Abstract
Although previous studies have demonstrated that the hippocampus plays a role in pain processing, the role of hippocampal subfields is uncertain. The goal of this study was to examine the relationship between hippocampal subfield volumes and chronic pain in nondemented older adults. The study sample included 86 community-residing adults age 70 or older who were free of dementia and recruited from the Einstein Aging Study. Chronic pain was defined as pain over the last 3 months, that was moderate or severe (minimum rating of 4 out of 10) most, or all of the time. Hippocampal subfield volumes were estimated using FreeSurfer software. We modeled the association between chronic pain and hippocampal and subfield volume using linear regression. The sample had a mean age of 80 and was 58% female. Chronic pain, present in 55% of the sample, was associated with smaller right and total hippocampal volumes, particularly in women, after adjusting for age, education, and intracranial volume (eTICV). In addition, in women, volume was significantly reduced in participants with chronic pain in right CA2–3 (β=−0.35, p=0.010), right CA4-DG (β=−0.35, p=0.011), left presubiculum (β=−0.29, p=0.030), and left fimbria (β=−0.30, p=0.023). In men, chronic pain was not associated with the volume of any of the hippocampal subfield volumes. Chronic pain in women is associated with a reduction in the volume of right hippocampus and also selected hippocampal subfields. Future studies should clarify the mechanisms underlying the association between regional hippocampal volumes and chronic pain, particularly in women.
Keywords: Hippocampal Subfields, Hippocampal Volume, Chronic Pain, MRI, Older Adults
1. INRODUCTION
Chronic pain occurs in more than half of older adults resulting in reductions in daily activities and decrements in health-related quality of life. The prevalence of disabling pain increases sharply amongst elderly individuals, especially the oldest-old (Thomas et al., 2004; Thomas et al., 2007). It is therefore important to understand both the factors that contribute to the development or acceleration of pain in the elderly as well as its consequences.
Reasons for the increasing prevalence of pain in older adults are not fully understood. Painful conditions such as osteoarthritis are more common in the elderly, which leads to increased peripheral nociception (Oliveria et al., 1995). In addition, aging is associated with widespread changes in the cellular and neurochemical substrates of the nervous system, including the nociceptive system. The functional consequences of biological age-related changes are difficult to extrapolate given the highly-integrated nature of pain processing, but it is clear that there is a relationship between changes in neurobiological structure and function and the experience of pain (Gibson and Farrell, 2004).
Overall pain prevalence is higher in females than males across the entire life span, though certain pain disorders have a male predilection (Fillingim et al., 2009). It has been suggested that different biological mechanisms such as gonadal hormones and endogenous pain modulatory systems, as well as psychological mechanisms including cognitive or affective factors may contribute to sex differences in pain and analgesic responses (Fillingim et al., 2009). However, it is still unclear which biological mechanisms contribute to this gender difference in pain perception.
The hippocampus plays an important role in a variety of physiological processes including memory, mood and stress (Price and Drevets, 2010; Zimmerman et al., 2008). Many investigators have evaluated the role of the hippocampus in pain processing in human and animal studies (Bingel et al., 2002; Duric and McCarson, 2006; Schweinhardt et al., 2006; Zimmerman et al., 2009). Neuroimaging studies have shown that the hippocampus is activated in response to painful stimuli in healthy volunteers (Bingel et al., 2002). Furthermore, adults with chronic pain syndromes have functional and anatomical alterations in brain regions involved in pain processing including the hippocampus, the thalamus, the basal ganglia and amygdala as well as cingulate, prefrontal and somatosensory cortex (Schweinhardt and Bushnell, 2010). In older adults, higher levels of pain severity have been associated with both reductions in hippocampal volume (HV) and lower NAA levels in the hippocampus (Zimmerman et al., 2009). Alterations in specific transmitters have been demonstrated. For example, patients with fibromyalgia have decreased presynaptic dopaminergic activity was evident in several brain regions, including the hippocampus (Schweinhardt et al., 2006).
The hippocampal formation consists of various subfields (subregions) including CA1, CA2, CA3, CA4, dentate gyrus (DG), fimbria, presubiculum, and subiculum. These subfields differ in histology, connectivity and function (Fanselow and Dong, 2010). Different hippocampal subfields have been implicated in different cognitive and psychiatric disorders (Elvsashagen et al., 2013; Hanseeuw et al., 2011; Wang et al., 2010). Though animal studies demonstrate involvement of specific hippocampal subfields in pain (for a review, see Liu et al (Liu and Chen, 2009)), the volumetric changes associated with chronic pain has rarely, if ever, been studied in humans. The goal of this study was to examine the association of pain with total HV as well as subfields in nondemented older women and men.
2. RESULTS
2.1. Demographic and Sample Characteristics (Table 1)
Table 1.
