Abstract
Background
In the era of destination continuous flow left ventricular assist devices (LVAD), the decision of whether a patient will tolerate isolated LVAD support or will need biventricular support (BIVAD) can be challenging. Incorrect decision making with delayed right ventricular (RV) assist device implantation results in increased morbidity and mortality. Continuous flow LVADs have been shown to decrease pulmonary hyper-tension and improve RV function. We undertook this study to determine predictors in the continuous flow LVAD era that identify patients who are candidates for isolated LVAD therapy as opposed to biventricular support.
Methods
We reviewed demographic, hemodynamic, laboratory, and echocardiographic variables for 218 patients who underwent VAD implant from 2003 through 2011 (LVAD = 167, BIVAD = 51), during the era of continuous flow LVADs.
Results
Fifty preoperative risk factors were compared between patients who were successfully managed with an LVAD and those who required a BIVAD. Seventeen variables demonstrated statistical significance by univariate analysis. Multivariable logistic regression analysis identified central venous pressure >15 mmHg (OR 2.0, “C”), severe RV dysfunction (OR 3.7, “R”), preoperative intubation (OR 4.3, “I”), severe tricuspid regurgitation (OR 4.1, “T”), heart rate >100 (OR 2.0, Tachycardia - “T”) -CRITT as the major criteria predictive of the need for biventricular support. Utilizing these data, a highly sensitive and easy to use risk score for determining RV failure was generated that outperformed other established risk stratification tools.
Conclusions
We present a preoperative risk calculator to determine suitability of a patient for isolated LVAD support in the current continuous flow ventricular assist device era.
Ventricular assist devices (VAD) have demonstrated marked success in rescuing patients from heart failure [1–3]. Outcomes are striking when compared to those of optimal medical management, and are quickly approaching those of the reference standard, cardiac transplantation. In particular, VADs have demonstrated major improvements in quality of life as well as survival [1–6]. A majority of the devices implanted currently are continuous flow devices, including the Food and Drug Administration–approved HeartMate II (Thoratec, Pleas-anton, CA) pump, given proven device durability and reliability [7]. Unfortunately, irreversible right ventricular (RV) failure remains a major contraindication for isolated left ventricular assist device (LVAD) implant. Currently, continuous flow devices are only available for left ventricular support, with anecdotal reports of utilization in the right ventricle. Therefore, patients with severe biventricular failure are effectively not candidates for destination VAD therapy.
Biventricular assist device (BIVAD) implantation is a reasonable strategy for patients being bridged to recovery or transplant but this is not a viable option for-patients who will be reliant on a VAD for destination therapy. Numerous reports have demonstrated increased mortality with delayed institution of biventricular support rather than early implant of a BIVAD [8–10]. Therefore, it is critical to identify the patients who will successfully tolerate isolated LVAD implant without RV failure at the outset of surgical decision making. Both our group and others have devised risks scores to determine whether a patient will tolerate isolated LVAD support or whether that patient will need biventricular support [11–14]. However, these risk stratification tools were devised from study populations in which pulsatile flow devices were the principal implants, before the advent of newer continuous flow devices.
It has been suggested that continuous flow devices may have a beneficial effect on improving pulmonary hyper-tension and unloading the right ventricle, thereby improving RV function [15–19]. Thus, it is possible that patients who traditionally were not candidates for LVAD implant may now be able to tolerate univentricular support. Additionally, with experience cardiac surgeons have learned operative and pharmacologic strategies to better improve or preserve RV function during the peri-operative period. Therefore, given the availability of newer, continuous flow devices and our enhanced understanding of mechanical circulatory support physiology, patients who may not have initially tolerated an isolated LVAD now may be candidates in the modern era. Kormos and colleagues [20] have very nicely demonstrated a very low incidence of RV failure after implant of the HeartMate II LVAD, based upon the HeartMate II bridge to transplant clinical trial [20]. But they also noted significantly worse outcomes with RV failure after continuous flow LVAD implant, stressing the importance of accurately predicting the need for biventricular support. We undertook this study to develop a simple and easily memorized risk stratification tool to determine whether a patient will tolerate an isolated LVAD, as opposed to needing biventricular support, (The CRITT SCORE: [C]VP, [R]V Dysfunction, [I]ntubation preoperatively, [T]ricuspid regurgitation, [T]achycardia) in the era of modern mechanical circulatory support devices.
