Abstract
Aims/hypothesis
The endogenous production of stromal cell-derived factor-1 (SDF-1) in beta cells in transgenic mice attenuates the development of diabetes in response to streptozotocin. Here we propose that beta cell injury induces SDF-1 production, and the SDF-1/chemokine (C-X-C motif) receptor 4 (CXCR4) interaction auto-activates Sdf1 expression, resulting in the autocrine production of SDF-1 by beta cells and the paracrine activation of glucagon-like peptide-1 (GLP-1) production by alpha cells.
Methods
SDF-1 production in adult mouse and human islets and rat INS-1 cells was measured in models of beta cell injury. The paracrine actions of SDF-1 on GLP-1 production in alpha cells were explored. The potential synergism between the growth-promoting actions of GLP-1 and the pro-survival actions of SDF-1 on the preservation of cell mass was evaluated by cell viability assays.
Results
In adult islets and INS-1 cells, Sdf1 expression was re-induced in response to injury. The interaction of SDF-1 with its receptor on alphaTC1 cells activated protein kinase Akt, stimulated cell proliferation and induced the expression of prohormone convertase 1/3 and the consequent production of GLP-1 in alpha cells. The combination of GLP-1 and SDF-1 additively enhanced both the growth and longevity of INS-1 beta cells.
Conclusions/interpretation
The results of these studies suggest that in response to beta cell injury and the ensuing induction of SDF-1, the biological function of alpha cells switches from the production of glucagon to the provision of the local growth factor GLP-1 which, in combination with SDF-1, promotes the growth, survival and viability of the beta cells.
Keywords: Alpha cells, Chemokine (C-X-C motif) receptor 4, Diabetes, GLP-1, Islets, Prohormone convertase, SDF-1, Stromal cell-derived factor-1
The prevalence of diabetes mellitus is increasing throughout the world [1]. A fundamental underlying cause of diabetes is an inadequate mass of insulin-producing beta cells in the islets of the pancreas, resulting in insufficient insulin to meet the body’s needs. In type 1 diabetes beta cells are nearly completely destroyed by autoimmunity. In type 2 diabetes, most commonly associated with obesity, beta cell mass is modestly reduced (~50%) and the remaining beta cells are functionally impaired by the acquired insulin resistance and resulting hyperlipidaemia and hyperglycaemia, so called gluco-lipotoxic stress. Gluco-lipotoxicity causes increased oxidative stress and shortens the lifespan of beta cells [2, 3]. A challenge in the treatment of diabetes is to find a means to preserve or enhance beta cell mass by stimulating the growth of new beta cells, prolonging the lifespan of beta cells, or both.
The architecture of the islets is remarkable in that the mass of insulin-producing beta cells is surrounded by non-beta endocrine cells: alpha, delta and pancreatic polypeptide-producing cells. In particular, in human islets the alpha cells are mixed in with the beta cells so that >70% of beta cells are in heterotypic contact with non-beta endocrine cells, most of which are the alpha cells that express the proglucagon gene (GCG) and produce the hormone glucagon in the islets [4]. This close proximity of endocrine cells to one another in the islets is highly suggestive of intra-islet autocrine and paracrine regulatory interactions among the different endocrine cell types [5].
Stromal cell-derived factor-1 (SDF-1; also known as chemokine [C-X-C motif] ligand 12 [CXCL12]) is a small peptide chemokine that regulates many essential biological processes, including stem cell motility, cardiac and neuronal development, neovascularisation and tissue repair and regeneration [6]. In particular, SDF-1 is produced in reactive stromal tissue in response to injuries of the liver [7] and heart [8], where it is believed to recruit bone-marrow-derived somatic stem cells involved in tissue repair [9]. Injury to the mouse pancreas in response to the transgenic overexpression of IFNγ activates production of SDF-1 in duct and stromal tissues [10]. In earlier studies we demonstrated that the SDF-1 receptor, chemokine (C-X-C motif) receptor 4 (CXCR4), is produced in the adult mouse pancreas. However, in adult islets the production of SDF-1 is restricted to vascular endothelial and stromal cells and is not detected in beta cells [11]. The forced endogenous production of SDF-1 in beta cells in transgenic mice (RIP-SDF-1) attenuates the development of diabetes in response to the ablation of beta cells by the administration of streptozotocin. An important and surprising finding was that 6 h after the administration of a high dose of streptozotocin to cause injury in beta cells, phospho-Akt, a pro-proliferative signal, appeared only in the alpha cells and not in the beta cells [11]. When the islets were examined 2 weeks after the single dose of streptozotocin the alpha cells had nearly completely replaced the beta cells in the islets in the normal mice, but many beta cells had persisted or regenerated in the SDF-1 transgenic mice that produce high levels of SDF-1 in beta cells [11]. These observations suggested the existence of paracrine cross-talk between beta and alpha cells as streptozotocin is known to be a beta cell-specific toxin that does not affect alpha cells [12, 13]. These findings led to our current hypothesis that injured beta cells might be communicating with alpha cells in the islets via SDF-1/CXCR4 signalling. Further, we showed that SDF-1 exerts cytoprotective actions on islet beta cells, and promotes their survival by the activation of the pro-survival kinase Akt and β-catenin/transcription factor 7-like 2 (T cell specific, HMG-box) (TCF7L2)-mediated wingless-related MMTV integration (WNT) signalling [14]. Likewise, the glucoincretin hormone glucagon-like peptide-1 (GLP-1) stimulates WNT signalling in beta cells resulting in an increase in their proliferation [15].
Here we report that in adult islets and in rat insulinoma INS-1 cells Sdf1 expression is re-induced in beta cells in response to injury invoked by cytokines, streptozotocin and thapsigargin. The SDF-1 receptor, CXCR4, is expressed on both alpha and beta cells. The paracrine actions of SDF-1 on its receptor on alpha cells activate protein kinase Akt, stimulate cell proliferation and induce the production of prohormone convertase (PC)1/3 and the consequent production of GLP-1 in alpha cells. GLP-1 predominantly promotes proliferation whereas SDF-1 exerts anti-apoptotic actions and promotes survival. We found the combination of GLP-1 and SDF-1 additively enhances both growth and longevity of cultured beta cells and thereby preserves beta cell mass against injury, glucotoxicity and nutrient deprivation.