Sample demographics, TPI scores, and hippocampal measurements
| Total sample, mean (SD) |
NCP group mean (SD) |
CP group mean (SD) |
p valueb | |
|---|---|---|---|---|
| Sample size | 86 | 39 | 47 | |
| % Women | 65.1 | 53.8 | 74.4 | 0.046 |
| % White | 54.7 | 59 | 51.1 | 0.515 |
| % Right handed | 89.5 | 92.3 | 87.2 | 0.444 |
| Age, years | 80.23 (4.82) | 80.02 (4.99) | 80.40 (4.72) | 0.722 |
| Education, years | 14.21 (3.57) | 15.18 (2.99) | 13.40 (3.83) | 0.021 |
| TPI | 4.34 (6.57) | 0.30 (.48) | 7.69 (7.37) | <0.001 |
| FCSRT IR free recall score | 32.06 (6.78) | 32.38 (7.25) | 31.79 (6.44) | 0.690 |
| FCSRT IR total recall score | 47.24 (2.55) | 47.54 (1.16) | 47.00 (3.29) | 0.334 |
| Left Hippocampal volume | 3.19 (0.40) | 3.28 (0.38) | 3.13 (0.41) | 0.076 |
| Right Hippocampal volume | 3.27 (0.44) | 3.39 (0.34) | 3.15 (0.49) | 0.010 |
| Total Hippocampal volume a | 6.46 (0.78) | 6.67 (0.63) | 6.28 (0.84) | 0.018 |
| eTICV | 1340 (202) | 1382 (197) | 1305 (201) | 0.078 |
MRI volumetric data are given in cubic centimeters.
Using t-test for continuous variables, and Chi-square test for categorical variables.
CP= Chronic pain, NCP= No chronic pain, TPI=Total Pain Index; FCSRT-IR = Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall; eTICV= estimated Total Intracranial Volume
The sample had a mean age of 80.2 years (SD=4.8). The sample was 53.8% women and 54.7% white, with a mean of 14.2 years (SD=3.5) of education. Participants with chronic pain (CP) represented 55% of the sample and those free of CP (NCP) represented 45%. Chronic pain groups did not differ in age or eTICV. The prevalence of CP was significantly higher in women (62%; n=35) than in men (40%; n=12; t=2.02, p=0.046). The CP group had a lower education level (t=2.4, p=0.021) than the NCP group. There was a negative correlation between age of participants and total HV (rs=−0.28, p=0.009) and women had smaller total HV than men (t=−3.6, p=0.001). There was no significant correlation between HVs and education level.
2.2. Total, Right and Left Hippocampal Volumes (Table 2)
Table 2.
Regression models assessing the effect of chronic pain (CP) on Hippocampal Volume (HV)
| Hippocampal Area | Predictor | Models for Total Sample | Models for Women | Models for Men | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | t | p value | Beta | t | Sig | Beta | t | p value | ||
| Models for Total HV | Chronic pain | −0.22 | −2.18 | 0.032 | −0.31 | −2.33 | 0.024 | −0.13 | −0.65 | 0.516 |
| Age | −0.20 | −2.03 | 0.046 | −0.26 | −1.95 | 0.056 | −0.21 | −1.12 | 0.272 | |
| Education | −0.16 | −1.58 | 0.118 | −0.07 | −0.58 | 0.563 | −0.32 | −1.66 | 0.108 | |
| eTICV | −0.01 | −0.01 | 0.995 | 0.07 | 0.56 | 0.577 | −0.15 | −0.81 | 0.424 | |
| Gender | 0.27 | 2.17 | 0.032 | |||||||
| Models for Left HV | Chronic pain | −0.15 | −1.45 | 0.150 | −0.22 | −1.65 | 0.105 | −0.07 | −0.40 | 0.691 |
| Age | −0.06 | −0.59 | 0.556 | −0.10 | −0.78 | 0.438 | −0.07 | −0.42 | 0.677 | |
| Education | −0.14 | −1.41 | 0.162 | −0.04 | −0.31 | 0.751 | −0.38 | −2.04 | 0.051 | |
| eTICV | −0.02 | −0.15 | 0.875 | 0.13 | 0.93 | 0.356 | −0.30 | −1.69 | 0.102 | |
| Gender | 0.34 | 2.64 | 0.010 | |||||||
| Models for Right HV | Chronic pain | −0.25 | −2.46 | 0.016 | −0.33 | −2.58 | 0.013 | −0.15 | −0.75 | 0.458 |
| Age | −0.30 | −3.00 | 0.004 | −0.35 | −2.77 | 0.008 | −0.29 | −1.48 | 0.150 | |
| Education | −0.14 | −1.45 | 0.151 | −0.09 | −0.73 | 0.464 | −0.22 | −1.11 | 0.276 | |
| eTICV | 0.01 | 0.13 | 0.892 | 0.01 | 0.08 | 0.930 | −0.01 | −0.01 | 0.991 | |
| Gender | 0.17 | 1.34 | 0.183 | |||||||
eTICV= estimated Total Intracranial Volume, HV=Hippocampal volume
In unadjusted models, total HV was significantly smaller in participants with CP (β=−0.25, p=0.018) in comparison with participants with NCP. Right HV was significantly reduced in the CP group (β=−027, p=0.012) relative to the NCP group; whereas, left HV volume differences did not reach significance (β=−0.19, p=0.076).
