Abstract
Objective
Among survivors of out-of-hospital cardiac arrest (OHCA), the functional outcomes of those with rapid early or with very delayed recoveries are known. For patients between those extremes early recovery is variable, and the probability of longer-term recovery and the implications for quality of life have not been clearly defined.
Methods
Twenty-five patients of a consecutive cohort of OHCA survivors with coma duration between 12 h and 7 days and a matched group with acute coronary syndrome underwent cognitive and disability assessments 3 and 12 months after OHCA. Correlations and regression analyses of demographic, clinical arrest variables, and cognitive tests with quality of life outcomes were performed.
Results
The OHCA group had impairments in all cognitive domains. There was little cognitive improvement. The OHCA group reported significantly greater health impact and lower quality of life at twelve months than the controls. Longer duration of coma (4–7 versus ≤ 3 days) and greater cognitive impairment at three months, particularly memory impairment, were both associated with reduced late quality of life.
Conclusions
These survivors of OHCA had persistent long-term cognitive deficits. Quality of life at one year after OHCA was reduced compared to cardiac controls. Coma duration and memory impairment at three months were harbingers of long term reduced quality of life.
Keywords: cardiac arrest, quality of life, anoxia, outcome assessment
INTRODUCTION
Only a minority of patients with out-of-hospital cardiac arrest (OHCA) survives to hospital discharge (1). Among survivors, about one-half have nearly immediate recovery (within hours) of consciousness with good neurological functional outcomes (2, 3); those with prolonged coma (more than 7 days) almost uniformly suffer severe disability or worse (2). These extreme cases tend to statistically overwhelm outcome and prediction studies. As such, most reports have been retrospective and have conflated all ranges of severity (4–6). Long-term quality of life (QoL) for the patients between those extremes has not been defined, and hence is more difficult to predict. We were interested in the cognitive deficits, disability and QoL of patients in this middle ground – those patients with enough deficits to impact society, but not severe enough to be dependent; the group most likely to require extensive rehabilitation. In order to prospectively identify such patients and eliminate selection bias, we relied on the objective, clinical parameter of coma duration to identify our target group. We previously reported on the 3 month neurological outcomes of this cohort (7). We now report an analysis of the one year cognitive deficits, disability and QoL of this prospective cohort of consecutive patients in the middle ground. Analysis also focused on factors that might predict QoL: demographic, clinical arrest variables and cognitive status at 3 months post injury. We had two hypotheses:
Cognition, mostly in the executive domain, will improve between 3 months and 12 months, but it is unclear whether this cognitive improvement will result in good QoL.
QoL at 12 months will correlate with severity of cognitive deficits.
To control for possible physical and psychological contributions to disability due to cardiac disease, a comparison group of matched patients with acute coronary events but no arrest were followed and tested in parallel manner.
METHOD
Approval for the study was obtained from the Institutional Review Board (IRB) of the Beth Israel Deaconess Medical Center (BIDMC), Boston, MA, Boston University School of Medicine, and the VA Boston Healthcare System.
Subjects
We screened all admissions to the coronary care and medical intensive care units at BIDMC from June 2005 to December 2008 for all patients with a diagnosis of OHCA. The hospital is a tertiary care center with a level one trauma center.
We excluded patients who regained consciousness within the first 12 h after arrest and all patients who were over 70, non-English speaking, with a history of prior OHCA, had had heart surgery within the previous year, brain trauma, stroke, dementia, any significant active medical disease, active depression or alcoholism. Patients were excluded if they remained in coma for more than 7 days, or if the clinical exam predicted a grim outcome – absent pupillary responses after 24 h, myoclonic status epilepticus within the first 48 h, or burst suppression or worse on any EEG.
Prior to discharge, we approached families of patients with eligible histories for permission to contact them in two months to recruit for the study. The families of all 32 patients who were eligible agreed. Testing occurred at 3 months (T1) and 12 months post-injury (T2). Of the 30 OHCA patients who were enrolled and tested at 3 months post injury (7), 3 declined to participate further when approached one year post, and two had died of non-cardiac causes, leaving 25 patients for the present report.
In our previous study, cognitive outcome at T1 was bimodal with significant differences in every cognitive domain between a group with mild impairment and a group with severe impairment (7). Of patients evaluated at T2, 17 were in the mild subgroup at T1 and 8 in the severe subgroup. These subgroups did not differ from each other or from the controls on most demographic variables, with the exception that the severe subgroup had lower ANART scores (American National Adult Reading Test) (105 vs. 118, t(23) = 3.3, p < 0.01).
