Abstract
Background
Tricyclic antidepressants (TCAs) have efficacy in treating irritable bowel syndrome (IBS). Some clinicians use TCAs to treat residual symptoms in inflammatory bowel disease (IBD) patients already on decisive IBD therapy or with quiescent inflammation, although this strategy has not been formally studied.
Goals
The aim of this study was to examine the efficacy of TCA therapy in IBD patients with residual symptoms, despite controlled inflammation, in a retrospective cohort study.
Study
Inclusion required initiation of TCA for persistent gastrointestinal symptoms. IBD patients had inactive or mildly active disease with persistent symptoms despite adequate IBD therapy as determined by their physician. Symptom response was compared with IBS patients. Established Likert scales were used to score baseline symptom severity (0 = no symptoms, 3 =severe symptoms) and TCA response (0= no improvement; 3 = complete satisfaction).
Results
Eighty-one IBD [41.3 ± 1.7y, 56F; 58 Crohn's disease/23 ulcerative colitis (UC)] and 77 IBS (46.2 ± 1.7y, 60F) patients were initiated on a TCA therapy. Baseline symptom scores (IBD, 2.06 ± 0.03; IBS, 2.12 ± 0.04; P = 0.15) and symptom response to TCA therapy (IBD, 1.46 ± 0.09; IBS, 1.30 ± 0.09; P = 0.2) were similar in both the groups. At least moderate improvement (Likert score ≥ 2) on TCA was achieved by comparable proportions of patients (59.3% IBD vs. 46% IBS; P = 0.09). Within IBD, response was better with UC than Crohn's disease (1.86 ± 0.13 vs. 1.26 ± 0.11, respectively, P = 0.003).
Conclusions
In a clinical practice setting, TCA use led to moderate improvement of residual gastrointestinal symptoms in IBD patients for whom escalation of IBD therapy was not planned. UC patients demonstrated higher therapeutic success. IBD symptom responses were similar to IBS patients.
Keywords: functional, amitriptyline, nortriptyline, quality of life, clinical decision making
A substantial percentage of inflammatory bowel disease (IBD) patients have gastrointestinal (GI) symptoms, which persist despite adequate IBD therapy and when their inflammatory disease is deemed to be in remission.1 3 Some have described this clinical phenomenon as irritable bowel syndrome (IBS)–like symptoms.4 Others have proposed that this symptom constellation reflects occult inflammation.3 In this context, anecdotally, many patients and providers do not want further escalation of IBD-specific therapy if minimal inflammatory disease is observed.5 Thus, the treatment of these residual GI symptoms remains an important clinical challenge with the limited literature to guide practice-based decision making.
Tricyclic antidepressants (TCAs), at doses below the traditional antidepressant range, have been used for symptom control in IBS.6,7 Meta-analyses combining controlled studies have reported numbers-needed-to-treat of 3 to 4 for symptomatic benefit over placebo in IBS patients.8,9 Diarrhea and pain-predominant symptoms often remain in treated IBD patients; these same features also comprise the symptom profiles of IBS patients who benefit from TCA therapy. In addition to their neuromodulatory actions, pre-clinical studies have suggested that TCAs may also exert some anti-inflammatory effects.10 Given these mechanisms of benefit, IBS therapies such as TCAs could constitute a reasonable adjunctive therapy for residual symptoms in IBD.
In a case-note review, antidepressants, in general, including TCAs, were used in approximately one third of the patients in an Australian IBD service.11 The most common reason for this prescription was depression or anxiety, with very few prescribed for somatic complaints. A second retrospective study found that high-dose TCAs prescribed for depression led to reduction of disease flares, endoscopies, and steroid use.12 Unlike the above studies, where depression was the indication for TCA use, our study evaluates the clinical outcome of taking low-dose TCA for treatment of residual, IBS-like symptoms in IBD patients.
In our institution, we have observed experiential success in using TCAs to treat this type of residual symptoms in IBD patients. To objectively validate this clinical experience and quantify its effect, we performed a retrospective review of our clinical records. Our hypothesis was that low-dose TCAs would provide comparable symptomatic benefit in IBD patients with residual symptoms as is achieved in patients with established IBS alone.
Materials and Methods
This retrospective observational study evaluating the outcomes of TCA therapy was conducted in 2 cohorts extracted from a multiphysician academic gastroenterology practice: (1) IBD patients in clinical remission or with mild inflammation according to the physician's global assessment (PGA) but with persistent GI symptoms, and (2) symptomatic IBS patients without IBD. The Human Research Protection Office (institutional review board) at Washington University School of Medicine granted approval for retrospective review of clinical records for this study.
