Abstract
Emotion regulation can be achieved in various ways, but few studies have evaluated the extent to which the neurocognitive substrates of these distinct operations overlap. In the study reported here, functional magnetic resonance imaging (fMRI) was used to measure activity in the amygdala and prefrontal cortex of ten participants who completed two independent tasks of emotion regulation – reappraisal, measuring intentional emotion regulation, and affect labeling, measuring incidental emotion regulation – with the objective of identifying potential overlap in the neural substrates underlying each task. Analyses focused on a priori regions of interest in the amygdala and inferior frontal gyrus (IFG).For both tasks, fMRI showed decreased amygdala activation during “emotion regulation” compared to “emotion” conditions. During reappraisal, this decrease in amygdala activation was accompanied by a proportional decrease in emotional intensity ratings; during affect labeling, the decrease in amygdala activation correlated with self-reported aggression. Importantly, across participants, the magnitude of decrease in amygdala activation during reappraisal correlated with the magnitude of decrease during affect labeling, even though the tasks were administered on separate days, and values indexing amygdala activation during each task were extracted independently of one another. In addition, IFG-amygdala connectivity, assessed via psychophysiological interaction analysis, overlapped between tasks in two regions within the right IFG. The results suggest that the two tasks recruit overlapping regions of prefrontal cortex, resulting in similar reductions in amygdala activation, regardless of the strategy employed. Intentional and incidental forms of emotion regulation, despite their phenomenological differences, may therefore converge on a common neurocognitive pathway.
Keywords: Emotion regulation, cognitive reappraisal, affect labeling, amygdala, prefrontal cortex, inferior frontal gyrus
Humans routinely engage in emotion regulation (ER) -- “the initiation of new, or alteration of ongoing, emotional responses through the action of regulatory processes” (Ochsner & Gross, 2005), p. 242 -- in an effort to modify their emotional trajectories. This regulation can be achieved in numerous ways(Hartley & Phelps, 2010; Koole, 2009), and although once assumed to only be initiated intentionally, evidence now suggests that some forms can occur automatically, without explicit intentions to modify emotions (Mauss, Bunge, & Gross, 2007). That is, under certain conditions, regulatory processes analogous to those elicited intentionally can be elicited incidentally (when individuals are not trying to regulate emotions) (Lieberman et al., 2007; Lieberman, Inagaki, Tabibnia, & Crockett, 2011), challenging prevailing assumptions. Overlap in neurobiological mechanisms across phenomenologically distinct forms of ER can offer some insight into commonalities in underlying processes. To this end, neuroimaging studies have compared neural processes across intentional ER strategies (Goldin, McRae, Ramel, & Gross, 2008; McRae et al., 2009), but commonalities with non-intentional forms of ER remain unexplored. As understanding such commonalities can refine and expand the definition of ER and encourage new avenues for research, we compared neural processes involved in intentional and incidental forms of ER, with the objective of determining whether processes overlap across this distinction.
The most commonly studied form of intentional ER is cognitive reappraisal, generally assumed to involve thedeliberate transformation of negative stimuli into less distressing terms by re-interpreting, rationalizing, or objectifying the material (but see (Mauss et al., 2007)). This strategy diminishes self-reported negative emotion and physiological markers of stress and arousal (Ochsner & Gross, 2008), and is associated with activation of prefrontal cortex (PFC) accompanied by decreased amygdala activity (Berkman & Lieberman, 2009; Ochsner & Gross, 2005, 2008). Aparticularly consistent finding across studies is involvement of ventrolateral PFC (Berkman & Lieberman, 2009), including inferior frontal gyrus (IFG). The IFG, particularly on the right, has been implicated in inhibitory control (Aron, Robbins, & Poldrack, 2004), spanning emotional as well as cognitive domains (Berkman, Burklund, & Lieberman, 2009; Tabibnia et al., 2011), and thus provides a possible neural mechanism underlying the decreased emotional experience with reappraisal.