After adjusting for age, gender, education, and eTICV in linear regression models, the association between chronic pain and hippocampal volumes persisted, showing significantly reduced total HV (β=−0.22, p=0.032) and right HV (β=−0.25, p=0.016), but not left HV (β=−0.15, p=0.15) in the CP versus the NCP group (See Table 2).
Next we investigated whether there was an interactive effect between gender and chronic pain on hippocampal volume. Inclusion of the interaction term eliminated the influence of gender and CP on hippocampal volumes (table S-1). However, given the well-known differences in pain in men and women, we reran the regression models stratifying by gender while adjusting for other covariates. Among women, results were similar to the total population showing an association between chronic pain and reduced total (β=−0.31, p=0.024) and right HV (β=−0.33, p=0.013), but not with left HV (β=−0.22, p=0.105). In men, associations between chronic pain and total, right and left hippocampal volumes were not significant. Table 2 shows the results of the adjusted linear regression models stratified by gender.
2.3. Hippocampal Subfield Volumes (Table 3)
Table 3.
Regression models assessing the effect of chronic pain (CP) on hippocampal subfield volumes.
| Hippocampal subfield | Models for CP in Total Sample* |
Models for CP in Women** |
CP Models for CP in Men** |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | t | p-value | Beta | t | p-value | Beta | t | p-value | ||
| CA1 | Left | 0.03 | 0.28 | 0.773 | −0.01 | −0.10 | 0.917 | −0.02 | −0.11 | 0.910 |
| Right | −0.13 | −1.26 | 0.211 | −0.24 | −1.86 | 0.069 | −0.04 | −0.18 | 0.858 | |
| CA2/CA3 | Left | −0.12 | −1.09 | 0.276 | −0.26 | −1.98 | 0.053 | 0.08 | 0.37 | 0.709 |
| Right | −0.20 | −1.94 | 0.055 | −0.34 | −2.65 | 0.011 | 0.02 | 0.13 | 0.895 | |
| CA4/DG | Left | −0.08 | −0.82 | 0.415 | −0.26 | −1.95 | 0.057 | 0.13 | 0.65 | 0.519 |
| Right | −0.17 | −1.66 | 0.101 | −0.34 | −2.66 | 0.010 | 0.07 | 0.34 | 0.731 | |
| Pre-subiculum | Left | −0.13 | −1.31 | 0.193 | −0.28 | −2.19 | 0.033 | 0.19 | 1.01 | 0.321 |
| Right | −0.07 | −0.65 | 0.515 | −0.14 | −1.03 | 0.307 | 0.18 | 0.93 | 0.360 | |
| Subiculum | Left | −0.04 | −0.44 | 0.656 | −0.26 | −1.95 | 0.056 | 0.29 | 1.43 | 0.164 |
| Right | −0.09 | −0.82 | 0.409 | −0.18 | −1.39 | 0.169 | 0.07 | 0.34 | 0.735 | |
| Fimbria | Left | −0.10 | −0.95 | 0.341 | −0.30 | −2.33 | 0.023 | 0.26 | 1.26 | 0.216 |
| Right | 0.02 | 0.25 | 0.799 | −0.02 | −0.13 | 0.892 | 0.03 | 0.163 | 0.872 | |
| Hippocampal Fissure | Left | 0.04 | 0.39 | 0.695 | −0.08 | −0.58 | 0.561 | 0.16 | 0.786 | 0.439 |
| Right | −0.04 | −0.35 | 0.726 | −0.08 | −0.67 | 0.505 | 0.01 | 0.048 | 0.962 | |
Models include age, gender, education, and eTICV as covariates.