A disease-matched control group was assembled by screening admissions to the Coronary Care Unit at BIDMC from June 2005 to December 2008 for patients with a diagnosis of any acute coronary syndrome. Demographic and clinical exclusion criteria were identical to those for the arrest patients. Of the 30 controls who were tested at 3 months, 3 declined participation at one year; the remaining 27 completed the study.
Informed consent was obtained from each participant at the time of initial testing.
Demographic and medical factors
For details regarding demographic and medical factors, see Table I. 24 h of hypothermia, if initiated, was started within 12 h of the cardiac arrest. Decisions about angiography and hypothermia were made by the Emergency and CCU staffs. Duration of coma was considered to last to any documentation of purposeful behavior. To allow for the variability of return to consciousness following the gradual rewarming phase after hypothermia and lifting of sedation, coma duration was coded as up to 3 days or more than 3 days.
Table I.
Demographics and medical factors
| OHCA n = 25 |
CC n = 27 |
p-value | |
|---|---|---|---|
| Gender, male, % (n) | 84 (21) | 81 (22) | χ2 = 81 |
| Age, years, mean | 56.2 | 58.7 | t(50) = 1.2, p = 0.22 |
| Education, years, mean | 14.0 | 15.2 | t(50) = 1.2, p = 0.22 |
| ANART, mean | 113 | 116 | t(50) = 1, p = 0.3 |
| Post index event to testing, years, mean | 399 | 384 | t(50) = 1.4; p = 0.16 |
| Therapeutic hypothermia, % (n) | 32 (8) | ||
| Coma duration > 3 days, % (n) | 24 (6) |
OHCA: out-of-hospital cardiac arrest; CC: cardiac control; ANART: American National Adult Reading Test.
Depression was assessed with the Beck Depression Inventory (8). This scale de-emphasizes the somatic symptoms of depression, a feature important for the evaluation of groups with significant medical problems.
Cognitive measures
Patients and controls had been tested at 3 months (T1) and were retested at one year (T2) with a series of neuropsychological tests, each tapping one dominant cognitive domain. The individual tests and dependent variables are the same as our previous study (7), see Appendix I for details. All tests have published normative data, and all raw scores were converted to z-scores based on age- (and when available education- and gender-) based norms. A composite z-score was computed for each domain.
Functional outcome measures
QoL was assessed with the Sickness Impact Profile short form (SIP68), based on the SIP (9, 10), a behaviorally-based measure of health status containing 136 items in 12 categories that has been used to measure QoL in survivors of cardiac arrest (11–14). It provides an overall score (maximum of 68), as well as physical (maximum of 29), psychological (maximum of 17), and social disability (maximum of 22) scores. A higher score indicates a higher level of dysfunction.
Functional status was further assessed using the Frenchay Activities Index (FAI) (15), a 15-item survey of common activities associated with home or leisure time that was developed as a QoL tool for stroke patients (16). It is answered in a semi-quantitative manner with intensity of activity rated on a 0–3-point scale (maximum score of 45). A higher score indicates a higher level of activity.
The patients completed the questionnaires, with help from a caregiver if necessary, to be certain that answers were not based on their status prior to the OHCA. Return to work was reviewed with patients and families for the patients who had been working prior to hospitalization. Return to work was judged as approximately at the level prior to illness based on patient and family report of hours and responsibility.
Statistical analysis
To evaluate cognitive outcomes T2, composite z-scores for each cognitive domain were calculated. Multivariate analysis of variance (MANOVA) was performed to examine the effect of group on cognitive outcomes. Group was the 2-level independent variable and cognitive domain was the 5-level dependent variable. Univariate follow up analyses were performed as needed. To directly compare the two OHCA subgroups to one another, the above analysis was repeated with OHCA subgroup as the independent variable. Each subgroup was also directly compared to the control group.
To examine changes in cognitive status from 3 months to 1 year post onset, composite z-scores for each domain were compared across groups using MANOVA. Group and time were included as two-level independent variables and cognitive domain was the 5-level dependent variable. Change in cognitive status was also evaluated for the mild and severe OHCA subgroups separately.
To evaluate QoL at 1-year post onset, the SIP68 scores were compared across groups using MANOVA with group as the two-level independent variable and SIP68 scores (physical, psychological, and social domain) as the 3-level dependent variable. The FAI score, and the rate of return to work were also compared across groups. For some measures we compared outcomes for just the mild OHCA subgroup to controls to prevent the results in the smaller severe group from yielding an exaggerated picture of impaired QoL in the overall group. The failure to maintain this distinction is a cause of confusion about the range of possible outcomes.
For the OHCA patients, we examined the relationship between functional outcomes and medical variables, demographic factors and T1 cognitive testing. Whenever a functional outcome score was correlated with more than one cognitive domain score, a stepwise regression analysis was performed to determine which variables account for variance in functional outcome.