In contrast, affect labeling -- the verbal labeling of emotional stimuli or one's reaction to them, i.e., “putting feelings into words” -- is an incidental, non-intentional form of ER. Like reappraisal, “emotion” conditions of this task can elicit markers of negative affect, including self-reported distress (Lieberman et al., 2011) and autonomic reactivity (Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003; Tabibnia, Lieberman, & Craske, 2008). Engaging in affect labeling diminishes these markers,and, like reappraisal, is associated with recruitment of right IFG and accompanying decreases in amygdala activity (Hariri, Bookheimer, & Mazziotta, 2000; Hariri et al., 2003; Lieberman et al., 2007; Payer, Lieberman, & London, 2011), i. e., neural activation patterns consistent with ER. This decreased amygdala activity was recently shown to relate to aggression (Payer et al., 2011), suggesting that despite being elicited incidentally, it indexes an individual's ER capacity.
Analogous neurobiological findings between reappraisal and affect labeling (i.e., IFG recruitment accompanied by reduced amygdala activation) suggest commonalities between tasks, yet no neuroimaging investigation has examined them in the same study. The only study to directly address their overlap measured self-reported distress, and found a correlation between reductions in distress achieved through reappraisal and affect labeling (Lieberman et al., 2011). The present study sought to explore commonalities at a neural systems level, using data from two functional magnetic resonance imaging (fMRI) studies (one investigating reappraisal (Baicy, 2008), the other affect labeling(Payer et al., 2011); also see Supplementary Materials) after identifying a subset of participants who completed both tasks. Here, we compared amygdala and IFG activation in these participants, with the expectation of revealing overlap in the neural substrates recruited by each task.
Method
All procedures were approved by the UCLA Office of the Human Research Protection Program.
Participants
The sample consisted of ten participants who completed independent studies of reappraisal(Baicy, 2008) and affect labeling (Payer et al., 2011) in the same laboratory, as part of a larger investigation of emotion processing in methamphetamine dependence (see Supplementary Materials). The participants described here were among the age- and education-matched healthy control group to which methamphetamine-dependent participants were compared. Participant overlap was assessed following conclusion of both studies, preventing expansion of the sample.
Inclusion/Exclusion criteria are described in detail in (Payer et al., 2011) and (Baicy, 2008). Briefly, volunteers aged 18 - 55 years provided written informed consent, and were screened for eligibility using questionnaires, psychiatric diagnostic interviewing (SCID-IV) (First, Spitzer, Gibbon, & Williams, 1995), and a medical examination. All participants were right-handed, fluent in English, and in good general health, and had no current Axis I diagnosis (except nicotine dependence), use of psychotropic medications or substances (except alcohol or marijuana use not qualifying for abuse or dependence), or positive urine drug screens on test days. Following completion of all sessions, participants were compensated in cash or vouchers.
The 10 participants described here (6 female) were on average 27.6 years old (SD = 8.09, range = 21-43 years), and had completed an average of 14.6 years of education (SD = 1.27). They completed Reappraisal and Affect Labeling tasks on separate test days, 1 - 61 days apart (mean = 24.3 days, SD = 24.5).
Tasks
For the Reappraisal Task (Figure 1B), participants were presented with images from the IAPS stimulus set (Lang, Bradley, & Cuthbert, 2005), each preceded by instructions to either experience the associated emotional response naturally (“Look” condition), or to decrease its intensity through reappraisal (“Decrease” condition). Participants confirmed their understanding via verbal description of their strategy on a practice trial. The stimulus set consisted of 32 highly aversive and 16 neutral images, matched for number of people/faces and visual complexity. Each trial consisted of a 2-sec instruction cue (“Look” or “Decrease”), 8 sec of stimulus presentation, 7 sec to rate emotional intensity on a scale from 1 (least intense) to 7 (most intense), and a 2-sec inter-trial-interval. The task contained a total of 96 trials, counterbalanced for instruction (“Look” or “Decrease”) across participants, and presented over two sequential functional runs.
Figure 1. Amygdala activation and emotional intensity ratings are lowered during both Reappraisal and Affect Label Tasks.
