Abstract
Sex-related differences in physiology and anatomy are responsible for profound differences in neuromuscular performance and fatigability between men and women. Women are usually less fatigable than men for similar intensity isometric fatiguing contractions. This sex difference in fatigability, however, is task specific because different neuromuscular sites will be stressed when the requirements of the task are altered, and the stress on these sites can differ for men and women. Task variables that can alter the sex difference in fatigue include the type, intensity and speed of contraction, the muscle group assessed, and the environmental conditions. Physiological mechanisms that are responsible for sex-based differences in fatigability may include activation of the motor neuron pool from cortical and subcortical regions, synaptic inputs to the motor neuron pool via activation of metabolically-sensitive small afferent fibres in the muscle, muscle perfusion, and skeletal muscle metabolism and fibre type properties. Non-physiological factors such as the sex bias of studying more males than females in human and animal experiments can also mask a true understanding of the magnitude and mechanisms of sex-based differences in physiology and fatigability. Despite recent developments, there is a tremendous lack of understanding of sex differences in neuromuscular function and fatigability, the prevailing mechanisms and the functional consequences. This review emphasises the need to understand sex-based differences in fatigability in order to shed light on the benefits and limitations that fatigability can exert for men and women during daily tasks, exercise performance, training and rehabilitation in both health and disease.
Keywords: sex differences, gender, central fatigue, peripheral fatigue, fibre types, metabolism, women, muscle fatigue
Introduction
Men and women differ in anatomy and physiology, which results in marked sex differences in neuromuscular performance and fatigability. In general, the skeletal muscles of men are larger and some muscles possess a greater proportional area of metabolically and functionally faster muscle fibres (Type II) than women [e.g. (Simoneau and Bouchard, 1989, Staron et al., 2000, Porter et al., 2002, Roepstorff et al., 2006)] due to sex-related differences in human skeletal muscle gene expression and interactions with sex-specific hormones (Roth et al., 2002, Welle et al., 2008, Maher et al., 2009, Liu et al., 2010). Consequently, the whole muscles of men are usually stronger and more powerful than women. When contractions are sustained or repeated, however, as they are during exercise, rehabilitation and many daily activities, the relative reduction in force and power can differ between men and women when performed at the same relative intensity of contraction. This activity-induced reduction in force or power is known as muscle fatigue (Gandevia, 2001, Enoka and Duchateau, 2008, Kent-Braun et al., 2012) or fatigability (Kluger et al., 2013). In this review the terminology is used interchangeably. Muscle fatigue develops soon after the onset of sustained physical activity and can occur despite continued and successful performance of submaximal exercise. However, if the submaximal task is maintained, task failure will eventually occur. Multiple mechanisms contribute to the force and power decrements, and range from an inadequate activation of the motor cortex to impairment of the contractile proteins within skeletal muscle fibres, however, the dominant mechanism is specific to the process that is stressed the most (Gandevia, 2001). While motor performance is ultimately limited by the output of the muscle, limitations in both activation within the central nervous system and muscle, can lead to fatigability and a decrement in performance of maximal and submaximal tasks in both men and women.
Despite recent developments in our understanding of the mechanisms of muscle fatigue [e.g. (Gandevia, 2001, Enoka and Duchateau, 2008, Kent-Braun et al., 2012)], there is still a tremendous lack of knowledge and appreciation of sex-based differences in fatigability and the prevailing mechanisms under different task conditions. This is, in part, because of the historical and current bias of studying proportionally more males than females in both human and animal-based physiology (Anonymous, 2010, Kim et al., 2010, Zucker and Beery, 2010, Beery and Zucker, 2011, Cahill, 2012, Miller, 2012) and the presumption that sex differences in fatigability do not exist. This review will provide a framework to understand the importance of sex differences in fatigability and its relevance for rehabilitation, training and daily function. The review will highlight: (1) known sex differences in fatigability for different task conditions, and (2) some of the physiological differences between men and women that may explain the sex differences in fatigability.
Sex Differences in Muscle Fatigue are Task Specific
Two experimental approaches have emerged over the last 20 years that, together, provide valuable insight into the mechanisms for the sex differences in fatigability. The classic approach is to measure the physiological mechanisms during maximal contractions performed by men and women before, during and after fatiguing exercise. A functionally relevant approach has been to vary the task requirements and environment of a fatiguing contraction in order to stress different sites (or the same site at a different rate) within the neuromuscular system (Hunter et al., 2004a, Enoka and Duchateau, 2008). This second approach is based on the concept that muscle fatigue is specific to the demands of the task (Enoka and Stuart, 1992) and this specificity can differ for men and women because of sex-related differences within the neuromuscular system (Hunter, 2009). Hence, the sex difference in fatigability and the rate limiting mechanisms can differ, for example, according to the contraction type, speed and intensity, the involved muscle group, environmental conditions and state of arousal. Following are examples of how the magnitude of the sex difference in fatigability will differ between single limb isometric, shortening and lengthening contractions, and multiple sprint exercise. In general, while much has been learned about sex differences in fatigability during isometric contractions over the last 20 years, less is known about the sex differences in fatigability during dynamic performance.
Single Limb Contractions
a. Isometric Contractions
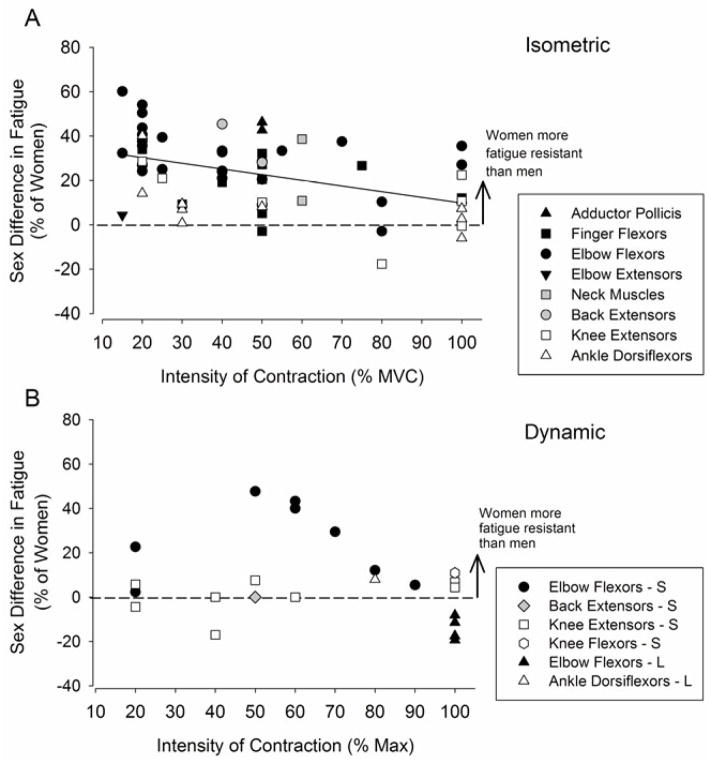
There can be large sex differences in muscle fatigue for isometric fatiguing contractions, especially for some muscle groups. In general, women are less fatigable than men for isometric sustained and intermittent contractions performed at the same relative intensity for several muscle groups, including the elbow flexors, finger flexors, adductor pollicis, back extensors, dorsiflexors, knee extensors and respiratory muscles [e.g. (Maughan et al., 1986, Fulco et al., 1999, Hunter and Enoka, 2001, Hunter et al., 2002, Clark et al., 2003, Russ and Kent-Braun, 2003, Hunter et al., 2006b, Hunter et al., 2009, Guenette et al., 2010)] (Figure 1A). Some muscle groups, such as the ankle dorsiflexors, demonstrate less of a sex difference in fatigability than the elbow flexor muscles (Kent-Braun et al., 2002, Hunter et al., 2008, Avin et al., 2010), and for the elbow extensor muscles there is no sex difference for a sustained contraction (Dearth et al., 2010). The explanation for the differences in the magnitude of the sex difference between muscle groups likely involves a combination of muscular mechanisms which include contractile properties, fibre type proportion and perfusion. These mechanisms are addressed in the second part of the review. Those muscle groups that exhibit the largest sex differences in fatigability however, for sustained isometric contractions also tend to show associations between strength and fatigability (e.g. elbow flexors and knee extensors) (Maughan et al., 1986, Hunter and Enoka, 2001, Avin et al., 2010). Further, the sex difference in muscle fatigue is diminished as the contraction intensity increases for some of these muscles (Maughan et al., 1986, West et al., 1995, Yoon et al., 2007). These observations provide insight into the role of strength-associated mechanisms and blood flow as potential mechanisms for the sex difference in muscle fatigue, especially at lower intensities of contraction (see blood flow section).
Figure 1. Sex differences in muscle fatigue for voluntary isometric contractions (A) and dynamic contractions (B).
Represented are mean data from 59 studies: 43 isometric contraction studies (A) and 16 dynamic contraction studies (B) that assessed muscle fatigue in men and women for various muscle groups. Plotted in both panels is the difference between the mean fatigue index or time to task failure of the men and women (as a percent of the women’s value) within a study as a function of the contraction intensity of the fatigue task. In both panels, upper limb muscles are in closed symbols and lower limb muscles represented in open symbols. Back and neck muscles are represented as grey symbols. A. Sex differences in muscle fatigue for sustained and intermittent isometric fatiguing contractions are plotted. Most data points are above the line indicating women are more fatigue resistant than men for most muscle groups. There was a significant negative relation between the relative contraction intensity and the magnitude of the sex difference for the isometric contractions when all muscle groups are included (r2 = 0.20). B. Sex differences in muscle fatigue for shortening (S) and lengthening (L, triangle-up symbols) fatiguing contractions are plotted. There was no relation between contraction intensity and the sex difference for dynamic contractions, although data from two studies for the elbow flexor muscles (between 50–90% max), however, showed a significant negative relation (r2 = 0.97) for shortening contractions. There are more data points than number of stated studies because some studies involved multiple contraction types or intensities.
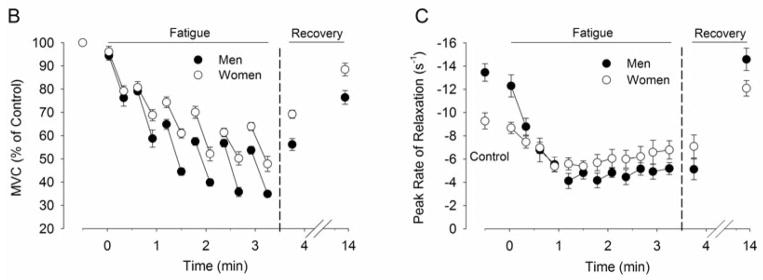
Comparison of fatigability in men and women during sustained versus intermittent isometric contractions demonstrates the reliance of the sex difference in fatigability on the details of the task. For example, men (who are usually stronger) have a briefer time to failure than women for the elbow flexor muscles (Hunter and Enoka, 2001, Hunter et al., 2004a, Yoon et al., 2007). For men and women who were matched for strength, however, there was no sex difference in the time to failure of a sustained submaximal contraction (Hunter et al., 2004b), indicating a strength–related mechanism contributed to the longer time to task failure. In contrast, the strength-matched women were able to perform an intermittent isometric task until failure almost three times longer than the strength-matched men (23.5 vs 8.6 mins) (Hunter et al., 2004c). The major difference between the fatiguing tasks is the muscle is more perfused during the intermittent contraction than the sustained contraction. Comparison of these studies with the elbow flexor muscles suggests that the mechanisms responsible for the sex difference in fatigability for an intermittent task: (1) differ from those responsible for the sex difference in fatigue during sustained isometric contractions; and (2) were independent of the absolute strength exerted by the men and women. More studies are needed to understand the magnitude and cause of the sex difference in fatigability during intermittent isometric tasks for many muscle groups (e.g. elbow extensor muscles). Comparisons with sustained contractions would also provide valuable insight into whether or not isometric contractions that are often prescribed in early rehabilitation need to be prescribed differently for men and women under different task conditions and for specific muscles.