All statistical tests were conducted using the SPSS software package (IBM SPSS Statistics for Windows, version 21.0. Armonk, NY, USA).
RESULTS
There was no significant difference between controls and OHCA patients in gender, age, education or ANART scores, or time to testing (see Table I).
Significant depression was uncommon in either group: Controls: 23/27 none (BDI < 10); 3/27 mild (10–19); 1/27 severe (≥ 20); Patients: 24/25 none; 1/25 severe (despite mild deficits). There was no group difference in the incidence of depression (χ2 = 2.95, p > 0.20).
T2 neuropsychological results
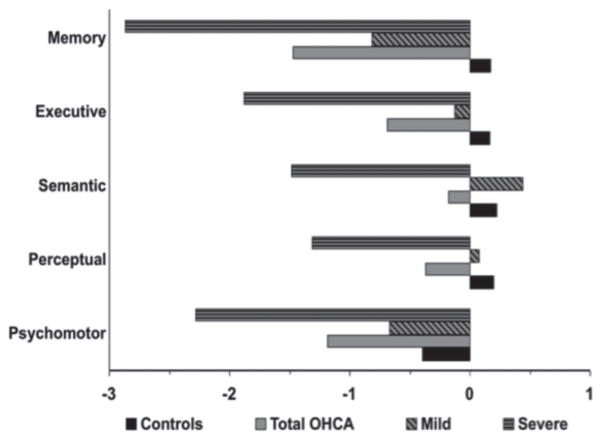
The analysis comparing the OHCA to the cardiac control group yielded a significant main effect of group (F(1,50) = 14.87, p < 0.01) and domain (F(4, 200) = 12.99, p < 0.01), as well as a group by domain interaction (F(4,200) = 5.88, p < 0.01) (Fig. 1). Post hoc comparisons indicated that the OHCA group differed significantly from the control group in all domains (F’s > 5.8, p’s < 0.02), with the exception of semantic functioning (F(1,50) = 2.1, p = 0.15). Further, there was a disproportionate impairment of memory in the OHCA group.
Fig. 1.
Composite z-scores at 12 months-injury. Mean composite z-scores of the 5 domains for controls, all out-of-hospital cardiac arrest (total OHCA), and mild and severe OHCA separately.
The analysis comparing performance of the mild and severe OHCA subgroups to each other revealed that those subgroups, determined at T1, remained significantly different from each other in every domain at T2 (main effect of group: F(1,23) = 17.1, p < 0.01). In comparison to controls, the severe subgroup was impaired in all domains (F > 24.31, p < 0.01), albeit it disproportionately in memory (group × domain interaction: F(4,132) = 4.73, p < 0.01). The mild subgroup performed more poorly than controls only in memory (group × domain interaction: (F(4,168) = 10.69, p < 0.01).
T1 to T2 recovery
The analysis comparing changes in performance over time in OHCA patients and cardiac controls revealed main effects of group (F(1,50) = 16.99, p < 0.01), time (F(1,50) = 15.00, p < 0.01), and domain (F(4,200) = 13.36, p < 0.01), as well as a significant group × time (F(1,50) = 4.60, p < 0.05) and group × domain interaction (F(4,200) = 4.34, p < 0.01). Critical to the question of recovery, the group × time interaction demonstrates that the OHCA group showed greater improvement from T1 to T2 (T1 z = −1.0; T2 z = −0.78, F(1,24) = 11.66, p < 0.01) than did the cardiac control group (T1 z = 0.0; T2 z = 0.1; F(1,26) = 2.86; p = 0.10).
The analysis comparing changes in performance over time in the severe OHCA subgroup and cardiac control group revealed a main effect of time (F(1,33) = 28.30, p < 0.01) and a group × time interaction (F(1,33) = 16.37, p < 0.01). The severe OHCA subgroup showed statistically significant improvement overall, indicative of recovery (T1 z = −2.53; T2 z = −1.96, F (1,7) = 11.77, p = 0.01), although the actual mean z-scores suggest minimal functional value to the change. Over time, the number of patients with moderate-severe impairments (z ≤ −2) in memory and executive functioning remained unchanged from T1 to T2, while there was a modest reduction in number of patients with moderate-severe impairments in the other domains (Fig. 2).
Fig. 2.
