Abstract
Chromosomal rearrangement polymorphisms are common and increasingly found to be associated with adaptive ecological divergence and speciation. Rearrangements, such as inversions, reduce recombination in heterozygous individuals and thus can protect favourable allelic combinations at linked loci, facilitating their spread in the presence of gene flow. Recently, we identified a chromosomal inversion polymorphism that contributes to ecological adaptation and reproductive isolation between annual and perennial ecotypes of the yellow monkeyflower, Mimulus guttatus. Here we evaluate the population genetic structure of this inverted region in comparison with the collinear regions of the genome across the M. guttatus species complex. We tested whether annual and perennial M. guttatus exhibit different patterns of divergence for loci in the inverted and noninverted regions of the genome. We then evaluated whether there are contrasting climate associations with these genomic regions through redundancy analysis. We found that the inversion exhibits broadly different patterns of divergence among annual and perennial M. guttatus and is associated with environmental variation across population accessions. This study is the first widespread population genetic survey of the diversity of the M. guttatus species complex. Our findings contribute to a greater understanding of morphological, ecological, and genetic evolutionary divergence across this highly diverse group of closely related ecotypes and species. Finally, understanding species relationships among M. guttatus sp. has hitherto been stymied by accumulated evidence of substantial gene flow among populations as well as designated species. Nevertheless, our results shed light on these relationships and provide insight into adaptation in life history traits within the complex.
Keywords: adaptation, chromosomal inversion, climate, cline, ecotypes, life history
Introduction
Chromosomal rearrangements are commonly revealed in interspecific and interpopulation comparisons and may play a major role in the processes of local adaptation and speciation (Dobzhansky 1951, 1970; Lewis 1953; Stebbins 1958; White 1978; King 1993). Polymorphic chromosomal rearrangements, such as inversions, are frequently found to be clinally associated with major environmental gradients (Dobzhansky 1951; Krimbas & Powell 1992; Umina et al. 2005; Ayala et al. 2011, 2013) and functional adaptive variation, such as in butterflies (Joron et al. 2011; Jones et al. 2012a), fish (Jones et al. 2012b), mosquitoes (Ayala et al. 2011, 2013) and plants (Lowry & Willis 2010; Fang et al. 2012). Multiple theoretical models have addressed the question of how chromosomal inversions contribute to adaptive evolution (Rieseberg 2001; Kirkpatrick & Barton 2006; Hoffmann & Rieseberg 2008; Feder & Nosil 2009; Kirkpatrick 2010; Joron et al. 2011; Kirkpatrick & Kern 2012). One key element of most inversion models is heavy suppression of meiotic recombination in heterozygotes, which may facilitate the capture of locally adaptive alleles across multiple linked loci and allow the inversion to spread through a local population (Dobzhansky 1970; Kirkpatrick & Barton 2006; Kirkpatrick 2010). Suppression of recombination within inversions can also lead to greater differentiation within an inverted region relative to collinear regions across the rest of the genome (Kolaczkowski et al. 2011; Cheng et al. 2012).
Recently, Lowry & Willis (2010) identified a large chromosome inversion polymorphism (DIV1) that contributes to local adaptation, reproductive isolation and life history divergence between annual and perennial populations of the yellow monkeyflower, Mimulus guttatus. Perennial populations of M. guttatus occur in habitats with year-round soil moisture, such as streams, wet grasslands and coastal seeps. These robust perennial plants produce stolons or rhizomes and flower during the summer months. Annual M. guttatus populations occur in drier habitats and the diminutive plants typically die when soil moisture availability is reduced during the summer. As a result, annual M. guttatus populations flower earlier in the season than perennial populations to escape from the summer drought (Vickery 1978; Dole 1992; Hall & Willis 2006; Lowry et al. 2008). Reciprocal transplant experiments have demonstrated that the morphological and flowering time differences between annual and perennial M. guttatus are locally adaptive (Hall & Willis 2006; Lowry et al. 2008; Hall et al. 2010; Lowry & Willis 2010).
Genetic mapping has revealed that for each of numerous morphological and life history trait differences between annual and perennial ecotypes of M. guttatus, there is a major quantitative trait locus (QTL) that colocalizes with the DIV1 inversion region of chromosome 8 (Hall et al. 2006; Lowry & Willis 2010), which recent mapping efforts have identified as ∼6 Mb in length (J. Friedman, unpublished data). To test whether the DIV1 inversion is involved in local adaptation, Lowry & Willis (2010) reciprocally introgressed alternative chromosomal variants into the annual and perennial genetic backgrounds and planted them along with controls in a reciprocal transplant design. This experiment demonstrated that the DIV1 inversion polymorphism contributes to local adaptation and ecological reproductive isolation in the wild between the annual and perennial populations. Unlike inversion polymorphism in many other species, the distribution of the DIV1 inversion is not clinal, but instead appears to be locally distributed in a mosaic between the respective habitats of annual and perennial populations (Lowry & Willis 2010).
Despite their morphological and life history differences, earlier studies have not found clear differentiation between annual and perennial M. guttatus for a variety of nuclear markers scattered across the genome (Sweigart & Willis 2003; Modliszewski & Willis 2012; Brandvain et al. 2013), although structure analysis of a limited sampling of perennials from the Pacific coast of California and Oregon found that they mainly clustered separately from inland annuals (Lowry et al. 2008). If the DIV1 inversion arose recently and swept to fixation in one ecotype (e.g. annuals) as suggested by mapping experiments in Lowry & Willis (2010), markers located within the inversion might exhibit reduced diversity within that ecotype as well as substantially greater differentiation between annuals and perennials than markers not associated with the inversion. Such a pattern might also result if the DIV1 inversion serves as a reproductive barrier across a wider sampling of M. guttatus annuals and perennials than hitherto examined (Lowry & Willis 2010).
Here, we undertake the first comparative analysis of the patterns of divergence for markers associated within the DIV1 inversion vs. markers distributed throughout the genome for a broad sampling of the M. guttatus species complex. We use population genetic methods, including estimates of genetic indices and clustering algorithms, to address the following questions: (i) Do we find, as have earlier studies, that annual and perennial M. guttatus fail to differentiate for markers located within the inversion? (ii) By contrast, is there differentiation between annual and perennial M. guttatus for the markers located within the inversion? (iii) Is the pattern of divergence for the inversion markers different from the overall pattern of shared genetic variation in the M. guttatus species complex as a whole? (iv) What is the magnitude of genetic, climatic, and morphological differentiation across the M. guttatus species complex? and (v) Is there an association between variation in the inverted vs. noninverted markers and climatic variation within M. guttatus? Addressing these questions will provide the first large-scale survey of genetic diversity and differentiation within a species complex that has become increasingly valuable for uncovering genetic basis of adaptation and divergence, and examine whether a recently identified chromosomal inversion is divergent across a much broader sample of annual and perennial accessions in this highly diverse species complex.
Materials and methods
Sampling of the Mimulus guttatus species complex
The M. guttatus complex is a highly diverse group of interfertile populations, ecotypes and species that are distributed over a broad latitudinal and altitudinal range across western North America, and occurs in a variety of habitats including alpine meadows, thermal springs, salt-spray zones, granite outcrops and serpentine soils (Vickery 1978; Ritland & Ritland 1989; Wu et al. 2008; Nesom 2012). Mimulus guttatus is the most widespread species in the complex and has a mixed mating system (Grant 1924; Hall et al. 2006; van Kleunen 2007; Lowry et al. 2008). There are also multiple other geographically restricted obligate selfers and edaphic endemics within the species complex (Kiang & Hamrick 1978; Fenster & Ritland 1994; Dudash & Carr 1998; Gardner & Macnair 2000; Sweigart & Willis 2003; Wright et al. 2013), with the bulk of the diversity located within the California Floristic Province (Grant 1924; Hitchcock 1959; Vickery 1978).
Characterizing genetic variation across a broad swathe of phenotypic diversity requires careful consideration of the sampling regime. Prior molecular work suggests that gene flow within the M. guttatus sp. complex is common (Sweigart & Willis 2003; Modliszewski & Willis 2012), which means that populations are unlikely to be regionally monophyletic. A scattered sampling design is less likely to produce biased estimates of population parameters than pooling multiple individuals from a few populations (Städler et al. 2009; Chikhi et al. 2010; Kalinowski 2011) and may also avoid biasing clustering algorithms (Kalinowski 2011). Thus, we sampled 1 individual per accession for a total of 120 accessions distributed across western North America, comprising populations from Alaska, British Columbia, Washington, Oregon, California, Colorado, Nevada, Idaho and Arizona (Table S1, Supporting information). The majority of populations sampled (N = 83) were of M. guttatus, the most geographically widespread and ecologically diverse species within the complex; this included 38 annual and 45 perennial M. guttatus populations. We note that designation of annual and perennial status is based on collective observations in the field and may be imperfect. The remaining samples comprise eight other species that generally occupy more restricted ranges and narrower ecological niches than M. guttatus. These include the annuals Mimulus arvensis, M. cupriphilus, M. laciniatus, M. micranthus, M. nasutus, M. nudatus and M. pardalis, as well as the perennial M. tilingii.
