Abstract
We developed a combined conditional cytotoxic, i.e., herpes simplex type 1-thymidine kinase (TK), plus immune-stimulatory, i.e., fms-like tyrosine kinase ligand-3–mediated gene therapy for glioblastoma multiforme (GBM). Therapeutic transgenes were encoded within high-capacity adenoviral vectors (HC-Ad); TK was expressed constitutively, while Flt3L was under the control of the TetOn regulatable promoter. We previously assessed efficacy and safety in intracranial GBM rodent models. But, since this approach involves expression of a cytokine within the brain, we chose the nonhuman primate, i.e., Callithrix jaccus (marmoset) as it has been established that its immune response shares similarities with man. We characterized the safety, cell-type specific expression, and doxycycline (DOX)-inducibility of HC-Ad-TetOn-Flt3L delivered within the striatum. We used allometrically scaled DOX doses delivered orally, twice daily for one month, mimicking the route and duration of DOX administration planned for the GBM trial. Flt3L was effectively expressed within astrocytes, microglia, oligodendrocytes, and neurons. No evidence of brain or systemic toxicities due to the treatment was encountered. Our data indicate that DOX doses equivalent to those used in humans to treat infections can be safely used “off-label” to turn “on” therapeutic gene expression from HC-Ad-TetOn-Flt3L; providing evidence for the safety of this approach in the clinic.
Introduction
The implementation of gene therapy strategies in the clinic requires stringent efficacy and safety assessment in preclinical animal models. Rodent models have been utilized to evaluate cytokine-mediated gene therapy approaches (reviewed in ref. 1), yet, these models may not predict outcomes in humans. Nonhuman primates have emerged as attractive models from the perspective of their pathological and pharmacokinetic responses. To this end, we have utilized the small New World, nonhuman primate, Callithrix jacchus (marmoset), which has the following useful characteristics: it is small; easy to breed and handle; they are available from inbred colonies, thus diminishing risk of transmitting infections to humans; and their immune system shares many similarities to man, making it an optimal model to test cytokine-mediated gene therapies.2 Marmosets have been used successfully to study the effects of human interleukin (IL)-6,3 IL-2, and IL-4.4 These studies have demonstrated that marmosets can be used to model the human immune system and its response to human cytokines. Therefore, we estimated that it would be the optimum preclinical model to evaluate the effects of expressing the human cytokine, Flt3L within the central nervous system; assessing its actions both locally and systemically as a prelude of the implementation of this strategy for the treatment of glioblastoma multiforme (GBM) in humans.
GBM is a commonly occurring and aggressive primary brain tumor, accounting for half of all brain tumors in adults.5 GBM is genetically heterogeneous, involving genes important for cell cycle regulation, growth and proliferation, cell invasion, and angiogenesis.6 The invasion of GBM cells prevents total resection, leading to tumor recurrence.6 The standard of care, including resection, radiotherapy, and chemotherapy, achieves a median survival of ~14 months.7 Thus, there is a need for the development and implementation of novel therapies. Adenovirus-mediated gene transfer presents a relatively novel therapeutic strategy for GBM, with only a handful of early clinical trials published. Strategies for treating glioma using adenoviral gene therapy have included cytokines, tumor suppressors, and conditional-cytotoxic genes.1 Cytotoxic gene therapy using herpes simplex virus type 1-thymidine kinase (TK) with ganciclovir or valaciclovir administration is the most common strategy. To date, however, clinical trials testing this approach have not shown significant improvement in patient survival, although the treatments have demonstrated good safety profiles.8–13
Our laboratory has developed a novel high-capacity adenovirus (HC-Ad)–based gene therapy for GBM.14,15 This therapy consists of a combined cytotoxic and immune-stimulatory strategy comprising two separate HC-Ads. The conditional cytotoxic vector (HC-Ad-TK) constitutively expresses TK to selectively kill proliferating tumor cells upon addition of ganciclovir or valaciclovir.9,14,16 The immune-stimulatory vector (HC-Ad-TetOn-Flt3L) expresses the cytokine fms-like tyrosine kinase ligand 3 (Flt3L) under the control of the doxycycline (DOX)-inducible rtTA2sM2/tTSkid promoter system.9,14,17,18 Flt3L mediates the recruitment of dendritic cells to the brain tumor microenvironment, where tumor antigens and the damage associated molecule high-mobility group protein B1, released via TK/prodrug-mediated cytotoxicity, trigger specific anti-GBM immunity and CD8+ T-cell–dependent tumor cell killing, leading to long-term survival in rodent models of GBM.14,19–23
For expression of Flt3L from the HC-Ad-TetOn-Flt3L vector, DOX, a common tetracycline antibiotic, is administered systemically. In the clinic, GBM patients receiving HC-Ad-TetOn-Flt3L as part of the combined therapy will be administered DOX orally to activate Flt3L transcription. This will be an “off-label” use of DOX, as it is currently only approved by the US Food and Drug Administration (FDA) to treat infections in humans.24 Thus, it is important to determine the amount of DOX required to turn “on” Flt3L expression from HC-Ad-TetOn-Flt3L in the central nervous system. This would be the first use of DOX to activate gene expression with therapeutic intent in humans. We have previously demonstrated robust Flt3L expression after intrastriatal injection of 1 × 109 viral particles (vp) of HC-Ad-TetOn-Flt3L in Lewis rats with oral DOX doses allometrically equivalent to human doses of 200 and 300 mg/day.25 In this report, we have investigated the effectiveness and safety of 1 × 109 vp HC-Ad-TetOn-Flt3L with a one-month administration of DOX to turn “on” Flt3L expression in the nonhuman primate C. jacchus (marmoset), which offers genetic26,27 and immunological characteristics4,28,29 similar to humans and has been previously used in immunological and gene therapy studies.2 In line with what is proposed for the clinical trial, DOX was administered orally twice a day for 1 month at 13.2 mg/kg/day (low-DOX) or 19.8 mg/kg/day (high-DOX). These doses are allometrically equivalent to 200 and 300 mg/day in humans.30 Our results in marmosets demonstrated that Flt3L was expressed at both DOX doses and that treatment was not associated with local or systemic toxicity. Double-labeling immunofluorescence showed Flt3L colocalization with astrocytes, microglia, olidendroglia, and neurons. These data support the use of either 200 or 300 mg DOX/day for the activation of Flt3L expression in human GBM patients enrolled in a phase 1 clinical trial using the HC-Ad-TK + HC-Ad-TetOn-Flt3L vectors.
