Abstract
Background
Human hearing is sensitive to sounds from as low as 20 Hz to as high as 20,000 Hz in normal ears. However, clinical tests of human hearing rarely include extended high frequency (EHF) threshold assessments, at frequencies extending beyond 8,000 Hz. EHF thresholds have been suggested for use monitoring the earliest effects of noise on the inner ear, although the clinical utility of EHF threshold testing is not well established for this purpose.
Purpose
The primary objective of this study was to determine if EHF thresholds in healthy, young adult college students vary as a function of recreational noise exposure.
Research Design
A retrospective analysis of a laboratory database was conducted; all participants with both EHF threshold testing and noise history data were included. The potential for “pre-clinical” EHF deficits was assessed based on the measured thresholds, with the noise surveys used to estimate recreational noise exposure.
Study Sample
EHF thresholds measured during participation in other ongoing studies were available from 87 subjects (34 male and 53 female); all participants had hearing within normal clinical limits (≤25 HL) at conventional frequencies (0.25 to 8 kHz).
Results
EHF thresholds closely matched standard reference thresholds [ANSI S3.6 (1996) Annex C]. There were statistically reliable threshold differences in subjects that used music players, with 3–6 dB worse thresholds at the highest test frequencies (10–16 kHz) in participants that reported long-term music player device use (longer than 5 years), or higher listening levels during music player use.
Conclusions
It should be possible to detect small changes in high frequency hearing for patients/participants that undergo repeat testing at periodic intervals. However, the increased population-level variability in thresholds at the highest frequencies will make it difficult to identify the presence of small but potentially important deficits in otherwise normal hearing individuals that do not have previously established baseline data.
Keywords: extended high frequency, EHF, music, hearing loss, personal music player
Human hearing has long been known to extend to at least 20,000 Hz (Fowler & Wegel, 1922; for reviews, see De Seta et al., 1985; Vogel et al., 2007). The frequency range from 10 to 20 kHz is now commonly referred to as the ultra-audiometric or “extended high frequency” (EHF) range of hearing. Elevated EHF thresholds have been linked with a history of noise exposure (Vassallo et al., 1968; Osterhammel, 1979; Erickson et al., 1980; Fausti et al., 1981a; Fausti et al., 1981b). Significant EHF deficits have also been reported in patients treated with ototoxic drugs such as the angioplast cisplatin (Fausti et al., 1984b; Tange et al., 1985) and aminoglycoside antibiotics (Fausti et al., 1984a). Consequently, EHF testing has been proposed as a potentially useful assay for identifying early changes in hearing following either physical or pharmacological trauma, thus providing an “early” warning for subsequent hearing loss.
Serial monitoring at EHF frequencies has been successfully applied for detecting the early onset of drug-induced ototoxicity (see, for example, Fausti et al., 1999; Vaughan et al., 2002; Knight et al., 2007; Konrad-Martin et al., 2010; Jacobs et al., 2012). Longitudinal studies assessing EHF thresholds as a function of noise, by contrast, are much more limited. Data from a single longitudinal study that followed a sample of 14-yr old students over a three-year time course revealed the largest threshold changes occurred between test 1 (at 14-yrs old) and test 2 (at 15-yrs old); the largest changes were 4 to 6 dB, and these threshold shifts were observed at the two highest frequencies tested (14 and 16 kHz) (Serra et al., 2005). Based on reported exposures, changes in hearing were attributed to music coming from a variety of sources, including live concert attendance and attendance at discothèques (Biassoni et al., 2005). Whereas serial studies are limited, cross-sectional studies are more common, and the most robust support for noise-induced deficits at EHF frequencies comes from a cross-sectional analysis of hearing thresholds in adult male factory workers, with varied exposure to noise (Ahmed et al., 2001). When the analysis was limited to just those workers with “normal” (≤20 dB HL) thresholds from 250 Hz to 8 kHz, the subset of workers exposed to noise had deficits at EHF frequencies from 12 to 20 kHz, whereas other workers not assigned to noisy areas did not have EHF threshold deficits. These data were taken to suggest EHF threshold deficits precede hearing loss at lower frequencies. Longitudinal studies, incorporating serial monitoring, are critical for determining whether workers with EHF deficits go on to develop hearing loss at lower frequencies over time. However, the cross-sectional data are both important and intriguing with respect to the utility of EHF deficits in providing an “early warning”. Changes at EHF frequencies may ultimately prove to be a useful tool for identifying individuals with increased vulnerability to noise insult (see Osterhammel, 1979), or those likely to develop noise-induced hearing loss (NIHL) at conventional test frequencies (250 Hz to 8 kHz).
The potential for changes at EHF frequencies have subsequently been explored in adolescents exposed to recreational music, an alternative source of sound overexposure. Use of personal music players has already been correlated with EHF deficits (Peng et al., 2007; Figueiredo et al., 2012); our own analysis of the relationship between thresholds at conventional test frequencies, ranging from 250 Hz to 8 kHz, is consistent with this literature in that deficits as a function of music player were observed only at the highest conventional test frequencies (6 and 8 kHz) (Le Prell et al., 2011). One of the main shortcomings of the existing literature that seeks to identify relationships between music player use and hearing deficits is the limited information on other sources of noise to which study participants are routinely exposed. Since our earlier report, we have continued to screen young adult hearing thresholds such that we now have access to EHF thresholds from a significant number of individuals who have participated in completed (Le Prell et al., 2012) and ongoing studies. Our noise survey yields information not only about music player use, but also concert attendance, attendance at loud sporting events, attending bars/clubs, playing a musical instrument, etc. Here, we test the hypothesis that recreational noise exposure is reliably related to “pre-clinical” changes in hearing, in the form of elevated EHF thresholds in young adults who report the greatest level of noise exposure.
Materials and Methods
Participants
EHF (10, 12.5, 14 and 16 kHz) thresholds were available from 87 young adult college students with hearing thresholds of 25 HL or better from 250 Hz to 8 kHz, as assessed during IRB-approved studies conducted from 2008 to 2012 (see Table 1 for demographic data). All participants had responded to advertisements posted on the University of Florida campus inviting “normal-hearing” young adults (ages 18–31) to participate in hearing studies. Prospective participants provided written informed consent, and were compensated $10–$15 per hour for their time. All protocols and procedures were approved by the appropriate Institutional Review Board (IRB) at the University of Florida.
Table 1.
General health/hearing health characteristics.
| Male n=34 (39.1%) |
Female n=53 (69.1%) |
All Subjects n=87 |
||
|---|---|---|---|---|
| Age | 21.4 yrs±2.4yrs Range=18–29 yrs |
21.7 yrs±2.6yrs Range=18–29 yrs |
21.6 yrs±2.5yrs Range=18–29 yrs |
|
| Height (feet) | 5.8 ft ±0.3 ft Range=5.2–6.4 ft |
5.4 ft ±0.3 ft Range=4.9–6 ft |
5.5 ft ±0.3 ft Range=4.9–6.4 ft |
|
| Weight (pounds) | 159 lbs±26 lbs Range=120–250 |
140 lbs±33 lbs Range=85–255 |
147 lbs±32 lbs Range=85–255 |
|
| Tobacco user (% yes) | 4 (12%) | 1 (2%) | 5 (6%) | |
| Cigarettes | 2 (6%) (plus 1 former) |
1 (2%) | 3 (3%) | |
| Cigars | 1 (3%) | 0 | 1 (1%) | |
| Pipe | 1 (3%) | 0 | 1 (1%) | |
| Chew | 0 | 0 | 0 | |
| Alcohol use (% yes) | 21 (62%) | 25 (47%) | 46 (53%) | |
| 0 drinks/week | 13 (38%) | 28 (53%) | 41 (47%) | |
| 1–5 drinks/week | 17 (50%) | 19 (36%) | 36 (41%) | |
| 6–10 drinks/week | 4 (12%) | 6 (11%) | 10 (12%) | |
| Hearing aids (% yes) | 0 | 0 | 0 | |
| Ear pain/Ear ache (% yes, previously) | 3 (9%) | 6 (11%) | 9 (10%) | |
| Ear drainage (% yes, previously) | 1 (3%) | 0 | 1 (1%) | |
| Ear infections (% yes, previously) | 8 (24%) | 12 (23%) | 20 (23%) | |
| Ringing in ears (% yes-currently, % yes-previously) | 2 (6%) 6 (18%) |
0 8 (15%) |
2 (2%, yes-currently) 14 (16%, yes-previously) |
|
| Balance Disturbance (% yes, previously) | 1 (3%) | 2 (4%) | 3 (3%) | |
| Seizures (% yes, previously) | 1 (3%) | 0 | 1 (1%) | |
| Freq severe headaches (% yes, previously) | 2 (6%) | 2 (4%) | 4 (5%) | |
| Stroke (% yes, previously) | 0 | 0 | 0 | |
| Fainting (% yes, previously) | 0 | 4 (8%) | 4 (5%) | |
| Disorientation (% yes, previously) | 1 (3%) | 0 | 1 (1%) | |
| Parent or Sibling with hearing loss? (% yes) | 2 (6%) | 10 (19%) | 12 (14%) | |
| Have you ever had an ear infection? (% yes) | 18 (53%) | 25 (47%) | 43 (49%); None were within past 3 months | |
| Have you ever had hearing loss? (% yes) | 1 (3%) | 4 (8%) | 5 (6%); all 5 reported “only after loud sound” | |
| Are you overly sensitive to loud sound? (% yes) | 1 (3%) | 1 (2%) | 2 (2%) | |
| Have you ever heard “ringing” in your ears after noise? (% yes) | 21 (62%) | 28 (53%) | 49 (56%) | |
| If yes, does this happen: | Always | 2/21=10% | 1/28=36% | 3/49=6% |
| Often | 4/21=19% | 4/28=24% | 8/49=16% | |
| Occasionally | 4/21=19% | 9/28=32% | 13/49=27% | |
| Rarely | 11/21=52% | 14/28=50% | 25/49=51% | |
| in absence of noise? (% yes) | 8/21=38% | 7/28=25% | 15/49=31% | |
Surveys
Participants completed health (Table 1) and hearing-related (Table 2) surveys after providing written informed consent. Because the study participants were drawn from two different study-specific volunteer populations, there was a subtle difference with respect to questions posed. In the first study population, participants were asked to report which loud sounds they are exposed to during their leisure time, including Bars/Clubs, Concerts/Discos, Walkman/iPod, Loud Music in a Vehicle, Hunting/Shooting Range, Sports Events, or other. There was no additional descriptive detail for any reported exposures. In the second study population, participants were asked which loud sounds they are typically exposed to in their leisure time, with the additional specification of once/month or more often; however, the same list of common exposures was provided. Survey responses are pooled here to provide a comprehensive array of common noise sources across the entire sample. Participants enrolled in this second study completed additional detailed questionnaires specific to musical instrument practice patterns, and personal music player use patterns. Data from the 54 participants who completed the more detailed surveys are provided in the bottom half of Table 2.
