Abstract
Tob1 (transducer of ERBB2-1, TOB1 is humans) is a member of the antiproliferative (APRO) family of proteins that controls cell cycle progression in several cell types. In addition, Tob1 has been implicated in diverse cellular mechanisms such as embryonic dorsal development, and T helper 17 (Th17) cell function. More recently, evidence linking Tob1 function to experimental and human immune related disorders has mounted, thus underscoring the potential of this molecule as a biomarker and as a therapeutic target. This article reviews these functions with an emphasis on their implications for human autoimmune diseases such as multiple sclerosis.
Keywords: Biomarker, cell proliferation, multiple sclerosis, quiescence, T lymphocyte.
Tob1 (Transducer of Erb-2, 1) is a member of the Tob/BTG antiproliferative (APRO) family of proteins, together with TOB2, BTG1, PC3/TIS21/BTG2, ANA/BTG3 and PC3/PC3K (1, 2). Like other members of this family, Tob1 plays important roles in suppressing tumor development. The common N-terminal domain characterizes all members of the APRO family. This domain (composed of ~120 residues), includes a nuclear localization signal (NLS) and a nuclear export signal (NES) and is presumably responsible for the antiproliferative effect of these genes. The NLS and NES enable translocation between nucleus and cytoplasm at different stages of the cell cycle. Indeed, Tob1 concentration is higher in the cytoplasm during G1/S phase, and then is reduced to enable cell division (3).
Tob1 is expressed in most cell types and in several species. Transcripts of zebrafish tob1a (4) and tob1b (5) and Xenopus Tob2 (6) are ubiquitously distributed during blastula and gastrula stages of development, suggesting a role in early embryogenesis in these species. At later stages of embryonic development, the expression of Tob genes occurs in distinct domains, such as the notochord, hatching gland, blood islands, and gut, depending on the species. During segmentation, Tob genes are expressed in somites, which ultimately give rise to axial skeleton, skeletal muscle, and dermis (7), in amphioxus (Tob; (8), zebrafish (tob1b; (5)), Xenopus (xTob2; (6)), and mouse (Tob2; (9)). In adults, expression of Tob1 (10) and Tob2 (9) and human TOB1 (2) and TOB2 (11) has been demonstrated in skeletal muscle.
Some Tob genes are also expressed in the central nervous system (CNS). For example, Tob is expressed in the embryonic CNS of Drosophila (12), and amphioxus Tob is expressed in the nerve cord during the late neurula and larva stages (8). However, putative functions for Tob genes in neurogenesis remain unknown. Tob transcripts are also present in adult mouse (9, 10), rat (13), and human (2, 11) brain tissues. Specifically, Tob1 transcripts have been detected in the hippocampus, a region related to learning and memory. Long-term potentiation in the hippocampal CA1 region is thought to be a cellular model of memory formation (14, 15). When mice received intra-CA1 infusion of Tob antisense oligonucleotides, they performed poorly in the water maze, and exhibited a deficit in long-term contextual fear memory, leaving short-term memory intact (13)).
Tob1 has also been detected at relatively high levels in the cerebellum of rats. Tob1 expression is up-regulated in the cerebellum after animals received training on a rotarod-running task. Interestingly, rats infused with Tob1 antisense oligonucleotides into the 4th ventricle exhibited a severe deficit in running on a rotating rod or walking across a horizontally elevated beam (16).
Tob genes are negative regulators of transcription and translation
In T lymphocytes Tob1 associates with Smad2 and Smad4 and enhances Smad4 DNA binding and Smad-dependent transcription (17). In contrast, in osteoblasts, Tob associates with Smads and enhances Smad DNA binding but inhibits Smad-mediated transcription (18). These data link Tob1 to the TGFβ family mediated signaling and regulation of transcription, which has a role in morphogenesis, as well as in cell survival, proliferation and differentiation
In zebrafish Tob1 acts upstream of B-catenin and competes with Lef/Tcf cofactors for binding to B-catenin, effectively blocking formation of a Lef1/Tcf/B-catenin protein complex that can stimulate the transcription of several genes. Additionally, Tob1a inhibits Smad3-induced embryonic dorsalization by physically interacting with and preventing Smad3 from binding to one of its cofactors, p300 (4).
In mammalian cells, mRNA decay begins with deadenylation. Regulation of the critical deadenylation step occurs at the RNA processing bodies (P-bodies), a site where poly (A)-shortened mRNA gets degraded. (19) TOB1 can simultaneously interact with the poly(A) nuclease complex CCR4-CAF1 (via its N-terminal domain) and the cytoplasmic poly(A)-binding protein PABPC1 and induced PABC (iPABP) (via its C-terminal domain), thus effectively enhancing mRNA decay and blocking translation of the target gene (20).