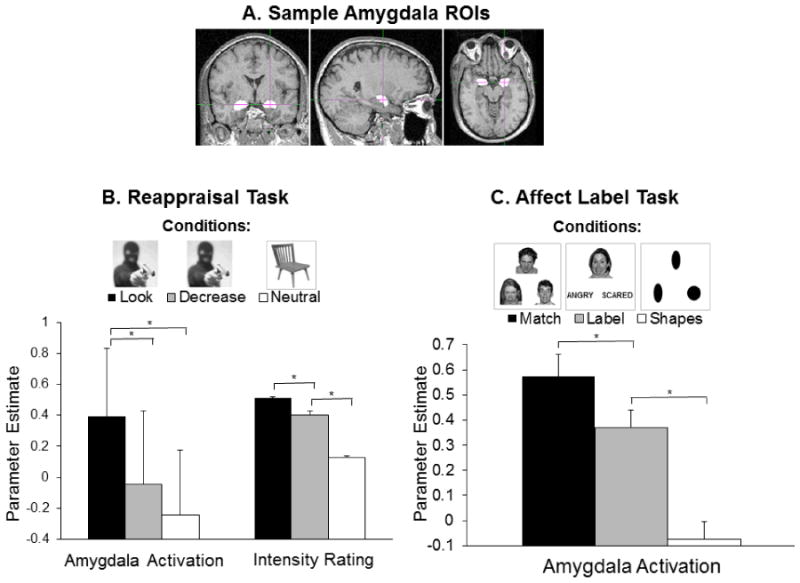
Panel A: Sample amygdala tracings from FSL FIRST. Voxels within these masks were used to calculate amygdala activation during each condition of the two tasks. Panel B: The figure legend displays sample stimuli from the Reappraisal Task. Bars represent means and standard errors for amygdala activation (parameter estimates in left and right amygdala combined), as well as emotional intensity ratings following image presentation (divided by 10 for display purposes). For both measures, values were lower during the “Decrease” than the “Look” condition of the task. Panel C: The figure legend displays sample stimuli from the Affect Label Task. Amygdala activation (parameter estimates in left and right amygdala combined) was lower during the affect labeling (“Label”) than the affect matching (“Match”) condition of the task.
The Affect Label Task (Figure 1C) included three conditions in which participants matched a target item at the top of the display to one of two choice items at the bottom: 1) Affect matching (“Match”), where participants matched faces(Tottenham et al., 2009) by emotional expression; 2) Affect labeling (“Label”), where participants chose a verbal label describing the facial expression (4 possibilities: “angry,” “scared,” “happy,” “surprised”); and 3) Shape matching (“Shapes”), where participants matched forms(10 possible geometric shapes). The task contained four blocks per condition, each consisting of five 5-sec trials (trial order randomized within blocks), presented over two sequential functional runs (order counterbalanced across participants). Each block was preceded by a 2-sec cue indicating the condition, and followed by 16 sec of fixation. For “Match” and “Label” conditions, 40 stimulus displays were chosen from the set used in (Lieberman et al., 2007) so that half the target faces for each condition displayed fear and half anger, and half were female. A total of 25 individuals comprised the stimulus set, and although some individuals appeared in more than one stimulus display, no individual-emotion combination was repeated across trials.
Apparatus and MRI Parameters
Functional MRI was performed on a 3.0 Tesla Siemens Allegra scanner (Erlangen, Germany), using a standard T2*-weighted gradient-echo echo-planar imaging pulse sequence to collect blood oxygen level dependent signal. Acquisition parameters for Reappraisal were: TR = 1500 msec, TE = 30 msec; flip angle = 70°; matrix = 64 × 64; voxel size = 3.1 × 3.1 × 4.0 mm. Each volume (608 total) consisted of 26 interleaved slices with a 25% distance factor, aligned parallel to the AC-PC line. Acquisition parameters for Affect Labeling were: TR = 2500 msec, TE = 28 msec; flip angle = 80°; matrix = 64 × 64; voxel size = 3.1 × 3.1 × 2.5 mm. Each volume (420 total) consisted of 36 interleaved slices with a 25% distance factor, aligned parallel to the AC-PC line. All images were later resampled into 3.125 mm isotropic voxels.Participants viewed stimuli through magnet-compatible video goggles(Resonance Technology, Northridge, CA),and indicated their choices through button presson a magnet-compatible keypad under their right hand. T2-weighted anatomical images in the same planes as the functional scansand a high-resolution MPRAGE image were also acquired for ROI generation and spatial normalization of functional images.
