Abstract
Purpose
To determine the functional role of M3 and M5 muscarinic acetylcholine receptor subtypes in ophthalmic arteries using gene-targeted mice.
Methods
Muscarinic receptor gene expression was quantified in murine ophthalmic arteries using real-time PCR. To test the functional relevance of M3 and M5 receptors, ophthalmic arteries from mice deficient in either subtype (M3R-/-, M5R-/-, respectively) and wild-type controls were isolated, cannulated with micropipettes and pressurized. Changes in luminal vessel diameter in response to muscarinic and nonmuscarinic receptor agonists were measured by video microscopy.
Results
Using real-time PCR, all five muscarinic receptor subtypes were detected in ophthalmic arteries. However, mRNA levels of M1, M3 and M5 receptors were higher than those of M2 and M4 receptors. In functional studies, after preconstriction with phenylephrine, acetylcholine and carbachol produced concentration-dependent dilations of ophthalmic arteries that were similar in M5R-/- and wild-type mice. Strikingly, cholinergic dilation of ophthalmic arteries was almost completely abolished in M3R-/- mice. Deletion of either M3 or M5 receptor did not affect responses to nonmuscarinic vasodilators, such as bradykinin or nitroprusside.
Conclusions
These findings provide the first evidence that M3 receptors are critically involved in cholinergic regulation of diameter in murine ophthalmic arteries.
Keywords: acetylcholine, muscarinic receptors, ophthalmic arteries, gene-targeted mice
Introduction
Disturbed ocular and retrobulbar hemodynamics have been observed in a variety of eye diseases, including age-related macular degeneration,1-3 diabetic retinopathy,4-6 nonarteritic anterior ischemic optic neuropathy,7;8 and glaucoma.9;10 Endothelial dysfunction, defined as impaired endothelium-dependent vasodilation to specific stimuli,11 has been implicated in the pathophysiology of these diseases.12-15 Acetylcholine is a powerful dilator of most vascular beds and a major investigative and diagnostic tool for the assessment of endothelial function.16-19 Its activity is mediated by endothelial muscarinic receptors triggering the release of vasorelaxing agents, such as nitric oxide (NO).20;21
Systemically or topically applied cholinergic agents, including muscarinic receptor agonists, were shown to increase ocular blood flow in experimental animals22;23 and humans,24 suggesting that acetylcholine is involved in regulation of ocular perfusion via activation of muscarinic receptors. Hence, it is important to define muscarinic receptor signaling at the molecular level in ocular arteries to understand the pathophysiological changes in the eye that occur with endothelial dysfunction.
Five muscarinic receptor subtypes, denoted M1 through M5, have been identified.25 They are generally grouped according to their functional coupling, either to the mobilization of intracellular calcium via the activation of phospholipase Cβ (M1, M3, M5) or to the inhibition of adenylyl cyclase (M2, M4).26 Remarkably, the expression pattern of muscarinic receptor subtypes and their role in mediating vascular responses differs substantially between individual vascular beds.27-30 Thus, they may represent an attractive therapeutic target for the selective treatment of local ischemic disorders.
To this date, muscarinic acetylcholine receptor expression has not been determined in ocular arteries. Therefore, we used real-time PCR to quantify mRNA expression of individual muscarinic receptor subtypes in isolated murine ophthalmic arteries.
Previous studies employing pharmacological approaches and electrical stimulation of parasympathetic nerve pathways suggest the involvement of M3 receptors in mediating choroidal vasodilation in pigeons,31 and of M3 and M5 receptors in chronically sympathectomized rats.32 However, conclusions regarding the physiological role of individual muscarinic receptor subtypes are hampered by the limited specificity of the pharmacological agents tested.25;33 For example, the pharmacological properties of the M3 receptor are very similar to those of the M5 subtype,34 raising the possibility that responses previously thought to be mediated by M3 receptors may involve the activation of M5 receptors. To circumvent these difficulties, we used mice deficient in the expression of M3 and M5 receptors to determine the role of either subtype in mediating cholinergic vasodilation in ophthalmic arteries.
