Abstract
Despite their inherent toxicity and the acquired bacterial resistance that continuously threaten their long-term clinical use, aminoglycosides (AGs) still remain valuable components of the antibiotic armamentarium. Recent literature shows that the AGs’ role has been further expanded as multi-tasking players in different areas of study. This review aims at presenting some of the new trends observed in the use of AGs in the past decade, along with the current understanding of their mechanisms of action in various bacterial and eukaryotic cellular processes.
Introduction
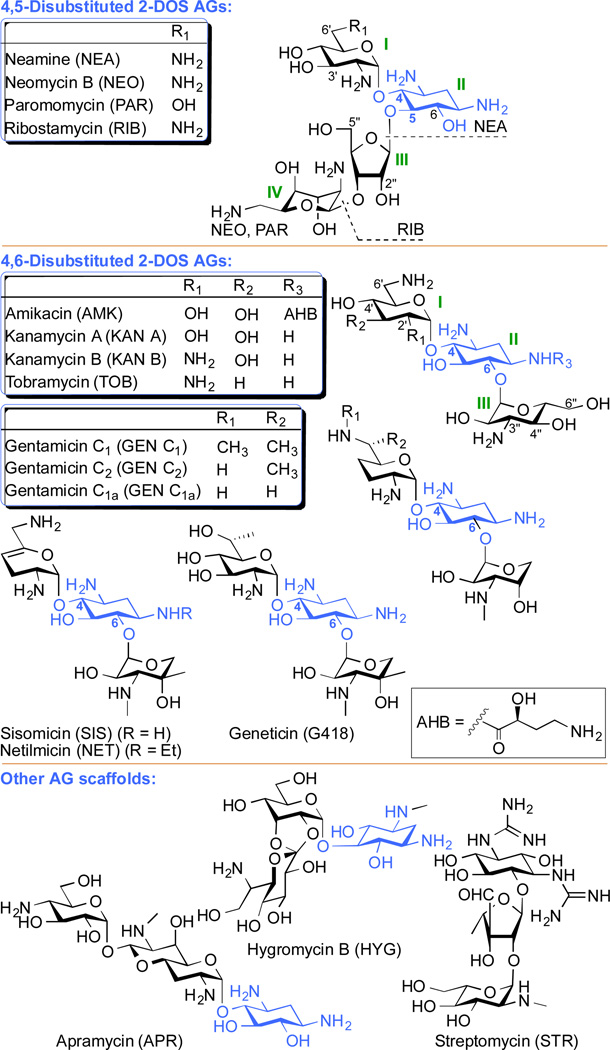
Aminoglycosides (AGs) are a group of naturally occurring and semi-synthetic amino-modified sugars (Fig. 1). AGs can be structurally classified as 4,5-disubstituted 2-deoxystreptamine (2-DOS) AGs (e.g., neomycin B (NEO), paromomycin (PAR), and ribostamycin (RIB); note: although neamine (NEA) is a 4-monosubtituted AG, we included it in the 4,5-disubstituted 2-DOS AGs in Fig. 1 as its structure is comprised in their scaffolds) as well as 4,6-disubstituted 2-DOS AGs (e.g., amikacin (AMK), kanamycin A and B (KAN A and KAN B), tobramycin (TOB), gentamicin (GEN), geneticin (G418), netilmicin (NET), and sisomicin (SIS)). Other AG scaffolds include the monosubstituted 2-DOS AGs (e.g., apramycin (APR) and hygromycin (HYG)), and streptomycin (STR).
Fig. 1.
Structures of parent AGs discussed in this review. The 2-deoxystreptamine (2-DOS) ring is depicted in blue.
AGs’ broad-spectrum of activity against pathogenic bacteria has favoured them over the past seventy years as a valuable class of antibiotics.1 Studies of their antibacterial mode of action have revealed that AGs bind both to the aminoacyl site (A-site) of the 16S ribosomal RNA (rRNA) of bacteria, where they perturb the “proof-reading” process that ensures protein translation fidelity,2 and to the 50S ribosomal subunit, inhibiting translocation and ribosome recycling.3, 4
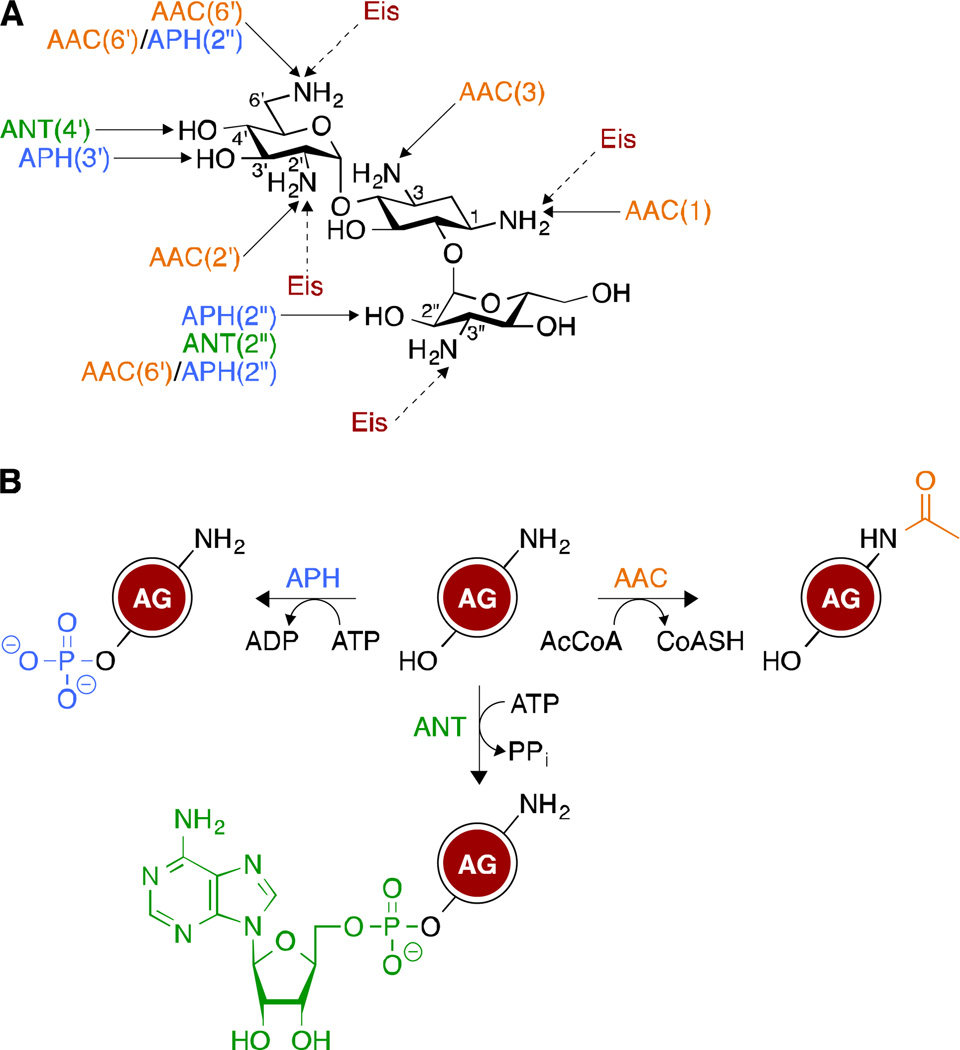
Naturally occurring AGs are produced by Streptomyces and Micromonospora soil bacteria,5 which proactively methylate their ribosome to survive the bactericidal action of their secondary metabolites.6 This mechanism, along with the decrease in AG uptake and the emergence of aminoglycoside-modifying enzymes (AMEs), has significantly plagued the clinical efficacy of AGs.7 AMEs, in particular, have been a serious threat to their long term use and more than 100 of them have been identified.8 These enzymes, which include AG acetyltransferases (AACs), AG phosphotransferases (APHs), and AG nucleotidyltransferases (ANTs) (Fig. 2A) act through chemical modifications of the structures of AGs. Indeed, AACs catalyze the transfer of an acetyl group from acetyl coenzyme A (AcCoA) to the amine functionalities of AGs, while APHs and ANTs use ATP (and in some cases GTP)9–12 to transfer a phosphate and an adenosine (guanidine) monophosphate moieties, respectively, to the hydroxyl groups of AGs (Fig. 2B). Unlike other AACs, which are regiospecific, the newly discovered enhanced intracellular survival (Eis) is a versatile enzyme that can acetylate different amine positions of AGs.13–22
Fig. 2.
A. Sites that are targeted by the different aminoglycoside-modifying enzymes (AMEs). Unlike other AMEs that are regiospecific, Eis can multi-acetylate AGs. B. Chemical modifications catalyzed by AMEs.
Soon after its introduction in the therapeutic regimen of tuberculosis, STR, the first AG ever discovered, displayed toxic side effects. Nephrotoxicity and ototoxicity, which are the most common adverse effects associated with AG antibiotics, have also hampered their clinical effectiveness. These serious shortages have sparked considerable interests in the scientific community. Our group has recently provided a comprehensive overview of AG antibiotics1 and the recent approaches that have been developed to triumph over AMEs’ actions.23 Of special note: the combination of AGs with AME inhibitors as a potentially effective tactic to revive the usefulness of these drugs against AG-resistant strains. This was inspired by the clinical success encountered by the co-administration of β-lactams and β-lactamase inhibitors.24 The search for Eis inhibitors enabled the development of a high-throughput screening (HTS) method that facilitated the identification of 25 active compounds out of 23,000 tested.22 While waiting for HTS to be applied to the other classes of AMEs, existing AME inhibitors could be utilized in the meantime. These include the APH(3')-IIIa inhibitor ankyrin repeat protein,25, 26 the APH(2")-IVa inhibitor quercetin,27 the APH(9)-Ia inhibitor CKI-7, which was co-crystallized with APH(3')-IIIa,28 and the bifunctional enzyme AAC(6')-Ie/APH(2")-Ia inhibitor aranosin.29 The 3-(dimethylamino)propylamine moiety was also found to be an essential scaffold for ANT(2")-Ia and APH(3')-IIIa inhibitors.30
Also worth mentioning is the development of AGs that could both tightly bind to the bacterial ribosome and disrupt the protein synthesis machinery, and also be poor substrates of AMEs. This has eventually led to the synthesis of:
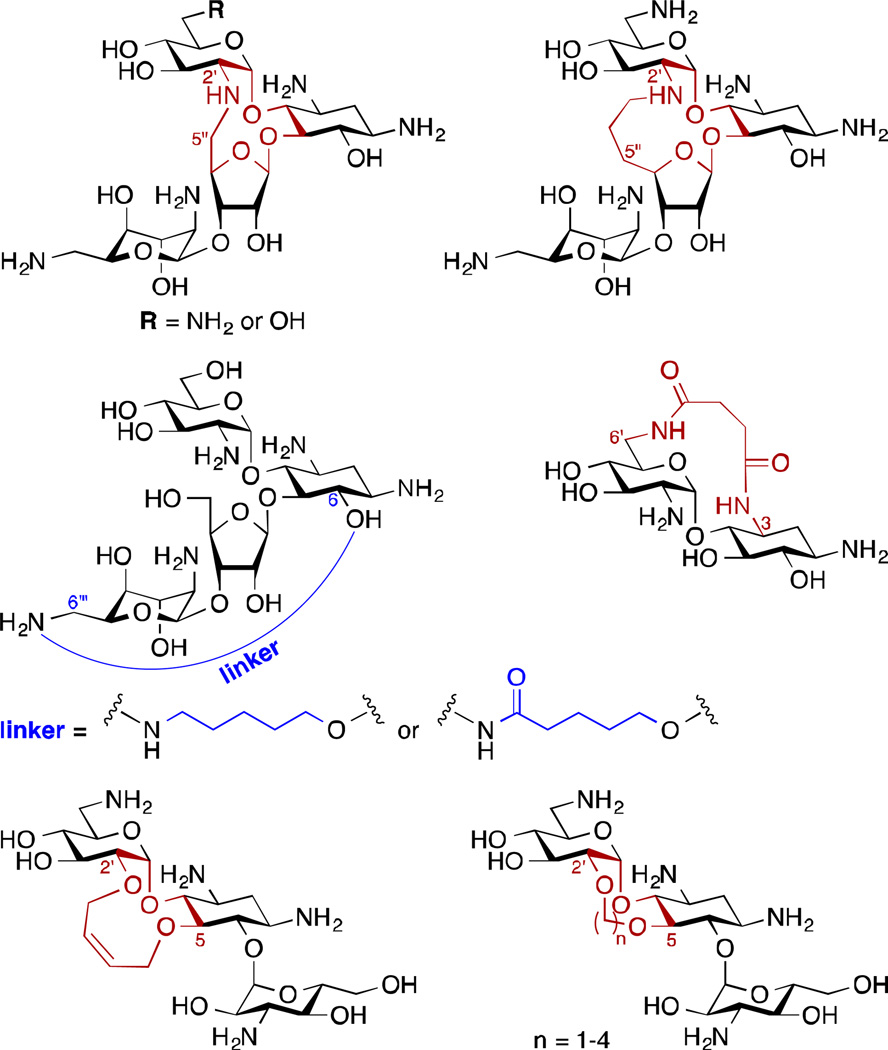
Structurally constrained AGs – Originally designed to resemble the locked conformation of AG when bound to the bacterial A-site, a variety of rigidified NEO, PAR, NEA, and KAN A derivatives were synthesized (Fig. 3).31–37 Although they all displayed a decreased antibacterial activity compared to the parent AGs, the NEO and the KAN A-restricted derivatives (through methylene linkers between the 2'-NH and 5"-C as well as the 2'-O and 5-O, respectively) were still quite active, with MIC values ranging from 2.5 to 64 µg/mL. Additionally, the NEO-restricted derivatives were poor substrates of S. aureus ANT(4') and M. tuberculosis AAC(2')-Ic.