Data Analysis
MRI preprocessing was performed using SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were spatially realigned to the mean of the functional images (using a least squares approach and 6-parameter rigid-body spatial transformation) to correct for movement, and spatially co-registered with individual structural templates.
Amygdala activation
Individual amygdala region-of-interest (ROI) masks were delineated using the FIRST tool (Patenaude, 2007) of the FSL software package (FMRIB Analysis Group, Oxford, UK), which uses an automated procedure to fit shape-and-appearance models of subcortical regions to individual MPRAGE images (Figure 1A). Following ROI generation for each participant, functional scans were spatially smoothed with a 5-mm full width at half maximum (FWHM) Gaussian kernel, and masked with the spatially co-registered amygdala ROIs. For each task, a general linear model (GLM) was applied at each voxel within the ROI mask, using the MarsBaR toolbox for SPM (Brett, Anton, Valabregue, & Poline, 2002).
For the Reappraisal task, the GLM consisted of regressors for instruction cues, each condition -- “Look” (negative images with LOOK instructions), “Decrease” (negative images with DECREASE instructions), and “Neutral” (neutral images collapsed across LOOK and DECREASE instructions) -- and the emotional intensity rating phase. Fixation between trials was the implicit baseline. Individual trials were modeled as 8-sec boxcar functions, convolved with the hemodynamic response function (HRF) provided by SPM. The GLM for the Affect Label task consisted of regressors for instruction cues and each condition – “Match,”“Label,” and“Shapes.” Fixation between blocks was the implicit baseline. Blocks were modeled as 25-sec boxcar functions, convolved with the standard HRF.
After fitting the models at each voxel within the ROI masks, the resulting parameter estimates were averaged across all voxels in the mask, providing an index of amygdala activation during each condition of each task. These values were exported to SPSS 16.0 (Chicago, IL) for further analysis.
IFG recruitment
To investigate effective connectivity between the amygdala and PFC, we used psychophysiological interaction (PPI) analysis, which employs multiple regression to isolate regions showing differential relationships with one another, depending on psychological context. Results can be interpreted as the context-specific influence one brain region exerts over another (Friston et al., 1997). Toisolate PFC regions showing the desired connectivity patterns (greater negative relationship with the amygdala during “regulation” than “emotion” conditions), we used individual FIRST-generated amygdala ROIs as “seed” regions from which activity time-courses were extracted, conditions of the two tasks as the manipulated context, and IFG as a potential region for connectivity.
For each participant, functional images were smoothed with an 8-mm FWHM Gaussian kernel, and activation time-courses during each task extracted from their amygdala ROIs. These time-courses, along witha regressor for task conditions of interest (Look vs. Decrease for Reappraisal, Match vs. Label for Affect Label) and the amygdala × condition interaction (product of the two time-courses), were entered into whole-brain multiple regression analyses. Given our a priori hypotheses and small sample size, analyses were restricted to left and right IFG, using anatomic masks defined by the PickAtlas toolbox for SPM (Tzourio-Mazoyer et al., 2002). After estimating the models and assessing the interaction for each participant, statistical maps were spatially normalized to the standard MNI template, using a 12-parameter affine transformation.
To assess whether IFG recruitment overlapped between tasks, we used a conjunction approach, searching for voxels that showed a significant effect during both tasks simultaneously. We performed one-sample t-tests for effects of each task, and used the resulting maps of t-values to create a third map, consisting of the lower of the two t-values at each voxel. This map was then subjected to a voxelwise threshold of p = .0025 (effective conjoint probability after subjecting each map to a threshold of p = .05, using Fisher's methods of combining probabilities (see (Kampe, Frith, & Frith, 2003; McRae et al., 2009)), with the assumption that surviving clusters would indicate areas in which both tasks produced effects.