Subjects and Study Design
Outpatient electronic medical records were reviewed to identify patients carrying clinical diagnoses of IBD and IBS over an 11-year period between July 2000 and June 2011. Word searches for “nortriptyline,” “amitriptyline,” “desipramine,” “doxepin,” and “tricyclic antidepressant” were performed on the outpatient clinical notes of patients with recorded diagnoses for IBD or IBS to identify the study cohorts. Inclusion required an established diagnosis of Crohn's disease (CD) and ulcerative colitis (UC) based on histologic, endoscopic, and/or radiographic evidence, unequivocal documentation of clinical remission or mild disease activity according to the PGA, persistent GI symptoms (diarrhea and/or constipation, abdominal discomfort/pain), at least one follow-up visit after initiating TCA therapy, and self-reported adherence to TCA therapy until follow-up. PGA was defined based on the American College of Gastroenterology practice outline criteria.13,14 Additional testing for IBD-related disease activity was performed at the discretion of the provider and was recorded when performed. Exclusion criteria for IBD patients were moderate to severe disease activity based on the PGA, TCA initiation before their first visit, or con-current initiation of anti-inflammatory IBD therapy such as anti-TNF therapy, immunomodulators, or steroids. IBD patients were also excluded if there was evidence of moderate or severe inflammation or active complications of IBD on diagnostic testing. Inclusion criteria for the IBS cohort included a clinical diagnosis of IBS (Rome III criteria), initiation of TCA therapy primarily for the management of GI symptoms, at least 1 follow-up visit after TCA initiation, and self-reported adherence to TCA therapy. Exclusion criteria in the IBS cohort were presence of organic disease, including IBD, celiac disease, or microscopic colitis. In both the groups, patients were also excluded if they had additional functional GI diagnoses, such as cyclic vomiting syndrome. The initial visit and up to 2 subsequent follow-up visits were reviewed for each patient. When only 1 follow-up visit was included, reasons for lack of a suitable second follow-up were recorded.
Chart Review/Data Collection
Authors not involved in the medical management of the patients (H.N.I., B.C., N.K.) used a consistent data extraction and chart review process to collect patient demographics, clinical symptoms, and data leading up to the time of treatment, IBD history, medication history, and previous documentation of comorbid medical and psychiatric disorders. TCA treatment data extracted included the type and dose used, treatment response, and patients' self-reported adherence to TCA. All clinical notations, including records from office visits, telephone contacts, or written communications, were considered potential sources of clinical data. Baseline symptom severity at the time of neuromodulator initiation was assessed on an established 4-point Likert-type scale from chart notations of severity, activity interference, and degree of needed intervention (Fig. 1). 15,17 Treatment response at follow-up was graded post hoc using chart descriptors extracted from the clinical record following a 4-point Likert-type response scale (Fig. 1).18,19 ‘Response’ was characterized as a treatment score of 2 or 3 on the Likert-type scale. These Likert-type scales have been applied in several studies assessing functional symptoms.7,15,17,18 Both the baseline symptom severity scale and the treatment response Likert scale have previously demonstrated reproducibility and high interobserver agreement upon comparison of separately rated, blinded scores (κ = 0.89 for the baseline scale and κ = 0.86 for the treatment response scale).15,19 In this study, 2 trained physicians (H.N.I. and B.C.) performed separate blinded grading of baseline symptoms and treatment response, yielding high interobserver agreement with a κ = 0.79 for the baseline symptom severity score and a κ = 0.83 for the treatment response score. Among the total number of IBD patient charts reviewed (n = 401), 20.2% met inclusion criteria (n = 81), 34.2% were excluded because of a lack of a second follow-up visit at the time of chart review (n = 137), and 45.6% were excluded according to the criteria mentioned above (n = 183).