Mimulus nudatus, an edaphic endemic with woody stems, narrow leaves and large corollas, which is restricted to the serpentine soil of Lake and Napa Counties, California, is the only annual that is primarily outcrossing. Mimulus laciniatus, M. micranthus and M. nasutus are all primarily selfing with cleistogamous flowers. Mimulus laciniatus has dissected, lobular leaves and is restricted to exposed granite outcrops in the Sierra Nevada foothills, while M. micranthus has tiny flowers and occurs only in the coastal range of central California. Mimulus nasutus is distributed across western North America, co-occurs with M. guttatus in streambeds and seeps and is distinguished by its quadrangular stem, its unusually small corolla and a peculiarly shaped calyx. Mimulus pardalis, another serpentine endemic restricted to the Sierra Nevada foothills, is primarily selfing but without cleistogamous flowers. Mimulus arvensis, which we believe to be misidentified as M. platycalyx in many recent papers (Ritland & Ritland 1989; Sweigart & Willis 2003; Wu et al. 2008; G. Nesom, personal communication), is distinguished by its short, even-sized and open calyx lobes, is patchily distributed across western North America and occurs in stream beds, often simultaneously with perennial M. guttatus (Nesom 2012). Also primarily selfing but with plesiogamous flowers, M. cupriphilus is an edaphic endemic tolerant of high concentrations of copper in mine tailings and occurs at copper mine sites in the Sierra Nevada foothills of California (Macnair 1989). Mimulus tilingii, a close relative of M. guttatus (Vickery 1978; Beardsley & Olmstead 2002), is restricted to high elevations (2000+ m) in California and Colorado and has a distinctive pincushion-like vegetative growth with radially arranged flowers.
DNA extraction and genetic markers
Tissue was collected from each individual and stored in 96-well plates at −80 °C. Genomic DNA was later extracted using a modified hexadecyl trimethyl-ammonium bromide chloroform extraction protocol (Kelly & Willis 1998). Sixteen codominant markers, including 1 microsatellite (AAT217; Kelly & Willis 1998) and 15 intron-length polymorphic MgSTS markers, were selected for genetic analyses. Development of the MgSTS markers is described in detail in Fishman et al. (2014). All of the markers used here have been physically mapped to linkage groups eight markers are distributed throughout the M. guttatus genome and the remaining eight are located within the chromosomal inversion identified by Lowry & Willis (2010) (see Table S2, Supporting information for primer sequences and genomic positions of microsatellite and MgSTS markers). Primer sequences are publicly available at www.mimulusevolution.org.
MgSTS markers were amplified using standard touchdown PCR protocols, as described in Sweigart et al. (2006). Microsatellites were amplified using reaction conditions given in Kelly & Willis (1998). Labelled products were subjected to electrophoresis and fragment analysis on an ABI 3730xI DNA Analyzer. Size of the amplified fragments was scored automatically by the program GENEMARKER (SoftGenetics, 2005, State College, PA) and was confirmed by eye. The markers used in our analyses have been utilized in multiple QTL mapping studies of several species within the complex (rendering null alleles an unlikely problem); we have included a supplementary table with our missing data (Table S3, Supporting information).
Genetic analyses
To compare patterns of differentiation between genomic regions (i.e. the inverted region vs. the remaining genome) and provide a visualization of structure between accessions across the species complex we used a Bayesian clustering algorithm implemented in structure v. 2.3.3 (Pritchard et al. 2000). structure attempts to assign individuals to clusters (K) that are in Hardy–Weinberg and linkage equilibrium, assigning each K a log probability given the data (Pr(X|K)). When a lack of HWE is detected, structure infers that K + 1 is a better fit to the data. While we cannot assume that the loci included in our analyses are evolving neutrally, our scattered sampling design provides the closest possible approximation of a panmictic population.
To assess whether linkage between markers within the inversion might affect our analyses, we conducted a series of runs that included the distances (previously determined by mapping experiments (Lowry & Willis 2010) between the markers in the inversion. We found that adding distance did not affect the likelihood of the data for any analyses. For this reason, and because some accessions are polymorphic for marker order (Lowry & Willis 2010), we did not include prior information for marker distance in the structure analyses reported here. We also did not include a priori knowledge of taxonomic assignment or geography in the structure analysis.
We ran structure 10 times each for K-values ranging from 2 to 10 for both the inversion and noninversion markers. In all analyses, we used the admixture model with independent allele frequencies among populations, λ = 1, and an MCMC with a 200 000-step burn-in and 200 000 steps for estimating parameters. Evidence suggests that gene flow is widespread (Sweigart & Willis 2003; Modliszewski & Willis 2012). High levels of admixture may produce misleading results when determining the most probable levels of structure (K) within a population, especially with the Evanno method (Evanno et al. 2005; Waples & Gaggiotti 2006). We rely on a Wilcoxon's signed-rank test to determine the K for each set of markers (Nordborg et al. 2005), such that an increase in likelihood for K + 1 was not significant. We note that we rely on structure primarily for comparative purposes (for example, to compare markers from two different genomic regions) and are not interested in K per se. We used Harvester to visualize our structure output (Earl & vonHoldt 2011), combined the results of multiple runs with CLUMPP 1.1.2 (Jakobsson & Rosenberg 2007) and visualized the combined data using DISTRUCT 1.1 (Rosenberg 2004). Finally, we examined whether divergence in the inverted and noninverted region was associated with increasing geographic distance with a Mantel test in GENEPOP 4.2 (Raymond & Rousset 1995; Rousset 2008).
Further, we compared patterns of divergence within the inverted and noninverted regions of the genome by calculating several genetic indices, including number of alleles (Na), observed heterozygosity (HO), expected heterozygosity (HE) and the inbreeding coefficient (FIS). We calculated these indices for annual and perennial M. guttatus as well as for species represented by multiple accessions (M. arvensis, M. laciniatus, M. nasutus and M. tilingii) using the program GENALEX v.6 (Peakall & Smouse 2006). We also calculated FST as part of an AMOVA with ARLEQUIN 3.5 (Excoffier & Lischer 2010) to contrast the extent of divergence between annuals and perennials and among species within and outside the inversion.
Phenotypic and environmental data
In addition to characterizing genomic variation, we sought to characterize the range of both climatic and morphological variation contained within the M. guttatus sp. complex. We compiled environmental data for each geo-referenced accession included in our study by extracting a total of 19 WorldClim climatic variables (Hijmans et al. 2005) for each location of origin using ARCGIS (ESRI, Redlands, California, USA; ESRI 2011); we combined these variables to characterize the environmental range of M. guttatus sp. To characterize phenotypic variation, we grew a representative of each of the individual accessions included in this study in climate-controlled growth chambers under 16-h days at 21 °C, 8-h nights at 16 °C and 30% relative humidity in 2.5-inch square pots in the Duke University greenhouses. All populations were selfed at least once in the greenhouse after field collection prior to inclusion in our study to control for maternal effects. We recorded the number of days until flowering postgermination (forthwith ‘flowering time’), length of the second internode, length and width of the first leaf, length and width of the first flower, diameter between first and second true leaves, number of leaf pairs upon flowering and the presence or absence of anthocyanin calyx spots. We utilized principal components to reduce the dimensionality of the quantitative phenotypic and environmental data and then visualized the distribution of variation across the complex using ordination plots of the first and second principal components (PC1 and PC2). Several authors have noted that annual and perennial forms of M. guttatus may differ morphologically (Grant 1924; Vickery 1974; van Kleunen 2007; Nesom 2012). We tested for differences between annual and perennial M. guttatus by performing a MANOVA on the principal components of our morphological data. We also tested whether these accessions differed in the presence of calyx spotting with a Fisher's exact test.
Redundancy analysis (RDA) is an extension of multivariate linear regression that performs constrained ordination between two matrices, seeking linear combinations of explanatory variables in one matrix that are correlated with linear combinations of response variables in the other matrix (Hair et al. 1998; Legendre et al. 2011). RDA and related constrained ordination methods have been employed extensively in the field of community ecology, where they are used to find associations between environmental data and ranges of multiple species (Legendre & Legendre 1998). We used RDA to determine whether climatic and spatial variation (the explanatory variables) can be said to explain significant portions of genomic variation (the response variables) (Ayala et al. 2011; Lasky et al. 2012). In our case, the explanatory variables are the WorldClim data as well as the elevation, latitude and longitude of each accession, while the response variables are allele frequency data for the inverted and noninverted microsatellite markers. RDA also enables us to control for the effects of geographic population structure by removing the effects of latitude and longitude and performing constrained ordination on the residual variation (partial RDA).
To avoid overparameterizing, a problem that arises when explanatory variables (i.e. the WorldClim variables) exhibit substantial intervariable correlation, we narrowed our search among the climatic variables by first performing a full RDA with stepwise forward addition of the WorldClim explanatory variables and assessing their significance using the Akaike information criterion (AIC). We retained any climatic variables that explained significant portions of genomic variation, after discarding any that were directly correlated to another variable (see Results). The remaining climate variables were combined with the latitude, longitude, elevation and annual/perennial status of each accession and tested for correlations with variation in the inverted and noninverted markers. We also performed partial RDA for both sets of markers after removing the effects of latitude and longitude to control for spatially derived population structure. We determined the individual significance of each explanatory variable using the ‘marginal’ testing method, which tests the effect of each term by removing it from a model that includes all of the terms (Legendre et al. 2011). We performed a similar analysis of associations between morphological and flowering time data and inversion and noninversion variation (see Supporting information). RDA with AIC was implemented with the ‘rda’ and ‘ordistep’ modules in ‘vegan’ (Oksanen et al. 2013), and significance of explanatory variables determined via ‘margin,’ with a minimum of 1000 permutations. All statistical analyses were performed in R 12.15.0.