We propose that injury to beta cells activates the SDF-1/CXCR4 axis in islets, resulting in the autocrine production of SDF-1 by beta cells and paracrine activation of GLP-1 production by alpha cells. The combination of the local intra-islet actions of GLP-1 and SDF-1 enhances the growth and survival of beta cells. Our findings suggest that SDF-1 agonists may be a means to stimulate the growth of alpha cells and their production of GLP-1 in the local intra-islet environment and thereby stimulate regeneration of injured beta cells in diabetes.
Methods
Reagents
Thapsigargin was obtained from Biomol (Ply-mouth Meeting, PA, USA). Cytokines, SDF-1 IFNγ, IL-1β and TNFα were obtained from R&D Systems (Minneapolis, MN, USA). Exendin-4 (EXD4) and streptozotocin were from Sigma-Aldrich (St Louis, MO, USA)
Culture of INS-1, MIN6 cells and alphaTC1 cells
Rat INS-1 cells (obtained from C. Wollheim, Geneva, Switzerland) were maintained in RPMI supplemented with 10% FBS, 1.0 mmol/l sodium pyruvate, 10 mmol/l HEPES, penicillin and streptomycin at 37°C under 5% CO2 and at 95% humidity. Mouse MIN6 cells (obtained from J. Miyazaki, University of Osaka, Japan) were cultured in DMEM containing 15% (vol./vol.) FBS. Mouse alphaTC1 cells (ATCC #2350, American Type Culture Collection, Gaithersberg, MD, USA) were maintained in RPMI supplemented with 10% (vol./vol.) FBS and 15 mmol/l HEPES.
Isolation of mouse pancreas and immunohistochemistry
Pancreases were removed from mice, fixed in formaldehyde or frozen, and immunostaining was performed on tissue sections as described by Yano et al. [11].
Isolation of mouse islets
Mouse islets were isolated from the pancreases of mice according to the standard protocol (see legend to Fig. 2). From each 12–18 week old male C57BL/6J mouse, 200–300 islets were obtained. Dispersed cells were prepared from islets isolated from 20–22 week old male mice for immunochemical studies by digestion of the islets in 0.05% (wt/vol.) trypsin at 37°C for 15 min with gentle shaking. The dispersed cells were plated on poly-D-lysine-coated slides (BD, Franklin Lakes, NJ, USA) and cultured overnight before treatment and fixation for immunostaining.
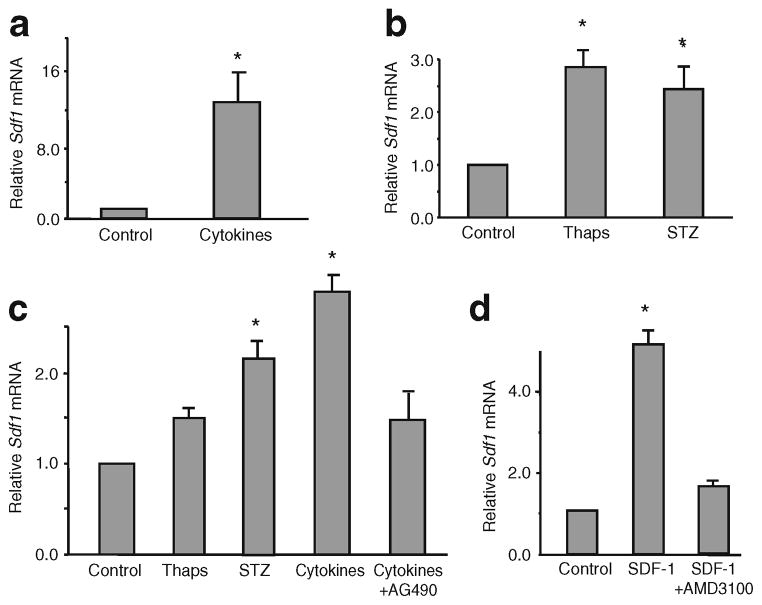
Fig. 2.
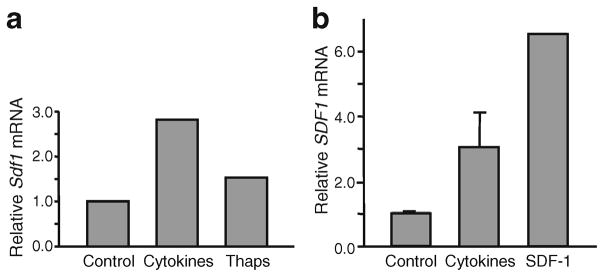
Beta cell injury induces expression of Sdf1 in islets. Sdf1 mRNA levels are induced in (a) mouse and (b) human islets by beta cell stress-activating agents. a Cytokines (50 ng/ml IL-1β, 10 ng/ml TNFα, 50 ng/ml IFNγ) and 50 nmol/l thapsigargin were added to mouse islets. b Cytokines and 20 nmol/l SDF-1 were added to human islets. In all experiments mRNA was extracted from islets and quantificative RT-PCR was performed. Islets were isolated by standard procedures [40]. All values are expressed relative to the value of the control-treated cells. Thaps, thapsigargin
Human islets from donors
Human islet tissue was obtained from the Integrated Islet Distribution Program (Madison, WI, USA). Use of human tissues was approved by the MGH Human Studies Committee. Dispersed cells were prepared from human islets by incubation in 0.01% trypsin (wt/vol.)-EDTA for 20 min at 37°C.
Treatment of islets and cells with cytokines and thapsigargin
Dispersed islet cells or INS-1 cells were cultured in 96 well plates or 10 cm dishes in the presence of 2 nmol/l EXD4 and/or 10 nmol/l SDF-1 on a background of nutrient deprivation by serum withdrawal or cell-stress-inducing reagent including thapsigargin 50 nmol/l or a cytokine cocktail of IL-1β 50 ng/ml, TNFα 10 ng/ml and IFNγ 50 ng/ml. Cell viability was measured by ATPlite assay (PerkinElmer, Waltham, MA, USA) or cell mass measurement.
