Abstract
Objective
Older adults with chronic kidney disease have a high rate of uncontrolled hypertension. Home monitoring of blood pressure (BP) is an integral part of management, but requires that patients bring records to clinic visits. Telemonitoring interventions, however, have not targeted older, less technologically-skilled populations.
Methods
Veterans with stage 3 or greater chronic kidney disease and uncontrolled hypertension were randomized to a novel telemonitoring device pairing a Bluetooth-enabled BP cuff with an Internet-enabled hub, which wirelessly transmitted readings (n= 28), or usual care (n= 15). Home recordings were reviewed weekly and telemonitoring participants were contacted if BP was above goal. The prespecified primary endpoints were improved data exchange and device acceptability. Secondary endpoint was BP change.
Results
Forty-three participants (average age 68 years, 75% white) completed the 6-month study. Average start-of-study BP was 147/78mmHg. Those in the intervention arm had a median of 29 (IQR 22, 53) transmitted BP readings per month, with 78% continuing to use the device regularly, whereas only 20% of those in the usual care group brought readings to in-person visits. The median number of telephone contacts triggered by the wireless monitoring was 2 (IQR 1, 4) per patient. Both groups had a significant improvement in systolic BP (P< 0.05, for both changes); systolic BP fell a median of 13 mmHg in monitored participants compared with 8.5mmHg in usual care participants (P for comparison 0.31).
Conclusion
This low-cost wireless monitoring strategy led to greater sharing of data between patients and clinic and produced a trend toward improvements in BP control over usual care at 6 months.
Keywords: home blood pressure monitoring, older adults, wireless
Introduction
Chronic kidney disease (CKD) and hypertension (HTN) are growing public health problems in older adults; 25–40% of individuals above the age of 60 have evidence of CKD, and 65–95% of these individuals have HTN [1]. HTN control is well-recognized to be one of the most effective interventions in patients with CKD [2]. Randomized trials have shown that optimal control of HTN prevents progression to kidney failure, cardiovascular events, and death. In practice, however, control is achieved in only 37–44% of CKD patients, reflecting a persistent gap between recommendations and practice [3]. Reports from the National Health and Nutrition Survey suggest that only 37% of those with kidney disease have adequately controlled HTN [4]. Even within the rarefied population of CKD patients who volunteered for a large cohort study of kidney disease in which 99% were aware of and receiving ongoing treatment for their HTN and kidney disease, a mere 44% had actually achieved control [5].
Although self-monitoring of blood pressure (BP) can be helpful for both treatment decisions and evaluating response to treatment [6], in older adults with CKD there are barriers to this practice, particularly inability to maintain accurate logs of home BP, and subsequently bring them to clinic. Hand transmitted BP logs have been shown in the past to often be inaccurate, with substantial under-reporting of BP [7]. Thus, common practice in BP management in the typical veterans’ administration (VA) CKD clinic setting is medication adjustment based on the intermittent in-clinic readings performed by a physician or a clinical assistant. Multiple studies, however, have shown this to be inadequate, both because of the lag between visits and specifically because of the pathophysiology and pattern of HTN in CKD patients [8]. BP is notoriously variable outside the clinic setting in CKD patients [9]. Both white-coat HTN and masked HTN are frequent [10] and are associated with adverse cardiovascular outcomes. CKD patients also have vascular calcification and episodes of relative hypotension [11], a major risk factor for falls and fractures. Complicating this situation further, CKD patients have high rates of frailty [12], cognitive impairment [13], and polypharmacy [14], making BP even more difficult to manage.
Efforts to improve BP management have included home BP monitoring programs, behavioral coaching, or reminder systems to improve adherence [15]. However, these efforts have largely been suboptimal due to its reliance on a high level of patient adherence with manually recording BP data and communicating data through telephone, fax, Internet, or hand-carried BP logs. There is evidence in the general hypertensive population that telemonitoring interventions can be effective [16–18], but the most effective of such interventions require Internet access and computer literacy as well as involvement of a pharmacist. Thus, in current practice, home BP readings are often not recorded, forgotten, or out-of-date by the next clinic visit.