Models include age, education, and eTICV as covariates
eTICV= estimated Total Intracranial Volume, HV=Hippocampal volume
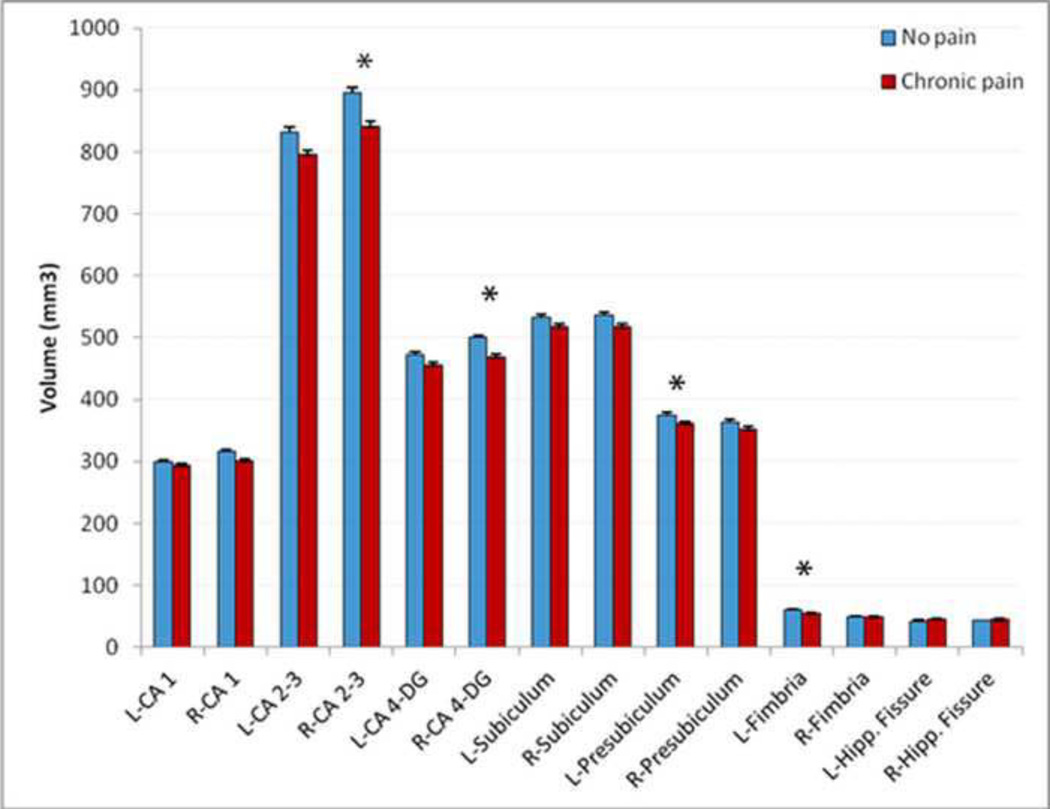
In unadjusted models, CP was associated with smaller right CA2–3 (β=−0.24, p=0.023) and CA4-DG volumes (β=−0.26, p=0.015). No other subfields demonstrated a significant association with chronic pain.
We therefore ran multiple linear regression analysis with the hippocampal subfield volumes as the dependent variable and the chronic pain groups (CP vs NCP), age, education, eTICV and gender as independent variables (Table 3). There were no significant effects of pain group on any subfield volume after controlling for all covariates. Similar to the analysis for total hippocampal volumes, we stratified data based on gender and repeated the regression models. In women, a significant effect of CP group was observed for right CA2–3 (β=−0.35, p=0.010), right CA4-DG (β=−0.35, p=0.011), left presubiculum (β=−0.29, p=0.030), and left fimbria (β=−0.30, p=0.023), with CP group consistently having smaller subfield volumes. In men, CP did not show any association with any of the hippocampal subfield volumes.
3. DISCUSSION
In this cross sectional study, we found a striking association between chronic pain and reduced hippocampal volume, especially in the right hippocampus and in women. Unilateral hippocampal volume loss has been previously reported in older adults with or without cognitive impairment (Shi et al., 2009), and mood disorders (Videbech and Ravnkilde, 2004). Studies using animal models have also showed lateralized pattern in response to painful stimuli in hippocampus (Belcheva et al., 2009). This asymmetric association between volume loss and pain may be due to asymmetric neurodegeneration and reorganization of neuronal pathways of the limbic system. However, little information is available about the role and differences of right or left hippocampus in pain processing.