Results
Genetic divergence
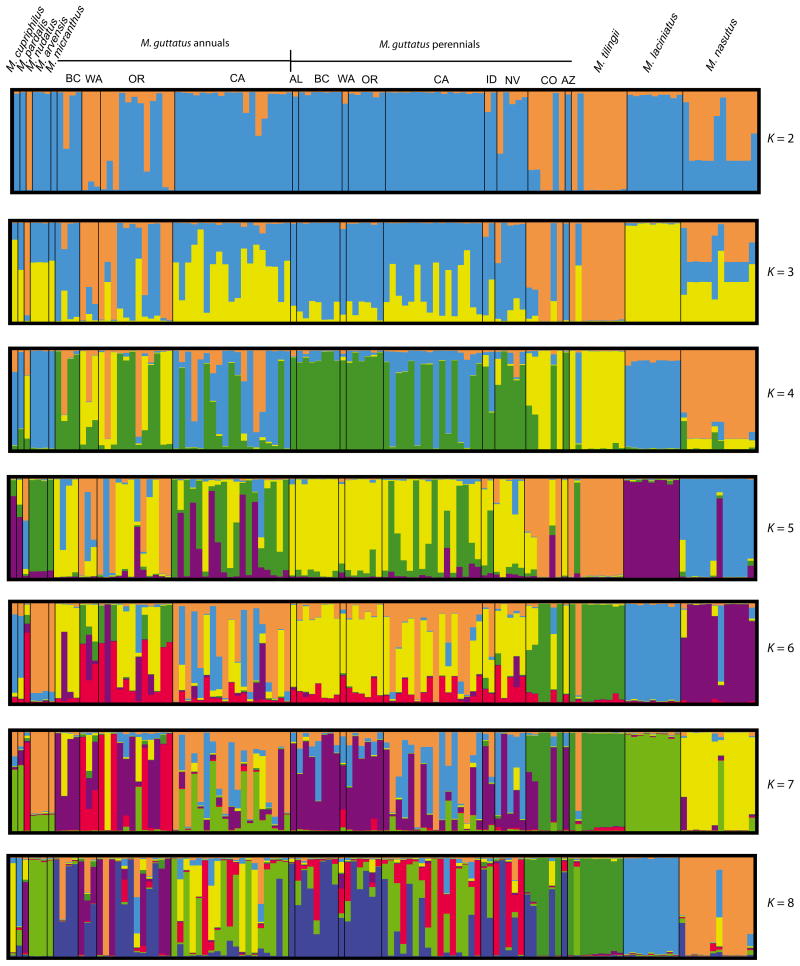
Several distinct patterns emerge from the structure analysis of the noninversion markers. The first is that there is no evidence that annual and perennial ecotypes of Mimulus guttatus form separate groups. Instead, variation in the noninversion markers is principally structured geographically (Fig. 1). At K = 8, the most likely K for the noninversion markers, three major groups emerge. One is comprised of northern annual and perennial accessions from British Columbia, Washington and Oregon. The other two are largely confined to California. One cluster is shared broadly across annual and perennial accessions, while the other is comprised of several annual accessions and a few coastal perennials. The second major pattern exhibited by the noninverted markers is that M. guttatus is extremely variable, with multiple K segregating across the 83 accessions. By contrast, the predominantly selfing taxa (M. arvensis, M. cupriphilus, M. laciniatus, M. micranthus, M. nasutus and M. pardalis) are considerably less variable. Third, M. guttatus accessions harbour, at low frequency, variation that co-occurs in all of the other species: M. arvensis, M. cupriphilus, M. laciniatus, M. micranthus, M. nasutus, M. nudatus, M. pardalis and M. tilingii. Additionally, the cluster that is largely composed of M. nasutus contains variation that segregates at low frequency in northern annual M. guttatus accessions. Fourth, M. nasutus and M. laciniatus, which are predominantly selfing, emerge distinct from each other at K = 4 and isolated from the rest of the complex at K = 5, while the selfing annual M. pardalis emerges separate from both and clusters with M. cupriphilus for K = 2 to K = 7. The latter finding supports Nesom (2012), who has argued that they are the same species (M. pardalis). Finally, the perennial M. tilingii clusters together with alpine perennial M. guttatus from Colorado.
Fig. 1.
Clustering analysis of population genetic differentiation across the Mimulus guttatus complex for eight microsatellite markers distributed throughout the genome. All analyses were carried out with N = 120 accessions with 10 runs each. Results of multiple runs combined with CLUMMP (Jakobsson & Rosenberg 2007) and visualized with DISTRUCT (Rosenberg 2004).
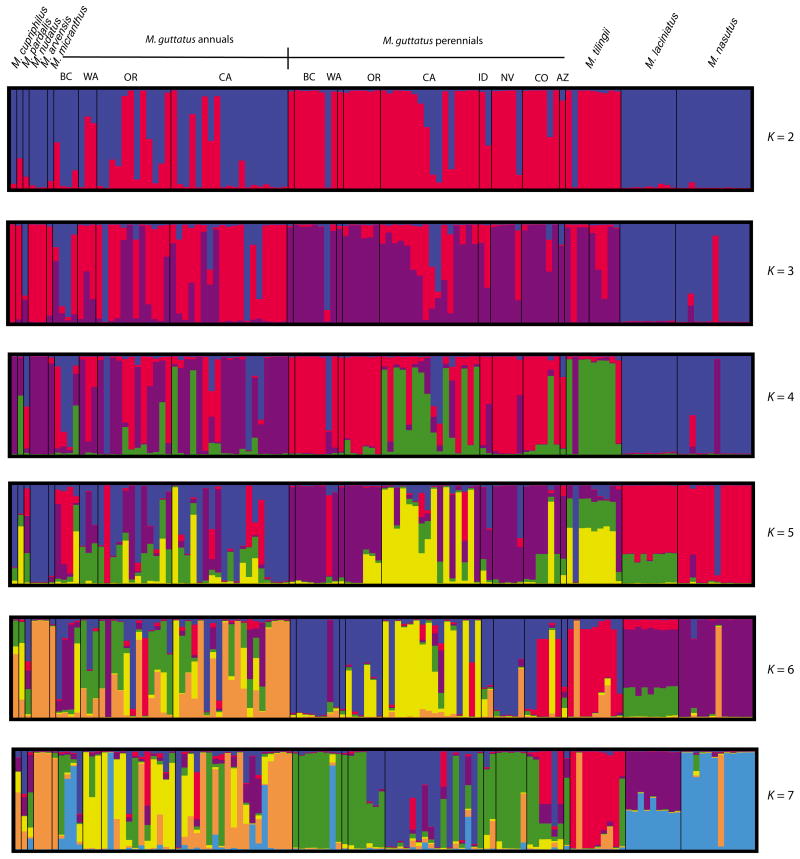
The pattern of emergence and divergence of other complex members differs somewhat in a structure analysis of the inverted markers vs. the noninverted markers (Fig. 2). Mimulus laciniatus and M. nasutus never fully differentiate from one another or from M. tilingii in the structure analysis of the inverted markers, while they are distinct as early as K = 4 for the noninverted markers. The other taxa (M. cupriphilus, M. pardalis, M. nudatus, M. micranthus and M. pardalis) largely cluster with annual M. guttatus for the inverted markers, as they did for the whole-genome markers. It is difficult to tell whether M. tilingii clusters more with the annuals or the perennials for the inverted markers, however, as it appears to harbour variation segregating within both.
Fig. 2.
Clustering analysis of population genetic differentiation across the Mimulus guttatus complex for eight microsatellite markers located within or proximate to the DIV1 inversion.
The inverted markers also reveal a very different pattern of differentiation within M. guttatus itself. While the primary axis of divergence for the noninversion markers is spatial, the inversion markers within M. guttatus cluster along a marked annual/perennial divide. Annual M. guttatus are nearly entirely distinct from perennial M. guttatus, although a few annual and perennial M. guttatus from California share some variation. Coastal perennials from California and Oregon cluster together. In general, perennial M. guttatus appear to be nearly distinct from annual M. guttatus and the other annual taxa, which share considerable variation and are altogether more diverse for this region than the perennials.
