Abstract
Rationale
Diffusion tensor imaging has been used before in testing associations between cigarette smoking and white matter integrity, with inconsistent results. Published reports indicate higher fractional anisotropy (FA) in some brain regions and lower FA in others in adult smokers compared to nonsmokers. Adolescent smokers exhibited elevated FA at several sites and a positive correlation of FA in the genu corpus callosum with exposure to smoking (pack-years).
Objective
To help resolve prior discrepancies, we studied adults, sampling multiple brain regions, and testing for relationships to clinical features of nicotine dependence and exposure to smoking.
Methods
Brain MRI scans (1.5T) were acquired, and FA and apparent diffusion coefficient (ADC) were assayed in corpus callosum and prefrontal white matter, corona radiata, internal capsule, cingulum bundle, and hippocampal perforant fibers in 18 smokers (33.7±7.9 years of age) and 18 age- and gender-matched nonsmokers.
Results
ADC showed no group difference, but smokers had higher (4.3-21.1%) FA than nonsmokers. The differences were significant in right prefrontal white matter, cingulum, and genu corpus callosum. FA in several regions was negatively correlated with nicotine dependence or cigarettes/day.
Conclusions
Combined with earlier findings, these results suggest a model of changing trajectories whereby FA is higher with tobacco exposure during adolescence, and declines with continued smoking in adulthood. This notion is supported by the observation that, at multiple sampling sites, participants who had started smoking earlier in life had higher FA than those who had started later.
Keywords: white matter, diffusion tensor imaging, nicotine dependence, magnetic resonance imaging, fractional anisotropy, tobacco
Introduction
As cigarette smoking is the largest preventable cause of disease and premature death in the United States (Danaei et al. 2009), neuroimaging studies have sought to identify its effects on the brain (Azizian et al. 2009). Smaller gray matter volumes have been observed in frontal, temporal, and occipital lobes in smokers than in nonsmokers (Brody et al. 2004; Gallinat et al. 2006), as has an inverse relationship between cortical volume and exposure to smoking (Brody et al. 2004; Kühn et al. 2010). In addition, lower gray matter density in prefrontal cortex and higher density in the insula were noted in smokers (Zhang et al. 2011). Lower cortical gray matter volumes measured with MRI, however, do not necessarily imply reduced gray matter since increased myelination of deeper cortical layers could shift the gray/white boundary on MRI into the cortex, reducing apparent gray-matter values (Bartzokis 2009; Bartzokis et al. 2009). How cigarette smoking affects brain white matter is therefore an important question (Bartzokis 2007).
Diffusion tensor imaging (DTI) previously has been used to examine white matter microstructure as related to smoking. One study found higher fractional anisotropy (FA) in the corpus callosum of adult smokers (age 38.6±14.2) than age-matched nonsmokers, but nicotine dependence as measured by Fagerström score was correlated negatively with FA in smokers (Paul et al. 2008). Another DTI study used tract-based spatial statistics (TBSS) to compare adult smokers (age 35.5±9.6) with age-matched nonsmokers, and found lower FA in prefrontal white matter of a subsample of highly nicotine-dependent smokers and a negative correlation of FA with nicotine dependence as measured by Fagerström score (Zhang et al. 2010). Finally, a study of adolescents revealed higher FA at several white matter sites in smokers (age 17.0±0.7) compared to age-matched nonsmokers, with no prenatal exposure, and a positive correlation of FA in the genu corpus callosum with pack-years of smoking (Jacobsen et al. 2007).
These discrepancies suggest that variables other than smoking history (e.g., age) must be considered in interpreting differences in FA and its relationship to nicotine dependence and tobacco exposure when comparing smokers and nonsmokers.
We report here on a DTI study that aimed to elucidate how chronic smoking affects white matter microstructure in the brain. The participants were adult men and women who were classified as smokers (age 33.7 ± 7.9) and nonsmokers (age 33.3 ± 10.1). To extend prior findings, we selected 30 brain regions for assessment. These included regions in which DTI findings related to smoking were previously observed (corpus callosum, internal capsule, and prefrontal white matter at multiple levels) (Jacobsen et al. 2007; Paul et al. 2008; Zhang et al. 2010), and other regions, such as the corona radiata, that exhibited smoking-related findings observed without DTI (Wang et al. 2009). We also examined the perforant path, a white matter tract of the hippocampal formation (Witter 2007), and the cingulum bundle, which connects the anterior thalamus with both hippocampus and cingulate cortex (Bürgel et al. 2006). Several lines of evidence suggest that smoking affects the hippocampus (Domino et al. 2004; Gallinat et al. 2006; Gallinat et al. 2007; Zubieta et al. 2005) and cingulate cortex (Domino et al. 2004; Gallinat et al. 2006; Zubieta et al. 2005); and the thalamus of the mammalian brain has a high density of nicotinic acetylcholine receptors (nAChRs) (Horti et al. 1997; Kimes et al. 2003;). We reasoned that white matter proximal to and/or innervating cingulate and mesial-temporal gray matter affected by smoking might itself be sensitive to smoking. This investigation asked two questions: How do FA and ADC compare between smokers and nonsmokers? How are FA and ADC related to clinical features of cigarette smoking (nicotine dependence, cigarettes/day, pack-years)?
