Abstract
Background
We and others have previously demonstrated that the μ-opioid receptor (MOR) is overexpressed in several human malignancies. There is a seven-fold increase in MOR in cell lines of human lung cancer. In animal models, overexpression of MOR promotes tumour growth and metastasis. We, therefore, examined whether MOR expression is increased in metastatic lung cancer.
Methods
In this study, we examined the association between MOR expression and metastasis in archived biopsy samples from patients with lung cancer. Paraffin-embedded patient material was stained using MOR antibody and scored qualitatively by two independent pathologists using a four-point scale.
Results
In human lung cancer and normal adjacent lung samples obtained from 34 lung cancer patients, MOR expression was increased significantly in cancer samples from patients with lung cancer compared with adjacent control tissue (P=0.0242). When the samples from patients with metastatic lung cancer were separated from the cohort of the total number of patients with lung cancer, we observed an approximately two-fold increase in MOR expression (P=0.0013).
Conclusions
The association between the expression of MOR and the progression of the tumour is consistent with the hypothesis of a direct effect of MOR on cancer progression.
Keywords: lung cancer, metastasis, methylnaltrexone, MOR, μ-opioid receptor
Editor's key points.
In laboratory cell lines and animal models, μ-opioid receptor (MOR) overexpression promotes tumour growth and metastasis.
This study evaluated the association between MOR expression and metastasis in archived samples of lung cancer.
MOR overexpression was evident in lung cancer samples of patients with metastasis compared with lung cancer patients who did not have metastasis.
The role of anaesthesia and analgesia in the recurrence and metastatic rate of malignancies has recently received considerable attention.1–4 Retrospective studies have demonstrated a lower incidence of cancer recurrence after regional anaesthesia with low doses of opioids after surgery for breast, prostate, colon cancer, and melanoma; other studies have failed to detect significant differences.5–8 Explanations for the differences in recurrence rates include immune suppression and direct effects on tumour cell growth.9–11 Our research has focused on the μ-opioid receptor (MOR) in directly regulating tumour growth and metastasis.12,13
Effective therapeutic strategies for lung cancer, the leading cause of cancer-associated mortality worldwide, are extremely limited exemplifying the need for early diagnosis and novel therapeutic interventions.14,15 We have previously reported that the MOR is upregulated in several types of human non-small-cell lung cancer (NSCLC).13 In xenograft models, overexpression of the MOR in human NSCLC increased primary tumour growth and metastasis.12 The correlation between the MOR and metastatic lung cancer, however, has not been previously reported in patient samples.
Several targeted therapies in NSCLC exist including epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (gefitinib and erlotinib) and monoclonal antibodies (cetuximab).16–19 The overall survival rate for NSCLC, however, still remains low.20–22 Fujioka and colleagues found that morphine can stimulate EGFR-signalling pathways such as the serine/threonine kinases Akt and MAP kinase in NSCLC. This result suggests that MOR inhibition is a potential therapeutic strategy for NSCLC.23 In another recent study, MOR expression correlated with progression-free and overall survival in prostate cancer.24
Based on the recent interest in the effects of anaesthesia and analgesia regimens on the recurrence and metastatic potential of various cancers,1–4 published data that the MOR is upregulated in lung tissue from patients with NSCLC,13 that overexpression of MOR promotes tumour growth and metastasis in human NSCLC xenograft models,12 and that MOR expression correlates with prostate cancer survival,24 we investigated MOR expression in lung cancer patient samples.
Methods
Lung cancer tissue samples
We used tissue samples from human subjects that were cytologically or histologically documented with NSCLC. The tissues were archival surgical specimens already banked in the University of Chicago Lung Cancer Tissue Bank or in the pathology department. The tissues were obtained with the subjects’ written informed consent under protocols 9571 and 13 473. The protocols give access to clinical information for the tissues in this study. The University of Chicago IRB grants a waiver of consent for archival tissues from patients who have died (10654N), and the protocol also gives access to the associated clinical data from the tissue bank database. Tumour tissues not associated with personal information (de-identified) that have already been banked were used under protocol 10653N. Use of these previously stored samples was granted exemption status through the University of Chicago IRB under PHS exemption category number 4. All data, which included patient, clinical, and treatment outcomes, were entered into our HIPAA regulated database.