Results
The main objective of this study was to evaluate whether allometrically scaled doses of DOX activate Flt3L gene expression from HC-Ad-TetOn-Flt3L in the marmoset brain; the second objective of this study was to evaluate its safety after chronic administration of DOX orally for 1 month. The study design was as follows: intrastriatal injection of 1 × 109 vp of HC-Ad-TetOn-Flt3L into naive brain (see Figure 1a for intrastriatal coordinates) on day 0; oral administration of 13.2 mg/kg/day (low-DOX) or 19.8 mg/kg/day (high-DOX) of DOX from day 2 until day 28. Blood was drawn on day 0 (presurgery), 7, 21, and 28 for complete blood counts (CBC) and serum chemistry. On day 28, animals were euthanized via transcardial perfusion and fixation; brains were collected for the analysis of Flt3L expression and neuropathology, liver and kidneys were collected for pathology, and plasma was collected for neutralizing antibody titer determination. A schematic of the study design is shown in Figure 1a.
Figure 1.
Determination of an effective dose of doxycycline (DOX) to turn on Flt3L expression in the brain encoded HC-Ad-TetOn-Flt3L. (a) Diagram of experimental design used. On day 0, 2.5 × 108 viral particles (vp) of HC-Ad-TetOn-Ftl3L was delivered at four sites within the striatum (total of 1 × 109 vp). From bregma, the four injection sites were (i) 5.0 mm anterior, 6.0 mm lateral, 8.0 ventral; (ii) 5.0 mm anterior, 6.0 mm lateral, 6.0 mm ventral; (iii) 5.0 mm anterior, 8.0 mm lateral, 8.0 mm ventral; and (iv) 5.0 mm anterior, 8.0 mm lateral, 6.0 mm ventral. DOX was orally administered twice daily, from day 2 through day 28. The low-DOX group received 13.20 mg/kg/day while the high-DOX group received 19.80 mg/kg/day. Brain, liver, kidney, blood, serum, and plasma were collected on day 28 for immunohistochemistry, histopathology, hematology and chemistry, antibody titer, and DOX concentration. Blood and serum were also collected on day 0 (presurgery), day 7, and day 21 for total blood cell counts and serum chemistry. Brains were processed for immunocytochemistry using primary antibodies against Flt3L to detect expression of the therapeutic transgene. Flt3L expression at (b) the low-DOX dose of 13.20 mg/kg/day and (c) the high-DOX dose of 19.80 mg/kg/day is shown within the striatum of all HC-Ad–injected animals. Scale bar: 1,000 µm for full brain sections, 100 µm for ×10 images, and 20 µm for ×40 images.
DOX-mediated Flt3L expression within the central nervous system in nonhuman primates
Because the proposed gene therapeutic strategy depends on the DOX-mediated expression of Flt3L encoded within HC-Ad-TetOn-Flt3L, we assessed Flt3L expression within the brain 1 month after vector delivery and DOX treatment. To this end, we performed immunohistochemistry on free-floating brain sections. As shown in Figure 1b,c, Flt3L expression was observed in both the low-DOX and the high-DOX groups, suggesting that the allometrically equivalent human doses, i.e., 200 or 300 mg/day orally delivered, will able to activate Flt3L expression encoded within the regulatable HC-Ad vector after delivery into the striatum.
To assess the identity of cells expressing Flt3L after induction with DOX, we performed double-labeling immunofluorescence. Figure 2a–d shows colabeling of astrocytes (glial fibrillary acidic protein (GFAP) positive) and Flt3L expressing cells. Another glial cell-type—microglia—revealed coexpression for IBA1 (ionized calcium-binding adapter molecule; upregulated in activated microglia) and Flt3L (Figure 2e–h). Oligodendrocytes (Olig2 immunoreactive cells) were also found to be infected and express Flt3L, as evidenced in Figure 2i–l. Finally, neurons were also transduced by the inducible HC-Ad vector, and expression of Flt3L was confirmed via immunohistochemistry using antibodies specific for Flt3L and MAP2, a common microtubule marker for the neuronal cytoskeleton (Figure 2m–p).
Figure 2.

Double-labeling immunofluorescence of cells transduced with HC-Ad-TetOn-Flt3L in the marmoset brain. Cell type specific expression of Flt3L within the marmoset brain was assessed using double-labeling immunofluorescence. All glial cell subtypes were shown to express the Flt3L transgene, as evidenced by coexpression within (a–d) GFAP+ astrocytes, (e–h) IBA1+ microglia, (i–l) and Olig-2+ oligodendrocytes. Additionally, neurons were also shown to coexpress MAP2 and Flt3L (m–p). Scale bars represent 20 μm.
