Abstract
The CAS family of scaffolding proteins has increasingly attracted scrutiny as important for regulation of cancer-associated signaling. BCAR1 (also known as p130Cas), NEDD9 (HEF1, Cas-L), EFS (Sin), and CASS4 (HEPL) are regulated by and mediate cell attachment, growth factor, and chemokine signaling. Altered expression and activity of CAS proteins is now known to promote metastasis and drug resistance in cancer, influence normal development, and contribute to the pathogenesis of heart and pulmonary disease. In this article, we provide an update on recently published studies describing signals regulating and regulated by CAS proteins, and evidence for biological activity of CAS proteins in normal development, cancer and other pathological conditions.
Comprehensive reviews in the past several years have discussed the important roles for the CAS (BCAR1, NEDD9, EFS, and CASS4) proteins in tumorigenesis and other pathological states (1–3). In this article, we provide an update on regulation of CAS proteins and interaction with partners important for CAS signaling, focusing most discussion on recently published papers. Most of this discussion focuses on BCAR1 and NEDD9, as only a limited number of studies have so far addressed the activity of CASS4/HEPL (4) and EFS/SIN (5). In brief introduction, CAS proteins have an amino terminal SH3 domain, an adjacent unstructured domain (substrate domain [SD]) containing multiple tyrosine phosphorylation sites that enable binding by SH2-domain containing proteins, a four-helix bundle (serine-rich region [SR]), and a second, highly conserved four-helix bundle (focal adhesion targeting [FAT] domain) (Figure 1A) (1, 28). Most of these domains mediate protein-protein interactions, causing the primary role of CAS proteins to be scaffolds regulating the magnitude and duration of cell signaling cascades. Initial studies of these proteins emphasized their roles as intermediaries in integrin-dependent signal transduction. Specifically interactions of CAS proteins with Focal Adhesion Kinase (FAK) and SRC family proteins at focal contacts to the extracellular matrix transmitted signals downstream that induced lamellipodia and cell migration, supported cell proliferation, and blocked anoikis. More recent work has demonstrated that CAS proteins are induced by multiple upstream stimuli, including hypoxia and activation of receptor tyrosine kinases (RTKs), and have additional functions in cell cycle, cell junctional control, and other processes, as discussed below. CAS proteins are rarely mutated, but frequently show altered expression or phosphorylation (associated with increased activity) in pathological conditions including immune cell dysfunction and cancer.
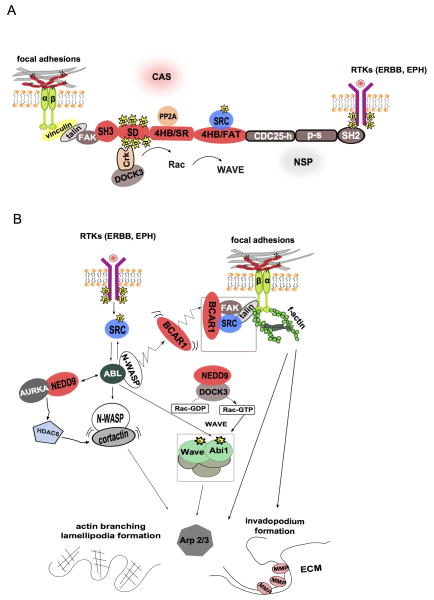
Figure 1. CAS/NSP interactions and CAS mediated pathways.
A. Structure and established interactions of CAS and NSP proteins. Binding of RTKs (ERbB and EPH) to the SH2 domain of NSP proteins leads to extracellular signal transduction through CAS proteins. β-integrin signaling is regulated by RTKs via the NSP proteins. B. Signaling interactions between BCAR1, NEDD9, and regulators of the cytoskeleton. See text for details.
CAS activation and signaling interactions
Phosphorylation of CAS proteins accompanies and is essential for the processes of cell adhesion, spreading, formation of lamellipodia (for migration) and maturation of invadopodia (for cancer cell invasion) (1). To date, the signal pathways regulating those processes appear to be similar. Upon activation of integrins, RTKs, or chemokine receptors or other upstream signals such as hypoxia, CAS proteins are direct targets of effectors in these signaling cascades such as the non-receptor focal adhesion kinases (FAK), SRC family kinases (SFKs), and ABL, with opposing signaling provided by cellular phosphatases (Figure 1B).
FAK, SRC, and phosphatases
Y925-phosphorylated FAK activates the BCAR1-DOCK180-RAC1 signaling pathway(6); mutating FAK to eliminate its recruitment to focal adhesions lead to a decline of both phospho-SRC and phospho-BCAR1 and reduces migration, cell adhesion and invasion(7). CAS proteins interact with FAK through their SH3 domains: Janoštiak et al have discovered that SH3 domain of BCAR1, phosphorylated on Y12, also binds to a proline-rich sequence of the hinge region of vinculin. This interaction is needed for both BCAR1 localization in focal adhesions, and stretch-induced phosphorylation of BCAR1 on Y410, supporting mechanotransduction (8). Interaction of the BCAR1 N-terminus with a complex containing FAK and N-WASP led to phosphorylation of the BCAR1 SD during cell spreading of fibronectin, dependent on actin polymerization and activity of SFKs. Both inhibition of N-WASP complex formation and inhibition of the downstream Arp2/3 complex blocked BCAR1 phosphorylation, confirming its role as responsive to mechanical stretching(9). SFK phosphorylation of Y189 on NEDD9 is analogous to Y253 phosphorylation on BCAR1, and is important for NEDD9 control of focal adhesion dynamics and cell migration (10).
Regulation of CAS protein functions depends on a balanced action of protein tyrosine kinases, protein tyrosine phosphatases, and interacting proteins that regulate the stability of these proteins based on their phosphorylation state. SRC kinase also phosphorylates BCAR1 at Y128, and colon cancer cell lines with high levels of BCAR1 phosphorylated at this site are highly sensitive to the SRC inhibitor dasatinib (11). Protein tyrosine phosphatase non-receptor 14 (PTPN14) was for the first time identified as a direct BCAR1 interactor in vitro, and PTPN14 was shown to dephosphorylate BCAR1 at the Y128 site (12). Another study has identified phosphorylated BCAR1 as a substrate of SOCS6 protein, an adaptor of Cullin-5-RING-E3-ubiquitin ligase complexes (Cul5-CRLs). SOCS6 binding to SRC-phosphorylated BCAR1 targets it for degradation by Cul5-CRLs and inhibits membrane ruffling (13).
