Abstract
Background
We previously demonstrated vagal neural pathways, specifically subdiaphragmatic afferent fibers, regulate expression of the intestinal sodium-glucose cotransporter SGLT1, the intestinal transporter responsible for absorption of dietary glucose. We hypothesized targeting this pathway could be a novel therapy for obesity. We therefore tested the impact of disrupting vagal signaling by total vagotomy or selective vagal de-afferentation on weight gain and fat content in diet-induced obese rats.
Methods
Male Sprague–Dawley rats (n = 5–8) underwent truncal vagotomy, selective vagal de-afferentation with capsaicin, or sham procedure. Animals were maintained for 11 months on a high-caloric Western diet. Abdominal visceral fat content was assessed by magnetic resonance imaging together with weight of fat pads at harvest. Glucose homeostasis was assessed by fasting blood glucose and HbA1C. Jejunal SGLT1 gene expression was assessed by qPCR and immunoblotting and function by glucose uptake in everted jejunal sleeves.
Results
At 11-months, vagotomized rats weighed 19% less (P = 0.003) and de-afferented rats 7% less (P = 0.19) than shams. Vagotomized and de-afferented animals had 52% (P < 0.0001) and 18% reduction (P = 0.039) in visceral abdominal fat, respectively. There were no changes in blood glucose or glycemic indexes. SGLT1 mRNA, protein and function were unchanged across all cohorts at 11-months postoperatively.
Conclusions
Truncal vagotomy led to significant reductions in both diet-induced weight gain and visceral abdominal fat deposition. Vagal de-afferentation led to a more modest, but clinically and statistically significant, reduction in visceral abdominal fat. As increased visceral abdominal fat is associated with excess morbidity and mortality, vagal de-afferentation may be a useful adjunct in bariatric surgery.
Keywords: Vagus, Bariatric surgery, Capsaicin, Vagotomy, Abdominal visceral fat
Introduction
Obesity, together with associated type 2 diabetes mellitus (T2DM), is a major public health and economic problem facing the developed world. Obesity in particular has become a surgical disease, with surgery providing the only treatment modality delivering sustained weight loss and resolution of co-morbidities, specifically T2DM [1]. The impressive impact of these procedures on glucose homeostasis has led to the recent interest in metabolic surgeries, where weight loss operations are offered to those with T2DM. Unfortunately, most bariatric surgeries are major interventions with appreciable morbidity and mortality, thus limiting their availability to only severely obese diabetics (BMI > 35) [1]. This consideration has led to increasing interest in vagal-targeted therapies because interventions such as laparoscopic truncal vagotomy are relatively minor surgical procedures [2]. Indeed, as early as the 1980s, vagotomy was promoted as a weight-loss procedure and resulted in improved glycemic control [3, 4], although subsequently lost favor with the development of gastric bypass and banding procedures. Whilst there is evidence for weight-loss benefits from vagotomy and vagal blockade [5], historically, experience with truncal vagotomy has been complicated by poor tolerance due to the complications of motor vagotomy, such as dumping syndrome and diarrhea. As a strategy to avoid these side effects, we examined the impact of selective vagal ablation therapy, using capsaicin to block the afferent fibers without any motor disruption, on total and visceral weight as well as intestinal nutrient transport [6].
As a novel therapeutic approach to treating obesity and associated T2DM, we focused on the intestinal Na+/glucose cotransporter SGLT1. This transporter is responsible for all active intestinal glucose transport under physiological conditions [7]. Hypothetically, reducing SGLT1-mediated glucose transport function would slow glucose absorption after meals, reducing post-prandial hyperglycemia, and increasing ileal delivery of glucose, with its ensuing satiety effects. Thus, suppressing SGLT1 might be expected to have anti-diabetic and anti-obesity effects. These have indeed been demonstrated in both rodents and patients, using phloridzin, an inhibitor of SGLT1, which is capable of improving oral glucose tolerance [8, 9].
In recent work, we and others have shown that afferent or total vagotomy [6] led to a reduction in SGLT1 protein circadian rhythmicity with a reduction in peak SGLT1 expression, through apparently post-transcriptional mechanisms. We therefore hypothesized that long-term vagotomy or vagal de-afferentation would suppress intestinal glucose transport capability and therefore prevent development of obesity in rats.
In this study, we set out to establish the effect of vagotomy or de-afferentation on development of obesity in a diet–induced obese rat model. Animals underwent afferent or total vagotomy (using capsaicin, the “hot” component of chilli peppers, directly applied to the vagus to induce de-afferentation [6]) or a sham procedure, and were then maintained for 11 months on a high-carbohydrate, high-fat diet. We now show that vagotomy or vagal de-afferentation lead to a reduction in the deposition of intra-abdominal fat in an age-associated obesity model.
Methods
Reagents
Chemicals were sourced from commercial suppliers as follows: Capsaicin, Tween 80, NaCl, KCl, CaCl2, KH2PO4, MgSO4, NaHCO3, KCl, glucose (Sigma, St Louis, MO); isoflurane (Webster Veterinary, Sterling, MA); buprenorphine (Bedford Laboratories, Bedford, OH); 14C-D-glucose and 3H-L-glucose (Moravek Biochemicals, Brea, CA); soluene 350, hyonic fluor (Perkin Elmer, Waltham, MA); chloroform, methanol (Fisher, Pittsburg, PA).
Animals and Experimental Design
All animal experiments were in accordance with protocols prospectively approved by the Harvard Medical Area Standing Committee on Animals. Male Sprague–Dawley (Harlan, Indianapolis, IN; n = 24) rats were acquired aged 7 weeks and acclimatized for 5 days in our animal facility under 12:12-h light:dark cycle (lights-on at 7 am) with ad libitum access to Purina LabDiet 5001 rat chow (Lab-Diet, PMI Nutrition International, Richmond, IN) and tap water. Animals were then switched to Western diet (43% carbohydrate, 41% fat; Western Diet, Research Diets, New Brunswick, NJ) and maintained for a further 5 days prior to surgery: total vagotomy, selective vagal de-afferentation, or sham procedure.