In this study, as in many others, the prevalence of chronic pain was higher in women than in men. In addition, HVs showed association with chronic pain only in women. Gender differences in pain perception are well established for clinical pain (Unruh, 1996) and experimental pain, both in humans (Riley et al., 1998) and animal models (Aloisi et al., 2000). Women show different responses to pain, including lower pain thresholds, less tolerance and a greater response to painful stimuli in comparison with men (Hurley and Adams, 2008). Perhaps this gender disparity is attributable to hormonal differences that influence brain functions by modulating neurotransmission (Aloisi and Bonifazi, 2006). The hormonal differences include both stress and sex hormones. The hippocampus regulates the hypothalamic-pituitary-adrenal (HPA) axis through a negative feedback mechanism via mineralocorticoid and glucocorticoid receptors, both of which are highly expressed in the dentate gyrus (Galea et al., 2013). Prior animal studies suggest that stress affect neural plasticity of hippocampus differently in females compared to men (Galea et al., 2013). Other animal studies demonstrate sex differences in the hippocampal responses to aversive stimuli and suggest that sex hormones may mediate these differences (Aloisi et al., 2000). Estrogens and androgens are neuroactive hormones that can be synthesized in the central nervous system de novo from endogenous cholesterol, especially in the hippocampus (Shibuya et al., 2003). Increases in estrogens can increase the numbers of dendrite spines and excitatory synapses in hippocampal neurons (Woolley and Schwartzkroin, 1998) and also excite neurons in the cerebral cortex, cerebellum and hippocampus by a non-genomic mechanism (McEwen and Alves, 1999). Although women experience fluctuations in estrogen and progesterone levels across the menstrual cycle and face a steep decline in sex hormone levels during menopause, men show a progressive and slow decline in testosterone with aging (Hogervorst, 2013). Testosterone levels continue to be much higher in men than women. There is plenty of evidence showing protective effects of testosterone on cognitive function and mood stability in older adults (Hogervorst, 2013), but studies on the effects of testosterone on perception of pain are limited. Such hormonal differences in men and women and their intermediate role on neurodegeneration and reorganization of neuronal pathways involved in pain and stress processing might underlie our women-specific finding of hippocampal volume loss with pain.
In addition to the lateralized and gender-specific effects, we also found differential effects in various subfields. Among older women, chronic pain was associated with smaller right CA2–3 and CA4-DG volumes. Animals, studies have shown that responses to painful stimuli differ among hippocampal subfields (Aloisi et al., 2000; McKenna and Melzack, 1992; McKenna and Melzack, 2001) with some reports of lateralized effects (Belcheva et al., 2009). Chronic inflammatory nociception significantly reduced the neurogenesis in the DG, providing a substrate for volume loss (Duric and McCarson, 2006). Injection of lidocaine or NMDA receptor antagonists into the DG reduces chronic pain, while injection in CA1 do not induce analgesia (McKenna and Melzack, 1992; McKenna and Melzack, 2001). Other studies have shown that injection of injection of vasoactive intestinal peptide (VIP) into CA1 in the rat elicits lateralized anti nociceptive effect (Belcheva et al., 2009). Our finding of a relationship between chronic pain and volume loss in CA4 DG is broadly compatible with these animal studies.
The subfield-specific association between HV and pain might be explained by anatomical differences among regions. Complex networks of afferent and efferent pathways connect the hippocampus to other parts of brain and are responsible for transmitting pain information. The entorhinal cortex is the major source of afferent pain stimuli to the hippocampus; these pathways terminate specifically on the dendrites of the CA1, CA3, DG, and subiculum (Liu and Chen, 2009). The fornix fibers comprise the efferent output from the hippocampus originating in the CA1 region and projecting to the anterior thalamus, the mammillary region, and limbic midbrain (Henke, 1982). The other efferent fibers include fimbria fibers that originate in CA2, CA3, CA4 and subiculum and project to the anterior thalamus, the preoptic area, and the hypothalamus (Meibach and Siegel, 1977). Our subfield findings predominantly indicate a change in the volume of regions that fimbria fibers originate from.
Another possible explanation could be molecular and biochemical differences between various hippocampal subfields. Stressful and noxious stimuli induce hormonal and behavioral modifications and lasting changes in many physiological parameters, which result in profound alterations in protein synthesis. Accordingly, it is possible that painful stimulation would probably lead to analogous changes in immediate early genes (IEG) expression in the hippocampus, to fulfill the necessary physiological and behavioral functions pertinent to pain. Immediate early genes (IEGs) are crucial intermediates in a cascade linking membrane stimulation to long term alterations of neuronal activity (Pennypacker et al., 1995). Several studies have shown a change in c-Fos (one of the IESs) expression following painful stimulation in the hippocampus specifically in CA1, CA3, and DG (Aloisi, 1997; Aloisi et al., 2000). Such changes in gene expression and subsequent protein synthesis, might lead to long-term changes in the function or volume of these subfields.
While our subfield findings are promising, a few limitations should be noted. We did not evaluate information about the duration of pain beyond the 3 month before acquiring images. Inclusion of the interaction term in models eliminated the influence of gender on hippocampal volumes, which is probably because our study was underpowered to detect this effect. Furthermore, consumption of various anti-inflammatory medications or pain medications is very common in this age group and might affect either perception of pain or volumetric changes. Finally, as this is a cross-sectional study we cannot determine the causal relationship of events and if the observed hippocampal volume reduction predisposes individuals to perception of pain or is it persistent pain that results in hippocampal atrophy.