Patients and Methods
Study Design
All patients who underwent either isolated LVAD (n = 167) or BIVAD (n = 51) implant from 2003 through 2011 at the Hospital of the University of Pennsylvania were retrospectively reviewed. The device profiles for these patients are presented in Tables 1 and 2. As many patients presented in acute cardiogenic shock, several patients were supported with short-term univentricular or biventricular mechanical devices. Patients in the BIVAD cohort included those who underwent planned BIVAD implantation and those who had right heart failure after initial isolated LVAD implantation requiring delayed insertion of a temporary or long-term RV assist device (n = 7). The decision to implant a BIVAD was made collectively by the heart failure team, which included a cardiac surgeon and heart failure cardiologist. This decision was based upon clinical presentation, hemodynamics as determined by a pulmonary artery catheter, and echocardiographic assessment of the right ventricle. Right ventricular function was measured echocardiographically by evaluation of regional myocar-dial contractility, tricuspid valvular competence, and tricuspid annular motion. We defined severe RV dysfunction based upon echocardiographic parameters, taking into account RV contractility, tricuspid regurgitation, and tricuspid annular motion. The RV function was initially determined by a cardiac anesthesiologist board certified in echocardiography and confirmed by an independent cardiologist evaluating the same study in a separate setting.
Table 1.
Type of Device Utilized in the Isolated Left Ventricular Assist Device Support Group
| Device | n |
|---|---|
| Abiomed BVS-5000 | 2 |
| Biomedicus | 1 |
| CentriMag | 8 |
| HeartMate II | 64 |
| HeartWare | 9 |
| TCI VE/HeartMate XVE | 36 |
| Thoratec PVAD | 41 |
| Ventricore VentrAssist | 6 |
| Total | 167 |
PVAD = paracorporeal ventricular assist device.
Table 2.
Device Combinations Utilized in the BIVAD Support Group
| RVAD Device | LVAD Device | n |
|---|---|---|
| Abiomed BVS-5000 | Abiomed BVS-5000 | 6 |
| CentriMag | CentriMag | 2 |
| Centrimag | TCI VE/HeartMate XVE | 1 |
| Centrimag | Thoratec PVAD | 2 |
| Thoratec PVAD | TCI VE/HeartMate XVE | 1 |
| Thoratec PVAD | Thoratec PVAD | 39 |
| Total | 51 |
LVAD = left ventricular assist device; PVAD = paracorporeal ventricular assist device; RVAD = right ventricular assist device.
Data Acquisition
All patient data were collected preoperatively and retrospectively analyzed. Hemodynamic variables were acquired immediately preoperatively from radial artery and pulmonary artery catheters. Echocardiographic data were obtained from preoperative transesophageal echo-cardiography studies. Preoperative circulatory support was defined as a need for an intraaortic balloon pump, temporary VAD, or extracorporeal membrane oxygenation preoperatively. Cardiac output and mixed venous oxygen saturation were obtained using a continuous cardiac output pulmonary artery catheter and confirmed using measured oxygen tension and manual calculation of cardiac output. Right ventricular stroke work index (RVSWI) was calculated as SVI × (MPAP – CVP) × 0.136, where SVI is stroke volume index, MPAP is mean pulmonary artery pressure, and CVP is central venous pressure. A total of 50 different variables were compared between the LVAD and BIVAD cohorts. In all, 196 of 218 patients had sufficient information to be included in the analysis; and 45 of 51 BIVAD patients had sufficient information to be included in the analysis.
The study was approved by the Institutional Review Board of the University of Pennsylvania. After initial data accrual, all patient identifiers were removed from the database, before statistical analysis.