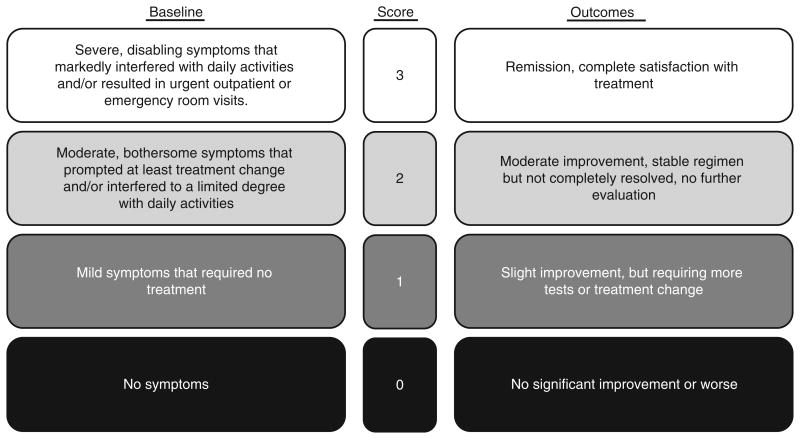
Figure 1.
Likert scale used for extracting baseline symptom severity and treatment outcome from chart review. The scoring system has documented reproducibility and high interobserver agreement (κ = 0.86-0.89).15 In the outcome scale, scores of 2 or 3 represented treatment response. Reproduced with permission from Schirbel et al.16 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Statistical Analysis
For demographic and clinical characteristics, χ2 test or the Fisher exact testing was used for dichotomous variables, and the Student t test or the Mann-Whitney U test was applied in the analysis of continuous variables. Continuous variables are reported as mean ± SEM. This was performed to assess differences in demographic, clinical, and symptom parameters between groups. Subgroup analysis comparisons were performed to assess IBD disease characteristics between CD and UC patients. To determine the independent significance of demographic data, comorbidities, and clinical parameters in predicting symptom response with low-dose tricyclic therapy, multivariable logistic regression was utilized, with the outcome variable of a symptom response (score of 2 or above; moderate improvement or complete satisfaction on the Likert scale). Predictors included in the analysis were used in a forced entry manner and included age, sex, diagnosis (IBS, UC, CD), presence of comorbid psychiatric or functional conditions, and the presence of abdominal pain, a target of TCA therapy. A second logistic regression with only the IBD patient subgroup was performed to determine if prior surgery or IBD medication regimen had an effect on the treatment response. Exponentiated β values were calculated as approximations of independent variable odds ratios (OR). In all cases, P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics v. 20.0 (SPSS Inc., Chicago, IL).
Results
A total of 158 subjects, 81 IBD patients (58CD/23 UC) and 77 IBS patients met the eligibility criteria and formed the study groups. Patient demographics are described in Table 1. Although age was slightly different statistically, it was clinically similar. Sex distributions were not significantly different between the 2 groups. The frequency and median dose of TCA use was similar in both the groups; nortriptyline was favored over amitriptyline and desipramine. Comparable proportions in both cohorts required TCA dose escalation. Although by design all study patients had at least 1 follow-up visit after starting the TCA therapy, over two thirds had multiple follow-up visits. Reasons for lack of inclusion for a second follow-up were discontinuation of TCA because of side effects (11% of IBD patients and 1.3% of IBS patients), lack of efficacy (3.7% of IBD patients, none of IBS patients), or because of symptom resolution (2.5% of IBD patients, none of IBS patients). In 3.7% of the IBD patients and 3.9% of the IBS patients, TCAs were discontinued for reasons not explicitly stated in the medical records. In 12.6% of the IBD group and 16% of the IBS group, there was no further contact with the patient in the chart, some lost to follow-up and some had not yet returned for follow-up. Overall mean follow-up time was comparable between the 2 cohorts (Table 1).
Table 1. Clinical Characteristics and Tricyclic Antidepressant (TCA) Use Among Study Groups.
| Patient Characteristics | IBD Group n = 81 | IBS Group n = 77 | P |
|---|---|---|---|
| Age (y) | 41.3 ± 1.7 | 46.2 ± 1.7 | 0.05 |
| Sex (% female) | 69.1 | 77.9 | 0.21 |
| Mean follow-up (mo) | 8.5 ± 0.8 | 10.1 ± 1.3 | 0.29 |
| TCA used [n (%)] | |||
| Amitriptyline | 19 (23.5) | 20 (26.0) | 0.85 |
| Nortriptyline | 54 (66.7) | 43 (55.8) | 0.19 |
| Desipramine | 8 (9.9) | 12 (15.6) | 0.34 |
| Doxepin | 0 (0) | 2 (2.6) | 0.24 |
| Median TCA dose (range in mg) at first follow-up | 25 (10-150) | 25 (10-200) | 0.26 |
| TCA dose increased by second follow-up [n (%)] | 29/81 (35.8) | 34/77 (44.2) | 0.28 |
| No. with multiple follow-up visits [n (%)]* | 54 (66.7) | 60 (77.9) | 0.16 |
All patients had 1 follow-up visit after the baseline visit.