To address this clinical problem in the management of HTN in older adults with CKD, we created a novel home-based, Bluetooth-enabled BP monitoring device and intervention protocol requiring minimal effort or technological skill from the participant. The feasibility and acceptability of this intervention protocol was tested in a randomized study in 43 older veterans with HTN and CKD. It was hypothesized that patients equipped with the novel home-based BP device would have improved processes of care (sharing of home data with physicians and interval medication titration by pharmacists and physicians) and better BP control over the course of the intervention compared with usual care.
Methods
Participants
Established patients attending the CKD/HTN clinic at VA San Diego were eligible to enroll in the study. Inclusion criteria were stage 3 CKD, as defined by an estimated glomerular filtration rate of less than 60 ml/ min/1.73 m2; established HTN [systolic blood pressure (SBP)>140 or diastolic blood pressure (DBP)>90 in-clinic or on reported home readings]; and age more than 50 years. In addition, participants had to be community-dwelling and currently self-managing their medications. Exclusion criteria were the presence of a clear secondary cause for HTN (e.g. aldosterone producing tumor), or estimation by clinic physicians that the individual was within 6 months of requiring dialysis or of dying from other causes. We screened the charts of patients attending the CKD/HTN clinic; potential participants were given study information at the time of their routine clinic visits, and were able to contact a study coordinator at the clinic if interested. Because the Bluetooth-enabled BP monitoring device and intervention protocol required minimal effort or technological skill from the participant and used a plug and play cellular technology, we did not exclude patients based on computer skills, literacy, or Internet access.
Design
This was a randomized, controlled device effectiveness study. Participants were randomized in a 2:1 manner (intervention:control), so as to maximize the number of participants receiving the device of interest over a short enrollment period. Random assignment occurred after the consent and initial enrollment interview, using opaque envelopes containing odd (intervention) or even (control) study numbers. Individuals randomized to the telemonitoring arm were provided with the equipment after randomization. Participants were followed for at least 6 months before the exit interview.
Participant-based data collection
All participants completed baseline in-person interviews to assess their comorbidities, self-rated current health, duration of HTN and CKD, and medication adherence [as measured by the eight-item Morisky Medication Adherence Scale (MMAS)] [19]. Laboratory records were reviewed to determine estimated glomerular filtration rate at baseline and at study close. Data were collected on baseline medication use from participants and from VA chart review. Start-of-study BP was taken from the clinic visit at which the participant had enrolled.
Using the VA’s electronic health record (CPRS VISTA), the number of clinic visits, emergency room visits, and hospitalizations at VASDHS were tracked for safety reasons and to monitor for medication changes outside of our protocol.
At the end of the study, BP was checked for each participant at a clinic visit or by the study coordinator. Questionnaires regarding medication use and MMAS were repeated at the study close along with questions regarding the acceptability of the device itself for those in the intervention arm. Questionnaires from the treating physicians in the HTN/CKD clinics were collected to gauge their opinions on the impact of the intervention.
Study device
The intervention device consisted of two integrated subunits: the A&D Medical UA-767PBT fully automatic oscillometric BP unit [20] (A&D Medical, San Jose, California, USA) and the home health hub (HHH). The BP units have a BP measuring range spread over 20–280mmHg and a pulse range of 40–200 beats/min. The HHH is a 1′ × 4′ × 6′-wall unit, which the participant was instructed to plug into any available outlet and leave there for the duration of the study. It receives BP and pulse data through Bluetooth from the BP unit, and relays that data through the Internet (using study-provided cellular modem) to a secure website, which was accessible through password to study personnel. The study device used its own cellular modem connection to transmit data. The website allowed for viewing of BP data sorted by participant, using unique study ID numbers. Also, each device sent a regular signal through the HHH even if BP was not being checked, so that study personnel could differentiate equipment failure from an absence of BP readings without additional calls to the participant. Patients were educated about appropriate use of the cuff, and those assigned to the device arm had BP checked before study initiation using a standard cuff as well as the device to ensure accuracy.