MOR immunohistochemistry
Samples for this study were acquired from paraffin-embedded, formalin-fixed tissue archived at The University of Chicago (Chicago, IL, USA) Human Tissue Resource Center. Immunohistochemistry for human MOR was conducted on tissue sections using rabbit anti-MOR antibody (GeneTex, San Antonio, TX, USA) that was previously validated in our laboratory.12,13,25–28 Following an antigen retrieval protocol, tissues were deparaffinized, blocked with BSA, probed with primary and secondary antibody. Tumour sections were evaluated by a pathologist who was blinded to the identity of the tissue as previously described.25,26 Briefly, IHC score was obtained by the semiquantitative method that accounts for staining intensity and percentage of cell stained. This estimation resulted in an IHC score of 0, 1, 2, or 3 denoting negative, weak, moderate, and strong expression, respectively. This score was correlated with available clinical information in future analyses. Images were captured at ×20 or ×40 using a Fisher Scientific Micromaster digital microscope (Pittsburg, PA, USA) and the Micron USB live-field capture software (Westover Scientific, Mill Creek, WA, USA) as previously described.25,26
Statistical analysis
For individual groups, normal distribution probabilities and P values were calculated using Lilliefors test for normality.27 For differences in MOR staining intensity in patient samples between normal adjacent control, total lung cancer, and subset of lung cancer with lymph node metastasis, data were compared by unpaired Student's t-test. P<0.05 was considered statistically significant. Statistical analysis was performed using the GraphPad Prism program (GraphPad Software Inc., La Jolla, CA, USA). Other graphical quantitation was performed using the Microsoft Excel™ software.
Results
MOR staining intensity was increased in lung cancer patient samples vs normal adjacent controls (1.24 vs 0.74, P=0.0242) (Table 1). The sets of MOR staining for normal adjacent control and lung cancer each had a normal distribution as determined by the Lilliefors test for normality27 (P=0.001 and P=0.001). When broken down into NSCLC subtypes, the largest differences in MOR staining, while statistically not significant, were observed in large cell carcinoma (1.60 vs 0.80, P=0.242), then in adenocarcinoma (1.00 vs 0.87, P=0.732), and finally in squamous cell carcinoma (1.07 vs 1.00, P=0.857).
Table 1.
Comparison of various parameters between normal adjacent control, total lung cancer, and subset of lung cancer with lymph node metastasis patient samples. MOR immunohistochemistry was performed on de-identified lung cancer patient samples and scored by two independent pathologists on a four-point scale (0, 1, 2, 3) as described in the Methods. Various parameters were analysed including MOR staining intensity, age, gender, NSCLC subtype histology, and MOR staining by histology. Adeno, adenocarcinoma; Large cell, large-cell carcinoma; SCC, squamous cell carcinoma. For certain parameters, data means, data ranges, and data P values are presented. P-value within groups: *P values of individual datasets using the Lilliefors test for normality.27 P-value between groups: **a comparison between either normal adjacent control and lung cancer or lung cancer and subset of lung cancer patients with lymph node metastasis using Student's t-test
| Normal adjacent control (n=34) |
Lung cancer (n=34) |
Subset of lung cancer patients with lymph node metastasis (n=7) |
||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean (range) | P-value within groups* | Mean (range) | P-value within groups* | P-value between groups** | Mean (range) | P-value within groups* | P-value between groups** |
| MOR staining intensity | 0.74 (0–2) | 0.001 | 1.24 (0–3) | 0.001 | 0.0242 | 2.0 (1–3) | 0.008 | 0.0013 |
| Age | 67.8 (46–90) | 0.186 | N/A | 70.4 (61–81) | 0.5 | 0.236 | ||
| Gender | Male=17 | Male=5 | ||||||
| Female=17 | Female=2 | |||||||
| Stage | 0–3 | 2–3 | ||||||
| Histology | Adeno=14 | Adeno=4 | ||||||
| Large cell=5 | SCC=3 | |||||||
| SCC=15 | ||||||||
| MOR staining/histology | Adeno=0.87 (0–2) | 0.022 | Adeno=1.00 (0–3) | 0.001 | 0.732 | Adeno=2.0 (1–3) | 0.5 | N/A |
| Large cell=0.80 (0–2) | 0.5 | Large cell=1.60 (0–3) | 0.470 | 0.242 | SCC=2.0 (2–2) | N/A | N/A | |
| SCC=1.00 (0–2) | 0.027 | SCC=1.07 (0–2) | 0.01 | 0.857 | ||||
We next examined the samples from a subset of total lung cancer patients with lymph node metastasis. The MOR staining for this subset of total patients with lung cancer had a normal distribution as determined by the Lilliefors test for normality27 (P=0.008). Samples from lung cancer patients with lymph node metastasis had higher MOR staining intensity than did the samples in the total lung cancer cohort (2.00 vs 1.24, P=0.0013). The average patient age was similar in the two groups (70.4 vs 67.8 yr, P=0.236). The metastatic portion was derived from adenocarcinoma and squamous cell carcinoma subtypes of NSCLC.