Postoperatively, animals were weighed daily or every other day through day 10 and subsequently weighed weekly or biweekly. Animals were maintained for 11 months after surgery. We monitored the animals for 11 months based on other rodent studies which had shown male Sprague–Dawley rats to reach their peak weight at just before 1 year old. At this age, the male rats had also reached plateau level for body fat levels too [10]. In the final 3 weeks of experimentation, animals underwent abdominal magnetic resonance imaging (detailed below) for assessment of retroperitoneal fat volume. Throughout the penultimate week, daily food intake was measured for all animals: animals were transferred to single cages, and the mass of food remaining at 7 pm was subtracted from that provided 24 h previously. At least 5 days prior to harvest, animals were fasted from 7 am, and underwent fasting blood glucose measurement under light isoflurane anesthesia (1–2% in oxygen) at 7 pm; blood was measured using a hand-held glucometer (one-Touch Ultra; Lifescan, Milpitas, CA) from a drop of blood from tail vein prick.
On the day of harvest, animals were anesthetized with 1–2% isoflurane at 10 pm (time of peak intestinal glucose transport function and SGLT1 protein expression). Tissue was harvested as below, and animals euthanized.
Surgical Procedures
Animals were randomized to either sham procedure, vagal de-afferentation with capsaicin, or total vagotomy (n = 7–8), as previously described [6]. Animals were anesthetized with 50 mg/kg IP sodium pentobarbital injection, and underwent midline laparotomy under aseptic conditions. Total subdiaphragmatic vagotomy was performed under 10× magnification. For de-afferentation, capsaicin 2 mg/mL in vehicle solution (90% olive oil and 10% Tween 80 v/v) was applied topically to the vagal trunks for 30 min, with parafilm preventing general dispersal in the peritoneal cavity. For sham procedure, the vehicle alone was applied. The abdomen was thoroughly lavaged with normal saline before closure with vicryl sutures. Buprenorphine 0.05 mg/kg SC was administered twice daily for the first 48 h after surgery as analgesia.
Magnetic Resonance Imaging
Animals were imaged using a 4.7-tesla Bruker BioSpin small animal magnetic resonance (MR) imager (Bruker, Billerica, MA), with T1-weighted spin (TR = 1,750 ms, TE = 14.4 ms, flip angle = 180, field of view = 70 mm, acquisition matrix = 256 × 256). Animals were anesthetized using 1–2% isoflurane in oxygen, placed in a custom-made cradle, and respiratory rate monitored throughout the procedure. The abdomen was imaged from the upper excursion of the diaphragm through to the femoral heads/bladder trigone, using 1 mm slices at 1.25 mm intervals (thus 250-μm interslice distance).
Image analysis was performed on publicly-available analytical software (3D Slicer; www.slicer.org) by an independent investigator (A.R., 2 years experience in image segmentation) blinded to the experimental treatment. Axial slices from dome of the diaphragm to the head of the right femur were reviewed. In each slice, retroperitoneal fat was outlined using 3D Slicer software. Sagittal and coronal slices were used as segmentation guides if needed. The mass of retroperitoneal fat was calculated from its volume, determined by integration with the 3D Slicer software, using a conversion factor of 0.9007 g/cm3.
Tissue Harvest
Tissue harvests were performed at 10 pm EST, the time of peak intestinal glucose transport capacity [11]. A midline laparotomy was performed under anesthesia. Blood (1 mL) was drawn from the inferior vena cava and placed in EDTA tubes for glycated hemoglobin measurement. The jejunum was transected at the ligament of Trietz, and flushed with ice-cold mammalian Ringer’s solution while still on the mesenteric pedicle. The jejunum was excised, and segments reserved for functional uptake studies. Mucosa was harvested from remaining sections by scraping with a glass slide over ice. Mesenteric and epididymal fat pads were carefully dissected and weighed. In some, retroperitoneal fat pads were also dissected solely for illustrative purposes. Finally, the liver was excised, blotted and weighed. Three samples (~1 cm3) were taken from each lobe, weighed to determine exact wet weights, and frozen for subsequent fat content analyses.
Functional Glucose Uptake Studies
We have previously described our method for measuring functional glucose uptake capacity using the everted sleeve technique [11]. Briefly, 1-cm lengths of proximal jejunum were everted over steel rods, equilibrated in ice-cold mammalian Ringer’s solution (MR; 128 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 20 mM NaHCO3, pH 7.38; oxygenated with 95%02/5% CO2), before warming to 38°C for 4 min. Tissue segments were then transferred to uptake solution, containing 50 mM D-glucose in oxygenated MR, together with tracer quantities of 14C-D-glucose and 3H-L-glucose as a volume control. Uptake was performed for 60 s, before washing with cold MR. Everted sections were solublized overnight in 500 μL of soluene 350 at 55°C, 4 mL hyonic fluor scintillation fluid was added and uptake measured on a dual-chamber scintillation counter (Beckman-Coulter, Fullerton, CA).
SGLT1 Analysis
For protein studies, we employed a standard protein extraction protocol as described previously [12]. Mucosa was homogenized in 1 mL of Triton X-100 lysis buffer containing 10 μL protease inhibitor and separated on a centrifuge at 11,000g for 15 min at 4°C. A total of 60 μg protein was denatured in LDS buffer and separated on a 10% bis–tris gel before transferring to PVDF membranes. These were blotted using α-SGLT1 antibodies (1:4,000, Chemicon) and antibodies detected using HRP-conjugated α-rabbit secondary antibodies (1:5,000, Vector Labs). Actin (1:500, Neomarkers) was used as a loading control. Semi-quantitative densitometry was performed using Image J, and SGLT1 protein expression quantified relative to actin expression [12].