IBD indicates inflammatory bowel disease; IBS, irritable bowel syndrome; TCA, tricyclic antidepressant.
The most frequent symptom reported was abdominal pain in the 2 groups (Table 2). Nausea and constipation were more prevalent in IBS patients, whereas diarrhea was more prevalent in IBS patients. Self-reported functional and psychiatric comorbidities were overall similar in the 2 groups. Depression was more commonly reported in IBD patients.
Table 2. Presenting Symptoms and Comorbidities in the Study Groups.
| Patient Characteristics | IBD Group n = 81 | IBS Group n = 77 | P |
|---|---|---|---|
| Clinical symptoms [n (%)] | |||
| Abdominal pain | 69 (85.2) | 67 (87.0) | 0.74 |
| Bloating | 25 (30.8) | 33 (46.5) | 0.16 |
| Diarrhea | 60 (74.1) | 45 (58.4) | 0.04 |
| Nausea | 19 (23.5) | 29 (37.7) | 0.05 |
| Constipation | 17 (21) | 37 (48.1) | < 0.01 |
| Functional comorbidities [n (%)]* | 32 (39.5) | 25 (32.9) | 0.39 |
| Chronic low back pain | 7 (8.6) | 3 (3.9) | 0.33 |
| Migraines | 11 (13.6) | 9 (11.7) | 0.81 |
| Chronic headaches | 2 (2.5) | 2 (2.6) | 1.00 |
| Fibromyalgia | 3 (3.7) | 8 (10.4) | 0.12 |
| Chronic pain syndrome | 3 (3.7) | 0 (0) | 0.20 |
| Temporomandibular joint pain | 1 (1.2) | 2 (2.6) | 0.61 |
| Interstitial cystitis | 1 (1.2) | 1 (1.3) | 1.00 |
| Chronic fatigue syndrome | 0 (0) | 1 (1.3) | 0.49 |
| Psychiatric comorbidities [n (%)]* | 39 (48.1) | 28 (36.4) | 0.13 |
| Depression | 23 (28.4) | 8 (10.4) | 0.01 |
| Anxiety | 5 (6.2) | 9 (11.7) | 0.27 |
| Both depression and anxiety | 10 (12.3) | 8 (10.4) | 0.80 |
| Bipolar disorder | 2 (2.5) | 2 (2.6) | 1.00 |
| Anorexia | 0 (0) | 2 (2.6) | 0.24 |
| Obsessive compulsive disorder | 0 (0) | 1 (1.3) | 0.49 |
Self-reported.
IBD indicates inflammatory bowel disease; IBS, irritable bowel syndrome.
Clinical characteristics of the IBD patients were further elucidated and are described in Table 3. Although there were slightly more female patients in the CD cohort, other baseline demographics were not different between the 2 groups. We tracked the frequency of recent endoscopy within 6 months (59.3%), imaging (11.1%), inflammatory markers (8.6%), and clinical impression without testing (remainder, 21%). Using the PGA, 45.6% of the IBD cohort (51.7% CD, 34.8% UC, P = 0.17) were in clinical remission and the remainder had mild disease activity (Table 3). As stated in the study inclusion criteria, the decision was made by the provider not to escalate IBD-specific anti-inflammatory therapy.
Table 3. Clinical Characteristics of the Inflammatory Bowel Disease Group.
| Clinical Characteristics | Crohn's Disease n = 58 | Ulcerative Colitis n = 23 | P |
|---|---|---|---|
| Age (y) | 40.2 ± 2.0 | 44.2 ± 3.5 | 0.31 |
| Sex (% female) | 76.2 | 52.3 | 0.04 |
| Prior surgery (%) | 50.0* | 43.5† | 0.60 |
| Psychiatric comorbidity (%) | 50.0 | 43.4 | 0.56 |
| Functional comorbidity (%) | 44.8 | 26.1 | 0.14 |
| Disease location/extent | 39.4% small bowel 13.8% colon 44.8% ileocolonic | 60.9% pancolitis 17.4% left-sided 17.4% pouchitis | |
| IBD medication regimen (%) | |||
| None | 29.3 | 21.7 | 0.78 |
| Biologics or combination | 22.4 | 0 (0) | 0.03 |
| Immune-modulator | 31.0 | 21.7 | 1.00 |
| 5-ASA | 12.1 | 47.8 | 0.30 |
| Other | 5.2% budesonide taper | 8.7% antibiotic | NA |
| Disease status (%) | |||
| Remission | 51.7 | 34.8 | 0.17 |
| Mild disease activity | 48.3 | 65.2 | |
| Presence of pain | 89.6 | 73.9 | 0.09 |
Ileocolic resection, n = 22; small-bowel resection, n = 6; colectomy, = 3; multiple surgeries n = 4.