Materials and Methods
Animals
All experiments were performed in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the federal animal rights committee. The generation of M3R-/-and M5R-/- mice has been described previously.29;35 Briefly, the M3 or the M5 receptor gene was inactivated using mouse embryonic stem cells derived from 129SvEv mice. The resulting chimeric mice were then mated with CF-1 mice to generate M3R-/-, M5R-/-, and wild-type mice with the following genetic contribution: 129SvEv (50%) × CF-1 (50%). In all experiments, male mice at the age of 3-5 months were used.
Real-Time PCR Analysis in Isolated Ophthalmic Arteries
Muscarinic receptor gene expression was quantified in isolated ophthalmic arteries of wild-type mice using real-time PCR. After mice were sacrificed by CO2 inhalation, ophthalmic arteries were carefully isolated by using fine-point tweezers under a dissecting microscope, added into a 1.5-ml tube and immediately snap frozen. To increase RNA yield, arteries were pooled from five mice. Subsequently, vessels were homogenized in lysis buffer using a FastPrep device (MP Biomedicals, Illkirch France). After homogenization, total RNA was extracted using the Absolutely RNA Nanoprep Kit (Stratagene, La Jolla, CA, USA) according to the manufacturers' protocol. After complete DNA digestion, the RNA was reverse transcribed using Superscript and random hexamers (Invitrogen, Karlsruhe, Germany). Quantitative PCR analysis was performed using a GeneAmp StepOne Plus (Applied Biosystems, Darmstadt, Germany). SYBR Green was used for the fluorescent detection of DNA generated during the PCR. The PCR reaction was performed in a total volume of 25μl with 0.4 pmol/μl of each primer, and 2 × SYBR Green Master Mix (Bioline, Luckenwalde, Germany); 2 μl cDNA corresponding to 10 ng RNA was used as template. Published sequences for mouse M1 (NM_007698), M2 (NM_203491), M3 (NM_033269), M4 (NM_007699) and M5 (NM_205783) were used to design primers for PCR amplification. Primer sequences were M1 sense 5′-TGA CAG GCA ACC TGC TGG TGC T-3′ and antisense 5′- AAT CAT CAG AGC TGC CCT GCG G-3′; M2 sense 5′- CGG ACC ACA AAA ATG GCA GGC AT-3′and antisense 5′- CCA TCA CCA CCA GGC ATG TTG TTG T-3′; M3 sense5′- CCT CTT GAA GTG CTG CGT TCT GAC C-3′ and antisense 5′- TGC CAG GAA GCC AGT CAA GAA TGC-3′; M4 sense 5′- TGT GGT GAG CAA TGC CTC TGT CAT G-3′ and antisense 5′- GGC TTC ATC AGA GGG CTC TTG AGG A-3′; M5 sense 5′- ACC ACT GAC ATA CCG AGC CAA GCG-3′ and antisense 5′- TTC CCG TTG TTG AGG TGC TTC TAC G-3′; ß-actin sense 5′-CAC CCG CGA GCA CAG CTT CTT T-3′ and antisense 5′-AAT ACA GCC CGG GGA GCA TC-3′. The expression levels of M1, M2, M3, M4 and M5 mRNA were normalized to β-actin using the ΔCt –method.