In summary, chronic pain is associated with a selective reduction in hippocampal subfields' volume. This reduction of volume is seen exclusively in women. Such gender exclusivity demonstrates the importance of gender stratification in studies investigating pain. This gender stratification could be of particular importance in translational and clinical studies for development of sex-specific pain treatments. Future, longitudinal studies are required to investigate the role of hippocampus subfields in the development and experience of pain in elderly population and the temporal relationship between chronic pain and hippocampal volume loss.
4. Experimental Procedure
4.1 Participants
The participants were 86 non-demented adults over the age of 70 years drawn from the Einstein Aging Study (EAS). The study design and methods of the EAS have been described in detail previously (Katz et al., 2012). Briefly, potential participants were recruited through systematic sampling from Medicare and voter registration lists for Bronx County, New York. Eligible participants were at least 70 years old, Bronx residents, non-institutionalized, and English-speaking. Exclusion criteria included visual or auditory impairments that preclude neuropsychological testing, active psychiatric symptomatology that interfered with the ability to complete assessments, and non-ambulatory status. Participants received annual in-person assessments which included medical histories, neuropsychological testing and general and neurologic examinations.
Participants who were demented or did not meet standard MRI eligibility criteria - metallic implants that obscure or interfere with MRI- were excluded from the MRI study. Dementia diagnosis was based on standardized clinical criteria from the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV., 2000) and required impairment in memory plus at least one additional cognitive domain, accompanied by evidence of functional decline. Diagnoses were assigned at consensus case conferences, which included a comprehensive review of cognitive test results, relevant neurological signs and symptoms, and functional status. All studies were approved by institutional review board of Albert Einstein College of Medicine.
4.2. Pain Measurements
Total pain and chronic pain was measured as a ubiquitous exposure in all body areas. The total pain index (TPI) is an inventory of pain symptoms that consists of questions concerning pain location, frequency, severity, and duration for eight body areas (head, face, neck and shoulder, back, arms and hands, legs and feet, chest, abdomen and pelvis, and other). For each body area, participants were asked: “In the past 3 months, how often did you have pain in the (insert one of the eight body areas)?” Participants had the option of choosing between “none of the time,” “a slight bit of the time,” “some of the time,” “most of the time,” and “all of the time” as their response. Participants then rated the intensity of their worst pain over the previous 3 months on a scale of 0 to 10, for each body area with pain (range 0–80). Chronic pain was defined by the presence of pain, in at least 1 location, that was moderate or severe (minimum rating of 4 out of 10) in the previous 3 months, some, most, or all of the time (McCarthy et al., 2009). This approximates the International Association for the Study of Pain definition of chronic pain (Breivik et al., 2006). This measure is valid and reliable in our elderly adult population [data not shown].
4.3. Neuropsychological Assessment
Verbal memory was assessed using the free recall score (range 0–48) from the Free and Cued Selective Reminding Test–Immediate Recall (FCSRT-IR) (Buschke, 1984). Details of neuropsychological tests have been previously described (Katz et al., 2012).
4.4. MRI Acquisition and Processing
Imaging was performed using a 3.0 T "short bore" MRI scanner (Achieva Quasar TX; Philips Medical Systems, Best, the Netherlands) with a 32-channel head coil (Sense Head Coil; Philips Medical Systems). T1-weighted whole-head structural imaging was performed using sagittal three-dimensional magnetization-prepared rapid acquisition gradient echo with parallel acceleration (MP-RAGE; TR/TE 9.9/4.6ms; 240 mm2 field of view; 240×240 mm matrix; slab thickness, 1 mm). In addition, a fluid-attenuated inversion recovery (FLAIR) acquisition was obtained with the following pulse sequence parameters: TR/TE/TI 11000/120/2800ms, 240 mm FOV, 0.6845×0.9758×2 mm, 70 slices, no gap, SENSE 2, and acquisition time of 3 min 18.0 sec.
4.5. Image Analysis
We processed all MRIs automatically using the FreeSurfer software package (version 5.2, available at http://surfer.nmr.mgh.harvard.edu/). Briefly, the processing stream starts with a hybrid watershed algorithm, which removes non brain tissue, automated transformation to the, Talairach reference space and segmentation of the subcortical white matter and deep gray matter. FLAIR images were used for pial surface refinement. The whole hippocampal formation was segmented using FreeSurfer’s standard segmentation procedure using a probabilistic brain atlas (Fischl et al., 2002). Additionally, for each subject the estimated intracranial volume (eTICV) was calculated based on the procedure described by Buckner et al (Buckner et al., 2004).