SGLT1 qPCR
SGLT1 mRNA quantification was as previously described [6]. Total mRNA was extracted using mirVana extraction kit (Ambion, Austin, TX), and 5 μg total RNA reversed using a Superscript III kit (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using SYBRgreen on an ABI7900HT thermal cycler (Applied Biosciences, Foster City, CA), using primers for SGLT1 (5′-CCAAGCCCATCCCAGACGTACACC-3′, 5′-CTTCCTTAGTCATCTTCGGTCCTT-3′) and Actin (5′-GGATCAGCAAGCAGGAGTACGA-3′, 5′-AACGCAGCTCAGTAACAGTCCG-3′). SGLT1 transcription was normalized to Actin expression.
Liver and Abdominal Visceral Fat Content
Liver fat content was assayed according to the Folch technique [13]. Assays were performed in triplicate for each animal. Frozen liver tissue (1 g) was homogenized in 2:1 v/v chloroform:methanol solution made up to 15 mL. The homogenate was then filtered through 0.22 μm filter paper, and the filter paper and residue washed with 5 mL of 2:1 chloroform:methanol. The filtrate was made up to 20 mL with chloroform:methanol, vortexed with 4 mL 0.74% KCl, and allowed to separate overnight. The upper phase was carefully removed and discarded, and the interphase carefully washed twice with 1.5 mL upper phase solvent (upper phase of 8:4:3 chloroform:methanol:0.74% KCl v/v). The lower phase was then placed into a pre-weighed aluminum fold dish and allowed to evaporate before weighing to calculate fat mass. Fat percentage was then expressed as a percentage of wet tissue mass:
As wet tissue mass of the whole liver was known, the total liver fat mass could then be calculated:
Mean intra-animal variation was calculated (4.3 ± 0.6%), suggesting acceptable assay replicability and accuracy.
Total intra-abdominal fat content was calculated from the sum of retroperitoneal fat (as measured by MRI), epididymal fat, mesenteric fat and calculated liver fat masstotal; this was then expressed as a percentage of body weight at the end of the study.
Glycated Hemoglobin Assays
Glycated hemoglobin assays were performed on 20 μL whole blood, using a direct enzymatic assay kit (Diazyme, Poway, CA) according to manufacturer’s instructions.
Data and Statistical Analysis
Weights were expressed as a percentage change from the start of the experiment. Two-tailed t-tests were used for planned comparison of all data to shams. Statistical analysis was performed with Graphpad Prism 5, with P ≤ 0.05 taken as significant.
Results
Two animals allocated to the de-afferentation arm died peri-operatively: one on induction of anesthesia, and the second of respiratory complications during the first postoperative night. Total animal numbers were therefore: sham, n = 7; vagotomy, n = 8; capsaicin, n = 5. One vagotomized animal died of anesthetic complications after fasting blood glucose assessment. Data from this animal are included in weight and feeding measurements, retroperitoneal fat assessment and liver fat quantification.
Vagotomy Reduces Age-Related Weight Gain
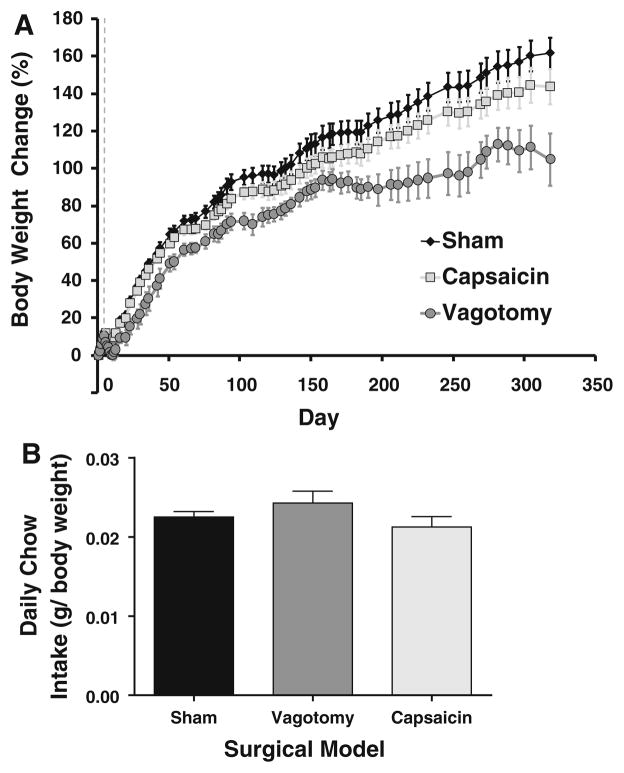
Initial animal weights were: 256 ± 3 g (sham), 253 ± 4 g (capsaicin) and 257 ± 4 g (vagotomy). There was no significant difference between groups (P > 0.45). Sham animals showed a normal growth rate, gaining 162 ± 8% of their initial weight by the end of the study. At this stage, animals were still gaining weight, albeit slowly (approximately 1.4% of start weight per week, Fig. 1a). Vagotomized animals gained significantly less weight than sham animals (maximum of 113 ± 9% weight gain, P = 0.0027). De-afferented animals trended towards a lower body weight than sham animals, although this did not reach significance (144 ± 9% weight gain, P = 0.19). Capsaicin-treated animals did however weigh more than vagotomized animals (P = 0.045).
Fig. 1.