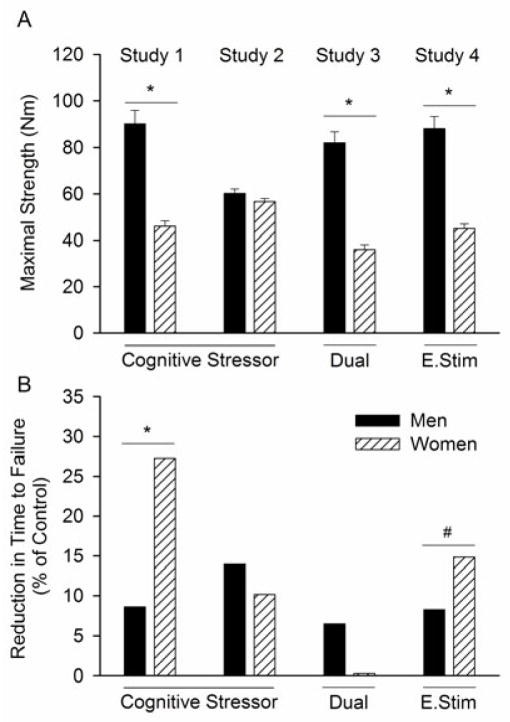
A functionally relevant strategy that provides insight into the influence of environment during a submaximal isometric fatiguing contraction is shown with experiments that have varied the cognitive demand and arousal (stress) imposed during a submaximal fatiguing task in men and women (Yoon et al., 2009b) (Figure 2). Women typically exhibit greater physiological responses to stress-inducing events than men, such as increased cognitive demand or unpredictable electric shocks to the back of the hand (Christou et al., 2004, Kajantie and Phillips, 2006). Sex differences in response to a stressor include different brain activation strategies (Wang et al., 2007) and reduced steadiness (larger force fluctuations) during light-load contractions (Christou et al., 2004). Further, when a cognitive stressor was imposed during performance of a fatiguing contraction sustained at 20% of maximal voluntary contraction (MVC), the time to failure was reduced for men (8.6 %), but more so for women (27.3 %) compared with their control fatiguing contraction that involved no imposed stressor (Yoon et al., 2009b) (Figure 2). The cognitive stressor involved performance of a mental-math task (counting backwards in increments of 13), which increased levels of reported anxiety and stress as well as salivary cortisol levels. Heart rate and blood pressure were also elevated, which increased cardiac work. The increased fatigability was not due to differences in the ability of young men and women to perform the mental-math, nor mental distraction (Yoon et al., 2009b) (Figure 2). This was corroborated by subsequent experiments that showed that when stress was induced in young men and women with unpredictable, but brief, electric shocks to the back of the hand of the non-exercising arm, the women exhibited larger reductions in the time to failure relative to control conditions compared with men (Yoon and Hunter, unpublished findings) (Figure 2).
Figure 2.
Summary of findings from 4 studies showing the influence of increased arousal on time to task failure of a fatiguing isometric contraction (20% MVC until failure) with the elbow flexor muscles in young men and women. In each study, two sessions were performed. A fatiguing contraction with no imposed stressor (control) and an experimental session where one of the following were imposed during the contraction: a difficult mental math task (counting backward by 13, cognitive stressor), easy mental math (dual) or brief electric shocks to the back of the non-exercising hand (E. Stim). A. Shown is the maximal voluntary isometric strength performed prior to the fatiguing contraction by young men and young women. B. Shown is the relative reduction in time to task failure (between the control and stressor contraction, i.e. the Stressor, Dual or E. Stim) for each study. Women had greater reductions than men for the cognitive stressor (study 1, n = 20) (Yoon et al., 2009b); but not when matched for strength (study 2, n = 10) (Keller-Ross et al., 2014). Study 3 showed minimal changes in time to failure when the subjects performed easy mental math (Dual, n = 20)(Yoon et al., 2009b). In study 4, reductions in time to failure were observed when the arousal was induced with unpredictable brief electric shocks to the back of the hand during the contraction (E.Stim, n = 20) (Hunter and Yoon, unpublished findings).
* P<0.05 between men and men, # P = 0.07 although, not included in the results, are 2 women who failed to complete the fatiguing contraction because of increased levels of anxiety.
The cause of the stress-induced increase in fatigability in the women is not fully understood. For the cognitive stressor experiments with the elbow flexor muscles, strength-related mechanisms may, in part, be responsible for the sex difference in the reduction in time to failure. There was an association between the initial maximal strength and the reduction in the time to failure when the cognitive stressor was imposed (Yoon et al., 2009b, Keller-Ross et al., 2014). Further, comparison of strength-matched men and women indicated a similar reduction in time to failure for men and women when the stressor was imposed (Keller-Ross et al., 2014) (Figure 2). The increased fatigability with exposure to the cognitive stressor in the elbow flexor muscles may involve altered perfusion via stress-induced changes in sympathetic activation of muscle that has greater effects in people who are weaker and therefore have a more perfused muscle at the start of low-force sustained contractions. Other mechanisms, however, also likely contribute to the sex difference in fatigability in response to a stressor because there was no association between initial strength and the time to failure (r = −0.09) for experiments that induced stress with the electric shocks (Yoon and Hunter, unpublished findings). Furthermore, the sex effect may only be relevant to the low force contractions. Fatigability was greater when a stressor was imposed during a sustained contraction at 50% MVC and an intermittent maximal task with the handgrip muscles and no sex differences were reported (Bray et al., 2008, Bray et al., 2012). More studies are required to determine the mechanism for the stress-induced increase in muscle fatigability for different muscle groups and task conditions, and the susceptibility of women.
b. Shortening Contractions
The sex difference for dynamic fatiguing contractions has been studied less than isometric contractions, but the findings indicate that women are either less fatigable than men or that the sex difference in fatigability is diminished (Figure 1B). Women were less fatigable than men for a protocol of 30 maximal dynamic contractions with the knee extensor and knee flexor muscles at a relatively constant speed of 3.14 rad·s−1 (180 deg/s) (Pincivero et al., 2003): the magnitude of fatigue was explained by the initial maximal torque, because stronger subjects (men) exhibited greater fatigue (decline in maximal torque). Similarly, the time to task failure was longer for women than men (9.7 ± 5.5 min vs. 6.1 ± 2.1 min, respectively) for a dynamic task that required the participant to lift and lower a load equivalent to 20% of MVC force for as long as possible (1 contraction every 3 seconds) (Yoon and Hunter, unpublished results). These studies indicate that women exhibited less fatigue than men for dynamic fatiguing contractions that assessed fatigue as either the time to task failure or as a loss of maximal torque when the velocity of contraction was relatively controlled. The intensity of the dynamic contraction, however, can alter the sex difference in fatigability because, as the load progressively increased between 50% and 90% of one repetition maximum (1RM), the sex difference lessened (Maughan et al., 1986). For these studies women were less fatigable than men for dynamic contractions.
The sex difference in fatigability of some dynamic tasks, however, can be diminished, and dependent on the contraction speed (Clark et al., 2003, Pincivero et al., 2004, Senefeld et al., 2013, Taipale and Hakkinen, 2013). For example, despite women having a longer time to task failure than men with the back extensor muscles for an isometric contraction sustained at 50% of maximal strength (28% difference), there was no sex difference in the number of repeated shortening contractions performed at 50% of maximal strength until failure (24.3 vs. 24.0 repetitions) (Clark et al., 2003). Furthermore, there was no sex difference in the relative reduction in velocity and power for a task that required the young men and women to contract their elbow flexor and knee extensor muscles as quickly as possible with a load equivalent to 20% of their maximal isometric strength over ~4.5 minutes (Senefeld et al., 2013). In contrast, the decline in MVC from initial values that were measured immediately following the dynamic exercise in this study was significantly greater for the men than the women for the knee extensor muscles (17.8% ± 2.8 vs 10.4 ± 2.3%), but similar for the elbow flexor muscles. Although a sex difference in fatigability was not apparent during the dynamic task, men experienced greater relative reductions in maximal force than women at the end of the dynamic task with the knee extensor muscles. Fatigue of maximal force is due to fewer high force cross-bridges and/or less force per cross-bridge, but reductions in maximal velocity contractions is related to the speed of cross-bridge cycling and calcium kinetics in the fibre [see (Kent-Braun et al., 2012) for review]. Greater insight into sex differences in the rate limiting mechanisms of dynamic contractions could be gained from experiments that fatigue human single fibres during shortening contractions in vitro. The implications of these findings for clinicians and scientists assessing fatigue of dynamic contractions in men and women are important because the mode of measurement chosen to assess fatigue may not reveal the underlying force decrements and potential differences between the sexes.
c. Lengthening Contractions
Many dynamic contractions involve lengthening of the muscle fibres while they are activated; this is also known as eccentric activation. Examples of such tasks include lowering an object with the elbow flexor muscles with the arm in the sagittal plane, or walking down a set of stairs so that the quadriceps muscles are activated while lengthening. A lengthening contraction is able to generate more force within the muscle fibre and, hence, greater muscle torque than maximal isometric and shortening contractions (Duchateau and Baudry, 2013, Herzog, 2013). Prior to fatigue, the sex difference in peak torque during a lengthening contraction is less than the sex difference in torque for a shortening contraction at the same velocity of contraction, although the mechanism is not fully understood [eg. (Seger and Thorstensson, 1994, Lindle et al., 1997)]. Further, voluntary activation is less during maximal lengthening contractions than shortening contractions prior to fatigue (Duchateau and Baudry, 2013), with no apparent differences between men and women (Spurway et al., 2000).
A bout of repeated lengthening contractions will elicit muscle fatigue and also muscle damage, the latter of which results in delayed on-set muscle soreness (DOMS) (Clarkson and Hubal, 2002). Muscle damage involves ultrastructural muscle fibre damage and inflammatory responses (Clarkson and Hubal, 2002). The initial loss of force due to muscle fatigue versus damage is difficult to differentiate, because both potentially reduce force generating capacity, but via different mechanisms. Fatigability of muscle is usually resolved within hours, while muscle damage can impair force generation for up to 7 days. Muscle damage, however, can increase fatigability of the elbow flexor muscles (Heroux and Gandevia, 2013, Semmler et al., 2013), although whether sex differences exist is not known. In contrast to the animal model (Tiidus and Enns, 2009, Enns and Tiidus, 2010), maximal force reductions in humans is either similar for men and women (Rinard et al., 2000, Hubal et al., 2008, Hubal and Clarkson, 2009, Power et al., 2010), or women have greater losses of force than men (Sayers and Clarkson, 2001, Sewright et al., 2008) (Figure 1B). Recently it was shown that women have slower recovery of power and rates of force development compared with men after 150 maximal lengthening actions performed at 60 deg·s−1 with the ankle dorsiflexor muscles (Power et al., 2013). A possible confounder is that women have lower pain thresholds than men (Fillingim et al., 2009, Racine et al., 2012), and pain will influence motor output and fatigability (Prasartwuth et al., 2005, Martin et al., 2008, Semmler et al., 2013). Pain and muscle damage from lengthening contractions also alters voluntary activation (Prasartwuth et al., 2005). Thus, sex differences in muscle fatigue with lengthening contractions may be masked by the pain accompanying muscle damage, which can influence men and women differently. Insight into any potential sex differences in fatigability could be gained by comparing the fatigability of lengthening contractions of men and women after several sessions of training or pre-conditioning (Chen et al., 2012). Because fatiguing lengthening contractions are important in optimising muscle hypertrophy during strength training and rehabilitation programs (Roig et al., 2009), addressing sex differences in fatigability may reveal information that is relevant to individualising such programs for men and women.
Multiple Sprint Exercise
The reduction in maximal power during, and in recovery from, sprint exercise on a cycle ergometer indicates that, in general, men experience greater reductions in power than women (Esbjornsson-Liljedahl et al., 2002, Billaut and Smith, 2009, Billaut and Bishop, 2012). [See (Billaut and Bishop, 2009) for review.] In each study, men were ~30% more powerful than women for the knee extensors (Esbjornsson et al., 2006, Billaut and Smith, 2009, Esbjornsson et al., 2012). The difference in decline in power between men and women with multiple high intensity sprints of short duration was associated with the initial power (Billaut and Bishop, 2012). Consequently, when men and women were matched for initial power, there was no sex difference in the reduction in power (Smith and Billaut, 2012). More mechanistic studies are needed to determine the role that maximal power plays in the difference in fatigability with multiple sprint exercise. Although the relative reduction in power is not always significantly different between men and women [e.g.(Esbjornsson et al., 2006, Esbjornsson et al., 2012, Smith and Billaut, 2012)], several of these studies have revealed sex differences in muscle and whole body metabolism that would likely have functional consequences for multiple sprint exercise of longer durations than several minutes. One functional consequence is that while men exert greater absolute power and speed than women, women recover more quickly than men (Laurent et al., 2010).
Mechanisms for Sex Differences in Muscle Fatigue
Sex differences in physiology and anatomy contribute to differences between men and women in muscle function and fatigue; although, there are other non-physiological factors that may also contribute and are addressed toward the end of this review.
Physiological Mechanisms
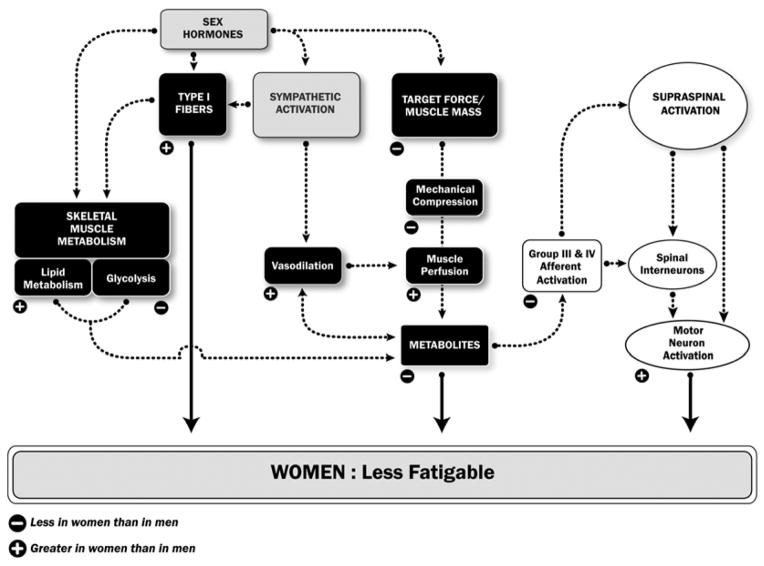
Following are mechanisms from physiological sources that potentially contribute to the sex difference in muscle fatigability and task specificity (Figure 3).