Colectomy, n = 8; other (cecal volvulus, gastric bypass), n = 1 each. 5-ASA indicates 5-aminosalicylic acid.
Mean baseline symptom severity scores were similar in the 2 groups (IBD: 2.06 ± 0.03, IBS: 2.12 ± 0.04; P = 0.15). Mean response scores following treatment using the Likert response scale were also similar (IBD: 1.46 ± 0.09, IBS 1.30 ± 0.09 in IBD, P = 0.2), indicating modest symptom improvement in both the cohorts (Fig. 2). Upon further analysis of response, 59.3% of the IBD and 46.0% of the IBS patients showed at least a moderate symptom response (score ≥ 2) to TCA therapy at the first visit. Overall, 52% of IBD and 62% of IBS patients had at least a moderate improvement (score 2 or 3) over the level of symptoms at first follow-up (Fig. 3).
Figure 2.
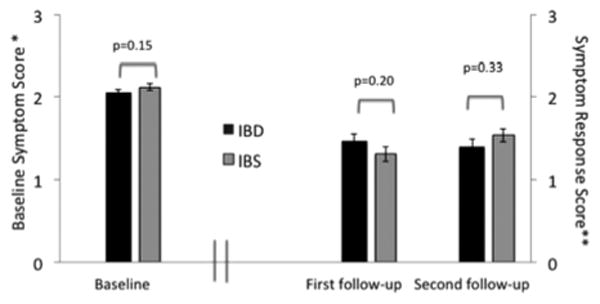
Baseline and symptom response Likert scores on 81 IBD and 77 IBS patients at baseline and first follow-up. *Higher baseline symptom scores indicate more severe symptoms. **Higher follow-up symptom response scores indicate better symptom improvement. Second follow-up symptom response (54 IBD and 60 IBS patients) represents further symptom improvement over the first follow-up. IBD indicates inflammatory bowel disease, IBS, irritable bowel syndrome.
Figure 3.
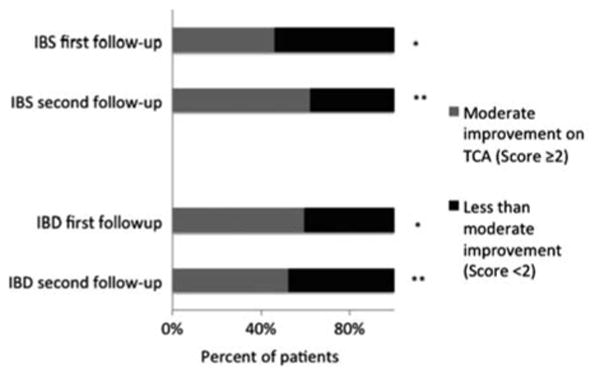
Proportion of IBD and IBS patients with at least moderate improvement on TCA therapy [≥ 2 on Likert scale (Fig. 1)]. *P = 0.09 between IBD and IBS at first follow-up; n = 81 IBD, 77 IBS. **P = 0.29 between IBD and IBS at second follow-up; n = 54 IBD, 60 IBS. IBD indicates inflammatory bowel disease, IBS, irritable bowel syndrome.
In the IBD group, mean treatment response scores were similar for the group where the PGA was remission versus mild disease (response score 1.53 ± 1.37 vs. 1.42 ± 1.33, respectively, P = 0.48). Subgroup analysis of the IBD group was performed comparing CD and UC patients. Despite comparable baseline severity scores (CD: 2.07 ± 0.03, UC: 2.03 ± 0.04, P = 0.67), UC patients responded significantly better to TCA therapy at the first visit, with a Likert response score of 1.86 ± 0.13 for UC and 1.26 ± 0.11 for CD (P = 0.003). This translated into 83% of UC patients having at least a moderate symptomatic improvement on TCA, compared with 50% of CD patients (P = 0.01). These differences in TCA response between CD and UC were nonsignificant for added benefit at the second follow-up visit with a mean response score of 1.31 ± 0.16 for CD and 1.47 ± 0.17 for UC, P = 0.76. At the second visit, 56% of CD group and 40% of UC group had at least a further moderate symptom response, P = 0.16.
