI.0000000000000086. http://www.ncbi.nlm.nih.gov/pubmed/24346638. [DOI] [PMC free article] [PubMed]
- 45.Rubio A, Monpoux F, Bailly C, Crenesse D, Albertini M. Pulmonary function in HIV-1 vertically infected children. J AIDS Clin Res. 2012;3:146. [Google Scholar]
- 46.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55:145–52. doi: 10.1093/cid/cis271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampériz G, Guerrero D, López M, et al. Prevalence of and risk factors for pulmonary abnormalities in HIV-infected patients treated with antiretroviral therapy. HIV Med. 2013 doi: 10.1111/hiv.12117. published online Dec 8. [DOI] [PubMed] [Google Scholar]
- 48.Kristoffersen US, Lebech AM, Mortensen J, Gerstoft J, Gutte H, Kjaer A. Changes in lung function of HIV-infected patients: a 4. 5-year follow-up study. Clin Physiol Funct Imaging. 2012;32:288–95. doi: 10.1111/j.1475-097X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 49.Drummond MB, Merlo CA, Astemborski J, et al. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS. 2013;27:1303–11. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camus F, de Picciotto C, Gerbe J, Matheron S, Perronne C, Bouvet E. Pulmonary function tests in HIV-infected patients. AIDS. 1993;7:1075–79. doi: 10.1097/00002030-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Shaw RJ, Roussak C, Forster SM, Harris JR, Pinching AJ, Mitchell DM. Lung function abnormalities in patients infected with the human immunodeficiency virus with and without overt pneumonitis. Thorax. 1988;43:436–40. doi: 10.1136/thx.43.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gingo MR, He J, Wittman C, et al. Contributors to diffusion impairment in HIV-infected persons. Eur Respir J. 2014;43:195–203. doi: 10.1183/09031936.00157712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzpatrick ME, Gingo MR, Kessinger C, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr. 2013;64:284–88. doi: 10.1097/QAI.0b013e3182a9213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64:271–78. doi: 10.1097/QAI.0b013e3182a9215a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moscato G, Maserati R, Marraccini P, Caccamo F, Dellabianca A. Bronchial reactivity to methacholine in HIV-infected individuals without AIDS. Chest. 1993;103:796–99. doi: 10.1378/chest.103.3.796. [DOI] [PubMed] [Google Scholar]
- 56.Aberle DR, Adams AM, Berg CD, et al. the National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sigel K, Wisnivesky J, Shahrir S, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS. 2014;287:1007–14. doi: 10.1097/QAD.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407–16. doi: 10.1001/jama.2008.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med. 2010;16:118–22. doi: 10.1097/MCP.0b013e328334c085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68:1105–13. doi: 10.1136/thoraxjnl-2012-203175. [DOI] [PubMed] [Google Scholar]
- 61.Brassard P, Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med. 2011;183:675–78. doi: 10.1164/rccm.201007-1099OC. [DOI] [PubMed] [Google Scholar]
- 62.Bernecker C, West TB, Mansmann G, Scherbaum WA, Willenberg HS. Hypercortisolism caused by ritonavir associated inhibition of CYP 3A4 under inhalative glucocorticoid therapy. 2 case reports and a review of the literature. Exp Clin Endocrinol Diabetes. 2012;120:125–27. doi: 10.1055/s-0031-1297993. [DOI] [PubMed] [Google Scholar]
- 63.Saberi P, Phengrasamy T, Nguyen DP. Inhaled corticosteroid use in HIV-positive individuals taking protease inhibitors: a review of pharmacokinetics, case reports and clinical management. HIV Med. 2013;14:519–29. doi: 10.1111/hiv.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zmeili O, Taha W, Abou-Samra A. Iatrogenic cushing syndrome with adrenal suppression in HIV-infected patient receiving inhaled fluticasone and ritonavir. 94th Annual Meeting of the Endocrine Society; Houston, Texas, USA. June 23–26, 2012; abstr. SAT-463. [Google Scholar]
- 65.Mahlab-Guri K, Asher I, Gradstein S, et al. Inhaled fluticasone causes iatrogenic cushing’s syndrome in patients treated with Ritonavir. J Asthma. 2011;48:860–63. doi: 10.3109/02770903.2011.606580. [DOI] [PubMed] [Google Scholar]
- 66.Kaviani N, Bukberg P, Manessis A, Yen V, Young I. Iatrogenic osteoporosis, bilateral HIP osteonecrosis, and secondary adrenal suppression in an HIV-infected man receiving inhaled corticosteroids and ritonavir-boosted highly active antiretroviral therapy. Endocr Pract. 2011;17:74–78. doi: 10.4158/EP09288.CR. [DOI] [PubMed] [Google Scholar]
- 67.Canalejo E, Pacheco MS. Cushing syndrome due to ritonavir-fluticasone interaction. CMAJ. 2012;184:1714. doi: 10.1503/cmaj.111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoganathan K, David L, Williams C, Jones K. Cushing’s syndrome with adrenal suppression induced by inhaled budesonide due to a ritonavir drug interaction in a woman with HIV infection. Int J STD AIDS. 2012;23:520–21. doi: 10.1258/ijsa.2011.011408. [DOI] [PubMed] [Google Scholar]
- 69.Blondin MC, Beauregard H, Serri O. Iatrogenic Cushing syndrome in patients receiving inhaled budesonide and itraconazole or ritonavir: two cases and literature review. Endocr Pract. 2013;19:e138–41. doi: 10.4158/EP13122.CR. [DOI] [PubMed] [Google Scholar]
- 70.Boyd SD, Hadigan C, McManus M, et al. Influence of low-dose ritonavir with and without darunavir on the pharmacokinetics and pharmacodynamics of inhaled beclomethasone. J Acquir Immune Defic Syndr. 2013;63:355–61. doi: 10.1097/QAI.0b013e31829260d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. [accessed Jan 29, 2014];Guidelines for the use ofantiretroviral agents in HIV-1-infected adults and adolescents. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 72.Cottrell ML, Hadzic T, Kashuba ADM. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet. 2013;52:981–94. doi: 10.1007/s40262-013-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ibarra-Barrueta O, Palacios-Zabalza I, Mora-Atorrasagasti O, Mayo-Suarez J. Effect of concomitant use of montelukast and efavirenz on neuropsychiatric adverse events. Ann Pharmacother. 2014;48:145–48. doi: 10.1177/1060028013510396. [DOI] [PubMed] [Google Scholar]
- 74.National Asthma Education and Prevention Program. . Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120 (suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]