Measurements of Vascular Reactivity
Mice were sacrificed by CO2 inhalation and the eyes were rapidly removed together with the retrobulbar tissue and placed in ice-cold Krebs buffer with the following ionic composition (in mM): 118.3 NaCl, 4.7 KCl , 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose (Carl Roth GmbH, Karlsruhe, Germany). Then, ophthalmic arteries from wild-type, M3R-/- and M5R-/- mice were isolated under a dissecting microscope, placed in an organ chamber filled with cold Krebs solution, cannulated onto glass micropipettes, and secured with 10-0 nylon monofilament suture as described previously for arteries of other vascular beds.29 Vessels were pressurized via the micropipettes to 50 mmHg under no-flow conditions using two reservoirs filled with Krebs solution and imaged using a video camera mounted on an inverted microscope (Leica DM IL, Wetzlar, Germany). Video sequences were captured to a personal computer for analysis using imaging software (Khoros, Khoral Research, Inc., Albuquerque, NM, USA). The organ chamber was continuously circulated with oxygenated and carbonated Krebs buffer at 37°C and pH 7.4. Arteries were allowed to equilibrate for 60 minutes before the experiments started. Viability of vessels was assessed as satisfactory when at least 50% constriction from resting diameter in response to membrane depolarization with KCl (100 mM) was achieved. Then, contractile responses to the α1-adrenergic receptor agonist phenylephrine (10-9-10-4 M, Sigma-Aldrich, Munich, Germany) were tested. Neither responses to KCl nor to phenylephrine differed between the three groups of mice (data not shown). Subsequently, arteries were preconstricted with phenylephrine to 50-60% of the initial vessel diameter and cumulative concentration-response curves to acetylcholine (10-9-10-3 M), carbachol (10-9-10-3 M), bradykinin (10-11-10-5 M), and nitroprusside (10-10-10-4 M) were obtained (all drugs from Sigma-Aldrich, Munich, Germany). Responses to acetylcholine were also compared before and after addition of atropine (3×10-5 M, Sigma-Aldrich, Munich, Germany), a nonselective muscarinic receptor antagonist.
Statistical Analysis
Data are presented as mean ± SE. Vascular responses are presented as percentage of change in diameter from the preconstricted diameter. When multiple vessels from a single mouse were studied, responses were averaged so that n represents the number of mice per group. Comparisons between concentration-response curves were made using ANOVA for repeated measures followed by the Bonferroni test to detect individual differences. For comparisons of vascular responses to acetylcholine before and after atropine treatment, the Wilcoxon signed-rank test was used. A value of P<0.05 was defined as significant.
Results
Muscarinic Receptor mRNA Expression in Ophthalmic Arteries
Expression of muscarinic receptor mRNA was determined in ophthalmic arteries from wild-type mice using real-time PCR. All five muscarinic receptor subtypes were found to be expressed in ophthalmic arteries. However, mRNA levels of M1, M3 and M5 receptors, which couple to phospholipase Cβ and intracellular calcium mobilization, were higher than those of M2 and M4 receptors, which couple to the inhibition of adenylyl cyclase (Fig.1).
Figure 1.
Relative mRNA expression of individual muscarinic receptor subtypes (M1-M5) normalized to β-actin transcripts in ophthalmic arteries pooled from five wild-type mice. The values are an average of three independent PCR measurements. Values are expressed as means ± SE.
Responses of Ophthalmic Arteries to Acetylcholine and Carbachol
Baseline luminal diameters of ophthalmic arteries (before preconstriction) were 109±8 μm, 111±8 μm, and 107±10 μm in M3R-/-, M5R-/-, and wild-type mice and did not differ between individual groups (P>0.05, ANOVA). To examine whether M3 or M5 receptors are involved in cholinergic responses of ophthalmic arteries, we compared vascular responses from M3R-/-, M5R-/-, and wild-type mice to acetylcholine (10-9-10-3 M) and carbachol (10-9-10-3 M). Acetylcholine elicited dose-dependent dilation of ophthalmic arteries from M5R-/- mice that was not different from vasodilation obtained in wild-type mice (Fig. 2A). Maximal dilation to 10-4 M acetylcholine was 44±7% (n=11) and 56±9% (n=11) in M5R-/- and wild-type mice, respectively.
Figure 2.
Responses of ophthalmic arteries from wild-type, M3R-/- and M5R-/- mice to acetylcholine. (A) Vasodilation to acetylcholine was almost completely abolished in ophthalmic arteries from M3R-/- mice. In contrast, deletion of the M5 receptor gene had no significant effect on relaxation of ophthalmic arteries in response to acetylcholine. Values are expressed as means ± SE (n=11 per concentration and genotype). *, P < 0.01 (M3R-/- vs. M5R-/- and wild-type mice). Absence of error bar indicates that the SE was less than the size of the symbol. (B) Responses of ophthalmic arteries to acetylcholine (10-4 M) were virtually abolished after addition of atropine (3×10-5 M). Values are expressed as means ± SE (n=8-10 per group). *, P < 0.01.