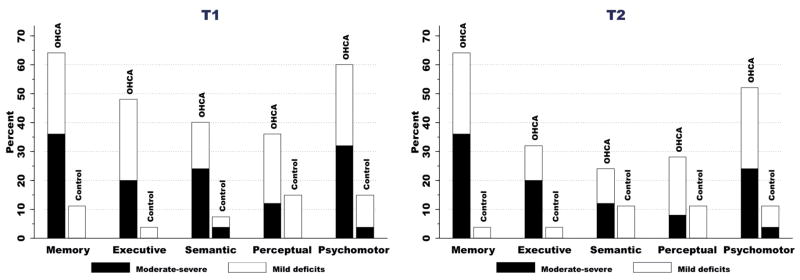
Neuropsychological deficits at 3 months (T1) (A) and 12 months (T″) (B) post-injury. Percentage of out-of-hospital cardiac arrest (OHCA) and control patients with mild (z-scores between −1 and −2) or moderate-severe (z-scores ≤ −2) deficits in each domain.
The analysis comparing changes over time in the mild OHCA subgroup and cardiac controls revealed a main effect of time (F(1,42) = 7.12, p < 0.05) but no group × time interaction (F < 1), indicating a similar (minimal) improvement in the mild OHCA subgroup (T1 z = −0.35; T2 z = −0.22) as in controls. The analysis of the two subgroups thus clarifies that the improvement in the OHCA group as a whole was driven by the severe subgroup.
T2 quality of life outcomes
SIP68
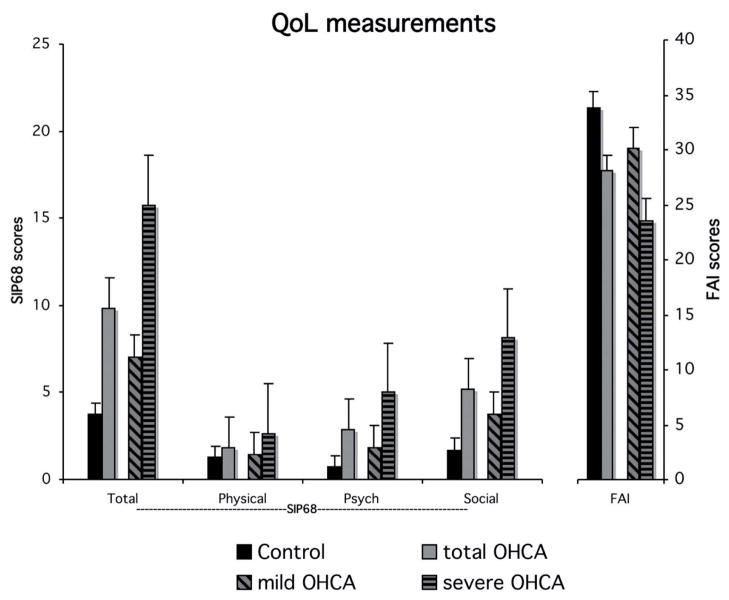
The analysis comparing the OHCA group to the cardiac controls revealed a main effect of group indicating that the OHCA group reported significantly greater health impact than the controls (OHCA X = 9.8; controls X = 3.7, F(1,50) = 10.77, p < 0.01). There was also a significant group × domain interaction (F(1,50) = 9.59, p < 0.01) (Fig. 3). One-way follow up analyses of variance indicated that the difference across groups was significant in the psychological (OHCA X = 2.8; controls X = 0.7, F(1,50) = 9.84, p < 0.01) and social domains (OHCA X = 5.2; controls X = 1.7, F(1,50) = 11.77, p < 0.01) but not in the physical domain (OHCA X = 1.8; controls X = 1.3, F < 1). Even the analysis of just the mildly impaired OHCA subgroup compared to cardiac controls revealed greater health impact for the mildly impaired patients (mild OHCA X = 7.0; F(1,42) = 4.15, p < 0.05).
Fig. 3.
Quality of Life measurements at T2. SIP68: sickness impact profile short form; FAI: Frenchay activities index; Psych: psychological; OHCA: out-of-hospital cardiac arrest.
FAI
Activities were significantly lower in the OHCA group than in controls (OHCA X = 28.0; controls X = 33.9, t(50) = 2.83, p < 0.01). The difference was not significant for the mild OHCA subgroup (mild OHCA X = 30.2, t(42) = 1.58; p = 0.12).
Return to work
Of 18 control participants who had worked before, 17 returned to work, one of those part-time. Of 25 OHCA patients who worked before, only 6 went back to work, a significantly lower rate of return (χ2 = 17.6 p < 0.001). Even considering the mild OHCA subgroup, return to work was significantly lower than in the cardiac controls (6/17 v. 16/18, χ2 = 10.76, p < 0.01). All patients who returned to work had done so by 3 months. OHCA patients who did not return to work had worse FAI and SIP68 scores than OHCA patients who did return to work, but the difference did not reach significance (SIP68 X = 10.8 vs. 6.7 and FAI X = 27.1 vs. 31.2; t < 1.2).
