The capacity of the heart to regenerate functional myocardium is extremely limited or absent. This lack of regenerative capacity contrasts with that of many other organs and tissues. Heart failure remains the leading cause of hospitalization in the United States, and its prevalence continues to grow as the population ages. In most patients, the underlying cause of heart failure is a loss of cardiomyocytes, accompanied by functional derangements in contraction and relaxation. The traditional view has held that the reparative ability of the heart is limited by the inability of terminally differentiated cardiomyocytes to undergo cell division after the first weeks of life and a failure in the mobilization of cardiac stem cells (if they exist). However, a recent study by Bergmann and colleagues1 calls this time-tested view into question.
The investigators performed a virtual pulse-chase experiment by measuring the incorporation of carbon-14, which was released during above-ground nuclear-bomb tests, into genomic DNA of human cardiomyocytes to calculate rates of turnover in these cells. Levels of carbon-14 in the atmosphere rose sharply as a result of nuclear testing and dropped precipitously once the Limited Nuclear Test Ban Treaty was signed in 1963. As a result, cells that were “born” during times of high carbon-14 levels can be precisely dated because subjects living during this period incorporated carbon-14 into the DNA of newly generated cardiomyocytes. The results indicate that, contrary to traditional teaching, cardiomyocytes renew throughout life at a very low rate. At the age of 25 years, approximately 1% of cardiomyocytes turn over annually, and the turnover rate decreases to 0.45% at the age of 75 years. During an average life span, fewer than 50% of cardiomyocytes renew. Remarkably, despite the substantial functional and metabolic demands placed on cardiomyocytes during the course of a lifetime, some of these cells survive for more than half a century.
Before this study, carbon-14 dating was used to resolve controversies regarding cell turnover in the brain and in fat cells. However, this methodology is particularly challenging when applied to the heart because of the heterogeneity of cardiomyocytes and nonmyocytic cells populating the heart. Bergmann et al. therefore isolated cardiomyocytes from rapidly dividing cardiac fibroblasts, endothelial cells, and vascular smooth-muscle cells. They also took into account that during the first decade of life, approximately 25% of cardiomyocytes undergo a final round of DNA synthesis without cell division, resulting in a sub-population of binucleated cardiomyocytes. As the heart undergoes hypertrophy (cell growth), cardiomyocytes may undergo additional rounds of DNA synthesis without division, resulting in tetraploidy (four sets of chromosomes) or polyploidy (many sets of chromosomes). The investigators therefore developed and applied a method to identify nuclei and DNA content of cardiomyocytes and so were able to measure only carbon-14 incorporation in diploid nuclei. This study provides the most definitive evidence to date that human cardiomyocytes are renewed during postnatal life.
However, the investigators were unable to determine the source of renewing cardiomyocytes. At least four potential sources of cells could account for “new” cardiomyocytes after birth. Adult cardiomyocytes, which are generally believed to be terminally differentiated, may reenter the cell cycle and divide. If this theory is validated, it suggests that the ancient regenerative program observed in the hearts of amphibians and fish may be exploited for therapeutic benefit in humans.2 Second, bone marrow–derived cardiac stem or progenitor cells have been described that possess the capacity to differentiate into cardiomyocyte-like cells in vitro and to functionally populate the heart after injury.3 However, substantial controversy persists regarding whether Don marrow cells can regenerate functional myocardium; clinical trials examining the efficacy of bone marrow–derived and circulating stem cell s in patients with acute myocardial infarction have shown limited, if any, long-term benefits. Third, recent studies suggest that cells that are derived from the embryonic epicardium may give rise to cardiomyocytes in rodents.4 Finally, niches of putative cardiac stem or progenitor cells have been identified in the murine and human heart.5 These populations of cells are notably heterogeneous, and the niches remain poorly characterized. Uniquely defined populations of “cardiac progenitor cells” remain elusive, as do unambiguous markers required to identify and track such cells. A critical unanswered question is why these cells, if they do exist, are not sufficiently mobilized after myocardial infarction or in the failing heart.
The demonstration that cardiomyocytes turn over in the adult heart highlights both the promise and the challenges of the nascent field of cardiac regenerative medicine (Fig. 1). The low rate of cardiomyocyte renewal that has been observed in the human heart reveals one formidable barrier that must be overcome to regenerate functional myocardium. Therefore, it is too soon to discount other approaches, including the potential application of embryonic stem cells, induced pluripotent stem cells, or mesenchymal progenitor cells that may be expanded and induced to differentiate into cardiomyocytes. Nevertheless, the activation of an autologous source of cells that can functionally repopulate the heart would be preferable, since it does not raise the concern associated with allogeneic-cell transplantation or the use of embryonic stem cells. It is ironic that the environmental devastation created by the testing of nuclear weapons during the Cold War may have provided a glimpse into the future of regenerative medicine. Ultimately, progress toward regenerative therapy for heart disease must be based on a foundation of basic cell and developmental biology.
Figure 1. Therapeutic Approaches to Cardiac Regeneration.
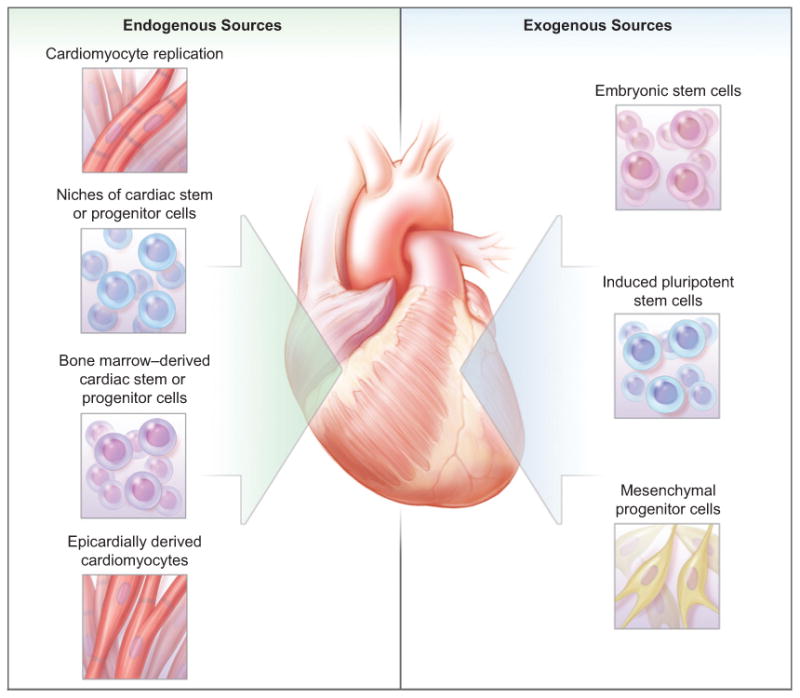
The demonstration that some cardiomyocytes are regenerated after birth highlights the promise and challenges of future regenerative cardiac therapies. Autologous and allogeneic sources of cells that may give rise to cardiomyocytes are under investigation.
Footnotes
Dr. Epstein reports receiving lecture fees from GlaxoSmithKline. No other potential conflict of interest relevant to this article was reported.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Cai CL, Martin JC, Sun Y, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmacek MS, Epstein JA. Pursuing cardiac progenitors: regeneration redux. Cell. 2005;120:295–8. doi: 10.1016/j.cell.2005.01.025. [DOI] [PubMed] [Google Scholar]