Strikingly, acetylcholine-induced vasodilation was almost completely abolished in M3R-/- mice. Dilation to 10-4 M acetylcholine was only 4±2% (n=11) in this group and not statistically different from baseline values (Fig. 2A).
To test whether cholinergic responses of ophthalmic arteries were mediated by muscarinic receptors, we examined responses to acetylcholine after addition of atropine (3×10-5 M), a nonselective muscarinic receptor blocker. Following atropine treatment, responses to acetylcholine were virtually abolished in all groups of mice (Fig. 2B), indicative of the involvement of muscarinic receptors.
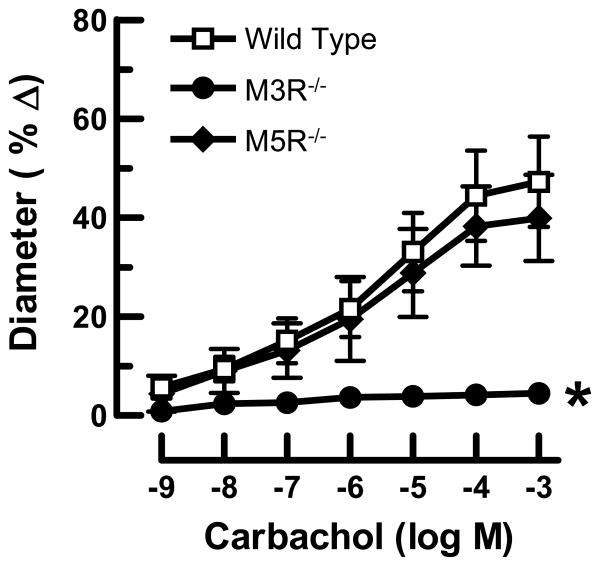
To exclude the possibility that the different responses to acetylcholine were caused by differences in acetylcholinesterase activity in the vascular wall, we tested responses of ophthalmic arteries to carbachol, another muscarinic receptor agonist, which in contrast to acetylcholine is resistant to degradation by acetylcholinesterase. Similar to acetylcholine, carbachol induced dose-dependent relaxation of opthalmic arteries from M5R-/- and wild-type mice that did not differ between these two groups (Fig. 3). For example, maximal dilation to 10-4 M carbachol was 38±8% (n=9) and 44±9% (n=11) in M5R-/- and wild-type mice, respectively. In contrast, vasodilation to carbachol was only 4±2% (n=11) in M3R-/- mice and not statistically different from baseline values (Fig. 3).
Figure 3.
Responses of ophthalmic arteries from wild-type, M3R-/- and M5R-/- mice to carbachol. Relaxation in response to carbachol was almost completely abolished in ophthalmic arteries from M3R-/- mice, while deletion of the M5 receptor gene had no significant effect on carbachol-induced vasodilation. Values are expressed as means ± SE (n=9-11 per concentration and genotype). *, P < 0.01 (M3R-/- vs. M5R-/- and wild-type mice). Absence of error bar indicates that the SE was less than the size of the symbol.
Responses of Ophthalmic Arteries to Bradykinin and Nitroprusside
To test whether deletion of the M3 and M5 receptor genes affected responses to nonmuscarinic vasodilators in ophthalmic arteries, we examined vascular responses in M3R-/-, M5R-/-, and wild-type mice to the endothelium-dependent vasodilator, bradykinin (10-11-10-5 M). Bradykinin elicited concentration-dependent dilatory responses in arteries from M3R-/-, M5R-/-, and wild-type mice that did not differ between the three groups (Fig. 4A). Maximal dilation to 10-6 M bradykinin was 18±3% (n=11), 22±5% (n=9) and 21±4% (n=11) in M3R-/-, M5R-/- and wild-type mice, respectively. Also, the endothelium-independent NO donor nitroprusside (10-10-10-4 M) produced concentration-dependent dilation of ophthalmic arteries, that did not differ between the three groups of mice (Fig. 4B). For example, maximal vasodilation to 10-5 M nitroprusside was 48±8% (n=11), 45±9% (n=9) and 53±8% (n=11) in M3R-/-, M5R-/- and wild-type mice, respectively.
Figure 4.