Annual and perennial M. guttatus do not differ significantly in number of alleles (Na) or expected heterozygosity (HE) for the inverted markers (Table 1; see Table S4, Supporting information for indices of individual loci). The inverted markers exhibit significantly reduced expected heterozygosity (HE) than the noninverted markers for a few of the intraspecific comparisons (Table 1, highlighted in bold), but most intraspecific comparisons have overlapping confidence intervals. While genetic differentiation between annual and perennial M. guttatus accessions is low overall, it is elevated within the DIV1 inverted region (FST of 0.061, 95% CI = 0.030–0.097) in comparison with the noninverted region (FST of 0.020, 95% CI = 0.012–0.028) (Table 2) (see Supporting information for individual marker diversity and FST). We detected a weak signal of isolation by distance among annual and perennial M. guttatus for the noninversion markers (annuals r2 = 0.080, P < 0.001; perennials r2 = 0.080; P = 0.017), but only among annuals for the inversion markers (annuals r2 = 0.100, P = 0.004; perennials r2 = 0.072, P = 0.074). There was no evidence of isolation by distance among all M. guttatus accessions (including annuals and perennials) for either the inversion or noninversion markers (inversion r2 = 0.001, P = 0.302; noninversion r2 = 0.048, P = 0.115), although there was a weak signal across the complex as a whole, which includes several taxa with restricted ranges (inversion r2 = 0.065, P < 0.001; noninversion r2 = 0.052, P < 0.001). Pairwise species divergence (i.e. FST) between M. guttatus and other members of the complex was lower than pairwise differences among the remaining complex species (Table 3).
Table 1. Genetic diversity indices for members Mimulus guttatus sp. complex within and outside of the DIV1 inverted region, including for M. guttatus, M. guttatus annuals and perennials separately, and other species represented by more than one accession in our data set.
| Na (Inv) | HE (Inv) | HO (Inv) | FIS (Inv) | |
|---|---|---|---|---|
| M. guttatus annuals | 14.75 ± 2.08 | 0.850 ± 0.023 | 0.368 ± 0.029 | 0.570 ± 0.028 |
| M. guttatus perennials | 13.12 ± 1.79 | 0.796 ± 0.034 | 0.300 ± 0.024 | 0.623 ± 0.025 |
| M. guttatus all accessions | 19.38 ± 2.79 | 0.853 ± 0.023 | 0.332 ± 0.020 | 0.613 ± 0.017 |
| Mimulus arvensis | 1.50 ± 0.189 | 0.222 ± 0.084 | 0.000 ± 0.000 | 1.000 ± 0.000 |
| Mimulus laciniatus | 4.00 ± 0.655 | 0.545 ± 0.072 | 0.222 ± 0.047 | 0.562 ± 0.112 |
| Mimulus nasutus | 4.25 ± 0.313 | 0.480 ± 0.055 | 0.146 ± 0.049 | 0.662 ± 0.122 |
| Mimulus tilingii | 4.88 ± 0.666 | 0.694 ± 0.045 | 0.306 ± 0.098 | 0.575 ± 0.143 |
|
| ||||
| Na (NI) | HE (NI) | HO (NI) | FIS (NI) | |
|
| ||||
| M. guttatus annuals | 20.12 ± 2.63 | 0.866 ± 0.045 | 0.408 ± 0.043 | 0.532 ± 0.041 |
| M. guttatus perennials | 17.38 ± 2.74 | 0.821 ± 0.064 | 0.300 ± 0.045 | 0.641 ± 0.039 |
| M. guttatus all accessions | 25.62 ± 3.74 | 0.855 ± 0.055 | 0.350 ± 0.040 | 0.595 ± 0.034 |
| M. arvensis | 2.12 ± 0.398 | 0.354 ± 0.108 | 0.125 ± 0.088 | 0.691 ± 0.160 |
| M. laciniatus | 4.62 ± 0.706 | 0.580 ± 0.090 | 0.154 ± 0.051 | 0.730 ± 0.077 |
| M. nasutus | 4.62 ± 0.498 | 0.552 ± 0.061 | 0.202 ± 0.056 | 0.653 ± 0.071 |
| M. tilingii | 7.12 ± 0.875 | 0.746 ± 0.058 | 0.361 ± 0.100 | 0.558 ± 0.120 |
Na, number of alleles; HE, expected heterozygosity; HO, observed heterozygosity; FIS, inbreeding coefficient; Inv, inverted region; NI, noninverted region.
Indices are given with standard error. Indices for which the inversion markers exhibit lower diversity than the noninversion markers are in bold.
Table 2. Genetic differentiation (FST) and AMOVA within the inverted and noninverted regions for annual vs. perennial Mimulus guttatus and for species within the complex represented by more than one accession (Mimulus laciniatus, M. nasutus, M. arvensis and M. tilingii).
| Source | d.f. | SS | Var. comp | % variance | FST |
|---|---|---|---|---|---|
| Inversion | |||||
| Among annual and perennial M. guttatus | 1 | 20.6 | 0.211 | 6.07 | 0.061** |
| Within annuals and perennials | 164 | 535.2 | 3.263 | 93.93 | |
| Total | 165 | 555.8 | 3.473 | ||
| Among species | 4 | 95.0 | 0.767 | 19.78 | 0.198* |
| Within species | 227 | 701.6 | 3.112 | 80.22 | |
| Total | 231 | 796.6 | 3.879 | ||
| Noninversion | |||||
| Among annual and perennial M. guttatus | 1 | 8.5 | 0.063 | 1.92 | 0.020* |
| Within annuals and perennials | 164 | 530.8 | 3.237 | 98.1 | |
| Total | 165 | 539.3 | |||
| Among species | 4 | 84.31 | 0.668 | 17.95 | 0.175* |
| Within species | 227 | 639.74 | 3.056 | 82.05 | |
| Total | 231 | 778.05 | |||
P ≤ 0.01
P ≤ 0.001.
Table 3. Pairwise divergence (FST) between species (Mimulus arvensis, M. guttatus, M. tilingii, M. laciniatus and M. nasutus) for the DIV1 inverted region and noninverted region.
| M. arvensis | M. guttatus | M. laciniatus | M. nasutus | |
|---|---|---|---|---|
| DIV1 inversion | ||||
| M. guttatus | 0.233** | |||
| M. laciniatus | 0.378** | 0.178** | ||
| M. nasutus | 0.504** | 0.202** | 0.334** | |
| M. tilingii | 0.454** | 0.116** | 0.293** | 0.328** |
| Noninversion | ||||
| M. guttatus | 0.190** | |||
| M. laciniatus | 0.348** | 0.135** | ||
| M. nasutus | 0.430** | 0.191** | 0.356** | |
| M. tilingii | 0.307* | 0.118** | 0.278** | 0.287** |
Significance assessed with 1000 permutations and Bonferonni-adjusted
P ≤ 0.01,
P ≤ 0.001.
Differentiation between species is also slightly pronounced within the inversion (FST of 0.198, 95% CI = 0.151–0.247) vs. the noninversion (FST of 0.175, 95% CI = 0.109–0.238), but not significantly so (Table 2). Selfing taxa (M. arvensis, M. laciniatus and M. nasutus) exhibited less genetic diversity than outcrossing taxa (M. guttatus and M. tilingii) and the difference in diversity between the inversion and noninversion markers was starker for the outcrossing taxa (Table 1).
Phenotypic variation
The first three principal components cumulatively summarize 89.5% of the variation in the environmental variables and 84.6% of the variation in the morphological traits for M. guttatus sp. All but one environmental variable (temperature seasonality) had moderately high loadings on the first and second principal components (climate PC1), with temperature loading positively and all but one of the precipitation variables loading negatively on PC1 (Table 4). This indicates that climate PC1 and PC2 summarize important variation in temperature and precipitation. All morphological traits, except distance between 1st and 2nd internodes, had positive and high loadings on the first principal component (morphological PC1) indicating that size and growth rate are the primary axes of variation captured by our grow-out experiment.
Table 4. Loadings of environmental and phenotypic traits on the first three principal components for all Mimulus guttatus complex accessions.
| Environmental variables | Phenotypic variables | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | ||
| % variation | 46.4 | 34.1 | 8.9 | 52.1 | 20.0 | 12.5 | |
| Annual mean temp. | 0.261 | 0.219 | 0.185 | Leaf length | −0.356 | 0.413 | −0.304 |
| Mean diurnal temp. range | 0.236 | −0.222 | 0.013 | Leaf width | −0.286 | 0.542 | −0.300 |
| Isothermality | 0.200 | 0.173 | −0.450 | Flower length | −0.389 | −0.065 | 0.551 |
| Temp. seasonality | 0.013 | −0.344 | 0.339 | Flower width | −0.405 | −0.074 | 0.496 |
| Max temp. warmest month | 0.293 | −0.040 | 0.354 | Internode distance | −0.025 | 0.589 | 0.394 |
| Min temp. coldest month | 0.150 | 0.339 | 0.075 | Height | −0.363 | −0.245 | −0.188 |
| Temp. annual range | 0.109 | −0.336 | 0.226 | No. leaves at flower | −0.400 | −0.329 | −0.193 |
| Mean temp. wettest quart. | 0.181 | 0.162 | 0.021 | Diameter btw. leaves | −0.427 | −0.093 | −0.200 |
| Mean temp. driest quart. | 0.268 | 0.132 | 0.350 | ||||
| Mean temp. warmest quart. | 0.286 | 0.073 | 0.368 | ||||
| Mean temp. coldest quart. | 0.207 | 0.301 | 0.034 | ||||
| Annual prec. | −0.233 | 0.253 | 0.168 | ||||
| Prec. wettest month | −0.181 | 0.297 | 0.120 | ||||
| Prec. driest month | −0.306 | −0.001 | 0.189 | ||||
| Prec. seasonality | 0.212 | 0.244 | −0.114 | ||||
| Prec. wettest quarter | −0.189 | 0.290 | 0.133 | ||||
| Prec. driest quarter | −0.314 | 0.029 | 0.205 | ||||
| Prec. warmest quarter | −0.307 | 0.025 | 0.202 | ||||
| Prec. coldest quarter | −0.177 | 0.296 | 0.116 | ||||
Prec, precipitation; temp., temperature.
