Abstract
Background
There are an increasing number of elderly patients with end-stage heart failure. Destination mechanical circulatory support is often the only therapy available for these patients who are not transplant candidates. The outcomes after continuous flow left ventricular assist device (CF LVAD) implant in older patients remains unclear. We undertook this multi-institutional study to quantify short-term and midterm outcomes after CF LVAD implant in the elderly.
Methods
We retrospectively analyzed all patients in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) national registry that underwent implant of a CF LVAD (June 2006 to April 2012). Patients were divided into 2 cohorts based upon age (<70 years [n = 4,439] and ≥70 years (n = 590]). Preoperative, intraoperative, and postoperative variables were analyzed. The primary endpoint, survival, was compared between cohorts.
Results
Patients age 70 and older were more hemodynamically stable pre-VAD implant as evidenced by INTERMACS profile and inotrope dependence. Perioperative outcomes, including median bypass time (89 vs 89 minutes) and length of stay (0.657 vs 0.657 months) were similar between cohorts (p = not significant). Kaplan-Meier analysis revealed a significant difference in 2-year survival between patients aged 70 years or greater (63%) and less than 70 (71%, p < 0.001). Multivariable Cox proportional hazard analysis revealed age as an independent predictor of mortality during follow-up (p < 0.001). Nonetheless, midterm cumulative survival in the older cohort was still reasonable (63% at 2 years).
Conclusions
Multi-institutional analysis revealed advanced age as a predictor of increased mortality after CF LVAD implantation. Careful patient selection is critical in the elderly to optimize long-term outcomes after CF LVAD implantation.
Mechanical circulatory support is becoming mainstream therapy for terminal heart failure. Though initially evaluated as a bridge to transplant strategy to support patients with unstable heart failure who could not survive on the wait list for a heart transplant, left ventricular assist devices (LVAD) are increasingly being used for destination therapy [1]. Large, multi-institutional, prospective, randomized clinical trials have clearly demonstrated markedly improved survival and quality of life with ventricular assist devices as compared with optimal medical therapy [2–5]. With an aging population, the incidence of heart failure is expected to dramatically increase [6]. Furthermore, there will be a very large number of elderly patients presenting with heart failure either failing medical therapy or temporarily stabilized medically. Given a severely limited supply of donor hearts, transplant is not a viable option for the aged population with end-stage heart failure.
Unfortunately, the decision to implant an LVAD in the elderly can be difficult to make. Often, older patients with heart failure have significant comorbidities that can adversely affect outcomes. Thus, questions are raised regarding the benefit of LVAD therapy in the elderly. Most major VAD centers have included destination LVAD therapy in their treatment algorithm. However, data on outcomes after LVAD implantation in the elderly patient cohort remain limited. Studies have identified advanced age as a risk factor for poor outcomes after VAD implant. Yet, most of these studies were not focused on the elderly population, and those that have are limited by small sample size [4, 7].
A recent single-center, retrospective review from Sharp-Memorial Hospital has demonstrated equivalent short and midterm outcomes and survival (70% at 2 years) after LVAD implant in patients greater than 70 years of age when compared with younger patients [8]. But the single-center nature and small sample size (n = 30 > 70 years old) of the study limit the generalizability of this study’s excellent results. Through analysis of the multiinstitutional Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) database, the present study was designed to determine contemporary clinical outcomes of patients 70 years of age and older after continuous flow LVAD implantation.