Materials and Methods
Subjects
Eighteen smokers (10 male, age 33.7 ± 7.9 years) and 18 nonsmokers (9 male, 33.3 ± 10.1 years) from the greater LA community were recruited through Craigslist ads, fliers, print ads, radio ads, and word of mouth and participated in the study, which was approved by the UCLA Institutional Review Board. Inclusion criteria were: a score of >85 on the Shipley Institute of Living Scale (Zachary et al. 1985), right-handedness (modified Edinburgh Inventory; Oldfield 1971), and absence of current Axis I psychiatric disorders (other than nicotine dependence for smokers) determined using the Structured Clinical Interview for DSM-IV (First et al. 2002). Exclusions were: prior head trauma involving loss of consciousness and/or requiring hospitalization; prior hospitalization for psychiatric illness; neurological, cardiovascular or pulmonary disease; HIV seropositivity; or other major medical conditions. Some marijuana (<1 cigarette/week) and alcohol (<10 drinks/week) consumption were permitted. Any other current use of any medications that affect cognitive functioning was exclusionary. Detailed drug use data were obtained using the Addiction Severity Index (McClellan et al. 1980). Any participant who met criteria for dependence on any drug of abuse or alcohol (other than nicotine in the smokers group) was excluded. On the day of the MRI scan, urine was tested for cocaine, methamphetamine, opioids, Δ9-tetrahydrocannabinol and benzodiazepines. Any positive drug test resulted in exclusion from the study.
Clinical Features of Cigarette Smoking
Inclusion in the Smokers group required a self-report of daily smoking for at least the 6 months before the study, with recent smoking verified by CO ≥10 ppm in expired air (Microsmokerlyzer®, Bedfont Scientific Ltd, Kent, UK) and the presence of urinary cotinine (Accutest NicAlert Strips; JANT Pharmacal Corporation, Encino, CA). Inclusion in the Nonsmokers group required a self-report of having smoked fewer than 5 cigarettes (lifetime), with ≤3 ppm CO in expired air and a negative cotinine test at the time of scan. For nonsmokers who reported ever having smoked a cigarette, the mean time since smoking the last cigarette was 40 months. Participants completed the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991) and other questionnaires regarding current consumption of cigarettes and cumulative exposure. Data on possible maternal smoking or second-hand smoke exposure of smokers and nonsmokers were not obtained.
MR Image Acquisition and Processing
The DTI acquisition and post-processing methods have been published (Ringman et al. 2007; Tobias et al. 2010). Briefly, data were acquired on a Siemens Sonata 1.5 T MR Scanner. High-resolution, T1-weighted, sagittal, whole-brain structural MRI (MPRAGE, 1×1×1 mm3 voxels) was used for positioning. Four sequential, six-direction -- [(1,0,1), (-1,0,1), (0,1,1), (0,1,-1), (1,1,0), (-1,1,0)] -- diffusion-weighted whole-brain DTI volumes were acquired using echo-planar imaging (EPI: TE=78 ms, TR=6000 ms) oriented parallel to the axial-oblique plane containing the anterior commissure-posterior commissure line (AC-PC). There were two b-values, 0 and 1000 s/mm2, and the nominal voxel size was 3×3×3 mm3. For each subject, the four volumes were co-registered off-line using FMRIB's Linear Image Registration Tool (Jenkinson and Smith 2004) and averaged together. Eddy current correction was applied using FMRIB's Diffusion Toolbox in FSL version 3.2 (Behrens et al. 2003; Smith et al. 2004). FA maps were created from the eddy-current corrected file with the FDT-DTIFIT package.
FA Sampling at Fiber-Tract Sites and in White-Matter Slabs
We used a region of interest (ROI) approach in native MRI space to avoid the distortions of warping DTI data to a common brain atlas. Two methods were used to extract regional FA and apparent diffusion coefficient (ADC) from the corresponding whole-brain maps. Both protocols were carried out by two investigators (MT, MH) blind to the sex and diagnoses of the participants. Inter-rater and intra-rater reliability tests were conducted to establish confidence in reproducibility. Ten participants were chosen randomly for these tests. Intra-rater reliability scores (intraclass correlation coefficients--ICC) were 0.97 (MT) and 0.95 (MH). The inter-rater reliability ICC was 0.88.
The first method, similar to previous studies (Ringman et al. 2007; Tobias et al. 2010), but with the addition of cingulum sites, was used to sample individual DTI voxels at 2 midline sites (genu and splenium corpus callosum) and 10 bilateral sites (anterior, midcaudal superior, and caudal superior corona radiata; anterior and posterior limbs and genu of the internal capsule; perforant path of the hippocampal formation; and anterior, middle, and posterior cingulum) for a total of 22 fiber-tract ROIs (Table 2). Anatomical locations of and criteria for picking the callosal, coronal, and internal capsular sites were presented previously (Tobias et al. 2010). Selection of the cingulum sites is shown in Figure 1. For each structure, the two highest adjacent FA values were recorded and their average taken as representative of the site, whereby FA color maps indicating fiber-tract direction were used to supplement voxel selection. These methods were used to avoid partial-volume effects by systematically selecting the highest FA and therefore likely purest white matter part of each tract, using the directional color maps to avoid cross-sampling, and also to enhance detection of the relatively slight differences (< 10%) that are usually seen in FA values. The average of the ADC values at the same two voxels was taken as representative of the site. Observer-independence of DTI voxel selection is claimed as an advantage of atlas-based approaches such as TBSS. In this regard, blinding of the operators, and using carefully defined anatomic criteria for selection of tracts and segments of tracts, and specific criteria (maximum FA, adjacency) for selection of voxels within tracts in the ROI approach mitigated any putative biases due to “observer dependence”. Furthermore, all fiber-tract sites were defined a priori, and data are reported for all sites sampled.