Neuropathology analysis
Due to the fact that gene therapy vectors will be delivered into the tumor bed after surgical resection of the tumor mass, it is important to evaluate the effects of vector delivery into normal brain parenchyma. Thus, to investigate the effects of HC-Ad-TetOn-Flt3L injection and DOX administration on the architecture and immune cellular infiltrates within the brain, we performed a detailed neuropathology analysis of brain sections 28 days postinjection (Figures 3 and 4; Supplementary Figures S1 and S2).
Figure 3.

Neuropathology assessment in the marmoset brain on day 28 after high-capacity adenoviral vectors (HC-Ad) delivery and high-doxycycline treatment. Architectural integrity of the brain was demonstrated with Nissl staining (a–c). No neuropathology was observed, as assessed using immunohistochemical stains for NeuN (neuronal bodies, d–f); tyrosine hydroxylase (dopaminergic fibers, g–i); myelin basic protein (oligodendrocytes and myelin sheaths, j–l); glial fibrillary acidic protein (astrocytes, m–o); and Olig2 (proliferating oligodendrocytes, p–r). Scale bar: 1,000 µm for full brain sections, 100 µm for ×10 images, and 20 µm for ×40 images.
Figure 4.

Immune cell infiltration examination in the marmoset brain on day 28 after high-capacity adenoviral vectors (HC-Ad) delivery and high-doxycycline treatment. Immunohistochemical staining was performed to identify (a–c) CD3+ T cells, (d–f) CD8+ T cells; microglia and activated macrophages (IBA1, f–i). Immunorective cells for all markers were identified at the site of injection. Scale bar: 1,000 µm for full brain sections, 100 µm for ×10 images and 20 µm for ×40 images.
In the high-DOX group, Nissl (Figure 3a–c) showed no gross tissue abnormalities. Staining for neuronal cell bodies (NeuN, Figure 3d–f), dopaminergic nerve terminals (tyrosine hydroxylase; Figure 3g–i), and myelin (myelin basic protein; Figure 3j–l) showed decreased intensity over the needle track but no large-scale defects. Staining for astrocytes (GFAP; Figure 3m–o) and activated oligoendrocytes (Olig2; Figure 3p–r) demonstrated increased staining near the injection site. CD3+ (Figure 4a–c), CD8+ (Figure 4d–f), and IBA1+ (activated macrophages and microglia; Figure 4g–i) cells were found near the injection site in the high-DOX group.
In the low-DOX group, Nissl (Supplementary Figure S1a–c), NeuN, (Supplementary Figure S1d–f). Tyrosine hydroxylase (Supplementary Figure S1g–i) and myelin basic protein (Supplementary Figure S1j–l) staining demonstrated largely unaffected architecture, with some disturbances observed over the needle track. GFAP staining showed areas of astrocyte activation restricted to the needle tract (GFAP; Supplementary Figure S1m–o), and Olig2 staining was also evident near the injection site (Olig2; Supplementary Figure S1p–r). Immunostaining for CD3+ (Supplementary Figure S2a–c), CD8+ (Supplementary Figure S2d–f), and IBA1+ (Supplementary Figure S2g–i) showed cellular infiltration near the injection site.
Pathology of liver and kidney
DOX is excreted through the kidneys, and the liver is a potential site for DOX metabolism31; therefore, we looked for pathology in these two organs. Histologic analysis of hematoxylin and eosin–stained liver and kidney tissue was performed on treated and saline-injected control animals (Figure 5). With the exception of two low-DOX animals that had extramedullary hematopoiesis in some parts of the liver (Supplementary Figure S3), each of the four lobes of the liver showed normal hepatocytes with no inflammation in either the control or DOX-treated groups (Figure 5a–c). Extramedullary hematopoiesis is a common finding affecting ~46.5% of marmosets held in captivity.32–34 Analysis of the kidneys showed significant variation in the control group and both DOX-treated groups, with areas of very mild inflammation in each group (Figure 5d–f), and areas of intense inflammation also present in each group (Figure 5g–i). Inflammation of the kidneys is also a common finding, affecting ~78.5% of captive marmosets.32 These results indicate that the HC-Ad-TetOn-Flt3/DOX treatment did not adversely affect the liver or kidneys.
Figure 5.

Histology of the liver and kidney. The livers and kidneys of control and experimental marmosets were analyzed with hematoxylin and eosin staining. The livers of the control animals, low-doxycycline (DOX), and high-DOX–treated animals exhibited no inflammation and had normal hepatocytes throughout the tissue (a–c, indicated by arrows). The kidneys of control, low-DOX, and high-DOX animals exhibited varying degrees of inflammation, from little to no inflammation (d–f), to high inflammation (g–i, indicated by arrows).