Remote monitoring and usual care protocols
To best approximate usual clinic practice, patients enrolled in both arms of the study were asked to measure and record their BP at home according to their physicians’ instructions; no study-specific instructions were given regarding the frequency of measurement so that the focus of study was on the new device and interactions. Participants in the intervention arm were instructed in proper use of the BP cuff. They were told that BP readings would be checked by study personnel on a weekly basis, and that they could expect to be contacted by telephone if their BP was out of range. They were further counseled that they would not be contacted if BP was at goal. Finally, they were told that although the device transmitted on a continuous basis, it would not be checked continuously, and was not a substitute for contacting medical personnel in case of severe hypertension or hypotension or other symptoms. Participants in the control arm were told to use their own home BP cuff as recommended by their physician and were told that study personnel would be checking in with them at the end of 6 months for an end-of-study visit related to BP.
On a weekly basis, the study physicians (D.E.R., C.M.M., J.A.A.) and pharmacist (C.L.) met to review BP logs of each participant (~30 min/week). If a participant had consistently above-goal readings during the prior week, one of the study physicians or pharmacist called to discuss the readings, provide counseling, or adjust medications as indicated. Each telephone encounter was recorded in the medical chart. Additional in-person follow-up with clinic physicians or urgent care was scheduled at the discretion of the study team if it was felt that telephone counseling was not sufficient. If participants in the intervention group did not have any readings during a given time period, the study coordinator checked to ensure that the device was still transmitting a check-in signal; if so, no additional contact with the participant was made so as not to change the participant’s usual behavior.
For participants in the intervention group, prior to scheduled in-person follow-up, the electronic medical record was updated by means of an addendum to the prior CKD clinic note with the full record of telemonitoring results since the prior visit, to allow clinic physicians to review these at the time of in-person visits. For all participants, we asked treating physicians to fill out a postvisit form for each in-person visit detailing the amount of home BP data brought to that visit by the participant, the number of medication changes at the visit, and whether BP was at goal.
Telemonitoring data collection
The number of BP readings transmitted by the system for each participant was totaled on a monthly basis, and monthly running averages created for each participant for SBP, DBP, and mean arterial pressure for the duration of the study.
The number of telephone-based counseling and medication interventions for each participant was totaled for all intervention participants. For the control arm, we reviewed any telephone-based or clinic-based HTN interaction for relevant changes.
Sample size
We planned to enroll 30 individuals in the intervention and 15 in the control arms in the study. Our primary prespecified aim was to detect a difference in the sum total of the number of BP readings from outside the clinic to assess the quantity of BP data exchanged. We calculated that we had more than 90% power at an α of 5% to detect a clinically significant average difference of four readings per month, assuming an average of two readings per month shared in the control group and six readings per month shared in the intervention group.
We also did an a priori power calculation for the secondary endpoint of difference in BP between groups. We calculated that we had ~60% power at α of 5% to detect a 10-point difference in SBP between groups, assuming a SD of 15 mmHg.
Statistical analysis
Baseline characteristics of study participants in the intervention and control groups were compared using χ2 and t-tests, as appropriate.
Between-group differences in in-clinic SBP and DBP at study start and end were compared using t-tests for each BP component. Within each group, paired t-tests were used to evaluate change in BP over the study period; between-group differences in change in BP were also compared using t-tests. Changes in the MMAS were compared as well using t-tests.
For those participants in the intervention arm, the telemonitoring BP values were plotted over the course of the study for each participant and overall slopes of change were calculated for each participant over the course of the study by fitting a least-squares regression line to the available data. For monthly averages in the intervention arm, we also tested for a trend in change in BP using least-squares linear regression and tested whether the β value for slope was different from 0.
The study was approved by the University of California, San Diego Institutional Review Board and by the VA Research and Development Committee. As this trial was designed as a single-site feasibility study of a new strategy for telemonitoring, registration at the clinicaltrials.gov was not required. A P value of 0.05 was considered significant. All statistical analyses were carried out using SAS (version 9.0; SAS Institute Inc., Cary, North Carolina, USA).