Statistical Analysis
Categorical variables are presented as proportions and continuous variables are expressed as the mean ± SD. Qualitative descriptions of diagnoses adhere to the definitions of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) unless otherwise defined. Differences between groups were analyzed using Fisher's exact test for categorical variables, the independent Student t test for normally distributed continuous variables, and the Mann-Whitney U test for nonnormally distributed continuous variables. All tests were two-tailed, and p less than 0.05 was considered statistically significant.
Multivariable logistic regression was used to model a binary outcome of LVAD or BIVAD support (planned or delayed) for each patient in the study population. Of 50 fixed-effect variables, 20 were identified in univariate analysis (threshold p < 0.15) for inclusion into a stepwise logistic regression model (entry limit p < 0.1). All continuous variables were converted into categorical variables before inclusion in the multivariable analysis. Conversion was performed with serial χ2 testing with stepwise threshold progression to determine maximal divergence between LVAD and BIVAD groups. To facilitate clinical utilization at the bedside, a simple risk score was generated where each of the five variables identified in the multivariable logistic regression model is assigned a score of 0 or 1 (Thus overall CRITT minimum score 0, maximum score 5). Model fit and predictive power were assessed using Hosmer-Lemeshow goodness-of-fit testing and receiver-operating characteristic curves. A separate subgroup analysis was performed to determine the predictive power of the scoring system for isolated continuous flow LVAD patients. The statistical analysis was performed using IBM SPSS Statistics for Macintosh, version 19.0 (SPSS, Armonk, NY).
Results
Univariate Analysis
Analysis of individual variables revealed several key criteria that were distinctly different between LVAD and BIVAD cohorts (Tables 3–6). Patients in the BIVAD cohort were more likely to be female and have pulmonary hypertension. Systemic hypertension, hypercholesterolemia, stroke, and chronic kidney disease were significantly more frequent in the LVAD cohort (Table 3). Patients who underwent BIVAD implant were more likely to require mechanical ventilation, have severe preoperative RV dysfunction, or require an intraaortic balloon pump or other circulatory support when compared with patients who tolerated isolated LVAD therapy (Table 3). Severe tricuspid regurgitation was also more common in the BIVAD cohort preoperatively (Table 4). Univariate analysis of preoperative hemodynamic variables revealed an elevated heart rate, elevated central venous pressure, and diminished RV stroke work index as variables indicative of the need for biventricular support (Table 5). With regard to laboratory values, an elevated white blood cell count, international normalized ratio, alanine aminotransferase, and aspartate aminotransferase were significantly more likely among patients in the BIVAD cohort when compared with the LVAD group (Table 6).
Table 3.
Demographics, Patient Characteristics, and Baseline Risk Profiles, Univariate Analysis
| Variable | LVAD Group | BIVAD Group | p Value |
|---|---|---|---|
| Age, years | 56.9 ± 16.0 | 56.8 ± 13.6 | 0.9 |
| Percent female | 17% | 28% | 0.09 |
| Body surface area, m2 | 27.6 ± 6.4 | 25.7 ± 5.9 | 0.06 |
| Ischemic cardiomyopathy (versus nonischemic) | 51% | 47% | 0.6 |
| Pulmonary hypertension | 59% | 82% | 0.008 |
| Chronic obstructive pulmonary disease | 11% | 10% | 0.7 |
| Diabetes mellitus | 35% | 29% | 0.4 |
| Smoking history | 44% | 35% | 0.3 |
| Hypertension | 66% | 51% | 0.05 |
| Hypercholesterolemia | 67% | 39% | < 0.001 |
| Carotid stenosis | 4% | 0% | 0.3 |
| Atrial fibrillation | 28% | 29% | 0.9 |
| Prior stroke | 13% | 2% | 0.03 |
| Prior myocardial infarction | 38% | 35% | 0.8 |
| Chronic kidney disease (Cr >2 mg/dL) | 35% | 20% | 0.03 |
| Reoperation | 32% | 41% | 0.3 |
| Mechanical ventilation | 25% | 57% | < 0.001 |
| Severe preoperative RV dysfunction | 29% | 65% | < 0.002 |
| Intraaortic balloon pump | 33% | 49% | 0.04 |
| Preoperative circulatory support | 5% | 20% | 0.002 |
BIVAD = biventricular assist device; Cr = creatinine; LVAD = left ventricular assist device; RV = right ventricle.