Table 2.
Noise history.
| Male n=34 (39.1%) |
Female n=53 (69.1%) |
All Subjects n=87 |
||
|---|---|---|---|---|
| Specific Noise Sources Surveyed | ||||
| Bar/Club | 11 (32%) | 25 (47%) | 36 (41%) | |
| Hunting/Shooting | 1 (3%) | 0 (0%) | 1 (1%) | |
| Sports | 10 (29%) | 8 (15%) | 18 (21%) | |
| Concert | 7 (21%) | 11 (21%) | 18 (21%) | |
| Music Rehearsal | 5 (15%) | 0 (0%) | 5 (6%) | |
| Music Player | 18 (53%) | 31 (58%) | 49 (56%) | |
| Music in Vehicle | 13 (38%) | 16 (30%) | 29 (33%) | |
| Workplace | 3 (9%) | 1 (2%) | 4 (5%) | |
| Total number of frequent or monthly noise sources reported | 2.0±1.6 Range=0–6 |
1.8±1.2 Range=0–4 |
1.9±1.4 Range=0–6 |
|
| Any Unprotected Impulse Noise (% yes) |
13 (38%) | 19 (36%) | 32 (37%) | |
| Use a music player? | 31 (91%) | 47 (89%) | 78 (90%) | |
| 54 subjects (21M, 33F) subjects were asked more detailed information. Of these, 46 subjects used music players (18M, 28F). Their responses are shown here. |
Hours per day | <1: 9/18=50% 1–2: 6/18=33% 3–5: 3/18=17% 5–8: 0/18 >8: 0/18 |
<1: 13/28=46% 1–2: 12/28=43% 3–5: 1/28=4% 5–8: 2/28=7% >8: 0/28 |
<1: 22/46=48% 1–2: 18/46=39% 3–5: 4/46=9% 5–8: 2/46=4% >8: 0/46 |
| Days per week | <1: 3/18=17% 1–2: 2/18=11% 3–5: 7/18=39% 5–7: 6/18=33% |
<1: 3/28=11% 1–2: 5/28=18% 3–5: 17/28=61% 5–7: 3/28=11% |
<1: 6/46=13% 1–2: 7/46=15% 3–5: 24/46=52% 5–7: 9/46=20% |
|
| Years of use | <1: 1/18=6% 1–2: 1/18=6% 3–5: 4/18=22% 5–8: 5/18=28% >8: 7/18=39% |
<1: 1/28=4% 1–2: 0/28 3–5: 11/28=39% 5–8: 6/28=21% >8: 10/28=36% |
<1: 2/46=4% 1–2: 1/46=2% 3–5: 15/46=33% 5–8: 11/46=24% >8: 17/46=37% |
|
| Type of earphones | Earbuds: 11/18=61% Inserts: 5/18=28% Headphones: 3/18=17% |
Earbuds: 21/28=75% Inserts: 5/28=18% Headphones: 3/28=11% Did not respond: 1/28=4% |
Earbuds: 32/45=71% Inserts: 10/45=22% Headphones: 6/45=13% |
|
| Common listening environment? | Noise: 6/18=33% Quiet: 7/18=39% Both: 4/18=22% Other: 1/18=6% (outdoors) |
Noise: 4/28=14% Quiet: 8/28=29% Both: 14/28=50% Other: 2/28=7% (outdoors, car) |
Noise: 10/46=22% Quiet: 15/46=33% Both: 17/46=37% Other: 3/46=7% (outdoors, in car) |
|
| Hear someone speaking to you? (% yes) | 14/18=78% | 18/28=64% | 32/46=70% | |
| Play a musical instrument? | 10/34=29% | 6/53=11% | 16/87=18% | |
| 54 subjects (21M, 33F) subjects were asked more detailed information. Of these, 13 subjects played musical instruments (7M, 27F). Their responses are shown here. | Hours per day, solo | <1: 1/7=14% 1–4: 4/7=57% 5–10: 2/7=29% >10: 0/7 |
<1: 2/6=33% 1–4: 2/6=33% 5–10: 1/6=17% >10: 1/6=17% |
<1: 3/13=23% 1–4: 6/13=46% 5–10: 3/13=23% >10: 1/13=8% |
| Hours per day, group rehearsal | <1: 3/7=43% 1–4: 3/7=43% 5–10: 1/7=14% >10: 0/7 |
<1: 3/6=50% 1–4: 2/6=33% 5–10: 0/6 >10: 1/6=17% |
<1: 6/13=46% 1–4: 5/13=38% 5–10: 1/13=8% >10: 1/13=8% |
|
| Years played | 1–4: 2/7=29% 5–10: 3/7=43% >10: 2/7=29% |
1–4: 1/6=17% 5–10: 5/6=83% >10: 0/13 |
1–4: 3/13=23% 5–10: 8/13=62% >10: 2/13=15% |
|
| Type of instrument | Brass: 1/7=14% Woodwind: 3/7=43% String: 6/7=86% Percussion: 4/7=57% Other: 1/7=14% (voice) |
Brass: 1/6=17% Woodwind: 1/6=17% String: 3/6=50% Percussion: 1/6=17% Other: 0 |
Brass: 2/13=15% Woodwind: 4/13=31% String: 9/13=69% Percussion: 5/13=38% Other: 1/13=8% (voice) |
|
Screening Procedures
Participants were asked to avoid loud sound for 48 hours prior to hearing screening. The screening tests included otoscopy to ensure normal external ear anatomy and the absence of obstructive debris such as occluding cerumen, followed by tympanometric testing and conventional pure-tone air conduction threshold testing from 250 Hz to 8 kHz. To proceed to the EHF testing protocol, subjects were required to pass both the otoscopic examination and tympanometric testing. Normal middle ear pressure and compliance was defined by tympanometric configurations with middle ear pressure (MEP) values from −140 to +40 daPa (based on the 90% range for adults, see Margolis & Hunter, 2000), compliance (Peak Ytm) values from 0.3 to 1.8 ml, and ear canal volume (Vea) values from 0.8 to 2.1 cm3. Participants were required to have air conduction thresholds no worse than 25 dB HL from 0.25 – 8 kHz. In addition, inclusion criteria required that thresholds for the right and left ears be no more than 15 dB HL apart. Finally, if air conduction thresholds were 15 dB HL or higher (worse), bone conduction testing was administered and the difference between air and bone thresholds was required to be 10 dB or less (see Le Prell et al., 2011; Le Prell et al., 2012). All of the participants described in this report met the aforementioned inclusion criteria. Participants who did not meet these criteria did not proceed to EHF testing. Approximately 20% of screened participants failed to meet the above criteria (Le Prell et al., 2011; 2012); it is worth noting that a previous study (Mills et al., 1979) had to screen 149 college student participants in order to find 60 subjects with thresholds of 10 dB HL or better (a 60% failure rate).
Audiometric threshold measurement was conducted using a Grason-Stadler model 61 (GSI 61) audiometer calibrated to ANSI 3.6 1996 with high frequencies calibrated according to Annex C. Participants were tested in a double-walled sound-treated test booth meeting ANSI/ASA S3.1-1999 (R2008) specifications for audiometric test rooms. Pure-tone air conduction thresholds were obtained using EAR 3A insert earphones for test frequencies of 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz, and Sennheiser HDA200 circum-aural headphones for test frequencies of 10, 12.5, 14, and 16 kHz. For each test frequency, the initial presentation level was 30 dB HL after which the intensity was decreased in 10-dB steps until the participant failed to respond. Presentation levels were then increased in 2-dB steps after each missed tone presentation, until correct responses were observed. Levels were then decreased by 6-dB after correct detection responses. Ascending runs using 2-dB increments were repeated three times, and threshold was operationally defined as the lowest level at which responses were obtained on two out of three ascending runs. Reliability was assessed using repeated tests at 2 and 8 kHz in each ear. Responses were considered reliable if the difference between test and retest thresholds was no more than 5 dB (a criterion previously used by Fausti et al., 1999). Only one subject was unable to meet the 5-dB test-retest criterion and was excluded from further assessment. All subjects contributing data to this report met the 5-dB test-retest criterion.