Association of demographic and medical factors with functional outcome in the OHCA group
Age, education, and depression did not correlate with any functional outcome measure. See Table II. In comparison to patients with a maximum duration of coma of 3 days (n = 19), those with a duration of coma greater than 3 days (n = 6) had more health-related effects (SIP68 X = 7 vs. 18.7, t(23) = 3.71, p < 0.01) and less functional activity (FAI X = 30.5 vs. 20.3, t(23) = 3.48, p < 0.01). Documented use of hypothermia (n = 8, SIP68 X = 11.9, FAI X = 26.1) or not (n = 17, SIP68 X = 8.8, FAI X = 28.9) had no impact on outcome (t <1).
Table II.
Association between functional outcome measures and demographic, medical, and cognitive variables
| SIP | FAI | |
|---|---|---|
| Demographic | ||
| Age | ns | ns |
| Education | ns | ns |
| Medical | ||
| Coma duration | r2 = 0.37 (p < 0.01) | r2 = 0.35 (p < 0.01) |
| Hypothermia | ns | ns |
| Depression | ns | ns |
| T1 Cognitive* | ||
| Memory | r2 = 0.48 (p < 0.01) | r2 = 0.37 (p < 0.01) |
| Executive | r2 = 0.31 (p < 0.01) | r2 = 0.27 (p < 0.01) |
| Semantic | r2 = 0.21 (p < 0.05) | r2 = 0.37 (p < 0.01) |
| Perceptual | r2 = 0.32 (p < 0.01) | r2 = 0.18 (p < 0.05) |
| Psychomotor | r2 = 0.42 (p < 0.01) | r2 = 0.15 (p < 0.05) |
Using stepwise regression, memory alone accounted for a significant proportion of the variance in functional outcome measures (for SIP, β = −3.87 and for FAI, β = 3.09).
Association of functional outcome with cognitive functions
As there was no functionally meaningful improvement from T1 to T2, either test time could be used to assess the relationship of cognitive impairment to QoL, but T1 might be predictive and, thus, useful for planning care.
SIP68
All T1 cognitive composite scores were significantly correlated with total SIP68 (see Table II). Stepwise regression indicated that memory alone accounted for a significant proportion of the variance in SIP scores (F(1,24) = 21.32, p < 0.001, r2 = 0.48).
FAI
All T1 cognitive composite scores also were significantly correlated with FAI scores (see Table II). In the regression model, memory alone again accounted for a significant proportion of the variance (F(1,24) = 13.65, p < 0.001; r2 = 0.37).
DISCUSSION
We examined the range of potential cognitive and functional outcomes at one year after OHCA in patients with post-anoxic coma duration between 12 h and 7 days. Although not anticipated and not an inclusion criterion, all of these patients had lengthy rehabilitation hospitalizations, but had none of the clinical markers already amply demonstrated to determine a very bad functional outcome.
OHCA survivors were, not surprisingly, significantly impaired one year later compared to cardiac controls, mostly in the cognitive domain of memory but also in the executive, visuospatial, and psychomotor domains. Deficits in the milder group persisted over time, with all recovery occurring before 3 months. Contrary to our hypothesis, no further improvement occurred between 3 and 12 months. The severe group had a longer course of improvement with continued gains between 3 and 12 months, but the recovery was not exclusively in executive function but rather in all domains, suggesting that the improvement may represent a recovery in general alertness rather than any specific cognitive function.
Whether measured as impact on health (SIP68), actual participation in activities (FAI questionnaire), or return to work, the OHCA patients had more disability and limitations in QoL than the controls. Disability at one year was predominantly in the psychological and social domains. The fact that physical QoL was not reduced following OHCA parallels our observations that these patients have few physical ailments. As hypothesized, QoL correlated with the severity of cognitive deficits, and the severity of memory impairments at 3 months, in particular, was a strong predictor of QoL at one year. The clinical evolution as early as one week also had predictive value: duration of coma longer than 3 days (but still less than 7 days) resulted in poorer QoL than duration of coma less than 3 days. This study with its narrow selection criteria was not constructed in a manner that would find an effect of hypothermia, and we did not find one.
The few studies of long-term cognitive consequences of cardiac arrest, most without disease matched controls (4, 17, 18), have similar results to ours. These studies suggest that survivors of OHCA who have confusion and memory difficulties immediately following the arrest (equivalent to cerebral performance category of 1–2) have a high risk of persistent deficits in memory (about 67%), psychomotor (about 50%), and executive (about 33%) functioning, and approximately one-third of these deficits will be moderate to severe.