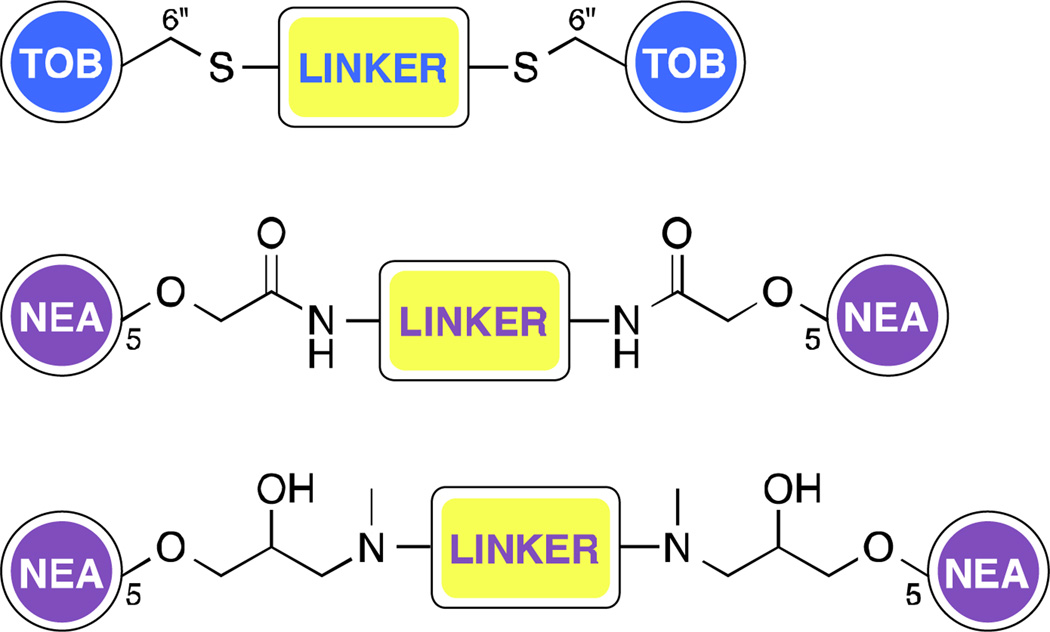
AG dimers – Following evidence that dimerized parent AGs may have improved binding affinity towards RNA,38 series of homo- and heterodimeric AGs were developed with the goal of investigating their ability to target the bacterial A-site.39–43 In addition, some NEA dimers, linked at the 5-position via amides and 1,2-hydroxyamines (Fig. 4), could evade the action of the AMEs AAC(6')-Ii, APH(3')-IIIa, and AAC(6')-Ie/APH(2")-Ia better than the parent compound.39 Furthermore, a TOB homodimer (Fig. 4) was shown to be a poor substrate of TOB-targeting AMEs AAC(6')-Ie/APH(2")-Ia, AAC(6")-Ib', and ANT(4').43 The use of AG dimers has however not been limited to targeting bacterial ribosome. In fact, AG dimers also found application as binders of RNA hairpin loops,44, 45 as binders of the dimerization initiation site of the HIV-1 genomic RNA,46 the HIV-1 Rev response element,47 and HIV-1 trans-acting responsive sequence.48 AG monomers and dimers have also found to be useful as inhibitors of anthrax lethal factor.49–51
Guanidinylated AGs – By replacing the amine or hydroxyl moieties with a guanidine functionality in TOB, AMK, KAN A, NEO, NEA, PAR, and APR, the Nizet and Tor groups were able to develop a library of AG derivatives, with most of them displaying an enhanced antibacterial activity, which correlated with higher affinity to bacterial A-site.52
Fig. 3.
Structures of conformationally constrained AGs.
Fig. 4.
A. Representative TOB and NEA dimers with different linker attachments. Note: NEO-NEO, NEO-TOB, KAN-KAN, and KAN-TOB dimers have also been reported in the literature.38, 41–43, 47
We will discuss herein the antibacterial modes of action of AGs as well as some of the novel applications of AGs that have been investigated in the last decade such as riboswitch binders, oncogenic microRNAs (miRNAs) targeting molecules, antileishmanial compounds, antifungal agents, amphiphiles, and as potential treatment of genetic diseases arising from premature termination codons (PTCs). New ways to alleviate AG-induced ototoxicity will also be examined, with reference to the solely non-toxic AG APR and the prospective clinical candidate plazomicin (PLZ).
Aminoglycosides mode of action: binding to the ribosome
AGs have long been known to exert their antibacterial functions by binding to the bacterial ribosome and interfering with protein translation. To further probe their mechanism of action, different approaches have been developed and employed in the past decades, including fluorescence-based assays,3, 53–56 computational simulations,57, 58 microarray assays,59–63 and X-ray crystallography.4, 64, 65 As a result, AGs have been demonstrated to bind not only to the ribosomal decoding A-site on the 16S rRNA, causing miscoding in the nascent polypeptide, but also to the helix 69 (h69) in the large 50S ribosomal subunit, which is critical in the processes of mRNA/tRNA translocation and ribosome recycling.
Aminoglycosides binding to the A-site
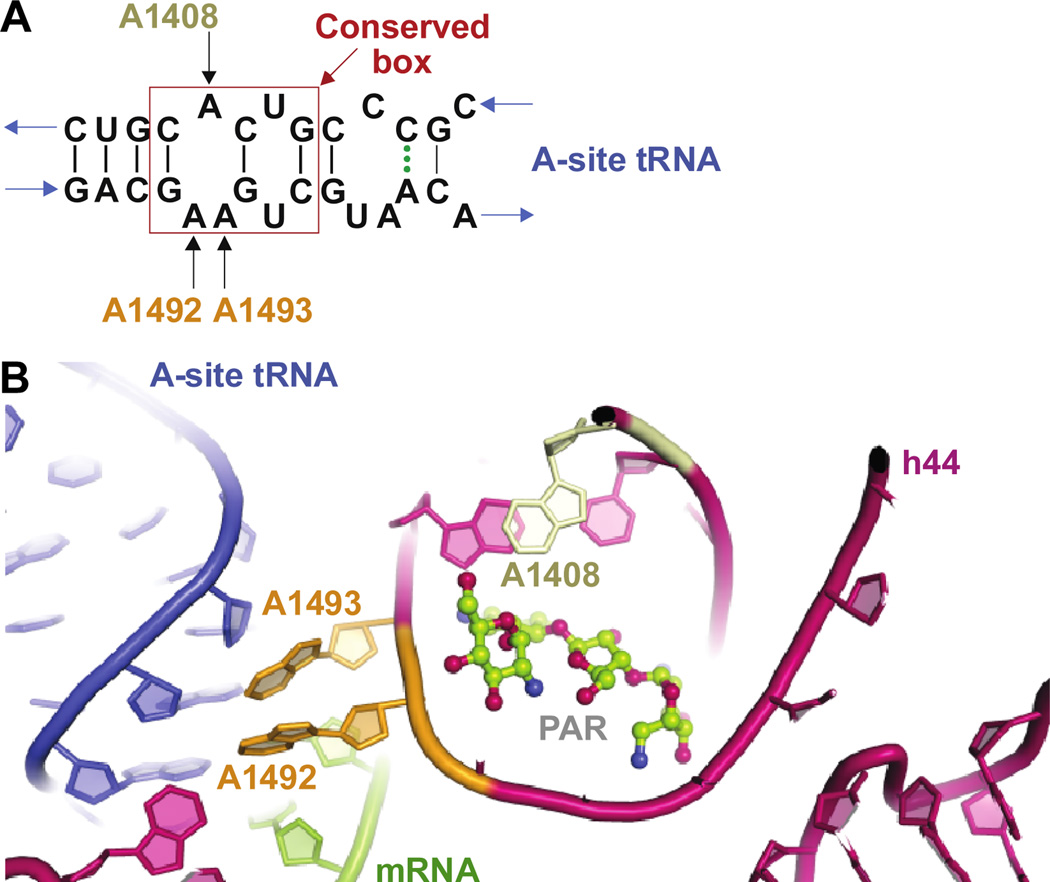
As a canonical model of AG binding, the interactions between AGs and the decoding A-site on the 30S ribosomal subunit have been extensively studied. AGs bind to a highly conserved set of nucleotides on helix 44 (h44) of the 16S rRNA (Fig. 5A). The carbohydrate rings of AGs stack to the RNA, thereby stabilizing the bound complex at the A-site by hydrogen-bonding and H2O-mediated interactions.57, 65–67 For 4,6-disubstituted 2-DOS AGs, the presence of a 4’-hydroxyl or a 2’-amino group on ring I was reported to be critical in assisting proper targeting of the ribosome by the antibiotics. However, the substitution pattern on ring III was found to have minimal effect on drug targeting.67 In the case of 4,5-disubstituted 2-DOS AGs, the stacking effect of ring I was complemented by rings III and IV, which enhanced AG-rRNA complex stabilization.66 Appending of an aromatic moiety at the 2"-position of PAR was found to improve the drug susceptibility of bacterial resistant strains, thereby providing further details for the mechanisms of AG binding.57
Fig. 5.
A. Representation of the internal loop structure of h44. B. Representation of h44 of the 16S rRNA showing residues destacking upon AG binding.
Binding of AGs to the 16S rRNA was reported to extrude the A1492 and A1493 residues on h44, conformationally rearranging them from an intra-helical to an extra-helical state in the rRNA. Such change disturbed the fidelity of aminoacyl-tRNA selection.68 rRNA mutagenesis, X-ray crystallography, and determination of MIC studies revealed the residues that were critical in maintaining proper AG binding (A1492 and A1493) and stabilizing the destacked helix (A1408) (Fig. 5B).54, 55 These results were also confirmed by fluorescence-based studies where S12, a ribosomal protein in the 30S complex, was fluorescently-tagged to investigate NEO and h44 interactions.56 Other residues, such as the purine/pyrimidine switches 1411•1489 and 1410•1490, were reported to have minimal effect on MIC values while 1409•1491 was found to be critical for AG susceptibility.69
In addition to the bacterial ribosomes, AGs have also been known to bind with lower affinity to the mammalian ribosomes. In the aim of achieving higher selectivity of AGs towards their bacterial target, both AG and ribosome modifications have been investigated. 4’,6’-O-acetal and 4’-O-ether modifications of PAR have been shown to impart greater sensitivity to A1408G, G1491C, and G1491A mutations, characteristic of prokaryotic-eukaryotic ribosome differences.70 Applying nucleobase-conjugated AGs as probes, the A-site was reported to discriminate its ligand structures and conformations.71 Conversely, in a study where KAN A, TOB, NEA, and NEO derivatives were examined in a microarray-based assay against a library of internal loops in a two-dimensional combinatorial screening analysis, AGs were found to be non-selective and each preferentially bound to a different internal loop. For instance, KAN A preferably bound to 1×1 internal loops with C•A mismatches, and the stability of the internal loop structure correlated with the binding affinity to AGs.60–63 Anderson and Mecozzi further investigated the minimum sequence required for PAR binding to the A-site via a computational approach.58 A combination of molecular dynamics, free-energy calculations, and in vitro binding assays showed that the 11-nucleotide:10-nucleotide duplex RNA was minimal to provide stable PAR binding, whereas smaller duplexes lost the complex stability and the accessibility for PAR.58
Aminoglycosides binding to h69
Although most AGs bind to the decoding A-site and introduce amino acids from non-cognate or near-cognate tRNAs into the nascent polypeptides, this effect alone was postulated to be insufficient justification of AGs’ antibacterial effect. Instead, some AGs, including NEO, PAR, and TOB, have been identified to have a secondary binding site at the major groove of h69 of the 23S rRNA of the 50S ribosomal subunit.64, 72 Crystallography data revealed that h69 forms direct contact with the A-/P-site tRNAs, as well as the decoding center, by looping around the interface of the two subunits and forming an inter-subunit bridge (B2). Due to the vital position of h69 in the 3D structure, the binding of AGs to h69 hinders the ribosomal movement/global conformational rearrangement when the 30S rotates around the 50S ribosomal subunit in racquet-like movement around the L1 stalk domain.73 This concerted movement, assisted by ribosome recycling factor (RRF) and GTPase elongation factor G (EF-G), occurs in multiple steps during protein translation, especially translocation and ribosome recycling processes. Therefore, AGs’ mechanism of inhibiting the global conformational rearrangement is a vital contribution to their antibacterial properties.4
During translocation, the aminoacyl-tRNA first orients itself perpendicularly to the 30S and 50S subunits at the A-site, which is considered the “classical” configuration. Post peptide bond formation and prior to translocation, intermediate states where the 3’-terminus and the acceptor stems of tRNAs proceed to the next site on the large subunit while the anticodon stems remain fixed on the 30S subunit are referred to as the “hybrid” states.74, 75 Two distinct hybrid-state intermediates have been elucidated by the Blanchard’s group via single-molecule fluorescence resonance energy transfer (smFRET).76 Studies have shown that AGs binding to h69 halted the pre-translocation complex at post peptidyl transfer. The dynamics of AG binding to pre-translocation complex revealed high-affinity (h44) and low-affinity (h69) binding sites on the ribosome. While most AGs bind to h44 only and stabilize the classical state, NEO was shown to stabilize the hybrid states at the low-affinity site upon saturation of h44.3 To further study NEO inhibition on the ribosomal global conformational rearrangement, pre-steady state smFRET and dynamics studies demonstrated that NEO binding to h69 excluded RRF binding to the ribosome and attenuated the 30S subunit rotation with respect to the large subunit that normally promotes the conformational switch from the classical to the hybrid configuration. Crystal structures also confirmed NEO binding to h69, where the latter forms an inter-subunit bridge B2 and interacts with helices h24 and h45 of the 30S subunit.64
At the end of each translation cycle is the dissociation of the ribosome complex, where the same racquet-like movement is involved as in translocation. RRF binds to h69 and extrudes it away from the inter-subunit surface, causing the 30S subunit to dissociate from the 50S. However, AG binding to h69 makes the complex inaccessible to the RRF and therefore locked in the bound form, inhibiting ribosome recycling.4 Confirming previous results, Agris and co-workers further compared the affinity of NEO and GEN to E. coli and human h69. They reported that AGs bind to human h69 with lower affinity than to E. coli h69, which shed light into AG antibiotics target selectivity.72
AGs are structurally diverse and therefore bound to function by different mechanisms. Puglisi and co-workers reported the various mechanisms of APR, GEN, and PAR, which they confirmed by nuclear magnetic resonance spectroscopy (NMR).53 APR was found not to displace A1492 and A1493 residues at the decoding center but rather to block the translocation process. GEN and PAR, on the other hand, exhibited a different mode of action whereby they destacked h44, leading to significant miscoding in the nascent polypeptide.
Tracing back the efforts that have been put forth in the investigation of how AGs perform their antibiotic functions, a greater picture has been revealed. However, the unobserved part of the iceberg still remains elusive yet inviting. Along this line, more untouched functions of AGs are starting to be discovered. In addition to causing amino acid misincorporation and inhibiting translocation and ribosome recycling, Foster and Champney reported that AGs hinder the 30S ribosome assembly, which brings up an unexplored mechanism of AG antibiotics.77 Any further knowledge we gain on the mechanism of action of AGs is certainly a step towards defeating bacterial infections, especially in the rapidly emerging resistant bacterial strains.
Aminoglycosides as universal RNA binders
AGs are known as universal RNA binders, for they not only bind to the rRNAs in the ribosomes, but also interact with other types of RNA structures.39 Two major types of RNAs that AGs interact with are the riboswitches and miRNAs, both of which are critical in regulating gene expression. The exploration of these interactions has recently gained popularity. Developing AGs as tools to target these non-coding RNA structures for engineering and therapeutic purposes clearly represents one of the newest trends of AG applications.