Analysis of clinical laboratory parameters
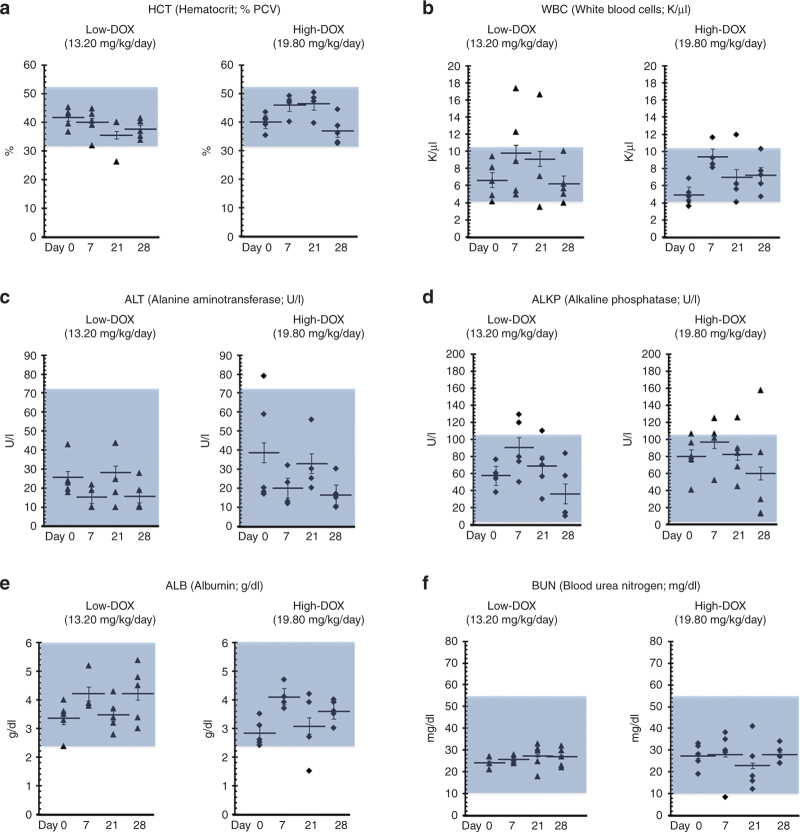
To evaluate any potential systemic toxicity, we measured several clinical parameters in whole blood and serum (Figure 6). The hematocrit (Figure 6a) and white blood cell (Figure 6b) counts were typically within the normal range for marmosets in both groups. Normal liver function was confirmed by analyzing alanine aminotransferase (Figure 6c), alkaline phosphatase (Figure 6d), and albumin (Figure 6e), and the kidney marker blood urea nitrogen (Figure 6f) was within normal range35,36 in both groups. In both groups, neutrophils, leukocytes, and eosinophils were within the normal range for marmosets (Supplementary Table S1). Monocyte and basophil counts reached higher than normal levels at 7 and 21 days postinjection but returned to normal levels 28 days postinjection (Supplementary Table S1). The overall safety of the HC-Ad-TetOn-Flt3L vector in both the low-DOX and high-DOX groups was consistent with our previous studies.14,15
Figure 6.
Hematology and serum chemistry. Blood and serum samples were drawn before surgery on day 0 and on day 7, 21, and 28 to assess clinical parameters. White blood cells, hematocrit, alanine aminotransferase, alkaline phosphatase, albumin, and blood urea nitrogen are shown, with a more complete panel shown in Supplementary Table S1. In both the low-doxycycline (DOX) and high-DOX groups, values for each parameter typically fell within normal ranges at each time point, with individual animals exhibiting some variations within all groups.
Circulating neutralizing antiadenovirus antibodies
It has been demonstrated that the presence of circulating antiadenovirus antibodies can hamper adenovirus-mediated transgene expression and therapeutic efficacy.37 Antibody titers between 8 and 32 were considered low, and titers at or above 128 were considered high.38,39 As shown in Figure 7, our results demonstrate that plasma levels of antiadenovirus neutralizing antibodies in both the low-DOX and the high-DOX groups were similar to those found in the saline-injection control group at all-time points except at day 28, where the mean titer of the high-DOX group was found to be higher than that of the saline group (Kruskall–Wallis test; P < 0.05). Titers from all groups at all-time points were below the high-titer threshold of 128.
Figure 7.
Antiadenovirus neutralizing antibodies in the plasma of high-capacity adenoviral vectors (HC-Ad)–treated marmosets. Plasma was collected on days 0, 7, 21, and 28 for testing. No significant differences were found between the saline group and either doxycycline (DOX) dose group (Kruskall–Wallis test, P > 0.05), with the exception of the high-DOX group on day 28, when statistical significance was found when comparing the saline-treated group with the high-DOX–treated group (P < 0.05).
DOX concentration in the plasma
Analysis of marmoset plasma after 1 month of DOX administration showed a DOX concentration in the high-DOX–treated group of 2.36 mg/l ± 0.223 (mean ± SEM). A DOX concentration of 2.54 mg/l ± 0.296 was found in the low-DOX–treated group (Supplementary Table S2). These data suggest that the plasma DOX concentration in marmosets can reach therapeutic levels with doses currently approved for use in humans. Product labeling for Monodox and Vibramycin DOX capsules indicate that plasma concentrations reach a peak of ~3 mg/l in healthy humans 2–3 hours after a single 200 mg oral dose.
Discussion
Our previous safety and efficacy studies with the combined HC-Ad-TK + HC-Ad-TetOn-Flt3L therapy have used the Lewis rat as a model system.14,15 For an assessment in an animal model with an immune system closer to humans, we chose the marmoset as a nonhuman primate model. The marmoset is a useful animal model for its relative ease of husbandry as well as its genetic26,27 and immunological4,28,29 similarity to humans. It has been used a model system to study viral infections,40–42 neurological disease,43,44 age-related disease,45 and gene therapy.2 Therefore, prior to initiating a phase 1 clinical trial in human patients with GBM, we examined transgene expression and overall safety of intracranial delivery HC-Ad-TetOn-Flt3L in naive marmosets.