Table 6.
Laboratory Parameters, Univariate Analysis
| Variable | LVAD | BIVAD | p Value |
|---|---|---|---|
| White blood cell count, 106/mL | 10.1 ± 5.1 | 11.9 ± 5.5 | 0.036 |
| Hemoglobin, g/dL | 11.4 ± 2.2 | 10.8 ± 2.2 | 0.1 |
| Platelet count, 106/mL | 203.6 ± 98.4 | 196.4 ± 171.6 | 0.02 |
| International normalized ratio | 1.5 ± 0.6 | 1.8 ± 0.9 | 0.01 |
| Partial thromboplastin time, s | 49.9 ± 22.2 | 53.3 ± 31.1 | 0.4 |
| Creatinine, mg/dL | 1.6 ± 0.7 | 1.8 ± 1.1 | 0.2 |
| Total bilirubin, mg/dL | 1.3 ± 1.0 | 1.5 ± 1.4 | 0.5 |
| Alanine aminotransferase, U/L | 120.9 ± 408.2 | 427.8 ± 1240.4 | 0.01 |
| Aspartate aminotransferase, U/L | 143.8 ± 329.5 | 631.7 ± 1757.3 | <0.001 |
| Albumin, g/dL | 3.2 ± 0.7 | 2.9 ± 0.7 | 0.2 |
| Sodium, mEq/L | 135.0 ± 5.0 | 134.0 ± 19.9 | 0.7 |
| Blood urea nitrogen, mg/dL | 33.6 ± 26.2 | 35.2 ± 25.5 | 0.7 |
| Bicarbonate, mEq/L | 26.5 ± 8.5 | 24.0 ± 4.5 | 0.05 |
BIVAD = biventricular assist device; LVAD = left ventricular assist device.
Table 4.
Preoperative Echocardiographic Parameters, Univariate Analysis
| Variable | LVAD | BIVAD | p Value |
|---|---|---|---|
| Left ventricular ejection fraction | 14.1 ± 9.5 | 14.2 ± 13.4 | 0.9 |
| Significant mitral regurgitationa | 31% | 24% | 0.296 |
| Significant tricuspid regurgitationa | 16% | 26% | 0.132 |
| Severe tricuspid regurgitation | 7% | 18% | 0.053 |
| Mild aortic regurgitation or greater | 19% | 18% | 0.882 |
| Patent foramen ovale | 13% | 14% | 0.776 |
Moderate-severe or greater.
BIVAD = biventricular assist device; LVAD = left ventricular assist device.
Table 5.
Hemodynamic Factors, Univariate Analysis
| Variable | LVAD | BIVAD | p Value |
|---|---|---|---|
| Heart rate, beats/min | 93.3 ± 20.3 | 102.8 ± 27.1 | 0.03 |
| Systolic blood pressure, mm Hg | 99.2 ± 17.4 | 99.3 ± 22.1 | 0.9 |
| Diastolic blood pressure, mm Hg | 57.1 ± 11.4 | 56.6 ± 11.6 | 0.8 |
| Mean arterial blood pressure, mm Hg | 71.8 ± 11.5 | 71.8 ± 14.7 | 0.9 |
| Central venous pressure, mm Hg | 12.5 ± 7.2 | 16.9 ± 9.1 | 0.001 |
| Mean pulmonary artery pressure, mm Hg | 29.8 ± 9.8 | 29.3 ± 9.7 | 0.8 |
| Cardiac index, (L/m2/min) | 2.1 ± 0.6 | 2.0 ± 0.6 | 0.5 |
| Mixed venous oxygen saturation, % | 60.1 ± 11.9 | 59.5 ± 18.5 | 0.9 |
| RVSWI, mmHg/L/m2 | 0.59 ± 0.42 | 0.44 ± 0.33 | 0.08 |
| Left ventricular ejection fraction, % | 14.1 ± 9.5 | 14.2 ± 13.4 | 0.9 |
BIVAD = biventricular assist device; LVAD = left ventricular assist device; RVSWI = right ventricular stroke work index.