Figure 3. Potential physiological mechanisms for the sex difference in muscle fatigue.
(or time to task failure).
The figure shows those potential mechanisms that can contribute to women being more fatigue resistant than men. The strength of a potential mechanism will vary with the task conditions so that one dominant mechanism does not fully explain the sex difference in performance of a fatiguing contraction. A negative sign indicates that the physiological variable or process is less in women than men and, conversely, a positive sign indicates it is greater in women than men. Ultimately, the differences in fatigue between men and women can be due to differences in: 1) motor neuron activation; 2) contractile function of the activated fibres; and 3) the magnitude of metabolites accumulating that interfere with contractile function. These mechanisms are stipulated with the large arrows. Black boxes indicate processes within the muscle, white boxes are processes in the nervous system, and the grey are hormonal and sympathetic actions.
a. Muscle Mass and Strength Differences
For some muscle groups and tasks, a greater initial strength is associated with increased fatigability, indicating the involvement of a strength-related mechanism (Maughan et al., 1986, West et al., 1995, Hunter and Enoka, 2001, Hunter et al., 2004a, Hunter et al., 2006a, Hunter et al., 2006b, Avin et al., 2010). Men are usually stronger than women because they have a larger skeletal muscle mass (Miller et al., 1993, Lindle et al., 1997, Ivey et al., 2000, Welle et al., 2008). The relative sex difference in muscle mass and strength is greater for some muscle groups than others, such as the elbow flexor muscles and finger flexors compared with the knee extensor muscles and dorsiflexor muscles (Miller et al., 1993, Russ and Kent-Braun, 2003, Hunter et al., 2006b, Senefeld et al., 2013). Because men and women are able to voluntarily activate their muscles to similar, and near maximal, levels during a maximal voluntary isometric contraction (MVC) of the upper limb muscles (Miller et al., 1993, Hunter et al., 2006a, Yoon et al., 2007) and lower limb (Russ and Kent-Braun, 2003) prior to fatiguing tasks, there are usually minimal reported sex differences in specific strength (ie force/unit of muscle). Thus, subsequent fatiguing contractions are usually performed at similar relative intensities for men and women, and any sex-difference in fatigue, therefore, is not because women activate relatively less muscle than men at the start of a fatiguing task. The sex difference in strength is mainly due to larger diameter muscle fibres in the men than women for both upper and lower limb muscles, and probably not due to a larger number of fibres in the men (Miller et al., 1993).
The larger absolute differences in muscle mass and greater forces exerted during a fatiguing contraction for men compared with women can have mechanical and metabolic consequences during fatiguing exercise, some of which are highlighted in Figure 3 and the following sections. Consequently, for some tasks, greater fatigability of men compared with women is a direct result of men exerting greater absolute strength during the contraction (see blood flow section). In other instances, the sex difference in fatigability is associated with the greater strength exerted by men, but is not the primary cause for the sex difference [e.g. (Hunter et al., 2006a)]. For other tasks, such as the intermittent isometric contraction, the sex difference in muscle fatigue is independent of strength differences [eg. (Fulco et al., 1999, Hunter et al., 2004c)]. Comparing men and women of the same absolute strength can determine the role of absolute strength in the observed sex differences in fatigue, but is not always easy to achieve given that, for most muscle groups, the majority of men are stronger than women.
b. Blood Flow and Muscle Perfusion
Reduced blood supply to an active muscle will result in accelerated fatigue and earlier task failure because of the reduced oxygen delivery to the muscle and rapid accumulation of metabolites that interfere with the contractile function (Russ and Kent-Braun, 2003, Clark et al., 2005). Accumulated metabolites may further accelerate fatigue by increasing peripheral afferent feedback and inhibitory inputs to the motor neuron pool, and further limit voluntary activation (Gandevia et al., 1996). Differences in muscle perfusion between men and women contribute to a sex difference in muscle fatigue and performance, but this mechanism is specific to certain tasks and muscle groups (Russ and Kent-Braun, 2003, Hunter et al., 2004b, Clark et al., 2005, Parker et al., 2007, Thompson et al., 2007, Saito et al., 2008). In general, women can have greater muscle perfusion than men for some muscle groups, due to a difference in mechanical compression onto the feed arteries during low-force sustained isometric contractions [e.g (Hunter and Enoka, 2001)], a sympathetically-mediated difference in vasodilation (Ettinger et al., 1996, Hogarth et al., 2007, Parker et al., 2007), and greater capillarization of the muscle bed. The contribution of each of these mechanisms to any sex difference in fatigability is task and muscle group dependent.
During low-to-moderate force sustained isometric contractions, when the muscle is not fully occluded (Barnes, 1980, Sadamoto et al., 1983), blood flow can be more restricted for men than women at the same contraction intensity. This is because men are typically stronger than women and, therefore, exert more intramuscular pressure onto the feed arteries (Hunter et al., 2006b, Hunter et al., 2009) (Figure 3). Thus, when men were stronger than women for the elbow flexor muscles and handgrip muscles, the time to failure of women was longer than the men (Hunter and Enoka, 2001, Hunter et al., 2004a, Hunter et al., 2006b, Yoon et al., 2007), but similar when the sexes were matched for strength (Hunter et al., 2004b, Hunter et al., 2006b). Accordingly, the metaboreflex [increase in mean arterial pressure due to activation of sensory fibres by muscle metabolites (Kaufman and Hayes, 2002)] was greater during the isometric contractions for the men compared with the women, and associated with greater fatigability (Hunter and Enoka, 2001). For high intensity sustained contractions, when the blood flow is similarly occluded for men and women, the difference in fatigability between men and women is reduced relative to that for low-force contractions for some muscle groups (Maughan et al., 1986, West et al., 1995, Yoon et al., 2007 ) (Figures 1 and 3). Under these task conditions, the absolute target force and resultant blood flow appear to be the primary causes for the differences in fatigability between men and women.
There are also widespread sympathetic-mediated actions on skeletal muscle (Joyner and Halliwill, 2000, Roatta and Farina, 2010), and sex differences in these actions potentially alter muscle fatigue of men and women. For example, a sex difference in sympathetic-mediated actions can influence muscle perfusion and may promote a sex difference in fatigability. Possibilities include sex differences in muscle sympathetic nerve activity at rest and during contraction (Ng et al., 1993, Hogarth et al., 2007), and β2 adrenergic receptor-mediated vasodilation. β2 receptors are localized with greater density on type I fibres than type II (Roatta and Farina, 2010). Women have greater type I fibre area than men in some muscles (see fibre type section) and, therefore, possibly greater β2 adrenergic receptor-mediated vasodilation than men during exercise (Figure 3). Although the interactions are not fully understood, the balance of vasoconstriction and vasodilation potentially alters the net perfusion of the active muscle, and, thus, muscle fatigability in men versus women. These altered interactions could be responsible for the greater fatigability during low force contractions when arousal is increased (Yoon et al., 2009b).
Greater vasodilatory responses of feed arteries to the skeletal muscle of women may also allow them to offset muscle fatigue during dynamic contractions compared with men. For example, vasodilatory responses of the femoral artery during dynamic knee extensor exercise were greater in women than men (Parker et al., 2007). These responses were not dependent on strength. Greater vasodilatory responses in women would promote increased muscle perfusion, less accumulation of metabolites and potentially offset the rate of muscle fatigue relative to men. Further studies are required to understand the contribution of these sex differences in vasodilation to fatigability and in men and women.
Last, perfusion can also depend on capillarization of skeletal muscle. There is a higher density of capillaries per unit of skeletal muscle (measurements made from muscles biopsies) in the vastus lateralis of women than men (Roepstorff et al., 2006), due to a greater proportional area of type I fibres. Such differences will increase muscle perfusion in the women relative to men. In contrast, capillary density within the fibres of the tibialis anterior muscle was similar for both sexes, although less in type II fibres than type I (Porter et al., 2002). The lack of differences in capillary density of the tibialis anterior fibres between men and women are consistent with a smaller sex difference in muscle fatigue in that muscle relative to other muscles (Avin et al., 2010).
Collectively, evidence indicates that women have greater muscle perfusion than men during exercise at the same relative intensity, which potentially explains some of the sex differences in muscle fatigability. However, the cause for the altered blood flow will differ with the details of the task, such as whether the contraction is sustained or intermittent.
c. Contractile Properties
Sex-based differences in muscle fibre types, their size, number, contractile properties and metabolism will influence muscle function and fatigability. Several studies show that less muscle fatigue in women was associated with slower contractile properties measured from contractions evoked by electrical and transcranial magnetic stimulation (TMS) (Hunter et al., 2006a, Wust et al., 2008, Keller et al., 2011). For example, young women demonstrated less muscle fatigue at the end of 2 minutes of electrically evoked intermittent contractions (1 s on at 30 Hz; 1 s off) of the quadriceps muscles than young men (30 ± 10% vs 38 ± 11% reduction respectively) (Wust et al., 2008). Lower initial peak rates of relaxation (slower muscle), which occurred in the women, were associated with less fatigue at the end of the electrically evoked fatiguing contraction (Wust et al., 2008). Similarly, for the elbow flexor muscles, greater fatigue exhibited by men compared with women during voluntary maximal and submaximal isometric fatiguing contractions was associated with faster peak relaxation rates (measured from contractions evoked with TMS, during a MVC) (Hunter et al., 2006a, Keller et al., 2011). For example, by the end of the six sustained MVCs (22 s duration each) with the elbow flexor muscles, young men exhibited greater absolute and relative reductions in torque (65 ± 3% of initial MVC) than young women (52 ± 9%) (Figure 4) (Hunter et al., 2006a). Supraspinal fatigue that was assessed with TMS, increased similarly for the men and women. The motor evoked potential (MEP, EMG response to TMS) increased to similar levels for the men and women, and the superimposed twitch torque (elicited with TMS during a MVC) was also similar for both sexes, indicating no sex difference in neural mechanisms. The sex difference in muscle fatigue, however, was attributed to muscular (peripheral) mechanisms because the reduction in the amplitude of the estimated resting twitch was greater for the men (59%) than the women (27%). Furthermore, the men had faster relaxation rates prior to fatigue than women and the muscle slowed more during the fatigue task in the men (53% from initial values) compared with the women (22% from initial values) (Figure 4). Together, these studies with the knee extensor and elbow flexor muscles underscore the involvement of contractile mechanisms contributing to the sex differences in muscle fatigue in several large muscle groups. Comparison of several studies, however, indicates that the contribution of muscular mechanisms to the sex difference in fatigability vary across muscle groups, such that there is less of a sex difference in the ankle dorsiflexors (Russ and Kent-Braun, 2003, Russ et al., 2005) than the elbow flexors and knee extensors (Hunter et al., 2006a, Martin and Rattey, 2007, Wust et al., 2008). In general, the tibialis anterior muscle (primary ankle dorsiflexor) has a greater proportion of slow type I fibres than the elbow flexor and knee extensor muscles (Johnson et al., 1973, Simoneau and Bouchard, 1989, Porter et al., 2002), possibly limiting the expression of any sex differences in contractile fatigability.
Figure 4.
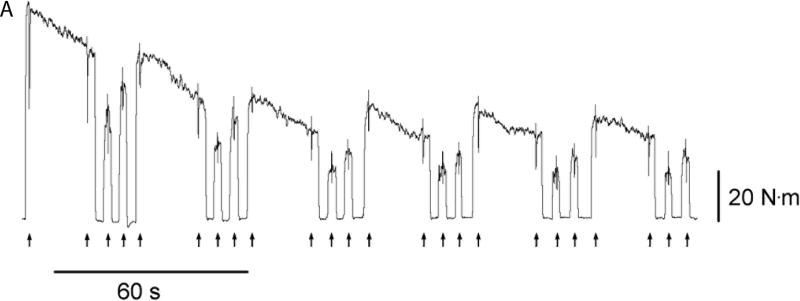
Young men (n = 9) and young women (n = 8) performed an isometric fatiguing protocol with the elbow flexor muscles. The protocol involved 6 × 22 s maximal voluntary contractions (MVC) with submaximal contractions at 60% and 80% MVC before, after and between the sustained MVCs. A. Representative torque data of a man. Arrows at the bottom of the panel show the timing of transcranial magnetic stimuli (Stim). B. MVC torque (mean ± SEM) relative to baseline control values for the men (closed circles) and women (open circles) during, and in recovery from, the fatiguing task. Relative torque is shown at the start and end of each sustained 22-s MVC, and during brief MVCs at the start and end of 10-min recovery. The men exhibited larger reductions in torque than the women during the intermittent fatiguing contractions (65% vs 52%). C. Peak relaxation rate of muscle measured from the fall in force immediately after the superimposed twitch during the MVC. Plotted are the mean (± SEM) from the 5 brief control MVCs, and then values at the start and end of each 22-s maximal contraction, and for the brief MVCs at the start and end of recovery. Peak relaxation rate became slower for both men and women as the muscle became more fatigued and then recovered during the 10-min recovery. However, the men had greater reductions in the peak relaxation rate than the women (P <0.05). Note that the y-axis is inverted. Larger negative numbers indicate faster relaxation. Adapted from (Hunter et al., 2006a).
d. Fibre Types
Skeletal muscles of men and women are comprised primarily of a combination of type I, type IIA and IIX fibres that are named based on the primary myosin heavy chain composition of the fibre, but whose metabolic, Ca2+ kinetics and functional contractile properties are generally coupled with the behaviour of the myosin proteins (Schiaffino and Reggiani, 2011). Hence, type II fibres, classified according to the dominant myosin isoform, have faster calcium kinetics, generate greater power, faster shortening velocities and relaxation, and are more fatigable than type I fibres (Schiaffino and Reggiani, 2011). While these categories are convenient for analysis, in reality there is a continuum of fibre properties based on a combination of myosin heavy and light chain isoforms, polymorphic expression of protein isoforms, metabolic potential, and Ca2+ handling properties (Ingalls, 2004). Furthermore, co-expression of myosin heavy chain isoforms within a fibre is not unusual (Klitgaard et al., 1990, Williamson et al., 2000, Caiozzo et al., 2003). Ultimately, the functional properties differ across the broad spectrum of fibre types. Because there are sex differences in the proportional area of fibre types within some muscles, the functional properties and fatigability of whole muscles can differ between men and women.
