Abstract
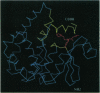
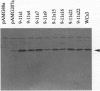
Glutathione S-transferases (EC 2.5.1.18) in mammalian cells catalyze the conjugation, and thus, the detoxication of a structurally diverse group of electrophilic environmental carcinogens and alkylating drugs, including the antineoplastic nitrogen mustards. We proposed that structural alteration of the nonspecific electrophile-binding site would produce mutant enzymes with increased efficiency for detoxication of a single drug and that these mutants could serve as useful somatic transgenes to protect healthy human cells against single alkylating agents used in cancer chemotherapy protocols. Random mutagenesis of three regions (residues 9-14, 102-112, and 210-220), which together compose the glutathione S-transferase electrophile-binding site, followed by selection of Escherichia coli expressing the enzyme library with the nitrogen mustard mechlorethamine (20-500 microM), yielded mutant enzymes that showed significant improvement in catalytic efficiency for mechlorethamine conjugation (up to 15-fold increase in kcat and up to 6-fold increase in kcat/Km) and that confer up to 31-fold resistance, which is 9-fold greater drug resistance than that conferred by the wild-type enzyme. The results suggest a general strategy for modification of drug- and carcinogen-metabolizing enzymes to achieve desired resistance in both prokaryotic and eukaryotic plant and animal cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton M. G., Hilton J., Robertson K. D., Streeper R. T., Colvin O. M., Noe D. A. Kinetic analysis of the reaction of melphalan with water, phosphate, and glutathione. Drug Metab Dispos. 1993 Nov-Dec;21(6):986–996. [PubMed] [Google Scholar]
- Bolton M. G., Hilton J., Robertson K. D., Streeper R. T., Colvin O. M., Noe D. A. Kinetic analysis of the reaction of melphalan with water, phosphate, and glutathione. Drug Metab Dispos. 1993 Nov-Dec;21(6):986–996. [PubMed] [Google Scholar]
- Buller A. L., Clapper M. L., Tew K. D. Glutathione S-transferases in nitrogen mustard-resistant and -sensitive cell lines. Mol Pharmacol. 1987 Jun;31(6):575–578. [PubMed] [Google Scholar]
- Ciaccio P. J., Tew K. D., LaCreta F. P. The spontaneous and glutathione S-transferase-mediated reaction of chlorambucil with glutathione. Cancer Commun. 1990;2(8):279–285. doi: 10.3727/095535490820874263. [DOI] [PubMed] [Google Scholar]
- Conney A. H., Chang R. L., Jerina D. M., Wei S. J. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab Rev. 1994;26(1-2):125–163. doi: 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- Deisseroth A. B. Current trends and future directions in the genetic therapy of human neoplastic disease. Cancer. 1993 Oct 1;72(7):2069–2074. doi: 10.1002/1097-0142(19931001)72:7<2069::aid-cncr2820720703>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Pegg A. E., Dumenco L. L., Moschel R. C., Gerson S. L. Comparison of the inactivation of mammalian and bacterial O6-alkylguanine-DNA alkyltransferases by O6-benzylguanine and O6-methylguanine. Carcinogenesis. 1991 Dec;12(12):2305–2309. doi: 10.1093/carcin/12.12.2305. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Gamcsik M. P., Hamill T. G., Colvin M. NMR studies of the conjugation of mechlorethamine with glutathione. J Med Chem. 1990 Mar;33(3):1009–1014. doi: 10.1021/jm00165a019. [DOI] [PubMed] [Google Scholar]
- Gulick A. M., Fahl W. E. Mammalian glutathione S-transferase: regulation of an enzyme system to achieve chemotherapeutic efficacy. Pharmacol Ther. 1995 May;66(2):237–257. doi: 10.1016/0163-7258(94)00079-i. [DOI] [PubMed] [Google Scholar]
- Gulick A. M., Goihl A. L., Fahl W. E. Structural studies on human glutathione S-transferase pi. Family of native-specific monoclonal antibodies used to block catalysis. J Biol Chem. 1992 Sep 15;267(26):18946–18952. [PubMed] [Google Scholar]