Western analysis of NEDD9 typically detects two phosphorylated isoforms, migrating at 105 kDa and 115 kDa; the 115 kDa form is typically associated with integrin-mediated adhesion, cell spreading and mesenchymal motility. Estradiol (E2) promoted and tamoxifen inhibited the accumulation of the less phosphorylated 105 kDa NEDD9 isoform, with accumulation of this form associated with failed cell spreading(14). The phosphatase PP2A has been proposed to mediate the 115 to 105 kDa interconversion; Bradbury et al disproved this idea, but showed that PP2A interacts with NEDD9 to limit cell spreading and induce a mesenchymal cell morphology by binding to the NEDD9 serine-rich region/four-helix bundle, with residue S369 important for this interaction (15).
Karginov at al described an elegant technical approach involving rapid, rapamycin-regulated activation of SRC targeted selectively to individual targets, specifically in the membrane or cytoplasm. Interestingly enough, SRC activation in the cytoplasm was enough to cause membrane protrusions formation that were stable even after prolonged activation but did not result in cell spreading. Activating membrane-anchored SRC, on the other hand, leads to cell spreading and adhesions induction. Specific activation of SRC by BCAR1 induced and lengthened filopodial and the number of focal adhesions, increased cell spreading and produced a limited burst of cell protrusions. In contrast, activation by FAK did not affect filopodia or protrusions, but altered the morphology of focal adhesions, and increased cell spreading (16).
ABL and the actin cytoskeleton
Although ABL was the first kinase shown to interact with NEDD9 (17), this interaction received relatively little subsequent study in comparison to the much more studied interactions of CAS proteins with FAK and SFKs. The BCR-ABL fusion protein formation drives chronic myelogenous leukemia CML, while non-fused ABL1 and ABL2 contribute to the development of many solid tumors (18). Crk-like protein (CrkL) is an important in vivo partner and substrate for the BCR-ABL tyrosine kinase, acting as a molecular linker connecting BCR-ABL with downstream signaling partners that bind the CrkL SH3 and SH2 domains. In the leukemic tissues of transgenic mice expressing BCR-ABL, NEDD9 is hyperphosphorylated and associated with CrkL in complex with BCR-ABL (19). ABL activation downstream of RTKs and chemokine receptors induces reorganization of the actin cytoskeleton and cellular invasion (Figure 1). This last process includes the formation of ABL2 invadopodia, actin-rich protrusive structures in cancer cells that promote remodeling of the extracellular matrix during tumor invasion, and are necessary for secretion of matrix metalloproteases. In the past several years, the intimate connection of these activities with NEDD9 and other CAS proteins has become apparent. Abl interactor 1 (ABI1) is a component of the WAVE protein complex. An interaction between NEDD9 and DOCK3 activates the RAC and WAVE proteins to decrease actomyosin contractility, promoting mesenchymal movement and invasion (20). ABI1 also regulates the interaction of ABL and BCAR1 (21), and BCAR1 has long been known to be regulated by BCR-ABL (22).
ABL interacts with N-WASP, noted above as recruiting BCAR1 to focal adhesions, and is likely to also interact with NEDD9, given the similarity between NEDD9 and BCAR1 in the interaction motifs (23, 24, 1). ABL also interacts with cortactin, a lamellipodially concentrated protein that activates both actin nucleation and F-actin branch juncture stabilization by binding to the ARP2/3 protein complex. These interactions provide a direct means for NEDD9 and ABL to collaboratively regulate activity of ARP2/3, which control actin polymerization in lamellipodial formation. Another group showed that BCAR1, the focal adhesion protein talin, and FAK regulate Arp2/3-independent cytoskeletal protrusions in fibrosarcoma cells (25).
A significant finding regarding NEDD9 in the past several years is the recognition that NEDD9 regulates cortactin through control of AURKA and HDAC6 (Figure 1) (26). Dynamic regulation of cortactin function is executed through the acetylation process: deacetylated cortactin is incapable of F-actin binding, and cannot support cell motility. Histone deacetylase 6 (HDAC6) deacetylation of cortactin restores its actin-binding capacity, stabilizing lamellipodia and increasing cell migration. NEDD9 had previously been shown to interact with and activate AURKA (27), which in turn activates HDAC6 (28), in control of cell cycle and ciliary dynamics. Kozyreva et al now show that NEDD9 or inhibition of AURKA and HDAC6 causes hyperacetylated cortactin or decreases the metastatic capability of NEDD9-overexpressing breast cancer cells, which implicates this protein as critical effectors of NEDD9 driven metastasis (26). NEDD9 activation by ABL was also shown to occur in the context of chemokine induction, and to be necessary for T cell migration (29). Whether these activities involving HDAC6 and cortactin also apply to other CAS proteins is currently unknown.
Complementing this work on the interaction between CAS proteins and direct regulation of the actin cytoskeleton, Yamauchi et al established a novel linkage between BCAR1 and the mitochondrial protease HtrA2/Omi. In this study, the authors found that oncogenic RAS transformation of cells induce accumulation of cytoplasmic p53, which signals through p38 to induces HtrA2/Omi phosphorylation concurrent with mitochondrial fragmentation. These processes release HtrA2/Omi into the cytoplasm, inducing F-actin disassembly thereby inhibiting BCAR1 activity in promoting formation of lamellipodia and the invasion of mouse embryonic fibroblasts (30). These connections with p53, p38, and RAS are likely to be relevant in the other settings of NEDD9 and BCAR1-dependent carcinogenesis and metastasis discussed below.
CAS/NSP interactions
One group of proteins emerging in the past several years of interest to CAS proteins is the novel SH2-containing protein family (NSPs)(31). Like the CAS proteins, the three NSP proteins are multidomain scaffolds (Figure 1A). Of the three family members (NSP1/SH2D3A; NSP2/BCAR3/AND-34/SH2D3B; NSP3/SHEP1/CHAT/SH2D3C), each contains an N-terminal SH2 domain and a C-terminal Cdc25-homology fold resembling the RAS guanine nucleotide exchange factor (GEF), although lacking any enzymatic activity due to a closed 3D conformation (32). In response to extracellular stimuli, NSP proteins bind through their SH2 domains to the phosphosites of activated RTKs, and recruit CAS proteins through interactions between the Cdc25-like domain of the NSP and the four-helix bundle (also known as focal adhesion targeting or FAT domain) of CAS proteins, directly connecting CAS proteins to RTK signaling (33). Both NEDD9 (34, 35) and BCAR1 (34, 36) interact with NSP2 and NSP3. At least some of the integrated signaling between these proteins is important for resistance to anti-estrogens (36), although it is clear that the NSPs also have CAS-independent functions (37).