Tob1 in T cells
Tob1 was found to be constitutively expressed in unstimulated peripheral blood T lymphocytes but strongly down-regulated after both antigen-specific and unspecific stimulation. Indeed, down-regulation of Tob1 was required for T cell activation and expansion (17). When expressed, Tob1 inhibits T cell proliferation presumably by suppressing transcription of IL2, IL4 and IFNg, and other positive regulators of the cell cycle such as cyclin E and cyclin A. As these cyclins directly interact with CAF1, it is possible that Tob1 regulates their expression through its interaction with CAF1-CCR4 complex (11, 21).
TGFβ inhibits T cell proliferation through a SMAD-dependent mechanism. TGFβ signal transduction is initiated by receptor phosphorylation of transcription factors Smad2 and Smad3. Then, phosphorylated Smad2 and Smad3 bind to Smad4 in the cytoplasm and translocate the nucleus where they exert their effect on transcription. Tob1 has been shown to interact with Smad2 and to enhance the ability of Smad4 to bind DNA, therefore increasing Smad-dependent gene transcription (17). However, since the IL-2 gene contains Smad-binding negative regulatory elements that block transcription when activated, the net effect of Tob1 in T cells is inhibition of IL-2 transcription and consequent blocking of cell division.
In addition to direct inhibition of IL-2 transcription, Tob1 also promotes transcription of the p27kip1 Cdk inhibitor (CDKN1B), which also blocks cell cycle progression (17).
Lymphocyte quiescence has been shown to be dependent on the enzymatic activity of dipeptidyl peptidase 2 (DPP2), a serine protease with an amino terminal dipeptidase activity (22). Indeed, inhibition of DPP2 in resting T cells results in apoptosis (23). Interestingly, the Dpp2 promoter contains a SMAD binding site, raising the possibility that SMADs are also involved in its transcriptional activation. Confirming this hypothesis, a recent article described the molecular mechanism by which TOB1 expression increases the Dpp2 promoter activity, effectively positioning Tob1 upstream of Dpp2 in the regulation of T cell quiescence (24).
In addition to its effect on IL-2 transcription (negative) and Dpp2 (positive), Tob1 has been recently shown to suppress the expression of twisted gastrulation (Tsg), a secrete d protein that interacts with Drosophila decapentaplegic (Dpp) and its vertebrate orthologs BMP2/4 and regulates morphogenetic effects in embryos. TSG mRNA was found to be expressed at very low levels in unstimulated T cells but highly up-regulated after activation by TCR/CD3 and either CD28, IL-2, or PMA (25). Tsg inhibits proliferation of pre-activated T cells by enhancing TGF-β signaling through phosphorylation of Smad2 and facilitation of DNA binding of Smad3/4.
A recent report described a higher expression of Tob1 in the IL-17 producing pro-inflammatory CD4 T helper (Th17) population, compared to its expression in IFN-g producing Th1 cells (26). A significant positive correlation between IL-4 induced gene 1 (IL4I1) and Tob1 mRNA expression in human Th17 cells was found. These data suggest that IL4I1 upregulation in human Th17 cells limits their TCR-mediated expansion not only by blocking the molecular pathway involved in the activation of the IL-2 promoter, but also by maintaining high levels of Tob1, which impairs entry into the cell cycle
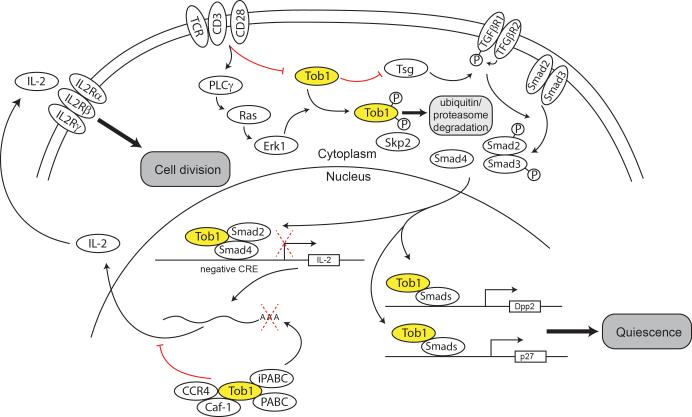
This article also shed some light on the possible mechanism of Tob1 degradation by identifying its interaction with Skp2, an inhibitor of the cyclin-dependent kinase inhibitor CDKN1B. Upon binding Tob1, Skp2 promotes its degradation via the ubiquitin-proteasome pathway (27). (Figure 1 summarizes the known processes of Tob1 in T cells)
Figure. Tob1 in T cells.