Flipping the switches of the riboswitches
Riboswitches are mRNA domains in the 5’-leader sequences and they control the expression of the downstream genes.78 Upon binding of a variety of ligands, ranging from metabolites, secondary messengers to xenobiotic, riboswitches can regulate gene expression via various mechanisms. Transcription termination and translation initiation are the mechanisms mostly observed upon ligand binding. Other mechanisms include alternative splicing, catalytic RNA activation, and trans-acting mechanism.79 In the last five years, AGs have been used as tools to discover new artificial and natural riboswitches to gain a better understanding of the resistance mechanisms.
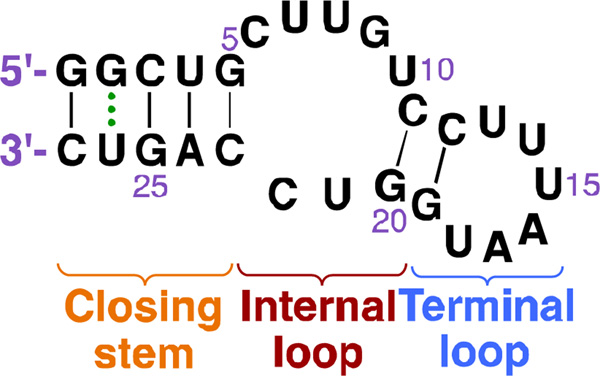
Different strategies have been utilized in order to engineer effective riboswitches, including antisense, ribozymes, and small molecule-binding aptamers. Aptamers are oligonucleotides that bind specific ligands and they have been used to develop an artificial NEO-binding riboswitch via a two-strategy approach. This 27-nucleotides (nt) aptamer was proposed to form an internal and a terminal loop secondary structure (Fig. 6). The binding of NEO caused extensive conformational rearrangement in the mRNA, which was confirmed by an observed change in the nuclease cleavage pattern. The binding between NEO and the riboswitch aptamer was explained to be the same as the AGs binding to their natural target at the decoding A-site.80, 81 As the smallest functional artificial riboswitch identified so far, the 27-nt riboswitch has been further investigated via structural and dynamics approaches, NMR, and pulsed electron-electron double resonance (PELDOR) spectroscopy. These studies revealed that in addition to NEO, the riboswitch element also responds to RIB and TOB.82–84
Fig. 6.
Structure of the 27-nucleotide riboswitch aptamer.
Naturally occurring riboswitches are essential gene regulators in pathogenic bacteria, and therefore are becoming cutting-edge targets for understanding resistance mechanisms and developing antibiotics.71 Recently, a natural riboswitch was discovered in the 5’-untranslated region (5’-UTR) of bacterial resistance genes aac/aad. Upon AG binding, the riboswitch induced the expression of encoded AAC and ANT. The binding of AG ligand alternates the secondary structure of the riboswitch and exposes a second ribosome-binding domain and was thus postulated to also promote the recruitment of the ribosome complex.85
Aminoglycoside conjugates targeting microRNAs
In addition to binding to the riboswitches, AGs have also been utilized to target miRNAs, which are often aberrantly expressed in various diseases. These single-stranded non-coding miRNAs are transcribed as pre-miRNA precursors and are later activated by the Dicer and Drosha nuclease complexes. Activated miRNAs hybridize to the UTRs of the target mRNAs, which trigger the degradation of the miRNA/mRNA complexes, and subsequently lead to targeted gene silencing.86
Efforts have been put forth in developing AG conjugates to target the oncogenic miRNAs precursors, pre-miR-372 and pre-miR-373 as a novel therapeutic approach.87 Oncogenic miRNAs, miR-372 and miR-373, are both upregulated in various tumors and suppress the expression of the Large Tumor Suppressor homologue 2 (LATS2) protein.88 By conjugating AGs with natural or synthetic nucleobases, the multimodal ligands were designed so that the nucleobase scaffolds provide specific recognition to the double-stranded portion of the pre-miRNAs and the AG structures, especially NEO, to ensure high affinity binding to the stem-loop structures. Applying fluorescence-based assays, adenine-NEO and uridine-NEO, together with six non-natural nucleobase-NEO conjugates were described to effectively suppress pre-miRNA activation, thus disabling the suppression of LATS2 expression in tumors.
Pursuing bioactive miRNA targeting small molecules can also be achieved by sequence-based molecule design, which takes advantage of the fact that RNA secondary structures are highly predictable from their primary sequences. Inforna, which designs lead small molecules for targeting RNA based on the RNA sequences, identified that 6’-N-hexynoylated KAN A would target oncogenic miRNA precursor miR-182 by blockage at the Drosha cleavage site.89 Inhibiting the activation of miR-182 subsequently restored the expression of the downstream tumor suppressor protein FOXO1, an apoptosis inducer in cancer cells.
RNA targeting strategies have found applications not only in cancer, but also in other diseases such as myotonic dystrophy (DM), which is caused by the splicing defect in the mutant pre-mRNA species when bound to the RNA splicing regulator muscleblind-like protein (MBNL1).90–92 6’-N-hexynoylated KAN A was also investigated and demonstrated to improve proper splicing of the pre-mRNA transcripts. Upon further development, 6’-N-hexynoylated KAN A conjugated to d-Arg9 was found to improve cellular permeability and localization in DM type 1 cell culture and animal model, signifying their application as a potential therapeutic option.93
Aminoglycosides as antifungal agents
AGs have long been used for the treatment of bacterial infections in humans and animals, but their ability to bind to the eukaryotic ribosomes, though with lower affinity when compared to the prokaryotic ribosomes, has been a continuous step back. This limitation has however been exploited to explore new targets of AGs. Recently, Lee and co-workers have investigated the activities of PAR, NEO, RIB, and STR against six crop pathogenic oomycetes (Phytophthora and Pythium species) and ten common fungi.94 All four AGs manifested modest to excellent antioomycete activity, with non-existent activity against several true fungal species. PAR displayed the strongest in vitro and in vivo inhibitory effect on various plant pathogens, including Phytophthora capsici and Phytophthora infestans, which are responsible for red pepper and tomato late blight diseases, respectively. Even though fungi are most often associated with crop diseases, they still remain an important problem in human and veterinary medicine that needs to be addressed.
Pythiosis is an infectious disease caused by the fungus Pythium insidiosum that affects dogs, cats, horses, and even humans. The in vitro susceptibility of 24 P. insidiosum isolates was measured against four naturally occurring AGs (PAR, GEN, NEO, and STR).95 With in vitro MIC values ranging from 32 to 128 µg/mL, which are all higher than the safe plasma concentration of 30 µg/mL, these AGs could not be used therapeutically. It has however been observed that, when used in conjunction with compounds that increase their cellular uptake in fungi, AGs could exert their fungicidal activity at lower, and possibly safer, concentrations. This is the case of HYG, a structurally unusual AG produced by Streptomyces hygroscopicus that kills bacteria and fungi through protein biosynthesis inhibition. The membrane-disrupting agent polymyxin B was found to work synergistically with HYG, enabling this latter to inhibit the growth of Saccharomyces cerevisiae cells at a lower concentration than that expected from its own MIC value.96
Fungal and oomycete infections are responsible for huge economic losses generated from plant diseases. Current strategies to control these infections include the direct application of chemical fungicides. Although potentially useful as agrofungicides, the majority of AG antibiotics are clinically used for the treatment of human bacterial pathogens. Their involvement in plant disease management could then promote bacterial resistance, reduce the soil concentration of the agrofungicide AG, or change its structure. To overcome this problem, AG derivatives possessing antifungal activities with no antibacterial capabilities were pursued.
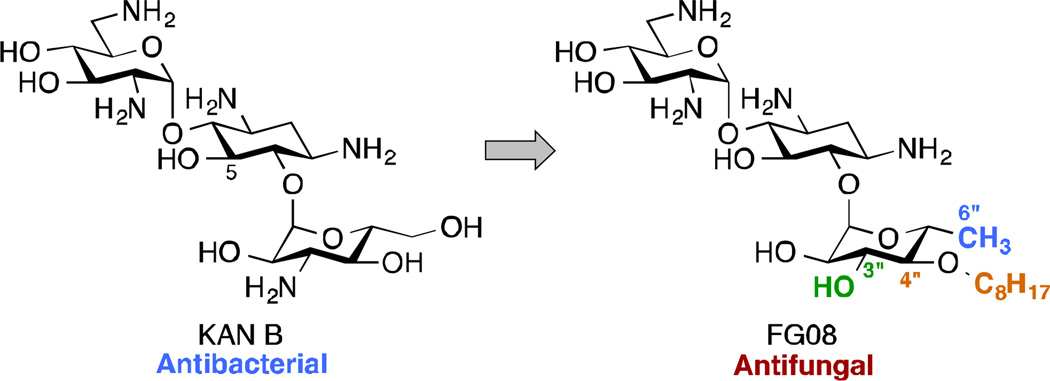
KAN B is a classical AG antibiotic isolated from the soil bacterium Streptomyces kanamyceticus and has long been used for its broad-spectrum activity against Gram-negative and Gram-positive bacteria. One of its analogue, FG08 (Fig. 7), which bears a 3"-hydroxyl group, a linear C8 alkyl chain at the 4"-position, and is deoxygenated at the 6"-position, showed little to no antibacterial activity, but inhibited the growth of various yeasts, oomycetes, and true fungi, with in vitro MIC values between 3.9 and 31.3 µg/mL.97 The antibacterial to antifungal activity “switch” in FG08 was attributed to the octyl chain, which conferred some amphiphilic properties to the AG. The susceptibility studies of Fusarium graminearum, a fungus responsible for the crop disease Fusarium Head Blight (FHB), to FG08 revealed that at concentration close to in vitro MIC, FG08 afforded prophylactic protection to FHB-susceptible wheat seedlings against F. graminearum. FG08 was also capable of suppressing FHB disease. FG08 was shown to exert its antifungal activity through permeation of the fungal membrane.97, 98 This property of certain AGs to disrupt the membrane of fungi has also been observed with bacteria.
Fig. 7.
Modifications at the 3"-, 4"-, and 6"-positions of the antibacterial KAN B result in the antifungal agent FG08.
Amphiphilic aminoglycosides
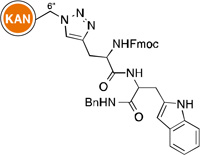
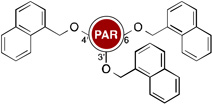
Owing to the recrudescence of antibiotic resistance by pathogenic bacteria and the promise of cationic amphiphiles as potent antimicrobial agents with new mechanisms of action, a novel class of antibacterial agents known as amphiphilic AGs came into being. Several groups have devoted tremendous efforts in the development of AG derivatives that bear in their structure hydrophobic moieties connected to the cationic hydrophilic AG. Linear alkyl chains, ranging from C6 to C20, have been introduced through an amide bond at the 5"-position of NEO,99–102 and the 6"-position of KAN103 and TOB.104 They have also been linked to TOB and PAR as thioethers at the 6"- and 5"-positions, respectively.105–107 Other hydrophobic residues investigated include (i) amino acids/peptides, which have been attached to the nitrogen atom of AGs through an amide bond,100 a lysine moiety,108 or a triazole ring;109, 110 and (ii) aromatic rings, attached at the oxygen atom, to form polyethers and/or polycarbamates of NEA, PAR, NEO, KAN, and AMK.111–114 This led to the discovery of potent amphiphilic AGs with revived antibacterial activity (Table 1). For example, the C16 and C18 lipid chains conferred to NEO a 32-fold improvement in antibacterial activity against MRSA, while the C16 lipid chain decreased the MIC of KAN from 128 to 2 µg/mL against Canadian clinical isolates of MRSE, and the C14 lipid chain rendered TOB 64 times more active against E. coli BL21 (DE3) (Eis) and S. mutans UA159. Aromatic rings also considerably ameliorated the MIC values of NEO, KAN, NEA, and PAR.
Table 1.
Activity of amphiphilic AGs.
| Structure | MIC (µg/mL) |
Strain | ↑a | Ref | Structure | MIC (µg/mL) |
Strain | ↑a | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 2.3 |
E. coli BL21 (DE3) (Eis)b |
64 | 105 | 8 |
P. aeruginosa CAN-ICU 62308 |
64 | 102 | ||
| 2.3 | B. subtilis 168 | 32 | 105 | 8 | MRSA ATCC33592 | 32 | 102 | ||
| 4.7 |
E. coli BL21 (DE3) (AAC(3)-IV)b |
32 | 105 |  |
4 | MRSA ATCC33592 | 64 | 102 | |
| 2 | S. mutans UA159 | 64 | 105 | (with all amino groups guanidinylated) |
16 |
P. aeruginosa CAN- ICU 62308 |
32 | 102 | |
| 2 |
S. pyogenes serotype M12 MGAS9429 |
32 | 105 |  |
1 | MRSA ATCC33592 | 256 | 113 | |
| 4 |
S. epidermidis ATCC35984 |
32 | 105 | 4 | S. maltophilia | 128 | 113 | ||
 |
8 | MRSA ATCC33592 | 64 | 109 | 4 8 |
MRSA ATCC33592 S. maltophilia |
64 >64 |
113 113 |
|
| 2 | MRSE CAN-ICU 61589 |
64 | 103,110 | 8 | MRSA ATCC33592 | 64 | 113 | ||
| (amino groups non- guanidinylated) |
16 | MRSA ATCC33592 | >32 | 103,110 | 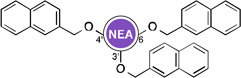 |
2 | MRSA ATCC33592 | >64 | 112, 114,117 |
| (with all amino groups guanidinylated) |
8 | MRSA ATCC33592 | 64 | 103 | 2 | S. aureus (APH(3'))b | >64 | 112, 114 | |
| 8 |
E. coli (TG1) (APH(3')-I)b |
250 | 100 | 4 |
P. aeruginosa PA22 (MexXY)c |
>32 | 117 | ||
| (amino groups non- guanidinylated) |
2–4 | MRSA ATCC33591 | 32–64 | 100,101 | 4 |
E. coli PAZ505H8101 (AAC(6’)-Ib) |
>32 | 117 | |
| 4–8 |
E. faecalis ATCC51299 (VRE) |
32–64 | 100,101 | 4 | VRSA-VRS-2 | >32 | 114, 117 | ||
| 2–4 |
E. faecalis ATCC29212 |
32 | 100,101 | 4 | S. aureus (ANT(4’))b | >32 | 114 | ||
| (with all amino groups guanidinylated) |
4 8 |
MRSA ATCC33592 P. aeruginosa CAN- ICU 62308 |
64 64 |
103 103 |
 |
2 |
E. coli PAZ505H8101 (AAC(6’)-Ib)b |
64 | 114 |
| 8 |
E. coli (TG1) (APH(3')-I)b |
250 | 100 | ||||||
| 4–8 | MRSA ATCC33591 | 16–32 | 100 | ||||||
| 8–16 |
E. faecalis ATCC51299 (VRE) |
16–32 | 100 | ||||||
Indicate increase (x-fold) in activity over that of the parent AG.