Responses of ophthalmic arteries from wild-type, M3R-/- and M5R-/- mice to bradykinin (A) and nitroprusside (B). Deletion of either M3 or M5 receptor did not affect responses to nonmuscarinic vasodilators, such as the endothelium-dependent agonist bradykinin or the endothelium-independent NO donor nitroprusside. Values are expressed as means ± SE (n=9-11 per concentration and genotype).
Discussion
The major goal of the present study was to identify the muscarinic receptor subtypes that mediate responses of ophthalmic arteries to acetylcholine. Using real-time PCR, we found all five muscarinic receptor subtypes to be expressed in ophthalmic arteries of wild-type mice. However, M1, M3 and M5 receptors were expressed at higher levels than M2 and M4 receptors. Since previous studies pointed towards an involvement of M3 and M5 receptors in cholinergic regulation of ocular perfusion,31;32 we used mice deficient in M3 or M5 receptors to assess the functional relevance of either subtype in isolated ophthalmic arteries. Strikingly, deletion of the M3 receptor almost completely abolished cholinergic vasodilation while the absence of M5 receptors had no effect on responses to acetylcholine. Responses to acetylcholine were primarily mediated by muscarinic receptors because blockade of muscarinic receptor activation with atropine abolished responses in wild-type and M5R-/- mice. In M3R-/- mice, dilation of ophthalmic arteries was also negligible in response to the muscarinic receptor agonist carbachol, which is resistant to degradation by acetylcholinesterase. Thus, an increased acetylcholinesterase activity in the vascular wall is not likely to account for the strongly reduced cholinergic dilation of ophthalmic arteries in M3R-/- mice. Deletion of either M3 or M5 receptor did not affect responses to nonmuscarinic vasodilators, such as the NO donor nitroprusside or the endothelium-dependent agonist bradykinin, suggesting that the absence of these receptors does not interfere with the downstream signaling cascades that ultimately mediate vasorelaxation. Thus, these data provide the first direct evidence that M3 receptors are critically involved in cholinergic vasodilation of ophthalmic arteries.
In a previous study employing intravenous administration of subtype-selective muscarinic receptor antagonists and electrical stimulation of parasympathetic nerve pathways, endothelial M3 receptors were suggested to mediate choroidal vasodilation in pigeons.31 In another study, where a similar experimental approach has been used, M3 and M5 receptors were proposed to be involved in parasympathetic-mediated choroidal vasodilation in chronically sympathectomized rats.32 However, the specificity of the pharmacological agents tested has shown to be limited.33;36 For example, the pharmacological properties of the M3 receptor are very similar to those of the M5 subtype,34 raising the possibility that responses previously thought to be mediated by M3 receptors may involve the activation of M5 receptors. Moreover, even selective M1 and M2 antagonists display high affinity for M3 and M4 receptors, respectively.25;33 Consequently, it is very difficult to discern the role of each muscarinic receptor subtype by using pharmacological agents of limited specificity when two or more subtypes are simultaneously involved in a specific functional response. The use of genetically engineered mice lacking specific muscarinic receptor subtypes allows a more definitive determination of the physiological roles of M3 and M5 receptors in ocular vessels. Recently, the function of muscarinic receptor subtypes has been examined in other arterial beds of gene-targeted mice. In these studies, the M3 subtype was shown to mediate cholinergic vasodilation in femoral37 and coronary arteries30 as well as in aorta30;37;38, while the M5 subtype mediated responses to acetylcholine in cerebral arteries and arterioles.29 Remarkably, similar to our present findings in ophthalmic arteries, no functional role of M5 receptors has been demonstrated for any extra-cerebral murine vascular bed tested so far.29
We found mRNA of all five muscarinic receptor subtypes to be expressed in ophthalmic arteries of wild-type mice. However, cholinergic responses were predominantly mediated by M3 receptors, raising the question about the physiological role of the other coexpressed receptor subtypes. One possibility is that the other subtypes are expressed by vascular smooth muscle or by autonomic nerve terminals rather than endothelial cells and play a role in regulating signaling pathways in vascular smooth muscle28 or in modulating transmitter release from autonomic nerves.31;32
Based on previous in vivo studies in healthy animals and humans, systemic pharmacological blockade of muscarinic receptors does not appear to significantly affect ocular blood flow under resting conditions and during isometric exercise.31;39-41 In contrast, pharmacological activation of muscarinic receptors was shown to increase pulsatile ocular blood flow in ocular hypertensive humans24 and long posterior ciliary artery blood flow in rabbits,22 suggesting that muscarinic mechanisms are involved in ocular blood flow regulation. In support of this concept, pharmacological blockade of muscarinic receptors was demonstrated to attenuate increases in choroidal blood flow induced by parasympathetic activation in pigeons.31 Hence, it remains to be established under which conditions muscarinic acetylcholine receptors contribute to ocular blood flow regulation.