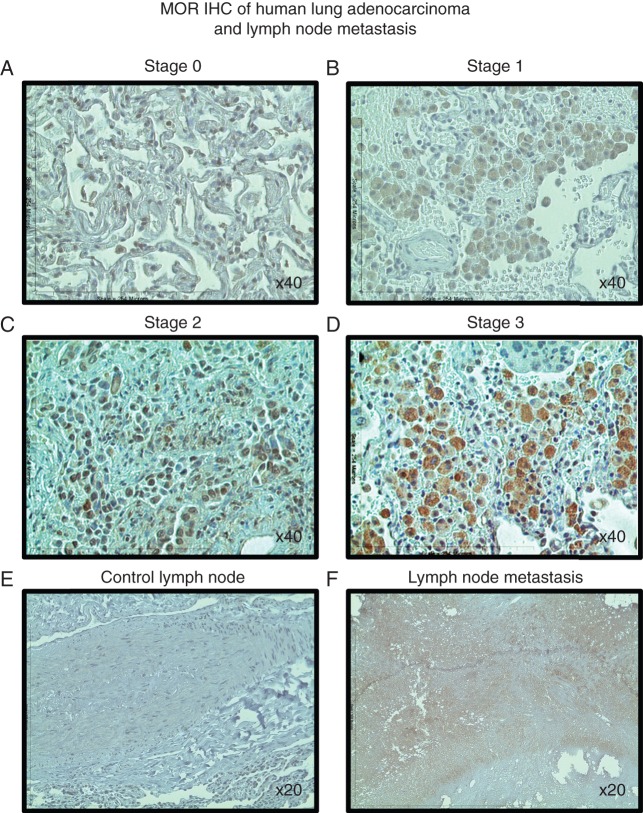
We examined MOR IHC stained sections from NSCLC patients with Stage 0, 1, 2 and 3 adenocarcinoma (Fig. 1a–d). MOR staining intensity increased with cancer stage. The pattern of MOR staining appeared to change from a nuclear (Fig. 1a) to cytosolic (Fig. 1b) to a cytosolic/membrane cellular localization (Fig. 1c and d). This is consistent with prior observations in prostate cancer.24 We also examined lymph nodes from NSCLC adenocarcinoma patients with or without cancer cell invasion. MOR staining intensity was also increased in metastatic lymph nodes (Fig. 1e vs f).
Fig 1.
MOR immunohistochemical staining intensity increases with NSCLC tumour stage and lymph node metastasis in patient samples. Patient NSCLC adenocarcinoma lung cancer samples were acquired from paraffin-embedded, formalin-fixed tissue archived at The University of Chicago (Chicago, IL, USA) Human Tissue Resource Center. Immunohistochemistry for human MOR was conducted on tissue sections and images were captured at ×20 or ×40 using a Fisher Scientific Micromaster digital microscope and the Micron USB live-field capture software. MOR staining intensity increases with cancer stage (a–d). The pattern of MOR staining appears to change from a nuclear (a) to cytosolic (b) to a cytosolic/membrane cellular localization (c and d). MOR staining intensity is also increased in metastatic lymph nodes (e vs f).
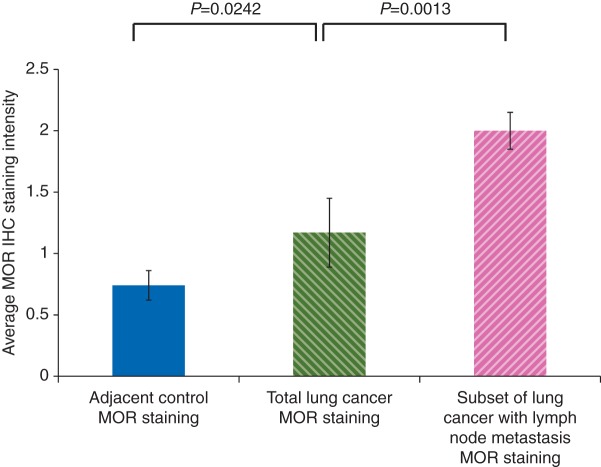
Although the difference between normal adjacent control and total lung cancer MOR staining was statistically significant, there was considerable overlap (0.74 vs 1.24, P=0.0242) (Table 1). We, therefore, performed a statistical analysis of the average MOR staining intensity of the patient samples between total lung cancer and the subset of total lung cancer with lymph node metastasis. Analysis in this fashion yielded a much larger significant difference in staining intensity between total lung cancer and the subset of total lung cancer with lymph node metastasis (1.24 vs 2.0, P=0.0013) (Fig. 2). Although our sample size was relatively small, our results are consistent with the hypothesis of a direct effect of MOR on cancer progression.