Cell mass assay
INS-1 cells were cultured for 6 days in media with and without combinations of thapsigargin or cytokines with and without SDF-1 and EXD4. At the end of the incubation the cells were harvested by scrape loading and weighed.
RNA isolation and real-time
RT-PCR RNA isolation and real-time RT-PCR were performed as previously reported by Yano et al. and Liu et al. [11, 15]. For each treatment, all samples were performed in triplicate and the data are presented as ratio of Sdf1 (also known as Cxcl12)/Gapdh compared with the normalised control.
Phospho-Akt assay
AlphaTC1 cells (5×105 cells/ml) were treated with 10 nmol/l SDF-1 or vehicle controls for the times indicated. Streptozotocin was dissolved in 50 mmol/l sodium citrate buffer (pH 4.7). Cell lysates were prepared and proteins were analysed on western immunoblots as described earlier by Liu et al. [14, 15]. Protein density on immunoblots was quantified by densitometric analysis using a Kodak Image Station 440 CF (Eastman Kodak, Rochester, NY, USA). The primary antibodies used were phospho-Akt (Ser473) (587F11) monoclonal antibody (catalogue no. 4051; Cell Signaling Technologies, Beverly, MA, USA) and Akt antibody, which recognises Akt1, 2 and 3 (catalogue no. 9272; Cell Signaling Technologies).
GLP-1 and insulin assays
GLP-1 content in the culture medium and cell lysates was measured using an ELISA kit from BioVender LLC (Candfer, NC, USA) according to the instruction manual. Insulin content in the culture medium was measured by ELISA kit from Millipore (Billerica, MA, USA) according to the instruction manual.
Cell viability assay
Cell viability was measured using the ATPlite one-step assay according to manufacturer’s manual (PerkinElmer).
Cell proliferation assay
Proliferation of alphaTC1 cells was determined by incorporation of BrdU into newly synthesised DNA of proliferating cells. Cells in 96 well plates were treated with SDF-1 (10 nmol/l) or PBS overnight, then pulse-labelled with BrdU for 4 h. BrdU staining was measured using the DELFIA cell proliferation kit (PerkinElmer).
Immunofluorescent staining of dispersed mouse islet cells
Trypsin-treated cells were plated on slides and cultured overnight in RPMI media (Gibco, Grand Island, NY, USA) containing 10% (vol./vol.) FBS. Cells were treated, washed and fixed in 10% formalin in PBS for 15 min at room temperature for fluorescence immunostaining using a rabbit antiserum to mouse PC1/3 (a gift from D. F. Steiner and M. Hara, Chicago, IL, USA) and antisera to insulin (Millipore) and glucagon (Sigma-Aldrich). A mouse anti-glucagon monoclonal antibody was used and the antiserum to insulin was from guinea pig. Secondary fluorophore antibodies used were Cy2 (green)-labelled donkey anti-rabbit IgG and Cy3 (red)-labelled donkey anti-mouse IgG (glucagon) and donkey anti-guinea pig IgG (insulin). Fluorescent images were captured with a Nikon Optiphot 2 microscope using Photometric Cool Snap HQ camera (Photometrics, Huntington Beach, CA, USA) and IP Lab 3.6.5 software (Scanalytics, Falls Church, VA, USA).
Statistical analyses
Data are presented as the mean±SD. Statistical analysis was performed using paired t test. Values of p<0.05 were considered statistically significant.
Results
Neonatal expression of SDF-1 in beta cells is extinguished in the adult pancreas
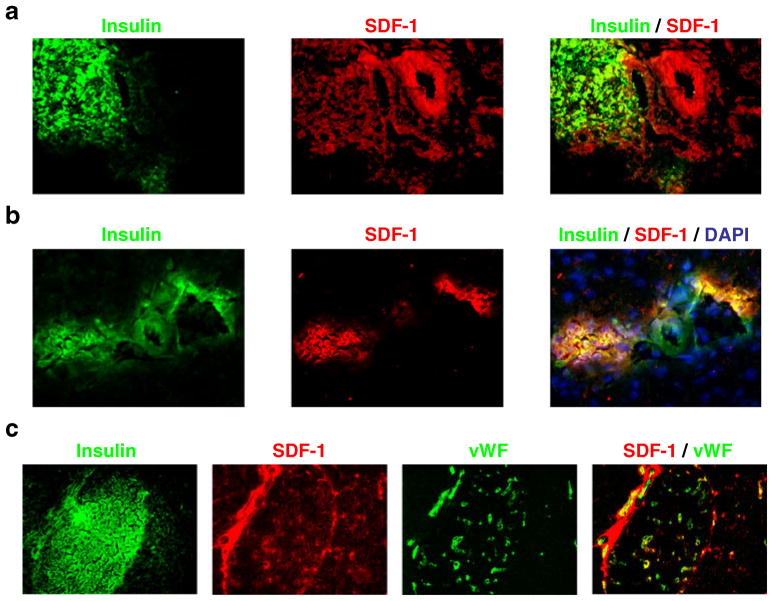
We previously reported that SDF-1 production in islets of the adult mouse pancreas is restricted to the vascular endothelial and stromal cells of the islets. As the SDF-1/CXCR4 axis signalling is implicated in the early developing pancreas [16], we examined neonatal pancreases for the production of SDF-1. We observed robust production of SDF-1 in beta cells of the islets at 3 (Fig. 1a) and 21 days (Fig. 1b) after birth but not in the adult islets at 60 days after birth (Fig. 1c). Notably, the pancreatic islets continue to develop and to remodel for 3–4 weeks after birth [17], suggesting that SDF-1 production in beta cells might be involved in their development and remodelling and that when they become fully differentiated Sdf-1 expression is suppressed.
Fig. 1.