Material and Methods
Study Design
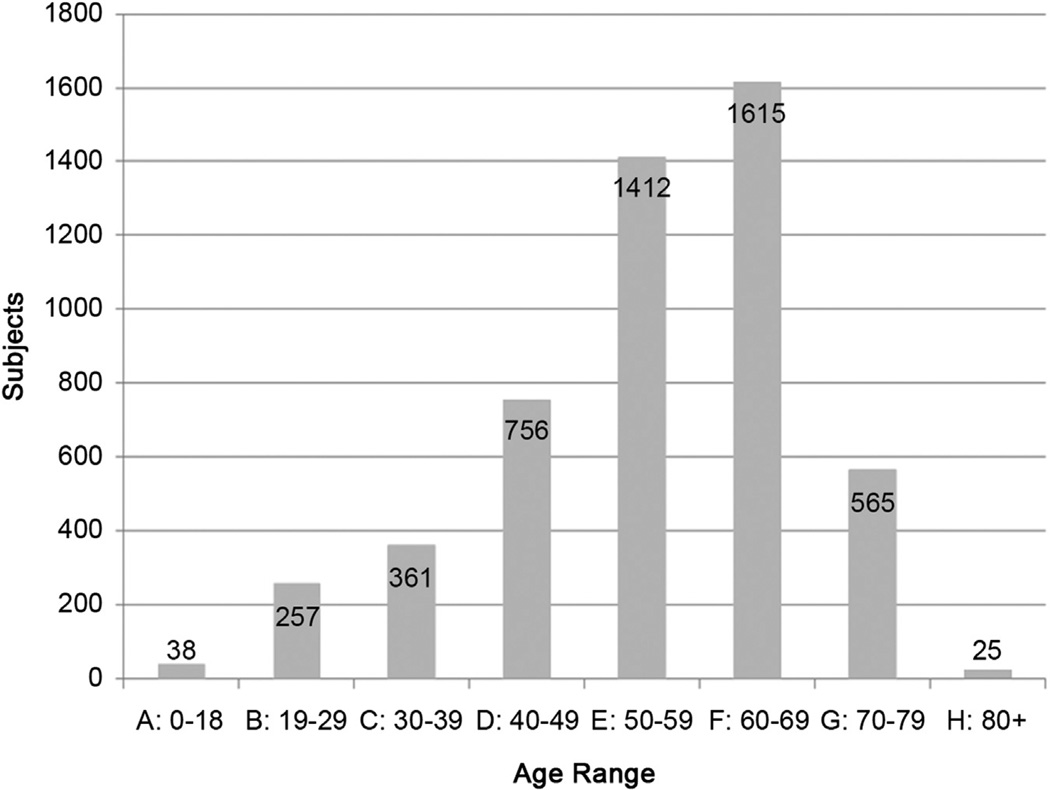
We retrospectively studied all patients in the INTERMACS database who underwent continuous flow LVAD (CF LVAD) implantation between June 2006 and April 2012. Only patients who underwent implant of isolated continuous flow LVADs were included in the study; all biventricular (BI)VAD and total artificial heart implant patients were excluded. The INTERMACS is a robust national registry of patients receiving mechanical circulatory support device therapy for heart failure. INTERMACS was created in 2005 through the collaborate efforts of the National Heart, Lung and Blood Institute, The Centers for Medicare and Medicaid Services, the Food and Drug Administration, physicians, industry, and the University of Alabama. At present there are 141 active participating sites. The goal of INTERMACS is to promote research and improve this innovative surgical therapy by collecting prospective clinical and laboratory data at defined intervals throughout a patient’s preoperative and postoperative course. Raw data were provided by the INTERMACS committee in a de-identified format according to National Institutes of Health guidelines. Only data, provided from participating sites, was available for analysis. Completeness of data is contingent on the reporting of participant sites. The breakdown of patients by decade of age of LVAD implant is provided in Figure 1. Patients were grouped into 2 cohorts; 70 years or greater (median follow-up: 9.1 months, interquartile range 3.384 to 16.263) and 69 or less (median follow-up 8.4 months, interquartile range 3.614 to 16.362) years of age. The age of 70 was chosen as the threshold for group stratification because this is the arbitrary age when most transplant centers will no longer consider a patient eligible for heart transplant. A total of 5,029 patients were included in the study; 4,439 patients in the younger cohort (≤69 years) and 590 patients in the older cohort (≥70 years).
Fig. 1.
Age range at time of left ventricular assist device implant.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation or median with interquartile range (if non-normally distributed), and categoric variables are presented as proportions. Comorbid diagnoses and perioperative outcome variables adhere to the definitions of the INTERMACS national registry. Differences between groups were assessed using the Fisher exact test for categoric variables, the independent Student t test for normally distributed continuous variables, and the Mann-Whitney U test for non-normally distributed continuous variables. Survival analyses were performed using Kaplan-Meier survival analysis with log-rank tests. A Cox proportional hazard multivariable regression model was generated to determine whether advanced age is an independent predictor of reduced survival. Variables were included in a stepwise multivariable analysis (threshold for inclusion, p < 0.05) if the p value is less than 0.05 on univariate analysis. Variables included in the multivariable analysis were the following: age greater than 70, creatinine greater than 1.7, albumin less than 3.0, VAD as destination therapy, inotrope dependence, etiology of heart failure, prior cardiac surgery, and smoking. Results are demonstrated as hazard ratios (HR) and 95% confidence intervals (CI). All tests were 2-tailed, and a p value less than 0.05 was considered statistically significant. The statistical analysis was performed using SAS for Windows (SAS Institute, Inc, Cary, NC) and IBM SPSS Statistics for Macintosh, version 19.0 (SPSS, Inc, Armonk, NY).