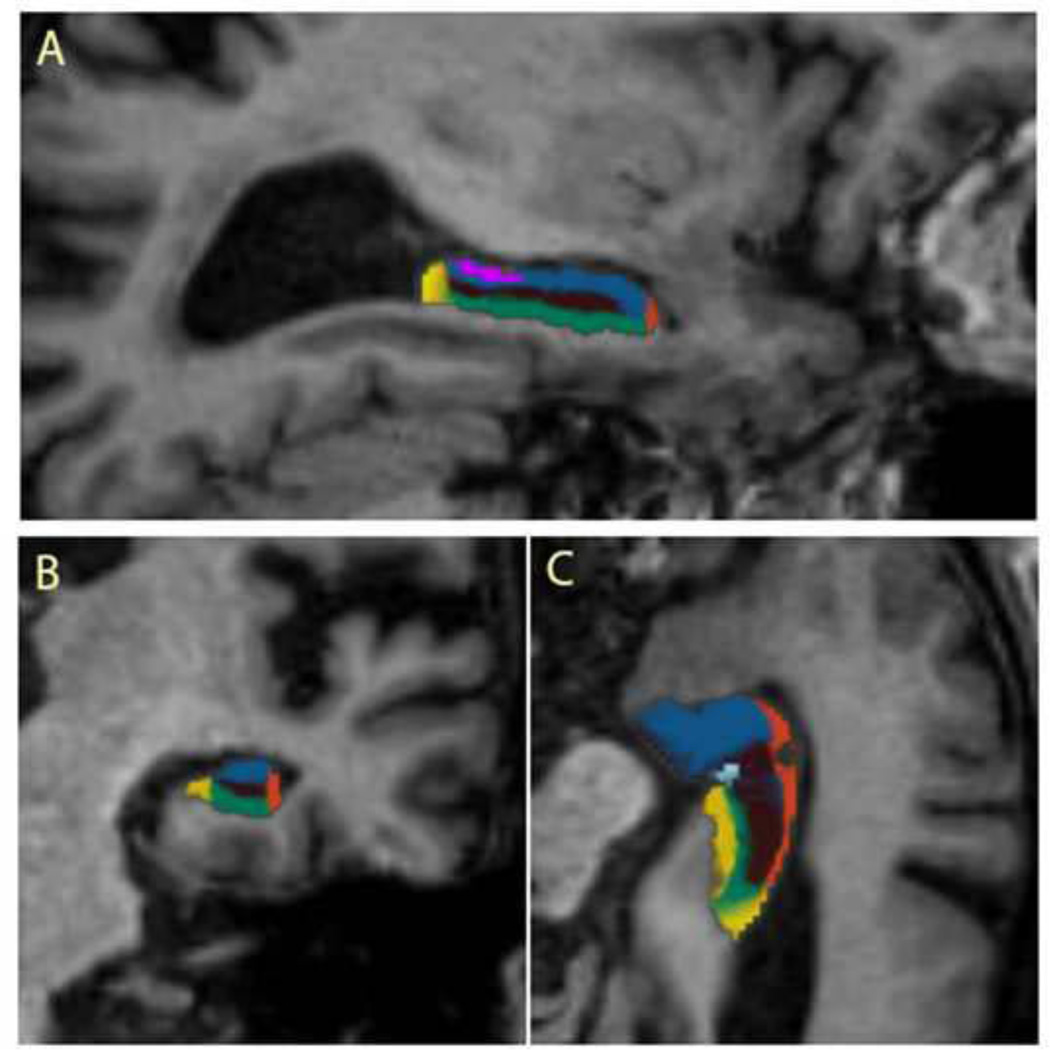
Subsequently, we performed automated subfield segmentation of the hippocampus using a recently added procedure within the FreeSurfer suite. This procedure uses Bayesian inference and a probabilistic atlas of the hippocampal formation which is based on manual delineations of subfields in T1-weighted MRI scans from a number of different subjects (Van Leemput et al., 2009). Seven subfield volumes were calculated for each side of the hippocampus: CA1, CA2–3, CA4-DG, presubiculum, subiculum, fimbria, and hippocampal fissure. Automated volume estimates of these subfields are shown to correlate well with manual volume estimates (Van Leemput et al., 2009). Segmentation results were visually inspected for errors in all datasets, but no manual edits were needed. Figure 1 shows the whole hippocampus and subfields segmentation results in one of the participants.
Figure 1.
Sagittal (A), Coronal (B) and Axial (C) images illustrating segmentation of subfields in the right hippocampus of a participant. Color code: purple = fimbria; brown = DG–CA4; blue = CA2–3; orange = CA1; green = subiculum; dark yellow = presubiculum. CA = cornu ammonis; DG; = dentate gyrus.