CAS proteins in development
Providing context for these findings, both Nedd9−/− and Sin−/− mice are vital and fertile, but have immunological abnormalities that result in pre-malignant conditions later in life. For Nedd9−/− mice, defects are initially subtle, but increase in later life; B cell homing to the spleen and lymphocyte trafficking are deficient (38, 39). In Sin−/− mice, thymocyte development is normal, but T-cell mediated immune response are enhanced and aged knockout mice develop severe T-cell infiltrated inflammatory lesions and tissue damage (40). BCAR1 null mice are embryonal lethals (41). To date, there is no knockout mouse for CASS4/HEPL.
BCAR1 in myogenesis
BCAR1 was identified as a negative regulator of the transcriptional factor Lim domain only 7 (Lmo7), inhibiting its activity in regulating genes important for development of muscles and heart(42). BCAR1 depletion in C2C12 myoblast cells inhibited F-actin assembly and disrupted the nuclear translocation of MAL, a co-activator for the serum response factor (SRF), inducing myogenic differentiation (43). BCAR1 also regulates myoblast determination protein 1 (MyoD1) has been described (44). Like MyoD1, the CAS proteins contain functionally active helix-loop-helix (HLH) domains in their C-terminal regions (45). Under some circumstances, CAS proteins are cleaved to release short C-terminal products (46, 44). HLH-mediated inhibitory binding of the 31-kDa BCAR1 cleavage product to MyoD1 activated myogenesis (44). Surprising, however, a recent genetic study found no significant defects in mice with a muscle-specific deletion of BCAR1 (47); more investigation is needed.
CAS proteins in neural differentiation
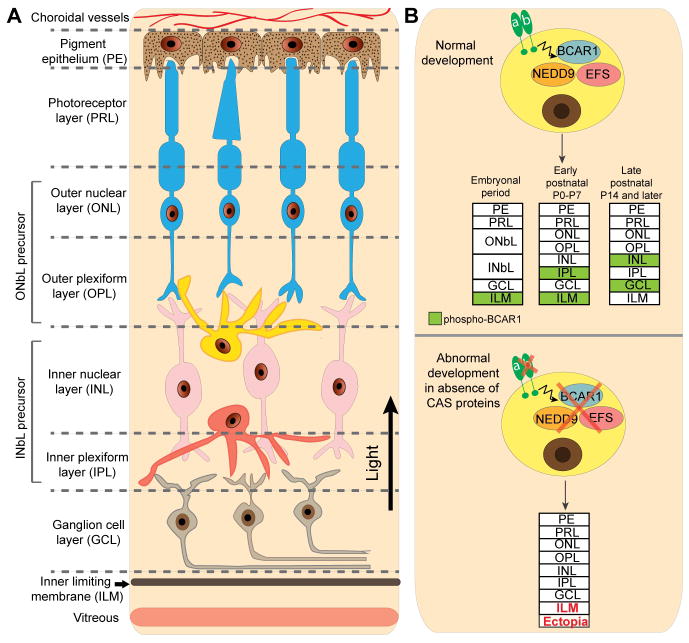
NEDD9 is expressed in neural progenitor cells and regulates the process of neuronal crest cell migration, and cellular differentiation, mediating integrin and TGF-β signaling (48, 49). Recent work from the Kolodkin group (50) has now found multiple CAS proteins (NEDD9, BCAR1 and EFS/SIN) have overlapping expression patterns and are each required in the outer and inner neuroblast layers (ONbL and INbL) during early retinal development (Figure 2). In contrast, detailed investigation of BCAR1 shows dynamic localization of the phosphorylated form during the stages of retinal development. BCAR1 phosphorylation is β1-integrin dependent. Mice with a conditional retinal neuron-specific knockout of BCAR1 do not experience appreciable developmental defects in retina development, but a combined triple conditional knockout of BCAR1 with NEDD9null and SINnull alleles resulted in dramatic disruption GCL structure, with formation of ectopic ganglion cell mass beyond the ILM, phenocopying the effect of loss of the β1-integrin (50). A separate study suggests that BCAR1 is a target of the SRC-family FYN kinase that is required for oligodendrocyte process outgrowth, cell migration and cell survival, with long term reduction of BCAR1 resulting in increased apoptosis; this implies a role for CAS proteins in myelin production, as myelination depends on functioning oligodendrocytes (51).
Figure 2. CAS proteins in retinal development.
Total BCAR1 is broadly expressed in the inner neuroblastic layer (INbL), but localization of phosphorylated BCAR1 changes during different stages of the retinal development. During embryonal development, phosphorylated BCAR1 is mainly accumulated close to the inner limiting membrane (ILM). In early neonatal period (postnatal days 0 to 7) it is detected in close proximity to ILM and forming inner plexiform layer (IPL). At the later developmental stages (starting P14 and later), phosphorylated BCAR1 becomes restricted to the ganglion cell layer (GCL) and a subset of the cells in the inner nuclear layer (INL).
A. Cell layers of the developing retina, shown at post-natal day P14 or later. B. Top, Changing expression of phosphorylated BCAR1 (green highlight). Bottom, in the absence of CAS proteins, growth of the ILM is disrupted, and ectopic growth is observed in adjacent tissue.
Update on CAS proteins in cancer
Overexpression of CAS proteins is common in and actively promotes the invasion and metastasis of many types of solid tumor. The seminal studies have been reviewed at length (52, 53, 1): we here highlight recent findings.
Hematological malignancies
Overexpression of BCAR1 in promyelocytic leukemia HL-60 cells induced cell adhesion and aggregation. The differentiation-promoting factor PMA induced BCAR1 expression together with its defined transcriptional inducer, EGR1 (Early Growth Response 1), an EGR1 co-regulator (NGFI-A binding protein (NAB2) and found that reciprocally, BCAR1 positively regulates expression of EGR1/NAB2 (54). In a surprising finding, Seo and colleagues analyzed the consequence of deleting NEDD9 in a transgenic model for BCR/-ABL leukemogenesis, and found that the disease was more aggressive in mice lacking NEDD9; the authors proposed this was due to a role of NEDD9 in limiting extramedullary hyperplasia (55).