Results
Patient Demographics and Baseline Risk Profiles
Analysis of preoperative patient demographics and comorbidities revealed several significant differences between patients 70 or greater years of age and the younger cohort (Table 1). A larger proportion of patients in the older cohort were male and Caucasian. Additionally, patients in the elderly cohort tended to be healthier and more hemodynamically stable at the time of LVAD implant as evidenced by INTERMACS profile and preoperative inotrope dependency. Elderly patients were more likely to have a chronic obstructive pulmonary disease, diabetes, and a history of cancer. Interestingly, a markedly higher percentage of patients 70 or greater had prior cardiac surgery, with over 50% of patients undergoing redo sternotomy at the time of CF LVAD implant. Patients in the older cohort tended to have worse renal function (Cr = 1.55 vs 1.43, p = <0.001) but had significantly lower transaminase levels.
Table 1.
Preoperative Demographics and Risk Profiles at the Time of Left Ventricular Assist Device Implant
| Variable | <70 Years | ≥70 Years | p Value |
|---|---|---|---|
| Gender (% male) | 77.76% | 86.78% | <0.001 |
| Race (%) | <0.001 | ||
| Caucasian | 68.44% | 87.63% | |
| Black | 24.54% | 8.76% | |
| Other | 7.02% | 3.61% | |
| INTERMACS profile (%) | <0.001 | ||
| 1 | 15.99% | 8.31% | |
| 2 | 41.02% | 36.95% | |
| 3 | 24.67% | 30.34% | |
| 4 | 12.37% | 17.63% | |
| 5 | 3.15% | 4.24% | |
| 6 | 1.82% | 1.86% | |
| 7 | 0.97% | 0.68% | |
| Etiology (%) | <0.001 | ||
| Idiopathic | 33.31% | 20.71% | |
| Ischemic | 35.71% | 53.48% | |
| Other | 30.98% | 25.81% | |
| Chronic obstructive Pulmonary disease (%) | 13.02% | 19.37% | <0.001 |
| Diabetes | 38.53% | 44.69% | 0.005 |
| Smoking history (%) | 61.75% | 64.26% | 0.3 |
| Prior cardiac surgery (%) | 33.12% | 52.54% | <0.001 |
| NYHA (%) | 0.7 | ||
| I | 0.32% | 0% | |
| II | 1.34% | 1.51% | |
| III | 19.39% | 18.71% | |
| IV | 78.94% | 79.77% | |
| Stroke (%) | 7.86% | 7.19% | 0.6 |
| Cancer (%) | 8.16% | 11.17% | 0.02 |
| Inotrope dependent | 81.69% | 75.59% | <0.001 |
| Height (cm) | 175 ± 11 | 174 ± 10 | 0.5 |
| Weight (kg) | 89 ± 23 | 81 ± 18 | <0.001 |
| BMI (kg/m2) | 29.2 ± 9.9 | 26.7 ± 9.1 | <0.001 |
| BUN (mg/dL) | 25 (IQR = 17–37) | 31 (IQR = 22–43) | <0.001 |
| Creatinine (mg/dL) | 1.3 (IQR = 1–1.7) | 1.4 (IQR = 1.1–1.8) | <0.0001 |
| AST (U/L) | 31 (IQR = 23–47) | 30 (IQR = 23–43, N) | 0.2 |
| ALT (U/L) | 30 (IQR = 20–54) | 28 (IQR = 19–44) | 0.002 |
| Total bilirubin (mg/dL) | 1.1 (IQR = 0.7–1.7) | 1 (IQR = 0.7–1.6) | 0.6 |
| Albumin (g/dL) | 3.4 ± 0.7 | 3.3 ± 0.6 | 0.1 |
| VO2 Max (mL/kg/min) | 11 (IQR = 9–13) | 11 (IQR = 8–13) | 0.7 |
ALT = alanine transaminase; AST = aspartate aminotransferase; BMI = body mass index; BUN = blood urea nitrogen; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; IQR = interquartile range; NYHA = New York Heart Association; VO2 = maximum oxygen consumption.