Tob1 gets downregulated following TCR signaling. In the nucleus Tob1 enhances binding of Smad4 to the negative cis regulatory element (CRE) of the IL-2 gene, thus negatively affecting IL2 expression. Concomitantly, the stability of IL2 mRNA is also regulated by the interaction of Tob1 and PABC and iPABC, a complex that favors deadenylation and subsequent mRNA decay. Through its interaction with CCR4 and Caf-1, Tob1 also inhibits IL2 mRNA translation. These coordinated actions by Tob1 result in quick IL2 downregulation and inhibition of cell division. In the cytoplasm, Tob1 also inhibits Tsg, which in turn, stimulates TGFb-mediated control of cell activation. TGFb acts through its heterodimeric receptor and phosphorylate Smad2 and Smad3, which when complexed with SMad4 translocate to the nucleus. Once there, Tob1 enhances Smad-mediated transcriptional activation of Dpp2 and p27, promoting T cell quiescence.
Tob1 in human and experimental autoimmune demyelination
Using a genome-wide transcriptome analysis, we recently reported downregulation of TOB1 was associated with higher risk of disease activity in patients with multiple sclerosis (MS), an autoimmune disease of the CNS (28). While more than 900 transcripts were differentially expressed in CD4+ T cells from subjects with early symptoms of MS and healthy controls, 108 genes were expressed at variable levels even within patients. This molecular signature obtained shortly after diagnosis defined 4 groups of patients, one of which was significantly associated with higher risk of disease activity in the subsequent 3 years of follow-up. The high-risk signature at baseline was characterized by 7-fold down-regulation of TOB1 (the largest differential expression of any transcript), down-regulation of the pro-apoptotic genes BRD7, PMAIP, SAT and CYCS and the cell cycle inhibitor CDKN1C, and up-regulation of the cell cycle inducer CDC34. This profile suggested that a significant fraction of CD4+ T cells from subjects at high risk of future disease activity were poised to enter a proliferative state, triggering concomitant disease flares. These results suggested TOB1 could be a valuable biomarker in MS, thus relating this gene with a human disease for the first time.
In a follow-up study, we later demonstrated that Tob1-deficient (Tob1−/−) mice experienced an earlier disease onset and a more aggressive disease when immunized with myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) to induce experimental autoimmune encephalomyelitis (EAE), a murine model of MS (29). These symptoms resembled the earlier results obtained in humans. T cells from Tob1−/− mice proliferated more than T cells from wild type (WT) mice in response to both antigen-specific and unspecific stimulation. Furthermore, when WT and Tob1−/− CD4+ T cells were stimulated under polarizing conditions, higher proportions of Th1 and Th17 were induced from Tob1−/− cells. Concomitantly, a lower proportion of CD4+ CD25+ FoxP3+ T regulatory (Treg) cells were seen in Tob1−/− mice. Taken together, these results indicate that absence of Tob1 results in a shift of immune responses towards a proinflammatory state.
In order to investigate whether Tob1 expression in T cells was sufficient to explain the symptoms, we reconstituted the CD4+ T cell compartment of Rag1 knockout mice (Rag−/−) with CD4+ T cells from either Tob1−/− or WT mice and induced EAE in these animals. Transfer of Tob1−/− CD4+ T cells was sufficient to reproduce the EAE symptoms observed with the conventional Tob1−/− mice, thus highlighting the profound effect of Tob1 on the ability of these cells to proliferate and eventually, to cause damage to the host. Interestingly, when Tob1−/− were crossed to a transgenic mouse line (2D2) in which all T cell receptors (TCR) recognize MOG35-55 at least 50% of the resulting offspring develop spontaneous EAE within 120 days. This phenotype can be explained by hyper-proliferation (induced by the absence of Tob1) of encephalitogenic T cells (from the 2D2), which eventually cross the blood brain barrier and get access to the CNS where they cause inflammation and demyelination associated with the observed symptoms.
Summary and conclusions
Several recent publications have significantly advanced our understanding of how the BTG/Tob protein family exerts controls over the cell cycle in health and disease. Elucidating the mechanism by which Tob1 influences quiescence by controlling mRNA degradation and translation has provided conclusive evidence one how this APRO molecule exerts if cellular effects. On the other hand, still little is known about upstream regulators of Tob1 and no research has been reported on whether Tob1 could be targeted pharmacologically. Given the recent implications of Tob1 in MS and potentially other autoimmune diseases, this seems a goal worth pursuing.