Bladder and prostate cancers
Increased intercellular reactive oxygen species (ROS) enhanced the BCAR1-dependent invasion and metastasis for bladder cancer cells (56). Morimoto et al. explored the role of NEDD9 in EMT and metastasis in PC-3 prostate cancer cells, and showed activation of invasion and spreading on TGF-β stimulation via a NEDD9 dependent process (57). Interestingly, work performed in hTERT-RPE1 (retinal pigmented epithelial) cells indicated a role of NEDD9 in binding to the TGF-β effector SMAD3, and demonstrated that the Wnt pathway regulator Dvl2 (phosphorylated by S143 and T224) competes with NEDD9 for binding to Smad3, bringing together multiple different oncogenic signaling pathways (58). NEDD9 is overexpressed in prostate tumors versus normal tissue. Tumor tissue from patients with more advanced stage of cancer (Gleason grade 5) are characterized by strong nuclear staining of NEDD9, while in normal prostate cells NEDD9 shows weak cytoplasmic staining (57); this shift in compartmentalization has been seen with other normally non-nuclear oncogenes such as EGFR or AURKA, and may indicate a neomorphic function.
Lung cancer
Recent studies have noted common overexpression of NEDD9 in non-small cell lung cancer (NSCLC), associated with poor prognosis, particularly in metastatic tumors (59–61). One reported mechanism for the elevated expression of NEDD9 is loss of transcriptional repression mediated by the liver kinase B1 (LKB1) tumor repressor, mediated by cytosolic translocation of CRTC1 (62). Elevation of NEDD9 is important for aggressive growth of LKB1-mutated tumors (62), and the NEDD9 partner SRC has been reported to be hyperactivated in these tumors (11). Simultaneous overexpression of LKB1 and another tumor suppressor gene, FUS1, have recently been shown to slow down lung cancer progression in a mouse model, and to downregulate NEDD9 (63). NEDD9 activity in lung cancer metastasis included FAK-mediated activation of EMT process and downregulation of E-cadherin (64). Finally, there was a first report of the overexpression and cytoplasmic accumulation of HEPL/CASS4 in NSCLC, correlated with lymph node metastasis and TNM stage (poor prognosis) (65).
Breast cancer
Nedd9 null mice have now been crossed to two independent models for mammary tumors, driven by the polyoma virus middle T oncogene, and the HER2/neu oncogene, each under the control of the MMTV promoter (MMTV-PyVT and MMTV-HER2). In the MMTV-PyVT mice, the Nedd9 null genetic background significantly increased the latency until tumor appearance (39); in the less aggressive MMTV-HER2/neu model, latency increased, and the incidence of tumors was sharply reduced (66). Analysis of signaling pathways in tumors from Nedd9−/− animals revealed decreased activation of multiple prooncogenic proteins including ERK, AKT, SRC and FAK (39). MMTV-PyVT Nedd9−/− cell lines were particularly sensitive to inhibition of the NEDD9 signaling partner SRC (67). Analysis of isolated mammary luminal progenitor cells from MMTV-HER2/neu mice lacking Nedd9 revealed quantitative reduction in the frequency of these progenitor cells, as well as impaired ability to form mammospheres, downregulation of Nedd9-interacting proteins such as FAK, and sensitization to small molecule inhibitors including those to SRC and FAK (66).
Overexpression of both NEDD9 and BCAR1 has been found to be associated with overexpression or activation of ErbB2 in mammary cells or acini (68, 69). Further, overexpression of BCAR1 increased the pool of luminal progenitor cells, causing aberrant differentiation and features of basal progenitor cells. Mouse mammary epithelial cells (MMEC) with high levels of BCAR1 had increased activity of c-kit (70), and an interaction between BCAR1 and c-kit was found to be crucial for the proper differentiation of MMEC. BCAR1 protein levels were elevated in 75% of analyzed tissue samples from the patients with triple negative breast cancer (44 out of 51) (70).
AURKA, a key partner of NEDD9, is overexpressed in 90% of malignant tumors and this overexpression is associated with poor prognosis (71). Overexpressed NEDD9 stabilized AURKA by limiting its binding to APC/C ubiquitin ligase, and AURKA and NEDD9 had correlated overexpression in breast cancer (72). NEDD9 depletion increases ubiquitination and proteasome-dependent degradation of AURKA, potentially explaining the correlation between NEDD9 expression and resistance to the AURKA inhibitor, alisertib (MLN8237) (72), and tumor aggressiveness (73) in a breast cancer xenograft model.
Cas proteins in other diseases
Beyond the much-studied context of cancer, several recent studies address the role of CAS proteins in other pathological signaling processes.
Pulmonary disease
Tu et al. investigated the role of BCAR1 in pulmonary diseases and showed that BCAR1 is increased in serum as well as in smooth muscle and epithelial cells of distal pulmonary arteries in patients with both heritable and idiopathic pulmonary arterial hypertension (PAH). Treatment of the patient-derived cultured cells with various receptor and non-receptor tyrosine kinase inhibitors (gefitinib, dovitinib, and imatinib) slowed both the cellular growth rate and BCAR1 expression. Furthermore, the authors showed that increased BCAR1 expression correlates with abnormal smooth muscle cell (SMC) and epithelial cell (EC) growth in animal models with monocrotaline or hypoxia-induced PAH. Based on these data, the researches proposed BCAR1 to be an amplifier of downstream RTK signals in pulmonary disease (74).
Coronary artery disease (CAD) and carotid artery atherosclerosis
Gertow et al. performed a meta-analysis of data from the IMPROVE project, focused on defining risk factors for vascular events. The BCAR1-CFDP1-TMEM170A locus was associated with an increase in cIMT (carotid intima-media thickness – a marker of subclinical atherosclerosis) and CAD in patients of European descent (75). Chen et al. proposed that cysteine rich protein 2 (CRP2) sequestered and redistributed BCAR1 in migrating vascular smooth muscle cells, affecting a major pathological step in development of atherosclerosis (31). Interestingly, the set of kinases shown to phosphorylate BCAR-1 has been extended to include Syk, a ZAP-70 related cytoplasmic tyrosine kinase that is essential for regulation of cellular adhesion, innate immune recognition, and vascular development (76).
NEDD9 and ciliopathies?
NEDD9, unique among the CAS group, has a second set of functions involving association with AURKA at the centrosome to regulate mitotic progression (27), and association with AURKA and HDAC6 at a centrosome-derived structure, the ciliary basal body, to regulate resorption of the primary cilium (28). The primary cilium is a non-motile, sensory organelle that is present on most mammalian cells. Ciliary abnormalities are associated with numerous severe “ciliopathies” (77), making these results of potential clinical significance. Extending the connections between NEDD9, ciliary disassembly, and these ciliopathies, Nek2, another NEDD9-regulated cell cycle kinase (27) has now been described to regulate ciliary disassembly (78). Wnt5a is a non-canonical Wnt pathway ligand recently found to induce ciliary disassembly. This activity involves formation of a complex by Dishevelled-2 (Dvl2) and Polo-like kinase 1 (Plk1) that stabilizes NEDD9 and hence activates AURKA (58). Finally, ciliopathies are often associated with abnormal intracellular calcium signaling; NEDD9 interactions with at least one partner, AURKA, were shown to be influenced by association with calcium-calmodulin (79), while NEDD9 regulation of AURKA has been found to influence cellular calcium response (80). It will be of interest to directly evaluate the genetic contribution of NEDD9 to clinically relevant ciliopathies.