Animals were weighed on a weekly basis though to harvest (a). Weight is expressed as percentage change compared to the initial weight of the animal before acclimatisation. The dashed grey line shows the weight on the day of surgery. Vagotomized animals weighed significantly less than both shams and de-afferented animals after day 4 postoperatively (P <0.001 at harvest). Chow intake, as measured over a week-long period at the end of the study, was reduced in the vagotomy and deafferented animals; however, when corrected for body weight (b), food intake was unchanged in both vagotomized and de-afferented animals compared to shams (P > 0.15)
Effect of Vagotomy and Selective Deafferentation on Food Intake
At the end of the study, when food intake was corrected for animal weight, there was no difference in food intake between the three groups (Fig. 1b). Sham animals consumed 2.3 ± 0.2% of their body weight each day, compared to 2.1 ± 0.3% and 2.4 ± 0.4% for capsaicin and vagotomized animals respectively (P > 0.16).
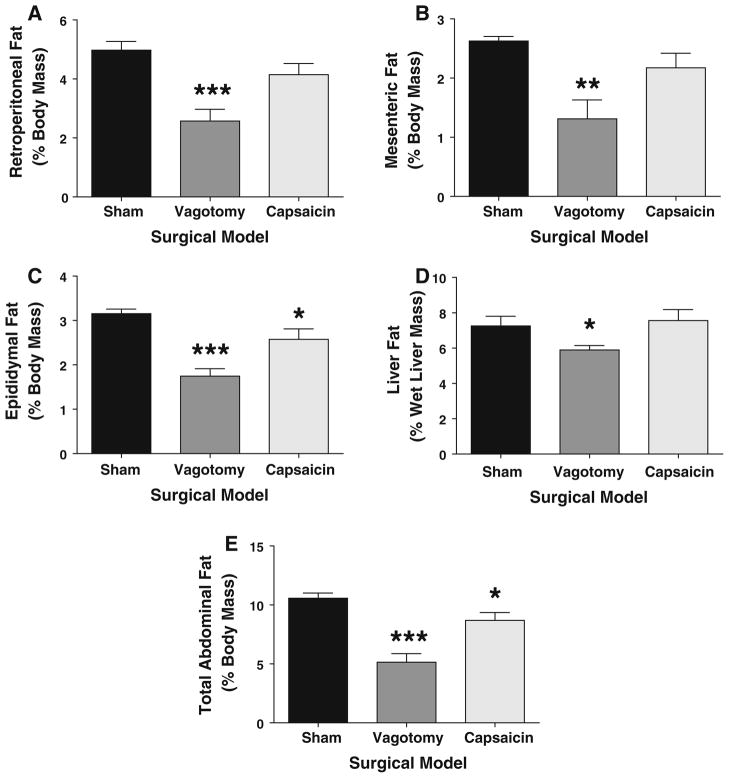
Vagotomy and De-afferentation Reduce Abdominal Fat Deposition
Vagotomized animals had significantly reduced intra-abdominal fat deposits compared to shams (see Figs. 2 and 3a–c for representative images). Retroperitoneal fat mass was reduced by 48% in vagotomized animals compared to shams (P = 0.0004; Figs. 2, 3a and 4b). Similarly, mesenteric fat mass was reduced from 2.6 ± 0.1% to 1.3 ± 0.3% of body weight by vagotomy (P = 0.0025, Figs. 3b and 4b), while epididymal fat was reduced after vagotomy by 45% (P < 0.0001, Figs. 3c and 4c). Finally, the fat content of the liver per unit weight was reduced (albeit more modestly than abdominal fat deposits) by 18% after vagotomy (7.3 ± 0.6% to 5.9 ± 0.2% of liver wet mass; P = 0.036, Fig. 4d). The relative hepatomegaly in the sham group translated to 45% more total liver fat compared to the vagotomized group, but this trend was not significant (P = 0.011). Total abdominal fat (mesenteric, epididymal, retroperitoneal, liver fat deposits) was reduced by 52% in vagotomized animals compared to shams (5.2 ± 0.6% vs. 10.6 ± 0.5%, P < 0.0001, Fig. 4e).
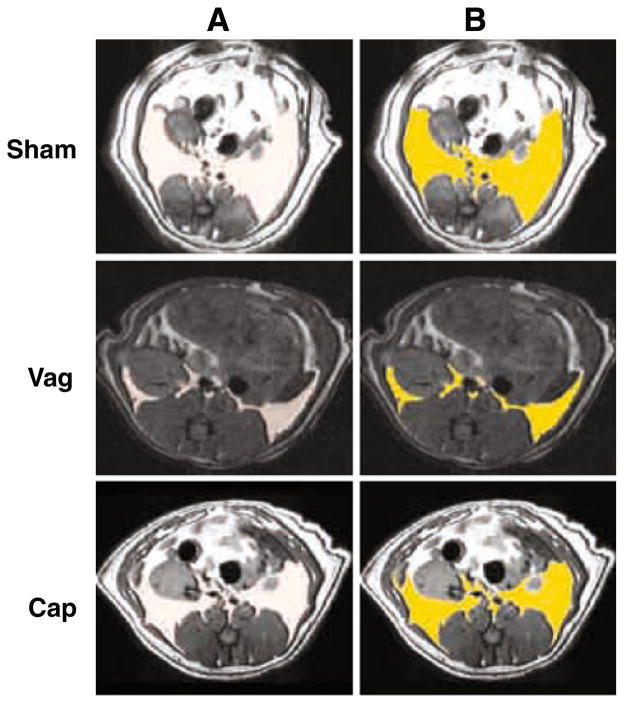
Fig. 2.
Representative magnetic resonance images for each cohort of animals are shown. The section is taken at the level of the upper pole of the left kidney (which, unlike humans is more caudal than the right). In T1-weighted spin imaging, fat density is bright white, while muscle appears dark grey and bone black. In (a), the output image from 3D Slicer is shown. This is falsely coloured in (b) to highlight retroperitoneal fat, with retroperitoneal fat coloured bright yellow. Retroperitoneal fat deposition was reduced in vagotomized (Vag) animals compared to shams (Sham). To a lesser extent, the same was true for de-afferented (Cap) animals versus shams. Images were selected by picking animals with median retroperitoneal fat volumes for their cohort
Fig. 3.