We have developed a novel HC-Ad-based gene therapy for GBM14,15 that overcomes some potential toxicities of adenovirus-mediated gene therapy by eliminating all Ad genes encoding for proteins that are required for viral replication. This greatly reduces the immune response to the vector, thus increasing safety and allowing long-term transgene expression.46 Further, in order to insert an added level of control within this therapeutic delivery platform, the expression of the Flt3L gene was placed under the control of a DOX-regulated promoter. In the clinic, DOX, a common tetracycline antibiotic, will be administered orally to “turn on” Flt3L expression, and, if desired, Flt3L gene transcription can be stopped by ceasing DOX administration. The use of DOX to activate therapeutic gene transcription has not been previously approved by the FDA and therefore constitutes an “off-label” use of DOX. Currently, DOX is approved by the FDA solely for bacterial infections. Thus, it was important to determine the amount of DOX required to “turn on” Flt3L expression from HC-Ad-TetOn-Flt3L using DOX doses allometrically equivalent to those used in humans, as the phase 1 trial for the combined HC-Ad therapy will be the first time DOX is used to activate gene expression with therapeutic intent in humans. We have previously demonstrated robust Flt3L expression after intrastriatal injection of 1 × 109 vp of HC-Ad-TetOn-Flt3L in Lewis rats and oral DOX doses allometrically equivalent to human doses of 200 and 300 mg/day.25
This study examined Flt3L gene expression from the HC-Ad-TetOn-Flt3L vector after an intrastriatal injection of 1 × 109 vp in an animal model that exhibits immunological responses to cytokines similar to man.3,4 For activation of transgene expression from the TetOn promoter system, we orally administered DOX twice a day for 1 month at either 13.2 mg/kg/day (low-DOX) or 19.8 mg/kg/day (high-DOX). This dosing regime was similar to that planned for the phase 1 clinical trial, and the doses were allometrically equivalent to 200 and 300 mg/day in humans.30 Flt3L exhibited robust expression in both the low-DOX and high-DOX groups, within astrocytes, microglia, oligodendroglia, and neurons. The average plasma DOX concentration measured from blood drawn shortly after oral DOX administration was 2.54 and 2.36 mg/l in the low-DOX and high-DOX animals, respectively. These plasma concentrations are similar to those observed in humans 2–3 hours after a single oral dose of 200 mg DOX in healthy adults, with peak serum concentrations of 2.6 mg/l (Vibramycin) and 3.61 mg/l (Monodox). The doses of 200 and 300 mg/day were chosen because these doses are FDA approved for severe infections with a number of different bacterial species (DRUGDEX, Monodox, Vibramycin). Therefore, we believe that either the 200 or 300 mg/day DOX doses would be acceptable for “off-label” use in a phase 1 GBM clinical trial.
With respect to safety, we found that intracranial HC-Ad-TetOn-Flt3L injection with oral DOX administration was not associated with local or systemic toxicity. Local toxicity in the brain was examined using immunohistochemistry, and no overt abnormalities were observed. Histological examination of the liver and kidneys, potential sites for toxicity relating to DOX metabolism, did show notable kidney inflammation in some animals, in all of the experimental groups, including the control group. However, because renal inflammation is a common spontaneous health issue in marmosets held in captivity, and because kidney inflammation was seen in the control group as well as the experimental groups, we do not believe this inflammation to be a result of the HC-Ad-TetOn-Flt3L/DOX treatment. Inflammation of the kidneys is very common in marmosets housed in captivity; more than three quarters of all captive marmosets exhibit inflammation within the kidneys.32 The assessment of systemic and hematologic toxicity with CBC and serum chemistry analysis showed no alterations indicative of toxicity when compared to reference ranges from other works35,36 or to the presurgery values within both the low-DOX and high-DOX groups. Neutralizing antibody titers were low at all-time points, although one animal in the high-DOX group exhibited a positive titer of 128 at day 28 which could be due to injection of the vector into the third ventricle. This would in turn elicit circulating anti-Ad antibodies. Overall, these results support the conclusion that the treatment is safe, both locally and systemically.
In conclusion, given our data in rats25 and marmosets related to the efficacy and safety of allometric DOX doses to activate Flt3L expression, we conclude that either one of these doses will prove to be safe for use in the proposed HC-Ad-TK + HC-Ad-TetOn-Flt3L–mediated phase 1 clinical trial for GBM. These data form an important aspect of experimental evidence which will be submitted to the FDA in support of an IND application. Taking into account that the proposed strategy involves the expression of a human cytokine (Flt3L) within the brain, the use of marmosets adds significance to the safety profile of this strategy. Marmosets have been shown to exhibit an immune system and a response to cytokines which share many similarities to man,3,4 thus they constitute an invaluable preclinical model with better predictive value than rodent models in relation to the safety of immune-mediated gene therapy strategies.
Materials and Methods
Animals
Marmosets were obtained from the University of Texas Primate Center. Animals were pair-housed, and husbandry and veterinary services were provided by the University of Michigan Unit for Laboratory Animal Medicine. The study protocol was approved by the University of Michigan Unit for Laboratory Animal Medicine and the Institutional Review Board.