- Hermes J. D., Parekh S. M., Blacklow S. C., Köster H., Knowles J. R. A reliable method for random mutagenesis: the generation of mutant libraries using spiked oligodeoxyribonucleotide primers. Gene. 1989 Dec 7;84(1):143–151. doi: 10.1016/0378-1119(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Huskey S. E., Wang R. W., Linemeyer D. L., Pickett C. B., Lu A. Y. Expression in Escherichia coli of rat liver cytosolic glutathione S-transferase Yc cDNA. Arch Biochem Biophys. 1990 May 15;279(1):116–121. doi: 10.1016/0003-9861(90)90470-j. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ziegler D. M. The enzymes of detoxication. J Biol Chem. 1990 Dec 5;265(34):20715–20718. [PubMed] [Google Scholar]
- Ji X., Armstrong R. N., Gilliland G. L. Snapshots along the reaction coordinate of an SNAr reaction catalyzed by glutathione transferase. Biochemistry. 1993 Dec 7;32(48):12949–12954. doi: 10.1021/bi00211a001. [DOI] [PubMed] [Google Scholar]
- Ji X., Johnson W. W., Sesay M. A., Dickert L., Prasad S. M., Ammon H. L., Armstrong R. N., Gilliland G. L. Structure and function of the xenobiotic substrate binding site of a glutathione S-transferase as revealed by X-ray crystallographic analysis of product complexes with the diastereomers of 9-(S-glutathionyl)-10-hydroxy-9,10-dihydrophenanthrene. Biochemistry. 1994 Feb 8;33(5):1043–1052. doi: 10.1021/bi00171a002. [DOI] [PubMed] [Google Scholar]
- Ji X., Zhang P., Armstrong R. N., Gilliland G. L. The three-dimensional structure of a glutathione S-transferase from the mu gene class. Structural analysis of the binary complex of isoenzyme 3-3 and glutathione at 2.2-A resolution. Biochemistry. 1992 Oct 27;31(42):10169–10184. doi: 10.1021/bi00157a004. [DOI] [PubMed] [Google Scholar]
- Johnson W. W., Liu S., Ji X., Gilliland G. L., Armstrong R. N. Tyrosine 115 participates both in chemical and physical steps of the catalytic mechanism of a glutathione S-transferase. J Biol Chem. 1993 Jun 5;268(16):11508–11511. [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Manoharan T. H., Gulick A. M., Puchalski R. B., Servais A. L., Fahl W. E. Structural studies on human glutathione S-transferase pi. Substitution mutations to determine amino acids necessary for binding glutathione. J Biol Chem. 1992 Sep 15;267(26):18940–18945. [PubMed] [Google Scholar]
- Manoharan T. H., Gulick A. M., Reinemer P., Dirr H. W., Huber R., Fahl W. E. Mutational substitution of residues implicated by crystal structure in binding the substrate glutathione to human glutathione S-transferase pi. J Mol Biol. 1992 Jul 20;226(2):319–322. doi: 10.1016/0022-2836(92)90949-k. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. High-dose cisplatin therapy in ovarian cancer. Semin Oncol. 1985 Dec;12(4 Suppl 6):21–30. [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Huber R., Lo Bello M., Federici G., Parker M. W. Three-dimensional structure of class pi glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 A resolution. J Mol Biol. 1992 Sep 5;227(1):214–226. doi: 10.1016/0022-2836(92)90692-d. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Schäffer J., Gallay O., Huber R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991 Aug;10(8):1997–2005. doi: 10.1002/j.1460-2075.1991.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinning I., Kleywegt G. J., Cowan S. W., Reinemer P., Dirr H. W., Huber R., Gilliland G. L., Armstrong R. N., Ji X., Board P. G. Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the Mu and Pi class enzymes. J Mol Biol. 1993 Jul 5;232(1):192–212. doi: 10.1006/jmbi.1993.1376. [DOI] [PubMed] [Google Scholar]
- Sorrentino B. P., Brandt S. J., Bodine D., Gottesman M., Pastan I., Cline A., Nienhuis A. W. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science. 1992 Jul 3;257(5066):99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994 Aug 15;54(16):4313–4320. [PubMed] [Google Scholar]
- Wang R. W., Newton D. J., Huskey S. E., McKeever B. M., Pickett C. B., Lu A. Y. Site-directed mutagenesis of glutathione S-transferase YaYa. Important roles of tyrosine 9 and aspartic acid 101 in catalysis. J Biol Chem. 1992 Oct 5;267(28):19866–19871. [PubMed] [Google Scholar]
- Wolf C. R., Hayward I. P., Lawrie S. S., Buckton K., McIntyre M. A., Adams D. J., Lewis A. D., Scott A. R., Smyth J. F. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987 Jun 15;39(6):695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]