Table 2.
Factional anisotropy in smokers and nonsmokers.
| ROI | ||
|---|---|---|
|
| ||
| Region of Interest | Fractional Anisotropy (dimensionless) | |
| Smokers | Non-Smokers | |
| Genu corpus callosum | 0.88 ± 0.04* | 0.84 ± 0.05 |
| Splenium corpus callosum | 0.90 ± 0.05 | 0.89 ± 0.04 |
| anterior corona radiata: | ||
| left | 0.60 ± 0.07 | 0.56 ± 0.07 |
| right | 0.58 ± 0.08 | 0.58 ± 0.08 |
| midcaudal superior corona radiata: | ||
| left | 0.44 ± 0.08 | 0.41 ± 0.07 |
| right | 0.43 ± 0.06 | 0.43 ± 0.07 |
| caudal superior corona radiata: | ||
| left | 0.51 ± 0.08 | 0.47 ± 0.07 |
| right | 0.48 ± 0.07 | 0.48 ± 0.08 |
| internal capsule anterior limb | 0 | |
| left | 0.54 ± 0.07 | 00.51 ± 0.09 |
| right | 0.53 ± 0.08 | 0.50 ± 0.08 |
| Genu internal capsule | ||
| Left | 0.63 ± 0.06 | 0.62 ± 0.07 |
| right | 0.63 ± 0.06 | 0.60 ± 0.06 |
| internal capsule posterior limb | ||
| left | 0.70 ± 0.06 | 0.70 ± 0.06 |
| right | 0.75 ± 0.04 | 0.74 ± 0.04 |
| perforant path | ||
| left | 0.54 ± 0.07 | 0.54 ± 0.09 |
| right | 0.54 ± 0.07 | 0.52 ± 0.08 |
| anterior cingulum | ||
| left | 0.49 ± 0.09 | 0.45 ± 0.11 |
| right | 0.42 ± 0.09 | 0.37 ± 0.11 |
| middle cingulum | ||
| left | 0.72 ± 0.06* | 0.66 ± 0.08 |
| right | 0.66 ± 0.08* | 0.61 ± 0.08 |
| posterior cingulum | ||
| left | 0.51 ± 0.09 | 0.54 ± 0.08 |
| right | 0.52 ± 0.10 | 0.49 ± 0.10 |
Values tabulated are group means ± SD.
Significant difference from nonsmoker group, p<*0.05, **0.01 protected post-hoc T-Test (rank-transformed, therefore nonparametric) following omnibus repeated-measures MANCOVA covarying age and years-of-education.
Fig. 1.
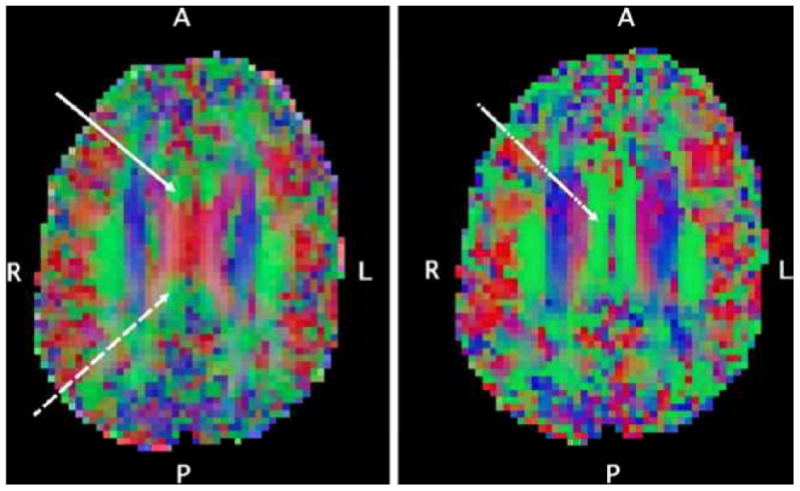
Axial-oblique (parallel to AC-PC line) DTI color maps of the brain at level of (left) and above (right) the upper lateral ventricles from a selected participant. Red denotes fibers in the left-right direction, green denotes fibers in the anterior-posterior direction, blue denotes fibers in the inferior-superior direction. In the left panel, the DTI voxel selection of the anterior site of the cingulum white matter tract (solid white arrow) and posterior site of the cingulum white matter tract (dashed white arrow) are shown. These are the relatively small anterior-posterior oriented tracts mesial and anterior/posterior respectively to the corpus callosum as it forms a prominent “X” at the level of the upper lateral ventricles. In the right panel, the DTI voxel selection of the right middle cingulum site (dot-dashed white arrow) on a supraventricular axial-oblique slice is shown, centered in the midcaudally situated anterior-posterior tract just mesial to the superior corona radiata.
The second method was similar to that of Chung et al. (2007), and for frontal white matter at least, provided a more expansive local sampling than the two voxels per ROI of the first method. These larger-slab ROIs were intended to capture possible effects of smoking on DTI indices that might be obscured by individual-subject variations in fiber crossing and mictrostructural anatomy. In this method, FA and ADC values were taken from and averaged across each of four axial-oblique (AC-PC-aligned) slab ROIs in each frontal lobe. Each ROI was 3×3 DTI voxels2 in-plane and 1 voxel deep. Each ROI was centered within prefrontal white matter and positioned to avoid contamination from neighboring gray matter. Within these constraints, the ROI position yielding the highest average FA value was selected. The four DTI slices on which the ROIs were selected are shown in Figure 2. The lowest slice was centered 6 mm inferior to the AC-PC plane, the next slice was at the level of the AC-PC plane, the third slice was 6 mm superior to the AC-PC plane, and the final slice was 9 mm above the AC-PC plane. Special care was taken to exclude the corpus callosum, identified with the help of FA color maps indicating the direction of fiber tracts.