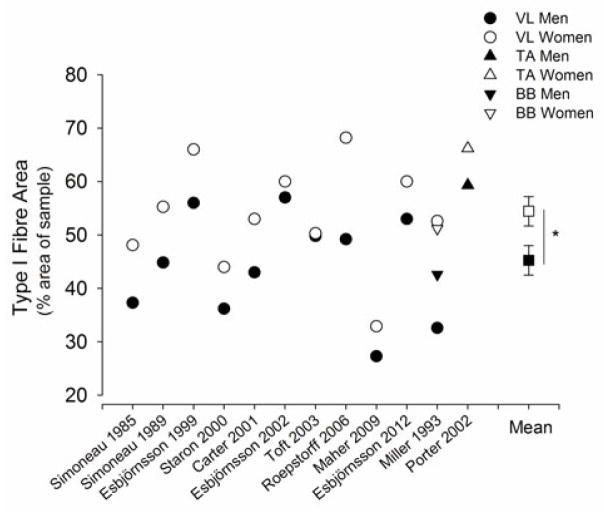
There is considerable evidence that women have a greater proportional area of the ‘slow’ type I fibres than men in several key muscles that are important to locomotion and daily function (Simoneau and Bouchard, 1989, Esbjornsson-Liljedahl et al., 1999, Staron et al., 2000, Carter et al., 2001b, Porter et al., 2002, Roepstorff et al., 2006, Welle et al., 2008) (Figure 5). Most studies also show that men have larger fibres than women across most of the fibre types with proportionally larger cross-sectional area (CSA) of type II fibres in the tibialis anterior, vastus lateralis and biceps brachii (Alway et al., 1989, Simoneau and Bouchard, 1989, Staron et al., 2000, Carter et al., 2001b, Porter et al., 2002). Hence, young women have smaller muscles that possess a greater proportional area of type I fibres compared with men.
Figure 5.
Type I fibre area (%, proportional area) of skeletal muscle histochemically analysed for myosin ATPase activity from muscle biopsy samples of vastus lateralis (VL), tibialis anterior (TA) and biceps brachii (BB) in young men (closed symbols) and women (open symbols) that were sampled in the same study. Shown are the mean proportional areas of the men and women in each of the 12 studies (Simoneau et al., 1985, Simoneau and Bouchard, 1989, Miller et al., 1993, Esbjornsson-Liljedahl et al., 1999, Staron et al., 2000, Carter et al., 2001b, Esbjornsson-Liljedahl et al., 2002, Porter et al., 2002, Toft et al., 2003, Roepstorff et al., 2006, Maher et al., 2009, Esbjornsson et al., 2012). The mean (± SEM) per cent area of type I fibres of all the muscles from the 12 studies is plotted on the right side. Women had greater type I fibre area (%) than men (P<0.05).
The muscle fibre properties and morphology of men and women are due to sex-related differences in human skeletal muscle gene expression and an interaction with sex-specific hormones (Roth et al., 2002, Welle et al., 2008, Maher et al., 2009, Liu et al., 2010). While genetic factors play an important role in determining variation in muscle fibre properties between men and women, specific genes are still being identified and further study is needed. Certainly, metabolically slower and more fatigue resistant fibres in women compared with men promote a sex difference in muscle fatigability. Greater insight into the sex differences in muscle fatigability can be gained by in vitro experiments of contractile properties and fatigability of various fibre types obtained from muscle biopsies of men and women. Several studies have shown that there are limited differences in the peak force, power and shortening velocity of single fibres (chemically skinned) between men and women relative to cell size (Krivickas et al., 2001, Trappe et al., 2003, Krivickas et al., 2006). Valuable information into sex differences in fatigability would be gained by including both men and women in single fibre studies.
Consistent with a sex difference in fibre properties, there is some evidence that the calcium (Ca2+) kinetics of the sarcoplasmic reticulum is slower in women compared with men. Analysis of muscle biopsy samples before and after sprint exercise showed that young women have a 24% lower maximal rate of sarcoplasmic reticulum Ca2+ATPase activity than men (Harmer et al., 2014) (Figure 6). Slower Ca2+ATPase activity and Ca2+ uptake into the sarcoplasmic reticulum is associated with slower rates of relaxation and muscle mechanics (Gollnick et al., 1991). Type II fibres have ~ three-fold higher Ca2+ATPase activity and two-fold higher calcium uptake than type I fibres (Li et al., 2002). Also, a significant relationship exists between the proportional area of type II fibres and Ca2+ATPase activity (Madsen et al., 1994, Hunter et al., 1999). Although this is the first study to directly compare the calcium kinetics in the skeletal muscle of men and women (Harmer et al., 2014), the values reported for men and women are similar to reports of Ca2+ATPase activity of men and women reported as separate cohorts (Booth et al., 1997, Hunter et al., 1999, Ortenblad et al., 2000, Thom et al., 2001, Li et al., 2002) (Figure 6). The lower Ca2+ATPase activity in the muscle of young women is not due to a less active muscle, because Ca2+ATPase activity was not altered after a 12 week high resistance strength training program (Hunter et al., 1999) or 10 days of immobilization (Thom et al., 2001). Thus, the sex difference in Ca2+ATPase activity of the sarcoplasmic reticulum is consistent with women possessing a slower and more fatigue resistant skeletal muscle profile than men. Studies the comparing calcium regulation of single fibres from the skeletal muscle of men and women are needed. Determining associations with fatigability in vitro and in vivo would provide valuable information into the contribution of Ca2+ kinetics to the sex difference in fatigability.
Figure 6.
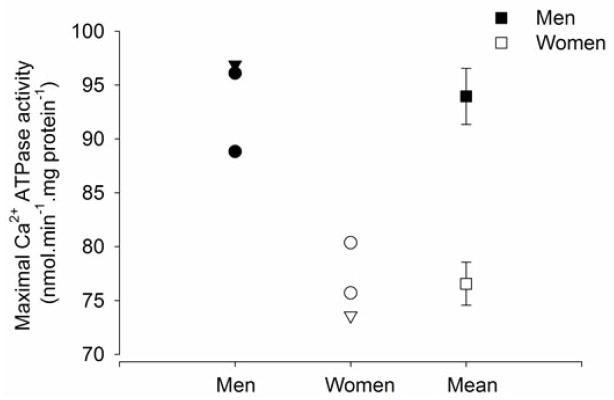
Maximal rates of sarcoplasmic reticulum Ca2+ATPase activity for whole muscle homogenates (obtained via needle biopsies of the vastus lateralis) of young men (n = 27) and women (n = 31) at rest. Men are represented in the closed symbols and women in the open symbols. Data plotted as circles, are from 4 different studies that examined men only (Booth et al., 1997, Li et al., 2002), women only (Hunter et al., 1999, Thom et al., 2001). The triangles are men and women in the same study (Harmer et al., 2014). The mean (±SEM) of the men and women from all the studies are plotted on the right side of the figure (squares) showing that men have faster maximal Ca2+ATPase activity than women.
e. Skeletal Muscle Metabolism
Sex-based differences in muscle fibre type and contractile properties have consequences for the skeletal muscle metabolism during dynamic and isometric fatiguing contractions in men and women. During high force isometric fatiguing contractions with the tibialis anterior (ankle dorsiflexors), men exhibited greater in vivo glycolysis, estimated from magnetic resonance spectroscopy of muscle, than women, with no sex differences in creatine kinase flux or oxidative capacity (Russ et al., 2005). During high intensity single and multiple sprints with the lower limb, men and women exhibit differences in metabolic pathways of quadriceps muscles. For example, in response to sprint exercise, women exhibited less increase of blood lactate concentration (Esbjornsson-Liljedahl et al., 1999), a smaller reduction of ATP and less accumulation of its breakdown products, IMP and inosine than men, especially in type II fibres (Esbjornsson-Liljedahl et al., 2002). These metabolic sex differences within a muscle are likely related to the greater proportional area of type I fibre of women compared with men (Esbjornsson et al., 1993, Esbjornsson-Liljedahl et al., 1999, Roepstorff et al., 2006, Maher et al., 2009), demonstrating the central contribution of skeletal muscle metabolism to the sex differences in fatigue. Despite differences in muscle metabolism between men and women during short duration isometric and single or multiple sprint exercise, a sex difference in muscle fatigue is not always observed [e.g (Russ et al., 2005, Esbjornsson et al., 2012)], indicating that, for those tasks, other rate limiting mechanisms were more important during the fatiguing exercise. Metabolic sex differences may influence men and women during longer duration high intensity exercise.
During whole body moderate-to-high intensity endurance exercise, women also oxidise more fat and less carbohydrate than men when compared at the same relative intensity of exercise (Horton et al., 1998, Carter et al., 2001a, Mittendorfer et al., 2002, Roepstorff et al., 2002, Roepstorff et al., 2006). Some of these differences originate from skeletal muscle metabolism, because women demonstrate a larger capacity for lipid metabolism than men, including greater mRNA levels of muscle lipoprotein lipase, membrane fatty acid transport protein-1, FAT/CD36 protein levels, and citrate synthase, irrespective of training status and age (Binnert et al., 2000, Kiens et al., 2004). Lipid metabolism in skeletal muscle of women is related to the presence of oestrogen (17-estradiol, E2) (Maher et al., 2010). Collectively, these studies demonstrate that the sex-differences in skeletal muscle properties during, and in recovery from, moderate and high intensity exercise can contribute to less muscle fatigue and faster recovery of force and power of women than men.
f. Voluntary Activation and the Role of the Central Nervous System
Activation of the motor neuron pool during voluntary contraction involves integration of synaptic inputs from descending pathways, spinal interneurons and peripheral afferent feedback (Enoka, 2012). The challenge during a fatiguing contraction is to maintain adequate and optimal voluntary activation as spinal and supraspinal centres modulate the changing muscle conditions during maximal and submaximal tasks (Enoka, 2012). Sex differences in voluntary activation and ultimately motor output during fatiguing exercise will arise if the synaptic inputs from descending pathways, spinal interneurons and peripheral afferent feedback differ between men and women.
Descending inputs from cortical centres that affect motor performance potentially differ between men and women because there are widespread sex differences in brain physiology, anatomy and functional activation throughout the lifespan (Becker et al., 2005, Hodes, 2013, Koolschijn and Crone, 2013). In general, during non-fatiguing motor tasks and comparable motor performance, such as finger tapping or preparation to reach, women tend to show greater activation levels of ipsilateral and bilateral cortical areas than men when assessed with functional magnetic resonance imaging (fMRI), which estimates the blood oxygenation level dependent (BOLD) as a correlate of neural activity in the brain (Lissek et al., 2007, Gorbet et al., 2010). Men, however, exhibited greater activation (fMRI signals) of subcortical areas, such as the basal ganglia (Lissek et al., 2007). Little is known about brain activation patterns during fatiguing tasks in men compared with women. A significant challenge is to determine the functional significance of these sex-based differences in the brain activity during motor control tasks and fatiguing exercise.