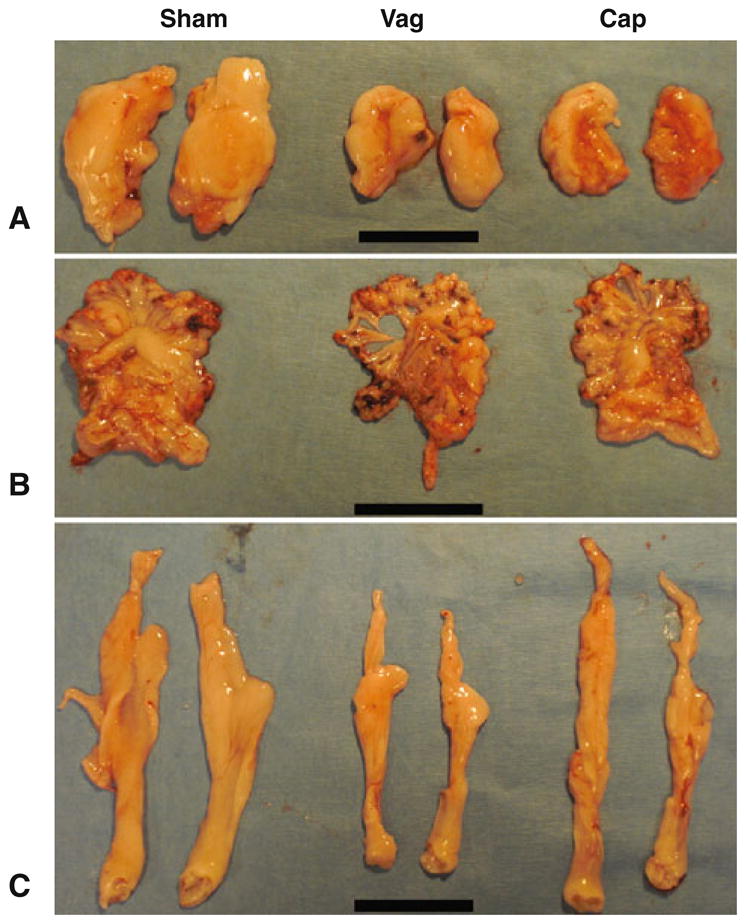
Representative abdominal visceral fat pads as dissected out at harvest. Images were selected by harvesting simultaneously animals with median body weights for their cohort. Cap de-afferented animals, Vag vagotomized animals, Sham shams. Scale bar = 5 cm. a Retroperitoneal fat pads were carefully dissected free for illustrative purposes, although these were not weighed as formal assessment was performed using magnetic resonance imaging. b Mesenteric fat pads, including both the mesentery and omentum. c Epididymal fat pads from each cohort, with clearly reduced fat deposition in both de-afferented and vagotomized animals compared to shams
Fig. 4.
Mean visceral abdominal fat deposition by abdominal fat compartment. Vagotomized animals had significantly less body fat content (as a proportion of body weight) compared to sham animals. This was seen in each intra-abdominal compartment, including mesenteric fat (a), epididymal fat pads (b), retroperitoneal fat pads (c) and total liver fat (d). This translated to 52% reduction in total abdominal visceral fat compared to shams (e). In contrast, de-afferentation only significantly reduced epididymal and total abdominal fat mass (b, e). Compared to shams: * P <0.05; ** P < 0.01; *** P <0.001
De-afferentation led to more modest reductions in body fat content, with trends to reduction in retroperitoneal fat (P = 0.11, Figs. 2, 3a and 4a) and mesenteric fat (P = 0.09, Figs. 3b and 4b) content in comparison with shams. There was no significant reduction in liver fat compared with shams (Fig. 4d). Epididymal fat mass did however significantly decline after de-afferentation (P = 0.0412, Figs. 3c and 4c), an 18% reduction. Total abdominal fat also significantly declined, with an 18% reduction (P = 0.039, Fig. 4e) compared to shams; this was also significantly different to vagotomized animals (P = 0.006).
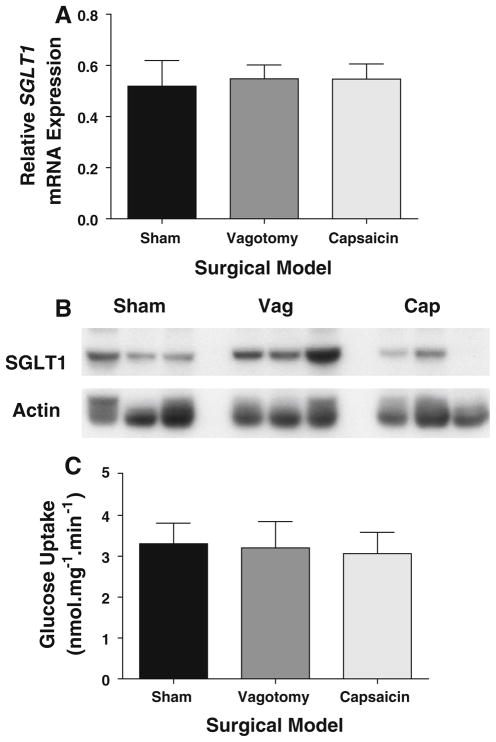
Body Mass Composition Changes Are Independent of Intestinal Glucose Handling
As we have previously reported changes in intestinal SGLT1 expression after afferent vagotomy, we measured SGLT1 transcription, expression and function. SGLT1 transcription was similar among the three surgical models (P > 0.8, Fig. 5a). Similarly, there was no significant difference among arms on protein abundance as measured by immunoblotting; however, vagotomized animals did trend towards higher whole-cell SGLT1 expression (P = 0.15) compared to shams. In contrast, de-afferented animals trended towards a lower expression of SGLT1 compared to vagotomized animals, although again this did not reach significance (P > 0.08), either compared to shams or vagotomized animals. Representative blots are shown in Fig. 5b. Functional glucose transport assays supported these findings: glucose uptake capacity in sham animals was 3.3 ± 0.5 nmol.mg−1 min−1 (Fig. 5c) with no significant difference between vagotomized and de-afferented animals (P > 0.7; 3.2 ± 0.6 and 3.1 ± 0.5 nmol mg−1 min−1, respectively). Similarly, there were no significant differences for fasting blood glucose levels (P > 0.2, data not shown), nor for Hba1C (P > 0.3; 5.5 ± 0.4% vs. 5.6 ± 0.2% for sham and vagotomized animals, respectively). This suggests that in these non-diabetic obese animals, glucose metabolism remains normal after vagal denervation procedures.