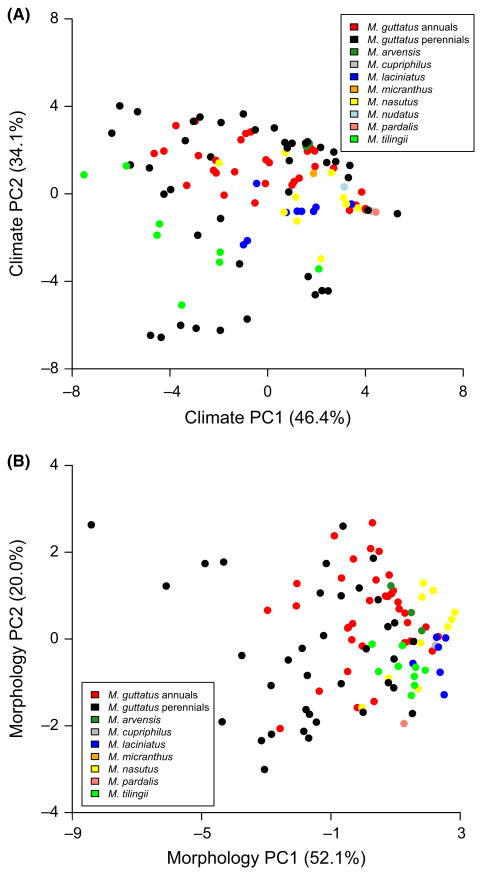
Plots of the first and second principal components from the climatic and morphological data illustrate the distribution of climatic and morphological variation within the complex. Not surprisingly, M. guttatus occupies a wider range of environmental conditions and exhibits greater morphological variation than any other member of the M. guttatus sp. complex. Within M. guttatus, there is considerable overlap between perennials and annuals in both climatic and morphological space; however, the perennials occupy a wider range of climatic conditions and exhibit more extreme morphological phenotypes than annuals (Fig. 3A, B). Annual and perennial M. guttatus differ significantly for both sets of variables (manova of three climate PCs: F1,78 = 7.00, Wilks' λ = 0.7878, P < 0.001; manova of three morphology PCs: F1,67 = 8.89, Wilks' λ = 0.71533, P « 0.001). While a lack of anthocyanin calyx spotting was the norm for M. guttatus, spotting was observed more on annual than perennial M. guttatus (annuals = 16, perennials = 5; Fisher's exact test, P = 0.015). For the complex as a whole, perennial M. guttatus overlap considerably in climate space with the other major perennial species in the complex, M. tilingii, while the annual selfers, in particular M. laciniatus and M. nasutus, are clustered with the annual M. guttatus. By contrast with their climatic range, M. nasutus, M. laciniatus and M. tilingii occupy a much narrower portion of morphological space.
Fig. 3.
(A) Plot of the first two principal components in an analysis of WorldClim variables obtained for 120 accessions within the Mimulus guttatus complex. (B) Plot of the first two principal components in an analysis of morphological variables collected from a common garden experiment under 16-h days for 120 accessions of the M. guttatus complex.
Redundancy analyses
Stepwise forward addition of climatic variables with AIC identified six environmental variables associated with variation in the inversion markers and five associated with the noninversion markers (Table S5, Supporting information) for M. guttatus. To avoid overparameterizing, we discarded temperature annual range, because it is correlated with minimum temperature of the coldest month, another variable identified for inclusion in our analyses.
Annual/perennial life history was associated with nearly twice as much variation in the inversion markers (R2 = 22.04%, P = 0.001) than in the noninversion markers (R2 = 13.70%, P = 0.001). Neither latitude, longitude, nor elevation exhibited a significant correlation with variation in the inversion or the noninversion markers. Two climatic variables (mean temperature of the coldest quarter and minimum temperature of the coldest month) exhibited significant association with variation in the whole-genome markers in a full RDA and remained significant after controlling for geographic distance (i.e. latitude and longitude) (Table 5). By contrast, three variables, all related to water availability, showed significant association with inversion variation: mean temperature of the driest quarter, precipitation of the driest quarter and precipitation of the wettest month (Table 5).
Table 5. Results of partial redundancy analysis (in which the effects of latitude and longitude are held constant) testing for associations between climate variables and variation in the inversion and noninversion markers within Mimulus guttatus.
| % variance | P | % variance | P | ||
|---|---|---|---|---|---|
| Inversion | Noninversion | ||||
| Annual/perennial | 22.04 | 0.001*** | Annual/perennial | 13.70 | 0.001* |
| Elevation | 8.81 | 0.064 | Elevation | 5.53 | 0.937 |
| Isothermality | 7.74 | 0.190 | Mean temp. coldest quarter | 10.19 | 0.040* |
| Mean temp. driest quarter | 9.52 | 0.028* | Min temp. coldest month | 10.43 | 0.032* |
| Precipitation driest quarter | 10.21 | 0.018* | Mean temp. driest quarter | 7.54 | 0.440 |
| Precipitation wettest month | 9.73 | 0.022* | Temp. seasonality | 7.94 | 0.347 |
Temp., temperature.
All terms remain significant when annual/perennial status is held constant. Significance assessed with a minimum of 1000 permutations:
P ≤ 0.05,
P ≤ 0.01,
P ≤ 0.001.
Plots of the climatic variables associated with whole-genome variation in M. guttatus, with structure-derived K superimposed, shed light on factors driving these associations (Fig. 4A, B). For example, structure has identified the M. guttatus annual accessions occurring in the California Central Valley region, which exhibits considerably higher mean temperatures than other sampling localities, as one cluster. Other clusters comprising perennial M. guttatus are associated with colder regions, such as Colorado and Idaho, which cluster together with perennials distributed across range of M. guttatus in colder climates.
Fig. 4.
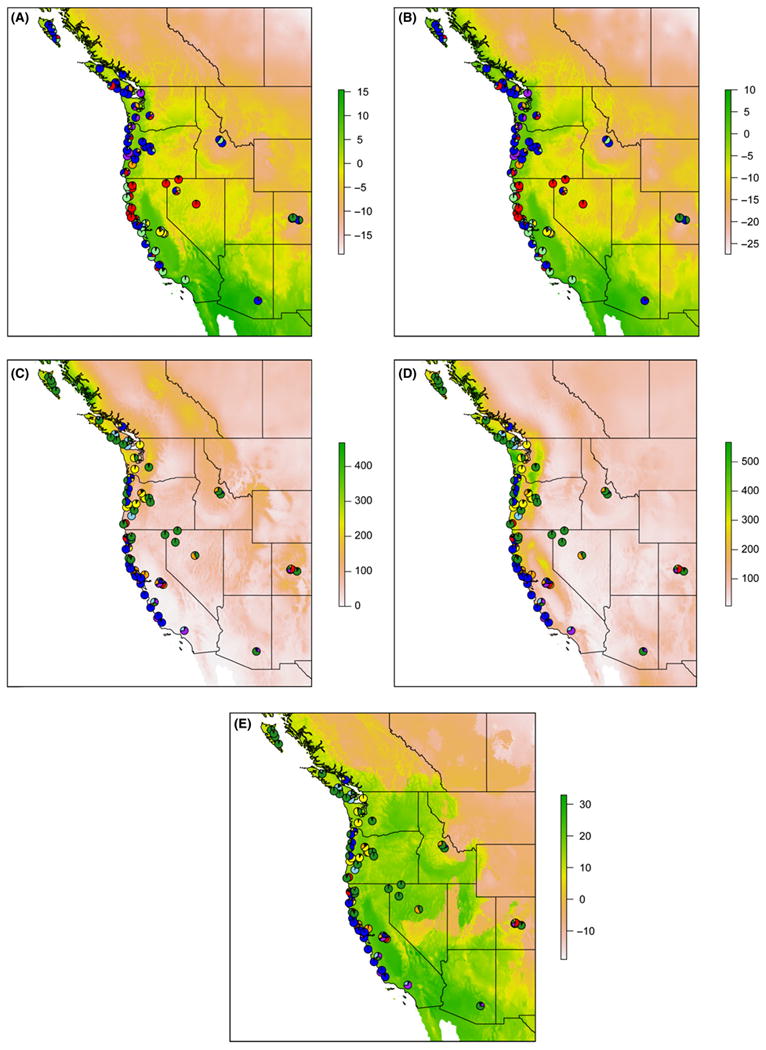
Heat maps of WorldClim variables with structure-derived clusters superimposed for 82 Mimulus guttatus accessions (one Alaska accession is excluded) for the noninverison (A, B) and inversion (C–E) markers. (A) Heat map of mean temperatures (°C) of the coldest quarter of the year with noninversion marker clusters at K = 8. (B) Heat map of minimum temperatures (°C) of the coldest month of the year with noninversion marker clusters at K = 8. (C) Heat map of precipitation levels (mm) of the driest quarter of the year with inversion marker clusters at K = 7. (D) Heat map of precipitation levels (mm) of the wettest month of the year with inversion marker clusters at K = 7. (E) Heat map of mean temperatures (°C) of the driest quarter of the year with inversion marker clusters at K = 7.