Fig. 2.
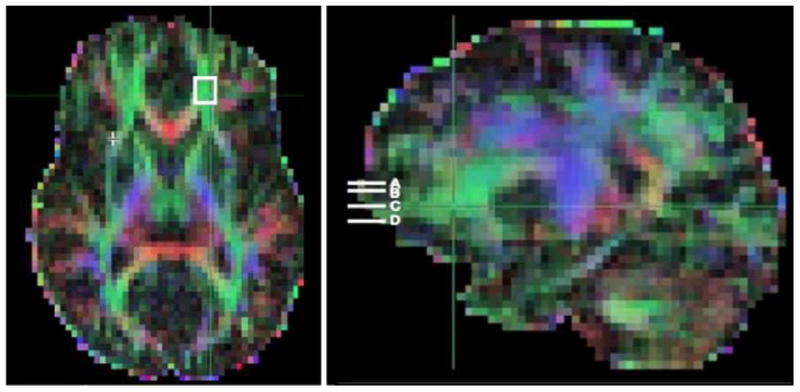
DTI FA whole-brain color map of data from a selected research participant. The image on the left shows an axial-oblique section (in the AC–PC plane). The white box in left prefrontal white matter (PFWM) indicates the boundaries of a 3-mm-deep, 9×9-mm2 slab, across which FA values were sampled and averaged in post-processing. A similar average was obtained for the right PFWM. The image on the right shows a parasagittal section of the same color map, depicting the positions of the four parallel prefrontal white-matter slabs (white bars) located at the level of the AC– PC plane (C), 6 mm below (D), and 6 (B) and 9 (A) mm above. Averages of left and right PFWM FA were obtained at each level.
Statistical Analysis
To control for multiple comparisons, we first performed omnibus testing across all white matter regions sampled before proceeding to post-hoc protected tests for the individual regions. The omnibus test was a repeated-measures multivariate analysis-of-covariance (R-MANCOVA) on the measures FA and ADC with participant's age and years of education as covariates. Potential differences in age and education were of concern, as their possible influences on DTI indices in normal adult populations have been reported (Hsu et al. 2008; Salat et al. 2005; Teipel et al. 2009). Therefore, they were included as covariates. In addition, effects of age on DTI indices for the combined smoker plus nonsmoker group were examined using Spearman correlation partialling out diagnosis. The R-MANCOVA had two within-subjects factors: ROI (15 levels: corpus callosum, anterior corona radiata, midcaudal superior corona radiata, caudal superior corona radiata, anterior limb internal capsule, genu internal capsule, posterior limb internal capsule, perforant path, anterior cingulum, middle cingulum, posterior cingulum, prefrontal white matter 6 mm inferior to AC-PC, prefrontal white matter at level of AC-PC, prefrontal white matter 6 mm superior to AC-PC, and prefrontal white matter 9 mm superior to AC-PC; Tables 2-3) and hemisphere (2 levels: left, right). In the case of the two midline corpus callosal sites, “genu” and “splenium” were used instead of left and right. There were two between-subjects variables smoker status (2 levels: smoker, nonsmoker) and gender (2 levels: male, female). The multivariate (i.e., effects on the combined FA and ADC measures) and univariate (i.e., effects on FA alone or on ADC alone) results of the R-MANCOVA were examined. For significant main effects or interactions involving smoking status, protected post-hoc T-Tests were then performed for FA and/or ADC as appropriate in each ROI with smoker status as a between-subjects factor. These tests were performed on rank-transformed data, a procedure that rendered them non-parametric.
Table 3.
Fractional anisotropy in prefrontal white matter in smokers and nonsmokers.
| Region of Interest | Fractional Anisotropy (dimensionless | |
|---|---|---|
|
| ||
| Smokers | Nonsmokers | |
| 6 mm inferior to ACPC plane: | 0.45 ± 0.08 | 0.44 ± 0.11 |
| left | ||
| right | 0.49 ± 0.10* | 0.44 ± 0.07 |
| at ACPC plane: | 0.49 ± 0.08 | 0.48 ± 0.06 |
| left | ||
| right | 0.50 ± 0.09 | 0.48 ± 0.05 |
| 6 mm superior to ACPC plane: | 0.44 ± 0.07 | 0.40 ± 0.07 |
| left | ||
| right | 0.46 ± 0.07*** | 0.38 ± 0.05 |
| 9 mm superior to ACPC plane: | 0.40 ± 0.05 | 0.39 ± 0.08 |
| left | ||
| right | 0.42 ± 0.06* | 0.38 ± 0.05 |
Values shown are group means ± SD.
Significant difference from nonsmoker group, p<*0.05, **0.01, ***0.001 protected post-hoc T-Test (rank-transformed, therefore non-parametric) following omnibus repeated-measures MANCOVA covarying age and years-of-education.