Statistical Analyses
Descriptive and inferential analyses of differences associated with the independent variables were conducted using SPSS version 20. For categorical data, the distribution of participants across groups was evaluated using the Pearson Chi-square statistic; Fischer’s exact test was used if sample size per cell was less than 5 in any cell. Normality of the distribution was assessed using the Levine test. Tests comparing paired data sets (such as right ear versus left ear comparisons) were conducted using paired-sample two-tailed t-tests if Levine tests indicated that the data were normally distributed. The Wilcoxin signed-rank test for related samples was used if the normality assumption was violated. Comparisons of two independent samples (such as male versus female) were conducted using two-tailed independent samples t-tests if the Levine tests revealed the data were normally distributed or the Mann Whitney U test was used if the normality assumption was violated. When there were more than two-levels of an independent variable, inferential comparisons were conducted using one-way analysis of variance (ANOVA) if the Levine tests revealed the data were normally distributed or the Kruskal Wallis test if the normality assumption was violated. A criterion of α = .05 was used for all analyses to determine significant effects. Inferential comparisons to assess effects of noise on threshold sensitivity were conducted for: total number of noise sources reported, individual noise sources (concerts, bars/clubs, sporting events, music player use, loud music in a vehicle, musical instrument use), and patterns of music player use (hours per day, days per week, years of use, background noise conditions).
Results
Participant Demographics: No Important Sex Differences
Descriptive characteristics are summarized in Table 1. There was no reliable difference in age of subjects when males and females were compared; however, males were generally taller and weighed more than females (p ≤ 0.05). There were no reliable differences in the distribution of “yes” responses on any of the categorical variables when males were compared to females. Tobacco use was minimal in both males and females, and the majority of male and female participants reported consumption of 0–5 alcoholic beverages per week. Of the participants, 23–24% reported a history of ear infections, percent yes responses increased to 47–53% when participants were asked if they had ever had an ear infection. None of the participants reported an ear infection within the past 3 months. There were 15–18% percent reporting a history of tinnitus; the proportion of participants reporting ever experiencing tinnitus after noise exposure was higher (53%–62%). Of those participants who reported experiencing tinnitus after noise, 50% reported tinnitus in the absence of noise as well. There were smaller numbers of participants reporting hyperacusis, history of ear pain or ear drainage, balance issues, seizures, frequent or severe headaches, fainting, disorientation, relatives with hearing loss, or any previous hearing loss. Of the 5 participants that reported ever experiencing any hearing loss, all 5 reported the hearing loss had only occurred after loud sound exposure.
Participant Noise History
Noise history is summarized in Table 2. For the total participant pool, there was no reliable difference with respect to total number of sound exposure sources reported by males and females, and there was no reliable difference with respect to any previous impulse noise exposure. The most commonly reported sources of loud sound exposures were personal music players (note that 56% reported the device was a frequent source of loud sound exposure, while 90% reported use of a device), followed by bar/club attendance (41% yes) and loud music played in vehicles (33% yes). A number of participants reported attending loud sporting events (21% yes) or concerts (21% yes). A smaller subset of participants reported music rehearsal (6% yes), workplace noise (5% yes), or firearms use (1% yes). The only source of exposure with a statistically reliable difference in the distribution of male and female participants reporting frequent loud sound exposure was music rehearsal, with 15% of males reporting yes compared to 0% of females (Pearson Chi-Square = 8.269, df = 1, p = 0.008). Male participants were the only ones to specifically report “yes” when asked if music rehearsal was a frequent loud sound exposure; however, there were female musicians in the participant pool. Among the subset of participants completing more detailed questionnaires about musical instrument use, there was a statistically reliable difference (Pearson Chi-Square = 4.517,df = 1, p = 0.047) between males and females with respect to playing an instrument, with 29% of males responding “yes” and 11% of females responding “yes”. Within those who played instruments, there was no sex difference in the distribution of responses regarding hours per day of solo practice, hours per day of group practice, years of playing experience, or type of instrument played.
Personal music players were the most common source of exposure, reported by more than half of the participants. Among the subset of participants completing more detailed questionnaires about personal music player use, participants were surveyed regarding both use of the device, as well as hours of device use per day, days of device use per week, and years of device use. Although preferred listening level was not measured, participants did report whether they used their device in noisy areas, quiet areas, or both, and also reported whether they could hear someone speaking to them while using the device. There were no statistically reliable differences in the distribution of the participants’ responses as a function of sex in any of the above categories.
Comparisons with existing normative literature
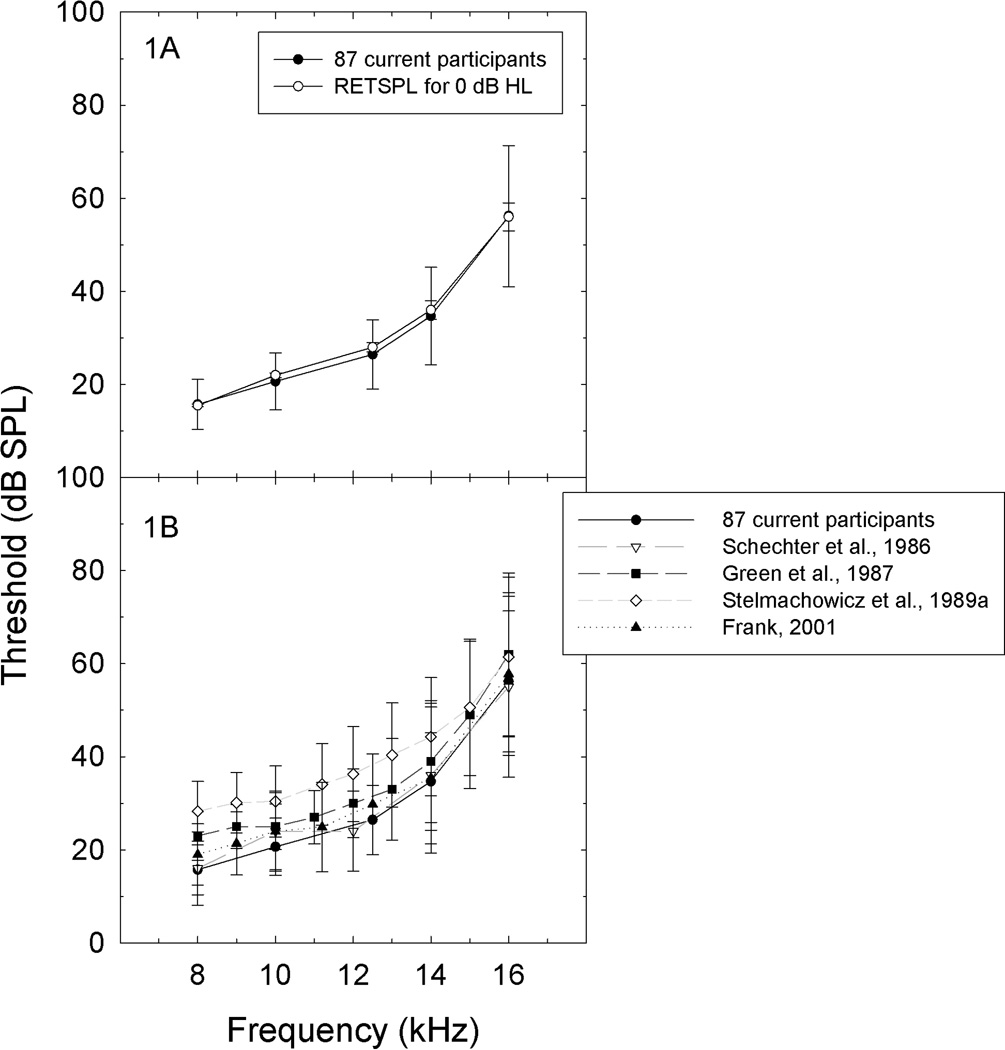
In this report, EHF thresholds from healthy young adults with hearing thresholds of 25 dB HL or better from 250 Hz to 8 kHz were consistent with the RETSPLs specified in Annex C of ANSI S3.6 (American National Standards Institute, 1996) (Figure 1A), as well as EHF thresholds for similarly aged subjects as described in other recent reports (Figure 1B). The observed increase in standard deviation with increasing frequency is consistent across studies (Figure 1B). That normative data derived from fairly large samples [100 subjects tested by Frank (2001) and the data from the 87 participants described here] are highly consistent across laboratories is an encouraging finding with respect to the development of normative databases.
Figure 1.
A. Average EHF thresholds measured from current subjects at frequencies from 8–16 kHz closely matched the RETSPL sound levels identified as normal hearing threshold level in ANSI S3.6 1996 Annex C. Figure 1B. EHF thresholds measured from current subjects tested at frequencies from 8–16 kHz were consistent with those reported by Schechter et al. (1986; mean and SD for subjects ages 16–20 years old, from their Tables I and II), Green et al. (1987; mean and SD for 37 subjects ages 18–26, from their Table II), Stelmachowicz et al. (1989; mean and SD for 160 subjects ages 10–19, from their Table I), and Frank (2001; mean and SD for 100 subjects ages 18–25, from his Table I).