Conclusion
Together, these studies indicate a more expansive view of CAS protein function, indicating roles in new biological processes and clarifying signaling interactions. It is likely that more roles will be identified; for example, a recent study identified for the first time a role for BCAR1 in osteoclast function and bone remodeling, opening a new area of activity for the CAS group (81). It also seems likely that one research focus of the next several years will be to shift to exploiting the biology of CAS proteins as prognostic biomarkers for therapeutic response in cancer and in other diseases. We look forward to seeing these developments.
Acknowledgments
The authors were supported by U54 CA149147, R01 CA63366 and P50 CA083638 from the NIH (to EAG), the PKD Foundation, and NIH core grant CA06927 (to Fox Chase Cancer Center).
References
- 1.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cellular and molecular life sciences: CMLS. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrero MS, Parsons Jt, Bouton AH. Cas and NEDD9 Contribute to Tumor Progression through Dynamic Regulation of the Cytoskeleton. Genes Cancer. 2012;5–6:371–380. doi: 10.1177/1947601912458585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P. p130Cas: a key signalling node in health and disease. Cellular signalling. 2013;25:766–777. doi: 10.1016/j.cellsig.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Singh MK, Dadke D, Nicolas E, Serebriiskii IG, Apostolou S, Canutescu A, Egleston BL, Golemis EA. A novel Cas family member, HEPL, regulates FAK and cell spreading. Molecular biology of the cell. 2008;19:1627–1636. doi: 10.1091/mbc.E07-09-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishino M, Ohba T, Sasaki H, Sasaki T. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene. 1995;11:2331–2338. [PubMed] [Google Scholar]
- 6.Deramaudt TB, Dujardin D, Hamadi A, Noulet F, Kolli K, De Mey J, Takeda K, Ronde P. FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion. Molecular biology of the cell. 2011;22:964–975. doi: 10.1091/mbc.E10-08-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deramaudt TB, Dujardin D, Noulet F, Martin S, Vauchelles R, Takeda K, Ronde P. Altering FAK-Paxillin Interactions Reduces Adhesion, Migration and Invasion Processes. PloS one. 2014;9:e92059. doi: 10.1371/journal.pone.0092059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janostiak R, Brabek J, Auernheimer V, Tatarova Z, Lautscham LA, Dey T, Gemperle J, Merkel R, Goldmann WH, Fabry B, Rosel D. CAS directly interacts with vinculin to control mechanosensing and focal adhesion dynamics. Cellular and molecular life sciences: CMLS. 2014;71:727–744. doi: 10.1007/s00018-013-1450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Moore SW, Iskratsch T, Sheetz MP. N-WASP-directed actin polymerization activates p130Cas phosphorylation and lamellipodium spreading. Journal of cell science. 2014 doi: 10.1242/jcs.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baquiran JB, Bradbury P, O’Neill GM. Tyrosine Y189 in the substrate domain of the adhesion docking protein NEDD9 is conserved with p130Cas Y253 and regulates NEDD9-mediated migration and focal adhesion dynamics. PloS one. 2013;8:e69304. doi: 10.1371/journal.pone.0069304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, McNamara KL, Brandstetter KA, Walton ZE, Gu TL, Silva JC, Crosby K, Shapiro GI, Maira SM, Ji H, Castrillon DH, Kim CF, Garcia-Echeverria C, Bardeesy N, Sharpless NE, Hayes ND, Kim WY, Engelman JA, Wong KK. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Guo A, Possemato A, Wang C, Beard L, Carlin C, Markowitz SD, Polakiewicz RD, Wang Z. Identification and functional characterization of p130Cas as a substrate of protein tyrosine phosphatase nonreceptor 14. Oncogene. 2013;32:2087–2095. doi: 10.1038/onc.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teckchandani A, Laszlo GS, Simo S, Shah K, Pilling C, Strait AA, Cooper JA. Cullin 5 destabilizes Cas to inhibit Src-dependent cell transformation. Journal of cell science. 2014;127:509–520. doi: 10.1242/jcs.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradshaw LN, Zhong J, Bradbury P, Mahmassani M, Smith JL, Ammit AJ, O’Neill GM. Estradiol stabilizes the 105-kDa phospho-form of the adhesion docking protein NEDD9 and suppresses NEDD9-dependent cell spreading in breast cancer cells. Biochimica et biophysica acta. 2011;1813:340–345. doi: 10.1016/j.bbamcr.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury P, Mahmassani M, Zhong J, Turner K, Paul A, Verrills NM, O’Neill GM. PP2A phosphatase suppresses function of the mesenchymal invasion regulator NEDD9. Biochimica et biophysica acta. 2012;1823:290–297. doi: 10.1016/j.bbamcr.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Karginov AV, Tsygankov D, Berginski M, Chu PH, Trudeau ED, Yi JJ, Gomez S, Elston TC, Hahn KM. Dissecting motility signaling through activation of specific Src-effector complexes. Nature chemical biology. 2014;10:286–290. doi: 10.1038/nchembio.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law SF, Estojak J, Wang B, Mysliwiec T, Kruh G, Golemis EA. Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae. Molecular and cellular biology. 1996;16:3327–3337. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nature reviews Cancer. 2013;13:559–571. doi: 10.1038/nrc3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong R, van Wijk A, Haataja L, Heisterkamp N, Groffen J. BCR/ABL-induced leukemogenesis causes phosphorylation of Hef1 and its association with Crkl. The Journal of biological chemistry. 1997;272:32649–32655. doi: 10.1074/jbc.272.51.32649. [DOI] [PubMed] [Google Scholar]