Preoperative Hemodynamics and Operative Data
Interestingly, the older cohort had lower right atrial and pulmonary capillary wedge pressures, suggesting improved volume optimization (Table 2). This may be due to a larger proportion of patients undergoing LVAD implant on an elective versus emergent basis. As expected, a significantly higher proportion of elderly patients underwent LVAD implant for destination therapy as opposed to bridge to transplant. Yet, 19% of the older patient group did receive an LVAD as bridge to transplant or recovery. A significantly larger proportion of patients in the older cohort underwent concomitant valvular surgery; however, there was no difference in mean cardiopulmonary bypass time between groups. Moreover, there was no difference in average length of stay between the 2 age cohorts. As expected, patients 70 or greater years of age were more likely to be discharged to rehab as opposed to home.
Table 2.
Hemodynamic, Operative, and Perioperative Data
| Variable | <70 Years | ≥70 Years | p value |
|---|---|---|---|
| Preoperative cardiac output (L/min) | 4.34 ± 1.45 | 4.12 ± 1.42 | 0.01 |
| Preoperative right atrial pressure (mm Hg) | 12.4 ± 7.0 | 11.0 ± 5.6 | <0.001 |
| Preoperative pulmonary capillary wedge pressure (mm Hg) | 24.4 ± 8.8 | 22.6 ± 7.7 | 0.005 |
| Preoperative heart rate (bpm) | 89 ± 18 | 82 ± 15 | <0.001 |
| Preoperative systolic blood pressure (mm Hg) | 102 ± 15 | 106 ± 16 | <0.001 |
| Preoperative diastolic blood pressure (mm Hg) | 64 ± 12 | 62 ± 11 | <0.001 |
| LVAD for destination therapy (%) | 21.65% | 80.85% | <0.001 |
| Concomitant surgery (%) | 0.002 | ||
| Valve | 21.62% | 28.31% | |
| Other | 16.75% | 15.76% | |
| Cardiopulmonary bypass time (minutes) | 89 (IQR = 65–119) | 89 (IQR = 62–127) | 0.8 |
| Length of stay (months) | 0.657 (IQR = 0.46–0.953) | 0.657 (IQR = 0.46–1.051) | 0.4 |
| Discharged to (%) | <0.001 | ||
| Home | 81.67% | 62.37% | |
| Rehab | 15.76% | 34.36% | |
| Other | 2.57% | 3.27% |
bpm = beats per minute; IQR = interquartile range; LVAD = left ventricular assist device.
Adverse Events After LVAD Implant
Studying the incidence of adverse events after LVAD implant reveals a significant increase in stroke (2.3% vs 0.9%, p = 0.01) in the elderly when compared with younger patients (Table 3). Additionally, the elderly appear to have a significantly increased risk of gastrointestinal bleeding. Interestingly, there is a much lower incidence of driveline infection among the elderly when compared with younger patients (5.7% vs 12.6%, p < 0.001). This may be related to the fact that more patients in the younger cohort are more ill and presumably underwent acute LVAD implant.
Table 3.