The resistance enzyme into parentheses is present in the bacterial strain studied.
This bacterial strain contains the efflux pump MexXY.
Amphiphiles, such as cationic peptides, have been shown to exert their antimicrobial activities by disrupting the bacterial membranes via three main models: the toroidal, barrel-stave, and carpet models.115 With the aim of investigating the mode of action of antibacterial amphiphilic AGs, the Garneau-Tsodikova and Fridman groups showed that amphiphilic AGs in the form of 6"-thioether TOB analogues target the bacterial membrane rather than the traditional bacterial ribosome.105 Similar results were also observed by the Chang116 and the Mingeot-Leclercq117 groups, who synthesized 5"-derivatized NEO as well as 3’- and 6-modified NEA, respectively.
Aminoglycosides as antiprotozoal agents: the fight against Leishmaniasis
The ability of AGs to bind to eukaryotic ribosomes to a lesser extent has also been exploited in the treatment of protozoan parasitic diseases. Leishmaniasis, a neglected tropical disease that affects 2 million people every year,118 has attracted special attention in the recent years due to the outbreak of Leishmania spp. resistant to current antimony-based therapy.119 The two main forms of leishmaniasis, cutaneous and visceral, are caused by the protozoa Leishmania major (L. major) and Leishmania donovi (L. donovi), respectively. In vitro growth inhibition studies of L. major and L. donovi showed that PAR and G418 were more potent than NEO, GEN, and APR.120–123 PAR even exhibited antileishmanial activity against antimony-resistant strains.124 This earned it a spot as a major ingredient of Leishcutan, a topical ointment used for the treatment of cutaneous leishmaniasis.125–127 In monotherapy or in combination with other drugs, PAR has also shown efficacy against visceral leishmaniasis (the most aggressive form of leishmaniasis),127–130 and is currently in Phase IV clinical trials in India.120
Efforts to elucidate the mechanisms of action of AGs against Leishmania spp. led to the finding that PAR might interact with the cytosolic ribosome,131, 132 where it induces codon misreading.121 The crystal structure of G418 bound to its putative Leishmania rRNA A-site shed some light on the molecular determinants involved in AG antiprotozoan mode of action.123 Extrusion of both A1492 and A1493 residues from the leishmanial site upon contact of G418 resembled the conformational change observed in the G418-bacterial binding site complex, reinforcing the miscoding character of antileishmanial AGs. PAR was also found to affect translation and vesicle-mediated trafficking in studies using PAR-susceptible and PAR-resistant L. donovani.133
Reading through the faulty “stops” at the premature termination codons
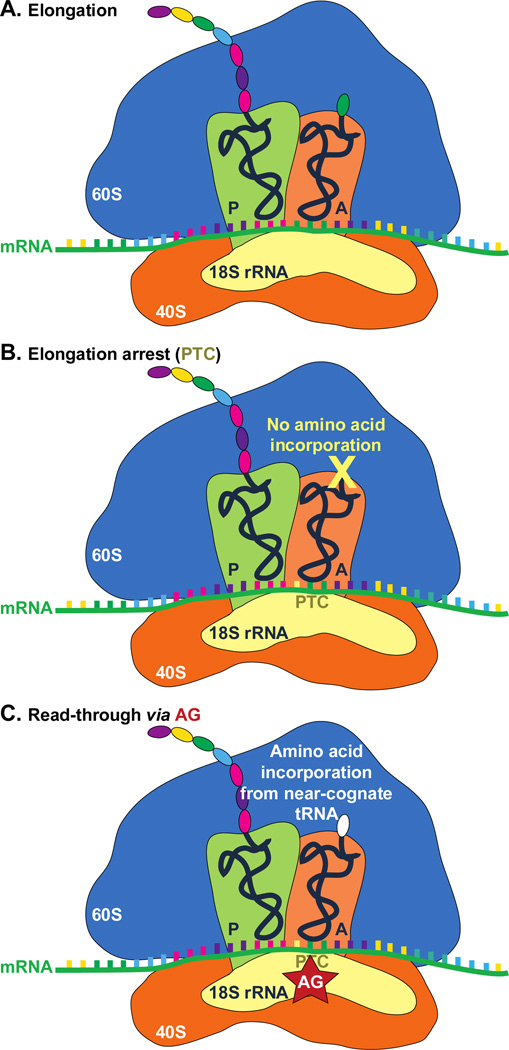
Pathogenic PTCs result from various mutations, including nonsense mutations, insertions or deletions, and/or alternative splicing events. In these diseases, PTCs are introduced prior to the natural stop codons, thereby leading to the production of truncated and, often times, defective proteins. AG interaction with eukaryotic ribosomes also sparkled their new application as PTC suppressing compounds. AG binding to the mammalian 18S rRNA A-site, which is homologous to the 16S rRNA in bacterial ribosome complex, has been proposed to promote near-cognate aminoacyl-tRNA misincorporation at PTC site and allow random read-through for full-length protein production in in-frame PTC diseases (Fig. 8).134, 135 Various AGs, including parent drugs and their derivatives, have been exploited for their read-through promoting activities in PTC diseases such as cystic fibrosis (CF), Duchenne muscular dystrophy (DMD), Rett syndrome (RTT), and other PTC disorders (Fig. 9 and Table 2).
Fig. 8.
Proposed mechanism of AG-induced PTC read-through. A. Eukaryotic ribosome complex during normal protein translation elongation. B. Elongation arrest at the PTC site due to nonsense mutations results in translation abortion and truncated protein product. C. Binding of AG to PTC site allows random incorporation of an amino acid from a near-cognate tRNA and read-through at the PTC site.
Fig. 9.
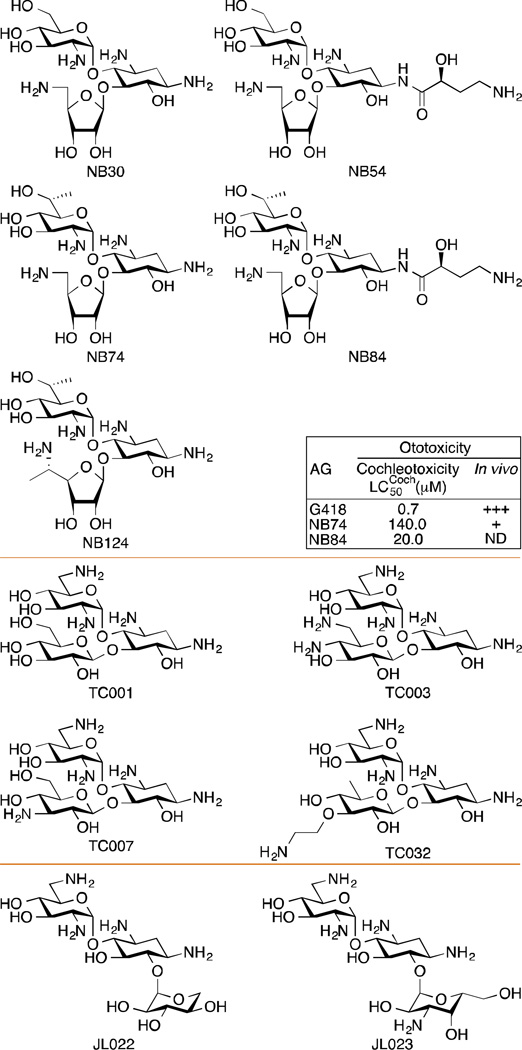
Structures of synthetic AG derivatives used for PTC-associated diseases. Note: Some of the NB series molecules have been shown to display reduced ototoxicity compared to G418.
Table 2.
AGs investigated to treat PTC mutations in various diseases.
| Disease | Gene | Mutations | AGs | Ref |
|---|---|---|---|---|
| CF | CFTR | Y122X | GEN | 136 |
| G521X | NB124 | 141 | ||
| G542X | AMK, GEN, TOB, NB54, NB74, NB84, NB124 | 138, 140–142, 154, 155 | ||
| R553X | GEN, NB54, NB84, NB124 | 141 | ||
| R1162X | GEN, G418, NB74, NB124 | 136, 141, 156 | ||
| W1282X | GEN, NB54, NB74, NB124 | 136, 140–142 | ||
| RTT | MECP2 | Y141X | GEN, G418 | 148 |
| R168X | GEN, G418, NB54, NB74 | 148–151 | ||
| Q170X | GEN, G418 | 148 | ||
| E205X | GEN, G418 | 148 | ||
| R255X | GEN, G418 | 148, 149 | ||
| R270X | GEN, NB54 | 149, 151 | ||
| R294X | AMK, GEN, G418, NB54 | 148, 149, 151, 153 | ||
| SMA | SMN1 | SMNΔ7 | TC001, TC003, TC007, TC032, JL022, JL023 | |
| Usher syndrome | PCDH15 | R3X | GEN, G418, PAR, NB54, NB74, NB84 | 142, 157 |
| R245X | GEN, G418, PAR, NB54, NB74, NB84 | 142, 157 | ||
| R643X | GEN, G418, PAR | 157 | ||
| R929X | GEN, G418, PAR | 157 | ||
| HS | IDUA | Q70X | GEN, NB54, NB74, NB84 | 142 |
| W402X | AMK, GEN, TOB | 158 | ||
| Factor VII deficiency | FVII | K316X W364X | GEN GEN | 159 159 |
| Obesity | MC4R | W16X | G418 | 160 |
| Y35X | G418 | 160 | ||
| Cancer | p53 | R192X | GEN, G418 | 161 |
| R213X | GEN, G418 | 161 | ||
| E298X | GEN, G418 | 161 | ||
| APC | R213X | GEN | 162 | |
| L360X | GEN | 162 | ||
| S811X | GEN | 162 | ||
| R1114X | GEN | 162 | ||
| Q1131X | GEN | 162 | ||
| Q1428X | GEN | 162 | ||
| R1450X | GEN | 162 | ||
| DMD | DMD | E1593X | GEN | 163 |
| Q60X | ||||
| Q988X | ||||
| mdx | ||||
| Q1240X | ||||
| Q1143X | ||||
| E2726X | ||||
| Q1437X | ||||
| Q2125X | ||||
| Q3149X | ||||
| W651X | ||||
| L1417X | ||||
| Q2522X | ||||
| E931X | ||||
| Q2264X | ||||
| Q673X | ||||
| R1326X | ||||
| C967X | ||||
| R3085X | ||||
| R744X | ||||
| R3190X | ||||
| R1549X | ||||
| R1967X | ||||
| R3381X | ||||
| R2098X | ||||
| R145X | ||||
| S319X | ||||
Cystic fibrosis (CF)
CF arises from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a transmembrane cAMP-gated chloride channel in the epithelium. Studies have shown that several parent AGs, such as AMK and GEN, promote read-through of the PTC and increase the level of functional CFTR in mouse models and patient samples.136–138 Furthermore, co-administration of AGs with poly-l-aspartic acid showed enhancement of the PTC suppression of GEN by 20–40%.139 A combination of the two drugs increased the level and duration of PTC suppression, as well as reduced the toxicity of GEN. In addition to parent AGs, their derivatives have also been exploited for higher efficacy and lower toxicity. Several AG derivatives, such as NB30, NB54, NB74, NB84, and NB124 (Fig. 9) have been reported to suppress PTC at higher potency and lower toxicity compared to parent AGs.140–142
Duchenne muscular dystrophy (DMD)
DMD presents lethal X-linked pathology that lacks the protein dystrophin in the muscle fibers, leading to progressive muscle degeneration. Nonsense mutations in the genetic sequence of dystrophin account for about 15% of all DMD cases.143 Glucocorticoids were the only beneficial treatment, but possessed significant side effects, calling for more efforts to develop AGs as treatments for DMD.144
As a result of the enormous size of dystrophin, an extensive number of PTCs has been identified (Table 2). GEN-treated mdx mouse showed rescued vascular parameters monitored by nitric oxide-dependent vascular functions.145 A study in patient samples also confirmed GEN as an effective PTC suppressing compound.146
As AGs exhibit various efficacies under distinct conditions, patients with different genetic variations at PTC can be subjected to different treatments. For example, PTCs with TGA mutations generally show better therapeutic response to GEN than patients with nonsense mutations TAA or TAG.147
Rett syndrome (RTT)
In contrast to DMD that only affects males, RTT as an X-linked PTC disorder solely impacts females. The PTCs for RTT are usually found in the MECP2 gene, which encodes methyl CpG-binding protein 2 (MeCP2) involved in epigenetic control of gene expressions. Common mutations in RTT were investigated in transiently transfected HEK293 cells. The read-through activity was recovered by multiple AGs, including GEN and G418, and weakly by AMK, while PAR was reported to have no read-through promoting activity for these mutations in RTT.148, 149 However, it was also reported that these AGs, though showing PTC suppressing properties in vitro, had no read-through activity effect at therapeutic dose.148 As the most prevalent mutation, R168X was also tested against PAR derivatives NB30, NB54, and NB84. Even though NB30 was not as promising, NB54 and NB84 both showed better efficiency than GEN as PTC suppressing drugs.150, 151
Spinal muscular atrophy (SMA)
SMA is a neurodegenerative disorder that results from the absence of survival motor neuron-1 (SMN1) and symptomized by progressive atrophy of the limb and trunk muscles. SMN genes are present in two nearly identical copies (SMN1 and SMN2) that differ by a C (SMN1) to T (SMN2) transition, leading to an alternative splicing pattern of SMN1 of the mRNA. The PTC in SMN1 results in a truncated non-functional protein that cannot be functionally compensated by SMN2.152 As AGs are used to suppress PTCs, studies have also utilized AGs to treat SMA to restore functional protein production.153 The Lorson’s group tested 20 synthetic AGs that belong to either the 4,5- or 4,6-disubstituted 2-DOS families. Six of them, TC001, TC003, TC007, TC032, JL022, and JL023 (Fig. 9) were shown to increase the number of “gems”, which is indicative of the alleviation of SMA disease progression, and functional protein level.153 One of the six compounds, TC007, was further characterized in an animal model and proved to partially restore phenotype.164, 165
Other PTC diseases
Type I Usher disease,157, 166 congenital muscular dystrophy (CMD),167 ataxia-telangiectasia (A–T),168 coagulation factor VII deficiency,159 mucopolysaccharidosis type I-Hurler (MPS I-H) disease,169 various types of cancer,161, 162 and other types of genetic disorders170, 171 have been investigated for the full-length protein production response after treating with AGs or their derivatives. Overall, GEN and G418 are the two most commonly tested AGs that improve PTC read-through. The response to AG treatment is dose and context dependent.147
Long-term AG treatment, which produces significant toxicity and bacterial resistance, has promoted the development of AG derivatives that possess higher potency and less toxicity. For instance, NB74 was reported to have a half-maximal lethal concentration (LC50) of 25.8 mM in HEK293 cells, which is about 10 times higher than the LC50 for GEN at 2.5 mM and 20 times higher than that for G418 at 1.3 mM.142 NB74 was also shown to decrease cochleotoxicity (LC50 = 140.0 µM) compared to G418 (LC50 = 0.7 µM) (Fig. 9).172 There have been discoveries made on other read-through promoting compounds in order to achieve lower toxicity than parent AGs, for instance the organic compound PTC124. However, the lower potency and large variations in the cellular response within each treatment has limited the use of PTC124 as a general treatment for multiple PTC diseases.173, 174 Therefore, the research on AGs supressing PTC, especially by their derivatization for improved potency and reduced toxicity, remains promising and inviting.