Our findings in ophthalmic arteries do not necessarily reflect the situation in all ocular vessels, since there exist substantial anatomical and functional differences within the ocular vascular bed.42;43 For example, retinal arteries as opposed to the ophthalmic artery, are not innervated by autonomic nerve fibers, which may result in differences in autonomic input between the ophthalmic artery and retinal vessels. Thus, acetylcholine-mediated vasodilation in the ophthalmic artery induced by activation of autonomic nerve fibers may transmit a higher blood pressure to the retinal vascular system, which in turn may respond with a myogenic constriction of arterioles to keep retinal blood flow constant. Furthermore, we cannot rule out the possibility that cholinergic responses of retinal arteries are mediated by another muscarinic receptor subtype than in ophthalmic arteries.
In a variety of cardiovascular diseases, acetylcholine-induced vasodilation was shown to be impaired. The factors underlying these altered responses include changes of post-receptor mechanisms, such as reduction of endothelial nitric oxide synthase (eNOS) expression and increase in protein kinase C (PKC) activity during hyperglycaemia, a mechanism, which has been implicated in the pathogenesis of diabetic retinopathy.44;45 However, in some pathologies, vasodilation to acetylcholine is selectively impaired, whereas responses to other endothelium-dependent vasodilators are barely affected.46;47 Thus, specific changes in muscarinic acetylcholine receptor function, for example, by receptor downregulation or uncoupling from intracellular signaling pathways may also contribute to abnormal cholinergic vasodilation in pathologic conditions.
Due to several technical limitations of in vivo measurements of ocular and retrobulbar blood flow48-50 and the limited availability of human vascular tissue, studies in ocular vascular preparations from animal models remain important in order to understand the mechanisms accounting for ischemic disorders in the eye. The use of gene-targeted mice offers an attractive opportunity to define the mechanisms leading to disturbed ocular perfusion at the molecular level. Moreover, such studies may help to design specific pharmacological approaches to treat abnormal ocular perfusion.
In conclusion, the data of the present study provide the first direct evidence that cholinergic vasodilation of ophthalmic arteries is mediated by M3 receptors. From a clinical point of view, selective M3 muscarinic receptor agonists may become therapeutically useful to increase ocular perfusion in some pathophysiological conditions, such as age-related macular degeneration and glaucoma.
Acknowledgments
The work was supported by a grant from the Ernst und Berta Grimmke Stiftung.