Adverse Events After Left Ventricular Assist Device Implant
| Incidence Patients | (%) | Event Rate (Events Per Patient-Month) |
||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event | <70 Years | ≥70 Years | p Value | <70 Years | (n = 3,906) | ≥70 Years | (n = 489) | p Value |
| Rehospitalization | 2,496 | (63.9%) | 304 | (62.17%) | 0.5 | 0.1841 ± 0.2428 | 0.1391 ± 0.1811 | <0.0001 |
| CVA | 36 | (0.92%) | 11 | (2.25%) | 0.02 | 0.0114 ± 0.2961 | 0.0103 ± 0.1262 | 0.9 |
| TIA | 199 | (5.09%) | 21 | (4.29%) | 0.5 | 0.0254 ± 0.5246 | 0.00841 ± 0.0691 | 0.5 |
| Infection | ||||||||
| Driveline | 491 | (12.57%) | 28 | (5.73%) | <0.001 | 0.0132 ± 0.0473 | 0.007 ± 0.0423 | 0.006 |
| Sepsis | 357 | (9.14%) | 49 | (10.02%) | 0.5 | 0.0236 ± 0.1627 | 0.0407 ± 0.2461 | 0.04 |
| Other | 1,667 | (42.68%) | 224 | (45.81%) | 0.2 | 0.1528 ± 0.4527 | 0.2319 ± 0.6312 | 0.0005 |
| Renal dysfunction | 499 | (12.78%) | 72 | (14.72%) | 0.2 | 0.1042 ± 0.9114 | 0.1175 ± 0.5206 | 0.7 |
| Bleeding | ||||||||
| Gastrointestinal | 523 | (13.39%) | 97 | (19.84%) | <0.001 | 0.0397 ± 0.238 | 0.0509 ± 0.1745 | 0.3 |
| Other | 1,318 | (33.74%) | 191 | (39.06%) | 0.02 | 0.3862 ± 2.8503 | 0.6193 ± 3.2675 | 0.09 |
| Right heart failure | 556 | (14.23%) | 70 | (14.31%) | 0.9 | 0.0905 ± 0.8796 | 0.079 ± 0.4069 | 0.8 |
| Respiratory failure | 784 | (20.07%) | 103 | (21.06%) | 0.6 | 0.1103 ± 0.4831 | 0.1611 ± 0.5931 | 0.03 |
CVA = cerebrovascular accident; TIA = transient ischemic attack.
Short-Term and Midterm Survival
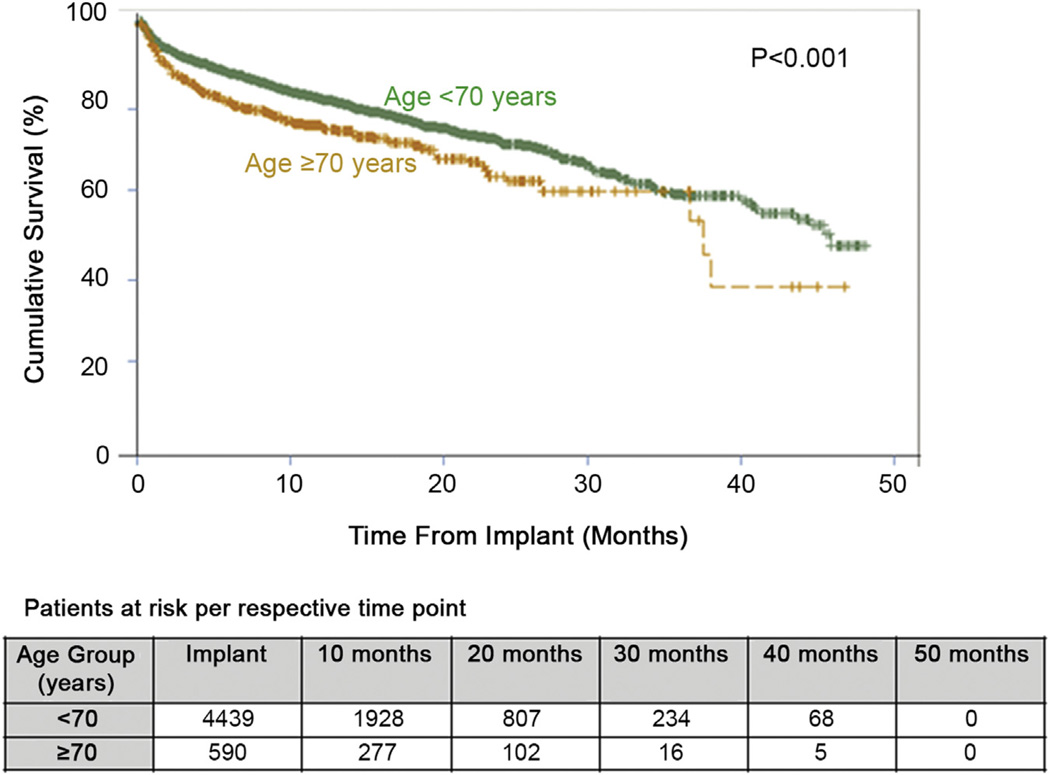
Analysis of short and midterm survival for all patients undergoing commercially available continuous flow devices revealed a significantly worse survival at 2 years for patients 70 or greater years of age when compared with those patients less than 70 (71% vs 63%, p < 0.001; Table 4). This significant decrease in survival appears to carry out to 36 months, though the number of elderly patients at risk is very limited and therefore generalized analysis is difficult. There appears to be a decrease in survival for the elderly patients at 30 days, 6 months, and 12 months (Fig 2). However, it should be noted that both the short (93% at 30 days) and midterm survival (75% at 12 months) are very reasonable for the older cohort and should not be considered a contraindication for LVAD implant. Cox proportional hazard multivariable analysis identified several independent risk factors for decreased survival, including age greater than 70 (HR 1.45), prior cardiac surgery (HR 1.49), inotrope dependence (1.32), serum albumin less than 3.0 g/dL (HR 1.25), serum creatinine greater than 1.7 mg/dL (HR 1.34) (Table 5).