There have been few reports that evaluated the extent of recovery after 3 months. Dougherty (19) reported no improvement in several memory, psychomotor and visual spatial tasks out to a year. Another longer follow-up study found no evidence that memory continued to improve from 3 months up to 3 years after the cardiac arrest (20). Roine et al. (18) noted no change in overall performance in the Wechsler Memory Scale-III from 3 months to 12 months but did note that moderate memory impairments dropped from 49% of the patients at 3 months to 33% at 12 months and that visuospatial impairments dropped from 43% to 30%. In the present study individual improvement was more modest, with recovery most notably in the semantic domain and no evidence of recovery in the memory or executive domain.
QoL studies have suggested that overall outcomes are good in survivors of cardiac arrest (21), but these studies are affected by inclusion of patients with good outcome following rapid recovery. Direct comparison to prior studies of QoL in survivors of cardiac arrest is difficult, because of methodological variability. Time since arrest has ranged from 3 months (22) to 15 years (23). Although the SIP68 or a closely related scale is commonly used to measure QoL or at least health impact, other measures have been reported, some fairly coarse such as the Glasgow Outcome Scale. The majority of studies comprise a retrospective collection of all available patients admitted to an acute or a rehabilitation hospital across some specified time span (4–6, 24–26). Retrospective studies may over-represent patients able and willing to respond, possibly producing an overly positive picture of the true range of outcomes (4, 5). We found 9 prospective studies of QoL in survivors of cardiac arrest. Seven lacked controls, relying on population norms (27–33). Cardiac disease by itself reduces QoL (34), so a disease-matched control population should better isolate the effect of cardiac arrest survival. Of the few studies that have taken advantage of a cardiac control population two were retrospective (3, 12) and one involved mostly in-hospital cardiac arrest (35), a much different clinical population. There is only one prospective study with a cardiac control population (13). That study found no difference in overall QoL as measure by SIP, but there were only 10 OHCA patients and there was large variability in outcome.
Return to work (prior level of employment) after OHCA has been reported to occur in 13% to 63% of patients (27, 30, 36), a range so large that only variability of included patients can account for it. Two cardiovascular controlled studies (3, 12), both retrospective, reported a return to work rate of 63% in OHCA patients compared to 88% and 79%, respectively, in controls. Only 24% of the patients in our study returned to work (compared to 89% of our controls) and none after the first 3 months. The discrepancies likely represent selection or survivor biases. Any report of outcome of OHCA survivors without attention to levels of severity will include many patients with good outcome following rapid recovery (21); about 50% of survivors have quick recovery of consciousness and immediately good outcome (2). Our study was designed to identify patients likely to have cognitive deficits but not profound impairment.
Limitations of this study include the small number of patients tested as well as the restriction to a single institution. In addition, because patients who were in coma less than 12 h or longer than 7 days were excluded, our study does not speak to the course of recovery for the small proportion of patients who did not meet our inclusion criteria, yet may also have mild to moderate deficits. As discussed above, our inclusion criteria may have resulted in an inability to determine the true effects of hypothermia.
In conclusion, our study is distinguished by its prospective nature, complete capture of the target population in one hospital, more patients, and more points of analysis. These patients had delayed recovery of consciousness after resuscitation. Cognitive impairments – confusion and amnesia – were severe enough to prevent home discharge. We did not anticipate the broad need for in-patient rehabilitation and did not use it as an inclusion criterion, but this group all went to rehabilitation. This appears to be the level of impairment that will require and benefit from early rehabilitation. In the initial weeks and months, corresponding to the time of rehabilitation, the patients had substantial but incomplete recovery. From 3 months to 12 months, however, there was very little additional improvement, and QoL at 12 months was strongly correlated with cognitive recovery at 3 months post-arrest. These patients are only a portion of those who survive out of hospital cardiac arrest, but their outcome has rarely been differentiated from the larger population of survivors with rapid recovery. Recognition of this discouraging natural history should serve as motivation to develop effective treatments for this population as recovery does not occur simply with passage of time.
Acknowledgments
We are grateful to Kate McNamara, Lily Wong, and Elana Anastasia for research assistance and to Douglas Katz for helpful comments on the manuscript. This research was supported by National Institutes of Health grant HD046442 from the National Center for Medical Rehabilitation Research (National Institute of Child Health and Human Development) and the Clinical Science Research and Development Service, Department of Veterans Affairs.