Multivariate Logistic Regression Analysis
A stepwise multivariable logistic regression model was created by incorporating significant variables identified by univariate analysis. Variables predictive of the need for biventricular support included severe RV dysfunction, severe tricuspid regurgitation, preoperative mechanical ventilation, an elevated central venous pressure (>15 mm Hg), and a heart rate more than 100 beats per minute (Table 7).
Table 7.
Results of Multivariable Logistic Regression Analysis
| Variable | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Severe right ventricular dysfunction | 3.7 | 1.7 – 8.1 | 0.001 |
| Severe tricuspid regurgitation | 4.1 | 1.4 – 12.4 | 0.011 |
| Preoperative mechanical ventilation | 4.3 | 1.9 – 9.6 | <0.001 |
| Central venous pressure >15 mm Hg | 2.0 | 0.9 – 4.2 | 0.089 |
| Heart rate >100 beats/min | 2.0 | 0.9 – 4.3 | 0.086 |
| Constant | 0.04 |
CI = confidence interval.
Quantitative Preoperative Risk Score— CRITT Score
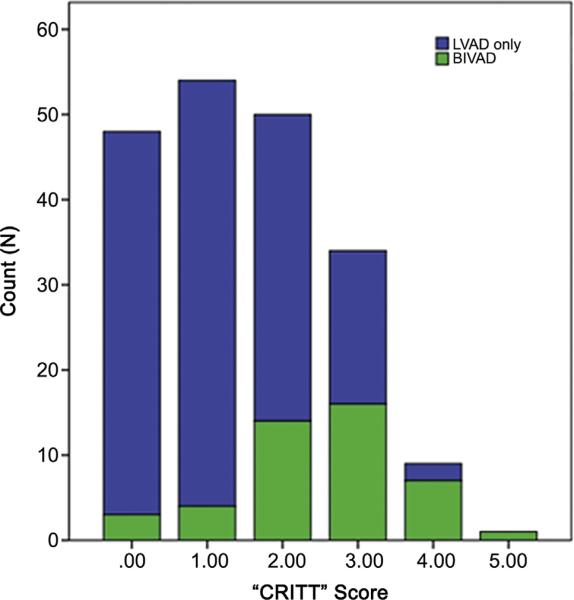
For simplicity and efficiency of use, a 5-point risk score was developed based on the clinical variables identified in the multivariable logistic regression model. Instead of weighting variables with coefficients based on their respective odds ratios, each variable is given a binary response. Therefore, if a patient satisfies the at-risk criterion (eg, preoperative central venous pressure >15 mm Hg), they are assigned a score of 1 for that variable. Alternatively, if a patient does not satisfy the at-risk criterion for a specific variable, they are assigned a score of 0 for that variable. Thus, 0 or 1 point is allotted for each of the five variables in the “CRITT” score: CVP greater than 15 mm Hg (C); severe RV dysfunction (R); preoperative mechanical ventilation/intubation (I); severe tricuspid regurgitation (T); and tachycardia (T). The model fit and predictive power of the 5-point risk score were excellent when applied to the University of Pennsylvania VAD experience (c statistic 0.8). A score of 2 or more points provided a sensitivity of 84%, specificity of 63%, and negative predictive value of 93%. Thus, 93% of patients with a score of 1 or less underwent successful isolated LVAD therapy (95 of 102 patients who could be scored). Additionally, 80% of patients with a score of 4 or higher required biventricular assistance (8 of 10 patients). Based on this model we recommend an isolated LVAD for a score of 0 or 1 and a BIVAD for a score of 4 or 5. Patients with a score of 2 or 3 are in the gray area and may be able to tolerate an isolated LVAD with appropriate pharmacologic or temporary RVAD support. The distribution of scores for the LVAD and BIVAD cohorts in this study has been summarized in Figure 1.