Grant: Supported by a grant from the Ernst und Berta Grimmke Stiftung
References
- 1.Friedman E, Krupsky S, Lane AM, Oak SS, Friedman ES, Egan K, et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102:640–6. doi: 10.1016/s0161-6420(95)30974-8. [DOI] [PubMed] [Google Scholar]
- 2.Pournaras CJ, Logean E, Riva CE, Petrig BL, Chamot SR, Coscas G, et al. Regulation of subfoveal choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:1581–6. doi: 10.1167/iovs.05-0434. [DOI] [PubMed] [Google Scholar]
- 3.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:358–63. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitrova G, Kato S, Yamashita H, Tamaki Y, Nagahara M, Fukushima H, et al. Relation between retrobulbar circulation and progression of diabetic retinopathy. Br J Ophthalmol. 2003;87:622–5. doi: 10.1136/bjo.87.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage HI, Hendrix JW, Peterson DC, Young H, Wilkinson CP. Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:4504–9. doi: 10.1167/iovs.04-0077. [DOI] [PubMed] [Google Scholar]
- 6.Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–3. doi: 10.1136/bjo.2003.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collignon-Robe NJ, Feke GT, Rizzo JF., III Optic nerve head circulation in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Ophthalmology. 2004;111:1663–72. doi: 10.1016/j.ophtha.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Kaup M, Plange N, Arend KO, Remky A. Retrobulbar haemodynamics in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2006;90:1350–3. doi: 10.1136/bjo.2006.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findl O, Rainer G, Dallinger S, Dorner G, Polak K, Kiss B, et al. Assessment of optic disk blood flow in patients with open-angle glaucoma. Am J Ophthalmol. 2000;130:589–96. doi: 10.1016/s0002-9394(00)00636-x. [DOI] [PubMed] [Google Scholar]
- 10.Emre M, Orgul S, Gugleta K, Flammer J. Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol. 2004;88:662–6. doi: 10.1136/bjo.2003.032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 12.Lip PL, Blann AD, Hope-Ross M, Gibson JM, Lip GY. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108:705–10. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 13.Malecki MT, Osmenda G, Walus-Miarka M, Skupien J, Cyganek K, Mirkiewicz-Sieradzka B, et al. Retinopathy in type 2 diabetes mellitus is associated with increased intima-media thickness and endothelial dysfunction. Eur J Clin Invest. 2008;38:925–30. doi: 10.1111/j.1365-2362.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 14.Potarazu SV. Ischemic optic neuropathy: models for mechanism of disease. Clin Neurosci. 1997;4:264–9. [PubMed] [Google Scholar]
- 15.Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2008 doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 16.Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–7. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM, Sigmund CD. Vascular biology in genetically altered mice : smaller vessels, bigger insight. Circ Res. 1999;85:1214–25. doi: 10.1161/01.res.85.12.1214. [DOI] [PubMed] [Google Scholar]
- 18.Klonizakis M, Tew G, Michaels J, Saxton J. Impaired microvascular endothelial function is restored by acute lower-limb exercise in post-surgical varicose vein patients. Microvasc Res. 2008 doi: 10.1016/j.mvr.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Henry E, Newby DE, Webb DJ, O'Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–4. [PubMed] [Google Scholar]
- 20.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 21.Leung HS, Leung FP, Yao X, Ko WH, Chen ZY, Vanhoutte PM, et al. Endothelial mediators of the acetylcholine-induced relaxation of the rat femoral artery. Vascul Pharmacol. 2006;44:299–308. doi: 10.1016/j.vph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Okubo H, Gherezhiher T, Koss MC. Long posterior ciliary arterial blood flow and systemic blood pressure. Invest Ophthalmol Vis Sci. 1990;31:819–26. [PubMed] [Google Scholar]
- 23.Mann RM, Riva CE, Stone RA, Barnes GE, Cranstoun SD. Nitric oxide and choroidal blood flow regulation. Invest Ophthalmol Vis Sci. 1995;36:925–30. [PubMed] [Google Scholar]
- 24.Shaikh MH, Mars JS. The acute effect of pilocarpine on pulsatile ocular blood flow in ocular hypertension. Eye. 2001;15:63–6. doi: 10.1038/eye.2001.15. [DOI] [PubMed] [Google Scholar]
- 25.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- 26.Hosey MM. Diversity of structure, signaling and regulation within the family of muscarinic cholinergic receptors. FASEB J. 1992;6:845–52. [PubMed] [Google Scholar]