Results
Reappraisal Task
Parameter estimates from left and right amygdala ROIs correlated with each other during all conditions of the task, all r(8) > .86, all p ≤ .001, and were combined by calculating their mean. A one-way repeated-measures ANOVA assessing differences in these values between task conditions (Look, Decrease, Neutral) showed a significant effect of condition, F(2,18) = 8.96, p = .002, and, as expected, follow-up paired-samples t-tests revealed lower amygdala activation during the Decrease than Look condition, t(9) = 3.41, p = .008, d = 1.12 (Figure 1A). Activation during the Neutral condition was lower than during the Look condition, t(9) = 3.97, p = .003, d = 1.27, but not the Decrease condition, t(9) = 1.17, p = .271.
A one-way repeated-measures ANOVA assessing emotional intensity ratings also showed a significant effect of condition, F(2,18) = 169.64, p < .001, echoing findings from the original study (Baicy, 2008) (Supplementary Materials). In the present sample, follow-up paired-samples t-tests revealed parallel patterns to those in the amygdala: Ratings were lower during the Decrease than the Look condition, t(9) = 4.53, p = .001, d = 1.67, and ratings during the Neutral condition were lower than during both Look, t(9) = 23.17, d = 8.98 and Decrease, t(9) = 11.90, d = 4.19, both p < .001 (Figure 1A).
To confirm that the observed decrease in amygdala activation indexed a change in emotional state, we assessed its relationship with emotional intensity ratings. Across participants, reduction in amygdala activation (difference between Look and Decrease conditions) marginally correlated with reduction in emotional intensity ratings, r(8) = .549, p = .100, suggesting a relationship between reduced amygdala activation and emotional state. When separated by hemisphere, reduced right amygdala activation significantly correlated with reduced emotional intensity, r(8) = .678, p = .031. The correlation was not statistically significant on the left, r(8) = .402, p = .250.
Affect Label Task
Parameter estimates from left and right amygdala ROIs correlated with each other during Match and Label conditions, both r(8) > .88, both p ≤ .001, and were combined by calculating their mean. A one-way repeated-measures ANOVA assessing differences in these values between task conditions (Match, Label, Shapes) showed a significant effect of condition, F(2,18) = 17.37, p < .001, and, as expected, follow-up paired-samples t-tests revealed lower amygdala activation during the Label than Match condition, t(9) = 3.04, p = .014< .05, d = .99 (Figure 1B), echoing findings from the original sample (Payer et al., 2011) (Supplementary Materials). Activation during the Shapes condition was lower than during the Match condition, t(9) = 4.79, p = .001, d = 1.52, and the Label condition, t(9) = 3.62, p = .006, d = 1.14.
Due to the incidental nature of the task, it was not possible to collect ratings of emotional intensity and measure changes in emotional state. However, we previously reported an inverse correlation between reduced amygdala activation and self-reported aggression (presumably indexing ER capacity) (Payer et al., 2011), and the correlation held true in the present sample, r(8) = -.801, p = .017.
Comparison of Reappraisal and Affect Label Tasks
Amygdala activity
To test for a relationship between the magnitudes of decrease in amygdala activation achieved through reappraisal and affect labeling, we assessed their correlation. The two difference measures (Look - Decrease for reappraisal, Match - Label for affect labeling) were significantly correlated across participants, r(8) = .765, p = .010 (Figure 2A). A follow-up “leave-one-out” analysis, iteratively removingone participantand assessing correlations among the remaining data points, ensured that this correlation was not driven by any single participant or outlier (for all iterations, .685 < r < .834, .005 < p < .042).
Figure 2. Overlap between neural correlates of emotion regulation during Reappraisal and Affect Labeling.