Fig 1.
Distribution of CRITT scores for the left ventricular assist device (LVAD) cohort (blue bars) and biventricular assist device (BIVAD) cohort (green bars). (CRITT = central venous pressure >15 mm Hg [C]; severe right ventricular dysfunction [R]; preoperative mechanical ventilation/intubation [I]; severe tricuspid regurgitation [T]; and tachycardia [T].)
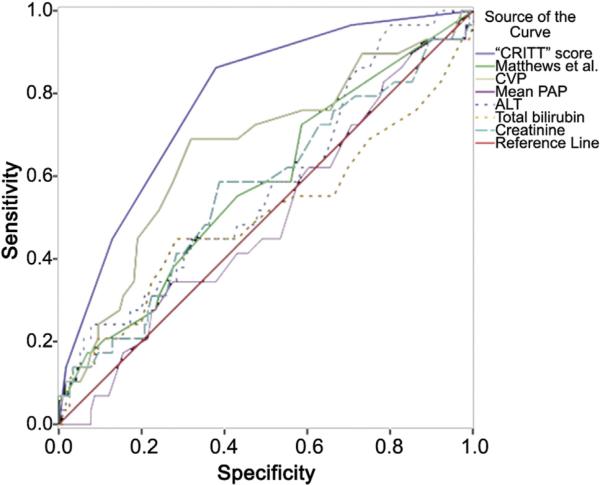
The area under the curve for the CRITT score was 0.80 ± 0.04 (Fig 2). Receiver-operating characteristic curves were also generated for previously reported predictors of RV failure, including central venous pressure, mean pulmonary arterial pressure, total bilirubin, creatinine, and aminotransferase levels (Fig 2, Table 8). Comparison of the area under the curve of the CRITT score with that of other previously reported predictors demonstrated the CRITT score to be most predictive of RV failure requiring mechanical support (p < 0.05 for all comparisons). When applied to the CF LVAD only subset, the predictive power was improved (c statistic 0.85 ± 0.04). The CRITT score demonstrated a sensitivity of 87%, specificity of 75%, and negative predictive value of 93% for continuous flow LVADs.
Fig 2.
Receiver-operating characteristic curves of the CRITT score (solid blue line) as well as other univariate and multivariable predictors of right ventricular failure. Refer to Table 8 for the area under the curve for each receiver-operating characteristic curve. Reference line is in red (null hypothesis); Matthews et al. [10], solid green line; central venous pressure, solid yellow line; mean pulmonary artery pressure, solid purple line; alanine aminotransferase, dashed blue line; total bilirubin, dashed yellow line; and creatinine, dashed aqua line. (CRITT = central venous pressure >15 mm Hg [C]; severe right ventricular dysfunction [R]; preoperative mechanical ventilation/intubation [I]; severe tricuspid regurgitation [T]; and tachycardia [T].) (ALT = alanine transaminase; CVP = central venous pressure; PAP = pulmonary artery pressure.)
Table 8.
Comparison Risk Discrimination Capability of CRITT Score With Other Commonly Used Predictors of Right Ventricular Failure
| Variable | AUC | 95% CI | p Valuea |
|---|---|---|---|
| CRITT score | 0.80 | 0.72 – 0.88 | - |
| Central venous pressure | 0.66 | 0.56 – 0.76 | 0.05 |
| Mean pulmonary artery pressure | 0.50 | 0.38 – 0.61 | <0.001 |
| Alanine aminotransferase | 0.59 | 0.46 – 0.69 | 0.03 |
| Total bilirubin | 0.51 | 0.37 – 0.64 | <0.001 |
| Creatinine | 0.57 | 0.45 – 0.69 | 0.001 |
| Matthews et al.10 | 0.61 | 0.50 – 0.71 | 0.008 |
Represents comparison to CRITT score (CRITT is an acronym for central venous pressure >15 mm Hg [C]; severe right ventricular dysfunction [R]; preoperative mechanical ventilation/intubation [I]; severe tricuspid regurgitation [T]; and tachycardia [T]).