SDF-1 produced in the beta cells of the neonatal (post-natal day [P]3 and P21) but not the adult (P60) mouse pancreas. SDF-1 production and its co-localisation with insulin in mouse islet beta cells at (a) P3 and (b) P21. c P60, adult pancreas. The majority of SDF-1 produced in the adult mouse pancreas is in cells within the interstitium surrounding the ducts and blood vessels in the islets (identified by staining of von Willebrand factor [vWF])
Injuries of islets and beta cells induces the production of SDF-1
Tissue injury induces regenerative processes that often recapitulate developmental programmes. Therefore, we asked whether injury to beta cells by various means would re-induce the neonatal pattern of SDF-1 production in islets. We subjected isolated mouse islets and human donor islets ex vivo (Fig. 2), and cultured beta cells in vitro to injuries by cytokines, thapsigargin, an inducer of ER stress [14], streptozotocin and hyperglycaemic glucotoxic stress (Fig. 3). In both mouse and human islets, treatment with cytokines (IL-1β, TNFα, IFNγ) or the inducer of endoplasmic reticulum stress thapsigargin increased levels of Sdf1 mRNA. Interestingly, the treatment of human islets with SDF-1 also increased the levels of SDF1 mRNA, indicating that the chemokine SDF-1 acts like the cytokines and induces its own expression in beta cells in islets by an autocrine mechanism (Fig. 2b). To examine actions of injurious agents more directly on beta cells than can be done on islets we then used relatively well-differentiated cultured beta cell lines: MIN6 (mouse) and INS-1 (rat). In both MIN6 (Fig. 3a, b) and INS-1 (Fig. 3c) cells cytokines induced Sdf1 mRNA levels by three- to 15-fold. Thapsigargin and streptozotocin were less potent than cytokines, increasing Sdf1 mRNA levels by two- to threefold. The addition of AMD3100, a specific antagonist of CXCR4, inhibited the increase in Sdf1 mRNA levels induced by SDF-1, indicating that the signalling responsible for activating Sdf1 gene expression is transduced via the SDF-1 receptor (Fig. 3d). Importantly, the induction of Sdf1 mRNA transcription by cytokines was inhibited by AG490, a potent inhibitor of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signalling pathway [18, 19], an important known signalling pathway used by CXCR4 in response to SDF-1 (Fig. 3c).
Fig. 3.
Beta cell injury induces expression of Sdf1 in beta cell lines. MIN6 and INS-1 beta cells were treated with stress/apoptosis inducers. MIN6 cells were treated with: (a) mixtures of cytokines (same concentration as indicated in the Fig. 2 legend), or (b) thapsigargin or streptozotocin. INS-1 cells were treated with: (c) thapsigargin (Thaps), streptozotocin (STZ) or cytokines with and without AG490, an inhibitor of JAK/STAT signalling; or (d) 10 nmol/l SDF-1 with and without 25 μmol/l AMD3100, an antagonist of the SDF-1 receptor, CXCR4. In all experiments the amount of Sdf1 mRNA was measured by quantitative RT-PCR. All values are means±SEM (n=8) relative to the value of the control-treated cells. *p<0.01 vs control values
SDF-1 stimulates the proliferation of alphaTC1 cells and induces the production of PC1/3 and GLP-1
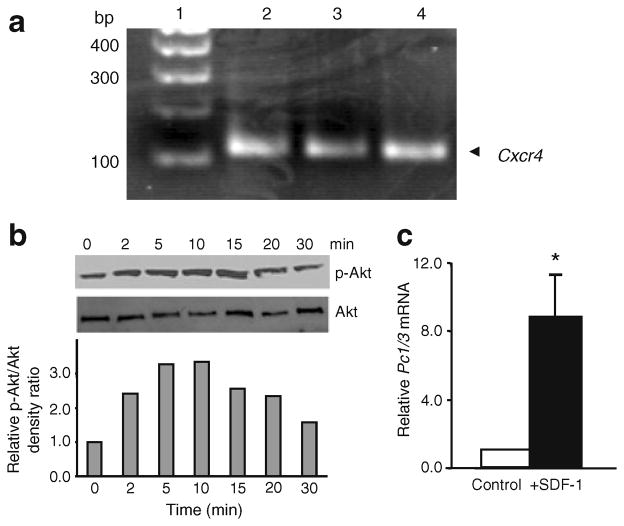
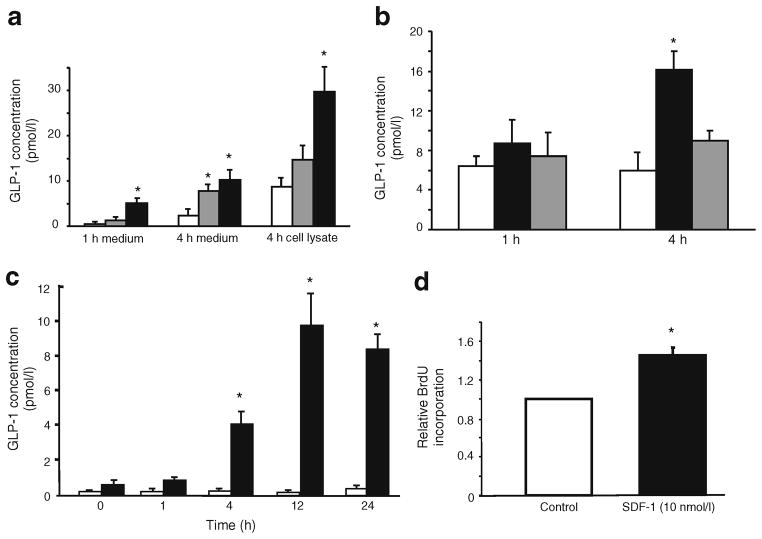
Because of our earlier findings that the administration of streptozotocin to mice activates Akt in the alpha and not the beta cells of islets [11] and the reported observation that the administration of streptozotocin to neonatal rats stimulates alpha cell hyperplasia and elevates plasma levels of GLP-1 [20], we wondered whether the SDF-1 induced by streptozotocin in beta cells might act on alpha cells. Therefore, we examined alphaTC1 cells, a well-studied alpha cell line [21, 22], for the presence of the SDF-1 receptor CXCR4. We found robust expression of CXCR4 on alphaTC1 cells (Fig. 4a) and that activation of the cells with SDF-1 stimulated the formation of active phospho-Akt within 2 min of its application to the cells (Fig. 4b). These findings were of interest because Akt and JAK/STAT signalling are known to activate the expression of Pc1/3 [23, 24]. PC1/3 is required for the processing of proglucagon, the product of the glucagon gene (Gcg), to GLP-1 [25]. We therefore examined mouse islets and alphaTC1 cells for their expression of Pc1/3 (also known as Pcsk1) and the production of GLP-1 in response to the application of SDF-1. As reported previously [22], we confirmed that Pc1/3 is barely detectable by quantificative PCR in alphaTC1 cells (Fig. 4c) and only trace amounts of GLP-1 peptide are detected by a sensitive GLP-1-specific ELISA in alphaTC1 cells (Fig. 5c) and in islets (Fig. 5a, b). The addition of SDF-1 to alphaTC1 cells induced the expression of Pc1/3 within 1 h (Fig. 4c) and stimulated the production and secretion of GLP-1 in both mouse and human islets (Fig. 5a, b) and in alphaTC1 cells (Fig. 5c). In a similar experiment the production of GLP-1 was unaffected by the GLP-1 receptor agonist EXD4 (data not shown).