Table 4.
Short-Term and Midterm Survival After Left Ventricular Assist Device Implant
| Survival | <70 Years | ≥70 Years | p Value |
|---|---|---|---|
| 30 Days | 95% | 93% | 0.02 |
| 6 Month | 87% | 80% | <0.001 |
| 1 Year | 81% | 75% | <0.001 |
| 2 Years | 71% | 63% | <0.001 |
| 3 Years | 59% | 54% | <0.001 |
Fig. 2.
Kaplan-Meier analysis demonstrating cumulative survival for patients less than 70 and 70 or greater years of age undergoing continuous flow left ventricular assist device implant.
Table 5.
Cox Proportional Hazards Multivariable Model to Identify Independent Risk Factors for Reduced Survival
| Variable | Coefficient β | HR | 95% CI | p-value |
|---|---|---|---|---|
| Prior cardiac surgery | 0.399 | 1.49 | (1.24–1.80) | <0.001 |
| Age >70 years | 0.371 | 1.45 | (1.13–1.85) | 0.003 |
| Creatinine >1.7 mg/dL | 0.294 | 1.34 | (1.10–1.63) | 0.003 |
| Inotrope dependence | 0.277 | 1.32 | (1.02–1.70) | 0.03 |
| Albumin <3.0 g/dL | 0.223 | 1.25 | (1.01–1.54) | 0.04 |
CI = confidence interval; HR = hazard ratio.
Comment
The data from this study are very encouraging for the field of mechanical circulatory support. This national, multi-institutional experience implies that continuous flow LVAD implant can be safely performed in the elderly (≥70 years of age). The short-term outcomes presented above (93% 30-day mortality) are very encouraging. Furthermore, 75% 1-year and 63% 2-year survivals for this critically ill cohort suggest that we can make a positive impact on this patient population with appropriate mechanical circulatory support. Medical therapy for endstage heart failure remains largely palliative with numerous studies suggesting very poor survival (8% to 10% at 2 years) without further intervention [3, 9, 10]. It appears that survival in the elderly (≥70 years of age) is diminished at 30 days, 1 month, 12 months, 1 year, and 2 years after CF LVAD implant when compared with younger patients (<70 years of age), but the outcomes remain very respectable. Even more interesting is the fact that the elderly cohort was “less ill” at the time of LVAD implant as determined by INTERMACS status when compared with the younger cohort. As the field of mechanical circulatory support advances, it will be important to continue to optimize outcomes through careful patient selection in this elderly patient population. A proportion of the increase in mortality is clearly related to the advanced comorbidities that often accompany this older cohort. This may further account for the significantly increased risk of stroke and gastrointestinal bleeding after LVAD implant in the 70 or greater group.