- 20.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Cleary RA, Wang R, Tang DD. Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J Biol Chem. 2013;288:20713–20722. doi: 10.1074/jbc.M112.439877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salgia R, Pisick E, Sattler M, Li JL, Uemura N, Wong WK, Burky SA, Hirai H, Chen LB, Griffin JD. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J Biol Chem. 1996;271:25198–25203. doi: 10.1074/jbc.271.41.25198. [DOI] [PubMed] [Google Scholar]
- 23.Alexandropoulos K, Donlin LT, Xing L, Regelmann AG. Sin: good or bad? A T lymphocyte perspective. Immunological reviews. 2003;192:181–195. doi: 10.1034/j.1600-065x.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 24.Donato DM, Ryzhova LM, Meenderink LM, Kaverina I, Hanks SK. Dynamics and mechanism of p130Cas localization to focal adhesions. The Journal of biological chemistry. 2010;285:20769–20779. doi: 10.1074/jbc.M109.091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giri A, Bajpai S, Trenton N, Jayatilaka H, Longmore Gd, Wirtz D. The Arp2/3 complex mediates multigeneration dendritic protrusions for efficient 3-dimensional cancer cell migration. FASEB J. 2013;10:4089–4099. doi: 10.1096/fj.12-224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozyreva VK, McLaughlin SL, Livengood RH, Calkins RA, Kelley LC, Rajulapati A, Ice RJ, Smolkin MB, Weed SA, Pugacheva EN. NEDD9 Regulates Actin Dynamics through Cortactin Deacetylation in an AURKA/HDAC6-dependent Manner. Molecular cancer research: MCR. 2014 doi: 10.1158/1541-7786.MCR-13-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nature cell biology. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu JJ, Lavau CP, Pugacheva E, Soderblom EJ, Moseley MA, Pendergast AM. Abl family kinases modulate T cell-mediated inflammation and chemokine-induced migration through the adaptor HEF1 and the GTPase Rap1. Sci Signal. 2012;5:ra51. doi: 10.1126/scisignal.2002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamauchi S, Hou YY, Guo AK, Hirata H, Nakajima W, Yip AK, Yu CH, Harada I, Chiam KH, Sawada Y, Tanaka N, Kawauchi K. p53-mediated activation of the mitochondrial protease HtrA2/Omi prevents cell invasion. The Journal of cell biology. 2014 doi: 10.1083/jcb.201309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallez Y, Mace PD, Pasquale EB, Riedl SJ. NSP-CAS Protein Complexes: Emerging Signaling Modules in Cancer. Genes & cancer. 2012;3:382–393. doi: 10.1177/1947601912460050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mace PD, Wallez Y, Dobaczewska MK, Lee JJ, Robinson H, Pasquale EB, Riedl SJ. NSP-Cas protein structures reveal a promiscuous interaction module in cell signaling. Nature structural & molecular biology. 2011;18:1381–1387. doi: 10.1038/nsmb.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balu K, Rajendran V, Sethumadhavan R, Purohit R. Investigation of binding phenomenon of NSP3 and p130Cas mutants and their effect on cell signalling. Cell biochemistry and biophysics. 2013;67:623–633. doi: 10.1007/s12013-013-9551-6. [DOI] [PubMed] [Google Scholar]
- 34.Sakakibara A, Hattori S. Chat, a Cas/HEF1-associated adaptor protein that integrates multiple signaling pathways. J Biol Chem. 2000;275:6404–6410. doi: 10.1074/jbc.275.9.6404. [DOI] [PubMed] [Google Scholar]
- 35.Cai D, Felekkis KN, Near RI, O’Neill GM, van Seventer JM, Golemis EA, Lerner A. The GDP exchange factor AND-34 is expressed in B cells, associates with HEF1, and activates Cdc42. Journal of immunology. 2003;170:969–978. doi: 10.4049/jimmunol.170.2.969. [DOI] [PubMed] [Google Scholar]
- 36.Wallez Y, Riedl SJ, Pasquale EB. Association of the Breast Cancer Antiestrogen Resistance Protein 1 (BCAR1) and BCAR3 Scaffolding Proteins in Cell Signaling and Antiestrogen Resistance. J Biol Chem. 2014;289:10431–10444. doi: 10.1074/jbc.M113.541839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanden Borre P, Near RI, Makkinje A, Mostoslavsky G, Lerner A. BCAR3/AND-34 can signal independent of complex formation with CAS family members or the presence of p130Cas. Cellular signalling. 2011;23:1030–1040. doi: 10.1016/j.cellsig.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo S, Asai T, Saito T, Suzuki T, Morishita Y, Nakamoto T, Ichikawa M, Yamamoto G, Kawazu M, Yamagata T, Sakai R, Mitani K, Ogawa S, Kurokawa M, Chiba S, Hirai H. Crk-associated substrate lymphocyte type is required for lymphocyte trafficking and marginal zone B cell maintenance. Journal of immunology. 2005;175:3492–3501. doi: 10.4049/jimmunol.175.6.3492. [DOI] [PubMed] [Google Scholar]
- 39.Izumchenko E, Singh MK, Plotnikova OV, Tikhmyanova N, Little JL, Serebriiskii IG, Seo S, Kurokawa M, Egleston BL, Klein-Szanto A, Pugacheva EN, Hardy RR, Wolfson M, Connolly DC, Golemis EA. NEDD9 promotes oncogenic signaling in mammary tumor development. Cancer research. 2009;69:7198–7206. doi: 10.1158/0008-5472.CAN-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danzl NM, Donlin LT, Alexandropoulos K. Regulation of medullary thymic epithelial cell differentiation and function by the signaling protein Sin. The Journal of experimental medicine. 2010;207:999–1013. doi: 10.1084/jem.20092384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 42.Wozniak MA, Baker BM, Chen CS, Wilson KL. The emerin-binding transcription factor Lmo7 is regulated by association with p130Cas at focal adhesions. PeerJ. 2013;1:e134. doi: 10.7717/peerj.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawauchi K, Tan WW, Araki K, Abu Bakar FB, Kim M, Fujita H, Hirata H, Sawada Y. p130Cas-dependent actin remodelling regulates myogenic differentiation. The Biochemical journal. 2012;445:323–332. doi: 10.1042/BJ20112169. [DOI] [PubMed] [Google Scholar]
- 44.Jeong da E, Lee EK, Song WK, Kim W. The 31-kDa caspase-generated cleavage product of p130Cas antagonizes the action of MyoD during myogenesis. Biochemical and biophysical research communications. 2014;444:509–513. doi: 10.1016/j.bbrc.2014.01.085. [DOI] [PubMed] [Google Scholar]