Acknowledgements
This study was supported by a grant from the National Institutes of Health (R01NS26799) and National Multiple Sclerosis Society (NMSS) grant PP1959. SEB is a Harry Weaver Neuroscience fellow from the NMSS.
Footnotes
Disclosure
No conflict of interest declared.
References
- 1.Jia S, Meng A. Tob genes in development and homeostasis. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236(4):913–21. doi: 10.1002/dvdy.21092. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda S, Kawamura-Tsuzuku J, Ohsugi M, Yoshida M, Emi M, Nakamura Y, et al. Tob, a novel protein that interacts with p185erbB2, is associated with anti-proliferative activity. Oncogene. 1996;12(4):705–13. [PubMed] [Google Scholar]
- 3.Kawamura-Tsuzuku J, Suzuki T, Yoshida Y, Yamamoto T. Nuclear localization of Tob is important for regulation of its antiproliferative activity. Oncogene. 2004;23(39):6630–8. doi: 10.1038/sj.onc.1207890. [DOI] [PubMed] [Google Scholar]
- 4.Xiong B, Rui Y, Zhang M, Shi K, Jia S, Tian T, et al. Tob1 controls dorsal development of zebrafish embryos by antagonizing maternal beta-catenin transcriptional activity. Dev Cell. 2006;11(2):225–38. doi: 10.1016/j.devcel.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Shi K, Zhang L, Meng A. Cloning and expression analysis of zebrafish tob1 gene. Dev Genes Evol. 2004;214(6):309–11. doi: 10.1007/s00427-004-0405-5. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, von Bubnoff A, Ikematsu N, Blitz IL, Tsuzuku JK, Yoshida EH, et al. Tob proteins enhance inhibitory Smad-receptor interactions to repress BMP signaling. Mechanisms of development. 2003;120(5):629–37. doi: 10.1016/s0925-4773(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 7.Pourquie O. Vertebrate somitogenesis. Annu Rev Cell Dev Biol. 2001;17:311–50. doi: 10.1146/annurev.cellbio.17.1.311. [DOI] [PubMed] [Google Scholar]
- 8.Holland ND, Zhang SC, Clark M, Panopoulou G, Lehrach H, Holland LZ. Sequence and developmental expression of AmphiTob, an amphioxus homolog of vertebrate Tob in the PC3/BTG1/Tob family of tumor suppressor genes. Developmental dynamics : an official publication of the American Association of Anatomists. 1997;210(1):11–8. doi: 10.1002/(SICI)1097-0177(199709)210:1<11::AID-AJA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Ajima R, Ikematsu N, Ohsugi M, Yoshida Y, Yamamoto T. Cloning and characterization of the mouse tob2 gene. Gene. 2000;253(2):215–20. doi: 10.1016/s0378-1119(00)00270-5. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida Y, Matsuda S, Yamamoto T. Cloning and characterization of the mouse tob gene. Gene. 1997;191(1):109–13. doi: 10.1016/s0378-1119(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 11.Ikematsu N, Yoshida Y, Kawamura-Tsuzuku J, Ohsugi M, Onda M, Hirai M, et al. Tob2, a novel anti-proliferative Tob/BTG1 family member, associates with a component of the CCR4 transcriptional regulatory complex capable of binding cyclin-dependent kinases. Oncogene. 1999;18(52):7432–41. doi: 10.1038/sj.onc.1203193. [DOI] [PubMed] [Google Scholar]
- 12.Bourbon HM, Gonzy-Treboul G, Peronnet F, Alin MF, Ardourel C, Benassayag C, et al. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mechanisms of development. 2002;110(1-2):71–83. doi: 10.1016/s0925-4773(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 13.Jin M, Wang XM, Tu Y, Zhang XH, Gao X, Guo N, et al. The negative cell cycle regulator, Tob (transducer of ErbB-2), is a multifunctional protein involved in hippocampus-dependent learning and memory. Neuroscience. 2005;131(3):647–59. doi: 10.1016/j.neuroscience.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 15.Morris RG, Willshaw DJ. Memory. Must what goes up come down? Nature. 1989;339(6221):175–6. doi: 10.1038/339175a0. [DOI] [PubMed] [Google Scholar]
- 16.Wang XM, Gao X, Zhang XH, Tu YY, Jin ML, Zhao GP, et al. The negative cell cycle regulator, Tob (transducer of ErbB-2), is involved in motor skill learning. Biochemical and biophysical research communications. 2006;340(4):1023–7. doi: 10.1016/j.bbrc.2005.12.125. [DOI] [PubMed] [Google Scholar]