Ear, sex, and age
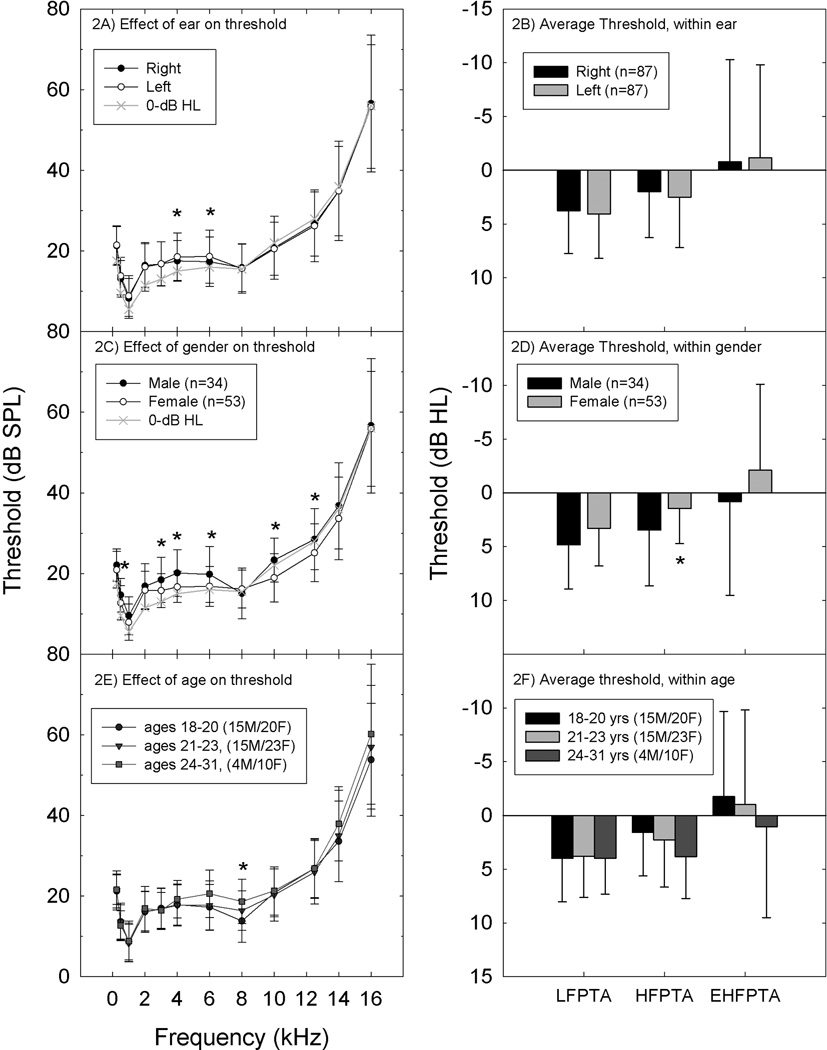
Right and left ear thresholds were highly correlated (Spearman’s Rho values ranging from 0.483–0.742, all p < 0.01); the only statistically reliable differences between right and left ear thresholds were at 4 and 6 kHz (p ≤ 0.05; Wilcoxon Signed Rank Test). Right ear thresholds were 1–1.5 dB better than left ear thresholds at those two frequencies (see Figure 2A). To reduce the effect of random test-retest variability and explore patterns of change, pure-tone-thresholds are often averaged at subsets of the lower and higher test frequencies during post-hoc analysis (see Niskar et al., 1998; Agrawal et al., 2008; Shargorodsky et al., 2010; Henderson et al., 2011). When pure-tone average thresholds were considered (LFPTA: 0.5, 1 and 2 kHz; HFPTA: 3, 4, and 6 kHz; EHFPTA: 10, 12, 14 and 16 kHz), there were no reliable differences between right and left ears (see Figure 2B; note that all PTA thresholds are plotted in dB HL). Given robust right-left correlations and small right-left asymmetries, thresholds for right and left ears were averaged for all subsequent analyses, such that each subject contributed a single survey response (per question) and a single average threshold (at each test frequency and for each pure-tone average).
Figure 2.
Factors that may influence thresholds include ear (2A,2B), gender, (2C,2D), and age (2D,2E). Ear. For these 87 subjects, small but statistically reliable differences were detected for right versus left comparisons at 4 and 6 kHz (2A), with no differences in pure-tone average threshold at 0.5, 1 and 2 kHz (LFPTA), 3, 4, and 6 kHz (HFPTA), or 10, 12, 14, and 16 kHz (EHFPTA) (2B). The thresholds defined as “0-dB HL” in the ANSI standard are plotted for comparisons purposes in Figure 2A, and pure-tone average thresholds are plotted using the dB HL convention (2B). Gender. Small but systematic differences were observed when males were compared to females, with males having poorer hearing at 0.5, 3, 4, 6, 10, and 12 kHz (2C). The effect of gender was observed for HFPTA comparisons, but not LFPTA or EHFPTA (2D). Age. There was a small but statistically reliable elevation in threshold at 8 kHz as a function of age (2E). Deficits did not extend to PTA comparisons (2F).
Male subjects had higher (worse) thresholds than female subjects at 0.5, 3, 4, and 6 kHz, as well as at 10 and 12 kHz (all p ≤ 0.05) (Figure 2C). Differences were relatively small, with male thresholds being ~3 dB worse than female thresholds. Differences in PTA thresholds were statistically reliable for the HFPTA comparison (p ≤ 0.05), but not LFPTA or EHFPTA comparisons (p > 0.05; see Figure 2D).
The possibility of age-related differences in threshold sensitivity was assessed using the age categories described by Green et al. (1987), who reported ~10 dB threshold differences during EHF tests of 18–20 year old subjects versus 21–23 year old subjects, with an additional deficit of ~20 dB in 24–26 year old subjects compared to 21–23 year old subjects. Here, the only frequency at which thresholds differed as a function of age was 8 kHz (p ≤ 0.05), with thresholds increasing by 2–3 dB with increasing age bin (see Figures 2E and 2F).
Effects of individual noise sources on hearing
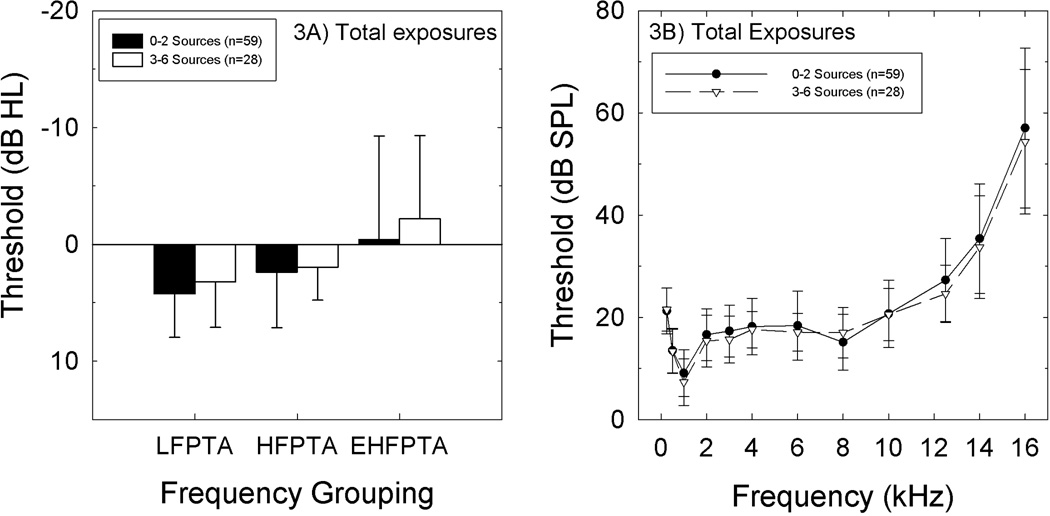
There was no statistically reliable relationship between either single-frequency threshold or PTA threshold and noise history when the analysis was based on a “total” risk metric (assessed as the total number of insults reported, which ranged from 0 to 6) (Figures 3A, 3B). When risk of any single insult was considered (i.e., thresholds of those reporting concert attendance compared to those that do not report attending concerts), there was no statistically reliable relationship between any individual noise insult and either single-frequency threshold or PTA threshold (all p values > 0.05).
Figure 3.
A. There was no relationship between pure-tone-average threshold and number of noise sources reported by the subjects. Figure 3B. There was no relationship between thresholds at individual EHF frequencies and number of noises sources reported by the subjects.