New ways to alleviate aminoglycoside toxicity
Toxicity has always been and still remains a serious impediment to the long-term clinical use of AGs. Indeed, a few years after the discovery of STR and its introduction in the therapeutic regimen of tuberculosis, patients’ kidneys and inner ear impairment were observed, resulting in AG-induced nephrotoxicity and ototoxicity, respectively.175
Nephrotoxicity arises from the small accumulation of AG drugs in the kidney cortex as a result of their uptake in the epithelial cells of the renal proximal tubules, following their glomerular filtration and excretion from the bloodstream by the urinary system.176 Nephrotoxicity is a reversible process due in part to the regeneration capability of tubular cells177 and the ability of dialysis to diminish AG accumulation in the kidneys. Ototoxicity, on the other hand, leads to permanent and irreversible hearing loss. AGs’ damage to the inner ear has been shown to be directed to the vestibular organ178 and the outer hair cells located in the cochlea.179, 180 It was reported that ≤15% of AG-treated patients develop vestibulotoxicity, while 2 to 25 % experienced cochleotoxicity.181, 182 Another estimate of ototoxicity incidence in patients administered AGs was shown to lie between 3 to 33%.180
Approaches to reduce or protect against AG-induced nephrotoxicity deleterious effects have previously been reviewed,183 and since it is less aggressive than its ototoxicity counterpart, we will briefly focus on understanding the mechanisms of AG-induced ototoxicity for a better appreciation of current ways utilized to alleviate it. A more detailed presentation of the mechanisms of AG ototoxicity has recently been reported.175
Mechanisms of ototoxicity
Following their uptake by inner ear sensory hair cells,184–188 parenterally and topically administered AGs appear to induce ototoxicity either through the formation of reactive oxygen species,189 activation of cochlear N-methyl-d-aspartate (NMDA) receptors,190 or increase of nitric oxide synthase activity.191, 192 Individuals carrying the maternally inherited mutations A1555G or C1494T, which are both localized on the A-site of the human mitochondrial 12S rRNA, have also been shown to be more vulnerable to AG ototoxicity.193–196 With the mitochondrion appearing to be a common denominator in all these mechanisms, mitochondrial protein synthesis may then be an essential target in AG ototoxicity.
Böttger and co-workers engineered hybrid bacterial ribosomes, whose decoding site mimicked that of the human mitochondrial 12S rRNA, and also contained either the A1555G or C1494T congenital deafness mutations.197, 198 The protein translation fidelity in these hybrid mitochondrial ribosomes (mitoribosomes) appeared to be disturbed in the presence of AGs. This misreading process could then lead to hair cell death i.e. AG ototoxicity. Francis and co-workers, however, recently showed that inhibition of the cytosolic, rather than mitochondrial, protein synthesis better compares to AG-induced hearing loss.199 In vivo studies reported by Baasov and co-workers tipped the scale towards mitochondrial protein synthesis inhibition as the major contributor to AG ototoxicity.172
Several antioxidants, including salicylate,200 aspirin,201 N-acetylcysteine,202, 203 mitoquinone,204 dexamethasone,205 melatonin,205 tacrolimus,205 SkQR1,206 have been shown to bear otoprotective properties when used in conjunction with AGs. The use of NMDA receptor antagonists has also been shown to alleviate AG-induced ototoxicity.207
Novel AG derivatives with reduced toxicity have also been designed based on the correlation that has been shown between the AGs’ structural scaffold and their toxicity. It was reported that reducing the number of amino groups on AGs may lead to reduced toxicity.208 Furthermore, a decrease in basicity of the amino groups, as a result of neighbouring group effect, was shown to also influence AG toxicity.209
Apramycin
Apramycin (APR) (Fig. 1) is an AG produced by the microorganism Streptomyces tenebrarius210 with unique structural features that allow it to escape bacterial resistance other legacy AGs are usually subjected to. It is a potent antibiotic with growth inhibitory action against Gram-positive and Gram-negative bacteria.211 It has found applications in veterinary medicine, but its use in humans is currently prohibited. Recent findings have however shown that APR does not display the characteristic ototoxicity generally observed with AGs.212 As a matter of fact, in a mice organ of Colti explant model, APR was found not to trigger the loss of hair cells in the base of cochlear at a concentration of 0.2 mM, dose at which GEN completely destroyed those hair cells. Minimal hair cells loss was only noticeable at 2 mM APR. Even in in vivo ototoxicity studies performed in guinea pig by measuring the auditory brain response at 12 kHz and the loss of hair cells, APR was less toxic than GEN. Since the ototoxicity of AGs has been correlated to their limited selectivity in preferentially binding bacterial ribosomes over mammalian ones,197, 198 it was interesting to find that APR does not significantly upset the mitochondrial ribosome as the other AG antibiotics do. Indeed, while APR exhibited comparable protein synthesis inhibitory activities in bacterial and hybrid human cytosolic ribosomes with GEN, TOB, KAN, and NEO, it perturbed the protein synthesis in hybrid human mitochondrial (wild-type and A1555G mutant) ribosomes to a much lesser extent. These results were further confirmed in eukaryotic rabbit reticulocyte ribosomes and in vitro mitochondrial in organelle translation assay. Analysis of the crystal structure of the APR-Thermus thermophiles 30S subunit h44 complex, together with the aprosamine-induced misreading of mitohybrid ribosomes, revealed that ring III of APR may prevent A1492 residue to puck out and may thus be essential in poorly inducing misreading in mitochondria. In the future, it will be fascinating to develop APR derivatives that retain these key structural features that will allow them to overcome ototoxicity and see if they will be able to find applications in the treatment of human bacterial infections.
New AGs: Plazomicin
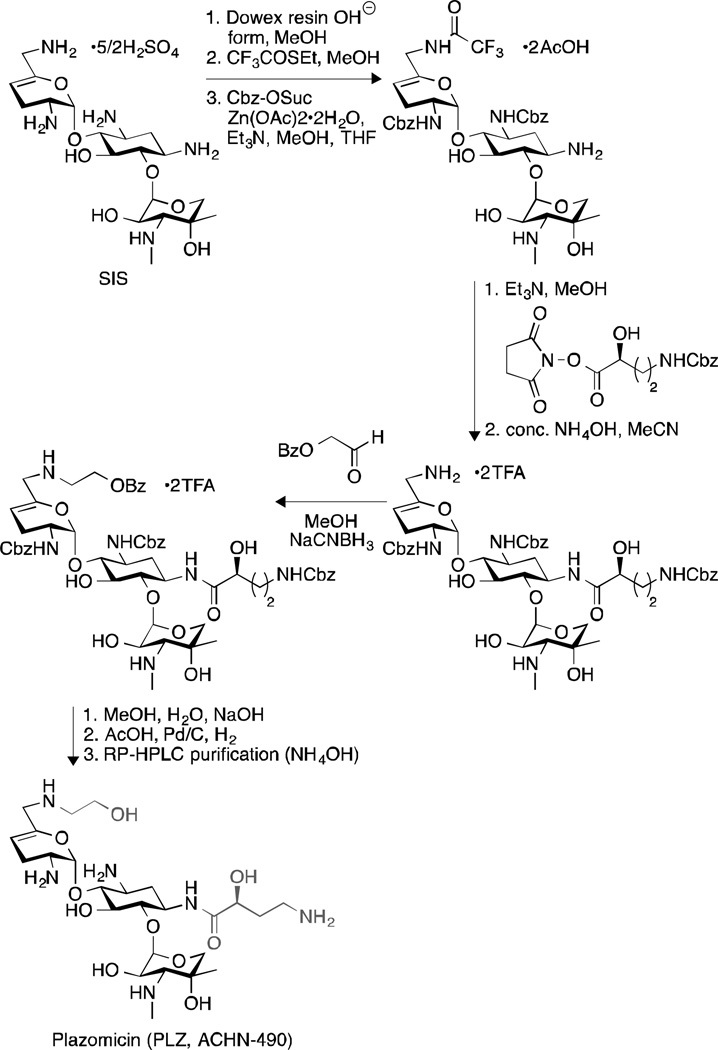
Plazomicin (PLZ), originally known as ACHN-490, is a semi-synthetic “neoglycoside” developed by Achaogen (San Francisco, CA, USA) that has shown great promise in alleviating nephrotoxicity and ototoxicity. Derived in eight steps from the AG sisomicin (SIS) (Fig. 10), PLZ has been shown to exhibit enhanced in vitro potency against AG-susceptible and AG-resistant pathogens.213 It was active against most Enterobacteriaceae species at a concentration below 4 µg/mL.213 Indeed, it displayed an MIC50/90 value of 0.5/1 µg/mL against 3050 E. coli clinical isolates from New York214 and 1/1 µg/mL against 26 Enterobacter spp. gathered from Athens, Greece.215 Interestingly, it even exhibited an MIC90 value of 1 µg/mL against a large collection of unique-patient Klebsiella pneumoniae isolates, including those producing carbapenemases (KPC) (blaKPC-containing isolates), which was at least 32-fold lower than the clinically used AGs AMK, GEN, and TOB.214, 216 However, PLZ was found to be inactive against any Enterobacteriaceae isolates carrying the ribosomal methyltransferase-encoding genes ArmA and/or RmtC.213, 217 It was also inactive against Providencia stuartii (MIC > 64 µg/mL), as a result of the inactivation by the AAC(2’)-I enzyme. In general, PLZ has been shown to overcome the action of most AG resistance enzymes.213
Fig. 10.
Synthesis of PLZ.
When evaluated against nocosomial pathogens Acinetobacter baumannii and Pseudomonas aeruginosa, PLZ was moderately active, with MIC50/90 of 8/16 µg/mL and 8/32 µg/mL, respectively;218 and quite active against AG-resistant staphylococci (MIC50/90 = 1/1 µg/mL).213
In light of these promising in vitro results, the in vivo efficacy of PLZ was tested in animals.219 In a septicemia model, PLZ was able to increase the survival rate of mice infected with E. coli ATCC25922 (ED50 = 0.6 mg/kg) and P. aeruginosa ATCC27853 (ED50 = 8.3 mg/kg) over seven days, as a function of the dose administered. A murine neutropenic thigh model also confirmed that PLZ was capable of treating bacterial infections caused by E. coli (susceptible and MDR), K. pneumoniae (susceptible, MDR, and strains expressing KPC), P. aeruginosa (susceptible), Serratia marcescens (with KPC phenotype), and Staphylococcus aureus (MDR).
All these satisfactory outcomes of in vitro and in vivo antibacterial studies led to the pharmacokinetic evaluation and the safety monitoring of PLZ injection, with no nephrotoxicity and ototoxicity observed so far in humans.220
Perspective and conclusions
AGs are broad-spectrum antibiotics that have long suffered from bacterial resistance and toxicity. While enormous efforts have been directed towards developing new strategies to minimize these accompanied side effects, novel applications of AGs as antifungal, antiprotozoan, and genetic regulating compounds have also being investigated.
The incessant need to better understand AGs’ mechanisms of action on the bacterial ribosomes has led researchers to the discovery of the h69 binding site, which could be another potential target of AGs and could be useful to develop novel AG drugs. The discovery of AME inhibitors and their use in combination with AGs also remain an interesting avenue to explore for counteracting bacterial resistance.
In addition to their traditional role as antibacterial agents, AGs have also emerged as potential antifungal agents and we believe that in the future, it will be important to pay attention to the derivatization of AGs as cationic amphiphiles for the development of novel fungicides. The capacity of certain AGs to bind to eukaryotic ribosomes has also been exploited for the treatment of parasitic diseases and PTC disorders. Although more attention had been directed towards cystic fibrosis and Duchenne muscular dystrophy, it will be worthy investigating how parent AGs and their derivatives could help in treating other PTC disorders. However, it should not be forgotten that ototoxicity is an intrinsic side effect of AGs. Priority should then be given to those parent compounds, such as APR, that have demonstrated limited toxicity to mammalian cells.
There are currently only six AGs (AMK, GEN, NEO, PAR, STR, and TOB) that are approved by the US Food and Drug Administration for clinical use. However, with the renaissance experienced by AGs in the past decades, notably with the discovery of PLZ and its satisfactory results in clinical trials, there is great hope that new clinically useful AGs can still be discovered. Together with the promising use of AGs described herein, it thus becomes apparent that AGs remain a valuable potential source of drugs.
Acknowledgements
Our work on aminoglycoside antibiotics is funded by a National Institute of Health (NIH) grant AI090048 (S.G.-T.) as well as startup funds from the College of Pharmacy at the University of Kentucky (S.G.-T.). We thank all of those working on aminoglycosides including those that we could not cite due to the scope of the review.