Higher levels of precipitation are similarly associated with perennial inversion clusters of M. guttatus from British Columbia, Washington, Oregon, Idaho, and Colorado (colours: blue and dark green), while lower levels are associated with clusters comprised of perennials and annuals from California (colours: red and light green) (Fig. 4C, D). The association between temperatures during the driest quarter and inversion variation is probably driven by accessions from Idaho, Nevada and Colorado (Fig. 4E).
Discussion
Chromosomal inversions are now well known to be involved in adaptive divergence and the formation of new species (Dobzhansky 1951, 1970; Noor et al. 2001; Rieseberg 2001; Hoffmann & Rieseberg 2008; Kirkpatrick 2010; Kirkpatrick & Kern 2012). Recent studies have found that there is elevated differentiation between opposite chromosomal inversion orientations in Drosophila (Kolaczkowski et al. 2011) and Anopheles mosquitoes (Cheng et al. 2012). Here, we evaluated whether there is evidence for elevated levels of population genetic structure across the Mimulus guttatus species complex for the DIV1 inversion, a geographically widespread chromosomal inversion known to play a major role in adaptive divergence between wet coastal perennial populations and dry inland annual populations (Lowry & Willis 2010). The DIV1 chromosomal inversion contributes to divergence in many traits, including locally adaptive flowering time and the perennial/annual life history transition (Lowry & Willis 2010). The scale of our study, which combines genomic, climatic and morphological data for 120 accessions, enables us to test whether a broader sample of annual and perennial M. guttatus are differentiated in the chromosomal inversion (DIV1) region, which has been linked to adaptive divergence between annuals and perennials.
Population structure and inversion variation
Mimulus guttatus is the only species of the complex that occurs in both annual and perennial forms, and due to their morphological differences, populations with different life histories have occasionally been split into separate species (Grant 1924; Pennell 1951; Nesom 2012). However, published gene trees show M. guttatus to be a polytomy of annuals and perennials (Sweigart & Willis 2003; Modliszewski & Willis 2012, Brandvain et al. 2013). Our results, which comprise a much larger sample of the complex, similarly emphasize a lack of divergence along the annual/perennial divide for most of the genome. The structure analysis of the noninverted markers indicates a clear role for geography in structuring genetic variation within M. guttatus and reveals no distinct annual/perennial groups. By contrast, a structure analysis of the inverted markers finds that annuals and perennials separate into mostly distinct clusters. Genetic divergence between annuals and perennials (as measured by FST) is slightly higher for the inversion markers than the noninversion markers, although still low. The structure results further suggest that there is less diversity for the inversion markers in perennial than annual M. guttatus. As early as K = 4, perennials separate into two major clusters (roughly corresponding to northern and southern groups) for the inversion, while the annuals are more evenly distributed across several clusters. Genetic indices, such as the number of alleles and expected heterozygosity, also indicate somewhat reduced diversity within perennials, although not significantly so. This is in contrast to the structure results and the expected heterozygosity for the noninversion markers, for which annual and perennial M. guttatus are equally diverse. More data (e.g. more microsatellite loci and/or genomic sequence data) are needed to confirm this pattern. If this pattern holds true with more data, it might suggest that the perennial form of the inversion is derived.
The structure results indicate limited introgression for the inversion, as compared with the noninversion markers, affirming that it serves as a reproductive barrier between annuals and perennials. Interestingly, earlier crossing studies involving 14 perennial and eight annual populations – ranging from British Columbia to southern California, and as far east as Wyoming – found a perfect association between annual/perennial life history and marker order within the DIV1 inversion. This led the authors to suggest that the inversion was a fixed difference between annuals and perennials (Lowry & Willis 2010). If this fixed difference had arisen recently, perhaps by a sweep of the derived form in the perennials, we might expect to see dramatic reductions in expected heterozygosity in perennials as compared to annuals. This is not the pattern we see, however. Rather, we find only subtle differences in expected heterozygosity between perennials and annuals. There are several nonexclusive explanations for the pattern of inversion variation that we observe. First, our study relied on microsatellite markers, which evolve very rapidly. What appears to be shared variation due to introgression may instead be the result of convergent mutation (i.e. homoplasy) among microsatellite loci. With their high mutability, microsatellite markers are not ideal for precisely determining the origin and spread of the derived form of the inversion. Second, double-crossovers and gene conversion events during meiosis could lead to shared marker variation between the arrangements and result in the pattern of limited shared variation between annuals and perennials for the inversion markers. An important caveat is that we currently have no direct evidence of the orientation of the inversion in the larger sample of the populations that we sampled here. Controlled crosses, as well as genomic sequence data, are needed to elucidate the orientation of the inversion across multiple populations and distinguish among these alternative hypotheses for history of emergence and spread of the DIV1 inverted region.
Ecological divergence of the M. guttatus species complex
The M. guttatus species complex has long been notable for its wide variety of floral, vegetative and life history characteristics, which occur over a wide range of habitats, including alpine meadows, hot springs, copper mine tailing, serpentine and granite outcrops, and the coastal salt-spray zone (Wu et al. 2008). Previous studies have focused on understanding the population genetic relationships of a subset of this diversity (Sweigart & Willis 2003; Lowry et al. 2008). Our study is the first attempt to expand population genetic analysis to a broader swath of the diversity of the species complex in conjunction with an analysis of morphological and environmental variation. PCA of the WorldClim variables emphasizes the broad climatic range occupied by the complex as a whole and by M. guttatus in particular. That species occupies more climatic space than the remaining complex members combined. Perennial M. guttatus, in particular, occur across a vast range of climatic conditions. We find more morphological than climatic differentiation across the complex. In general, other species (e.g. Mimulus laciniatus, M. nasutus and M. tilingii) occupy narrower morphological than climatic spaces and are more morphologically than environmentally differentiated from M. guttatus.
Seasonal variation in soil water availability, which varies geographically, is known to be an important selective pressure for M. guttatus populations (Kiang & Hamrick 1978; Hall & Willis 2006; Lowry et al. 2008; Lowry & Willis 2010; Wu et al. 2010). Our principal components results confirm this, and the redundancy analyses further suggest that adaptation to local climatic regimes, and particularly water and temperature stress, has shaped genomic variation in M. guttatus. Specifically, the association between variation in the noninversion markers and climatic variables is probably a consequence of differential gene flow, such that populations adapted to similar climates experience greater pairwise introgression than those adapted to different climates. It is unlikely that noninversion markers sampled for this study are physically linked to loci underlying adaptation to water availability and temperature regime. The association between inversion variation and temperature and precipitation is likely due both to adaptation within annuals and perennials as well as the differential effects of gene flow. Notably, while we found some evidence for isolation by distance within annuals and perennials, latitude and longitude (proxies for geographic distance) did not explain a significant portion of the variation in inversion or noninversion markers. This indicates that associations between climate and genomic variation are unlikely to be due to the retention of ancestral polymorphism by geographically proximate populations. Rather, climatic variables linked to stressful conditions are important selective factors that have shaped the genomic landscape of M. guttatus.
Understanding species relationships among M. guttatus sp
The highly divergent taxonomic treatments of the M. guttatus species complex, from four to more than 20 species (Grant 1924; Campbell 1950; Pennell 1951; Thompson 1993; Nesom 2012), emphasize the difficulty of confidently assigning species relationships to this morphologically diverse and complicated group. Our results suggest that even though considerable phenotypic differentiation exists between identifiable ‘species,’ there also exists extensive shared neutral genetic variation across the complex, which may ultimately undermine any clear taxonomy of the group. This common genetic variation is probably the result of shared ancestral polymorphism or ongoing gene flow, which has occurred despite high levels of reproductive isolation (Lowry et al. 2008; Martin & Willis 2010) between members of the complex. Some regions of the genome, such as the DIV1 inversion region, do show some elevated differentiation among members of the complex, but are unlikely to reflect the genome at large.
A recent taxonomy of M. guttatus and its relatives by Nesom (2012) splits the complex into many different species and hypothesizes a major division within M. guttatus between annual (Microphyllus group) and perennial (Guttatus group) species. We did find a trend towards larger size of morphological traits for M. guttatus perennials than in annuals. However, the overall low levels of population structure in our study between annual and perennial populations for markers across the genome do not support Nesom's life history-based division of the complex. Even for markers within the inversion, there appears to be shared variation between annual and perennial members of the complex, likely due to retention of ancestral variation and ongoing, if restricted, gene flow.