Effects of music player use on hearing: detailed analysis
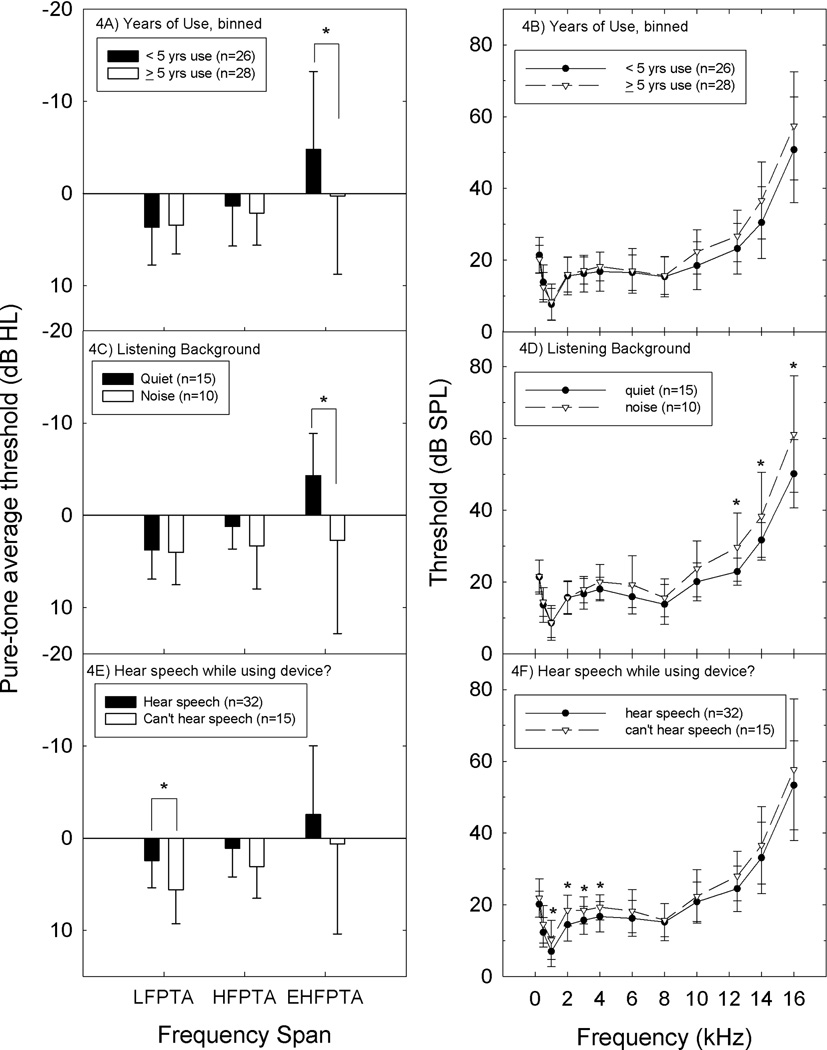
There was no statistically reliable relationship between either PTA threshold or single-frequency threshold and music player use when it was dichotomized as a yes/no variable (not shown). Because only the subset of users that choose higher listening levels and/or longer listening durations are likely to be at risk for hearing loss, thresholds were evaluated for potential relationships with hours of device use per day, days of device use per week, and years of device use. There were no statistically reliable relationships between PTA thresholds or thresholds at single test frequencies using any of these metrics (not shown). Because robust group differences have been reported for subjects that have used music players for 5 years or longer (Peng et al., 2007), long-term device users were compared to shorter term users and nonusers. Using the same 5-year criteria as Peng et al. (2007), there were no LFPTA or HFPTA differences and no threshold differences at any individual frequency at or below 8 kHz. However, there was a statistically significant group difference using the EHFPTA metric (p ≤ 0.05; see Figure 4A). At and above 10 kHz, there were small but statistically reliable elevations in thresholds in those that had used the devices for longer periods of time compared to non-users and those that had used the devices for shorter periods of time, with 3–4 dB deficits at 10 and 12.5 kHz (10 kHz: p ≤ 0.05; 12.5 kHz: 0.5 < p ≤ 0.10), and 6–7 dB deficits at 14 and 16 kHz (14 kHz: p ≤ 0.05; 16 kHz: 0.5 < p ≤ 0.11) (Figure 4B).
Figure 4.
A. There was a statistically reliable relationship between PTA threshold and long-term use of a music player device, defined as greater than 5-years of device use, using the EHFPTA metric (average threshold at 10, 12, 14, and 16 kHz). Figure 4B. Single frequency comparisons revealed statistically reliable differences at 10 and 14 kHz. Group differences at 12 and 16 kHz were not statistically reliable at the α=0.05 level. Figure 4C. Average PTA thresholds at EHF frequencies were reliably worse in those subjects that used their devices in noisy backgrounds (p<0.05). Figure 4D. Single frequency comparisons revealed statistically reliable differences at 12, 14, and 16 kHz. Figure 4E. Average PTA threshold for low frequencies (0.5, 1, 2 kHz) was reliably worse in those subjects that could not hear others speaking to them (p<0.05) with a similar trend observed for conventional high frequencies (3, 4, and 6 kHz: HFPTA p=0.055). Figure 4F. Single-frequency comparisons revealed statistically reliable differences at 1, 2, 3 and 4 kHz.
Although preferred listening level was not measured, subjects did report whether they used their device in noisy areas, quiet areas, or both, and they also reported whether they could hear someone speaking to them while using the device. Subjects that used their devices in noisy backgrounds had worse EHPTA thresholds than subjects that used their devices in quiet backgrounds (Figure 4C). Single-frequency analyses revealed statistically reliable differences at 12, 14, and 16 kHz (Figure 4D). In addition, subjects that choose listening levels that allow them to hear others speaking to them during device use had better LFPTA thresholds than device users who reported they could not hear others speak to them while using their devices (Figure 4E). Single-frequency comparisons revealed statistically reliable differences at 1, 2, 3, and 4 kHz (Figure 4F). Taken together, the evidence suggesting an effect of music player use on thresholds was limited to an effect of long-time use (> 5 years), and use of the device at higher listening levels.
Discussion
In this retrospective analysis assessing the effects of recreational noise exposure on EHF thresholds in young adults, the one source of sound exposure that was reliably related to higher thresholds was music player use. Statistically significant group differences were shown for long-term music player device users, and device users that select higher listening levels, with approximately 3–6 dB deficits detected in those user groups in this sample. The effect size reported here is within the range established by earlier literature. The largest deficits to date were measured in a study of students at Wuhan University (ages 19–23 years). In those participants, threshold deficits were approximately 6 dB at 10 kHz, and approximately 15 dB at 16 kHz, when participants who had used personal music players for greater than 5 years were compared to control subjects who did not use these devices (Peng et al., 2007). Group differences reported by others have been smaller. Deficits in the range of 2–4 dB were reported at 10–16 kHz in Brazilian secondary school students, teachers, and staff who use MP3 players regularly (defined as at least 1 hr use per day for at least one year) (Figueiredo et al., 2012). Based on these observations, it is reasonable to conclude that EHF deficits have the potential to increase with additional years of device use.
In the current study, music player listening level appeared to have a robust relationship with the observed threshold differences at EHF frequencies. While preferred listening level was not explicitly measured, subjects did report whether they used their device in noisy areas, quiet areas, or both. Most of the available data suggest that listening levels increase when devices are used in a noisy background (Hodgetts et al., 2007; Hodgetts et al., 2009; Epstein et al., 2010; McNeill et al., 2010; Muchnik et al., 2011; Portnuff et al., 2011), supporting the use of background noise levels as a rough metric for listening level. Subjects also reported whether they could hear someone speaking to them while using the device. Because all of these subjects had “normal” thresholds (i.e., at least 25 dB HL or better), ability to hear someone speaking would be strongly influenced by the volume of the device. Such data directly lead to the interpretation that listening at levels which preclude detection of environmental sound increases the risk for higher thresholds, but it should be stressed that threshold differences averaged only 3 dB at the lower frequencies (Figures 4C, 4D), while growing to 6 dB at the EHF frequencies (Figures 4E, 4F). Other studies describing thresholds at conventional test frequencies report similarly small deficits in pure-tone audiometric thresholds (e.g., 2–3 dB; see Meyer-Bisch, 1996; Kim et al., 2009), or no threshold deficits (Wong et al., 1990; Mostafapour et al., 1998; Kumar et al., 2009; Shah et al., 2009).
The fact that the multiple groups have measured threshold compromise at frequencies beyond 10 kHz due to long-term music player use is interesting. One possibility is that this low-dose chronic noise exposure potentiates age-like changes in the cochlea. Age-related increases in EHF thresholds are well documented (Osterhammel & Osterhammel, 1979; Schechter et al., 1986; Green et al., 1987; Stelmachowicz et al., 1989; Lee et al., 2012). The effects of aging have been attributed to four mechanisms of cochlear pathology based on data from animal models and human temporal bones. The proposed categories include sensory ARHL (hair cell loss), neural ARHL (primary ganglion cell loss), metabolic ARHL (strial atrophy) and cochlear conductive ARHL (as a consequence of stiffening of the basilar membrane), with the caveat that most cases of ARHL are of mixed origin in humans (Schuknecht, 1955; Schuknecht, 1964; Schuknecht & Gacek, 1993; Gates & Rees, 1997; Chisolm et al., 2003; Ohlemiller, 2004; Gates & Mills, 2005; Ohlemiller & Frisina, 2008; Ohlemiller, 2009). The steeply sloping pattern of high-frequency hearing loss has historically been attributed to sensory cell loss. It is clear that noise insult damages hair cells not only at the frequencies associated with the noise insult, but also at higher frequencies (i.e., the so-called ‘half-octave shift’, as well as other, higher, "unexpected" frequencies, see Davis et al., 1950; Mitchell et al., 1977; Cody & Johnstone, 1980; Cody & Johnstone, 1981; Yamashita et al., 2004; Le Prell et al., 2007). Importantly, there is increasing suggestion that some of the high-frequency hearing loss attributed to ARHL may in fact reflect the effects of noise insult in addition to age-related cell loss. Taken together, long-term music player use, and use of the device at higher listening levels, may result in harm to the high-frequency basal end of the cochlea, evident here as poorer EHF hearing.