To further characterize symptom response, a multivariable logistic regression model was generated. Presentation variables including age, sex, medical diagnosis, functional and psychiatric comorbidities, and abdominal pain as one of the presenting complaints were assessed as predictors of response to TCA in the entire study cohort (Table 4). UC patients responded significantly better than CD patients; presence of abdominal pain and presence of a comorbid functional diagnosis were associated with a significantly lower likelihood of response. Further multivariable logistic regression analysis was performed within the IBD subgroup to determine whether prior surgery or IBD medication regimen had an effect on symptom response. Only a diagnosis of UC was found to be a significant predictor of treatment response in this model (OR = 4.5, 95% confidence interval, 1.3-15.5, P = 0.02). Prior surgery and IBD medication regimen were not significant predictors of treatment response to TCAs in this cohort.
Table 4. Predictors of Symptom Response* to TCA Among All Patient Included Using Multivariable Logistic Regression†.
| Predictor Variable | OR | 95% CI | P |
|---|---|---|---|
| Male sex | 1.8 | 0.8-4.1 | 0.18 |
| Age | 1.0 | 0.97-1.0 | 0.84 |
| Diagnosis | |||
| IBS | 1 | ||
| CD | 1.3 | 0.6-2.7 | 0.50 |
| UC | 4.7 | 1.4-16.3 | 0.01 |
| Presence of abdominal pain | 0.3 | 0.1-0.98 | 0.048 |
| Presence of psychiatric diagnosis | 1.6 | 0.8-3.2 | 0.20 |
| Presence of functional diagnosis | 0.5 | 0.2-0.9 | 0.035 |
Response defined as the Likert score ≥ 2.
Logistic regression χ2 = 26, df = 7, P-value <0.0001, the Hosmer-Lemeshow lack-of-fit test showed that the model fit the data well, P = 0.72, C-statistic = 0.74.
CD indicates Crohn's disease; CI, confidence interval; IBS, irritable bowel syndrome; OR, odds ratio; TCA, tricyclic antidepressant; UC, ulcerative colitis.
Discussion
In IBD patients, not all symptoms reflect IBD disease activity or failure of immune-suppressive therapy.20 There is a need for agents to address these symptoms in patients who do not require, or are not candidates for, further escalation of immunosuppression.1 Our study now indicates that TCAs may be a beneficial therapeutic option for residual bowel symptoms in IBD patients without structural abnormalities. Patients with UC had a significantly better response to TCA therapy than CD. TCA symptom response was comparable in IBD patients to that seen in an IBS cohort at similar doses. TCAs were effective in producing symptom response in IBD even in the presence of mild inflammatory disease activity. This suggests that TCAs could be used by clinicians irrespective of whether these residual symptoms are functional in etiology or related to occult inflammation. Confirmation of these observations will require prospective study.
A functional etiology is an explanatory mechanism for residual symptoms in IBD patients responsive to TCAs.5 IBS occurs in approximately10% to 15% of adults in North America.21 Given this prevalence, it is unlikely that IBD patients are protected from IBS.2 A recent meta-analysis identified a greater pooled prevalence of IBS in IBD patients as compared with non-IBD controls.4 Symptoms in IBD may not always be linked to structural or inflammatory findings, which are supported by the poor correlation between pain symptoms and IBD disease activity indices.16 Although there is continued need for more objective and less-invasive measures of inflammatory disease activity,22,23 perhaps the noted discordance comes from the existence of overlapping IBS in IBD patients without overt inflammation.24,25 A recent study showed that the prevalence of IBS-like symptoms was approximately 30% in IBD patients in remission with a normal fecal calprotectin. Calprotectin did not differ with the presence or absence of IBS symptoms in these IBD patients.26
Occult inflammation represents another mechanistic explanation for residual symptoms in IBD patients without structural abnormalities.5 A study evaluating IBD patients in clinical remission noted that Rome II symptoms correlated with increased calprotectin suggesting that occult inflammation may be driving these symptoms.3 However, TCA therapy should not worsen inflammation and may even reduce inflammation as evidence in preclinical models suggests.10 Irrespective of the mechanism of residual symptoms in IBD patients, our study suggests that TCAs provided a global symptom benefit in this IBD patient population.