Nesom (2012) argued that his hypothesized taxonomy of the species complex could be tested with a molecular phylogeny, but this is unlikely for two reasons. First and foremost, gene flow is widespread across the complex, and no phylogenetic methods currently exist which satisfactorily account for the confounding factor of hybridization (Eckert & Carstens 2008; Liu et al. 2009; Meng & Kubatko 2009), although one possible way forward is through new methods that evaluate population splits and mixtures in a tree-based framework (Pickrell & Pritchard 2012). Second, we have demonstrated convincingly that different regions of the genome, particularly the inversion, experience different patterns of introgression and shared ancestry. Together, these features suggest that the difficulty inherent in resolving relationships among the diverse members of the M. guttatus species complex is not merely a technical problem, but instead reflects the true nature of the speciation process, whereby clear genome-wide divergence does not occur until well after species are first identifiable.
Conclusion
The scale of our study, with 120 independent population accessions grown simultaneously, provides insight into the structuring of genetically based morphological and phenological variation across the Mimulus guttatus species complex, better defines the climatic range and extent of morphological and genetic differentiation among previously identified taxonomic groups and sheds light on the history of adaptation within M. guttatus sp. We demonstrate that a chromosomal inversion region, previously implicated in life history shifts and adaptation within a subset of annual and perennial M. guttatus, may be associated with ecological and life history divergence across a broader range of M. guttatus populations. Further evidence for this role is revealed by broadly different patterns of genetic structure within and outside of this inversion. We confirm the importance of water availability as a primary adaptive trait. Finally, we shed light on species relationships within the complex and provide evidence that shifts between outcrossing and selfing have probably occurred multiple times within the complex.
Supplementary Material
Table S1 Sampling localities for accessions from the Mimulus guttatus complex included in our genetic, phenotypic and redundancy analyses.
Table S2 Primers for the 1 microsatellite and 15 intron-length polymorphic markers used in our genetic analyses.
Table S3 Missing alleles for Mimulus guttatus sp. ‘–’ indicates the number of accessions with missing data.
Table S4 Genetic diversity indices for each locus for members of the Mimulus guttatus sp. complex and for M. guttatus annuals and perennials.
Table S5 Results of stepwise forward selection of variables for inclusion in RDA.
Table S6 Results of stepwise forward selection of morphological variables and flowering time for inclusion in RDA.
Table S7 Results of redundancy analysis testing for associations between morphology variables and variation in the inversion and noninversion markers within Mimulus guttatus.
Appendix S1 Results of a redundancy analysis testing for associations between morphology and flowering time and variation in noninversion and inversion markers.
Appendix S2 We investigated whether FST and allelic variation (Na) in the DIV1 inversion markers could be a function of distance to the breakpoints. We detected no significant correlation (Spearman rank correlation coefficient).
Fig. S1 Plot of FST and position of inversion markers on linkage group 8. There is little evidence for an association between FST and distance to the breakpoints of the DIV1 inversion.
Fig. S2 Plot of allelic variation (Na) vs. position of inversion markers on linkage group 8. There is little evidence for an association..
Acknowledgments
The authors would like to thank C. Sheng and the Duke University greenhouse staff for help with experiments. K. Behrman provided climate variables for all populations. G. Nesom helped to confirm identification of many collection samples. Ben Blackman, Jannice Friedman, and Alex Twyford and three anonymous reviewers gave helpful comments on earlier manuscripts. Funding was provided by the National Science Foundation grants EF-0328636 and EF-0723814 and IOS-1024966 to J.W. and a Doctoral Dissertation Improvement Grants to D.L. (DEB-0710094). An NIH NRSA fellowship (1F32GM097929-01) to E.O. provided funding during data analysis and the writing of the manuscript. A USDA NIFA-AFRI postdoctoral fellowship (2011-67012-30696) to D.L. provided funding during the writing of the manuscript.
Footnotes
Supporting information: Additional supporting information may be found in the online version of this article.
E.O., D.B.L., K.M.W. and J.H.W. conceived of and designed the study. E.O. collected molecular data. D.B.L., K.M.W., and Z.Z. collected morphological data. E.O. analysed the data. E.O. and D.B.L. wrote the paper with contributions from K.M.W. and J.H.W.
References
- Ayala D, Fontaine MC, Cohuet A, et al. Chromosomal inversions, natural selection and adaptation in the malaria vector Anopheles funestus. Molecular Biology and Evolution. 2011;28:745–758. doi: 10.1093/molbev/msq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala D, Guerrero RF, Kirkpatrick M. Reproductive isolation and local adaptation quantified for a chromosome inversion in a malaria mosquito. Evolution. 2013;67:946–958. doi: 10.1111/j.1558-5646.2012.01836.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Olmstead RG. Redefining Phrymaceae: the placement of Mimulus, tribe Mimuleae, and Phryma. American Journal of Botany. 2002;89:1093–1102. doi: 10.3732/ajb.89.7.1093. [DOI] [PubMed] [Google Scholar]
- Brandvain Y, Kenney AM, Flagel L, Coop G, Sweigart AL. Speciation and introgression between Mimulus nasutus and Mimulus guttatus. PLoS Genetics. 2013;1310:7131. doi: 10.1371/journal.pgen.1004410. in press. arXiv preprint ar Xiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR. Mimulus guttatus and related species. El Aliso. 1950;2:319–337. [Google Scholar]
- Cheng C, White BJ, Kamdem C, et al. Ecological genomics of Anopheles gambiae along a latitudinal cline: a population-resequencing approach. Genetics. 2012;190:1417–1432. doi: 10.1534/genetics.111.137794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhi L, Sousa VC, Luisi P, Goossens B, Beaumont MA. The confounding effects of population structure, genetic diversity and the sampling scheme on the detection and quantification of population size changes. Genetics. 2010;186:983–995. doi: 10.1534/genetics.110.118661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics and the Origin of Species. 3rd. Columbia University Press; New York City, New York: 1951. [Google Scholar]
- Dobzhansky T. Genetics of the Evolutionary Process. Columbia University Press; New York City, New York: 1970. [Google Scholar]
- Dole JA. Reproductive assurance mechanisms in three taxa of the Mimulus guttatus complex (Scrophulariaceae) American Journal of Botany. 1992;1992:650–659. [Google Scholar]
- Dudash MR, Carr DE. Genetics underlying inbreeding depression in Mimulus with contrasting mating systems. Nature. 1998;393:682–684. [Google Scholar]
- Earl DA, vonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2011;4:359–361. [Google Scholar]
- Eckert AJ, Carstens BC. Does gene flow destroy phylogenetic signal? The performance of three methods for estimating species phylogenies in the presence of gene flow. Molecular Phylogenetics and Evolution. 2008;49:832–842. doi: 10.1016/j.ympev.2008.09.008. [DOI] [PubMed] [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10. Environmental Systems Research Institute; Redlands, California: 2011. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Fang Z, Pyhäjärvi T, Weber AL, et al. Megabase-scale inversion polymorphism in the wild ancestor of maize. Genetics. 2012;191:883–894. doi: 10.1534/genetics.112.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JL, Nosil P. Chromosomal inversions and species differences: when are genes affecting adaptive divergence and reproductive isolation expected to reside within inversions? Evolution. 2009;63:3061–3075. doi: 10.1111/j.1558-5646.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Ritland K. Evidence for natural selection on mating system in Mimulus (Scrophulaiaceae) International Journal of Plant Sciences. 1994;155:588–596. [Google Scholar]
- Fishman L, Willis JH, Wu CA, Lee YW. Comparative linkage maps suggest that fission, not polyploidy, underlies near-doubling of chromosome number within monkey-flowers (Mimulus; Phrymaceae) Heredity. 2014;112:562–568. doi: 10.1038/hdy.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Macnair M. Factors affecting the co-existence of the serpentine endemic Mimulus nudatus Curran and its presumed progenitor, Mimulus guttatus Fischer ex DC. Biological Journal of the Linnean Society. 2000;69:443–459. [Google Scholar]
- Grant AL. A monograph of the genus Mimulus. Annals of the Missouri Botanical Garden. 1924;11:99–389. [Google Scholar]
- Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. Prentice Hall International; Englewood, New Jersey: 1998. Canonical correlation analysis; pp. 442–465. [Google Scholar]
- Hall MC, Willis JH. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution. 2006;60:2466–2477. [PubMed] [Google Scholar]
- Hall MC, Basten CJ, Willis JH. Pleiotropic quantitative trait loci contribute to population divergence in traits associated with life history variation in Mimulus guttatus. Genetics. 2006;172:1829–1844. doi: 10.1534/genetics.105.051227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MC, Lowry DB, Willis JH. Is local adaptation in Mimulus guttatus caused by trade-offs at individual loci? Molecular Ecology. 2010;19:2739–2753. doi: 10.1111/j.1365-294X.2010.04680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Hitchcock CL. In: Vascular plants of the Pacific Northwest: Ericaceae through Campanulaceae. Hitchcock CL, Cronquist A, Ownbey M, editors. Vol. 17. University of Washington Press; Seattle, Washington: 1959. [Google Scholar]
- Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annual Review of Ecology and Systematics. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Jones FC, Grabherr MG, Chan YF, et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012a;484:55–61. doi: 10.1038/nature10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, Salazar PA, ffrench-Constant RH, Jiggins CD, Joron M. Evolution of a mimicry supergene from a multilocus architecture. Proceedings of the Royal Society of London. Series B, Biological Sciences. 2012b;279:316–325. doi: 10.1098/rspb.2011.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron M, Frezal L, Jones RT, et al. Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature. 2011;477:203–206. doi: 10.1038/nature10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski ST. The computer program STRUCTURE does not reliably identify the main genetic clusters within species: simulations and implications for human population structure. Heredity. 2011;106:625–632. doi: 10.1038/hdy.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AJ, Willis JH. Polymorphic microsatellite loci in Mimulus guttatus and related species. Molecular Ecology. 1998;7:769–774. [Google Scholar]
- Kiang YT, Hamrick JL. Reproductive isolation in the Mimulus guttatus-M. nasutus complex. American Midland Naturalist. 1978;100:269–276. [Google Scholar]
- King M. Species Evolution: The Role of Chromosomal Change. Cambridge University Press; Cambridge, UK: 1993. [Google Scholar]
- Kirkpatrick M. How and why chromosome inversions evolve. PLoS Biology. 2010;8:e1000501. doi: 10.1371/journal.pbio.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton N. Chromosome inversion, local adaptation and speciation. Genetics. 2006;173:419–434. doi: 10.1534/genetics.105.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Kern A. Where's the money? Inversions, genes, and the hunt for genomic targets of selection. Genetics. 2012;190:1153–1155. doi: 10.1534/genetics.112.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleunen M. Adaptive genetic differentiation in life-history traits between populations of Mimulus guttatus with annual and perennial life-cycles. Evolutionary Ecology. 2007;21:185–199. [Google Scholar]
- Kolaczkowski B, Kern AD, Holloway AK, Begun DJ. Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics. 2011;187:245–260. doi: 10.1534/genetics.110.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimbas CB, Powell JR. Drosophila Inversion Polymorphism. CRC Press; Boca Raton, Florida: 1992. [Google Scholar]
- Lasky JR, Des Marais DL, McKay JK, et al. Characterizing genomic variation of Arabidopsis thaliana: the roles of geography and climate. Molecular Ecology. 2012;21:5512–5529. doi: 10.1111/j.1365-294X.2012.05709.x. [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre L. Numerical Ecology. 2nd. Elsevier; New York City, New York: 1998. [Google Scholar]
- Legendre P, Oksanen J, ter Braak CJF. Testing the significance of canonical axes in redundancy analysis. Methods in Ecology and Evolution. 2011;2:269–277. [Google Scholar]
- Lewis H. The mechanism of evolution in the genus Clarkia. Evolution. 1953;7:1–20. [Google Scholar]
- Liu L, Yu L, Kubatko L, Pearl DK, Edwards S. Coalescent methods for estimating phylogenetic trees. Molecular Phylogenetics and Evolution. 2009;53:320–328. doi: 10.1016/j.ympev.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Lowry DB, Willis JH. A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biology. 2010;8:e1000500. doi: 10.1371/journal.pbio.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry DB, Rockwood RC, Willis JH. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution. 2008;62:2196–2214. doi: 10.1111/j.1558-5646.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnair M. A new species of Mimulus endemic to copper mines in California. Botanical Journal of the Linnean Society. 1989;100:1–14. [Google Scholar]
- Martin NH, Willis JH. Geographical variation in postzygotic isolation and its genetic basis within and between two Mimulus species. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2010;365:2469–2478. doi: 10.1098/rstb.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng C, Kubatko LS. Detecting hybrid speciation in the presence of incomplete lineage sorting using gene tree incongruence: a model. Theoretical Population Biology. 2009;75:35–45. doi: 10.1016/j.tpb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Modliszewski JL, Willis JH. Allotetraploid Mimulus sookensis are highly interfertile despite independent origins. Molecular Ecology. 2012;21:5280–5298. doi: 10.1111/j.1365-294X.2012.05706.x. [DOI] [PubMed] [Google Scholar]
- Nesom GL. Taxonomy of Erythranthe sect. Simiola (Phrymaceae) in the USA and Mexico. Phytoneuron. 2012;40:1–123. [Google Scholar]
- Noor MA, Grams KL, Bertucci LA, Reiland J. Chromosomal inversions and the reproductive isolation of species. Proceedings of the National Academy of Sciences. 2001;98:12084–12088. doi: 10.1073/pnas.221274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg M, Hu TT, Ishino Y, et al. The pattern of polymorphism in Arabidopsis thaliana. PLoS biology. 2005;3:e196. doi: 10.1371/journal.pbio.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Oksanen MJ, Suggests MASS. Package ‘vegan’. Community ecology package Version. 2013;2:0–0. [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell FW. Mimulus. In: Abrams L, editor. Illustrated Flora of the Pacific States. Vol. 3. Stanford University Press; Palo Alto, California: 1951. pp. 688–731. [Google Scholar]
- Pickrell JK, Pritchard JK. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genetics. 2012;8:e1002967. doi: 10.1371/journal.pgen.1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Rieseberg LH. Chromosomal rearrangements and speciation. Trends in Ecology and Evolution. 2001;16:351–358. doi: 10.1016/s0169-5347(01)02187-5. [DOI] [PubMed] [Google Scholar]
- Ritland C, Ritland K. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae) American Journal of Botany. 1989;76:1731–1739. [Google Scholar]
- Rosenberg NA. Distruct: a program for the graphical display of population structure. Molecular Ecology Notes. 2004;4:137–138. [Google Scholar]
- Rousset F. Genepop'007: a complete reimplementation of the Genepop software for Windows and Linux. Molecular Ecology Resources. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Städler T, Haubold B, Merino C, Stephan W, Pfaffelhuber P. The impact of sampling schemes on the site frequency spectrum in nonequilibrium subdivided populations. Genetics. 2009;182:205–216. doi: 10.1534/genetics.108.094904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. The inviability, weakness, and sterility of interspecific hybrids. Advances in Genetics. 1958;9:147–215. doi: 10.1016/s0065-2660(08)60162-5. [DOI] [PubMed] [Google Scholar]
- Sweigart AL, Willis JH. Patterns of nucleotide diversity in two species of Mimulus are affected by mating system and asymmetric introgression. Evolution. 2003;57:2490–2506. doi: 10.1111/j.0014-3820.2003.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Sweigart AL, Fishman L, Willis JH. A simple genetic incompatibility causes hybrid male sterility in Mimulus. Genetics. 2006;172:2465–2479. doi: 10.1534/genetics.105.053686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM. Mimulus. In: Hickman JC, editor. The Jepson Manual: Higher Plants of California. University of California Press; Berkeley, California: 1993. pp. 1037–1046. [Google Scholar]
- Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffmann AA. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science. 2005;308:691–693. doi: 10.1126/science.1109523. [DOI] [PubMed] [Google Scholar]
- Vickery RK., Jr Crossing barriers in the yellow monkey flowers in the genus Mimulus (Scrophulariaceae) Genetics Lectures. 1974;3:33–82. [Google Scholar]
- Vickery RK. Case studies in the evolution of species complexes in Mimulus. Evolutionary Biology. 1978;11:405–507. [Google Scholar]
- Waples RS, Gaggiotti O. What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Molecular Ecology. 2006;15:1419–1439. doi: 10.1111/j.1365-294X.2006.02890.x. [DOI] [PubMed] [Google Scholar]
- White MJD. Modes of Speciation. W.H. Freeman; San Francisco, California: 1978. [Google Scholar]
- Wright KM, Lloyd D, Lowry DB, Macnair MR, Willis JH. Indirect evolution of hybrid lethality due to linkage with a selected locus in Mimulus guttatus. PLoS Biology. 2013;11:e1001497. doi: 10.1371/journal.pbio.1001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CA, Lowry DB, Cooley AM, et al. Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity. 2008;100:220–230. doi: 10.1038/sj.hdy.6801018. [DOI] [PubMed] [Google Scholar]
- Wu CA, Lowry DB, Nutter LI, Willis JH. Natural variation for drought response traits in the Mimulus guttatus species complex. Oecologia. 2010;162:23–33. doi: 10.1007/s00442-009-1448-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sampling localities for accessions from the Mimulus guttatus complex included in our genetic, phenotypic and redundancy analyses.
Table S2 Primers for the 1 microsatellite and 15 intron-length polymorphic markers used in our genetic analyses.
Table S3 Missing alleles for Mimulus guttatus sp. ‘–’ indicates the number of accessions with missing data.
Table S4 Genetic diversity indices for each locus for members of the Mimulus guttatus sp. complex and for M. guttatus annuals and perennials.
Table S5 Results of stepwise forward selection of variables for inclusion in RDA.
Table S6 Results of stepwise forward selection of morphological variables and flowering time for inclusion in RDA.
Table S7 Results of redundancy analysis testing for associations between morphology variables and variation in the inversion and noninversion markers within Mimulus guttatus.
Appendix S1 Results of a redundancy analysis testing for associations between morphology and flowering time and variation in noninversion and inversion markers.
Appendix S2 We investigated whether FST and allelic variation (Na) in the DIV1 inversion markers could be a function of distance to the breakpoints. We detected no significant correlation (Spearman rank correlation coefficient).
Fig. S1 Plot of FST and position of inversion markers on linkage group 8. There is little evidence for an association between FST and distance to the breakpoints of the DIV1 inversion.
Fig. S2 Plot of allelic variation (Na) vs. position of inversion markers on linkage group 8. There is little evidence for an association..