DOX administration
DOX was administered at 13.2 or 19.8 mg/kg/day, divided into two doses. Powdered DOX was purchased from Sigma-Aldrich (St Louis, MO, Cat# D9891), dissolved in deionized water, and administered orally with Ensure (Abbot Laboratories, Abbott Park, IL, Cat# 57231). DOX solution was prepared fresh weekly. The marmoset doses were found by dividing the human dose by a human weight of 45.45 kg, then multiplying by the allometric conversion factor of 3.30 For example, a human dose of 200 mg DOX per day is thus (200 mg/day ÷ 45.45 kg) × 3 = 13.2 mg/kg/day in the marmoset. Similarly, a 300 mg/day human dose is equivalent to a 19.8 mg/kg/day marmoset dose.
Blood draws
Animals were restrained using a custom-made restraint device to isolate one hind limb of the animal for blood draws. Blood was drawn from the femoral vein with a 26-gauge needle (BD Medical, Franklin Lakes, NJ, Cat# 305111) and a 1-ml syringe (BD Medical, Franklin Lakes, NJ, Cat# 309656) and placed immediately in an ethylenediaminetetraacetic acid tube (BD Medical, Cat# 367842) for CBC or plasma isolation or in a serum separation tube (Sarstedt, Newton, NC, Cat# 411378005).
Surgery
Anesthesia was initiated with an intramuscular injection of 20 mg/kg ketamine (Fort Dodge Animal Health, Overland Park, KS, Cat# NDC 59390-198-50). An endotracheal tube with 0.5–3% isoflurane (MWI Supply, Boise, ID, Cat# 502017) was used to maintain the animal under anesthesia. The animal was placed on a heating pad and secured into a stereotactic frame (Stoelting, Wood Dale, IL, Cat# 51600). Heart rate, respiration, and temperature were monitored throughout the procedure using a SurgiVet Advisor (Smiths Medical, Dublin, OH). Under sterile conditions, a midline incision over the skull was made, and two burr holes were drilled through the cranium at the two injection sites. 2.5 × 108 vp of HC-Ad-TetOn-Ftl3L were injected by Hamilton syringe (Hamilton, Reno, NV, Cat# 7635-01) at four sites in the brain, for a total of 1 × 109 vp. The four stereotactic injection sites, with respect to the bregma, were 5.0 mm anterior, 6.0 mm lateral, 8.0 ventral; 5.0 mm anterior, 6.0 mm lateral, 6.0 mm ventral; 5.0 mm anterior, 8.0 mm lateral, 8.0 mm ventral; and 5.0 mm anterior, 8.0 mm lateral, 6.0 mm ventral. At each site, the vector was slowly injected over a period of 2 minutes, with a 3-minute waiting period between each injection.
Fixation and sectioning
Perfusion and fixation of tissues was performed as previously described.47 Brains were postfixed for 2 days in 4% paraformaldehyde prior to sectioning on a Leica VT 1000S (Buffalo Grove, IL) vibratome at 50 µm. The liver and kidneys were postfixed for 2 days in 4% paraformaldehyde prior to dehydration on a Leica ASP 300 processor, embedded on a Tissue-Tek embedding station, and sectioned on a Leica RM 2135 microtome at 5 µm. Liver and kidney sections were stained with hematoxylin and eosin.
3,3′-Diaminobenzidine-peroxidase immunohistochemistry and Nissl staining
Free-floating brain sections were stained using the 3,3′-diaminobenzidine-peroxidase method for NeuN (1:1,000; Chemicon/Millipore, Billerica, MA, Cat# MAB337), tyrosine hydroxylase (1:5,000; Calbiochem/Millipore, Cat# 657012), myelin basic protein (1:1,000; Chemicon, Cat# MAB1580), GFAP (1:1,000; Chemicon, Cat# AB5804), Olig2 (1:500; Santa Cruz Biotechnology, Dallas, TX, Cat# SC19969), CD3 (Ventana, Tucson, AZ, Cat# 7904341), CD8 (Ventana, Cat# 7904460), IBA1 (1:1,000; Wako, Richmond, VA, Cat# 01919741), and Flt3L (1:1,000; Rabbit polycloncal, custom made) as previously described.47 Nissl staining was performed also as described.47
Immunofluorescence colabeling experiments
Free-floating colabeling immunofluorescence was performed as described elsewhere16,48,49 to determine the identity of cells transduced by the HC-Ad-TetOn-Flt3L and turned “on” by DOX to express Flt3L. Briefly, to identify reactive astrocytes expressing Flt3L, we used a mouse anti-GFAP antibody (1:250; Cell Signaling, Danvers, MA, Cat# 3670) in combination with a rabbit anti-Flt3L antibody (1:1,000; custom made). Primary antibodies were detected using goat anti-mouse Alexa-Fluor 546 (1:1,000; Molecular Probes/Life Technologies, Grand Island, NY) for GFAP and goat anti-rabbit Alexa-Fluor 488 (1:1,000; Molecular Probes) for Flt3L. To identify activated microglia and macrophages expressing Flt3L, we used a goat anti-IBA1 antibody (1:1,000; Abcam, Cambridge, MA, Cat# ab107159) in combination with the rabbit anti-Flt3L antibody. Primary antibodies were detected using chicken anti-goat Alexa-Fluor 488 (1:1,000; Molecular Probes) for IBA1 and chicken anti-rabbit Alexa-Fluor 546 (1:1,000; Molecular Probes) for Flt3L. To identify oligodendrocytes expressing Flt3L, we used a goat anti-Olig2 antibody (1:500; Santa Cruz, Dallas, TX, Cat# SC-19969) in combination with the rabbit anti-Flt3L antibody. Primary antibodies were detected using chicken anti-goat Alexa Fluor 488 (1:1,000; Molecular Probes) for Olig2 and goat anti-rabbit Alexa Fluor 546 (1:1,000; Molecular Probes) for Flt3L. To identify neurons expressing Flt3L, we used a chicken anti-MAP2 antibody (1:10,000; Abcam, Cat# ab5392) for neuron-specific cytoskeletal proteins, in combination with the rabbit anti-Flt3L antibody. Primary antibodies were detected using goat anti-chicken Alexa-Fluor 594 (1:1,000; Molecular Probes) for MAP2 and goat anti-rabbit Alexa-Fluor 488 (1:1,000; Molecular Probes) for Flt3L. Confocal images were taken using a Leica DMIRE2 microscope with a ×40 oil objective with additional ×4 magnification using Leica confocal software. Confocal images of each section were taken at a 0.5 μm thick resolution and were overlaid to produce merged images.