- 45.Law SF, Zhang YZ, Fashena SJ, Toby G, Estojak J, Golemis EA. Dimerization of the docking/adaptor protein HEF1 via a carboxy-terminal helix-loop-helix domain. Exp Cell Res. 1999;252:224–235. doi: 10.1006/excr.1999.4609. [DOI] [PubMed] [Google Scholar]
- 46.Law SF, O’Neill GM, Fashena SJ, Einarson MB, Golemis EA. The docking protein HEF1 is an apoptotic mediator at focal adhesion sites. Molecular and cellular biology. 2000;20:5184–5195. doi: 10.1128/mcb.20.14.5184-5195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akimoto T, Okuhira K, Aizawa K, Wada S, Honda H, Fukubayashi T, Ushida T. Skeletal muscle adaptation in response to mechanical stress in p130cas−/− mice. American journal of physiology Cell physiology. 2013;304:C541–547. doi: 10.1152/ajpcell.00243.2012. [DOI] [PubMed] [Google Scholar]
- 48.Aquino JB, Lallemend F, Marmigere F, Adameyko, Golemis EA, Ernfors P. The retinoic acid inducible Cas-family signaling protein Nedd9 regulates neural crest cell migration by modulating adhesion and actin dynamics. Neuroscience. 2009;162:1106–1119. doi: 10.1016/j.neuroscience.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel T, Ahrens S, Buttner N, Krieglstein K. Transforming growth factor beta promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb Cortex. 2010;20:661–671. doi: 10.1093/cercor/bhp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riccomagno MM, Sun LO, Brady CM, Alexandropoulos K, Seo S, Kurokawa M, Kolodkin AL. Cas adaptor proteins organize the retinal ganglion cell layer downstream of integrin signaling. Neuron. 2014;81:779–786. doi: 10.1016/j.neuron.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonsior C, Biname F, Fruhbeis C, Bauer NM, Hoch-Kraft P, Luhmann HJ, Trotter J, White R. Oligodendroglial p130Cas is a target of Fyn kinase involved in process formation, cell migration and survival. PloS one. 2014;9:e89423. doi: 10.1371/journal.pone.0089423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Neill GM, Seo S, Serebriiskii IG, Lessin SR, Golemis EA. A new central scaffold for metastasis: parsing HEF1/Cas-L/NEDD9. Cancer research. 2007;67:8975–8979. doi: 10.1158/0008-5472.CAN-07-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nature reviews Cancer. 2010;10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 54.Kumbrink J, Kirsch KH. p130Cas acts as survival factor during PMA-induced apoptosis in HL-60 promyelocytic leukemia cells. The international journal of biochemistry & cell biology. 2013;45:531–535. doi: 10.1016/j.biocel.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo S, Nakamoto T, Takeshita M, Lu J, Sato T, Suzuki T, Kamikubo Y, Ichikawa M, Noda M, Ogawa S, Honda H, Oda H, Kurokawa M. Crk-associated substrate lymphocyte type regulates myeloid cell motility and suppresses the progression of leukemia induced by p210Bcr/Abl. Cancer Sci. 2011;102:2109–2117. doi: 10.1111/j.1349-7006.2011.02066.x. [DOI] [PubMed] [Google Scholar]
- 56.Hempel N, Bartling TR, Mian B, Melendez JA. Acquisition of the metastatic phenotype is accompanied by H2O2-dependent activation of the p130Cas signaling complex. Molecular cancer research: MCR. 2013;11:303–312. doi: 10.1158/1541-7786.MCR-12-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morimoto K, Tanaka T, Nitta Y, Ohnishi K, Kawashima H, Nakatani T. NEDD9 crucially regulates TGF-beta-triggered epithelial-mesenchymal transition and cell invasion in prostate cancer cells: Involvement in cancer progressiveness. The Prostate. 2014;74:901–910. doi: 10.1002/pros.22809. [DOI] [PubMed] [Google Scholar]
- 58.Lee KH, Johmura Y, Yu LR, Park JE, Gao Y, Bang JK, Zhou M, Veenstra TD, Yeon Kim B, Lee KS. Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. The EMBO journal. 2012;31:3104–3117. doi: 10.1038/emboj.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JX, Gao F, Zhao GQ, Zhang GJ. Role of NEDD9 in invasion and metastasis of lung adenocarcinoma. Experimental and therapeutic medicine. 2012;4:795–800. doi: 10.3892/etm.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondo S, Iwata S, Yamada T, Inoue Y, Ichihara H, Kichikawa Y, Katayose T, Souta-Kuribara A, Yamazaki H, Hosono O, Kawasaki H, Tanaka H, Hayashi Y, Sakamoto M, Kamiya K, Dang NH, Morimoto C. Impact of the integrin signaling adaptor protein NEDD9 on prognosis and metastatic behavior of human lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:6326–6338. doi: 10.1158/1078-0432.CCR-11-2162. [DOI] [PubMed] [Google Scholar]
- 61.Miao Y, Li AL, Wang L, Fan CF, Zhang XP, Xu HT, Yang LH, Liu Y, Wang EH. Overexpression of NEDD9 is associated with altered expression of E-Cadherin, beta-Catenin and N-Cadherin and predictive of poor prognosis in non-small cell lung cancer. Pathology oncology research: POR. 2013;19:281–286. doi: 10.1007/s12253-012-9580-2. [DOI] [PubMed] [Google Scholar]
- 62.Feng Y, Wang Y, Wang Z, Fang Z, Li F, Gao Y, Liu H, Xiao T, Li F, Zhou Y, Zhai Q, Liu X, Sun Y, Bardeesy N, Wong KK, Chen H, Xiong ZQ, Ji H. The CRTC1-NEDD9 signaling axis mediates lung cancer progression caused by LKB1 loss. Cancer research. 2012;72:6502–6511. doi: 10.1158/0008-5472.CAN-12-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Yu C, Ren J, Ye S, Ou W, Wang Y, Yang W, Zhong G, Chen X, Shi H, Su X, Chen L, Zhu W. Synergistic effects of eukaryotic coexpression plasmid carrying LKB1 and FUS1 genes on lung cancer in vitro and in vivo. Journal of cancer research and clinical oncology. 2014 doi: 10.1007/s00432-014-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin Y, Li F, Zheng C, Wang Y, Fang Z, Guo C, Wang X, Liu H, Deng L, Li C, Wang H, Chen H, Feng Y, Ji H. NEDD9 promotes lung cancer metastasis through epithelial-mesenchymal transition. International journal of cancer Journal international du cancer. 2014;134:2294–2304. doi: 10.1002/ijc.28568. [DOI] [PubMed] [Google Scholar]