Omnibus testing was used to control for multiple comparisons when evaluating possible associations of nicotine dependence, cigarettes/day, and cumulative tobacco exposure on FA and ADC within the smoker sample. For nicotine dependence, an R-MANCOVA was performed on the measures FA and ADC with FTND score as covariate. Within-subjects factors were ROI (15 levels as above) and hemisphere (2 levels as above). For current tobacco use, a similar R-MANCOVA was performed with cigarettes smoked per day as covariate. For cumulative smoking exposure, a similar R-MANCOVA was performed with pack-years as covariate. For each of these R-MANCOVAs, the multivariate and univariate results of the R-MANCOVA were examined as above. For significant effects involving the covariate in question, protected post-hoc Spearman correlation analyses within the smoking group were then performed in each ROI between the smoking variable in question (FTND score, cigarettes/day, or pack-years) and FA and/or ADC, as appropriate. Participant's age was partialled out for correlations involving pack-years.
Results
Research Participants
Table 1 lists demographics and drug use parameters for smokers and nonsmokers. The groups did not differ significantly in gender, age, IQ, alcohol or marijuana use. Smokers had, on average, significantly fewer years of education [t(33.4)=2.5, p<0.05]; therefore, years of education was included as a covariate in later analyses. There were no significant differences between male and female participants in age, cigarettes consumed per day, years of smoking, or pack-years.
Table 1. Demographics of Smoker and Nonsmoker Groups.
| Subjects | Smokers (n=18, 10 men) | Nonsmokers (n=18, 9 men) | ||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Age (years) | 33.7 | 7.9 | 33.3 | 10.1 |
| Education (years) | 13.7 | 1.5 | 15.3 | 2.2 |
| IQ (Shipley score) | 112.9 | 8.1 | 110.3 | 7.2 |
| Smoking Measures: | ||||
| Daily consumption (cigarettes/day) | 16.9 | 5.7 | -- | -- |
| Smoking history (years) | 15.7 | 8.1 | -- | -- |
| Exposure (pack-years) | 14.2 | 9.2 | -- | -- |
| Nicotine Dependence (Fagerström score) | 4.3 | 1.8 | -- | -- |
| Alcohol (drinks/week) | 2.8 | 2.7 | 1.4 | 2.6 |
| Cannabis (days used in last 30) | 0.3 | 1.2 | 0.1 | 0.3 |
Smoking and DTI Indices in White Matter
Mean regional FA values for the smoker and nonsmoker groups are shown in Table 2 (single-voxel fiber tract sites) and Table 3 (prefrontal slab ROIs). R-MANCOVA on FA and ADC across all regions yielded a significant multivariate main effect of smoker status [F(2,29)=3.6, p<0.05]. There were a significant univariate main effect of smoker status [F(1,30)=7.2, p<0.05], but no significant main effect or interactions for ADC. Therefore, post-hoc tests were conducted for FA but not for ADC. There were no significant multivariate or univariate effects of years of education, so this variable was not included in further post-hoc T-Tests. Spearman correlations of age with FA in the combined smoker plus non-smoker group, partialling out diagnosis, showed a significant negative correlation at four tract sites: genu and splenium corpus callosum, left genu internal capsule, and right caudal superior corona radiata, as well as in prefrontal white matter 9 mm superior to the AC-PC plane.
In post-hoc T-Tests, FA was significantly higher in smokers than in nonsmokers in midline genu corpus callosum [4.3%; t(33.8)=2.3], left middle cingulum [8.3%; t(31.8)=2.2], right middle cingulum [9.1%; t(34)=2.0], right prefrontal white matter 6 mm inferior to the AC-PC plane [13.1%; t(33.3)=2.5], right prefrontal white matter 6 mm superior to AC-PC [21.1%; t(34)=3.9], and right prefrontal white matter 9 mm superior to AC-PC [11.8%; t(32.8)=2.3] (Tables 2-3, Figure 3).
Fig. 3.
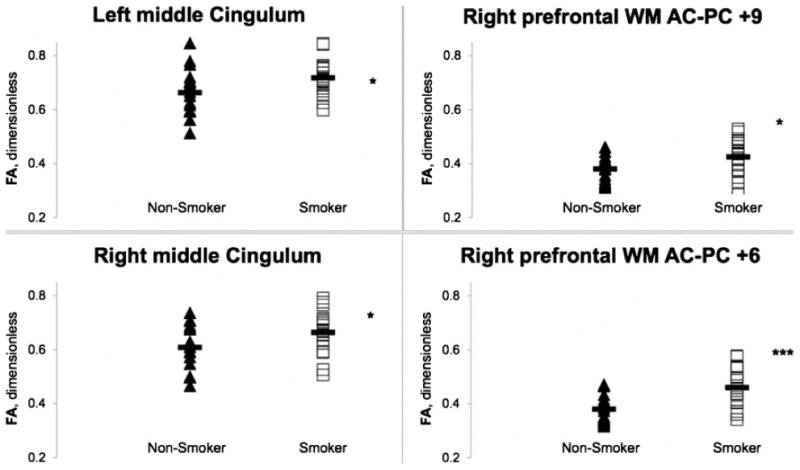
Fractional anisotropy (FA) in nonsmokers (filled triangles) and smokers (open squares) at sites in left (upper left) and right (lower left) middle cingulum white-matter tracts. Data also shown (right) for FA averaged across each of two the 3-mm-thick, 9 × 9 mm2, axial-oblique, right prefrontal white-matter slabs. The slabs were oriented parallel to the AC-PC plane and centered 9 mm (upper right) and 6 mm (lower right) superior to it. Group mean FA (solid bar) was significantly higher for smokers than for nonsmokers in all four regions. Several other brain regions exhibited this elevation of FA in smokers (see Tables 2 and 3). p<*0.05, ***0.001 (protected post-hoc rank-transformed T-Test after omnibus repeated-measures MANCOVA covarying age and years of education).