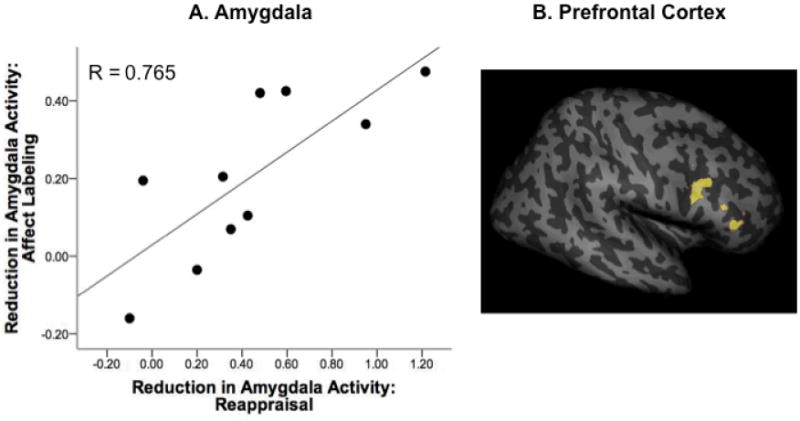
Panel A: Across participants, reduced amygdala activation achieved through reappraisal (Look – Decrease) correlated with reduced amygdala activation achieved through affect labeling (Match – Label). Panel B: Regions of IFG associated with these decreases in amygdala activation (assessed with PPI) overlapped in the right IFG (coordinates [x, y, z] = 54, 34, 20 mm, cluster size = 139 voxels; coordinates [x, y, z] = 38, 10, 28 mm, cluster size = 118 voxels). Data are rendered onto a structural template provided by SPM using the SurfRend toolbox.
IFG recruitment
To examine whether IFG recruitment overlapped between reappraisal and affect labeling, we performed a “minimum t-value” conjunction analysis between PPI maps from the two tasks (each map showing IFG regions that had greater connectivity with the amygdala during “regulation”than“emotion” conditions). Task-related IFG recruitment overlapped in two clusters in the right IFG (Figure 2B), with a peak “minimum t-value” of 3.44.
Discussion
The results provide evidence that intentional and incidental ER share neural substrates. Specifically, the magnitude of decrease in amygdala activation achieved through reappraisal related to that achieved through affect labeling, and both tasks recruited common areas in right IFG. To our knowledge, this study is the first to show this relationship in a within-subjects design, and provides a neural basis for the recently reported correlation between subjective emotional consequences of reappraisal and affect labeling (Lieberman et al., 2011).
Taken separately, each task produced amygdala and IFG activation patterns that are consistent with ER. During reappraisal, participants decreased amygdala activation and reported proportional decreases in emotional intensity, consistent with previous neuroimaging studies (Berkman & Lieberman, 2009; Ochsner & Gross, 2005, 2008). Similarly, participants decreased amygdala activation through affect labeling, which correlated with self-reported aggression, consistent with studies suggesting affect labeling as a form of incidental ER(Hariri et al., 2000; Hariri et al., 2003; Lieberman et al., 2007; Payer et al., 2011).
Importantly, neural activation patterns during the two tasks showed commonalities with one another. Across participants, the extent to which amygdala activation was reduced through reappraisal correlated with the extent of reduction achieved through affect labeling. Further, PFC activity associated with these reductions overlapped between tasks in right IFG. These findings suggest convergence of the disparate neurocognitive processes necessary to perform each task, resulting in similar amygdala regulation across individuals, regardless of strategy employed. Together, the results are consistent with the role of right IFG in cognitive and emotional inhibitory control (Berkman et al., 2009; Tabibnia et al., 2011), and provide the first direct evidence for the previously suggested neural overlap between intentional and incidental ER(Berkman & Lieberman, 2009).