At rest, there are minimal sex differences in motor cortical input-output properties characterized with the MEP in healthy young men and women (Pitcher et al., 2003). During contraction, one method to assess adequacy of voluntary activation within the central nervous system involves stimulating the nervous system during maximal efforts at either the muscle or motor neuron (Merton, 1954, Belanger and McComas, 1981, Gandevia, 2001). Similarly, adequacy of voluntary drive to the motor cortex can be estimated with a transcranial magnetic stimulus during voluntary contractions (Todd et al., 2003, Todd et al., 2004). Any observed increase in the increment in force evoked by a superimposed stimulation at the muscle or cortex during the voluntary contraction implies a failure of voluntary drive at one or more sites proximal to the site of stimulation. During brief maximal efforts, men and women are able to similarly activate and drive their motor cortex during upper limb maximal efforts before fatiguing exercise (Hunter et al., 2006a, Keller et al., 2011, Molenaar et al., 2013). Similarly, there is no sex difference in voluntary activation of the elbow flexor muscles (Miller et al., 1993, Yoon et al., 2007) and dorsiflexor muscles (Russ and Kent-Braun, 2003). Hence, during brief maximal efforts in a non-fatigued and healthy muscle, there appear to be minimal sex differences in neural drive to skeletal muscles when assessed with these techniques.
During fatiguing exercise, there is often a failure of voluntary activation by both men and women. A failure in voluntary activation during maximal efforts means that the level of neural drive to the muscle is less than optimal because either the motor units were not all recruited voluntarily, or they were discharging at rates that were not high enough to maximize the force capacity of the muscle (Gandevia, 2001). This fall in voluntary activation is sometimes referred to as central fatigue. Supraspinal fatigue is a component of central fatigue and is attributable to suboptimal output from the motor cortex (Gandevia, 2001). It is seen as an exercise-related fall in voluntary activation measured with cortical stimulation. Despite women sustaining a low-force isometric contraction with the elbow flexor muscles for a longer duration than the men, the sexes demonstrated a similar reduction in voluntary activation at task failure for superimposed stimulations at the muscle (14% decline) (Yoon et al., 2007) and the motor cortex (14% decline to ~79%) (Keller et al., 2011). Comparison of these results indicates that a reduction in voluntary drive during the maximal contraction at task failure is due to a failure to generate output from the motor cortex in both men and women. Men and women also had similar levels of voluntary activation (assessed with TMS) at the end of six 22 s sustained maximal contractions (~77% for both sexes), despite the men exhibiting greater decrements in maximal force than the women (Hunter et al., 2006a). The greater fatigue exhibited by the men than the women was explained by muscular mechanisms (Figure 4). Thus, there was no sex difference in central and supraspinal fatigue at the end of the low- and high-force isometric fatiguing contractions sustained with the elbow flexor muscles, despite greater fatigue exhibited by the men.
For lower limb exercise, however, greater fatigability of men than women during maximal contractions was associated with a larger loss of voluntary activation (Russ and Kent-Braun, 2003, Martin and Rattey, 2007). Men, for example, had greater decrements in maximal force and voluntary activation than women when assessed with peroneal nerve stimulation during intermittent maximal contractions with the ankle dorsiflexor muscles (Russ and Kent-Braun, 2003). Similarly, men had larger reductions in knee extensor force during a sustained maximal contraction (100 s) than women (24% vs 16%), and this difference was associated with the larger reductions in voluntary activation in men than women (22% vs 9%) when assessed with evoked contractions elicited at the femoral nerve (Martin and Rattey, 2007). It is unknown if this sex difference is universal for submaximal tasks or dynamic contractions.
The fatigue-related reduction in voluntary activation can be increased by peripheral afferent feedback, and is possibly greater for men than women in lower limb muscles (Russ and Kent-Braun, 2003). The firing of group III and IV muscle afferents, for example, is sensitive to ischemia and metabolite accumulation associated with fatigue and pain (Martin et al., 2008, Kaufman, 2011, Murphy et al., 2011). Their effect on increased excitation of motor neurone output with fatigue is not fully understood, but they depress cortical excitation in response to pain [eg. (Martin et al., 2008)]. At the spinal cord, they can excite or inhibit motor neurones, and this may depend on the muscle group (extensors vs. flexors) and whether the excitation is from pain or metabolites [see (Martin et al., 2006, Martin et al., 2008)]. Excitation of group III and IV afferents, however, appears to result in a net decrease of motor unit discharge rates and impaired voluntary activation [e.g (Woods et al., 1987, Gandevia, 2001, Martin et al., 2006, Martin et al., 2008, Dideriksen et al., 2010, Amann, 2012, Rossman et al., 2012)], although evidence for their actions are quite indirect in humans Higher intramuscular pressure in stronger muscles, or sex differences in muscle metabolism and accumulation of metabolic by-products, (Ettinger et al., 1996, Russ et al., 2005) however, may lead to a greater discharge of group III and IV muscle afferents in men than women during similar intensity exercise. While it is possible that excitation of group III and IV afferents and their actions in the central nervous system in some muscle could reduce voluntary activation differently in men and women, more direct evidence is needed to support their role.
A loss of voluntary activation during maximal tasks, however, may not represent the impairments or sex differences in voluntary activation that occur during submaximal fatiguing tasks. Comparison of submaximal isometric fatiguing tasks that are similar in intensity but differ in the load compliance, for example (force vs position task) [e.g.(Hunter et al., 2002, Madeleine et al., 2002, Maluf et al., 2005, Hunter et al., 2008, Yoon et al., 2009a, Rudroff et al., 2010)], suggest that the limitations during submaximal contractions are probably more related to loss of activation rather than the capacity of muscle to develop maximal force or power (Enoka, 2012). The difference between the force and position task involves greater activation of gamma motor neurons, increased excitation of muscle spindles and 1a afferent feedback to correct deviations of limb position, and differential modulation of presynaptic Ia inhibition of the position task (Enoka et al., 2011). Men and women have similar reductions in time to failure for a position task compared with a force task in upper and lower limb muscles (Hunter et al., 2003, Hunter et al., 2005, Hunter et al., 2008), indicating that deficits in activation and the involved Ia afferent spinal networks during a position task are likely similar for men and women.
Because inputs from descending pathways and the periphery are modulated in the spinal cord, any sex–related differences in modulation at this level will influence the motor output. While these inputs may differ between men and women for some tasks and muscles (e.g. greater group III and IV input to spinal centres by men under some task conditions), it is not clear whether spinal modulation differs between men and women once the inputs are received. While one study indicated no sex differences in electrophysiological indices [Hoffmann reflex (H-reflex)] of spinal excitability for the lower leg muscles at rest (Christie et al., 2004), another showed recurrent inhibition was greater in the men than women at rest for motor neuron pools of the soleus muscle (Johnson et al., 2012). The lack of sex differences in response to the position task versus force task (see above) would suggest modulation is similar in spinal excitability that involves Ia afferent inputs. Whether there is a sex difference in recurrent inhibition or other indices of spinal excitability that are modulated differently for men and women (i.e. differences in modulation of the circuitry) during fatiguing tasks is not known.
g. Menstrual Cycle and Reproductive Hormone Fluctuations
There is no clear evidence that monthly fluctuations in hormones associated with the menstrual cycle will substantially alter fatigability and contractile function in young women at moderate environmental temperatures (Janse de Jonge, 2003). Initial reports suggested that women were stronger (greater specific tension), but also exhibited increased fatigability and slowing of relaxation in mid-cycle when oestrogen levels were high (Sarwar et al., 1996). More recent and well controlled studies, however, indicate there are minimal differences across the menstrual cycle in strength and fatigability (Ettinger et al., 1998, Janse de Jonge et al., 2001, Friden et al., 2003, Hoeger Bement et al., 2009a). Furthermore, we have repeatedly found no association between the day of menstrual cycle and strength or fatigability of isometric fatiguing contractions e.g. (Hunter et al., 2004a, Hunter et al., 2004c, Hunter et al., 2006b, Hunter et al., 2009, Keller et al., 2011). While differences in muscle sympathetic activity may be greater during the late follicular phase (mid cycle when oestrogen levels were high) than the luteal phase, there were no differences in metabolite accumulation (H+ and H2PO−4 concentrations) during isometric fatiguing contractions (Ettinger et al., 1998).
Whole body substrate utilization during endurance exercise can be influenced by menstrual cycle phase and use of oral contraceptives (Tarnopolsky, 2008). The effects of altered substrate utilization on performance, however, are small and the fluctuations in metabolism during the menstrual cycle are relatively small compared with the larger differences between men and women (Casazza et al., 2002, Suh et al., 2003, Casazza et al., 2004, Devries et al., 2006, Tarnopolsky, 2008, Fu et al., 2009). Menstrual cycle phase may, however, be more important to performance during longer duration exercise in hot and humid conditions. Time to exhaustion for submaximal exercise on a cycle ergometer (60% of maximal oxygen consumption and > 60 mins duration) was reduced, and heart rate, ventilation and perceived exertion greater for young women during the luteal phase (days 19–25 of the cycle) compared with follicular (days 1–5) during hot, humid conditions but not during temperate conditions (Janse de Jonge et al., 2012). Hence, menstrual cycle may influence long duration fatiguing exercise in hot and humid conditions.
The long term reductions in reproductive hormones associated with age provides an opportunity to determine their influence on fatigability and function in both men and women. The sex difference in fatigability with advanced age is generally reduced (Hunter, 2009), although the role of the hormonal reductions are not entirely clear. Despite the possible anti-catabolic effects of hormone replacement therapy on skeletal muscle in older women and men (Brown, 2008, Greising et al., 2009, Ronkainen et al., 2009, Ahtiainen et al., 2012, Qaisar et al., 2013), the limited number of studies to date indicate there are minimal differences in fatigability and endurance in older women on hormone replacement therapy compared with those who are not (Cheng et al., 2003, Finni et al., 2011). More, well-designed human studies are required to understand and clarify the effects of reproductive hormonal reductions on muscle fatigue and the possible impact of hormone replacement therapy for both men and women.
Beyond Physiology
There are several sociological factors that potentially mask a true understanding of the sex-based differences in performance and fatigability. They not only contribute to a lack of knowledge of the female response during fatiguing motor tasks but distort the sex differences that may exist and attributed to physiology alone. These factors include: (1) the experimental bias of under-reporting non-significant differences between men and women; (2) the past and present sex bias of studying and reporting the physiology and function of predominantly males in both human and animal, and (3) sex differences in physical activity levels.
An accurate understanding of the sex differences in performance and fatigability due to physiology alone can be limited because of the sampling bias of studying lower numbers of females than males in animal and human laboratory experiments (Anonymous, 2010, Kim et al., 2010, Zucker and Beery, 2010, Beery and Zucker, 2011, Cahill, 2012, Miller, 2012). The assumption that the underlying mechanisms of fatigability apply to one sex only is limiting; every cell in the human body has a sex which potentially impacts function (Miller, 2012). Generally, women are underrepresented in biomedical research studies (Kim et al., 2010) and this is also true in the fatigability literature. Fatigability is the foundation of neuromuscular adaptations during training and rehabilitation, but information on the female response is lacking. More studies are needed that examine neuromuscular function and fatigability that include both men and women, and also are statistically powered to determine whether sex differences exist. In cases where both men and women were previously included in data sets that are already published, re-examining the data for sex differences, where power is sufficient, may reveal new findings.
In general, women tend to be less active than men (Bassett et al., 2010), and this difference may promote sex differences in muscle performance and fatigability. Certainly, large reductions in activity and activation of a limb results in muscle atrophy and impaired muscle function for both men and women (Narici and de Boer, 2011, Hackney and Ploutz-Snyder, 2012). There is some, but limited, evidence that sex differences in muscle function and fatigability exist after a period of disuse (Ploutz-Snyder et al., in review). For example, women demonstrated greater decrements in strength (isometric and dynamic) compared with men after 7 days of lower limb unloading (Deschenes et al., 2009, Deschenes et al., 2012), although the susceptibility to fatigability was not examined. Further, four weeks of disuse of elbow flexor muscles resulted in large reductions in muscle strength and paradoxically an increased time to failure of a low-force fatiguing contraction (Semmler et al., 2000) but more so in women than men (Semmler et al., 1999). While sex differences may exist in response to large reductions in muscle activity (e.g. casting or unloading models), little is known about the more subtle, but daily, and long term reductions in physical activity on muscle function and fatigability in men and women. Physical activity levels, therefore, need to be controlled and reported in studies to expose the sex differences due to physiology alone.
Relevance to Clinical Populations, Training and Rehabilitation
Understanding sex-based differences in muscle fatigue across different maximal and submaximal tasks and the prevailing mechanisms is relevant to designing best strategies and practices: (1) for training and rehabilitation; (2) to offset limitations in sports performance and the limitations in daily tasks for some older and clinical populations; and (3) to address sex differences in pain often observed during and after fatiguing contractions. Muscle fatigability, for example, is a primary vehicle for promoting overload and the subsequent adaptation of the neuromuscular system that results in improved muscle performance in both men and women (Staron et al., 1991, Fiatarone et al., 1994, Hunter et al., 1999, Adams et al., 2004, Munn et al., 2005, Burd et al., 2012). Because there are sex differences in muscle fatigue and the contributing mechanisms, then neuromuscular adaption and optimal training regimes that adopt different contraction types will differ between men and women. In the push for greater individualization of medicine and rehabilitation, and more emphasis on genomics (Bouchard, 2012), the sex of the individual and the fundamental presence of XX or XY chromosome pairs in each cell is surely one of the basic individual differences that needs to be considered (Miller, 2012). One potent example is osteoarthritis, which increases in incidence with age but has greater prevalence and severity in women than men across all ages (Boyan et al., 2012, Boyan et al., 2013). Strength and endurance training that rely on fatiguing exercise can be beneficial to treatment and offset pain, however, whether there are sex differences in the response to different exercise regimes is not known (Golightly et al., 2012). The assumption that rehabilitation based on fatiguing exercise at relative intensities after injury or neuromuscular disorder should be similar for men and women is inappropriate, until it is otherwise known.