Unlike in younger patients, heart transplant is not an option in the vast majority of centers for patients older than 70. Therefore, it is critical that only patients who can truly tolerate an isolated LVAD undergo this procedure. Unfortunately, right ventricular failure remains a major concern in this subset of patients. As opposed to the younger cohort that can be converted to biventricular support as a bridge to transplant, right ventricular failure in the elderly after LVAD implant often means subsequent end-organ damage and a high likelihood of death. There are several scoring systems that attempt to preoperatively predict the mortality associated with LVAD implant or the likelihood of right ventricular failure and the ability to tolerate an isolated LVAD that can be utilized in trying to decide the appropriateness for CF LVAD implant in the elderly [4, 11–17]. There have been several anecdotal reports in the literature utilizing continuous flow devices as a long-term right (R)VAD [18–20]. Unfortunately, there still is not a widely applicable CF RVAD platform that is available. In the meantime, it may be possible to utilize temporary right ventricular support to bridge patients with intermediate risk for right ventricular failure for a short period of time with a temporary CF RVAD tunneled through the chest wall [21, 22]. This strategy to unload the right ventricle may allow the ventricle to recover enough to support the LVAD long term.
Although several scoring systems have been devised to predict the outcomes after LVAD implant and appropriateness for mechanical circulatory support, these scoring systems are all focused on younger patients [4, 11–17]. Based upon the present study, it is clear that the vast majority of patients (88%) in whom CF LVADs are implanted are less than 70 years of age. The elderly patient population has many issues that are germane only to this group. Some of these major challenges include cerebrovascular disease, renal insufficiency, chronic obstructive pulmonary disease, psychosocial support, and potential frailty. These age specific issues need to be accounted for in a risk score specific to the elderly population with heart failure.
The concept of frailty has not been very well explored in the surgical literature. With the advent of transcatheter aortic valve technology, the concept of frailty has become a cornerstone of patient evaluation. It is believed that patients who are severely debilitated may not have the reserve to either withstand a surgical intervention or have a good long-term outcome. Recent data suggest that frailty may adversely impact long-term outcomes after transcatheter aortic valve replacement [23]. It is difficult to know without statistical analyses, but it is likely that frailty would also have an adverse impact on the elderly undergoing LVAD implant. It is imperative that as a field we clearly delineate those elderly patients who will be positively impacted by mechanical circulatory support. We should be able to preoperatively identify those patients who are too frail, have too many comorbidities, or have inappropriate physiology to benefit from LVAD implant. A risk score specifically focused on patients greater than 70 years of age will help to select the elderly patients most suitable for CF LVAD implantation.
As LVAD implant for destination therapy remains a relatively young field, more experience is needed before we can optimize patient selection and outcomes. Already in the 8 years since the advent of the HeartMate II VAD, we have made significant strides in refining patient selection and improving clinical outcomes. A recent report by Kirklin and colleagues [7] demonstrated improving outcomes with modern CF LVADs that are beginning to rival heart transplant, suggesting significant education and improvements in patient selection, management, and technique. We can expect that as our experience improves so too will outcomes after destination therapy LVAD implantation. Even though this study demonstrates significantly worse outcomes in the elderly after LVAD implant when compared with the younger and sicker patients, a 63% 2-year survival is still a very good result, especially when compared with that of medical management. Moreover, even though multivariable Cox proportional hazard analysis in this study and other studies demonstrates age to be a risk factor for poor outcome after LVAD implant [4, 7, 14], with appropriate patient selection we can attain as excellent results as have been reported in other studies [8].
When the INTERMACS data in this study are analyzed, it is noted that nearly 76% of those patients 70 or greater were inotrope dependent at the time of LVAD implant. Patients who are inotrope dependent or have a worse INTERMACS status have the highest mortality [24]. We should strive to continue to identify patients prior to progression of heart failure and the need for inotropic support. This will help to ensure improved outcomes. Hopefully, studies such as ROADMAP and REVIVE-IT, designed to study the role of early LVAD implant will shed light on this topic. There have been significant advances made in the field of mechanical circulatory support. Much optimism exists for the present and future therapeutic role of LVADs in elderly and young patients alike.
This study is a retrospective review of data from the INTERMACS registry. The data in this registry are extremely valuable for future patient care but do have limitations. As these data include outcomes from numerous centers, it is difficult to control for physician and center experience. Additionally, there are a limited number of patients in the 70 or greater age group that have remained at risk at 36 months; therefore it is difficult to make a reasonable long-term conclusion and rather we are limited to midterm data. Moreover, as patients less than 70 were more likely to undergo LVAD implant as bridge to transplant as opposed to destination therapy, direct comparability is not as clear as a randomized, multi-center prospective trial. Finally, because the INTERMACS registry includes only patients who had FDA-approved devices implanted, there are several patients who underwent implant of experimental continuous flow devices who were not captured with this dataset. Taking into account these limitations, we feel that there are valuable data to be gathered from this study that can be utilized in optimizing outcomes after LVAD surgery.