Notes and references
- 1.Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. ChemBioChem. 2010;11:880–902. doi: 10.1002/cbic.200900779. [DOI] [PubMed] [Google Scholar]
- 2.Magnet S, Blanchard JS. Chem. Rev. 2005;105:477–498. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- 3.Feldman MB, Terry DS, Altman RB, Blanchard SC. Nat. Chem. Biol. 2010;6:54–62. doi: 10.1038/nchembio.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovinskaya MA, Pai RD, Zhang W, Schuwirth BS, Holton JM, Hirokawa G, Kaji H, Kaji A, Cate JH. Nat. Struct. Molec. Biol. 2007;14:727–732. doi: 10.1038/nsmb1271. [DOI] [PubMed] [Google Scholar]
- 5.Park SR, Park JW, Ban YH, Sohng JK, Yoon YJ. Nat. Prod. Rep. 2013;30:11–20. doi: 10.1039/c2np20092a. [DOI] [PubMed] [Google Scholar]
- 6.Galimand M, Courvalin P, Lambert T. Antimicrob. Agents Chemother. 2003;47:2565–2571. doi: 10.1128/AAC.47.8.2565-2571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker B, Cooper MA. ACS Chem. Biol. 2013;8:105–115. doi: 10.1021/cb3005116. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez MS, Tolmasky ME. Drug Resist. Updates. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi K, Berghuis AM. J. Biol. Chem. 2012;287:13094–13102. doi: 10.1074/jbc.M112.349670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakya T, Wright GD. Antimicrob. Agents Chemother. 2010;54:1909–1913. doi: 10.1128/AAC.01570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CA, Toth M, Frase H, Byrnes LJ, Vakulenko SB. J. Biol. Chem. 2012;287:12893–12903. doi: 10.1074/jbc.M112.341206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter VR, Green KD, Zolova OE, Houghton JL, Garneau-Tsodikova S. Biochem. Biophys. Res. Commun. 2010;403:85–90. doi: 10.1016/j.bbrc.2010.10.119. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Biswas T, Porter VR, Tsodikov OV, Garneau-Tsodikova S. Proc. Nat. Acad. Sci., U.S.A. 2011;108:9804–9808. doi: 10.1073/pnas.1105379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton JL, Green KD, Pricer RE, Mayhoub AS, Garneau-Tsodikova S. J. Antimicrob. Chemother. 2013;68:800–805. doi: 10.1093/jac/dks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KH, An DR, Song J, Yoon JY, Kim HS, Yoon HJ, Im HN, Kim J, Kim do J, Lee SJ, Kim KH, Lee HM, Kim HJ, Jo EK, Lee JY, Suh SW. Proc. Nat. Acad. Sci., U.S.A. 2012;109:7729–7734. doi: 10.1073/pnas.1120251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Green KD, Tsodikov OV, Garneau-Tsodikova S. Biochemistry. 2012;51:4959–4967. doi: 10.1021/bi3004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pricer RE, Houghton JL, Green KD, Mayhoub AS, Garneau-Tsodikova S. Molec. Biosyst. 2012;8:3305–3313. doi: 10.1039/c2mb25341k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsodikov OV, Green KD, Garneau-Tsodikova S. PloS one. 2014;9:e92370. doi: 10.1371/journal.pone.0092370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton JL, Biswas T, Chen W, Tsodikov OV, Garneau-Tsodikova S. ChemBioChem. 2013;14:2127–2135. doi: 10.1002/cbic.201300359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings BC, Labby KJ, Green KD, Garneau-Tsodikova S. Biochemistry. 2013;52:5125–5132. doi: 10.1021/bi4002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Green KD, Garneau-Tsodikova S. Antimicrob. Agents Chemother. 2012;56:5831–5838. doi: 10.1128/AAC.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green KD, Chen W, Garneau-Tsodikova S. ChemMedChem. 2012;7:73–77. doi: 10.1002/cmdc.201100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labby KJ, Garneau-Tsodikova S. Future Med. Chem. 2013;5:1285–1309. doi: 10.4155/fmc.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drawz SM, Bonomo RA. Clin. Microbiol. Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amstutz P, Binz HK, Parizek P, Stumpp MT, Kohl A, Grutter MG, Forrer P, Pluckthun A. J. Biol. Chem. 2005;280:24715–24722. doi: 10.1074/jbc.M501746200. [DOI] [PubMed] [Google Scholar]
- 26.Kohl A, Amstutz P, Parizek P, Binz HK, Briand C, Capitani G, Forrer P, Pluckthun A, Grutter MG. Structure. 2005;13:1131–1141. doi: 10.1016/j.str.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Shakya T, Stogios PJ, Waglechner N, Evdokimova E, Ejim L, Blanchard JE, McArthur AG, Savchenko A, Wright GD. Chem Biol. 2011;18:1591–1601. doi: 10.1016/j.chembiol.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Fong DH, Xiong B, Hwang J, Berghuis AM. PloS one. 2011;6:e19589. doi: 10.1371/journal.pone.0019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suga T, Ishii T, Iwatsuki M, Yamamoto T, Nonaka K, Masuma R, Matsui H, Hanaki H, Omura S, Shiomi K. J. Antibiot. 2012;65:527–529. doi: 10.1038/ja.2012.53. [DOI] [PubMed] [Google Scholar]
- 30.Welch KT, Virga KG, Whittemore NA, Ozen C, Wright E, Brown CL, Lee RE, Serpersu EH. Bioorg. Med. Chem. 2005;13:6252–6263. doi: 10.1016/j.bmc.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 31.Blount KF, Zhao F, Hermann T, Tor Y. J. Am. Chem. Soc. 2005;127:9818–9829. doi: 10.1021/ja050918w. [DOI] [PubMed] [Google Scholar]
- 32.Zhao F, Zhao Q, Blount KF, Han Q, Tor Y, Hermann T. Angew. Chem. 2005;44:5329–5334. doi: 10.1002/anie.200500903. [DOI] [PubMed] [Google Scholar]
- 33.Asensio JL, Hidalgo A, Bastida A, Torrado M, Corzana F, Chiara JL, Garcia-Junceda E, Canada J, Jimenez-Barbero J. J. Am. Chem. Soc. 2005;127:8278–8279. doi: 10.1021/ja051722z. [DOI] [PubMed] [Google Scholar]
- 34.Bastida A, Hidalgo A, Chiara JL, Torrado M, Corzana F, Perez-Canadillas JM, Groves P, Garcia-Junceda E, Gonzalez C, Jimenez-Barbero J, Asensio JL. J. Am. Chem. Soc. 2006;128:100–116. doi: 10.1021/ja0543144. [DOI] [PubMed] [Google Scholar]
- 35.Kling D, Hesek D, Shi Q, Mobashery S. J. Org. Chem. 2007;72:5450–5453. doi: 10.1021/jo0707636. [DOI] [PubMed] [Google Scholar]
- 36.Hanessian S, Szychowski J, Campos-Reales Pineda NB, Furtos A, Keillor JW. Bioorg. Med. Chem. Lett. 2007;17:3221–3225. doi: 10.1016/j.bmcl.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Chen Y, Liang Q, Li H, Jin H, Zhang L, Meng X, Li Z. J. Org. Chem. 2013;78:400–409. doi: 10.1021/jo302247x. [DOI] [PubMed] [Google Scholar]
- 38.Michael K, Wang H, Tor Y. Bioorg. Med. Chem. 1999;7:1361–1371. doi: 10.1016/s0968-0896(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 39.Sucheck SJ, Wong AL, Koeller KM, Boehr DD, Draker K-A, Sears P, Wright GD, Wong C-H. J. Am. Chem. Soc. 2000;122:5230–5231. [Google Scholar]
- 40.Agnelli F, Sucheck SJ, Marby KA, Rabuka D, Yao SL, Sears PS, Liang FS, Wong CH. Angew. Chem. 2004;43:1562–1566. doi: 10.1002/anie.200353225. [DOI] [PubMed] [Google Scholar]
- 41.Santana AG, Batisda A, Del Campo TM, Asensio JL, Revuelta J. Synlett. 2011;2:219–222. [Google Scholar]
- 42.Kumar S, Xue L, Arya DP. J. Am. Chem. Soc. 2011;133:7361–7375. doi: 10.1021/ja108118v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkov-Zrihen Y, Green KD, Labby KJ, Feldman M, Garneau-Tsodikova S, Fridman M. J. Med. Chem. 2013;56:5613–5625. doi: 10.1021/jm400707f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas JR, Liu X, Hergenrother PJ. J. Am. Chem. Soc. 2005;127:12434–12435. doi: 10.1021/ja051685b. [DOI] [PubMed] [Google Scholar]
- 45.Thomas JR, Liu X, Hergenrother PJ. Biochemistry. 2006;45:10928–10938. doi: 10.1021/bi0607296. [DOI] [PubMed] [Google Scholar]
- 46.Bodlenner A, Alix A, Weibel JM, Pale P, Ennifar E, Paillart JC, Walter P, Marquet R, Dumas P. Org. Lett. 2007;9:4415–4418. doi: 10.1021/ol701760k. [DOI] [PubMed] [Google Scholar]
- 47.Luedtke NW, Liu Q, Tor Y. Biochemistry. 2003;42:11391–11403. doi: 10.1021/bi034766y. [DOI] [PubMed] [Google Scholar]
- 48.Riguet E, Desire J, Boden O, Ludwig V, Gobel M, Bailly C, Decout JL. Bioorg. Med. Chem. Lett. 2005;15:4651–4655. doi: 10.1016/j.bmcl.2005.07.082. [DOI] [PubMed] [Google Scholar]
- 49.Lee LV, Bower KE, Liang FS, Shi J, Wu D, Sucheck SJ, Vogt PK, Wong CH. J. Am. Chem. Soc. 2004;126:4774–4775. doi: 10.1021/ja0495359. [DOI] [PubMed] [Google Scholar]
- 50.Fridman M, Belakhov V, Lee LV, Liang FS, Wong CH, Baasov T. Angew. Chem. 2005;44:447–452. doi: 10.1002/anie.200462003. [DOI] [PubMed] [Google Scholar]
- 51.Numa MM, Lee LV, Hsu CC, Bower KE, Wong CH. ChemBioChem. 2005;6:1002–1006. doi: 10.1002/cbic.200500009. [DOI] [PubMed] [Google Scholar]
- 52.Fair RJ, Hensler ME, Thienphrapa W, Dam QN, Nizet V, Tor Y. ChemMedChem. 2012;7:1237–1244. doi: 10.1002/cmdc.201200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai A, Uemura S, Johansson M, Puglisi EV, Marshall RA, Aitken CE, Korlach J, Ehrenberg M, Puglisi JD. Cell Rep. 2013;3:497–508. doi: 10.1016/j.celrep.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaul M, Barbieri CM, Pilch DS. J. Am. Chem. Soc. 2004;126:3447–3453. doi: 10.1021/ja030568i. [DOI] [PubMed] [Google Scholar]
- 55.Kaul M, Barbieri CM, Pilch DS. J. Am. Chem. Soc. 2006;128:1261–1271. doi: 10.1021/ja056159z. [DOI] [PubMed] [Google Scholar]
- 56.Llano-Sotelo B, Hickerson RP, Lancaster L, Noller HF, Mankin AS. RNA. 2009;15:1597–1604. doi: 10.1261/rna.1681609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.François B, Szychowski J, Adhikari SS, Pachamuthu K, Swayze EE, Griffey RH, Migawa MT, Westhof E, Hanessian S. Angew. Chem. 2004;43:6735–6738. doi: 10.1002/anie.200462092. [DOI] [PubMed] [Google Scholar]
- 58.Anderson PC, Mecozzi S. Biopolymers. 2007;86:95–111. doi: 10.1002/bip.20707. [DOI] [PubMed] [Google Scholar]
- 59.Liang FS, Greenberg WA, Hammond JA, Hoffmann J, Head SR, Wong CH. Proc. Nat. Acad. Sci., U.S.A. 2006;103:12311–12316. doi: 10.1073/pnas.0605264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aminova O, Paul DJ, Childs-Disney JL, Disney MD. Biochemistry. 2008;47:12670–12679. doi: 10.1021/bi8012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paul DJ, Seedhouse SJ, Disney MD. Nucl. Acids Res. 2009;37:5894–5907. doi: 10.1093/nar/gkp594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tran T, Disney MD. Biochemistry. 2010;49:1833–1842. doi: 10.1021/bi901998m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran T, Disney MD. Biochemistry. 2011;50:962–969. doi: 10.1021/bi101724h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Pulk A, Wasserman MR, Feldman MB, Altman RB, Cate JH, Blanchard SC. Nat. Struct. Molec. Biol. 2012;19:957–963. doi: 10.1038/nsmb.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.François B, Russell RJ, Murray JB, Aboul-ela F, Masquida B, Vicens Q, Westhof E. Nucl. Acids Res. 2005;33:5677–5690. doi: 10.1093/nar/gki862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobbie SN, Pfister P, Bruell C, Sander P, François B, Westhof E, Böttger EC. Antimicrob. Agents Chemother. 2006;50:1489–1496. doi: 10.1128/AAC.50.4.1489-1496.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hobbie SN, Pfister P, Brull C, Westhof E, Böttger EC. Antimicrob. Agents Chemother. 2005;49:5112–5118. doi: 10.1128/AAC.49.12.5112-5118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poehlsgaard J, Douthwaite S. Nat. Rev. Microbiol. 2005;3:870–881. doi: 10.1038/nrmicro1265. [DOI] [PubMed] [Google Scholar]
- 69.Akshay S, Bertea M, Hobbie SN, Oettinghaus B, Shcherbakov D, Böttger EC, Akbergenov R. Antimicrob. Agents Chemother. 2011;55:4096–4102. doi: 10.1128/AAC.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez-Fernandez D, Shcherbakov D, Matt T, Leong NC, Kudyba I, Duscha S, Boukari H, Patak R, Dubbaka SR, Lang K, Meyer M, Akbergenov R, Freihofer P, Vaddi S, Thommes P, Ramakrishnan V, Vasella A, Böttger EC. Nat. Commun. 2014;5:3112. doi: 10.1038/ncomms4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blount KF, Tor Y. ChemBioChem. 2006;7:1612–1621. doi: 10.1002/cbic.200600109. [DOI] [PubMed] [Google Scholar]
- 72.Scheunemann AE, Graham WD, Vendeix FA, Agris PF. Nucl. Acids Res. 2010;38:3094–3105. doi: 10.1093/nar/gkp1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trabuco LG, Schreiner E, Eargle J, Cornish P, Ha T, Luthey-Schulten Z, Schulten K. J. Molec. Biol. 2010;402:741–760. doi: 10.1016/j.jmb.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorner S, Brunelle JL, Sharma D, Green R. Nat. Struct. Molec. Biol. 2006;13:234–241. doi: 10.1038/nsmb1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunkle JA, Wang L, Feldman MB, Pulk A, Chen VB, Kapral GJ, Noeske J, Richardson JS, Blanchard SC, Cate JH. Science. 2011;332:981–984. doi: 10.1126/science.1202692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munro JB, Altman RB, O’Connor N, Blanchard SC. Molec. Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foster C, Champney WS. Arch. Microbiol. 2008;189:441–449. doi: 10.1007/s00203-007-0334-6. [DOI] [PubMed] [Google Scholar]