It is important to note that age and noise can interact. Studies in mice demonstrate that exposures producing a single robust TTS early in life can result in long-term spiral ganglion degeneration and increased hearing loss over the course of the mouse’s life span, which is approximately 2 years (Kujawa & Liberman, 2006). More recent data from rodent models show rapidly decreased synaptic connections between inner hair cells and the auditory nerve dendrites associated with a single robust temporary threshold shift (TTS) following noise insult (Kujawa & Liberman, 2009; Lin et al., 2011). Multiple episodes of TTS have the potential for increasing this damage (Wang & Ren, 2012). In our sample of young adults, 6% of the subjects reported that they previously experienced a change in hearing after exposure to loud sound, and 56% reported that they had previously experienced tinnitus after loud sound (Table 1). We do not have any measures of the extent or duration of the previous TTS, and, moreover, the point at which TTS insult has the potential to result in neural change has not been identified (for detailed discussion, see Le Prell et al., 2012). Thus, the significance of this work with respect to potential synaptic trauma in young adults that use music players is unclear, but, electrophysiological tests that document the integrity of the neural population are critically needed. The small EHF deficits observed here could reflect a slowly-progressive accumulated insult triggered by multiple periods of noise stress and synaptic trauma. More importantly, it is possible that the modest change in thresholds could indicate significant auditory nerve fiber deterioration.
Variability in thresholds at EHF frequencies, across subjects, has been noted by multiple groups (Schechter et al., 1986; Green et al., 1987; Stelmachowicz et al., 1989; Frank, 2001; Schmuziger et al., 2004). Frank (2001) has demonstrated that within-subject test-retest reliability can be just as good as at lower frequencies, and our own in-house test-retest data from a smaller number of subjects are consistent with his systematic assessment (unpublished). One possible explanation for increased variability at EHF thresholds across individuals is that inter-subject differences in noise history could contribute to deficits in EHF thresholds, in the absence of changes at conventional test frequencies. In other words, the increased variability may reflect effects of noise. Here, we tested the hypothesis that EHF thresholds in participants with a history of recreational noise exposure would be reliably worse than EHF thresholds from participants reporting less recreational noise exposure. The current analysis revealed that long-term (>5-year) music player use or use of the device at higher listening levels may result in higher thresholds at EHF frequencies.
With respect to the broader question, integrating multiple noise sources for an overall recreational noise risk metric, there was no reliable relationship between the number of reported noise sources and pure-tone-average thresholds (LFPTA, HFPTA, EHFPTA) or thresholds at individual frequencies for this sample of young adults. The potential for differences in LFPTA, HFPTA, and EHFPTA thresholds, and thresholds at individual frequencies, was specifically examined as a function of frequent bar/club attendance and concert attendance, given that the average measured sound level in bars and clubs, and at concerts, commonly reaches or exceeds 100 dBA (Cabot et al., 1979; Gunderson et al., 1997; Smith et al., 2000; Serra et al., 2005; Opperman et al., 2006; Müller et al., 2010; Williams et al., 2010). However, we found no statistically reliable differences in thresholds as a consequence of these activities. Sports events sound levels have been reported to reach or exceed 90 dBA, although the literature is not extensive (Hodgetts & Liu, 2006; Engard et al., 2010). We similarly failed to find any statistically reliable relationship between threshold and sporting event attendance. Although it has been suggested that EHF thresholds are more vulnerable to noise insult than other frequencies within the conventional 250 Hz to 8 kHz range, the current data largely fail to support this hypothesis, with the exception of music player use for long periods of time, or at high listening levels.
Because most of these exposures (concerts, bars, sports events) are recreational in nature, they are limited with respect to duration and frequency, and would not by themselves meet the definition of hazardous noise as described in the federal noise regulations (29 CFR 1910.95). These regulations, enforced by the Occupational Safety & Health Administration (OSHA), mandate the use of hearing protection devices for any worker that is exposed to sounds exceeding personal exposure limits (PEL) of 90 dBA × 8 hours per day, based on the increased probability of hearing impairment with exposures 8 hours per day, 5 days per week, over the course of a 40-year occupational career. Thus it is not surprising that the young adult college students presented here did not have significant threshold deficits as a consequence of recreational exposures. Given the well-documented increase in variability in thresholds (for populations, not individuals) at EHF frequencies, and multiple suggestions that EHF frequencies are more vulnerable to noise insult than other lower frequencies, it was reasonable to explore the potential relationship between recreational noise exposure and EHF thresholds. Although the present dataset largely failed to support the proposed relationship between most recreational noise sources and “pre-clinical” damage (assessed using EHF thresholds), the statistically reliable relationship between music player use factors and elevated EHF thresholds provides a cautionary note for device users regarding the potential for increasing effects with long-term use and high listening levels. The potential utility of EHF thresholds for monitoring early effects of noise cannot be excluded.
Whereas the utility of an early warning is clear, and several studies clearly support the use of EHF testing to detect subtle deficits in noise exposed populations (Ahmed et al., 2001; Biassoni et al., 2005; Serra et al., 2005; Peng et al., 2007), the literature is in fact highly mixed with respect to effects of noise at EHF frequencies. Several groups of military personnel have been tested at EHF frequencies, with no clear utility for the testing. For example, Balatsouras et al. (2005) compared thresholds in 18–21 year-old male soldiers not yet exposed to military weapons noise (n=30) with thresholds from 39 young soldiers seen after acute acoustic trauma. Deficits in noise-exposed patients were greatest at 4–8 kHz, and although they extended to 11.2 kHz, in the EHF range, there were no threshold differences from 12.5 to 20 kHz when noise-exposed soldiers were compared to soldiers not yet exposed to noise (Balatsouras et al., 2005). Kuronen et al. (2003) similarly compared conventional and EHF thresholds in Finnish Air Force Military Personnel, 19–48 years old (50 male, 1 female) to Finnish normative data and found no hearing deficits in either conventional or EHF test outcomes. They considered whether EHF tests might reveal TTS deficits after exposure to flight noise. Comparison of pre- and post-flight hearing tests indicated small (1 to 3 dB) but statistically significant TTS at both conventional and EHF frequencies, suggesting no additional benefit was obtained by adding EHF testing to the conventional test paradigm (Kuronen et al., 2003). In a study on TTS after music player use, EHF deficits did not accompany TTS measured at lower frequencies (Le Prell et al., 2012). Data from musicians are akin to those of military personnel. EHF threshold deficits (12.5 and 14 kHz) accompanied deficits at conventional test frequencies (3–8 kHz) in one group of musicians (Schmuziger et al., 2006). However, EHF threshold deficits were minimal (Axelsson & Lindgren, 1978; Axelsson et al., 1995), or not detected at all (Johnson et al., 1985; Johnson et al., 1986), in other groups of musicians. When TTS after music rehearsal was evaluated, TTS was detected at frequencies at and below 8 kHz but not at or above 9 kHz (Schmuziger et al., 2007). Musicians, military personnel, and music player users will have significantly different exposure to noise, with respect to frequency and duration of exposure, sound levels, as well spectral content, and kurtotic distribution. It may ultimately prove to be the case that some patterns of exposure are more likely to result in slowly progressive changes in the basal cochlea than other patterns of exposure, a finding that would explain the diverse outcomes regarding the utility of EHF monitoring.
Summary and Conclusions
Despite the potential for “early warning” benefits, the literature is mixed with respect to the utility of EHF thresholds for identifying early effects of noise. The present data clearly suggest that music player use can drive threshold changes during EHF tests. Howevr, the present data provide no compelling evidence that most normal recreational noise exposure (including periodic concert attendance, bar/club attendance, sporting event attendance, or combinations of the above) induce EHF threshold changes in healthy young adult populations. Longitudinal data are critically needed to determine the extent to which EHF deficits are, or are not, followed by changes in conventional audiometry.
Although the utility of EHF threshold for monitoring “pre-clinical” changes in auditory function remains unclear, we encourage clinicians to consider routine testing at EHF frequencies. An effort to detect the earliest changes in hearing may offer an opportunity to provide information about the possible consequences of continued risky listening behavior, particularly when audiologic history reveals a history of noise exposure. RETSPLs are a reliable reference against which hearing among young adults can be compared. However, as older populations are compared to the RETSPL normative hearing levels, increasing divergence is expected as age-related increases in EHF thresholds are well documented (Osterhammel & Osterhammel, 1979; Schechter et al., 1986; Green et al., 1987; Stelmachowicz et al., 1989; Lee et al., 2012).
Serial monitoring at EHF frequencies has been readily possible for new patients entering therapeutic treatment with ototoxic drugs. Baseline testing is implemented prior to the first drug administration, and EHF monitoring provides physicians with valuable information regarding potential permanent hearing loss. EHF monitoring is routinely used to help guide drug titration to preserve hearing, or if the drug therapy cannot be modified, to guide the onset of rehabilitation services (see, for example, Fausti et al., 1999; Vaughan et al., 2002; Knight et al., 2007; Konrad-Martin et al., 2010; Jacobs et al., 2012). If emerging noise-induced deficits at EHF thresholds could be similarly routinely identified, this may provide parallel opportunities for intervention. With respect to hearing conservation programs, employers may be able to choose to move workers to less noisy workstations, or implement engineering controls. On an individual level, employees might begin use of or be refit with hearing protection devices (HPDs), and receive counseling about noise outside the workplace. Finally, if EHF deficits could be routinely identified in adolescents, at-risk individuals could perhaps be encouraged to attend educational programs such as Dangerous Decibels (Griest et al., 2007), Sound Sense™ (Neufeld et al., 2011), or other hearing conservation educational programs (Lass et al., 1986; Lukes & Johnson, 1998). Although there is not yet a significant database documenting long-term change in adolescent listening behaviors as a function of such educational outreach programs, early efforts to document long-term improvements are encouraging.
Acknowledgments
Data collection was supported by U01 DC 008423 and R44 DC009106, from the National Institute on Deafness and other Communication Disorders, National Institutes of Health; additional support for subject payments was provided by the Center for Hearing Research at the University of Florida. We thank Sebastian de la Calle, Shawna Dell, Diana Guercio, Brittany Hensley, Vicki Ledon, Kari Morgenstein, Marissa Rosa, Jason Schmitt, and Lindsey Willis-Banks, for their time consenting and testing subjects.