Footnotes
Presented at the Poster Session of the Forty-ninth Annual Meeting of The Society of Thoracic Surgeons, Los Angeles, CA, Jan 26–30, 2013.
Dr Acker discloses a financial relationship with Thoratec, Inc.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 5.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584–603. doi: 10.1016/j.jtcvs.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamson RM, Stahovich M, Chillcott S, et al. Clinical strategies and outcomes in advanced heart failure patients older than 70 years of age receiving the HeartMate II left ventricular assist device: a community hospital experience. J Am Coll Cardiol. 2011;57:2487–2495. doi: 10.1016/j.jacc.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs337. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Fang JC. Rise of the machines–left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–2285. doi: 10.1056/NEJMe0910394. [DOI] [PubMed] [Google Scholar]
- 11.Imamura T, Kinugawa K, Shiga T, et al. Novel risk scoring system with preoperative objective parameters gives a good prediction of 1-year mortality in patients with a left ventricular assist device. Circ J. 2012;76:1895–1903. doi: 10.1253/circj.cj-12-0182. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 13.Rao V, Oz MC, Flannery MA, Catanese KA, Argenziano M, Naka Y. Revised screening scale to predict survival after insertion of a left ventricular assist device. J Thorac Cardiovasc Surg. 2003;125:855–862. doi: 10.1067/mtc.2003.111. [DOI] [PubMed] [Google Scholar]
- 14.Holman WL, Kormos RL, Naftel DC, et al. Predictors of death and transplant in patients with a mechanical circulatory support device: a multi-institutional study. J Heart Lung Transplant. 2009;28:44–50. doi: 10.1016/j.healun.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Schaffer JM, Allen JG, Weiss ES, et al. Evaluation of risk indices in continuous-flow left ventricular assist device patients. Ann Thorac Surg. 2009;88:1889–1896. doi: 10.1016/j.athoracsur.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Cheng RK, Deng MC, Tseng CH, Shemin RJ, Kubak BM, MacLellan WR. Risk stratification in patients with advanced heart failure requiring biventricular assist device support as a bridge to cardiac transplantation. J Heart Lung Transplant. 2012;31:831–838. doi: 10.1016/j.healun.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Klotz S, Vahlhaus C, Riehl C, Reitz C, Sindermann JR, Scheld HH. Pre-operative prediction of post-VAD implant mortality using easily accessible clinical parameters. J Heart Lung Transplant. 2010;29:45–52. doi: 10.1016/j.healun.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Potapov E, Schweiger M, Vierecke J, et al. Discontinuation of HeartWare RVAD support without device removal in chronic BIVAD patients. Asaio J. 2012;58:15–18. doi: 10.1097/MAT.0b013e3182376b7b. [DOI] [PubMed] [Google Scholar]
- 19.Krabatsch T, Potapov E, Stepanenko A, et al. Biventricular circulatory support with two miniaturized implantable assist devices. Circulation. 2011;124(11 Suppl):S179–S186. doi: 10.1161/CIRCULATIONAHA.110.011502. [DOI] [PubMed] [Google Scholar]
- 20.Saito S, Sakaguchi T, Miyagawa S, et al. Biventricular support using implantable continuous-flow ventricular assist devices. J Heart Lung Transplant. 2011;30:475–478. doi: 10.1016/j.healun.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Haneya A, Philipp A, Puehler T, et al. Temporary percutaneous right ventricular support using a centrifugal pump in patients with postoperative acute refractory right ventricular failure after left ventricular assist device implantation. Eur J Cardiothorac Surg. 2012;41:219–223. doi: 10.1016/j.ejcts.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito S, Sakaguchi T, Miyagawa S, et al. Recovery of right heart function with temporary right ventricular assist using a centrifugal pump in patients with severe biventricular failure. J Heart Lung Transplant. 2012;31:858–864. doi: 10.1016/j.healun.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: patient selection and outcomes. Curr Opin Cardiol. 2011;26:232–236. doi: 10.1097/HCO.0b013e328345aff4. [DOI] [PubMed] [Google Scholar]