- 27.Phillips JK, Vidovic M, Hill CE. Variation in mRNA expression of alpha-adrenergic, neurokinin and muscarinic receptors amongst four arteries of the rat. J Auton Nerv Syst. 1997;62:85–93. doi: 10.1016/s0165-1838(96)00114-2. [DOI] [PubMed] [Google Scholar]
- 28.Elhusseiny A, Cohen Z, Olivier A, Stanimirovic DB, Hamel E. Functional acetylcholine muscarinic receptor subtypes in human brain microcirculation: identification and cellular localization. J Cereb Blood Flow Metab. 1999;19:794–802. doi: 10.1097/00004647-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, et al. Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98:14096–101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamping KG, Wess J, Cui Y, Nuno DW, Faraci FM. Muscarinic (M) receptors in coronary circulation: gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler Thromb Vasc Biol. 2004;24:1253–8. doi: 10.1161/01.ATV.0000130661.82773.ca. [DOI] [PubMed] [Google Scholar]
- 31.Zagvazdin Y, Fitzgerald ME, Reiner A. Role of muscarinic cholinergic transmission in Edinger-Westphal nucleus-induced choroidal vasodilation in pigeon. Exp Eye Res. 2000;70:315–27. doi: 10.1006/exer.1999.0791. [DOI] [PubMed] [Google Scholar]
- 32.Steinle JJ, Smith PG. Presynaptic muscarinic facilitation of parasympathetic neurotransmission after sympathectomy in the rat choroid. J Pharmacol Exp Ther. 2000;294:627–32. [PubMed] [Google Scholar]
- 33.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 34.Watson N, Daniels DV, Ford AP, Eglen RM, Hegde SS. Comparative pharmacology of recombinant human M3 and M5 muscarinic receptors expressed in CHO-K1 cells. Br J Pharmacol. 1999;127:590–6. doi: 10.1038/sj.bjp.0702551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–12. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 36.Eglen RM, Watson N. Selective muscarinic receptor agonists and antagonists. Pharmacol Toxicol. 1996;78:59–68. doi: 10.1111/j.1600-0773.1996.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 37.Beny JL, Nguyen MN, Marino M, Matsui M. Muscarinic receptor knockout mice confirm involvement of M3 receptor in endothelium-dependent vasodilatation in mouse arteries. J Cardiovasc Pharmacol. 2008;51:505–12. doi: 10.1097/FJC.0b013e31816d5f2f. [DOI] [PubMed] [Google Scholar]
- 38.Khurana S, Chacon I, Xie G, Yamada M, Wess J, Raufman JP, et al. Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R-/- mice. Eur J Pharmacol. 2004;493:127–32. doi: 10.1016/j.ejphar.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp Eye Res. 1999;69:413–29. doi: 10.1006/exer.1999.0717. [DOI] [PubMed] [Google Scholar]
- 40.Jandrasits K, Polak K, Luksch A, Stark B, Dorner GT, Eichler HG, et al. Effects of atropine and propranolol on retinal vessel diameters during isometric exercise. Ophthalmic Res. 2001;33:185–90. doi: 10.1159/000055668. [DOI] [PubMed] [Google Scholar]
- 41.Polska E, Luksch A, Schering J, Frank B, Imhof A, Fuchsjager-Mayrl G, et al. Propranolol and atropine do not alter choroidal blood flow regulation during isometric exercise in healthy humans. Microvasc Res. 2003;65:39–44. doi: 10.1016/s0026-2862(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 42.Delaey C, Van d V. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 43.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Chakravarthy U, Hayes RG, Stitt AW, McAuley E, Archer DB. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998;47:945–52. doi: 10.2337/diabetes.47.6.945. [DOI] [PubMed] [Google Scholar]
- 45.Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye. 2002;16:242–60. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- 46.Holdright DR, Clarke D, Fox K, Poole-Wilson PA, Collins P. The effects of intracoronary substance P and acetylcholine on coronary blood flow in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 1994;15:1537–44. doi: 10.1093/oxfordjournals.eurheartj.a060427. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto T, Horie H, Minai K, Yokohama H, Takashima H, Ohira N, et al. Coronary vasomotor responses to bradykinin and acetylcholine in patients with coronary spastic angina. Jpn Circ J. 2001;65:1052–6. doi: 10.1253/jcj.65.1052. [DOI] [PubMed] [Google Scholar]
- 48.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it - myth and reality. Prog Retin Eye Res. 2001;20:563–93. doi: 10.1016/s1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 49.Feke GT. Retrobulbar haemodynamics in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2006;90:1334–5. doi: 10.1136/bjo.2006.0101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmetterer L, Garhofer G. How can blood flow be measured? Surv Ophthalmol. 2007;52(Suppl 2):S134–S138. doi: 10.1016/j.survophthal.2007.08.008. [DOI] [PubMed] [Google Scholar]