- 78.Grundy FJ, Henkin TM. Crit. Rev. Biochem. Molec. Biol. 2006;41:329–338. doi: 10.1080/10409230600914294. [DOI] [PubMed] [Google Scholar]
- 79.Deigan KE, Ferre-D’Amare AR. Acc. Chem. Res. 2011;44:1329–1338. doi: 10.1021/ar200039b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weigand JE, Sanchez M, Gunnesch EB, Zeiher S, Schroeder R, Suess B. RNA. 2008;14:89–97. doi: 10.1261/rna.772408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weigand JE, Schmidtke SR, Will TJ, Duchardt-Ferner E, Hammann C, Wohnert J, Suess B. Nucl. Acids Res. 2011;39:3363–3372. doi: 10.1093/nar/gkq946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duchardt-Ferner E, Weigand JE, Ohlenschlager O, Schmidtke SR, Suess B, Wohnert J. Angew. Chem. 2010;49:6216–6219. doi: 10.1002/anie.201001339. [DOI] [PubMed] [Google Scholar]
- 83.Schmidtke SR, Duchardt-Ferner E, Weigand JE, Suess B, Wohnert J. Biomolec. NMR Assign. 2010;4:115–118. doi: 10.1007/s12104-010-9223-z. [DOI] [PubMed] [Google Scholar]
- 84.Krstic I, Frolow O, Sezer D, Endeward B, Weigand JE, Suess B, Engels JW, Prisner TF. J. Am. Chem. Soc. 2010;132:1454–1455. doi: 10.1021/ja9077914. [DOI] [PubMed] [Google Scholar]
- 85.Jia X, Zhang J, Sun W, He W, Jiang H, Chen D, Murchie AI. Cell. 2013;152:68–81. doi: 10.1016/j.cell.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 86.Ambros V. Nat. Med. 2008;14:x–xiv. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 87.Vo DD, Staedel C, Zehnacker L, Benhida R, Darfeuille F, Duca M. Chem Biol. 2014 doi: 10.1021/cb400668h. [DOI] [PubMed] [Google Scholar]
- 88.Belair C, Baud J, Chabas S, Sharma CM, Vogel J, Staedel C, Darfeuille F. Silence. 2011;2:7. doi: 10.1186/1758-907X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velagapudi SP, Gallo SM, Disney MD. Nat. Chem. Biol. 2014;10:291–297. doi: 10.1038/nchembio.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee MM, Pushechnikov A, Disney MD. ACS Chem. Biol. 2009;4:345–355. doi: 10.1021/cb900025w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Childs-Disney JL, Wu M, Pushechnikov A, Aminova O, Disney MD. ACS Chem. Biol. 2007;2:745–754. doi: 10.1021/cb700174r. [DOI] [PubMed] [Google Scholar]
- 92.Disney MD, Childs-Disney JL. ChemBioChem. 2007;8:649–656. doi: 10.1002/cbic.200600569. [DOI] [PubMed] [Google Scholar]
- 93.Childs-Disney JL, Parkesh R, Nakamori M, Thornton CA, Disney MD. ACS Chem. Biol. 2012;7:1984–1993. doi: 10.1021/cb3001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee HB, Kim Y, Kim JC, Choi GJ, Park SH, Kim CJ, Jung HS. J. Appl. Microbiol. 2005;99:836–843. doi: 10.1111/j.1365-2672.2005.02684.x. [DOI] [PubMed] [Google Scholar]
- 95.Mahl DL, de Jesus FP, Loreto E, Zanette RA, Ferreiro L, Pilotto MB, Alves SH, Santurio JM. Antimicrob. Agents Chemother. 2012;56:4021–4023. doi: 10.1128/AAC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yutani M, Ogita A, Fujita K-I, Tanaka T. Int. J. Life Sci. Med. Res. 2013;3:193–199. [Google Scholar]
- 97.Chang CW, Fosso M, Kawasaki Y, Shrestha S, Bensaci MF, Wang J, Evans CK, Takemoto JY. J. Antibiot. 2010;63:667–672. doi: 10.1038/ja.2010.110. [DOI] [PubMed] [Google Scholar]
- 98.Shrestha S, Grilley M, Fosso MY, Chang CW, Takemoto JY. PloS one. 2013;8:e73843. doi: 10.1371/journal.pone.0073843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bera S, Zhanel GG, Schweizer F. J. Med. Chem. 2008;51:6160–6164. doi: 10.1021/jm800345u. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Chiang FI, Wu L, Czyryca PG, Li D, Chang CW. J. Med. Chem. 2008;51:7563–7573. doi: 10.1021/jm800997s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J, Keller K, Takemoto JY, Bensaci M, Litke A, Czyryca PG, Chang CW. J. Antibiot. 2009;62:539–544. doi: 10.1038/ja.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bera S, Dhondikubeer R, Findlay B, Zhanel GG, Schweizer F. Molecules. 2012;17:9129–9141. doi: 10.3390/molecules17089129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bera S, Zhanel GG, Schweizer F. J. Antimicrob. Chemother. 2010;65:1224–1227. doi: 10.1093/jac/dkq083. [DOI] [PubMed] [Google Scholar]
- 104.Herzog IM, Feldman M, Eldar-Boock A, Satchi-Fainaro R, Fridman M. MedChemComm. 2013;4:120–124. [Google Scholar]
- 105.Herzog IM, Green KD, Berkov-Zrihen Y, Feldman M, Vidavski RR, Eldar-Boock A, Satchi-Fainaro R, Eldar A, Garneau-Tsodikova S, Fridman M. Angew. Chem. 2012;51:5652–5656. doi: 10.1002/anie.201200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berkov-Zrihen Y, Herzog IM, Feldman M, Sonn-Segev A, Roichman Y, Fridman M. Bioorg. Med. Chem. 2013;21:3624–3631. doi: 10.1016/j.bmc.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 107.Berkov-Zrihen Y, Herzog IM, Feldman M, Fridman M. Org. Lett. 2013;15:6144–6147. doi: 10.1021/ol4030138. [DOI] [PubMed] [Google Scholar]
- 108.Bera S, Zhanel GG, Schweizer F. Carbohydr. Res. 2011;346:560–568. doi: 10.1016/j.carres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 109.Bera S, Zhanel GG, Schweizer F. Bioorg. Med. Chem. Lett. 2010;20:3031–3035. doi: 10.1016/j.bmcl.2010.03.116. [DOI] [PubMed] [Google Scholar]
- 110.Dhondikubeer R, Bera S, Zhanel GG, Schweizer F. J. Antibiot. 2012;65:495–498. doi: 10.1038/ja.2012.59. [DOI] [PubMed] [Google Scholar]
- 111.Hanessian S, Szychowski J, Adhikari SS, Vasquez G, Kandasamy P, Swayze EE, Migawa MT, Ranken R, François B, Wirmer-Bartoschek J, Kondo J, Westhof E. J. Med. Chem. 2007;50:2352–2369. doi: 10.1021/jm061200+. [DOI] [PubMed] [Google Scholar]
- 112.Baussanne I, Bussiere A, Halder S, Ganem-Elbaz C, Ouberai M, Riou M, Paris JM, Ennifar E, Mingeot-Leclercq MP, Decout JL. J. Med. Chem. 2010;53:119–127. doi: 10.1021/jm900615h. [DOI] [PubMed] [Google Scholar]
- 113.Bera S, Zhanel GG, Schweizer F. J. Med. Chem. 2010;53:3626–3631. doi: 10.1021/jm1000437. [DOI] [PubMed] [Google Scholar]
- 114.Zimmermann L, Bussiere A, Ouberai M, Baussanne I, Jolivalt C, Mingeot-Leclercq MP, Decout JL. J. Med. Chem. 2013;56:7691–7705. doi: 10.1021/jm401148j. [DOI] [PubMed] [Google Scholar]
- 115.Brogden KA. Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 116.Udumula V, Ham YW, Fosso MY, Chan KY, Rai R, Zhang J, Li J, Chang CW. Bioorg. Med. Chem. Lett. 2013;23:1671–1675. doi: 10.1016/j.bmcl.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 117.Ouberai M, El Garch F, Bussiere A, Riou M, Alsteens D, Lins L, Baussanne I, Dufrene YF, Brasseur R, Decout JL, Mingeot-Leclercq MP. Biochim. Biophys. Acta. 2011;1808:1716–1727. doi: 10.1016/j.bbamem.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 118.Bhargava P, Singh R. Interdisciplinary perspectives on infectious diseases. 2012;2012:626838. doi: 10.1155/2012/626838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Croft SL, Sundar S, Fairlamb AH. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jhingran A, Chawla B, Saxena S, Barrett MP, Madhubala R. Molec. Biochem. Parasitol. 2009;164:111–117. doi: 10.1016/j.molbiopara.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandez MM, Malchiodi EL, Algranati ID. Antimicrob. Agents Chemother. 2011;55:86–93. doi: 10.1128/AAC.00506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zufferey R, Bibis SS, Zhu T, Dhalladoo S. Exp. Parasitol. 2012;130:200–204. doi: 10.1016/j.exppara.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Shalev M, Kondo J, Kopelyanskiy D, Jaffe CL, Adir N, Baasov T. Proc. Nat. Acad. Sci., U.S.A. 2013;110:13333–13338. doi: 10.1073/pnas.1307365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kulshrestha A, Singh R, Kumar D, Negi NS, Salotra P. Antimicrob. Agents Chemother. 2011;55:2916–2921. doi: 10.1128/AAC.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ben Salah A, Buffet PA, Morizot G, Ben Massoud N, Zaatour A, Ben Alaya N, Haj Hamida NB, El Ahmadi Z, Downs MT, Smith PL, Dellagi K, Grogl M. PLoS Neglec. Trop. Dis. 2009;3:e432. doi: 10.1371/journal.pntd.0000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, Gharbi A, Belhadj Hamida N, Boukthir A, Chlif S, Abdelhamid K, El Ahmadi Z, Louzir H, Mokni M, Morizot G, Buffet P, Smith PL, Kopydlowski KM, Kreishman-Deitrick M, Smith KS, Nielsen CJ, Ullman DR, Norwood JA, Thorne GD, McCarthy WF, Adams RC, Rice RM, Tang D, Berman J, Ransom J, Magill AJ, Grogl M. New Engl. J. Med. 2013;368:524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- 127.Sundar S, Jha TK, Thakur CP, Sinha PK, Bhattacharya SK. New Engl. J. Med. 2007;356:2571–2581. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- 128.Musa AM, Younis B, Fadlalla A, Royce C, Balasegaram M, Wasunna M, Hailu A, Edwards T, Omollo R, Mudawi M, Kokwaro G, El-Hassan A, Khalil E. PLoS Neglec. Trop. Dis. 2010;4:e855. doi: 10.1371/journal.pntd.0000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hailu A, Musa A, Wasunna M, Balasegaram M, Yifru S, Mengistu G, Hurissa Z, Hailu W, Weldegebreal T, Tesfaye S, Makonnen E, Khalil E, Ahmed O, Fadlalla A, El-Hassan A, Raheem M, Mueller M, Koummuki Y, Rashid J, Mbui J, Mucee G, Njoroge S, Manduku V, Musibi A, Mutuma G, Kirui F, Lodenyo H, Mutea D, Kirigi G, Edwards T, Smith P, Muthami L, Royce C, Ellis S, Alobo M, Omollo R, Kesusu J, Owiti R, Kinuthia J g. Leishmaniasis East Africa Platform. PLoS Neglec. Trop. Dis. 2010;4:e709. doi: 10.1371/journal.pntd.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Das S, Rani M, Pandey K, Sahoo GC, Rabidas VN, Singh D, Das P. J. Antimicrob. Chemother. 2012;67:2373–2378. doi: 10.1093/jac/dks220. [DOI] [PubMed] [Google Scholar]
- 131.Hobbie SN, Kalapala SK, Akshay S, Bruell C, Schmidt S, Dabow S, Vasella A, Sander P, Böttger EC. Nucl. Acids Res. 2007;35:6086–6093. doi: 10.1093/nar/gkm658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hobbie SN, Kaiser M, Schmidt S, Shcherbakov D, Janusic T, Brun R, Böttger EC. PLoS Neglec. Trop. Dis. 2011;5:e1161. doi: 10.1371/journal.pntd.0001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chawla B, Jhingran A, Panigrahi A, Stuart KD, Madhubala R. PloS one. 2011;6:e26660. doi: 10.1371/journal.pone.0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Linde L, Kerem B. Trends Genet. 2008;24:552–563. doi: 10.1016/j.tig.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 135.Zingman LV, Park S, Olson TM, Alekseev AE, Terzic A. Clin. Pharmacol. Ther. 2007;81:99–103. doi: 10.1038/sj.clpt.6100012. [DOI] [PubMed] [Google Scholar]
- 136.Sermet-Gaudelus I, Renouil M, Fajac A, Bidou L, Parbaille B, Pierrot S, Davy N, Bismuth E, Reinert P, Lenoir G, Lesure JF, Rousset JP, Edelman A. BMC Med. 2007;5:5. doi: 10.1186/1741-7015-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Linde L, Boelz S, Nissim-Rafinia M, Oren YS, Wilschanski M, Yaacov Y, Virgilis D, Neu-Yilik G, Kulozik AE, Kerem E, Kerem B. J. Clin, Invest. 2007;117:683–692. doi: 10.1172/JCI28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du M, Keeling KM, Fan L, Liu X, Kovacs T, Sorscher E, Bedwell DM. J. Molec. Med. 2006;84:573–582. doi: 10.1007/s00109-006-0045-5. [DOI] [PubMed] [Google Scholar]
- 139.Du M, Keeling KM, Fan L, Liu X, Bedwell DM. J. Biol. Chem. 2009;284:6885–6892. doi: 10.1074/jbc.M806728200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rowe SM, Sloane P, Tang LP, Backer K, Mazur M, Buckley-Lanier J, Nudelman I, Belakhov V, Bebok Z, Schwiebert E, Baasov T, Bedwell DM. J. Molec. Med. 2011;89:1149–1161. doi: 10.1007/s00109-011-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xue X, Mutyam V, Tang L, Biswas S, Du M, Jackson LA, Dai Y, Belakhov V, Shalev M, Chen F, Schacht J, Bridges R, Baasov T, Hong J, Bedwell DM, Rowe SM. Am. J. Resp. Cell Molec. Biol. 2013 doi: 10.1165/rcmb.2013-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nudelman I, Glikin D, Smolkin B, Hainrichson M, Belakhov V, Baasov T. Bioorg. Med. Chem. 2010;18:3735–3746. doi: 10.1016/j.bmc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 143.Pichavant C, Aartsma-Rus A, Clemens PR, Davies KE, Dickson G, Takeda S, Wilton SD, Wolff JA, Wooddell CI, Xiao X, Tremblay JP. Molec. Ther. 2011;19:830–840. doi: 10.1038/mt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Malik V, Rodino-Klapac LR, Viollet L, Mendell JR. Ther. Adv. Neurol. Dis. 2010;3:379–389. doi: 10.1177/1756285610388693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loufrani L, Dubroca C, You D, Li Z, Levy B, Paulin D, Henrion D. Arterioscler. Thromb. Vasc. Biol. 2004;24:671–676. doi: 10.1161/01.ATV.0000118683.99628.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malik V, Rodino-Klapac LR, Viollet L, Wall C, King W, Al-Dahhak R, Lewis S, Shilling CJ, Kota J, Serrano-Munuera C, Hayes J, Mahan JD, Campbell KJ, Banwell B, Dasouki M, Watts V, Sivakumar K, Bien-Willner R, Flanigan KM, Sahenk Z, Barohn RJ, Walker CM, Mendell JR. Ann. Neurol. 2010;67:771–780. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- 147.Kimura S, Ito K, Miyagi T, Hiranuma T, Yoshioka K, Ozasa S, Matsukura M, Ikezawa M, Matsuo M, Takeshima Y, Miike T. Brain Dev. 2005;27:400–405. doi: 10.1016/j.braindev.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 148.Popescu AC, Sidorova E, Zhang G, Eubanks JH. J. Neurosci. Res. 2010;88:2316–2324. doi: 10.1002/jnr.22409. [DOI] [PubMed] [Google Scholar]