Neutralizing antiadenovirus antibody assay
The levels of adenovirus-specific neutralizing antibodies were assessed as described previously (see ref. 14,47; see Supplementary Materials and Methods) .
DOX plasma concentration
DOX plasma determination was performed by the University of Michigan Pharmacokinetics Core. Briefly, one volume of plasma was mixed with one volume of methanol, then with three volumes of acetonitrile prior to liquid chromatography-tandem mass spectrometry analysis with a Shimadzu LC-20AD liquid chromatograph (Columbia, MD) and an AB SCIEX QTRAP 5500 dual-mass spectrophotometer (Framingham, MA). Samples were run with a tetracycline internal standard, and the DOX concentration was calculated using a DOX standard curve.
CBC and serum chemistry
CBC was performed on an IDEXX Procyte Dx Hematology Analyzer (Westbrook, ME) within 15 minutes of blood draw according to the manufacturer’s instructions. Serum chemistry was performed on an IDEXX VetTest 8008 Chemistry Analyzer according to the manufacturer’s instructions.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants U01-NS052465, U01-NS052465-S1, R01-NS074387, R01-NS057711, and MICHR Pilot R12 to M.G.C.; NIH/NINDS Grants R01-NS054193, R01-NS061107, R01-NS082311, and R21-NS084275 to P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH UL1-TR000433; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863. M.C. was supported by an NIH/NINDS 1F32 NS058156 fellowship.
N.V., C.P., and M.G.C. wrote the manuscript. M.G.C., P.R.L., C.P., and N.V. designed the research; N.V., C.P., J.K., R.D., H.A., and A.A. performed the research; N.V., C.P., A.A., J.K., H.A., R.D., P.R.L., and M.G.C. analyzed the data; D.P. and P.N. contributed the HC-Ad-TetOn-Flt3L; all the authors read and reviewed the manuscript and made comments related to its content.
The authors declare no conflict of interest.
References
- Castro MG, Candolfi M, Kroeger K, King GD, Curtin JF, Yagiz K. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2011;11:155–180. doi: 10.2174/156652311795684722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Tani K, Ikebuchi K, Wu MS, Sugiyama H, Nakazaki Y. The common marmoset as a target preclinical primate model for cytokine and gene therapy studies. Blood. 1999;93:2839–2848. [PubMed] [Google Scholar]
- Ryffel B, Car BD, Woerly G, Weber M, DiPadova F, Kammüller M. Long-term interleukin-6 administration stimulates sustained thrombopoiesis and acute-phase protein synthesis in a small primate–the marmoset. Blood. 1994;83:2093–2102. [PubMed] [Google Scholar]
- Quint DJ, Buckham SP, Bolton EJ, Solari R, Champion BR, Zanders ED. Immunoregulation in the common marmoset, Calithrix jaccus: functional properties of T and B lymphocytes and their response to human interleukins 2 and 4. Immunology. 1990;69:616–621. [PMC free article] [PubMed] [Google Scholar]
- CDC. United States Cancer Statistics. (ed. Prevention, C.f.D.C.a.) (National Program of Cancer Registries, 2009).
- Omuro A, DeAmngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Colombo F, Barzon L, Franchin E, Pacenti M, Pinna V, Danieli D. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen A, Vapalahti M, Tyynelä K, Hurskainen H, Sandmair A, Vanninen R. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- Floeth FW, Shand N, Bojar H, Prisack HB, Felsberg J, Neuen-Jacob E. Local inflammation and devascularization–in vivo mechanisms of the “bystander effect” in VPC-mediated HSV-Tk/GCV gene therapy for human malignant glioma. Cancer Gene Ther. 2001;8:843–851. doi: 10.1038/sj.cgt.7700382. [DOI] [PubMed] [Google Scholar]
- Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Muhammad AK, Puntel M, Candolfi M, Salem A, Yagiz K, Farrokhi C. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther. 2010;88:204–213. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad AK, Xiong W, Puntel M, Farrokhi C, Kroeger KM, Salem A. Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: implications for a glioma phase 1 clinical trial. Hum Gene Ther Methods. 2012;23:271–284. doi: 10.1089/hgtb.2012.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Lamartina S, Silvi L, Roscilli G, Casimiro D, Simon AJ, Davies ME. Construction of an rtTA2(s)-m2/tts(kid)-based transcription regulatory switch that displays no basal activity, good inducibility, and high responsiveness to doxycycline in mice and non-human primates. Mol Ther. 2003;7:271–280. doi: 10.1016/s1525-0016(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Xiong W, Goverdhana S, Sciascia SA, Candolfi M, Zirger JM, Barcia C. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Curtin JF, Yagiz K, Assi H, Wibowo MK, Alzadeh GE. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011;13:947–960. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUGDEX. Thompson Reuters.