Results
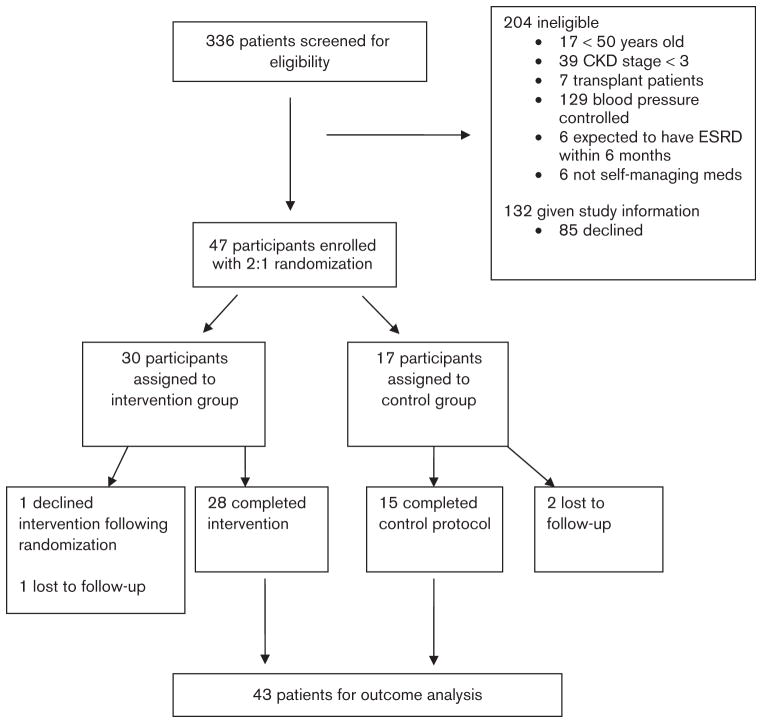
We screened 336 patients who had CKD/HTN visits at the VASDHS during the study enrollment period (Fig. 1). Forty-seven participants enrolled in the study; two participants who completed the entrance interview and were assigned to the control group were unable to be contacted again, and two participants who completed the interviews and enrolled in the intervention group never activated the cuffs and could not be contacted again. The remaining 43 participants are presented in this analysis. The average age was 68 years and 26% were black; all but two were male, reflecting the sex distribution in our VA clinic. There was a high burden of comorbid disease (Table 1) with more than half having diabetes and 44% having sleep apnea in addition to CKD and HTN. Baseline characteristics and average starting BP were similar between the randomization groups.
Fig. 1.
CONSORT figure summarizing flow of the trial.
Table 1.
Baseline characteristics among telemonitoring and usual care groups
| Characteristics | Telemonitoring (n = 28) | Usual care (n = 15) | P value |
|---|---|---|---|
| Age (years) | 68.5±7.5 | 67.9±8.4 | 0.61 |
| Male (%) | 93 | 100 | 0.28 |
| African–American (%) | 25 | 27 | 0.61 |
| Serum creatinine (mg/dl) | 2.13±0.66 | 1.96±0.53 | 0.42 |
| eGFR at start (mg/dl) | 37.3±14.2 | 39.4±10.6 | 0.64 |
| Education (%) | |||
| High school graduate | 100 | 87 | 0.05 |
| College graduate | 28 | 13.3 | 0.24 |
| Reported that cost of medications a problem (%) | 14 | 6.7 | 0.44 |
| BMI (kg/m2) | 30.1 (26.5–38) | 29.2 (25–39.3) | 0.79 |
| Diabetes (%) | 57 | 53.3 | 0.94 |
| Obstructive sleep apnea (%) | 46.4 | 40 | 0.63 |
| Congestive heart failure (%) | 10.7 | 6.7 | 0.64 |
| Coronary artery disease (%) | 35.7 | 13.3 | 0.11 |
| History of stroke (%) | 13.8 | 13.3 | 0.9 |
| Hyperlipidemia (%) | 82 | 66.7 | 0.43 |
| Smoking – any history (%) | 57 | 73 | 0.19 |
| Prostate disease (%) | 35.7 | 46.7 | 0.54 |
| COPD (%) | 0 | 20 | 0.02 |
| Number of blood pressure medications | 3.9±1.4 | 3.9±1.4 | 0.92 |
| Total number of medications at start | 11.2±4.1 | 11.3±4.6 | 0.91 |
| Total number of pills per day | 14.1±6.5 | 14.6±5.6 | 0.93 |
| Systolic blood pressure (mmHg) | 149±16.2 | 147±8.6 | 0.87 |
| Diastolic blood pressure (mmHg) | 78±12.4 | 81±11.2 | 0.35 |
| α-Blocker (%) | 14.2 | 27 | 0.35 |
| β-Blocker (%) | 71 | 73 | 0.67 |
| Diuretic (%) | 82 | 73 | 0.53 |
| ACE inhibitor (%) | 46 | 47 | 0.91 |
| ARB (%) | 28.5 | 13.3 | 0.24 |
| Calcium channel blocker (%) | 68 | 67 | 0.99 |
| Clonidine (%) | 17.9 | 33.3 | 0.13 |
| Hydralazine (%) | 25 | 0 | 0.03 |
| Minoxidil (%) | 3.6 | 6.7 | 0.66 |
| Other | 7.1 | 0 | 0.28 |
Data are presented as mean±SD, median and IQR, or percentages.
ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Over the course of the study, participants in the intervention arm shared a median of 29 (IQR 22, 59) readings per month (Table 2), compared with minimal sharing in the control group; only three of 15 (20%) participants of the control group ever brought a BP log to their follow-up visits, with a reported number of readings varying from two to 21 per week. No spontaneous between-visit communications with the clinic were noted. Participants in the intervention arm continued to share data throughout the course of the study with 78% of participants continuing to use the cuff at least four times monthly throughout the 6 months (Table 2) with minimal decline in the number of readings per participant per month. The number of intervention contacts was, as expected, highest in the initial months of the study and decreased thereafter. There was a trend towards improved monthly mean SBP among the intervention group (P for monthly trend<0.01).
Table 2.
Monthly results of blood pressure monitoring
| Month
|
|||||||
|---|---|---|---|---|---|---|---|
| 0 (clinic) | 1 (n = 28) | 2 (n = 28) | 3 (n = 28) | 4 (n = 28) | 5 (n = 28) | 6 (n = 22) | |
| Median number of readings | – | 36.5 (26.5–56.5) | 35.5 (23.5–59) | 30 (19.5–50) | 31 (13–51.5) | 35 (23.5–77) | 14 (4–28) |
| Percentage of patients with at least 4 readings | – | 97 | 100 | 100 | 93 | 86 | 78 |
| Systolic blood pressure (mmHg) | 147±17.5 | 139.2±12.2 | 137.4±11.7 | 137.4±11.3 | 137±10.3 | 139.4±10.6 | 136±13.9 |
| Diastolic blood pressure (mmHg) | 77±12.3 | 74.3±9.6 | 74±9.7 | 72.5±9.9 | 72.4±10.8 | 73.3±10.7 | 71.6±12.2 |
| Mean arterial pressure (mmHg) | 100±11.3 | 95.7±7.9 | 94.9±7.6 | 93.9±7.9 | 93.6±8.3 | 95.1±8.4 | 92.8±10.4 |
| Number of patients receiving telephone interventions | – | 13 | 18 | 12 | 4 | 8 | 3 |
| Total number of medication changes | – | 9 | 14 | 15 | 5 | 7 | 3 |
| Number of counseling calls | – | 10 | 6 | 3 | 1 | 1 | 2 |
Data are presented as number, mean±SD, median and IQR, or percentages.
The intervention participants received an average of 2.7 contacts in total over the 6-month study, with 1.9 medication changes per participant. Typical transmitted data and study events are shown for a representative patient (Fig. 1). Considering only home-based readings, the median decrease in home SBP over the course of the study was 2.5mmHg (IQR 15.2, − 3.5) with a median decrease in home DBP of 3.5 (IQR 8.2, 0).
Of note, the average number of antihypertensive medications was not different between study groups, despite the trend toward better control in the intervention group (Table 3). There were no significant differences in renal function between groups. There were no hospitalizations or emergency room visits attributable to changes instituted because of this study. No additional in-person visits were scheduled because of telemonitoring results. We detected hypotensive episodes in two participants in the study and contacted them because of these episodes. In one instance, a patient had been suffering a gastrointestinal illness, and recorded SBPs in the 70–80 range. Another case involved a participant who had been bitten by a spider, with subsequent SBPs under 90. These participants were advised to hold antihypertensive agents and seek urgent care and both recovered from these illnesses.