Fig. 5.
SGLT1 mRNA, as determined by qPCR relative to Actin, was unchanged between the three cohorts (a). This was reflected by unchanged levels of SGLT1 protein on Western blotting (b) and very similar levels of functional glucose transport capacity (c) as determined by radiolabelled glucose uptake by everted sleeves. Cap de-afferented animals, Vag vagotomized animals, Sham shams
Discussion
We have shown that total vagotomy leads to a significant reduction in age-associated obesity, as measured both by total body weight, and by abdominal fat assessments, with 52% reduction in visceral fat content compared to shams. Hepatic steatosis was also reduced by 31%. In contrast, vagal de-afferentation led to more modest changes in obesity as assessed by total body weight and individual abdominal fat measures. Nevertheless, it does achieve a significant reduction in total visceral fat, with an 18% reduction in fat mass per unit weight.
These results support previous findings showing that vagotomy enhances weight loss, both as a unique weight loss procedure [2–4, 14, 15], and as an adjunct to other bariatric procedures such as gastric banding or sleeve gastrectomy [16, 17]. Indeed, these data from rodents (showing an 18% body weight reduction) match remarkably well with the data from these human trials. Our results for capsaicin also support the much smaller body of extant work with long-term survival after de-afferentation in rodents, with previous reports suggesting reductions in body-weight and fat deposition [10, 18]. Our experiments further the field by providing more detailed analysis of the changes in fat distribution and intestinal glucose transport.
Of particular interest are our data showing a preferential reduction in visceral fat following both vagotomy and de-afferentation; in both procedures visceral fat reduction was achieved at a higher percentage than the reduction in total weight. Although the reduction in visceral fat following de-afferentation may seem rather modest, it is worth noting that visceral abdominal fat content is highly associated with metabolic syndrome [19, 20], dyslipidemia [20, 21], atherosclerotic disease [22], insulin resistance [23] and all cause mortality [24]. In this context, even a relatively small reduction in visceral abdominal fat can lead to significant reductions in morbidity. This is made more profound by the exponential nature of the relationship between visceral fat content and mortality, such that in patients with very high levels of visceral abdominal fat, even small changes in visceral fat mass may lead to large changes in overall risk of death [24]. In fact many of the metabolic improvements seen after weight loss surgery are linked to changes in visceral fat rather than total weight; in a study of patients 6 months after laparoscopic gastric banding, there were significant improvements in many cardiovascular and metabolic risk markers, which was strongly associated with visceral fat reduction, but not total body weight loss [25].
It seems clear from these results that vagal de-afferentation alone has an effect on body fat deposition, although this is not to the same extent as truncal vagotomy. This implies that both efferent and afferent vagal fibers contribute to the resistance to development of age-associated obesity observed in rats. Our data however has some limitation as we did not confirm the completeness of our truncal or afferent vagotomy. We have previously validated our surgical technique for the truncal vagotomy using fluorogold, demonstrating very high rates of completeness [6]. To avoid the stress of this procedure in the rodents in the last week of life when they were also subjected to MR scans, we omitted this confirmatory test. However, given the prolonged follow-up period, a degree of vagal truncal regeneration is possible. Other groups have examined neural regeneration after truncal vagotomy, and have shown afferent fibers, unlike efferent fibers, can regenerate up to 45 weeks post-vagotomy in rats and mice [26–28]. However, this is incomplete and not comparable to the intact animal [27].
One limitation of our study is that we did not perform confirmatory tests for our selective de-afferentation, using tests such as the CCK suppression test [29]. Although we have previously reported our technique for successful selective deafferentation with capsaicin, the follow-up period for those studies was shorter. Acknowledging this limitation, it should however be pointed out that incomplete deafferentation, or regeneration of fibers, would be expected to reduce the efficacy of these procedures, hence biasing the results towards accepting the null hypothesis. Significant results were obtained despite this possibility.
The mechanisms by which either neural pathway might be acting remain unclear. It has been long appreciated that truncal vagotomy reduces food intake in rats [30]. Although gastric distension is a pronounced feature of such animals, delayed gastric emptying does not seem to be the etiological factor, as evidenced by no change with pyloroplasty [30]. Indeed, in our animals (at least at the end of the study), there was no difference in quantity of food consumed per unit body weight by vagotomized animals compared to shams. It would have been interesting to review the quantity of food consumed at earlier time points during the experiment, but unfortunately these data were not collected.