- VanderVeen N, Paran C, Krasinkiewicz J, Zhao L, Palmer D, Hervey-Jumper S. Effectiveness and preclinical safety profile of doxycycline to be used “off label” to induce therapeutic transgene expression in a phase I clinical trial for glioma. Human Gene Ther Clin Develop. 2013;24:116–126. doi: 10.1089/humc.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumoto S, Adati N, Tohtoki Y, Sakaki Y, Boroviak T, Habu S. Development and characterization of cDNA resources for the common marmoset: one of the experimental primate models. DNA Res. 2013;20:255–262. doi: 10.1093/dnares/dst007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock JK, Griffin DK, Delhanty JD, Parrington JM. Homologies between human and marmoset (Callithrix jacchus) chromosomes revealed by comparative chromosome painting. Genomics. 1996;33:214–219. doi: 10.1006/geno.1996.0186. [DOI] [PubMed] [Google Scholar]
- Crawford DH, Janossy G, Hetherington CM, Francis GE, Edwards AJ, Hoffbrand AV. Immunological characterization of hemopoietic cells in the common marmoset, rhesus monkey, and man. In search of a model for human marrow transplantation. Transplantation. 1981;31:245–250. doi: 10.1097/00007890-198104000-00003. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Kitaura K, Matsutani T, Shirai K, Suzuki S, Takasaki T. Immune-related gene expression profile in laboratory common marmosets assessed by an accurate quantitative real-time PCR using selected reference genes. PloS one. 2013;8:e56296. doi: 10.1371/journal.pone.0056296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin EM, Ruthsatz M, Collins JM, Hoyle PC. Extrapolation of animal toxicity to humans: interspecies comparisons in drug development. Regul Toxicol Pharmacol. 1990;12:107–116. doi: 10.1016/s0273-2300(05)80052-2. [DOI] [PubMed] [Google Scholar]
- Böcker R. Analysis and quantitation of a metabolite of doxycycline in mice, rats, and humans by high-performance liquid chromatography. J Chromatogr. 1983;274:255–262. doi: 10.1016/s0378-4347(00)84428-x. [DOI] [PubMed] [Google Scholar]
- Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G. Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Exp Toxicol Pathol. 2006;57:405–410. doi: 10.1016/j.etp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Tucker MJ. A survey of the pathology of marmosets (Callithrix jacchus) under experiment. Lab Anim. 1984;18:351–358. doi: 10.1258/002367784780865397. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Kurata Y, Makinodan F, Kidachi F, Yokoyama M, Wako Y. Spontaneous lesions detected in the common cotton-eared marmosets (Callithrix jacchus) J Vet Med Sci. 1996;58:181–190. doi: 10.1292/jvms.58.181. [DOI] [PubMed] [Google Scholar]
- Hawkey C, Hart M, Jones D. Clinical hematology of the common marmoset callithrix jacchus. American Journal of Primatology. 1982;3:21. doi: 10.1002/ajp.1350030117. [DOI] [PubMed] [Google Scholar]
- Willenbrock D, Lewis R, Ponzio B, Sis R, Stein F. Blood chemistry of the common marmoset (callithrix jaccus) maintained in an indoor-outdoor environment: primate comparisons. Primates. 1984;25:7. [Google Scholar]
- Barcia C, Jimenez-Dalmaroni M, Kroeger KM, Puntel M, Rapaport AJ, Larocque D. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntel M, A K M GM, Farrokhi C, VanderVeen N, Paran C, Appelhans A. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol Appl Pharmacol. 2013;268:318–330. doi: 10.1016/j.taap.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R, Jr, Patterson JL. An animal model that reflects human disease: the common marmoset (Callithrix jacchus) Curr Opin Virol. 2012;2:357–362. doi: 10.1016/j.coviro.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Bird BH, Lewis B, Johnston SC, McCarthy S, Keeney A. Development of a novel nonhuman primate model for Rift Valley fever. J Virol. 2012;86:2109–2120. doi: 10.1128/JVI.06190-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Yagi S, Carrion R, Chen EC, Liu M, Brasky KM. Experimental cross-species infection of common marmosets by titi monkey adenovirus. PloS one. 2013;8:e68558. doi: 10.1371/journal.pone.0068558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Obayashi S, Nagai Y, Oh-Nishi A, Minamimoto T, Higuchi M. PET analysis of dopaminergic neurodegeneration in relation to immobility in the MPTP-treated common marmoset, a model for Parkinson’s disease. PLoS ONE. 2012;7:e46371. doi: 10.1371/journal.pone.0046371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagessar SA, Gran B, Heijmans N, Bauer J, Laman JD, ‘t Hart BA. Discrepant effects of human interferon-gamma on clinical and immunological disease parameters in a novel marmoset model for multiple sclerosis. J Neuroimmune Pharmacol. 2012;7:253–265. doi: 10.1007/s11481-011-9320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52:54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- Puntel M, Kroeger KM, Sanderson NS, Thomas CE, Castro MG, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. Curr Protoc Neurosci. 2010;Chapter 4:Unit 4.24. doi: 10.1002/0471142301.ns0424s50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.