Fatigability can also limit exercise performance, ergonomic tasks and daily activities, especially for older men and women and people with chronic disease or disability [eg. (Sjogaard et al., 2010, Skurvydas et al., 2011, Vedsted et al., 2011)]. For example, women exhibit less fatigue than men for maximal sustained contractions in healthy populations due to a more fatigue resistant muscle of the women (Bilodeau et al., 2001, Hunter et al., 2006a). However, this sex difference was diminished in the quadriceps of people with multiple sclerosis. Both men and women with multiple sclerosis demonstrated large and similar decrements in voluntary activation and similar changes in fatigue within the muscle when assessed with evoked contractions using electrical stimulation (Skurvydas et al., 2011). Thus, the mechanisms contributing to sex differences in muscle fatigue in healthy men and women differed to those contributing to fatigue in people with multiple sclerosis. Multiple sclerosis is one of many chronic diseases that results in muscle atrophy and weakness, leading to greater muscle fatigue that can limit daily activities of living.
Despite women being less fatigable than men during many fatiguing tasks, women can experience greater perception of pain during exercise, and there are sex differences in pain perception before and after exercise (Fillingim et al., 2009, Racine et al., 2012). The sex differences in fatigability, interactions with increased pain during fatiguing exercise (Ge et al., 2005, Falla et al., 2008, Johansen et al., 2013), and the potential for exercise to decrease pain in healthy and clinical populations (Hoeger Bement et al., 2008, Hoeger Bement et al., 2009b, Hoeger Bement et al., 2011) deserves greater attention. Understanding the mechanisms involved in sex differences in muscle fatigue will provide information for targeted strategies to offset fatigue and pain that differ between men and women.
Conclusions
Fatigability is the foundation for effective training and rehabilitation, but also limits performance during exercise and daily tasks for some populations. However, there is a superficial understanding of the origins of the sex differences in fatigability under different task conditions, and the responsible physiological mechanisms. This is in part due to the predominance of male only studies in the physiology, fatigability and exercise training literature, and the false assumption that sex differences do not exist. There is a clear need for more high quality and well-designed studies to examine sex differences in muscle fatigue and performance under differing task conditions. Progress toward a true understanding of the sex differences in fatigability and the involved mechanisms will promote more tailored and effective strategies to enhance neuromuscular adaptations or offset fatigability in both men and women.
Acknowledgments
The author thanks Mr Jeffrey Rainwater for his contribution to Figure 3.
Funding
Funding was provided by the National Institute of Health, USA (R15AG039697).
Footnotes
Conflict of Interest
I have no conflict of interest.
References
- Adams GR, Cheng DC, Haddad F, Baldwin KM. Skeletal muscle hypertrophy in response to isometric, lengthening, and shortening training bouts of equivalent duration. J Appl Physiol. 2004;96:1613–8. doi: 10.1152/japplphysiol.01162.2003. [DOI] [PubMed] [Google Scholar]
- Ahtiainen M, Pollanen E, Ronkainen PH, Alen M, Puolakka J, Kaprio J, Sipila S, Kovanen V. Age and estrogen-based hormone therapy affect systemic and local IL-6 and IGF-1 pathways in women. Age (Dordr) 2012;34:1249–60. doi: 10.1007/s11357-011-9298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alway SE, Grumbt WH, Gonyea WJ, Stray-Gundersen J. Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol. 1989;67:24–31. doi: 10.1152/jappl.1989.67.1.24. [DOI] [PubMed] [Google Scholar]
- Amann M. Significance of Group III and IV muscle afferents for the endurance exercising human. Clin Exp Pharmacol Physiol. 2012;39:831–5. doi: 10.1111/j.1440-1681.2012.05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Putting gender on the agenda. Nature. 2010;465:665. doi: 10.1038/465665a. [DOI] [PubMed] [Google Scholar]
- Avin KG, Naughton MR, Ford BW, Moore HE, Monitto-Webber MN, Stark AM, Gentile AJ, Law LA. Sex differences in fatigue resistance are muscle group dependent. Med Sci Sports Exerc. 2010;42:1943–50. doi: 10.1249/MSS.0b013e3181d8f8fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes WS. The relationship between maximum isometric strength and intramuscular circulatory occlusion. Ergonomics. 1980;23:351–357. doi: 10.1080/00140138008924748. [DOI] [PubMed] [Google Scholar]
- Bassett DR, Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42:1819–25. doi: 10.1249/MSS.0b013e3181dc2e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–5. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Billaut F, Bishop D. Muscle fatigue in males and females during multiple-sprint exercise. Sports Med. 2009;39:257–78. doi: 10.2165/00007256-200939040-00001. [DOI] [PubMed] [Google Scholar]
- Billaut F, Bishop DJ. Mechanical work accounts for sex differences in fatigue during repeated sprints. Eur J Appl Physiol. 2012;112:1429–36. doi: 10.1007/s00421-011-2110-1. [DOI] [PubMed] [Google Scholar]
- Billaut F, Smith K. Sex alters impact of repeated bouts of sprint exercise on neuromuscular activity in trained athletes. Appl Physiol Nutr Metab. 2009;34:689–99. doi: 10.1139/H09-058. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Erb MD, Nichols JM, Joiner KL, Weeks JB. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24:98–106. doi: 10.1002/1097-4598(200101)24:1<98::aid-mus11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Binnert C, Koistinen HA, Martin G, Andreelli F, Ebeling P, Koivisto VA, Laville M, Auwerx J, Vidal H. Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am J Physiol Endocrinol Metab. 2000;279:E1072–9. doi: 10.1152/ajpendo.2000.279.5.E1072. [DOI] [PubMed] [Google Scholar]
- Booth J, McKenna MJ, Ruell PA, Gwinn TH, Davis GM, Thompson MW, Harmer AR, Hunter SK, Sutton JR. Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. J Appl Physiol. 1997;83:511–521. doi: 10.1152/jappl.1997.83.2.511. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Genomic predictors of trainability. Exp Physiol. 2012;97:347–52. doi: 10.1113/expphysiol.2011.058735. [DOI] [PubMed] [Google Scholar]
- Boyan BD, Tosi L, Coutts R, Enoka R, Hart DA, Nicolella DP, Berkley K, Sluka K, Kwoh K, O’Connor MI, Kohrt W. Sex differences in osteoarthritis of the knee. J Am Acad Orthop Surg. 2012;20:668–9. doi: 10.5435/JAAOS-20-10-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyan BD, Tosi LL, Coutts RD, Enoka RM, Hart DA, Nicolella DP, Berkley KJ, Sluka KA, Kwoh CK, O’Connor MI, Kohrt WM, Resnick E. Addressing the gaps: sex differences in osteoarthritis of the knee. Biol Sex Differ. 2013;4:4. doi: 10.1186/2042-6410-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SR, Graham JD, Martin Ginis KA, Hicks AL. Cognitive task performance causes impaired maximum force production in human hand flexor muscles. Biol Psychol. 2012;89:195–200. doi: 10.1016/j.biopsycho.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Bray SR, Martin Ginis KA, Hicks AL, Woodgate J. Effects of self-regulatory strength depletion on muscular performance and EMG activation. Psychophysiology. 2008;45:337–43. doi: 10.1111/j.1469-8986.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32:120–6. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- Burd NA, Andrews RJ, West DW, Little JP, Cochran AJ, Hector AJ, Cashaback JG, Gibala MJ, Potvin JR, Baker SK, Phillips SM. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol. 2012;590:351–62. doi: 10.1113/jphysiol.2011.221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology. 2012;153:2541–3. doi: 10.1210/en.2011-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiozzo VJ, Baker MJ, Huang K, Chou H, Wu YZ, Baldwin KM. Single-fiber myosin heavy chain polymorphism: how many patterns and what proportions? Am J Physiol Regul Integr Comp Physiol. 2003;285:R570–80. doi: 10.1152/ajpregu.00646.2002. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab. 2001a;280:E898–907. doi: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001b;79:386–92. [PubMed] [Google Scholar]
- Casazza GA, Jacobs KA, Suh SH, Miller BF, Horning MA, Brooks GA. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol. 2004;97:302–9. doi: 10.1152/japplphysiol.00050.2004. [DOI] [PubMed] [Google Scholar]
- Casazza GA, Suh SH, Miller BF, Navazio FM, Brooks GA. Effects of oral contraceptives on peak exercise capacity. J Appl Physiol. 2002;93:1698–702. doi: 10.1152/japplphysiol.00622.2002. [DOI] [PubMed] [Google Scholar]
- Chen TC, Chen HL, Pearce AJ, Nosaka K. Attenuation of eccentric exercise-induced muscle damage by preconditioning exercises. Med Sci Sports Exerc. 2012;44:2090–8. doi: 10.1249/MSS.0b013e31825f69f3. [DOI] [PubMed] [Google Scholar]
- Cheng A, Ditor DS, Hicks AL. A comparison of adductor pollicis fatigue in older men and women. Can J Physiol Pharmacol. 2003;81:873–9. doi: 10.1139/y03-084. [DOI] [PubMed] [Google Scholar]
- Christie A, Lester S, LaPierre D, Gabriel DA. Reliability of a new measure of H-reflex excitability. Clin Neurophysiol. 2004;115:116–23. doi: 10.1016/s1388-2457(03)00306-7. [DOI] [PubMed] [Google Scholar]
- Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol. 2004;97:225–35. doi: 10.1152/japplphysiol.00066.2004. [DOI] [PubMed] [Google Scholar]
- Clark BC, Collier SR, Manini TM, Ploutz-Snyder LL. Sex differences in muscle fatigability and activation patterns of the human quadriceps femoris. Eur J Appl Physiol. 2005;94:196–206. doi: 10.1007/s00421-004-1293-0. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM, The DJ, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol. 2003;94:2263–72. doi: 10.1152/japplphysiol.00926.2002. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- Dearth DJ, Umbel J, Hoffman RL, Russ DW, Wilson TE, Clark BC. Men and women exhibit a similar time to task failure for a sustained, submaximal elbow extensor contraction. Eur J Appl Physiol. 2010;108:1089–98. doi: 10.1007/s00421-009-1323-z. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, McCoy RW, Holdren AN, Eason MK. Gender influences neuromuscular adaptations to muscle unloading. Eur J Appl Physiol. 2009;105:889–97. doi: 10.1007/s00421-008-0974-5. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, McCoy RW, Mangis KA. Factors relating to gender specificity of unloading-induced declines in strength. Muscle Nerve. 2012;46:210–7. doi: 10.1002/mus.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries MC, Hamadeh MJ, Phillips SM, Tarnopolsky MA. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1120–8. doi: 10.1152/ajpregu.00700.2005. [DOI] [PubMed] [Google Scholar]
- Dideriksen JL, Farina D, Baekgaard M, Enoka RM. An integrative model of motor unit activity during sustained submaximal contractions. J Appl Physiol. 2010;108:1550–62. doi: 10.1152/japplphysiol.01017.2009. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Baudry S. Insights into the neural control of eccentric contractions. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00002.2013. (1985) [DOI] [PubMed] [Google Scholar]
- Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40:41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Muscle fatigue - from motor units to clinical symptoms. J Biomech. 2012;45:427–33. doi: 10.1016/j.jbiomech.2011.11.047. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol. 2011;21:208–19. doi: 10.1016/j.jelekin.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–48. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Esbjornsson-Liljedahl M, Bodin K, Jansson E. Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. J Appl Physiol. 2002;93:1075–83. doi: 10.1152/japplphysiol.00732.1999. [DOI] [PubMed] [Google Scholar]
- Esbjornsson-Liljedahl M, Sundberg CJ, Norman B, Jansson E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol. 1999;87:1326–32. doi: 10.1152/jappl.1999.87.4.1326. [DOI] [PubMed] [Google Scholar]
- Esbjornsson M, Bulow J, Norman B, Simonsen L, Nowak J, Rooyackers O, Kaijser L, Jansson E. Adipose tissue extracts plasma ammonia after sprint exercise in women and men. J Appl Physiol. 2006;101:1576–80. doi: 10.1152/japplphysiol.01119.2004. [DOI] [PubMed] [Google Scholar]
- Esbjornsson M, Rundqvist HC, Mascher H, Osterlund T, Rooyackers O, Blomstrand E, Jansson E. Sprint exercise enhances skeletal muscle p70S6k phosphorylation and more so in women than in men. Acta Physiol. 2012;205:411–22. doi: 10.1111/j.1748-1716.2012.02404.x. [DOI] [PubMed] [Google Scholar]
- Esbjornsson M, Sylven C, Holm I, Jansson E. Fast twitch fibres may predict anaerobic performance in both females and males. Int J Sports Med. 1993;14:257–63. doi: 10.1055/s-2007-1021174. [DOI] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol. 1996;80:245–51. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol. 1998;85:2075–81. doi: 10.1152/jappl.1998.85.6.2075. [DOI] [PubMed] [Google Scholar]