We examined various predictors of response to TCA in our study population, including IBD type and the presence of psychiatric and extraintestinal functional comorbidities. UC patients responded better than CD patients. This was an unexpected finding. Small-bowel involvement in CD patients may have led to inflammation or even mild structural abnormalities that were not detected by routine testing. Both mood disorders and non-GI functional diagnoses are common in IBS patients.27,28 In our IBD and IBS cohorts, functional and psychiatric disorders were equally common, perhaps because of our center's experience in treating GI patients with these comorbid diagnoses. Furthermore, mood disorders are found more frequently in patients with IBD in several epidemiologic studies, when compared with the general population.29–31 These comorbidities are implicated in poorer response to therapy in IBS patients and predict premature discontinuation of antidepressant therapies.32 Similarly, in our entire study population, functional diagnoses predicted poorer response to TCA therapy, whereas a mood disturbance diagnoses did not affect the therapeutic response. In our multivariable analysis, psychiatric comorbidity may have not been a statistically significant predictor because of a type II error.
Although mood itself can influence the experience of pain within limbic regions of the brain implicated in processing pain and emotions, it is unlikely that the response to low-dose TCA therapy is a consequence of improvements in mood symptoms.33 Further, effects of TCA on gut motility have not proven as important as TCA effects on global improvement.34,35 In a retrospective study of UC patients, 50% of patients experienced pain, with coexisting mood disorders and female sex identified as significant predictors of pain, similar to functional bowel disorders.36 We postulate that global improvement mechanisms may explain why the presence of pain in our study was not a significant independent predictor of response to TCA in our model. Further prospective study will be required to delineate individual symptom responses and define the mechanism for improvement with TCA.
Several limitations to our study exist. Differences in TCA choice, dosing variety, patient follow-up intervals, and available testing are inherent to this retrospective study. This retrospective design was the most practical exploratory design and enabled us to review a large number of charts; however, the study is strengthened by the presence of a control group of IBS patients. Because our study examined treatment response, exclusion of patients who did not have a follow-up introduced an inevitable selection bias in our sample. As for disease severity, we relied on the PGA, ensuring that categorization was not restricted by the performance of, or specific findings on tests; nevertheless, guidelines for CD management stress the importance of this global assessment, which correlates with CD activity index scores.14 The grading system we used includes some subjective elements; however, high interobserver agreement was seen in our study and others.15,17,19 Because of our retrospective design, we could not assess patients symptoms directly; we relied on the provider's notes for our outcome. Our conclusions were based on global symptom improvement, as quantitative improvement in specific symptoms could not be tracked using this grading system. Still, this assessment of TCA outcomes is reasonable as they have been previously observed to have a larger global effect than on any individual GI symptom.34 It is encouraging that even in this challenging tertiary care patient population, TCAs were found to exert a measurable symptomatic benefit. However, patient expectation of tertiary center physician recommendations could have increased the likelihood of positive response.
In conclusion, our data suggests that low-dose TCAs may be a viable option in the management of residual symptoms in IBD patients with minimal inflammation. We propose that this is a low-risk/high-benefit intervention in the right patient as TCA effects can improve symptoms but are unlikely to mask a true IBD flare. These findings will provide guidance in the design of a prospective multicenter study to validate the symptomatic benefit of TCA therapy in IBD patients.
Acknowledgments
The authors thank Dr David Alpers for his valuable input.
Supported by 5T32DK007130-36 (H.I.); UL1TR000448 (M.C.); 1K08DK089016; and K23 DK084113 (G.S.S.).
Footnotes
M.A.C. and G.S.S. contributed equally to the article.
Presented in preliminary form at the Annual Meeting of the American College of Gastroenterology, Las Vegas, October 2012.
H.I.: study design, data collection, analysis, and interpretation, manuscript preparation; B.C.: data collection and analysis; N.K.: data collection; C.P.G.: study concept and critical revision of the manuscript; A.G.: critical revision of the manuscript; T.D.: critical revision of the manuscript; M.C. and G.S.S.: study concept and design, data analysis, and critical revision of manuscript.
The authors declare that they have nothing to disclose.