Abbreviations
- ANSI
American National Standards Institute
- EHF
extended high frequency
- EHFPTA
extended high-frequency pure-tone average/average threshold at 10, 12, 14, and 16 kHz
- dB HL
decibels hearing level
- dB SPL
decibels sound pressure level
- HFPTA
high-frequency pure-tone average/average threshold at 3, 4, and 6 kHz
- Hz
Hertz
- kHz
kilohertz
- LFPTA
low-frequency pure-tone average/average threshold at 0.5, 1, and 2 kHz
- NIHL
noise-induced hearing loss
- PTA
pure-tone average
- RETSPL
Reference Equivalent Threshold Sound Pressure Level
- TTS
temporary threshold shift
Literature Cited
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- Ahmed HO, Dennis JH, Badran O, Ismail M, Ballal SG, Ashoor A, Jerwood D. High-frequency (10–18 kHz) hearing thresholds: reliability, and effects of age and occupational noise exposure. Occup Med (Lond) 2001;51:245–258. doi: 10.1093/occmed/51.4.245. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute. American national standard specifications for audiometers, S3.6–1996. American National Standards Institute, New York. 1996 [Google Scholar]
- Axelsson A, Lindgren F. Hearing in pop musicians. Acta Otolaryngol (Stockh) 1978;85:225–231. doi: 10.3109/00016487809111929. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Eliasson A, Israelsson B. Hearing in pop/rock musicians: a follow-up study. Ear Hear. 1995;16:245–253. doi: 10.1097/00003446-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Balatsouras DG, Homsioglou E, Danielidis V. Extended high-frequency audiometry in patients with acoustic trauma. Clin Otolaryngol. 2005;30:249–254. doi: 10.1111/j.1365-2273.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- Biassoni EC, Serra MR, Richtert U, Joekes S, Yacci MR, Carignani JA, Abraham S, Minoldo G, Franco G. Recreational noise exposure and its effects on the hearing of adolescents. Part II: development of hearing disorders. Int J Audiol. 2005;44:74–85. doi: 10.1080/14992020500031728. [DOI] [PubMed] [Google Scholar]
- Cabot RC, Genter R, II, Lucke T. Sound levels and spectra of rock music. Journal of the Audio Engineering Society. 1979;27:267–284. [Google Scholar]
- Chisolm TH, Willott JF, Lister JJ. The aging auditory system: anatomic and physiologic changes and implications for rehabilitation. Int J Audiol. 2003;42(Suppl 2):2S3–2S10. [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Single auditory neuron response during acute acoustic trauma. Hear Res. 1980;3:3–16. doi: 10.1016/0378-5955(80)90004-0. [DOI] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Acoustic trauma: Single neuron basis for the "half octave shift". J Acoust Soc Am. 1981;70:707–711. doi: 10.1121/1.386906. [DOI] [PubMed] [Google Scholar]
- Davis H, Morgan CT, Hawkins JE, Jr, Galambos R, Smith FW. Temporary deafness following exposure to loud tones and noise. Acta Otolaryngol Suppl (Stockh) 1950;88:1–57. [PubMed] [Google Scholar]
- De Seta E, Bertoli GA, Filipo R. High-frequency audiometry above 8 kHz. Comparative results of normative thresholds obtained with a headphone system and a quasi-free-field system. Audiology. 1985;24:254–259. doi: 10.3109/00206098509070109. [DOI] [PubMed] [Google Scholar]
- Engard DJ, Sandfort DR, Gotshall RW, Brazile WJ. Noise exposure, characterization, and comparison of three football stadiums. J Occup Environ Hyg. 2010;7:616–621. doi: 10.1080/15459624.2010.510107. [DOI] [PubMed] [Google Scholar]
- Epstein M, Marozeau J, Cleveland S. Listening habits of iPod users. J Speech Lang Hear Res. 2010;53:1472–1477. doi: 10.1044/1092-4388(2010/09-0059). [DOI] [PubMed] [Google Scholar]
- Erickson DA, Fausti SA, Frey RH, Rappaport BZ. Effects of steady-state noise upon human hearing sensitivity from 8000 to 20 000 Hz. Am Ind Hyg Assoc J. 1980;41:427–432. doi: 10.1080/15298668091424979. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Erickson DA, Frey RH, Rappaport BZ. The effects of impulsive noise upon human hearing sensitivity (8 to 20 kHz) Scand Audiol. 1981a;10:21–29. doi: 10.3109/01050398109076158. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Erickson DA, Frey RH, Rappaport BZ, Schechter MA. The effects of noise upon human hearing sensitivity from 8000 to 20 000 Hz. J Acoust Soc Am. 1981b;69:1343–1347. doi: 10.1121/1.385805. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Rappaport BZ, Schechter MA, Frey RH, Ward TT, Brummettt RE. Detection of aminoglycoside ototoxicity by high frequency auditory evaluation: Selected case studies. Am J Otolaryngol. 1984a;5:177–182. doi: 10.1016/s0196-0709(84)80009-5. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Schechter MA, Rappaport BZ, Frey RH, Mass RE. Early detection of cisplatin ototoxicity. Selected case reports. Cancer. 1984b;53:224–231. doi: 10.1002/1097-0142(19840115)53:2<224::aid-cncr2820530207>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, Phillips DS, Frey RH, Noffsinger D, Larson VD, Fowler CG. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Figueiredo RR, Azevedo AA, Oliveira PM, Amorim SP, Rios AG, Baptista V. Incidence of tinnitus in mp3 player users. Braz J Otorhinolaryngol. 2012;77:293–298. doi: 10.1590/S1808-86942011000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler EP, Wegel RL. Audionmetric methods and their applications. Transactions of the American Laryngological, Rhinological, and Otological Society. 1922:98-. [Google Scholar]
- Frank T. High frequency (8 to 16 kHz) reference thresholds and intrasubject threshold variability relative to ototoxicity criteria using a sennheiser HAD 200 earphone. Ear Hear. 2001;22:161–168. doi: 10.1097/00003446-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Gates GA, Rees TS. Hear ye? Hear ye! Successful auditory aging. Western Journal of Medicine. 1997;167:247–252. [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Green DM, Kidd G, Jr, Stevens KN. High-frequency audiometric assessment of a young adult population. J Acoust Soc Am. 1987;81:485–494. doi: 10.1121/1.394914. [DOI] [PubMed] [Google Scholar]
- Griest SE, Folmer RL, Martin WH. Effectiveness of "Dangerous Decibels," a school-based hearing loss prevention program. Am J Audiol. 2007;16:S165–S181. doi: 10.1044/1059-0889(2007/021). [DOI] [PubMed] [Google Scholar]
- Gunderson E, Moline J, Catalano P. Risks of developing noise-induced hearing loss in employees of urban music clubs. Am J Ind Med. 1997;31:75–79. doi: 10.1002/(sici)1097-0274(199701)31:1<75::aid-ajim11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Henderson E, Testa MA, Hartnick C. Prevalence of noise-induced hearing-threshold shifts and hearing loss among US youths. Pediatrics. 2011;127:e39–e46. doi: 10.1542/peds.2010-0926. [DOI] [PubMed] [Google Scholar]
- Hodgetts W, Szarko R, Rieger J. What is the influence of background noise and exercise on the listening levels of iPod users? Int J Audiol. 2009;48:825–832. doi: 10.3109/14992020903082104. [DOI] [PubMed] [Google Scholar]
- Hodgetts WE, Liu R. Can hockey playoffs harm your hearing? CMAJ. 2006;175:1541–1542. doi: 10.1503/cmaj.060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts WE, Rieger JM, Szarko RA. The effects of listening environment and earphone style on preferred listening levels of normal hearing adults using an MP3 player. Ear Hear. 2007;28:290–297. doi: 10.1097/AUD.0b013e3180479399. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Silaski G, Wilmington D, Gordon S, Helt W, McMillan G, Fausti S, Dille M. Development and evaluation of a portable audiometer for high frequency screening of hearing loss from ototoxicity in homes/clinics. IEEE Trans Biomed Eng. 2012 doi: 10.1109/TBME.2012.2204881. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Sherman RE, Aldridge J, Lorraine A. Effects of instrument type and orchestral position on hearing sensitivity for 0.25 to 20 kHz in the orchestral musician. Scand Audiol. 1985;14:215–221. doi: 10.3109/01050398509045944. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Sherman RE, Aldridge J, Lorraine A. Extended high frequency hearing sensitivity. A normative threshold study in musicians. Ann Otol Rhinol Laryngol. 1986;95:196–202. doi: 10.1177/000348948609500219. [DOI] [PubMed] [Google Scholar]
- Kim MG, Hong SM, Shim HJ, Kim YD, Cha CI, Yeo SG. Hearing threshold of Korean adolescents associated with the use of personal music players. Yonsei Medical Journal. 2009;50:771–776. doi: 10.3349/ymj.2009.50.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. Journal of Clinical Oncology. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, James KE, Gordon JS, Reavis KM, Phillips DS, Bratt GW, Fausti SA. Evaluation of audiometric threshold shift criteria for ototoxicity monitoring. J Am Acad Audiol. 2010;21:301–314. doi: 10.3766/jaaa.21.5.3. quiz 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Mathew K, Alexander SA, Kiran C. Output sound pressure levels of personal music systems and their effect on hearing. Noise Health. 2009;11:132–140. doi: 10.4103/1463-1741.53357. [DOI] [PubMed] [Google Scholar]