Associations of Clinical Features of Smoking with FA
Within the smoker group, R-MANCOVA on the measures FA and ADC across all white matter regions yielded a significant multivariate association with FTND score [F(2,15)=5.7, p<0.05]. There was a significant univariate effect of FTND score for FA [F(1,16)=4.5, p<0.05]. In post-hoc protected Spearman correlations, FTDN score was correlated negatively with FA in left anterior internal capsule, left genu internal capsule, and right anterior cingulum (Figure 4).
Fig. 4.
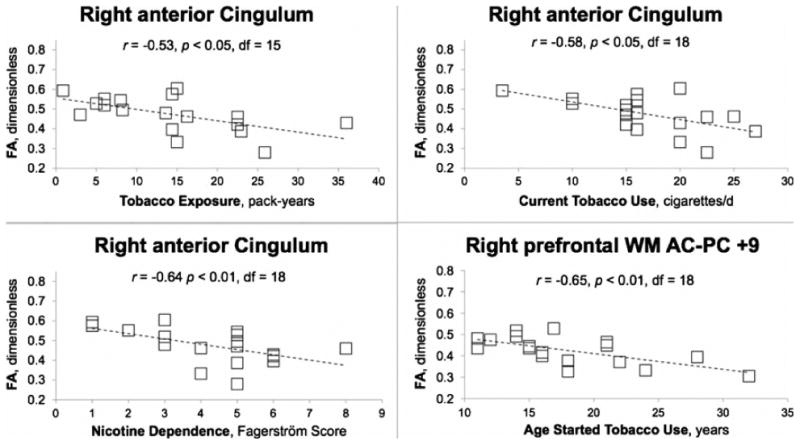
Fractional anisotropy (FA) at sites in the right anterior cingulum bundle as a function of long-term tobacco exposure (pack-years, upper left), nicotine dependence (FTND score, lower left), and current tobacco use (cigarettes/day, upper right) in smokers. Data are also shown (lower right) for FA averaged across the 3-mm thick, 9 × 9 mm2, axial-oblique, right prefrontal white matter slab oriented parallel to the AC-PC plane and centered 9 mm superior to it, as a function of age when tobacco use began. Note significant negative correlations of FA with all three measures of smoking in same region of cingulum. Negative correlation of FA with FTND was observed at 2 other white matter sites; with current cigarette consumption at 2 other sites; and with long-term exposure at 2 other sites (Table 4). Protected post-hoc Spearman correlation following repeated-measures MANOVA for nicotine dependence, current cigarette consumption, and long-term smoking exposure, exploratory for age when smoking began.
Within the smoker group, R-MANCOVA on FA and ADC across all regions yielded a significant univariate association of cigarettes/day with FA [F(1,16)=4.8, p<0.05]. In post-hoc Spearman correlations, cigarettes/day correlated negatively with FA in right anterior corona radiata, right anterior cingulum, and right posterior cingulum (Figure 4).
Within the smoker group, R-MANCOVA covarying age on FA and ADC across all regions yielded a significant hemisphere-by-cumulative smoking exposure (pack-years) interaction [F(2,14)=7.4, p<0.01] and a significant univariate effect for this interaction for FA [F(1,15)=15.6, p<0.001] but not for ADC. In post-hoc Spearman correlations, partialling out age, pack-years was correlated negatively with FA in right anterior cingulum (Figure 4). Pack-years was correlated positively with FA in left midcaudal superior corona radiata and right prefrontal white matter 9 mm superior to AC-PC. Thus, with few exceptions, FA at several diffuse white-matter sites declined with increasing values of smoking-related parameters. The most common regions across the three variables were in the internal capsule and anterior cingulum, and the most robust correlations were found in the anterior cingulum (Table 4).
Table 4. Correlations of fractional anisotropy (FA) with measures of smoking behavior.
| Region-of-interest | Current Smoking Dependence, FTND score |
|---|---|
| Left anterior internal capsule | -0.66** |
| Left genu interal capsule | -0.52* |
| Right anterior cingulum | -0.64** |
| Current Tobacco Use, cigarettes/day | |
| Right anterior corona radiata | -0.52* |
| Right posterior cingulum | -0.62** |
| Right anterior cingulum | -0.58* |
| Lifetime Tobacco Exposure, pack-years | |
| Left midcaudal superior corona radiata | +0.56* |
| Right anterior cingulum | -0.53* |
| Right prefrontal white matter 9 mm superior to AC-PC plane | +0.61** |
p<*0.05, **0.01, protected post-hoc Spearman correlations following omnibus repeated-measures MANCOVA. For pack-years, correlations partial age.
None of the above R-MANCOVA analyses showed any significant effects for ADC (data not shown, p's>.3); therefore, further analyses of ADC were not pursued.
The higher levels of FA in smokers despite negative correlations of FA with smoking-related parameters was puzzling. A rise in FA during early smoking years as observed by Jacobsen et al. (2007) suggested itself as a possible resolution. Therefore, exploratory Spearman correlations were run between FA and age when smoking began, partialling out subject age at time of DTI scan. Significant negative correlations were found in the left midcaudal corona radiata [r=-0.66, p<0.01], left prefrontal white matter 6 mm inferior to AC-PC [r=-0.48, p<0.05], left caudal corona radiata [r=-0.51, p<0.05], and right prefrontal white matter 9 mm superior to AC-PC [r=-0.61, p<0.01].