Appendix I. Cognitive test battery and dependent measures for each test
Table III.
| Domain | Test(s) | Dependent measure(s) |
|---|---|---|
| Premorbid IQ estimate | National Adult Reading Test (ANART) | Total correct |
| Memory | Rey Auditory Verbal Learning Test (RAVLT) | Total learned in 5 trials |
| Brief Visual Memory Test-Revised (BVMT-R) | Delayed recall | |
| Delayed recognition (RAVLT: corrected recognition BVMT–R: discrimination index) | ||
| Executive function | Trail Making Test B | Time |
| Wisconsin Card Sorting Test | Number of categories, % perseverations | |
| Verbal Fluency | Number | |
| Lexical-semantic | Boston Naming Test (alternate items) | Number correct without cues |
| Peabody Picture Vocabulary | Total correct | |
| Visuoperceptual | Judgment of Line Orientation | Total correct |
| Number location | Total correct | |
| Visual discrimination | Total correct | |
| Psychomotor | Trail Making Test A | Time |
| Grooved Pegboard | Time for each hand | |
| Finger Tapping | Time for each hand |
Footnotes
The authors report no conflicts of interest.
References
- 1.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longstreth WT, Inui TS, Cobb LA, Copass MK. Neurologic recovery after out-of-hospital cardiac arrest. Ann Intern Med. 1983;98:588–592. doi: 10.7326/0003-4819-98-5-588. [DOI] [PubMed] [Google Scholar]
- 3.Bertini G, Giglioli C, Giovannini F, Bartoletti A, Taiti A. Neuropsychological outcome of survivors of out-of-hospital cardiac arrest. J Emerg Med. 1990;8:407–412. doi: 10.1016/0736-4679(90)90166-s. [DOI] [PubMed] [Google Scholar]
- 4.Torgersen J, Strand K, Bjelland TW, Klepstad P, Kvale R, Soreide E, et al. Cognitive dysfunction and health-related quality of life after a cardiac arrest and therapeutic hypothermia. Acta Anaesthesiol Scand. 2010;54:721–728. doi: 10.1111/j.1399-6576.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 5.Wachelder EM, Moulaert VRMP, van Heugten C, Verbunt JA, Bekkers SCAM, Wade DT. Life after survival: Long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009;80:517–522. doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Reinhard V, Pärna K, Lang K, Pisarev H, Sipria A, Starkopf J. Long-term outcome of bystander-witnessed out-of-hospital cardiac arrest in estonia from 1999 to 2002. Resuscitation. 2009;80:73–78. doi: 10.1016/j.resuscitation.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Alexander MP, Lafleche G, Schnyer D, Lim C, Verfaellie M. Cognitive and functional outcome after out of hospital cardiac arrest. J Int Neuropsychol Soc. 2011;17:364–368. doi: 10.1017/S1355617710001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technic. Postgrad Med. 1972;52:81–85. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- 9.Bergner M, Bobbitt BA, Carter WB, Gilson BS. The sickness impact profile: Development and final revision of a health status measure. Medical Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Pollard WE, Bobbitt RA, Bergner M, Martin DP, Gilson BS. The sickness impact profile: Reliability of a health status measure. Medical Care. 1976;14:146–155. doi: 10.1097/00005650-197602000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bergner L, Bergner M, Hallstrom AP, Eisenberg MS, Cobb LA. Service factors and health status of survivors of out-of-hospital cardiac arrest. Am J Emerg Med. 1983;3:259–263. doi: 10.1016/0735-6757(83)90101-8. [DOI] [PubMed] [Google Scholar]
- 12.Bergner L, Hallstrom AP, Bergner M, Eisenberg MS, Cobb LA. Health status of survivors of cardiac arrest and of myocardial infarction controls. Am J Public Health. 1985;75:1321–1323. doi: 10.2105/ajph.75.11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillis M, Sinclair D, Butler G, CaiN E. Prehospital cardiac arrest survival and neurologic recovery. T Emerg Med. 1993;11:245–252. doi: 10.1016/0736-4679(93)90041-5. [DOI] [PubMed] [Google Scholar]
- 14.de Vos R, de Haes H, Koster RW, de Haan RJ. Quality of life after cardiopulmonary rescuscitation. Arch Intern Med. 1999;159:249–254. doi: 10.1001/archinte.159.3.249. [DOI] [PubMed] [Google Scholar]
- 15.Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age Ageing. 1983;12:166–170. doi: 10.1093/ageing/12.2.166. [DOI] [PubMed] [Google Scholar]
- 16.Wade DT, Legh-Smith J, Langton Hewer R. Social activities after stroke: Measurement and natural history using the frenchay activities index. Int Rehabil Med. 1985;7:176–181. doi: 10.3109/03790798509165991. [DOI] [PubMed] [Google Scholar]
- 17.Pusswald G, Fertl E, Faltl M, Auff E. Neurological rehabilitation of severely disabled cardiac arrest survivors. Part II. Life situation of patients and families after treatment. Resuscitation. 2000;47:241–248. doi: 10.1016/s0300-9572(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 18.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA. 1993;269:237–242. [PubMed] [Google Scholar]