- Falla D, Arendt-Nielsen L, Farina D. Gender-specific adaptations of upper trapezius muscle activity to acute nociceptive stimulation. Pain. 2008;138:217–25. doi: 10.1016/j.pain.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finni T, Noorkoiv M, Pollanen E, Ronkainen PH, Alen M, Kaprio J, Kovanen V, Sipila S. Muscle function in monozygotic female twin pairs discordant for hormone replacement therapy. Muscle Nerve. 2011;44:769–75. doi: 10.1002/mus.22162. [DOI] [PubMed] [Google Scholar]
- Friden C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sport Med. 2003;13:238–41. doi: 10.1097/00042752-200307000-00007. [DOI] [PubMed] [Google Scholar]
- Fu MH, Maher AC, Hamadeh MJ, Ye C, Tarnopolsky MA. Exercise, sex, menstrual cycle phase, and 17beta-estradiol influence metabolism-related genes in human skeletal muscle. Physiol Genomics. 2009;40:34–47. doi: 10.1152/physiolgenomics.00115.2009. [DOI] [PubMed] [Google Scholar]
- Fulco CS, Rock PB, Muza SR, Lammi E, Cymerman A, Butterfield G, Moore LG, Braun B, Lewis SF. Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand. 1999;167:233–239. doi: 10.1046/j.1365-201x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–36. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge HY, Arendt-Nielsen L, Farina D, Madeleine P. Gender-specific differences in electromyographic changes and perceived pain induced by experimental muscle pain during sustained contractions of the upper trapezius muscle. Muscle Nerve. 2005;32:726–33. doi: 10.1002/mus.20410. [DOI] [PubMed] [Google Scholar]
- Golightly YM, Allen KD, Caine DJ. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys Sportsmed. 2012;40:52–65. doi: 10.3810/psm.2012.11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Korge P, Karpakka J, Saltin B. Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiol Scand. 1991;142:135–6. doi: 10.1111/j.1748-1716.1991.tb09139.x. [DOI] [PubMed] [Google Scholar]
- Gorbet DJ, Mader LB, Staines WR. Sex-related differences in the hemispheric laterality of slow cortical potentials during the preparation of visually guided movements. Exp Brain Res. 2010;202:633–46. doi: 10.1007/s00221-010-2170-1. [DOI] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–81. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette JA, Romer LM, Querido JS, Chua R, Eves ND, Road JD, McKenzie DC, Sheel AW. Sex differences in exercise-induced diaphragmatic fatigue in endurance-trained athletes. J Appl Physiol. 2010;109:35–46. doi: 10.1152/japplphysiol.01341.2009. [DOI] [PubMed] [Google Scholar]
- Hackney KJ, Ploutz-Snyder LL. Unilateral lower limb suspension: integrative physiological knowledge from the past 20 years (1991–2011) Eur J Appl Physiol. 2012;112:9–22. doi: 10.1007/s00421-011-1971-7. [DOI] [PubMed] [Google Scholar]
- Harmer AR, Ruell PA, Hunter SK, McKenna MJ, Thom JM, Chisholm DJ, Flack JR. Effects of type 1 diabetes, sprint training and sex on skeletal muscle sarcoplasmic reticulum Ca2+ uptake and Ca2+-ATPase activity. J Physiol. 2014;592:523–35. doi: 10.1113/jphysiol.2013.261172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux ME, Gandevia SC. Human muscle fatigue, eccentric damage and coherence in the EMG. Acta Physiol. 2013;208:294–5. doi: 10.1111/apha.12133. [DOI] [PubMed] [Google Scholar]
- Herzog W. Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J Appl Physiol. 2014;116(11):1407–1417. doi: 10.1152/japplphysiol.00069.2013. [DOI] [PubMed] [Google Scholar]
- Hodes GE. Sex, stress, and epigenetics: regulation of behavior in animal models of mood disorders. Biol Sex Differ. 2013;4:1. doi: 10.1186/2042-6410-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger Bement M, Weyer A, Hartley S, Drewek B, Harkins AL, Hunter SK. Pain perception after isometric exercise in women with fibromyalgia. Archives of Physical Medicine and Rehabilitation. 2011;92:89–95. doi: 10.1016/j.apmr.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40:1880–1889. doi: 10.1249/MSS.0b013e31817eeecc. [DOI] [PubMed] [Google Scholar]
- Hoeger Bement MK, Rasiarmos RL, Dicapo JM, Lewis A, Keller ML, Harkins AL, Hunter SK. The role of the menstrual cycle phase in pain perception before and after an isometric fatiguing contraction. Eur J Appl Physiol. 2009a;106:105–112. doi: 10.1007/s00421-009-0995-8. [DOI] [PubMed] [Google Scholar]
- Hoeger Bement MK, Weyer A, Hartley S, Yoon T, Hunter SK. Fatiguing exercise attenuates pain-induced corticomotor excitability. Neurosci Lett. 2009b;452:209–13. doi: 10.1016/j.neulet.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci. 2007;112:353–61. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1998;85:1823–32. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- Hubal MJ, Clarkson PM. Counterpoint: Estrogen and sex do not significantly influence post-exercise indexes of muscle damage, inflammation, and repair. J Appl Physiol. 2009;106:1012–4. doi: 10.1152/japplphysiol.90848.2008a. discussion 1014, 1022. [DOI] [PubMed] [Google Scholar]
- Hubal MJ, Rubinstein SR, Clarkson PM. Muscle function in men and women during maximal eccentric exercise. J Strength Cond Res. 2008;22:1332–8. doi: 10.1519/JSC.0b013e31817392ec. [DOI] [PubMed] [Google Scholar]
- Hunter SK. Sex differences and mechanisms of task-specific muscle fatigue. Exerc Sport Sci Rev. 2009;37:113–22. doi: 10.1097/JES.0b013e3181aa63e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol. 2006a;101:1036–44. doi: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol. 2004a;97:1723–1732. doi: 10.1152/japplphysiol.00460.2004. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol. 2004b;96:195–202. doi: 10.1152/japplphysiol.00893.2003. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004c;96:2125–2132. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol. 2001;91:2686–94. doi: 10.1152/jappl.2001.91.6.2686. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Griffith EE, Schlachter KM, Kufahl TD. Sex differences in time to task failure and blood flow for an intermittent isometric fatiguing contraction. Muscle Nerve. 2009;39:42–53. doi: 10.1002/mus.21203. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Lepers R, MacGillis CJ, Enoka RM. Activation among the elbow flexor muscles differs when maintaining arm position during a fatiguing contraction. J Appl Physiol. 2003;94:2439–2447. doi: 10.1152/japplphysiol.01038.2002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Rochette L, Critchlow A, Enoka RM. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve. 2005;31:730–740. doi: 10.1002/mus.20325. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol. 2002;88:3087–96. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Schletty JM, Schlachter KM, Griffith EE, Polichnowski AJ, Ng AV. Active hyperemia and vascular conductance differ between men and women for an isometric fatiguing contraction. J Appl Physiol. 2006b;101:140–50. doi: 10.1152/japplphysiol.01567.2005. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol. 1999;86:1858–65. doi: 10.1152/jappl.1999.86.6.1858. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Yoon T, Farinella J, Griffith EE, Ng AV. Time to task failure and muscle activation vary with load type for a submaximal fatiguing contraction with the lower leg. J Appl Physiol. 2008;105:463–72. doi: 10.1152/japplphysiol.90398.2008. [DOI] [PubMed] [Google Scholar]
- Ingalls CP. Nature vs. nurture: can exercise really alter fiber type composition in human skeletal muscle? J Appl Physiol. 2004;97:1591–2. doi: 10.1152/classicessays.00010.2004. [DOI] [PubMed] [Google Scholar]
- Ivey FM, Tracy BL, Lemmer JT, NessAiver M, Metter EJ, Fozard JL, Hurley BF. Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A Biol Sci Med Sci. 2000;55:B152–7. doi: 10.1093/gerona/55.3.b152. discussion B158–9. [DOI] [PubMed] [Google Scholar]
- Janse de Jonge XA. Effects of the menstrual cycle on exercise performance. Sports Med. 2003;33:833–51. doi: 10.2165/00007256-200333110-00004. [DOI] [PubMed] [Google Scholar]
- Janse de Jonge XA, Boot CR, Thom JM, Ruell PA, Thompson MW. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530:161–6. doi: 10.1111/j.1469-7793.2001.0161m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse de Jonge XA, Thompson MW, Chuter VH, Silk LN, Thom JM. Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med Sci Sports Exerc. 2012;44:2190–8. doi: 10.1249/MSS.0b013e3182656f13. [DOI] [PubMed] [Google Scholar]
- Johansen TI, Samani A, Antle DM, Cote JN, Madeleine P. Gender effects on the coordination of subdivisions of the trapezius muscle during a repetitive box-folding task. Eur J Appl Physiol. 2013;113:175–82. doi: 10.1007/s00421-012-2425-6. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles:an autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Johnson ST, Kipp K, Hoffman MA. Spinal motor control differences between the sexes. Eur J Appl Physiol. 2012;112:3859–64. doi: 10.1007/s00421-012-2363-3. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Halliwill JR. Sympathetic vasodilatation in human limbs. J Physiol. 2000;526(Pt 3):471–80. [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kaufman MP. The exercise pressor reflex in animals. Exp Physiol. 2011;97:51–8. doi: 10.1113/expphysiol.2011.057539. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–39. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Keller-Ross ML, Pereira HM, Pruse J, Yoon T, Schlinder-DeLap B, Nielson KA, Hunter SK. Stress-induced increase in muscle fatigability of young men and women is predicted by strength but not voluntary activation. J Appl Physiol. 2014;116(7):767–78. doi: 10.1152/japplphysiol.01129.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller ML, Pruse J, Yoon T, Schlinder-Delap B, Harkins A, Hunter SK. Supraspinal fatigue is similar in men and women for a low-force fatiguing contraction. Med Sci Sports Exerc. 2011;43:1873–83. doi: 10.1249/MSS.0b013e318216ebd4. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Fitts RH, Christie A. Skeletal muscle fatigue. Compr Physiol. 2012;2:997–1044. doi: 10.1002/cphy.c110029. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–23. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol. 2004;97:1209–18. doi: 10.1152/japplphysiol.01278.2003. [DOI] [PubMed] [Google Scholar]
- Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465:688–9. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140:55–62. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–16. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5C:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivickas LS, Fielding RA, Murray A, Callahan D, Johansson A, Dorer DJ, Frontera WR. Sex differences in single muscle fiber power in older adults. Med Sci Sports Exerc. 2006;38:57–63. doi: 10.1249/01.mss.0000180357.58329.b1. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–455. doi: 10.1097/00002060-200106000-00012. quiz 456–7. [DOI] [PubMed] [Google Scholar]
- Laurent CM, Green JM, Bishop PA, Sjokvist J, Schumacker RE, Richardson MT, Curtner-Smith M. Effect of gender on fatigue and recovery following maximal intensity repeated sprint performance. J Sports Med Phys Fitness. 2010;50:243–53. [PubMed] [Google Scholar]
- Li JL, Wang XN, Fraser SF, Carey MF, Wrigley TV, McKenna MJ. Effects of fatigue and training on sarcoplasmic reticulum Ca(2+) regulation in human skeletal muscle. J Appl Physiol. 2002;92:912–22. doi: 10.1152/japplphysiol.00643.2000. [DOI] [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–7. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- Lissek S, Hausmann M, Knossalla F, Peters S, Nicolas V, Gunturkun O, Tegenthoff M. Sex differences in cortical and subcortical recruitment during simple and complex motor control: an fMRI study. Neuroimage. 2007;37:912–26. doi: 10.1016/j.neuroimage.2007.05.037. [DOI] [PubMed] [Google Scholar]
- Liu D, Sartor MA, Nader GA, Gutmann L, Treutelaar MK, Pistilli EE, Iglayreger HB, Burant CF, Hoffman EP, Gordon PM. Skeletal muscle gene expression in response to resistance exercise: sex specific regulation. BMC Genomics. 2010;11:659. doi: 10.1186/1471-2164-11-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeleine P, Jorgensen LV, Sogaard K, Arendt-Nielsen L, Sjogaard G. Development of muscle fatigue as assessed by electromyography and mechanomyography during continuous and intermittent low-force contractions: effects of the feedback mode. Eur J Appl Physiol. 2002;87:28–37. doi: 10.1007/s00421-002-0578-4. [DOI] [PubMed] [Google Scholar]
- Madsen K, Franch J, Clausen T. Effects of intensified endurance training on the concentration of Na,K-ATPase and Ca-ATPase in human skeletal muscle. Acta Physiol Scand. 1994;150:251–8. doi: 10.1111/j.1748-1716.1994.tb09684.x. [DOI] [PubMed] [Google Scholar]
- Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics. 2010;42:342–7. doi: 10.1152/physiolgenomics.00016.2010. [DOI] [PubMed] [Google Scholar]