- Kuronen P, Sorri MJ, Paakkonen R, Muhli A. Temporary threshold shift in military pilots measured using conventional and extended high-frequency audiometry after one flight. Int J Audiol. 2003;42:29–33. doi: 10.3109/14992020309056082. [DOI] [PubMed] [Google Scholar]
- Lass NJ, Woodford CM, Lundeen C, Lundeen DJ, Everly-Myers DS. The prevention of noise-induced hearing loss in the school-aged population: a school educational hearing conservation program. J Aud Res. 1986;26:247–254. [PubMed] [Google Scholar]
- Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Hensley BN, Campbell KCM, Hall JWI, Guire K. Hearing outcomes in a “normally-hearing” college-student population: Evidence of hearing loss. Int J Audiol. 2011;50:S21–S31. doi: 10.3109/14992027.2010.540722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Dell S, Hensley BN, Hall JWI, Campbell KCM, Antonelli PA, Green GE, Miller JM, Guire K. Digital music exposure reliably induces temporary threshold shift (TTS) in normal hearing human subjects. Ear Hear. 2012 doi: 10.1097/AUD.0b013e31825f9d89. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, Banakis R, Grolley E, Zecker S, Siegel J. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear. 2012;33:315–329. doi: 10.1097/AUD.0b013e31823d7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011 doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukes E, Johnson M. Hearing conservation. Community outreach program for high school students. AAOHN J. 1998;46:340–343. [PubMed] [Google Scholar]
- Margolis RH, Hunter LL. Acoustic immittance measurements. In: Roeser RJ, Valente M, Hosford-Dunn H, editors. Audiology Diagnosis. New York: Thieme; 2000. pp. 381–342. [Google Scholar]
- McNeill K, Keith SE, Feder K, Konkle AT, Michaud DS. MP3 player listening habits of 17 to 23 year old university students. J Acoust Soc Am. 2010;128:646–653. doi: 10.1121/1.3458853. [DOI] [PubMed] [Google Scholar]
- Meyer-Bisch C. Epidemiological evaluation of hearing damage related to strongly amplified music (personal cassette players, discotheques, rock concerts)--high-definition audiometric survey on 1364 subjects. Audiology. 1996;35:121–142. doi: 10.3109/00206099609071936. [DOI] [PubMed] [Google Scholar]
- Mills JH, Gilbert RM, Adkins WY. Temporary threshold shifts in humans exposed to octave bands of noise for 16 to 24 hours. J Acoust Soc Am. 1979;65:1238–1248. doi: 10.1121/1.382791. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Brummett RE, Vernon JA. Frequency effects of temporary N1 depression following acoustic overload. Arch Otolaryngol. 1977;103:117–123. doi: 10.1001/archotol.1977.00780200043001. [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Lahargoue K, Gates GA. Noise-induced hearing loss in young adults: the role of personal listening devices and other sources of leisure noise. Laryngoscope. 1998;108:1832–1839. doi: 10.1097/00005537-199812000-00013. [DOI] [PubMed] [Google Scholar]
- Muchnik C, Amir N, Shabtai E, Kaplan-Neeman R. Preferred listening levels of personal listening devices in young teenagers: Self reports and physical measurements. Int J Audiol. 2011 doi: 10.3109/14992027.2011.631590. [DOI] [PubMed] [Google Scholar]
- Müller J, Dietrich S, Janssen T. Impact of three hours of discotheque music on pure-tone thresholds and distortion product otoacoustic emissions. J Acoust Soc Am. 2010;128:1853–1869. doi: 10.1121/1.3479535. [DOI] [PubMed] [Google Scholar]
- Neufeld A, Westerberg BD, Nabi S, Bryce G, Bureau Y. Prospective, randomized controlled assessment of the short- and long-term efficacy of a hearing conservation education program in Canadian elementary school children. Laryngoscope. 2011;121:176–181. doi: 10.1002/lary.21185. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes A, Esteban E, Rubin C, Brody DJ. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA. 1998;279:1071–1075. doi: 10.1001/jama.279.14.1071. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Age-related hearing loss: the status of Schuknecht's typology. Current Opinion in Otolaryngology Head and Neck Surgery. 2004;12:439–443. doi: 10.1097/01.moo.0000134450.99615.22. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Frisina RD. Age-related hearing loss and its cellular and molecular bases. In: Schacht J, Popper AN, Fay RR, editors. Auditory trauma, protection, and repair: Springer handbook of auditory research, volume 31. New York: Springer Science+Business Media LLC; 2008. pp. 145–194. [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Research. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman DA, Reifman W, Schlauch R, Levine S. Incidence of spontaneous hearing threshold shifts during modern concert performances. Otolaryngol Head Neck Surg. 2006;134:667–673. doi: 10.1016/j.otohns.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Osterhammel D. High-frequency audiometry and noise-induced hearing loss. Scand Audiol. 1979;8:85–90. doi: 10.3109/01050397909076306. [DOI] [PubMed] [Google Scholar]
- Osterhammel D, Osterhammel P. High-frequency audiometry. Age and sex variations. Scand Audiol. 1979;8:73–81. doi: 10.3109/01050397909076304. [DOI] [PubMed] [Google Scholar]
- Peng JH, Tao ZZ, Huang ZW. Risk of damage to hearing from personal listening devices in young adults. J Otolaryngol. 2007;36:181–185. [PubMed] [Google Scholar]
- Portnuff CD, Fligor BJ, Arehart KH. Teenage use of portable listening devices: a hazard to hearing? J Am Acad Audiol. 2011;22:663–677. doi: 10.3766/jaaa.22.10.5. [DOI] [PubMed] [Google Scholar]
- Schechter MA, Fausti SA, Rappaport BZ, Frey RH. Age categorization of high-frequency auditory threshold data. J Acoust Soc Am. 1986;79:767–771. doi: 10.1121/1.393466. [DOI] [PubMed] [Google Scholar]
- Schmuziger N, Patscheke J, Probst R. Hearing in nonprofessional pop/rock musicians. Ear Hear. 2006;27:321–330. doi: 10.1097/01.aud.0000224737.34907.5e. [DOI] [PubMed] [Google Scholar]
- Schmuziger N, Patscheke J, Probst R. An assessment of threshold shifts in nonprofessional pop/rock musicians using conventional and extended high-frequency audiometry. Ear Hear. 2007;28:643–648. doi: 10.1097/AUD.0b013e31812f7144. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Presbycusis. Laryngoscope. 1955;65:402–419. doi: 10.1097/00005537-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Further observations on the pathology of presbycusis. Arch Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Serra MR, Biassoni EC, Richter U, Minoldo G, Franco G, Abraham S, Carignani JA, Joekes S, Yacci MR. Recreational noise exposure and its effects on the hearing of adolescents. Part I: an interdisciplinary long-term study. Int J Audiol. 2005;44:65–73. doi: 10.1080/14992020400030010. [DOI] [PubMed] [Google Scholar]
- Shah S, Gopal B, Reis J, Novak M. Hear today, gone tomorrow: an assessment of portable entertainment player use and hearing acuity in a community sample. J Am Board Fam Med. 2009;22:17–23. doi: 10.3122/jabfm.2009.01.080033. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772–778. doi: 10.1001/jama.2010.1124. [DOI] [PubMed] [Google Scholar]
- Smith PA, Davis A, Ferguson M, Lutman ME. The prevalence and type of social noise exposure in young adults in England. Noise Health. 2000;2:41–56. [PubMed] [Google Scholar]
- Stelmachowicz PG, Beauchaine KA, Kalberer A, Jesteadt W. Normative thresholds in the 8- to 20-kHz range as a function of age. J Acoust Soc Am. 1989;86:1384–1391. doi: 10.1121/1.398698. [DOI] [PubMed] [Google Scholar]
- Tange RA, Dreschler WA, van der Hulst RJ. The importance of high-tone audiometry in monitoring for ototoxicity. Arch Otorhinolaryngol. 1985;242:77–81. doi: 10.1007/BF00464411. [DOI] [PubMed] [Google Scholar]
- Vassallo L, Sataloff J, Menduke H. Very high frequency audiometric technique. Arch Otolaryngol. 1968;88:251–253. doi: 10.1001/archotol.1968.00770010253007. [DOI] [PubMed] [Google Scholar]
- Vaughan NE, Fausti SA, Chelius S, Phillips D, Helt W, Henry JA. An efficient test protocol for identification of a limited, sensitive frequency test range for early detection of ototoxicity. J Rehabil Res Dev. 2002;39:567–574. [PubMed] [Google Scholar]
- Vogel DA, McCarthy PA, Bratt GW, Brewer C. The clinical audiogram: its history and current uses. Communicative Disorders Review. 2007;1:81–94. [Google Scholar]
- Wang Y, Ren C. Effects of repeated "benign" noise exposures in young CBA mice: shedding light on age-related hearing loss. J Assoc Res Otolaryngol. 2012;13:505–515. doi: 10.1007/s10162-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W, Beach EF, Gilliver M. Clubbing: the cumulative effect of noise exposure from attendance at dance clubs and night clubs on whole-of-life noise exposure. Noise Health. 2010;12:155–158. doi: 10.4103/1463-1741.64970. [DOI] [PubMed] [Google Scholar]
- Wong TW, Van Hasselt CA, Tang LS, Yiu PC. The use of personal cassette players among youths and its effects on hearing. Public Health. 1990;104:327–330. doi: 10.1016/s0033-3506(05)80524-4. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang H, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Research. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]