Fig. 4.
Beta cell injury activates SDF-1/CXCR4 axis signalling in alpha cells. a The SDF-1 receptor CXCR4 is produced in alphaTC1 cells. Messenger RNA for Cxcr4 is detected in alphaTC1 cells by PCR. The known Cxcr4 expression in MIN6 cells is shown as a control. Lanes: 1, molecular markers; 2, alphaTC1 cells; 3, alphaTC1 cells with disruption of the Pc2 gene; 4, MIN6 cells. bp, base pairs. b SDF-1 activates protein kinase Akt in alphaTC1 cells. Phosphorylated Akt (p-Akt), the active form of Akt, is increased within 2 min after the addition of SDF-1 to the cells. Shown are the phospho-immunoblot and a semi-quantificative bar graph of the relative ratios of p-Akt to total Akt protein with time after the addition of SDF-1. c SDF-1 induces the expression of Pc1/3. Shown is the induction of Pc1/3 mRNA levels in alphaTC1 cells 1 h after the addition of SDF-1 (10 nmol/l) to the cells. No mRNA is detectable in alphaTC1 cells without added SDF-1. White bars, control medium; black bars, SDF-1 added. Values are shown relative to the value of the control-treated cells. Statistical significance is depicted as *p<0.01 when compared with control values
Fig. 5.
Cytokines and SDF-1 induce the production of GLP-1 protein in islets and alphaTC1 cells. a SDF-1 and cytokines stimulate the production of GLP-1 protein in isolated mouse islets. White bars, control medium; grey bars, cytokines added (see Methods); black bars, SDF-1 (10 nmol/l) added. b SDF-1 stimulates GLP-1 production in isolated human islets and production is inhibited by the CXCR4 antagonist AMD3100. White bars, control medium; black bars, SDF-1 (10 nmol/l) added; grey bars, SDF-1+AMD3100 added. c SDF-1 stimulates the production of GLP-1 in alphaTC1 cells during a 24 h incubation and production is not affected by the GLP-1 receptor agonist EXD4. GLP-1 levels were determined by ELISA in islet culture media and in extracts of the islets (cell lysate). White bars, control medium; black bars, SDF-1 (10 nmol/l) added. d SDF-1 stimulates the proliferation of alphaTC1 cells. Cells were incubated with 10 nmol/l SDF-1 for 24 h and relative proliferation was assayed by BrdU incorporation into the cells. White bars, control medium; black bars, SDF-1 added. Values are shown relative to the value of the control-treated cells. *p<0.01 vs control values in all panels (n=8)
To investigate production of PC1/3 in primary mouse alpha cells, dispersed mouse islet cells were prepared and cultured overnight before immunostaining with antisera to PC1/3, glucagon and insulin. Immunocytochemical analyses of alpha and beta cells in dispersed mouse islet cells showed weak, but definite, specific production of PC1/3 in alpha cells compared with stronger production in beta cells (electronic supplementary material [ESM] Fig. 1a, b). Dispersed mouse islet cells were treated with SDF-1 (100 nmol/l) for 6 h. Examination of several hundred cells by immunocytofluorography with antisera to PC1/3, glucagon and insulin suggested that the intensity of PC1/3 fluorescence increased in alpha cells with the addition of SDF-1 (ESM Fig. 1c). However, the paucity of cells precluded attempts at quantificative assessment of PC1/3 production in alpha cells. It remains possible, however, that the production of PC1/3 in alpha cells is perturbed by the stresses induced in the cells by islet isolation and the enzymatic dispersal of the islet cells. The effect of SDF-1 on GLP-1 production is an important finding because GLP-1 is known to stimulate the growth [14, 26] and promote the survival [27, 28] of beta cells. The production of GLP-1 locally within the islet could exert short-range tropic actions on adjacent beta cells. The addition of SDF-1 to alphaTC1 cells appears to stimulate their proliferation as determined by BrdU incorporation into the cells in response to SDF-1 (Fig. 5d). This finding suggests that the production of SDF-1 by injured beta cells might be a mechanism for the development of alpha cell hyperplasia seen in mice after the ablation of beta cells by the administration of streptozotocin [11, 12].