4.6. Statistical Analyses
Total HV volume was normally distributed in our population (p=0.178). We examined the bivariate associations of hippocampal measures with demographic variables such as age, education, sex and eTICV using Spearman rank correlation coefficients. Linear regression models with hippocampal volumes as the outcome, were used to assess the association between chronic pain and MRI measures, firstly unadjusted, and then adjusted for age, sex, education, and eTICV. Furthermore, to evaluate whether the association between chronic pain and hippocampal volumes was driven by gender differences, we stratified the data by gender and reran all the previous models separately for each gender. Chi-square, t-tests and ANOVAs were used as appropriate. All statistical analyses were conducted using SPSS, version 20 (Chicago, IL: SPSS Inc.)
Supplementary Material
Figure 2.
Differences between groups in hippocampal subfield volumes. CA: cornus armonis; DG: dentate gyrus; Hipp: hippocampal. Bar graphs present data distribution with average means and 95% confidence interval of hippocampal subfield volumes among participants with and without chronic pain. (*) Indicates significant results. Error bars represent standard error of mean.
Highlights.
Chronic pain is associated with a selective hippocampal subfields' volume loss.
This association is asymmetric and right hippocampus shows stronger correlations.
Gender exclusivity shows the importance of gender stratification in pain studies.
ACKNOWLEDGMENTS
Funding: This research was supported by National Institute on Aging Grant AG03949 and AG026728.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors declare that there are no financial, personal, or other potential conflicts of interest to report.
Author Contributions: MEZ, MLL, RBL: Study concept and design. MJK, MLL: Data acquisition. AE, EES, JLS: MRI data-analysis, AE: Statistical analysis, interpretation and initial manuscript preparation. AE, MEZ, MJK, EES, JLS, MLL, RBL: Critical revision of manuscript for important intellectual content. All authors contributed to and approved the final manuscript.
REFERENCES
- Aloisi AM. Sex differences in pain-induced effects on the septo-hippocampal system. Brain Res Brain Res Rev. 1997;25:397–406. doi: 10.1016/s0165-0173(97)00030-1. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I, Herdegen T. Gonadectomy and persistent pain differently affect hippocampal c-Fos expression in male and female rats. Neurosci Lett. 2000;281:29–32. doi: 10.1016/s0304-3940(00)00819-3. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav. 2006;50:1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Belcheva I, Ivanova M, Tashev R, Belcheva S. Differential involvement of hippocampal vasoactive intestinal peptide in nociception of rats with a model of depression. Peptides. 2009;30:1497–1501. doi: 10.1016/j.peptides.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- Elvsashagen T, Westlye LT, Boen E, Hoi PK, Andersson S, Andreassen OA, Boye B, Malt UF. Evidence for reduced dentate gyrus and fimbria volume in bipolar II disorder. Bipolar Disord. 2013;15:167–176. doi: 10.1111/bdi.12046. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013 doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- Hanseeuw BJ, Van Leemput K, Kavec M, Grandin C, Seron X, Ivanoiu A. Mild cognitive impairment: differential atrophy in the hippocampal subfields. AJNR Am J Neuroradiol. 2011;32:1658–1661. doi: 10.3174/ajnr.A2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke PG. The telencephalic limbic system and experimental gastric pathology: a review. Neurosci Biobehav Rev. 1982;6:381–390. doi: 10.1016/0149-7634(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Hogervorst E. Effects Of Gonadal Hormones On Cognitive Behavior In Elderly Men And Women. J Neuroendocrinol. 2013 doi: 10.1111/jne.12080. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth Analg. 2008;107:309–317. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull. 2009;25:237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009;57:115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Melzack R. Analgesia produced by lidocaine microinjection into the dentate gyrus. Pain. 1992;49:105–112. doi: 10.1016/0304-3959(92)90195-H. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Melzack R. Blocking NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test. Exp Neurol. 2001;172:92–99. doi: 10.1006/exnr.2001.7777. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- Pennypacker KR, Hong JS, McMillian MK. Implications of prolonged expression of Fos-related antigens. Trends Pharmacol Sci. 1995;16:317–321. doi: 10.1016/s0165-6147(00)89061-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Lee M, Tracey I. Imaging pain in patients: is it meaningful? Curr Opin Neurol. 2006;19:392–400. doi: 10.1097/01.wco.0000236620.89710.63. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Bushnell MC. Pain imaging in health and disease--how far have we come? J Clin Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori TA, Kawato S. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Thomas E, Mottram S, Peat G, Wilkie R, Croft P. The effect of age on the onset of pain interference in a general population of older adults: prospective findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2007;129:21–27. doi: 10.1016/j.pain.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. doi: 10.1002/hipo.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Schwartzkroin PA. Hormonal effects on the brain. Epilepsia. 1998;39(Suppl 8):S2–S8. doi: 10.1111/j.1528-1157.1998.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, Katz MJ, Verghese J, Buschke H, Derby CA, Lipton RB. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology. 2008;70:1594–1600. doi: 10.1212/01.wnl.0000306314.77311.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009;73:1567–1570. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.