Table 3.
Comparison of telemonitoring and usual care groups at start and conclusion
| Telemonitoring | Usual care | P for two-group comparison | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | |||
| Start of study | 147±17.5 | 146±8.8 | 0.87 |
| End of study | 136±15.6 | 140±14.4 | 0.48 |
| Change | − 13 (0, − 31); P = 0.009 vs. start | − 8.5 (0, − 18); P = 0.031 vs. start | 0.32 |
| Diastolic blood pressure (mmHg) | |||
| Start of study | 77±12.3 | 81±10.9 | 0.35 |
| End of study | 73±10.3 | 73±12.6 | 0.93 |
| Change | − 3.5 (− 3, 12.5); P = 0.02 vs. start | − 8 (3, 13); P = 0.0079 vs. start | 0.35 |
| Mean arterial pressure (mmHg) | |||
| Start of study | 101±11 | 102.9±9.6 | 0.46 |
| End of study | 93.9±8.6 | 95.2±11.7 | 0.67 |
| Change | −7.13 (− 16.5, 0.05) | −7.67 (− 12.3, − 1.7) | 0.87 |
| Creatinine (mg/dl) | |||
| Start of study | 2.13±0.66 | 1.96±0.53 | 0.42 |
| End of study | 2.17±0.76 | 2.32±0.84 | 0.64 |
| Change | 0.04 (− 0.09, 0.34) | 0.28 (0, 0.34) | 0.12 |
| eGFR (ml/min/1.73 m2) | |||
| Start of study | 37.3±14.2 | 39.4±10.6 | 0.64 |
| End of study | 37.9±16.7 | 34.5±13.2 | 0.51 |
| Change | 0.6 (− 3.4, 1.8) | − 3.69 (− 6.2, 0) | 0.14 |
| Total number of medications | |||
| Start of study | 11.2±4.1 | 11.1±5.6 | 0.91 |
| End of study | 12±4.6 | 12.8±5.1 | 0.62 |
| Change | 0.96 (0, 2) | 1.53 (0, 3) | 0.33 |
| Number of blood pressure medications | |||
| Start of study | 3.9±1.4 | 3.9±1.4 | 0.92 |
| End of study | 4±1.2 | 3.9±1.3 | 0.61 |
| Change | 0.89 (− 1, 4) | 1 (0, 2) | 0.91 |
| Total number of interventions | 4.6±2.1 | 1.7±0.97 | < 0.01 |
| Morisky Medication Adherence Scale | |||
| Start of study | 6.9±1.3 | 6.4±1.7 | 0.35 |
| End of study | 7±1.2 | 7.2±1.4 | 0.58 |
| Change | 0.11 (0, 0.5) | 0.67 (0, 2) | 0.17 |
Data are presented as means±SD, median and IQR, or percentages.
Morisky Medication Adherence Scale, 8 represents perfect adherence, 6–7 represent medium adherence, 5 and below represent poor adherence.
eGFR, estimated glomerular filtration rate.
Overall, satisfaction with the device was high among participants with 96% reporting that they would continue using the device (Box 1). Similarly, clinic physicians also rated the device as a highly acceptable intervention, with 7/7 regular clinic physicians surveyed stating that they felt the patients benefited from the system in terms of BP control.
Box 1. Participant feedback.
The whole thing is so simple and easy. If I have any blood pressure problems, the doctor knows right away. I’m sad that I don’t get to keep using it.
It made me check my blood pressure more because I knew people were looking at the readings.
Really liked it. It was easy for me to hook it up and keep track.
It’s like special treatment because the doctor was able to call and fix my medications.
In surveys during the exit interview, 57% of patients reported experiencing minor technical problems with the device or nontechnical problems with the actual BP cuff. Of this group, four of 28 (14%) participants described their problems as pertaining to the hub occasionally falling out of the wall outlet. The major nontelemonitoring related problem experienced was reinflating of the cuff multiple times while checking BPs, which was noted by eight participants (29%).