- Maher AC, Fu MH, Isfort RJ, Varbanov AR, Qu XA, Tarnopolsky MA. Sex differences in global mRNA content of human skeletal muscle. PLoS One. 2009;4:e6335. doi: 10.1371/journal.pone.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluf KS, Shinohara M, Stephenson JL, Enoka RM. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res. 2005;167:165–77. doi: 10.1007/s00221-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch. 2007;454:957–69. doi: 10.1007/s00424-007-0243-1. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–89. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ, Harmon M, Leiper JB, Sale D, Delman A. Endurance capacity of untrained males and females in isometric and dynamic muscular contractions. Eur J Appl Physiol. 1986;55:395–400. doi: 10.1007/BF00422739. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol. 1993;66:254–62. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- Miller VM. In pursuit of scientific excellence: sex matters. J Appl Physiol. 2012;112:1427–8. doi: 10.1152/japplphysiol.00303.2012. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab. 2002;283:E58–65. doi: 10.1152/ajpendo.00504.2001. [DOI] [PubMed] [Google Scholar]
- Molenaar JP, McNeil CJ, Bredius MS, Gandevia SC. Effects of aging and sex on voluntary activation and peak relaxation rate of human elbow flexors studied with motor cortical stimulation. Age. 2013;35:1327–37. doi: 10.1007/s11357-012-9435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn J, Herbert RD, Hancock MJ, Gandevia SC. Resistance training for strength: effect of number of sets and contraction speed. Med Sci Sports Exerc. 2005;37:1622–6. doi: 10.1249/01.mss.0000177583.41245.f8. [DOI] [PubMed] [Google Scholar]
- Murphy MN, Mizuno M, Mitchell JH, Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol. 2011;301:H1191–204. doi: 10.1152/ajpheart.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, de Boer MD. Disuse of the musculo-skeletal system in space and on earth. Eur J Appl Physiol. 2011;111:403–20. doi: 10.1007/s00421-010-1556-x. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Ortenblad N, Lunde PK, Levin K, Andersen JL, Pedersen PK. Enhanced sarcoplasmic reticulum Ca(2+) release following intermittent sprint training. Am J Physiol Regul Integr Comp Physiol. 2000;279:R152–60. doi: 10.1152/ajpregu.2000.279.1.R152. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol. 2007;103:1583–91. doi: 10.1152/japplphysiol.00662.2007. [DOI] [PubMed] [Google Scholar]
- Pincivero DM, Coelho AJ, Campy RM. Gender differences in perceived exertion during fatiguing knee extensions. Med Sci Sports Exerc. 2004;36:109–17. doi: 10.1249/01.MSS.0000106183.23941.54. [DOI] [PubMed] [Google Scholar]
- Pincivero DM, Gandaio CM, Ito Y. Gender-specific knee extensor torque, flexor torque, and muscle fatigue responses during maximal effort contractions. Eur J Appl Physiol. 2003;89:134–41. doi: 10.1007/s00421-002-0739-5. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol. 2003;546:605–13. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploutz-Snyder L, Bloomfield S, Bemben D, Hunter S, Smith S, Templeton K. Effects of sex and gender on adaptation to space: musculoskeletal health. J Womens Health. doi: 10.1089/jwh.2014.4910. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MM, Stuart S, Boij M, Lexell J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J Appl Physiol. 2002;92:1451–7. doi: 10.1152/japplphysiol.00744.2001. [DOI] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Rice CL, Vandervoort AA. Delayed recovery of velocity-dependent power loss following eccentric actions of the ankle dorsiflexors. J Appl Physiol. 2010;109:669–76. doi: 10.1152/japplphysiol.01254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Rice CL, Vandervoort AA. Peak power is reduced following lengthening contractions despite a maintenance of shortening velocity. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2013;38(12):1196–205. doi: 10.1139/apnm-2013-0092. [DOI] [PubMed] [Google Scholar]
- Prasartwuth O, Taylor JL, Gandevia SC. Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J Physiol. 2005;567:337–48. doi: 10.1113/jphysiol.2005.087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaisar R, Renaud G, Hedstrom Y, Pollanen E, Ronkainen P, Kaprio J, Alen M, Sipila S, Artemenko K, Bergquist J, Kovanen V, Larsson L. Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J Physiol. 2013;591:2333–44. doi: 10.1113/jphysiol.2012.250092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153:602–18. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Rinard J, Clarkson PM, Smith LL, Grossman M. Response of males and females to high-force eccentric exercise. J Sports Sci. 2000;18:229–36. doi: 10.1080/026404100364965. [DOI] [PubMed] [Google Scholar]
- Roatta S, Farina D. Sympathetic actions on the skeletal muscle. Exerc Sport Sci Rev. 2010;38:31–5. doi: 10.1097/JES.0b013e3181c5cde7. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282:E435–47. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol. 2006;574:125–38. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, O’Brien K, Kirk G, Murray R, McKinnon P, Shadgan B, Reid WD. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009;43:556–68. doi: 10.1136/bjsm.2008.051417. [DOI] [PubMed] [Google Scholar]
- Ronkainen PH, Kovanen V, Alen M, Pollanen E, Palonen EM, Ankarberg-Lindgren C, Hamalainen E, Turpeinen U, Kujala UM, Puolakka J, Kaprio J, Sipila S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol. 2009;107:25–33. doi: 10.1152/japplphysiol.91518.2008. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, Venturelli M, McDaniel J, Amann M, Richardson RS. Muscle mass and peripheral fatigue: a potential role for afferent feedback? Acta Physiol. 2012;206:242–50. doi: 10.1111/j.1748-1716.2012.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SM, Ferrell RE, Peters DG, Metter EJ, Hurley BF, Rogers MA. Influence of age, sex, and strength training on human muscle gene expression determined by microarray. Physiol Genomics. 2002;10:181–90. doi: 10.1152/physiolgenomics.00028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Matthews S, Zuo R, Enoka RM. Muscle activity differs with load compliance during fatiguing contractions with the knee extensor muscles. Exp Brain Res. 2010;203:307–16. doi: 10.1007/s00221-010-2233-3. [DOI] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94:2414–22. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
- Russ DW, Lanza IR, Rothman D, Kent-Braun JA. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve. 2005;32:647–55. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- Sadamoto T, Bonde-Petersen F, Suzuki Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur J Appl Physiol. 1983;51:395–408. doi: 10.1007/BF00429076. [DOI] [PubMed] [Google Scholar]
- Saito Y, Iemitsu M, Otsuki T, Maeda S, Ajisaka R. Gender differences in brachial blood flow during fatiguing intermittent handgrip. Med Sci Sports Exerc. 2008;40:684–90. doi: 10.1249/MSS.0b013e3181614327. [DOI] [PubMed] [Google Scholar]
- Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493:267–72. doi: 10.1113/jphysiol.1996.sp021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers SP, Clarkson PM. Force recovery after eccentric exercise in males and females. Eur J Appl Physiol. 2001;84:122–6. doi: 10.1007/s004210000346. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Seger JY, Thorstensson A. Muscle strength and myoelectric activity in prepubertal and adult males and females. Eur J Appl Physiol Occup Physiol. 1994;69:81–7. doi: 10.1007/BF00867932. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Ebert SA, Amarasena J. Eccentric muscle damage increases intermuscular coherence during a fatiguing isometric contraction. Acta Physiol. 2013;208:362–75. doi: 10.1111/apha.12111. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Kutzscher DV, Enoka RM. Gender differences in the fatigability of human skeletal muscle. J Neurophysiol. 1999;82:3590–3. doi: 10.1152/jn.1999.82.6.3590. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Kutzscher DV, Enoka RM. Limb immobilization alters muscle activation patterns during a fatiguing isometric contraction. Muscle and Nerve. 2000;23:1381–92. doi: 10.1002/1097-4598(200009)23:9<1381::aid-mus9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Senefeld J, Yoon T, Bement MH, Hunter SK. Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle Nerve. 2013;48:436–9. doi: 10.1002/mus.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewright KA, Hubal MJ, Kearns A, Holbrook MT, Clarkson PM. Sex differences in response to maximal eccentric exercise. Med Sci Sports Exerc. 2008;40:242–51. doi: 10.1249/mss.0b013e31815aedda. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257:E567–72. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Lortie G, Boulay MR, Thibault MC, Theriault G, Bouchard C. Skeletal muscle histochemical and biochemical characteristics in sedentary male and female subjects. Can J Physiol Pharmacol. 1985;63:30–5. doi: 10.1139/y85-005. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Rosendal L, Kristiansen J, Blangsted AK, Skotte J, Larsson B, Gerdle B, Saltin B, Sogaard K. Muscle oxygenation and glycolysis in females with trapezius myalgia during stress and repetitive work using microdialysis and NIRS. Eur J Appl Physiol. 2010;108:657–69. doi: 10.1007/s00421-009-1268-2. [DOI] [PubMed] [Google Scholar]
- Skurvydas A, Brazaitis M, Andrejeva J, Mickeviciene D, Streckis V. The effect of multiple sclerosis and gender on central and peripheral fatigue during 2-min MVC. Clin Neurophysiol. 2011;122:767–76. doi: 10.1016/j.clinph.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Billaut F. Tissue oxygenation in men and women during repeated-sprint exercise. Int J Sports Physiol Perform. 2012;7:59–67. doi: 10.1123/ijspp.7.1.59. [DOI] [PubMed] [Google Scholar]
- Spurway NC, Watson H, McMillan K, Connolly G. The effect of strength training on the apparent inhibition of eccentric force production in voluntarily activated human quadriceps. Eur J Appl Physiol. 2000;82:374–80. doi: 10.1007/s004210000221. [DOI] [PubMed] [Google Scholar]
- Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–9. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol. 1991;70:631–40. doi: 10.1152/jappl.1991.70.2.631. [DOI] [PubMed] [Google Scholar]
- Suh SH, Casazza GA, Horning MA, Miller BF, Brooks GA. Effects of oral contraceptives on glucose flux and substrate oxidation rates during rest and exercise. J Appl Physiol. 2003;94:285–94. doi: 10.1152/japplphysiol.00693.2002. [DOI] [PubMed] [Google Scholar]
- Taipale RS, Hakkinen K. Acute hormonal and force responses to combined strength and endurance loadings in men and women: the “order effect”. PLoS One. 2013;8:e55051. doi: 10.1371/journal.pone.0055051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc. 2008;40:648–54. doi: 10.1249/MSS.0b013e31816212ff. [DOI] [PubMed] [Google Scholar]
- Thom JM, Thompson MW, Ruell PA, Bryant GJ, Fonda JS, Harmer AR, Janse de Jonge XA, Hunter SK. Effect of 10-day cast immobilization on sarcoplasmic reticulum calcium regulation in humans. Acta Physiol Scand. 2001;172:141–7. doi: 10.1046/j.1365-201X.2001.00853.x. [DOI] [PubMed] [Google Scholar]
- Thompson BC, Fadia T, Pincivero DM, Scheuermann BW. Forearm blood flow responses to fatiguing isometric contractions in women and men. Am J Physiol Heart Circ Physiol. 2007;293:H805–12. doi: 10.1152/ajpheart.01136.2006. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Enns DL. Point:Counterpoint: Estrogen and sex do/do not influence post-exercise indexes of muscle damage, inflammation, and repair. J Appl Physiol. 2009;106:1010–2. doi: 10.1152/japplphysiol.90848.2008. discussion 1014–15, 1021. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–71. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol. 2004;97:236–42. doi: 10.1152/japplphysiol.01336.2003. [DOI] [PubMed] [Google Scholar]
- Toft I, Lindal S, Bonaa KH, Jenssen T. Quantitative measurement of muscle fiber composition in a normal population. Muscle Nerve. 2003;28:101–8. doi: 10.1002/mus.10373. [DOI] [PubMed] [Google Scholar]
- Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedsted P, Sogaard K, Blangsted AK, Madeleine P, Sjogaard G. Biofeedback effectiveness to reduce upper limb muscle activity during computer work is muscle specific and time pressure dependent. J Electromyogr Kinesiol. 2011;21:49–58. doi: 10.1016/j.jelekin.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS One. 2008;3:e1385. doi: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West W, Hicks A, Clements L, Dowling J. The relationship between voluntary electromyogram, endurance time and intensity of effort in isometric handgrip exercise. European J Appl Physiol. 1995;71:301–305. doi: 10.1007/BF00240408. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol. 2000;88:627–33. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–37. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol. 2008;93:843–50. doi: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- Yoon T, Hawe R, Hunter SK. Variation in limb support influences the time to task failure for a postural contraction. J Mot Behav. 2009a;41:393–5. doi: 10.3200/35-09-003. [DOI] [PubMed] [Google Scholar]
- Yoon T, Keller ML, De-Lap BS, Harkins A, Lepers R, Hunter SK. Sex differences in response to cognitive stress during a fatiguing contraction. J Appl Physiol. 2009b;107:1486–96. doi: 10.1152/japplphysiol.00238.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Schlinder Delap B, Griffith EE, Hunter SK. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve. 2007;36:512–524. doi: 10.1002/mus.20844. [DOI] [PubMed] [Google Scholar]
- Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]