Discussion
This investigation yielded two major findings. The first is that FA was higher in multiple white matter regions in adult smokers (age 33.7±7.9) than in age-matched nonsmokers. The second finding is that FA at several sites correlated negatively with nicotine dependence and daily cigarette consumption. One way to reconcile these two seemingly inconsistent observations would be if smoking led to above-normal FA in adolescent years when most people begin, that then declined with continued smoking in adulthood. The ensuing discussion cites evidence from this paper, other work in our laboratory and the literature for this possibility.
Higher FA in Smokers than in Nonsmokers
Our observation of higher FA in smokers than in nonsmokers is concordant with observations on adolescents (Jacobsen et al. 2007) and adults (Paul et al. 2008). We expand these findings to additional brain regions not previously examined, in particular the cingulum bundle, which connects cingulate cortex and hippocampus (Nolte and Angevine 2000). Smoking-related findings in cingulate cortex and hippocampus have been reported (Almeida et al. 2008; Due et al. 2002; Gallinat et al. 2007; Zubieta et al. 2005), Our new observation points to effects of smoking on white matter connecting these two regions. Elevated FA in smokers has now been observed in three independent investigations (Jacobsen et al. 2007; Paul et al. 2008; present study).
The finding of higher FA in smokers conflicts with Zhang et al. (2010), who observed lower than normal FA in a section of left prefrontal white matter in a subsample of highly dependent adult smokers, and no smoking-related differences in other white matter areas. A possible explanation of this conflict is that our study targeted only brain regions with previously suggested effects of smoking. Zhang et al. (2010), in contrast, used a data-driven approach that sampled the entirety of brain white matter over the full range of FA values, imposing a higher statistical threshold to be exceeded by effects that are often modest. Anatomical warping to standard atlas space can also introduce subtle effects due to volume differences that have the potential to alter measured differences in subcortical DTI. Differences between the samples of smokers (e.g., age, gender, smoking history) may also have contributed to differences between studies.
It is notable that high FA in white matter is widely but not universally regarded as beneficial. The literature presents examples of positive correlation between FA and cognitive function in some subject samples (Medina et al. 2005; Lim et al. 2006; Grieve et al 2007). However, there are also reports of functional deficits associated with high regional FA (e.g., Hoeft et al. 2007), and elevated FA has been observed in the white matter of children who were exposed to methamphetamine in utero (Cloak et al. 2009).
A possible mechanism by which smoking could raise FA draws from the following observations: 1) white matter matures through late adolescence faster than in adulthood (Benes 1989; Bartzokis and Lu 2009;); 2) nicotine acting at nAChRs can promote glial activity or proliferation (Garrido et al. 2003; Liu et al. 2005; Opanashuk et al. 2001); and 3) nAChRs exist in subcortical white matter (Ding et al. 2004). Oligodendrocyte precursor cells but not the oligodendrocytes and astrocytes into which these precursors differentiate do express nAChRs (Rogers et al. 2001; reviewed Bartzokis 2007). Thus, as suggested by Paul et al. (2008), nicotine exposure in adolescence could stimulate glial development, leading to greater white matter volume (Gazdzinski et al. 2005) and organization or maturity.
Correlations of FA with Clinical Features of Cigarette Smoking
Despite higher mean FA in nearly all ROIs, a general negative correlation was found between FA and smoking parameters, though this effect varied by ROI. These results resemble those of Paul et al. (2008), who reported higher FA in the body of the corpus callosum of adult smokers with low FTDN scores than in those with high scores, and those of Zhang et al. (2010) that FA in left prefrontal white matter was negatively correlated with FTND in highly dependent adult smokers. Negative FA correlations are discordant with the positive correlation between FA and pack-years in adolescent smokers (Jacobsen et al. 2007). A model of a changing trajectory of FA is a possible explanation for these inconsistencies. According to this model, FA would rise during early years of smoking, and then decline with continued smoking in later years. The negative correlations between age when smoking began and FA observed here suggest that smokers who begin at earlier ages experience higher FA during adolescence, relative to those who begin smoking later. In this regard, we have a preliminary finding of positive association between FA (genu and splenium of corpus callosum, corona radiata, internal capsule, and superior and inferior longitudinal fascicule) with pack-years in adolescent smokers (Kohno et al. 2010). Within-subject prospective study designs are needed to test this model more directly.
The functional significance of the findings regarding smoking and FA, including whether elevated FA is beneficial or detrimental to overall neurological health, is unknown. The model of changing trajectory in FA values suggests a negative effect on white matter due to continued smoking and more severe dependence during adulthood. Finally, a distinction should be made between the effects of nicotine specifically and the effects of chronic smoking overall. The finding of higher FA in smokers supports the suggestion that cholinergic stimulation has promyelinating effects that could underlie beneficial effects of nicotine as well as cholinesterase inhibitors on cognitive functions (Bartzokis 2007).
Limitations
This study has important limitations, including small sample size and limited age range within the sample, though the latter also helps diminish possible effects of aging on DTI indices. The sample of smokers also showed relatively mild dependence. In addition, newer DTI protocols feature improved acquisition with increased resolution, EPI distortion correction, additional measures such as radial and axial diffusivity that may provide more specific data than the composite measure of FA, and an increased number of directions, which leads to more uniform space sampling and a higher signal to noise ratio. Despite carefully constructed voxel-picking criteria, some of the tracts we sampled were small enough that partial-volume effects cannot be entirely ruled out, though almost all of our significant findings did come from larger and more robust tracts.