Discussion
In this randomized clinical effectiveness trial, we were able to show an ongoing exchange of data between home and clinic in the intervention group with excellent acceptability of the intervention by both participants and physicians. Data exchange in the intervention group was markedly different from the pattern in the usual care group, with participants in the intervention group sharing an average of 30 readings per month and with more than 75% ongoing participation throughout the 6-month study, whereas only 20% of the control group brought home logs to the clinic visits. We found that this wireless telemonitoring device paired with telephone contact from study physicians and pharmacists improved BP both in terms of individual trend toward goal as well as in comparison with a control population without the intervention cuff. Improvement in BP occurred in both groups; the difference of 4.5mmHg between the improvement in SBP seen in the intervention vs. control groups is a clinically significant one and is similar in magnitude to the change seen with adding a new medication in many antihypertensive drug studies, although our study did not have the scale to detect a statistically significant difference of this magnitude. For this reason, BP change was designated as a secondary endpoint.
Our study results are in line with other larger studies in younger populations [16], which have shown that a combined approach using telemonitoring and pharmacist contact is effective in improving BP control. Our intervention is novel in that from the standpoint of the participant it requires no additional training or effort beyond the ability to use a standard BP cuff, and thus is potentially more feasible in an older, less affluent, or less technologically sophisticated population. As checking home BP is already a part of the recommendations for care of older adults with HTN [21], from the standpoint of the participant this protocol required no additional behavior changes and no additional burden other than having the HHH in the home.
It is also notable that the improvement in BP in the intervention group came with a minimal change in the number of antihypertensive agents prescribed. It is possible that this improvement occurred because of improved adherence with already prescribed medications and other recommendations such as dietary changes or use of continuous positive airway pressure machines for sleep apnea. Several participants noted that they enjoyed being monitored, and this may have prompted better adherence, although we do not have daily medication data or behavioral logs to confirm this.
Advantages of the device and monitoring plan we have proposed include the lack of reliance on participants’ computer skills, data entry, or Internet access. Another advantage is that our intervention required only periodic monitoring by study personnel and a relatively small number of telephone contacts to improve BP control. Cost of the intervention was relatively modest, with the combination of BP cuff ($130) and HHH ($99) being the major fixed cost. Modem service continues to decline in cost; and although this varies based on location, it would be available at $20–30/month in most areas. The ongoing cost of this intervention, therefore, compares favorably with the cost of one or two clinic visits for BP management. The HHH is compatible with WiFi or local area networks, and so as these services become more ubiquitous, this cost would likely be lower. The data being transmitted was deidentified and so could be sent using nonsecure communications, with all personal data being stored separately from the BP data.
We did not examine BP logs on a daily basis and so did not provide any real-time monitoring for urgent illnesses. Of note, we did not contact any participants because of sustained severe HTN or initiate any emergent visits for HTN, but did detect two patients with hypotension during our weekly reviews. One unintended result of the study was that participants could be counseled and directed to seek medical assistance for these hypotensive episodes. Further research should examine whether the detection of hypotension, wide variability in BP, or other unusual home BP patterns would prompt changes in treatment of these patients or whether these patterns should influence clinical care of HTN in this population.
Limitations of the current study include the small sample size and short duration; we cannot predict whether the intervention would be robustly effective over longer periods of time. Given our small sample, our results do not reach statistical significance for BP between groups, although we believe the magnitude of the difference we found is clinically important.
It is well recognized that in general monitoring interventions tend to decline in effectiveness with time, and we cannot comment on whether this intervention would continue to be effective after a 6-month time period; however, the protocol we used, with telephone contacts only when readings were out of range, would tend to promote ongoing contact when appropriate, and telephone contacts could be a cost-effective way to promote ongoing self-monitoring and medication adherence. As the telemonitoring device could easily be reassigned to new patients, it might in fact be most effective as a short-term intervention for patients with difficult to control BP so that changes could be made without multiple in-clinic interventions.