- 17.Tzachanis D, Freeman GJ, Hirano N, van Puijenbroek AA, Delfs MW, Berezovskaya A, et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nature immunology. 2001;2(12):1174–82. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida Y, Tanaka S, Umemori H, Minowa O, Usui M, Ikematsu N, et al. Negative regulation of BMP/Smad signaling by Tob in osteoblasts. Cell. 2000;103(7):1085–97. doi: 10.1016/s0092-8674(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 19.Ezzeddine N, Chang TC, Zhu W, Yamashita A, Chen CY, Zhong Z, et al. Human TOB, an antiproliferative transcription factor, is a poly(A)-binding protein-dependent positive regulator of cytoplasmic mRNA deadenylation. Molecular and cellular biology. 2007;27(22):7791–801. doi: 10.1128/MCB.01254-07. PMCID: 2169145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okochi K, Suzuki T, Inoue J, Matsuda S, Yamamoto T. Interaction of anti-proliferative protein Tob with poly(A)-binding protein and inducible poly(A)-binding protein: implication of Tob in translational control. Genes Cells. 2005;10(2):151–63. doi: 10.1111/j.1365-2443.2005.00826.x. [DOI] [PubMed] [Google Scholar]
- 21.Bogdan JA, Adams-Burton C, Pedicord DL, Sukovich DA, Benfield PA, Corjay MH, et al. Human carbon catabolite repressor protein (CCR4)-associative factor 1: cloning, expression and characterization of its interaction with the B-cell translocation protein BTG1. The Biochemical journal. 1998;336(Pt 2):471–81. doi: 10.1042/bj3360471. PMCID: 1219893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood R, Chiravuri M, Lee H, Schmitz T, Kabcenell AK, Yardley K, et al. Sequence, purification, and cloning of an intracellular serine protease, quiescent cell proline dipeptidase. The Journal of biological chemistry. 1999;274(48):34053–8. doi: 10.1074/jbc.274.48.34053. [DOI] [PubMed] [Google Scholar]
- 23.Chiravuri M, Schmitz T, Yardley K, Underwood R, Dayal Y, Huber BT. A novel apoptotic pathway in quiescent lymphocytes identified by inhibition of a post-proline cleaving aminodipeptidase: a candidate target protease, quiescent cell proline dipeptidase. Journal of immunology. 1999;163(6):3092–9. [PubMed] [Google Scholar]
- 24.Bista P, Mele DA, Baez DV, Huber BT. Lymphocyte quiescence factor Dpp2 is transcriptionally activated by KLF2 and TOB1. Mol Immunol. 2008;45(13):3618–23. doi: 10.1016/j.molimm.2008.05.001. PMCID: 2597389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzachanis D, Li L, Lafuente EM, Berezovskaya A, Freeman GJ, Boussiotis VA. Twisted gastrulation (Tsg) is regulated by Tob and enhances TGF-beta signaling in activated T lymphocytes. Blood. 2007;109(7):2944–52. doi: 10.1182/blood-2006-03-006510. PMCID: 1852213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santarlasci V, Maggi L, Mazzoni A, Capone M, Querci V, Rossi MC, et al. IL-4-induced gene 1 maintains high Tob1 expression that contributes to TCR unresponsiveness in human T helper 17 cells. Eur J Immunol. 2014;44(3):654–61. doi: 10.1002/eji.201344047. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, J KT, Ajima R, Nakamura T, Yoshida Y, Yamamoto T. Phosphorylation of three regulatory serines of Tob by Erk1 and Erk2 is required for Ras-mediated cell proliferation and transformation. Genes & development. 2002;16(11):1356–70. doi: 10.1101/gad.962802. PMCID: 186319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corvol JC, Pelletier D, Henry RG, Caillier SJ, Wang J, Pappas D, et al. Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proc Natl Acad Sci U S A. 2008;105(33):11839–44. doi: 10.1073/pnas.0805065105. PMCID: 2504481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulze-Topphoff U, Casazza S, Varrin-Doyer M, Pekarek K, Sobel RA, Hauser SL, et al. Tob1 plays a critical role in the activation of encephalitogenic T cells in CNS autoimmunity. The Journal of experimental medicine. 2013;210(7):1301–9. doi: 10.1084/jem.20121611. PMCID: 3698524. [DOI] [PMC free article] [PubMed] [Google Scholar]