Notably, significant findings were obtained despite these limitations, suggesting that effects of smoking on white matter FA are robust. Our interpretations also inferred white matter changes over the course of smoking history from a cross-sectional study design, and causality could best be determined through prospective studies. That FA elevations in smokers could have existed premorbidly is also a consideration. Prospective studies could address this possibility as well.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P20DA022539, R01DA020726 – EDL; R03DA20512, R21DA023192 - JON; and MOIRR00865 (UCLA General Clinical Research Center). Additional funding was provided by endowments from the Katherine K. and Thomas P. Pike Chair in Addiction Studies and the Marjorie Green Family Trust (EDL).
Research support for projects other than the one reported here was supplied to Dr. Edythe London under UCLA contract (number 20063287) with Philip Morris USA. There was no involvement of Philip Morris USA in this project.
Footnotes
Financial Disclosures: None of the other authors have any conflicts of interest or financial disclosures to report.
References
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- Azizian A, Monterosso J, O'Neill J, London ED. Magnetic resonance imaging studies of cigarette smoking. Handb Exp Pharmacol. 2009;192:113–143. doi: 10.1007/978-3-540-69248-5_5. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Acetylcholinesterase inhibitors may improve myelin integrity. Biol Psychiatry. 2007;62:294–301. doi: 10.1016/j.biopsych.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH. Brain Volume: Age-Related Changes. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 2. Academic Press; Oxford: 2009. pp. 417–447. [Google Scholar]
- Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113:322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelker MA, Jarvik ME, Lee GS, Smith E, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72(24):2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Fowler JS, Logan J, Wang GJ, Telang F, Garza V, et al. 6-[18F]Fluoro-A-85380, a new PET tracer for the nicotinic acetylcholine receptor: studies in the human brain and in vivo demonstration of specific binding white matter. Synapse. 2004;53:184–189. doi: 10.1002/syn.20051. [DOI] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol There. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, version 2.0) 2002 [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AM J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Lang U, Jacobsen L, Bajbouj M, Kalus P, von Haebler D, et al. Abnormal Hippocampal Neurochemistry in Smokers: Evidence from Proton Magnetic Resonance Spectroscopy at 3 T. J Clin Psychopharmacol. 2007;27:80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- Garrido R, King-Pospisil K, Son KW, Hennig B, Toborek M. Nicotine upregulates nerve growth factor expression and prevents apoptosis of cultured spinal cord neurons. Neurosci Res. 2003;47:349–355. doi: 10.1016/s0168-0102(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, Korenberg J, Bellugi U, Galaburda A, Reiss A. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams Syndrome. J Neurosci. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horti A, Scheffel U, Stathis M, Finley P, Ravert HT, London ED, Dannals RF. Fluorine-18-FPH for PET imaging of nicotinic acetylcholine receptors. J Nucl Med. 1997;38:1260–1265. [PubMed] [Google Scholar]
- Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, Chen WH. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, et al. 2-[18F]F-A85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB J. 2003;17:1331–1333. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- Kohno M, Morales A, London ED. Cigarette exposure associated with abnormal white matter development in adolescent smokers. Soc Neurosci Abst 2010 [Google Scholar]
- Kühn S, Schubert F, Gallinat J. Reduced Thickness of Medial Orbitofrontal Cortex in Smokers. Biol Psychiatry. 2010;68:1061–1065. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lim KO, Ardekani BA, Nierenberg J, Butler PD, Javitt DC, Hoptman MJ. Voxelwise correlational analyses of white matter integrity in multiple cognitive domains in schizophrenia. Am J Psychiatry. 2006;163:2008–2010. doi: 10.1176/appi.ajp.163.11.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Mohila CA, Gong Y, Govindarajan N, Onn SP. Chronic nicotine exposure during adolescence differentially influences calcium-binding proteins in rat anterior cingulate cortex. Eur J Neurosci. 2005;22:2462–2474. doi: 10.1111/j.1460-9568.2005.04423.x. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The addiction severity index. Journal of Nervous and Mental Diseases. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, et al. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Nolte J, Angevine JB. The Human Brain: In photographs and diagrams. Mosby; St. Louis: 2000. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Opanashuk LA, Pauly JR, Hauser KF. Effect of nicotine on cerebellar granule neuron development. Eur J Neurosci. 2001;13:48–56. [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, et al. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob Res. 2008;10:137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, O'Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Gregori NZ, Carlson N, Gahring LC, Noble M. Neuronal nicotinic acetylcholine receptor expression by O2A/oligodendrocyte progenitor cells. Glia. 2001;33:306–313. [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann NY Acad Sc. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Kohl T, Bürger K, Reiser MF, et al. White matter microstructure in relation to education in aging and Alzheimer's disease. J Alzheimers Dis. 2009;17:571–583. doi: 10.3233/JAD-2009-1077. [DOI] [PubMed] [Google Scholar]
- Tobias MC, O'Neill J, Hudkins M, Bartzokis G, Dean AC, London ED. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacol. 2010;209:13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Durazzo TC, Gazdzinski S, Yeh PH, Mon A, Meyerhoff DJ. MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed. 2009;22:516–522. doi: 10.1002/nbm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- Zachary RA, Paulson MJ, Gorsuch RL. Estimating WAIS IQ from the Shipley Institute of Living Scale using continuously adjusted age norms. J Clin Psychol. 1985;41:820–831. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stein EA, Hong LE. Smoking and schizophrenia independently and additively reduce white matter integrity between striatum and frontal cortex. Biol Psychiatry. 2010;68:674–677. doi: 10.1016/j.biopsych.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, Domino EF. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]