AUC = area under receiver-operating characteristic curve; CI = confidence interval.
Comment
We have developed a reliable risk stratification tool that can be applied to patients in acute or chronic heart failure to determine suitability for univentricular support, or alternatively, need for biventricular support. Whereas many prior published risk models for right heart failure are cumbersome and often involve complex calculations, the CRITT score is very easy to use and remember. The CRITT score can be quickly calculated at the bedside without a complex calculation, and a score less than 2 is highly predictive of the ability to tolerate isolated LVAD therapy (negative predictive value of 93%). This score is not only applicable to patients with long-standing heart failure who are about to undergo placement of a continuous flow LVAD for destination therapy, but also to previously healthy patients who present in acute cardiogenic shock.
The development of RV failure after LVAD implantation is multifactorial, and includes factors such as increased preload, ventricular ischemia, and ventricular geometric mechanical interdependence. Numerous studies have closely examined both measures reflective of the consequences of RV dysfunction, as well as those that are directly diagnostic of reduced RV contractility [9, 11, 12, 21–28]. These studies have identified many logical preoperative hemodynamic and laboratory measures as predictors of RV failure, including a decreased pulmonary artery pressure [9, 21–23] or RVSWI [9, 11, 22, 23], and elevated central venous pressure [24, 28], hepatic transaminases [12, 21, 23], bilirubin [12, 25–27], or creati-nine [11, 12, 21]. Clearly, hemodynamic variables directly reflect increases in preload and afterload, and reductions in RV contractility, whereas consequences of RV failure such as venous congestion and organ hypoperfusion are reflected in hepatic and renal abnormalities.
Unfortunately, the variables (or combinations thereof) identified as significant risk factors for RV failure are not consistent across the number of studies in the literature. For example, preoperative elevations in central venous pressure was associated with a higher risk of RV failure in our study as well as others [24, 28], but was not confirmed in others [12, 23, 25–27]. In a study examining predictors of RV failure that occurred in 11 of 100 HeartMate IP and VE patients, Fukamachi and colleagues [23] showed that a lower mean pulmonary artery pressure and RVSWI, and elevated aminotransferase levels conferred an increased risk of RV failure. In the present study, although such laboratory parameters were significant on univariate analysis (eg, aminotransferases and creatinine), the final multivariable model consisted mainly of direct hemodynamic and echocardiographic measurements of RV performance. When the CRITT score was compared with previously published risk factors for RV failure, the CRITT score emerged as a superior risk stratification tool in our study population that included newer devices and postoperative management techniques.
Differences in risk stratification tools between studies can be attributed to selection bias, time period over which the study was conducted, and indications for VAD therapy. Limitations of prior studies include a low incidence of RV failure [9, 22, 23], vague definitions of RV failure [26], as well as a lack of multivariable analysis [21, 23, 25–27]. Although studies vary in the preoperative variables each identifies as predictors of RV failure, the overall group and type of variables remain the same.
It is important to note that as practice patterns and patient selection vary across institutions, and as VAD technology evolves, each physician should adopt a particular way of stratifying risk for RV failure. However, such risk stratification tools should probably include variables that are directly diagnostic of RV performance and reflective of consequences of RV dysfunction. As we move forward into newer and more advanced continuous flow VADs, new risk scores should ultimately be created and validated against national data. Until that time, we present a simple, easy to remember risk stratification tool that can guide clinicians and heart failure teams in the surgical management of end-stage heart failure in an era of continuous flow LVADs.
Footnotes
Presented at the Fifty-ninth Annual Meeting of the Southern Thoracic Surgical Association, Naples, FL, Nov 7–10, 2012.
Dr Acker discloses a financial relationship with Thoratec.