Several limitations of the study should be noted. First, since reappraisal task instructions preceded stimulus onsets, the task measured biased appraisal (rather than initial appraisal/subsequent re-reappraisal) of stimuli, making “reappraisal” somewhat of a misnomer. However, we wished to remain consistent with the literature, and therefore adopted these traditional design and naming conventions (but see (Eippert et al., 2007)). Second, the affect labeling task does not allow for emotional intensity ratings, preventing distinction between emotion-regulation and potential non-emotion effects (e.g., instruction-sensitivity of the amygdala, participant inattention). However, we present evidence throughout that amygdala/IFG patterns associated with affect labeling at least in part reflect modulation of emotions. Further, it is possible that during affect labeling, participants spontaneously adopted intentional ER strategies. However, the task was introduced as a “visual processing task,” and did not require consideration of emotional content beyond task instructions. Third, the sample was small, potentially limiting statistical power and enhancing the probability of beta errors (e.g., marginal correlations may have reached significance in a larger sample, as the smallest detectable correlation with n=10 is r=.63). We took measures to minimize multiple comparisons, but recognize the importance of replicating the findings in a larger sample. Finally, the two tasks were completed on separate days, using differing task designs and MRI acquisition parameters. Although correlation and conjunction analyses allow for some comparison, the findings should be replicated using parallel designs during a single session.
These limitations notwithstanding, the present findings not only reinforce that affect labeling, like reappraisal, elicits PFC-amygdala connectivity consistent with ER, but also show that these seemingly disparate approaches can recruit overlapping neural substrates and result in similar outcomes. Although the present results are specific to the down-regulation of negative emotions, and relationships to up-regulation or positive emotions (e.g., (Kim & Hamann, 2007; Ochsner et al., 2004)) remain to be explored, the findings help refine the definition of emotion regulation, and lend support to the utility of putting feelings into words in alleviating negative affect.
Supplementary Material
Acknowledgments
Research was supported by NIH research grants R01DA020726, R01DA015179, P20DA022539 (EDL), and R01MH084116 (MDL), individual fellowships F31DA025422 (DEP) and F30DA021961 (KB), and institutional training grants T90DA022768 and T32DA024635. Additional funding was provided by endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies and the Marjorie Green Family Trust (EDL).
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baicy K. Ph D Dissertation. (Ph.D.), University of California; Los Angeles, CA: 2008. Dissociation between neural processing and negative emotion in methamphetamine dependence. [Google Scholar]
- Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. Neuroimage. 2009;47(2):705–712. doi: 10.1016/j.neuroimage.2009.04.084. S1053-8119(09)00485-6 [pii] 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: Theoretical and methodological considerations. Social and Personality Psychology Compass. 2009;3/4:475–403. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2):1140–1141. [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IP) Washington, D.C.: American Psychiatric Press; 1995. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:219–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. S0006-3223(07)00592-6 [pii] 10.1016/j.biopsych.2007.05.031 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. npp2009121 [pii] 10.1038/npp.2009.121 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Frith U. “Hey John”: signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(12):5258–5263. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koole S. The psychology of emotion regulation: An integrative review. Cognition & Emotion. 2009;23(1):4–41. 10.1080/02699930802619031 Pii 906986882. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings: Technical Report A-6. The Center for Research in Psychophysiology, University of Florida; 2005. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. PSCI1916 [pii] 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki T, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion. 2011;11(3):468–480. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, Gross JJ. Automatic Emotion Regulation. Social and Personality Psychology Compass. 2007;1(1):146–167. [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The Neural Bases of Distraction and Reappraisal. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive Emotion Regulation: Insights From Social Cognitive and Affective Neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. S1053-8119 (04)00340-4 [pii] 10.1016/j.neuroimage.2004.06.030 [doi] [DOI] [PubMed] [Google Scholar]
- Patenaude B. Bayesian statistical models of shape and appearance for subcortical brain segmentation. (D.Phil.), University of Oxford; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED. Neural correlates of affect processing and aggression in methamphetamine dependence. Archives of General Psychiatry. 2011;68(3):271–282. doi: 10.1001/archgenpsychiatry.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: words may facilitate exposure effects to threatening images. Emotion. 2008;8(3):307–317. doi: 10.1037/1528-3542.8.3.307. 2008-06717-001 [pii] 10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, et al. London ED. Different forms of self-control share a neurocognitive substrate. Journal of Neuroscience. 2011;31(13):4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. Nelson CA. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