References
- 1.Meng J, Agrawal A, Whorwell PJ. Refractory inflammatory bowel disease-could it be an irritable bowel? Nat Rev Gastro-enterol Hepatol. 2013;10:58–61. doi: 10.1038/nrgastro.2012.173. [DOI] [PubMed] [Google Scholar]
- 2.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 3.Keohane J, O'Mahony C, O'Mahony L, et al. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788, 1789–1794. doi: 10.1038/ajg.2010.156. quiz 1795. [DOI] [PubMed] [Google Scholar]
- 4.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 5.Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what?: a challenge to the functional-organic dichotomy. Am J Gastroenterol. 2010;105:1796–1798. doi: 10.1038/ajg.2010.162. [DOI] [PubMed] [Google Scholar]
- 6.Clouse RE. Antidepressants for irritable bowel syndrome. Gut. 2003;52:598–599. doi: 10.1136/gut.52.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clouse RE, Lustman PJ, Geisman RA, et al. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 1994;8:409–416. doi: 10.1111/j.1365-2036.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Jackson JL, O'Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 9.Rahimi R, Nikfar S, Rezaie A, et al. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–2218. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikocka-Walus AA, Gordon AL, Stewart BJ, et al. The role of antidepressants in the management of inflammatory bowel disease (IBD): a short report on a clinical case-note audit. J Psychosom Res. 2012;72:165–167. doi: 10.1016/j.jpsychores.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Goodhand JR, Greig FI, Koodun Y, et al. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm Bowel Dis. 2012;18:1232–1239. doi: 10.1002/ibd.21846. [DOI] [PubMed] [Google Scholar]
- 13.Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of G. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 15.Sawhney MS, Prakash C, Lustman PJ, et al. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–424. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 16.Schirbel A, Reichert A, Roll S, et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J Gastroenterol. 2010;16:3168–3177. doi: 10.3748/wjg.v16.i25.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakash C, Lustman PJ, Freedland KE, et al. Tricyclic antidepressants for functional nausea and vomiting: clinical outcome in 37 patients. Dig Dis Sci. 1998;43:1951–1956. doi: 10.1023/a:1018878324327. [DOI] [PubMed] [Google Scholar]
- 18.Clouse RE, Sayuk GS, Lustman PJ, et al. Zonisamide or levetiracetam for adults with cyclic vomiting syndrome: a case series. Clin Gastroenterol Hepatol. 2007;5:44–48. doi: 10.1016/j.cgh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Patel A, Sayuk GS, Kushnir VM, et al. Sensory neuromodulators in functional nausea and vomiting: predictors of response. Postgrad Med J. 2013;89:131–136. doi: 10.1136/postgradmedj-2012-131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruining DH, Sandborn WJ. Do not assume symptoms indicate failure of anti-tumor necrosis factor therapy in Crohn's disease. Clin Gastroenterol Hepatol. 2011;9:395–399. doi: 10.1016/j.cgh.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Saito YA, Schoenfeld P, Locke GR., III The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 22.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minderhoud IM, Oldenburg B, Wismeijer JA, et al. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469–474. doi: 10.1023/b:ddas.0000020506.84248.f9. [DOI] [PubMed] [Google Scholar]
- 25.Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:778–788. doi: 10.1002/ibd.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berrill JW, Green JT, Hood K, et al. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment Pharmacol Ther. 2013;38:44–51. doi: 10.1111/apt.12335. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead WE, Palsson OS, Levy RR, et al. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102:2767–2776. doi: 10.1111/j.1572-0241.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 29.Walker JR, Ediger JP, Graff LA, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989–1997. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 30.Kurina LM, Goldacre MJ, Yeates D, et al. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55:716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflamm Bowel Dis. 2006;12:697–707. doi: 10.1097/00054725-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Sayuk GS, Elwing JE, Lustman PJ, et al. Predictors of premature antidepressant discontinuation in functional gastrointestinal disorders. Psychosom Med. 2007;69:173–181. doi: 10.1097/PSY.0b013e318031391d. [DOI] [PubMed] [Google Scholar]
- 33.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. NeuroImage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 34.Clouse RE, Lustman PJ. Use of psychopharmacological agents for functional gastrointestinal disorders. Gut. 2005;54:1332–1341. doi: 10.1136/gut.2004.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coates MD, Lahoti M, Binion DG, et al. Abdominal pain in ulcerative colitis. Inflamm Bowel Dis. 2013;19:2207–2214. doi: 10.1097/MIB.0b013e31829614c6. [DOI] [PMC free article] [PubMed] [Google Scholar]