SDF-1 and GLP-1 additively enhance INS-1 cell mass
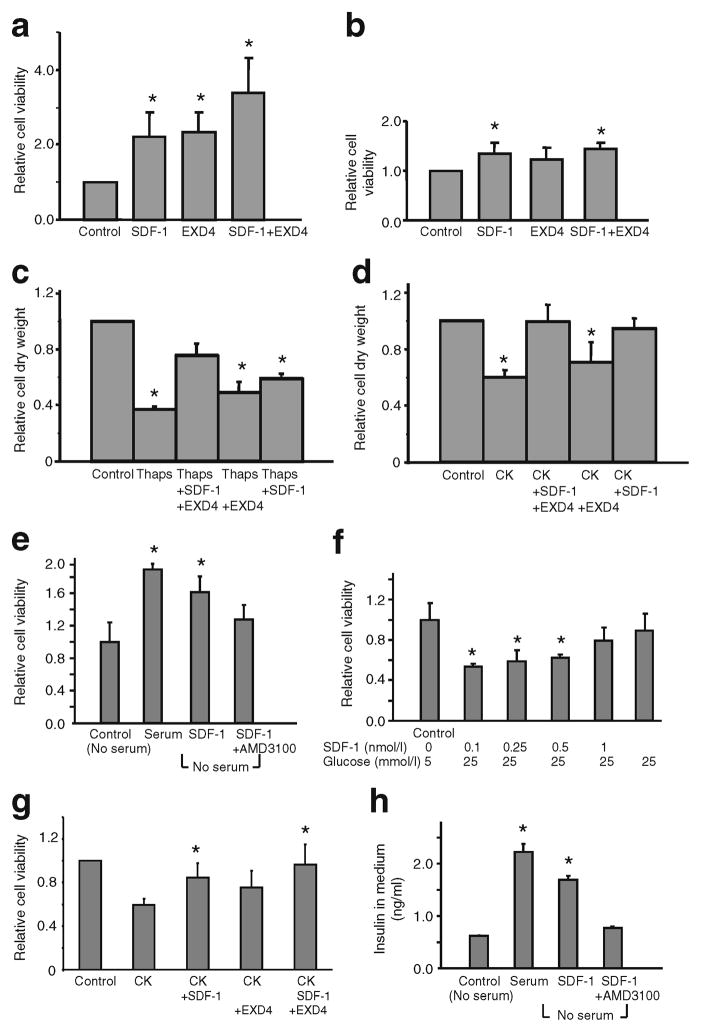
We next explored potential synergism between the growth-promoting actions of GLP-1 and the pro-survival actions of SDF-1 on the efficacy of the regeneration of beta cell mass in response to beta cell injury (Fig. 6). We hypothesised that the combination of GLP-1 and SDF-1 enhance both growth and longevity of INS-1 cells and thereby preserve beta cell mass against ongoing injury. We used the more stable long-acting GLP-1 receptor agonist EXD4. INS-1 cells were cultured in the presence of 2 nmol/l EXD4 and/or 10 nmol/l SDF-1 on a background of injury induced by serum withdrawal and repletion (Fig. 6a, b), thapsigargin (Fig. 6c), or cytokines (IL-1β, TNFα, IFNγ) (Fig. 6d, g). EXD4 and SDF-1 were replenished at 2 days. At 4 days after treatment cell viability was measured by the ATPlite assay (Fig. 6a, b). In parallel, INS-1 cell mass was assessed at the end of the culture by scrape-harvest and weighing of the cell mass (Fig. 6c, d). Under glucotoxicity (25 mmol/l glucose), SDF-1 protects cell survival in a dose-dependent fashion (Fig. 6f) and in serum deprivation conditions SDF-1 partially restores insulin secretion from INS-1 cells (Fig. 6h). The viability of primary dispersed human islet cells treated with cytokines was also enhanced additively by the co-addition of SDF-1 and EXD4 (Fig. 6g). Importantly, the actions of SDF-1 on cell survival (Fig. 6a) and on insulin secretion (Fig. 6h) in conditions of serum deprivation are inhibited by the SDF-1 receptor antagonist AMD3100.
Fig. 6.
SDF-1 and the GLP-1 receptor agonist EXD4 act additively to preserve and enhance beta cell mass. INS-1 cells were cultured in 96 well plates in the presence of 2 nmol/l EXD4 and/or 10 nmol/l SDF-1 on a background of (a) serum deprivation or (b) serum repletion (0.8%). EXD4 and SDF-1 were replenished every 2 days. Cell viability was measured 6 days after treatment by ATPlite assay. INS-1 cells treated with (c) thapsigargin or (d) cytokines were incubated with vehicle or 10 nmol/l SDF-1, 10 nmol/l EXD4 or SDF-1+EXD4 for 6 days and their dry weight was then measured. Data are expressed as mass relative to the mass for vehicle treatment. e INS-1 cells were cultured in 96 well plates in the presence of 10 nmol/l SDF-1 with or without AMD3100 (25 μmol/l) on a background of nutrient deprivation by serum withdrawal (no serum). f INS-1 cells were cultured in 96 well plates in the presence of increasing doses of SDF-1 on a background of normal (5 mmol/l) or high glucose concentration (25 mmol/l). Reagents were added at day 0 and day 2, and 4 days after treatment, cell viability was measured using the ATPlite assay. g Dispersed human islets were cultured in 96 well plates (~100 islets/well) in the presence of 2 nmol/l EXD4, and/or 10 nmol/l SDF-1 on a background of cytokines (IL-1β 50 ng/ml, TNFα 10 ng/ml, IFNγ 50 ng/ml). Cell viability was measured 3 days after treatment by ATPlite assay. The viable beta cell number was enhanced additively by EXD4 and SDF-1. Therefore the combination of GLP-1 and SDF-1 increased human islet cell viability against cytokine-stress-induced cell death. h For the insulin secretion assay, INS-1 cells were plated in 96 well plates. SDF-1 (2 nmol/l) and AMD3100 were added at day 0, and the insulin concentration of culture medium was measured at day 6. Statistical significance is depicted as *p<0.01 when compared with control values in all panels (n=6). CK, cytokines; Thaps, thapsigargin
Discussion
We demonstrate that when islet beta cells are injured by cytokines, streptozotocin, thapsigargin and glucotoxicity, SDF-1 production resembling neonatal production is reinduced. The SDF-1 receptor CXCR4 is expressed on alpha cells and is capable of activating the Akt protein kinase. Importantly, the apparent paracrine actions of SDF-1 on its receptor in alpha cells stimulate the production of GLP-1 in islets. We further explored the potential synergism between the growth-promoting actions of GLP-1 and the pro-survival actions of SDF-1 on the preservation of INS-1 cell mass, and found that the combination of GLP-1 and SDF-1 additively enhances both growth and longevity of beta cells.