Conclusion
This clinical effectiveness study in a group of older adults with CKD and HTN demonstrates a clinically significant improvement in data exchange and a trend toward a statistically significant difference in BP in the intervention arm. We showed that participants using this device transmitted more than 30 BP readings per month as a part of their routine self-care behaviors, whereas those patients receiving usual care continued to exchange minimal data with their providers regarding out-of-clinic BP. A low-burden device such as this in combination with clinical oversight by trained pharmacists and physicians may be a useful intervention in the care of older patients with HTN. Further work should determine the appropriate role for this technology in the clinical care of older adults.
Acknowledgments
The authors acknowledge the assistance of Bernadette Bauer, VASDHS clinic nurse, and the support of Cal-IT2 in the development of the platform and device used in this study.
This study was funded by the UCSD Clinical/Translational Research Institute’s Innovative Technology Pilot Grant (Grant UL RR031980 and UL1TR000100). D.E.R. received additional support from K23DK091521-01 and C.M.M. received support from KO8 DK078580.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.Parikh NI, Hwang S-J, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884–1891. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 4.Peralta CA, Hicks LS, Chertow GM, Ayanian JZ, Vittinghoff E, Lin F, Shlipak MG. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering TG White WBGroup on behalf of the AS of HW. ASH position paper: home and ambulatory blood pressure monitoring when and how to use self (home) and ambulatory blood pressure monitoring. J Clin Hypertens. 2008;10:850–855. doi: 10.1111/j.1751-7176.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mengden T, Hernandez Medina RM, Beltran B, Alvarez E, Kraft K, Vetter H. Reliability of reporting self-measured blood pressure values by hypertensive patients. Am J Hypertens. 1998;11:1413–1417. doi: 10.1016/s0895-7061(98)00241-6. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R. Home and ambulatory blood pressure monitoring in chronic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:507–512. doi: 10.1097/MNH.0b013e3283319b9d. [DOI] [PubMed] [Google Scholar]
- 9.Fischer MJ, O’Hare AM. Epidemiology of hypertension in the elderly with chronic kidney disease. Adv Chronic Kidney Dis. 2010;17:329–340. doi: 10.1053/j.ackd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2009;4:656–664. doi: 10.2215/CJN.05391008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson LA, Holt SG, Leslie AR, Rajkumar C. Prevalence of ambulatory hypotension in elderly patients with CKD stages 3 and 4. Nephrol Dial Transplant. 2009;24:3751–3755. doi: 10.1093/ndt/gfp357. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Kurella Tamura M, Muntner P, Wadley V, Cushman M, Zakai NA, Bradbury BD, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis. 2011;58:756–763. doi: 10.1053/j.ajkd.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rifkin DE, Laws MB, Rao M, Balakrishnan VS, Sarnak MJ, Wilson IB. Medication adherence behavior and priorities among older adults with CKD: a semistructured interview study. Am J Kidney Dis. 2010;56:439–446. doi: 10.1053/j.ajkd.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AbuDagga A, Resnick HE, Alwan M. Impact of blood pressure telemonitoring on hypertension outcomes: a literature review. Telemed J E Health. 2010;16:830–838. doi: 10.1089/tmj.2010.0015. [DOI] [PubMed] [Google Scholar]
- 16.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857–2867. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinfret S, Lussier M-T, Peirce A, Duhamel F, Cossette S, Lalonde L, et al. The impact of a multidisciplinary information technology-supported program on blood pressure control in primary care. Circ Cardiovasc Qual Outcomes. 2009;2:170–177. doi: 10.1161/CIRCOUTCOMES.108.823765. [DOI] [PubMed] [Google Scholar]
- 18.Magid DJ, Ho PM, Olson KL, Brand DW, Welch LK, Snow KE, et al. A multimodal blood pressure control intervention in 3 healthcare systems. Am J Manag Care. 2011;17:e96–e103. [PubMed] [Google Scholar]
- 19.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed 12 May 2012];UA-767PBT. Available at: http://www.andonline.com/medical/products/details.php?catname=&product_num=UA-767PBT.
- 21.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the council on high blood pressure research professional and public education subcommittee. J Clin Hypertens (Greenwich) 2005;7:102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]