- 149.Brendel C, Klahold E, Gartner J, Huppke P. Ped. Res. 2009;65:520–523. doi: 10.1203/PDR.0b013e31819d9ebc. [DOI] [PubMed] [Google Scholar]
- 150.Brendel C, Belakhov V, Werner H, Wegener E, Gartner J, Nudelman I, Baasov T, Huppke P. J. Molec. Med. 2011;89:389–398. doi: 10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vecsler M, Ben Zeev B, Nudelman I, Anikster Y, Simon AJ, Amariglio N, Rechavi G, Baasov T, Gak E. PloS one. 2011;6:e20733. doi: 10.1371/journal.pone.0020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wolstencroft EC, Mattis V, Bajer AA, Young PJ, Lorson CL. Hum. Molec. Genet. 2005;14:1199–1210. doi: 10.1093/hmg/ddi131. [DOI] [PubMed] [Google Scholar]
- 153.Mattis VB, Rai R, Wang J, Chang CW, Coady T, Lorson CL. Hum. Genet. 2006;120:589–601. doi: 10.1007/s00439-006-0245-7. [DOI] [PubMed] [Google Scholar]
- 154.Du M, Jones JR, Lanier J, Keeling KM, Lindsey JR, Tousson A, Bebok Z, Whitsett JA, Dey CR, Colledge WH, Evans MJ, Sorscher EJ, Bedwell DM. J. Molec. Med. 2002;80:595–604. doi: 10.1007/s00109-002-0363-1. [DOI] [PubMed] [Google Scholar]
- 155.Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. Proc. Nat. Acad. Sci., U.S.A. 2008;105:2064–2069. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rowe SM, Varga K, Rab A, Bebok Z, Byram K, Li Y, Sorscher EJ, Clancy JP. Am. J. Resp. Cell Molec. Biol. 2007;37:347–356. doi: 10.1165/rcmb.2006-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rebibo-Sabbah A, Nudelman I, Ahmed ZM, Baasov T, Ben-Yosef T. Hum. Genet. 2007;122:373–381. doi: 10.1007/s00439-007-0410-7. [DOI] [PubMed] [Google Scholar]
- 158.Keeling KM, Bedwell DM. J. Molec. Med. 2002;80:367–376. doi: 10.1007/s00109-001-0317-z. [DOI] [PubMed] [Google Scholar]
- 159.Pinotti M, Rizzotto L, Pinton P, Ferraresi P, Chuansumrit A, Charoenkwan P, Marchetti G, Rizzuto R, Mariani G, Bernardi F, International Factor VIIDSG. J. Thromb. Haemost. 2006;4:1308–1314. doi: 10.1111/j.1538-7836.2006.01915.x. [DOI] [PubMed] [Google Scholar]
- 160.Brumm H, Muhlhaus J, Bolze F, Scherag S, Hinney A, Hebebrand J, Wiegand S, Klingenspor M, Gruters A, Krude H, Biebermann H. Obesity. 2012;20:1074–1081. doi: 10.1038/oby.2011.202. [DOI] [PubMed] [Google Scholar]
- 161.Floquet C, Deforges J, Rousset JP, Bidou L. Nucl. Acids Res. 2011;39:3350–3362. doi: 10.1093/nar/gkq1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Floquet C, Rousset JP, Bidou L. PloS one. 2011;6:e24125. doi: 10.1371/journal.pone.0024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bidou L, Hatin I, Perez N, Allamand V, Panthier JJ, Rousset JP. Gene Ther. 2004;11:619–627. doi: 10.1038/sj.gt.3302211. [DOI] [PubMed] [Google Scholar]
- 164.Mattis VB, Ebert AD, Fosso MY, Chang CW, Lorson CL. Hum. Molec. Genet. 2009;18:3906–3913. doi: 10.1093/hmg/ddp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mattis VB, Fosso MY, Chang CW, Lorson CL. BMC Neurosci. 2009;10:142. doi: 10.1186/1471-2202-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, Belakhov V, Hainrichson M, Chen F, Schacht J, Pilch DS, Ben-Yosef T, Baasov T. J. Med. Chem. 2009;52:2836–2845. doi: 10.1021/jm801640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Allamand V, Bidou L, Arakawa M, Floquet C, Shiozuka M, Paturneau-Jouas M, Gartioux C, Butler-Browne GS, Mouly V, Rousset JP, Matsuda R, Ikeda D, Guicheney P. J. Gene Med. 2008;10:217–224. doi: 10.1002/jgm.1140. [DOI] [PubMed] [Google Scholar]
- 168.Lai CH, Chun HH, Nahas SA, Mitui M, Gamo KM, Du L, Gatti RA. Proc. Nat. Acad. Sci., U.S.A. 2004;101:15676–15681. doi: 10.1073/pnas.0405155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gunn G, Dai Y, Du M, Belakhov V, Kandasamy J, Schoeb TR, Baasov T, Bedwell DM, Keeling KM. Molec. Genet. Metab. 2013 doi: 10.1016/j.ymgme.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perez B, Rodriguez-Pombo P, Ugarte M, Desviat LR. Molec. Syndromol. 2012;3:230–236. doi: 10.1159/000343086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moosajee M, Gregory-Evans K, Ellis CD, Seabra MC, Gregory-Evans CY. Hum. Molec. Genet. 2008;17:3987–4000. doi: 10.1093/hmg/ddn302. [DOI] [PubMed] [Google Scholar]
- 172.Shulman E, Belakhov V, Wei G, Kendall A, Meyron-Holtz EG, Ben-Shachar D, Schacht J, Baasov T. J. Biol. Chem. 2014;289:2318–2330. doi: 10.1074/jbc.M113.533588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yu H, Liu X, Huang J, Zhang Y, Hu R, Pu J. Int. J. Molec. Med. 2014;33:729–735. doi: 10.3892/ijmm.2013.1601. [DOI] [PubMed] [Google Scholar]
- 174.Goldmann T, Overlack N, Wolfrum U, Nagel-Wolfrum K. Hum. Gene Ther. 2011;22:537–547. doi: 10.1089/hum.2010.067. [DOI] [PubMed] [Google Scholar]
- 175.Huth ME, Ricci AJ, Cheng AG. Int. J. Otolaryngol. 2011;2011:937861. doi: 10.1155/2011/937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nagai J, Takano M. Drug Metab. Pharmacokinet. 2004;19:159–170. doi: 10.2133/dmpk.19.159. [DOI] [PubMed] [Google Scholar]
- 177.Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME. Drugs. 2011;71:2277–2294. doi: 10.2165/11597020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 178.Rizzi MD, Hirose K. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15:352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- 179.Guthrie OW. Toxicology. 2008;249:91–96. doi: 10.1016/j.tox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 180.Rybak LP, Ramkumar V. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 181.Fee WE., Jr The Laryngoscope. 1980;90:1–19. doi: 10.1288/00005537-198010001-00001. [DOI] [PubMed] [Google Scholar]
- 182.Mulheran M, Degg C, Burr S, Morgan DW, Stableforth DE. Antimicrob. Agents Chemother. 2001;45:2502–2509. doi: 10.1128/AAC.45.9.2502-2509.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mingeot-Leclercq MP, Tulkens PM. Antimicrob. Agents Chemother. 1999;43:1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marcotti W, van Netten SM, Kros CJ. J. Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Hear. Res. 2006;213:64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Q, Steyger PS. J. Assoc. Res. Otolaryngol. 2009;10:205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. PloS one. 2011;6:e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee JH, Park C, Kim SJ, Kim HJ, Oh GS, Shen A, So HS, Park R. Exp. Molec. Med. 2013;45:e12. doi: 10.1038/emm.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Warchol ME. Curr. Opin. Otolaryngol. Head Neck Surg. 2010;18:454–458. doi: 10.1097/MOO.0b013e32833e05ec. [DOI] [PubMed] [Google Scholar]
- 190.Harvey SC, Skolnick P. J. Pharmacol. Exp. Ther. 1999;291:285–291. [PubMed] [Google Scholar]
- 191.Hong SH, Park SK, Cho YS, Lee HS, Kim KR, Kim MG, Chung WH. Hear. Res. 2006;211:46–53. doi: 10.1016/j.heares.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 192.Liu HY, Chi FL, Gao WY. Neuroreport. 2008;19:117–120. doi: 10.1097/WNR.0b013e3282f3b0ec. [DOI] [PubMed] [Google Scholar]
- 193.Zhao H, Li R, Wang Q, Yan Q, Deng JH, Han D, Bai Y, Young WY, Guan MX. Am. J. Hum. Genet. 2004;74:139–152. doi: 10.1086/381133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xing G, Chen Z, Cao X. Cell Res. 2007;17:227–239. doi: 10.1038/sj.cr.7310124. [DOI] [PubMed] [Google Scholar]
- 195.Guan MX. Mitochondrion. 2011;11:237–245. doi: 10.1016/j.mito.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 196.Wei Q, Xu D, Chen Z, Li H, Lu Y, Liu C, Bu X, Xing G, Cao X. Int. J. Audiol. 2013;52:98–103. doi: 10.3109/14992027.2012.743046. [DOI] [PubMed] [Google Scholar]
- 197.Hobbie SN, Akshay S, Kalapala SK, Bruell CM, Shcherbakov D, Böttger EC. Proc. Nat. Acad. Sci., U.S.A. 2008;105:20888–20893. doi: 10.1073/pnas.0811258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hobbie SN, Bruell CM, Akshay S, Kalapala SK, Shcherbakov D, Böttger EC. Proc. Nat. Acad. Sci., U.S.A. 2008;105:3244–3249. doi: 10.1073/pnas.0707265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Francis SP, Katz J, Fanning KD, Harris KA, Nicholas BD, Lacy M, Pagana J, Agris PF, Shin JB. J. Neurosci. 2013;33:3079–3093. doi: 10.1523/JNEUROSCI.3430-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mazurek B, Lou X, Olze H, Haupt H, Szczepek AJ. Neurosci. Lett. 2012;506:107–110. doi: 10.1016/j.neulet.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 201.Sha SH, Qiu JH, Schacht J. New Engl. J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 202.Feldman L, Efrati S, Eviatar E, Abramsohn R, Yarovoy I, Gersch E, Averbukh Z, Weissgarten J. Kidney Int. 2007;72:359–363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 203.Tokgoz B, Ucar C, Kocyigit I, Somdas M, Unal A, Vural A, Sipahioglu M, Oymak O, Utas C. Nephrol. Dial. Transplant. 2011;26:4073–4078. doi: 10.1093/ndt/gfr211. [DOI] [PubMed] [Google Scholar]
- 204.Ojano-Dirain CP, Antonelli PJ. The Laryngoscope. 2012;122:2543–2548. doi: 10.1002/lary.23593. [DOI] [PubMed] [Google Scholar]
- 205.Bas E, Van De Water TR, Gupta C, Dinh J, Vu L, Martinez-Soriano F, Lainez JM, Marco J. Br. J. Pharmacol. 2012;166:1888–1904. doi: 10.1111/j.1476-5381.2012.01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jankauskas SS, Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Skulachev VP, Zorov DB. Biochemistry. Biokhimiia. 2012;77:666–670. doi: 10.1134/S0006297912060144. [DOI] [PubMed] [Google Scholar]
- 207.Basile AS, Brichta AM, Harris BD, Morse D, Coling D, Skolnick P. Neurosci. Lett. 1999;265:71–74. doi: 10.1016/s0304-3940(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 208.Hainrichson M, Nudelman I, Baasov T. Org. Biomolec. Chem. 2008;6:227–239. doi: 10.1039/b712690p. [DOI] [PubMed] [Google Scholar]
- 209.Chen L, Hainrichson M, Bourdetsky D, Mor A, Yaron S, Baasov T. Bioorg. Med. Chem. 2008;16:8940–8951. doi: 10.1016/j.bmc.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 210.O’Connor S, Lam LK, Jones ND, Chaney MO. J. Org. Chem. 1976;41:2087–2092. doi: 10.1021/jo00874a003. [DOI] [PubMed] [Google Scholar]
- 211.Ryden R, Moore BJ. J. Antimicro. Chemother. 1977;3:609–613. doi: 10.1093/jac/3.6.609. [DOI] [PubMed] [Google Scholar]
- 212.Matt T, Ng CL, Lang K, Sha SH, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Böttger EC. Proc. Nat. Acad. Sci., U.S.A. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. Antimicrob. Agents Chemother. 2010;54:4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Landman D, Babu E, Shah N, Kelly P, Backer M, Bratu S, Quale J. J. Antimicrob. Chemother. 2010;65:2123–2127. doi: 10.1093/jac/dkq278. [DOI] [PubMed] [Google Scholar]
- 215.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, Panagea T, Argyropoulou A, Stefanou I, Plakias G, Giamarellou H, Petrikkos G. J. Chemother. 2012;24:191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Endimiani A, Hujer KM, Hujer AM, Armstrong ES, Choudhary Y, Aggen JB, Bonomo RA. Antimicrob. Agents Chemother. 2009;53:4504–4507. doi: 10.1128/AAC.00556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. J. Antimicrob. Chemother. 2011;66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 218.Landman D, Kelly P, Backer M, Babu E, Shah N, Bratu S, Quale J. J. Antimicrob. Chemother. 2011;66:332–334. doi: 10.1093/jac/dkq459. [DOI] [PubMed] [Google Scholar]
- 219.Reyes N, Aggen JB, Kostrub CF. Antimicrob. Agents Chemother. 2011;55:1728–1733. doi: 10.1128/AAC.00862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cass RT, Brooks CD, Havrilla NA, Tack KJ, Borin MT, Young D, Bruss JB. Antimicrob. Agents Chemother. 2011;55:5874–5880. doi: 10.1128/AAC.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]