- 65.Miao Y, Wang L, Liu Y, Li AL, Liu SL, Cao HY, Zhang XP, Jiang GY, Liu D, Wang EH. Overexpression and cytoplasmic accumulation of Hepl is associated with clinicopathological parameters and poor prognosis in non-small cell lung cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:107–114. doi: 10.1007/s13277-012-0517-x. [DOI] [PubMed] [Google Scholar]
- 66.Little JL, Serzhanova V, Izumchenko E, Egleston BL, Parise E, Klein-Szanto AJ, Loudon G, Shubina M, Seo S, Kurokawa M, Ochs MF, Golemis EA. A requirement for Nedd9 in luminal progenitor cells prior to mammary tumorigenesis in MMTV-HER2/ErbB2 mice. Oncogene. 2014;33:411–420. doi: 10.1038/onc.2012.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh MK, Izumchenko E, Klein-Szanto AJ, Egleston BL, Wolfson M, Golemis EA. Enhanced genetic instability and dasatinib sensitivity in mammary tumor cells lacking NEDD9. Cancer research. 2010;70:8907–8916. doi: 10.1158/0008-5472.CAN-10-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fashena SJ, Einarson MB, O’Neill GM, Patriotis C, Golemis EA. Dissection of HEF1-dependent functions in motility and transcriptional regulation. Journal of cell science. 2002;115:99–111. doi: 10.1242/jcs.115.1.99. [DOI] [PubMed] [Google Scholar]
- 69.Pincini A, Tornillo G, Orso F, Sciortino M, Bisaro B, del Leal MP, Lembo A, Brizzi MF, Turco E, De Pitta C, Provero P, Medico E, Defilippi P, Taverna D, Cabodi S. Identification of p130Cas/ErbB2-dependent invasive signatures in transformed mammary epithelial cells. Cell Cycle. 2013;12:2409–2422. doi: 10.4161/cc.25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tornillo G, Elia AR, Castellano I, Spadaro M, Bernabei P, Bisaro B, del Camacho-Leal MP, Pincini A, Provero P, Sapino A, Turco E, Defilippi P, Cabodi S. p130Cas alters the differentiation potential of mammary luminal progenitors by deregulating c-Kit activity. Stem Cells. 2013;31:1422–1433. doi: 10.1002/stem.1403. [DOI] [PubMed] [Google Scholar]
- 71.Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack RL, Jr, Golemis EA. Aurora A kinase (AURKA) in normal and pathological cell division. Cellular and molecular life sciences: CMLS. 2013;70:661–687. doi: 10.1007/s00018-012-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ice RJ, McLaughlin SL, Livengood RH, Culp MV, Eddy ER, Ivanov AV, Pugacheva EN. NEDD9 depletion destabilizes Aurora A kinase and heightens the efficacy of Aurora A inhibitors: implications for treatment of metastatic solid tumors. Cancer research. 2013;73:3168–3180. doi: 10.1158/0008-5472.CAN-12-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLaughlin SL, Ice RJ, Rajulapati A, Kozyulina PY, Livengood RH, Kozyreva VK, Loskutov YV, Culp MV, Weed SA, Ivanov AV, Pugacheva EN. NEDD9 depletion leads to MMP14 inactivation by TIMP2 and prevents invasion and metastasis. Molecular cancer research: MCR. 2014;12:69–81. doi: 10.1158/1541-7786.MCR-13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu L, De Man FS, Girerd B, Huertas A, Chaumais MC, Lecerf F, Francois C, Perros F, Dorfmuller P, Fadel E, Montani D, Eddahibi S, Humbert M, Guignabert C. A critical role for p130Cas in the progression of pulmonary hypertension in humans and rodents. American journal of respiratory and critical care medicine. 2012;186:666–676. doi: 10.1164/rccm.201202-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gertow K, Sennblad B, Strawbridge RJ, Ohrvik J, Zabaneh D, Shah S, Veglia F, Fava C, Kavousi M, McLachlan S, Kivimaki M, Bolton JL, Folkersen L, Gigante B, Leander K, Vikstrom M, Larsson M, Silveira A, Deanfield J, Voight BF, Fontanillas P, Sabater-Lleal M, Colombo GI, Kumari M, Langenberg C, Wareham NJ, Uitterlinden AG, Gabrielsen A, Hedin U, Franco-Cereceda A, Nyyssonen K, Rauramaa R, Tuomainen TP, Savonen K, Smit AJ, Giral P, Mannarino E, Robertson CM, Talmud PJ, Hedblad B, Hofman A, Erdmann J, Reilly MP, O’Donnell CJ, Farrall M, Clarke R, Franzosi MG, Seedorf U, Syvanen AC, Hansson GK, Eriksson P, Samani NJ, Watkins H, Price JF, Hingorani AD, Melander O, Witteman JC, Baldassarre D, Tremoli E, de Faire U, Humphries SE, Hamsten A. Identification of the BCAR1-CFDP1-TMEM170A locus as a determinant of carotid intima-media thickness and coronary artery disease risk. Circulation Cardiovascular genetics. 2012;5:656–665. doi: 10.1161/CIRCGENETICS.112.963660. [DOI] [PubMed] [Google Scholar]
- 76.Kim JY, Huh K, Jung R, Kim TJ. Identification of BCAR-1 as a new substrate of Syk tyrosine kinase through a determination of amino acid sequence preferences surrounding the substrate tyrosine residue. Immunology letters. 2011;135:151–157. doi: 10.1016/j.imlet.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Pan J, Seeger-Nukpezah T, Golemis EA. The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cellular and molecular life sciences: CMLS. 2013;70:1849–1874. doi: 10.1007/s00018-012-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spalluto C, Wilson DI, Hearn T. Nek2 localises to the distal portion of the mother centriole/basal body and is required for timely cilium disassembly at the G2/M transition. European journal of cell biology. 2012;91:675–686. doi: 10.1016/j.ejcb.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA. Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Molecular biology of the cell. 2012;23:2658–2670. doi: 10.1091/mbc.E11-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plotnikova OV, Pugacheva EN, Golemis EA. Aurora A kinase activity influences calcium signaling in kidney cells. The Journal of cell biology. 2011;193:1021–1032. doi: 10.1083/jcb.201012061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagai Y, Osawa K, Fukushima H, Tamura Y, Aoki K, Ohya K, Yasuda H, Hikiji H, Takahashi M, Seta Y, Seo S, Kurokawa M, Kato S, Honda H, Nakamura I, Maki K, Jimi E. p130Cas, Crk-associated substrate, plays important roles in osteoclastic bone resorption. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28:2449–2462. doi: 10.1002/jbmr.1936. [DOI] [PubMed] [Google Scholar]