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 5.Atluri P, Goldstone AB, Kobrin DM, et al. Ventricular assist device implant in the elderly is associated with increased, but respectable risk. Ann Thor Surg. 2013 May 31; doi: 10.1016/j.athoracsur.2013.04.010. doi:10.1016/j.athoracsur.2013.04.010. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaczorowski Dj, Woo Yj. Who needs an RVAD in addition to an LVAD. Cardiology Clinics. 2011;29:599–605. doi: 10.1016/j.ccl.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Kormos RL, et al. The fourth INTER-MACS annual report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–26. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick JR, Frederick JR, Hiesinger W, et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg. 2009;137:971–7. doi: 10.1016/j.jtcvs.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan JA, John R, Lee BJ, Oz MC, Naka Y. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg. 2004;77:859–63. doi: 10.1016/j.athoracsur.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 10.Deng MC, Edwards LB, Hertz MI, et al. Mechanical circula-tory support device database of the International Society for Heart and Lung Transplantation: third annual report—2005. J Heart Lung Transplant. 2005;24:1182–7. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick JR, 3rd, Frederick JR, Hsu VM, et al. Risk score derived from preoperative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008;27:1286–92. doi: 10.1016/j.healun.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a preoperative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–72. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105:1030–5. doi: 10.1016/j.amjcard.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 15.Etz CD, Welp HA, Tjan TD, et al. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg. 2007;83:1697–705. doi: 10.1016/j.athoracsur.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Pauwaa S, Bhat G, Tatooles AJ, et al. How effective are continuous flow left ventricular assist devices in lowering high pulmonary artery pressures in heart transplant candidates? Cardiol J. 2012;19:153–8. doi: 10.5603/cj.2012.0027. [DOI] [PubMed] [Google Scholar]
- 17.Elhenawy AM, Algarni KD, Rodger M, et al. Mechanical circulatory support as a bridge to transplant candidacy. J Card Surg. 2011;26:542–7. doi: 10.1111/j.1540-8191.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- 18.Torre-Amione G, Southard RE, Loebe MM, et al. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. J Heart Lung Transplant. 2010;29:195–200. doi: 10.1016/j.healun.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Kamdar F, Madlon-Kay R, et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J Heart Lung Transplant. 2010;29:209–15. doi: 10.1016/j.healun.2009.11.599. [DOI] [PubMed] [Google Scholar]
- 20.Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–24. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Santambrogio L, Bianchi T, Fuardo M, et al. Right ventricular failure after left ventricular assist device insertion: preoperative risk factors. Interact Cardiovasc Thorac Surg. 2006;5:379–82. doi: 10.1510/icvts.2006.128322. [DOI] [PubMed] [Google Scholar]
- 22.Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106:I198–202. [PubMed] [Google Scholar]
- 23.Fukamachi K, McCarthy PM, Smedira NG, Vargo RL, Starling RC, Young JB. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181–4. doi: 10.1016/s0003-4975(99)00753-5. [DOI] [PubMed] [Google Scholar]
- 24.Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Kavarana MN, Pessin-Minsley MS, Urtecho J, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. 2002;73:745–50. doi: 10.1016/s0003-4975(01)03406-3. [DOI] [PubMed] [Google Scholar]
- 26.Farrar DJ, Hill JD, Pennington DG, et al. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the Thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113:202–9. doi: 10.1016/S0022-5223(97)70416-1. [DOI] [PubMed] [Google Scholar]
- 27.Kormos RL, Gasior TA, Kawai A, et al. Transplant candidate's clinical status rather than right ventricular function defines need for univentricular versus biventricular support. J Thorac Cardiovasc Surg. 1996;111:773–83. doi: 10.1016/s0022-5223(96)70337-9. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani S, Thomas JD, Savage RM, Vargo RL, Smedira NG, McCarthy PM. Prediction of right ventricular dysfunction after left ventricular assist device implantation. Circulation. 1996;94:II216–21. [PubMed] [Google Scholar]