GLP-1, an incretin hormone initially recognised for its glucose-dependent insulin-releasing actions, is now known to stimulate both the proliferation [15, 26] and the survival [27] of beta cells. GLP-1 agonists given systemically are currently used to stimulate insulin secretion in type 2 diabetic individuals. Alpha cells normally produce PC2, which cleaves proglucagon to release glucagon, a hormone that stimulates hepatic glucose production to maintain euglycaemia during fasting. GLPs are cleaved from proglucagon by PC1/3, another member of the PC protein family not normally produced in alpha cells. Activation of PC1/3 production and the ensuing GLP-1 production were recently found in alpha cells of prediabetic NOD mice, pregnant mice, ob/ob mice and db/db mice [29]. Therefore the stimulation of GLP-1 production from alpha cells within islets, specifically by SDF-1 agonists, might provide high local paracrine concentrations of GLP-1 for adjacent beta cells at a critical time during their injury and thereby might promote beta cell regeneration. We propose that a mechanism for the induction of GLP-1 production in alpha cells of islets is via the induction of PC1/3 by the paracrine actions of SDF-1. PC1/3 production and resultant GLP-1 production convert the alpha cell from a hyperglycaemia-promoting cell to one that promotes beta cell growth and survival.
In addition to a role for alpha cells in exerting paracrine tropic influences on beta cells via GLP-1 secretion, alpha cells might actually transdifferentiate into beta cells, given the appropriate stimulus. Recent seminal findings by Collombat et al. [30] and Thorel et al. [31] provide proof-of-concept evidence that the alpha cell lineage is endowed with an unusual plasticity and that alpha cells can become functional beta cells in response to beta cell injury. Based on our findings, we propose that alpha cells might also play active role in the preservation and regeneration of beta cells in the adult pancreas in response to injury invoked by means such as autoimmunity in type 1 diabetes and gluco-lipotoxicity in type 2 diabetes. As such, we refer to this mechanism as the B>A>B hypothesis: injured beta cells signal to alpha cells, which in turn signal to beta cells to stimulate their regeneration (Fig. 7). In our model, in response to beta cell injury (resulting in hypoinsulinaemia and hyperglycaemia) the biological functions of the alpha cells switch from regulation of glucose metabolism (glucagon) to providing a local growth factor, GLP-1, which is involved in the regeneration of the injured beta cells. This switch is mediated through the intra-islet signalling mediated by the SDF-1/CXCR4 axis, which has been shown to play an important role in pancreas development [16] and regeneration [11] and, particularly, in the growth and survival of beta cells [10, 11, 14]. The effects of GLP-1-induced protection against apoptosis might be mediated by IGF-1 as GLP-1 is reported to increase the activity of an IGF-1/IGF-1 receptor loop in beta cells [32].
Fig. 7.
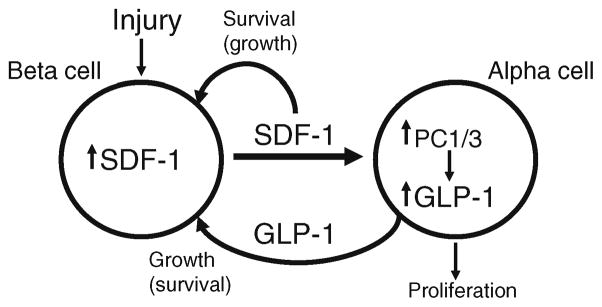
Model depicting the proposed autocrine and paracrine crosstalk between beta and alpha cells in response to beta cell injury: the B>A>B hypothesis. See text for explanation
In pathophysiological conditions, such as exposure to sustained high glucose because of the development of insulin resistance in type 2 diabetes, the beta cells increase in number (hyperplasia) in an attempt to produce more insulin to counteract the hyperglycaemia. Likewise, sustained hypoglycaemia induced by the experimental disruption of glucagon signalling, e.g. knockout of the glucagon receptor gene [33, 34], or knockout of the gene encoding PC2, which is required for the post-translational cleavage of proglucagon to produce glucagon [35–37], resulting in impaired gluconeogenesis, leads to marked alpha cell hyperplasia.
Paradoxically, alpha cell hyperplasia also occurs in conditions of insulin deficiency resulting from injury of beta cells, for example in mice given the beta cell toxin streptozotocin [11, 12, 37, 38], or in the NOD mouse in which beta cells are injured by autoimmune attack [39]. By all physiological arguments of feedback regulation of glucagon secretion, hyperglycaemia should suppress glucagon secretion and alpha cell functions, and not cause hyperplasia of the alpha cells. We propose that this paradox of alpha cell hyperplasia might be explained by the fact that in response to beta cell injury (resulting in hypoinsulinaemia and hyperglycaemia) the injured beta cells produce SDF-1 and the activation of the SDF-1/CXCR4 axis in alpha cells adjacent to beta cells in islets. The activation of CXCR4-mediated signalling stimulates alpha cell proliferation and switches the biological functions of the alpha cells from the regulation of glucose metabolism to that of providing local growth factors, such as GLP-1, involved in the regeneration of the injured beta cells. Whether or not SDF-1 stimulates the proliferation of primary alpha cells in vivo or in vitro remains uncertain. Although we have demonstrated increased proliferation of cultured alphaTC1 cells by SDF-1, attempts at demonstrating SDF-1-mediated stimulation of primary mouse and human islet cells in vitro were equivocal because of the sparsity of alpha cells in dispersed mouse islet cell preparations and the rapid (1 day in culture) de-differentiation of dispersed cells prepared from donor human islets. The validation of the hypothesis that SDF-1 might stimulate the growth of alpha cells awaits appropriate studies in vivo, such as using the transgenic RIP-SDF-1 mouse that constitutively expresses Sdf1 in islet beta cells [11]. Our results, and the previous study in Pc2 (also known as Pcsk2)-null mice [34], demonstrate that the alpha cells are endowed with an unusual plasticity in their abilities to switch their hormone production from glucagon to GLP-1, which has functions within islets proposed to promote the growth and the survival of beta cells.
Supplementary Material
Acknowledgments
We thank K. McManus and L. Brindamour for expert experimental assistance. We thank M. Hara and D. F. Steiner for the generous gift of the PC1/3 antiserum. The studies were supported in part by research grants (J. F. Habener) and a postdoctoral fellowship (Z. Liu) from the Juvenile Diabetes Research Foundation and a grant from the Charles H. Hood Foundation of the Medical Research Foundation to Z. Liu.