- 19.Dougherty CM. Longitudinal recovery following sudden cardiac arrest and internal cardioverter defibrillator implantation: Survivors and their families. Am J Crit Care. 1994;3:145–154. [PubMed] [Google Scholar]
- 20.Drysdale EE, Grubb NR, Fox KAA, O’Carroll RE. Chronicity of memory impairment in long-term out-of-hospital cardiac arrest survivors. Resuscitation. 2000;47:27–32. doi: 10.1016/s0300-9572(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 21.Elliott VJ, Rodgers DL, Brett SJ. Systematic review of quality of life and other patient-centred outcomes after cardiac arrest survival. Resuscitation. 2011;82:247–256. doi: 10.1016/j.resuscitation.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 22.de Vos R. Quality of survival after cardiopulmonary resuscitation. Arch Intern Med. 1999;159:249–254. doi: 10.1001/archinte.159.3.249. [DOI] [PubMed] [Google Scholar]
- 23.Harve H, Tiainen M, Poutiainen E, Maunu M, Kajaste S, Roine RO, et al. The functional status and perceived quality of life in long-term survivors of out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2007;51:206–209. doi: 10.1111/j.1399-6576.2006.01214.x. [DOI] [PubMed] [Google Scholar]
- 24.Lim C, Alexander MP, LaFleche G, Schnyer DM, Verfaellie M. The neurological and cognitive sequelae of cardiac arrest. Neurology. 2004;63:1774–1778. doi: 10.1212/01.wnl.0000144189.83077.8e. [DOI] [PubMed] [Google Scholar]
- 25.Nunes B. Cardiac arrest: Long-term cognitive and imaging analysis. Resuscitation. 2003;57:287–297. doi: 10.1016/s0300-9572(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 26.Grubb NR, O’Carroll RE, Cobbe SM, Sirel J, Fox KAA. Chronic memory impairment after cardiac arrest outside hospital. BMJ. 1996;313:143–146. doi: 10.1136/bmj.313.7050.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunch TJ, White RD, Smith GE, Hodge DO, Gersh BJ, Hammill SC, et al. Long-term subjective memory function in ventricular fibrillation out-of-hospital cardiac arrest survivors resuscitated by early defibrillation. Resuscitation. 2004;60:189–195. doi: 10.1016/j.resuscitation.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 28.van Alem AP, Waalewijn RA, Koster RW, de Vos R. Assessment of quality of life and cognitive function after out-of-hospital cardiac arrest with successful resuscitation. Am J Cardiol. 2004;93:131–135. doi: 10.1016/j.amjcard.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Horsted TI, Rasmussen LS, Meyhoff CS, Nielsen SL. Long-term prognosis after out-of-hospital cardiac arrest. Resuscitation. 2007;72:214–218. doi: 10.1016/j.resuscitation.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren-Nilsson, Rosén H, Hofgren C, Sunnerhagen KS. The first year after successful cardiac resuscitation: Function, activity, participation and quality of life. Resuscitation. 2005;66:285–289. doi: 10.1016/j.resuscitation.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Stiell I, Nichol G, Wells G, De Maio V, Nesbitt L, Blackburn J, et al. Health-related quality of life is better for cardiac arrest survivors who received citizen cardiopulmonary resuscitation. Circulation. 2003;108:1939–1944. doi: 10.1161/01.CIR.0000095028.95929.B0. [DOI] [PubMed] [Google Scholar]
- 32.Nichol G, Stiell IG, Hebert P, Wells GA, Vandemheen K, Laupacis A. What is the quality of life for survivors of cardiac arrest? A prospective study. Acad Emerg Med. 1999;6:95–102. doi: 10.1111/j.1553-2712.1999.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 33.Bro-Jeppesen J, Kjaergaard J, Horsted T, Wanschler M, Nielsen S, Rasmussen L, et al. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscitation. 2009;80:171–176. doi: 10.1016/j.resuscitation.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Lukkarinen H, Hentinen M. Assessment of quality of life with the nottingham health profile among patients with coronary heart disease. J Adv Nurs. 1997;26:73–84. doi: 10.1046/j.1365-2648.1997.1997026073.x. [DOI] [PubMed] [Google Scholar]
- 35.Granja C, Cabral G, Pinto AT, Costa-Pereira A. Quality of life 6-months after cardiac arrest. Resuscitation. 2002;55:37–44. doi: 10.1016/s0300-9572(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 36.Middelkamp W, Moulaert VR, Verbunt JA, van Heugten CM, Bakx WG, Wade DT. Life after survival: Long-term daily life functioning and quality of life of patients with hypoxic brain injury as a result of a cardiac arrest. Clin Rehabil. 2007;21:425–431. doi: 10.1177/0269215507075307. [DOI] [PubMed] [Google Scholar]