The mechanisms underlying the effect of vagal de-afferentation on weight-loss remain unclear. However, it is becoming increasingly recognized that vagal afferents play an important part in energy homeostasis. The nucleus tractus solitarius, the vagal afferent nucleus, senses afferent inputs to regulate energy balance [31, 32]. Some of these afferent inputs convey satiety, through CCK-mediated stimulation, and thus their ablation would be assumed to impart an adverse effect on weight. However these pathways seem to be dysregulated in obesity, and vagal afferent fibers also appear to play a role in other pathways that could be beneficial targets in fighting obesity. For example, preliminary reports have suggested that intact intestinal mucosal afferent fibers are required for the normal accumulation of fat in visceral tissues [33]. Supporting this, there are early descriptions of weight loss accompanying chronic cervical vagal stimulation as a treatment for major depression [34]. It should be highlighted that we took several steps to ensure that capsaicin was selectively demyelinating the vagal afferent fibers, without a more systemic impact. To achieve this, we avoided systemic, or intra-peritoneal injection of capsaicin. Capsaicin was applied topically to the nerve and the gastroesophageal junction wrapped in parafilm, before carefully lavaging the peritoneal cavity at the end of the procedure to ensure there was no peritoneal spillage or contamination.
We found no changes in intestinal SGLT1 expression or glucose transport capacity after either vagotomy or de-afferentation. This was not however surprising, as we were using a non-diabetic rat model. Unlike such disease models, where there is an elevation of SGLT1 expression and therefore vagotomy may have a therapeutic role by reducing the baseline expression, such finding was not expected in a non-disease model. Although we, and others, had previously shown disruption of the circadian rhythm of SGLT1 expression in normal rodents with vagal manipulation, these experiments were performed at a single time point and thus circadian disruptions were not assessed. We were thus encouraged to see that in a non-diabetic model, vagal manipulation does not adversely impact intestinal glucose transport. The data also suggest that the observed weight loss is unlikely to be due to changes in carbohydrate absorption. This is due to functional data presented, as well as lack of diarrhea seen. It should however be also considered that either a non-neurally mediated adaptive process has returned glucose transport function to that of shams, or that vagal afferents have regenerated as discussed earlier. Furthermore, we used aged rats in this study. Aging has been shown to reduce intestinal glucose transport both in rats [35] and humans [36]. Indeed, the intestine in this study was notably very atrophic, with relatively low glucose transport rates of 3.1–3.3 nmol mg−1 min−1 observed compared to rates approximating 4.5 nmol mg−1 min−1 in historical controls (young sham-operated animals maintained on high-carbohydrate high-fat diet) [37]. Thus, it is possible that both aging and vagotomy cause a loss of some of the reserve capacity for glucose transport. Given that intestinal glucose transport is up-regulated in T2DM by three- to four-fold [38], it is now important to repeat these experiments in a T2DM model to examine whether de-afferentation or vagotomy influences or normalizes intestinal glucose transport during these disease states. Indeed, this may explain the greater magnitude of effect of vagal de-afferentation in Zucker rats, both in terms of weight [18] and glucose homeostasis [18, 39–41], compared to our data reported here.
Of note, the test diet provided to the animals was both rich in simple sugars and in fats, representing the typical Western diet. It is possible that the fat content influenced the results we observed, either reducing or increasing the weight loss effects. In particular, experiments using knockout mice for the neuronal vanilloid receptor TRPV1 (which mediates the acute effects of capsaicin, and is present on capsaicin-sensitive afferents) are resistant to diet-induced obesity only in the context of a high-fat diet [42]. It clearly will be important to examine whether capsaicin-sensitive vagal afferents confer the same susceptibility to diet-induced obesity with differing dietary constituents, for example, high-sugar versus high-fat.
Summary
In summary, we have shown that both efferent and afferent vagal neural pathways are involved in the development of diet-induced obesity in the context of a high-carbohydrate high-fat diet. In particular, both influence abdominal visceral adiposity, with consequent implications for the risk of development of metabolic syndrome and cardiovascular disease.
Acknowledgments
Funding sources include: Harvard Clinical Nutrition Center Grant P30-DK040561 (AT); Berkeley Fellowship and George Herbert Hunt Travelling Fellowship (ATS); and Nutricia Foundation Fellowship (AB). We would like to thank Dr Sharon Peled, and Dr Clare Tempany-Afdhal for technical advice and support with magnetic resonance image acquisition, and Jan Rounds for invaluable managerial support.
Footnotes
Conflict of interest No authors have any conflicts of interest to declare.
Contributor Information
A. T. Stearns, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA. Department of Physiology, Anatomy and Genetics, University of Oxford, South Parks Road, Oxford, UK
A. Balakrishnan, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA. Division of Gastroenterology, School of Clinical Sciences, University of Liverpool, Crown Street, Liverpool L69 3GE, UK
A. Radmanesh, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA
S. W. Ashley, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA
D. B. Rhoads, Pediatric Endocrine Unit, MassGeneral Hospital for Children, Harvard Medical School, Fruit Street, Boston, MA 02114, USA
A. Tavakkolizadeh, Email: atavakkoli@partners.org, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Boss TJ, Peters J, Patti MG, Lustig RH, Kral JG. Laparoscopic truncal vagotomy for severe obesity: six month experience in 10 patients from a prospective, two-center study. Surg Obes Relat Dis. 2007;3:292. [Google Scholar]
- 3.Kral JG. Vagotomy for treatment of severe obesity. Lancet. 1978;1:307–308. doi: 10.1016/s0140-6736(78)90074-0. [DOI] [PubMed] [Google Scholar]