Abbreviations
- CXCR4
Chemokine (C-X-C motif) receptor 4
- GLP-1
Glucagon-like peptide-1
- JAK
Janus kinase
- PC
Prohormone convertase
- SDF-1
Stromal cell-derived factor-1
- STAT
Signal transducer and activator of transcription
- WNT
Wingless-related MMTV integration
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00125-011-2181-x) contains supplementary material, which is available to authorised users.
Duality of interest The authors declare there is no duality of interest associated with this manuscript.
Contributor Information
Z. Liu, Laboratory of Molecular Endocrinology, Massachusetts General Hospital, Boston, MA, USA
V. Stanojevic, Laboratory of Molecular Endocrinology, Massachusetts General Hospital, Boston, MA, USA
S. Avadhani, Laboratory of Molecular Endocrinology, Massachusetts General Hospital, Boston, MA, USA
T. Yano, Laboratory of Molecular Endocrinology, Massachusetts General Hospital, Boston, MA, USA
J. F. Habener, Email: jhabener@partners.org, Laboratory of Molecular Endocrinology, Massachusetts General Hospital, Boston, MA, USA. Thier 306, 55 Fruit Street, Boston, MA 02114, USA
References
- 1.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17 (suppl 1):S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a. [DOI] [PubMed] [Google Scholar]
- 2.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2349. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagner JI, Samols E, Bonner-Weir S. Beta–alpha–delta pancreatic islet cellular perfusion in dogs. Diabetes. 1988;37:1715–1721. doi: 10.2337/diab.37.12.1715. [DOI] [PubMed] [Google Scholar]
- 6.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 7.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 8.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 9.Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 10.Kayali AG, van Gunst K, Campbell IL, et al. The stromal cell-derived factor-1alpha/CXCR4 ligand–receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–869. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano T, Liu Z, Donovan J, Thomas MK, Habener JF. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes. 2007;56:2946–2957. doi: 10.2337/db07-0291. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. J Endocrinol. 2000;165:93–99. doi: 10.1677/joe.0.1650093. [DOI] [PubMed] [Google Scholar]
- 13.Park IS, Che YZ, Bendayan M, Kang SW, Min BH. Up-regulation of clusterin (sulfated glycoprotein-2) in pancreatic islet cells upon streptozotocin injection to rats. J Endocrinol. 1999;162:57–65. doi: 10.1677/joe.0.1620057. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Habener JF. Stromal cell-derived factor-1 promotes survival of pancreatic beta cells by stabilization of beta-catenin and activation of TCF7L2. Diabetologia. 2009;52:1589–1598. doi: 10.1007/s00125-009-1384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem. 2008;283:8723–8735. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 17.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 18.Vila-Coro AJ, Rodríguez-Frade JM, Martín de Ana A, et al. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- 19.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 20.Thyssen S, Arany F, Hill DJ. Ontogeny of regeneration of beta cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006;147:2346–2356. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 21.Webb GC, Dey A, Wang J, Stein J, Milewski M, Steiner DF. Altered proglucagon processing in an alpha-cell line derived from prohormone convertase 2 null mouse islets. J Biol Chem. 2004;279:31068–31075. doi: 10.1074/jbc.M404110200. [DOI] [PubMed] [Google Scholar]
- 22.Wideman RD, Yu IL, Webber TD, Verchere CB, Johnson JD, Cheung AT, Kieffer TJ. Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1. Proc Natl Acad Sci USA. 2006;103:13468–13473. doi: 10.1073/pnas.0600655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lankat-Buttgereit B, Müller S, Schmidt H, Parhofer KG, Gress TM, Göke R. Knockdown of Pdcd4 results in induction of proprotein convertase 1/3 and potent secretion of chromogranin A and secretogranin II in a neuroendocrine cell line. Biol Cell. 2008;100:703–715. doi: 10.1042/BC20080052. [DOI] [PubMed] [Google Scholar]
- 24.Fox DL, Good DJ. Nhlh2 interacts with STAT3 to regulate transcription of prohormone convertase 1/3. Mol Endocrinol. 2008;22:1438–1448. doi: 10.1210/me.2008-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. A switch from prohormone convertase PC2 to PC1/3 expression in transplanted alpha cells is accompanied by differential processing of proglucagon and improved glucose homeostasis in mice. Diabetes. 2007;56:2744–2752. doi: 10.2337/db07-0563. [DOI] [PubMed] [Google Scholar]
- 26.Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, Nielsen JH, Møldrup A. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188:481–492. doi: 10.1677/joe.1.06160. [DOI] [PubMed] [Google Scholar]
- 27.Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144:1444–1455. doi: 10.1210/en.2002-220897. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 29.Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets. 2010;2:149–155. doi: 10.4161/isl.2.3.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B. Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes. 2009;58:1816–1825. doi: 10.2337/db09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedees MH, Guz Y, Vuguin PM, et al. Nestin expression in pancreatic endocrine and exocrine cells of mice lacking glucagon signaling. Dev Dyn. 2007;236:1126–1133. doi: 10.1002/dvdy.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vuguin PM, Kedees MH, Cui L, et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb GC, Akbar MS, Zhao C, Swift HH, Steiner DF. Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes. 2002;51:398–405. doi: 10.2337/diabetes.51.2.398. [DOI] [PubMed] [Google Scholar]
- 36.Vincent M, Guz Y, Rozenberg M, et al. Abrogation of protein convertase 2 activity results in delayed islet cell differentiation and maturation, increased alpha-cell proliferation, and islet neogenesis. Endocrinology. 2003;144:4061–4069. doi: 10.1210/en.2003-0088. [DOI] [PubMed] [Google Scholar]
- 37.Nie Y, Nakashima M, Brubaker PL, et al. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J Clin Invest. 2000;105:955–965. doi: 10.1172/JCI7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones CW, Reynolds WA, Hoganson GE. Streptozotocin diabetes in the monkey: plasma levels of glucose, insulin, glucagon, and somatostatin, with corresponding morphometric analysis of islet endocrine cells. Diabetes. 1980;29:536–546. doi: 10.2337/diab.29.7.536. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53:1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- 40.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.