- 4.Kral JG. Effects of truncal vagotomy on body weight and hyperinsulinemia in morbid obesity. Am J Clin Nutr. 1980;33:416–419. doi: 10.1093/ajcn/33.2.416. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Stearns AT, Balakrishnan A, Rounds J, Rhoads DB, Ashley SW, Tavakkolizadeh A. Capsaicin-sensitive vagal afferents modulate posttranscriptional regulation of the rat Na+/glucose cotransporter SGLT1. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1078–G1083. doi: 10.1152/ajpgi.00591.2007. [DOI] [PubMed] [Google Scholar]
- 7.Martin MG, Lostao MP, Turk E, Lam J, Kreman M, Wright EM. Compound missense mutations in the sodium/D-glucose cotransporter result in trafficking defects. Gastroenterology. 1997;112:1206–1212. doi: 10.1016/s0016-5085(97)70132-x. [DOI] [PubMed] [Google Scholar]
- 8.Johnston KL, Clifford MN, Morgan LM. Possible role for apple juice phenolic, compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J Sci Food Agric. 2002;82:1800–1805. [Google Scholar]
- 9.Rossetti L, Smith D, Shulman GI, Papachristou D, Defronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melnyk A, Himms-Hagen J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes Res. 1995;3:337–344. doi: 10.1002/j.1550-8528.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 11.Balakrishnan A, Stearns AT, Rounds J, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1) Surgery. 2008;143:813–818. doi: 10.1016/j.surg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakk-olizadeh A. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery. 2009;145:294–302. doi: 10.1016/j.surg.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Kral JG, Gortz L. Truncal vagotomy in morbid obesity. Int J Obes. 1981;5:431–435. [PubMed] [Google Scholar]
- 15.Kral JG. Vagotomy as a treatment for morbid obesity. Surg Clin North Am. 1979;59:1131–1138. doi: 10.1016/s0039-6109(16)41991-2. [DOI] [PubMed] [Google Scholar]
- 16.Kral JG, Gortz L, Hermansson G, Wallin GS. Gastroplasty for obesity: long-term weight loss improved by vagotomy. World J Surg. 1993;17:75–78. doi: 10.1007/BF01655710. [DOI] [PubMed] [Google Scholar]
- 17.Angrisani L, Cutolo PP, Ciciriello MB, et al. Laparoscopic adjustable gastric banding with truncal vagotomy versus laparoscopic adjustable gastric banding alone: interim results of a prospective randomized trial. Surg Obes Relat Dis. 2009;5:435–438. doi: 10.1016/j.soard.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari B, Arnold M, Carr RD, et al. Subdiaphragmatic vagal deafferentation affects body weight gain and glucose metabolism in obese male Zucker (fa/fa) rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1027–R1034. doi: 10.1152/ajpregu.00736.2004. [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. 2007;116:39–48. doi: 10.1161/circulationaha.106.675355. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–E1071. doi: 10.1152/ajpendo.00442.200200442.2002. [DOI] [PubMed] [Google Scholar]
- 21.Banerji MA, Buckley MC, Chaiken RL, Gordon D, Lebovitz HE, Kral JG. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obes Relat Metab Disord. 1995;19:846–850. [PubMed] [Google Scholar]
- 22.Diamant M, Lamb HJ, van de Ree MA, et al. The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1495–1501. doi: 10.1210/jc.2004-1579. [DOI] [PubMed] [Google Scholar]
- 23.Despres JP, Lemieux S, Lamarche B, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19:S76–S86. [PubMed] [Google Scholar]
- 24.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 25.Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47–55. doi: 10.1007/s11695-008-9642-4. [DOI] [PubMed] [Google Scholar]
- 26.Powley TL, Chi MM, Baronowsky EA, Phillips RJ. Gastrointestinal tract innervation of the mouse: afferent regeneration and meal patterning after vagotomy. Am J Physiol Regul Integr Comp Physiol. 2005;289:R563–R574. doi: 10.1152/ajpregu.00167.2005. [DOI] [PubMed] [Google Scholar]
- 27.Phillips RJ, Baronowsky EA, Powley TL. Long-term regeneration of abdominal vagus: efferents fail while afferents succeed. J Comp Neurol. 2003;455:222–237. doi: 10.1002/cne.10470. [DOI] [PubMed] [Google Scholar]
- 28.Phillips RJ, Baronowsky EA, Powley TL. Regenerating vagal afferents reinnervate gastrointestinal tract smooth muscle of the rat. J Comp Neurol. 2000;421:325–346. doi: 10.1002/(SICI)1096-9861(20000605)421:3<325:AID-CNE3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol. 1985;248:R501–R504. doi: 10.1152/ajpregu.1985.248.4.R501. [DOI] [PubMed] [Google Scholar]
- 30.Mordes JP, Lozy ME, Herrera MG, Silen W. Effects of vagotomy with and without pyloroplasty on weight and food-intake in rats. Am J Physiol. 1979;236:R61–R66. doi: 10.1152/ajpregu.1979.236.1.R61. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 32.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 33.Leung FW, Go VL, Scremin OU, et al. Pilot studies to demonstrate that intestinal mucosal afferent nerves are functionally linked to visceral adipose tissue. Dig Dis Sci. 2007;52:2695–2702. doi: 10.1007/s10620-006-9645-8. [DOI] [PubMed] [Google Scholar]
- 34.Pardo JV, Sheikh SA, Kuskowski MA, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond) 2007;31:1756–1759. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esposito G, Faelli A, Tosco M, Orsenigo MN, Battistessa R. Age-related changes in rat intestinal transport of D-glucose, sodium, and water. Am J Physiol. 1985;249:G328–G334. doi: 10.1152/ajpgi.1985.249.3.G328. [DOI] [PubMed] [Google Scholar]
- 36.Vincenzini MT, Iantomasi T, Stio M, et al. Glucose transport during ageing by human intestinal brush-border membrane vesicles. Mech Ageing Dev. 1989;48:33–41. doi: 10.1016/0047-6374(89)90023-7. [DOI] [PubMed] [Google Scholar]
- 37.Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297:G950–G957. doi: 10.1152/ajpgi.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 39.Gram DX, Ahren B, Nagy I, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 40.Gram DX, Hansen AJ, Wilken M, et al. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol. 2005;153:963–969. doi: 10.1530/eje.1.02046. [DOI] [PubMed] [Google Scholar]
- 41.Gram DX, Hansen AJ, Deacon CF, et al. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases insulin secretion in Zucker Diabetic Fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase IV. Eur J Pharmacol. 2005;509:211–217. doi: 10.1016/j.ejphar.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]