Fig 2.
Graphical representation of the average MOR immunohistochemical staining intensity of normal adjacent, total lung cancer, and subset of lung cancer with lymph node metastasis patient samples. MOR immunohistochemistry was performed on de-identified normal adjacent control and lung cancer patient samples, scored by two independent pathologists on a four-point scale (0, 1, 2, 3) and box plots were generated as described in the Methods. There was a statistically significant difference between normal adjacent control and total lung cancer (P=0.0242) and also total lung cancer and subset of lung cancer with lymph node metastasis (P=0.0013).
Discussion
Our most important finding that samples from patients with metastatic disease exhibited a dramatically higher MOR expression serves as a bridge between prior molecular studies and recent clinical observations in humans. Our observations specifically support other molecular, cellular, and animal evidence demonstrating that the μ-opiate receptor plays an important role in the progression of lung cancer.
In preclinical models, μ-opioids stimulate angiogenesis and tumour progression through the MOR.11,13,29,30 Gupta and colleagues initially reported that μ-opioids at clinically relevant doses were proangiogenic in a model of breast cancer xenografts.28 Support for the hypothesis that the MOR is involved in cancer progression comes from other work showing a reciprocal transactivation of the VEGF receptor,31 and potentiation of bevacizumab and 5-fluorouracil30 and also mTOR inhibitors29 in human endothelial cells by the peripheral opiate antagonist methylnaltrexone (MNTX). In MOR knockout mice, there was markedly diminished progression of Lewis lung carcinoma, and MNTX or naltrexone infusions blocked tumour growth and metastasis.13 Tumour progression appeared not to be mediated through the effects of opioids on the immune system as it was not significantly influenced in nude mice.12,13 Although several studies have failed to implicate an association between the MOR and progression, in at least one of the studies, pharmacologic rather than clinical doses of opioids were used.30 MNTX blocked several intermediaries of cancer progression including Src even absent exogenous opiates,32 suggesting a dual effect on the angiogenic pathway.
In addition to the laboratory evidence supporting involvement of the MOR in cancer progression, results of some human studies are consistent with this finding. Recently, in a retrospective study of 113 patients with advanced prostate cancer, μ-opiate receptor expression and opioid requirement were independently associated with reduced progression-free survival and overall survival.24 These observations in prostate cancer patients are remarkably consistent with what we have demonstrated in our cohort of lung cancer patients and suggest a broader applicability of the hypothesis that the MOR is involved in cancer progression. In our study, the increase in the MOR was especially prominent in samples with metastatic disease.
Further human evidence supporting the role of the MOR in cancer progression in humans comes from a recent study examining the A118G gene mutation of the MOR.33 In that retrospective study of 2039 women with breast cancer, the A118G gene polymorphism, which decreases response to opioid receptors, was associated with a two-fold improvement in survival in heterozygotes and a four-fold difference in survival in homozygotes at all stages of disease. The role of the A118G MOR gene mutation in tumour progression was confirmed in oesophageal cancer.34
Finally, support for an effect of opioids on human cancer comes from a study35 of palliative care patients: intrathecal fentanyl markedly improved survival compared with medical treatment with systemic opiates. In a study of three patients with pancreatic cancer treated with α-lipoic acid and low-dose naltrexone, the proliferation of malignant cells was attenuated.36
Although there is growing evidence for an effect of the MOR in mediating tumour metastasis, the reason for this effect remains unclear. Our studies of tumour progression were performed in the absence of exogenous opiates, suggesting an intrinsic process. Studies in human melanoma showed that endogenous opioid expression is associated with tumour progression.37 μ-Opioids reduced cell–cell adhesion in a concentration-dependent manner in layers of human pulmonary endothelial cells and facilitated capillary leakage in animal models.38 Opioids at clinically relevant doses potentiated epithelial mesenchymal transition, and opioid receptor antagonists blocked both opioid- and EGF-mediated changes in that process.39 Thus, it is possible that the endothelial barrier dysfunction in cellular and animal models may permit further seeding of the existing tumours.
Although our sample sizes were small, the individual sets of MOR staining samples for normal adjacent control, lung cancer, and subset of lung cancer with lymph node metastasis each had a normal distribution as determined by the Lilliefors test for normality.27 Further, there was a statistically significant difference using Student's t-test between the groups. However, a larger sample size will be needed to conduct advanced statistical analyses.
The present study has focused on cancer patients, but our findings could provide an explanation for the observations that cancer recurrence is associated with the type of anaesthesia used in surgery. There are important differences between opioid use for surgery in opiate naive individuals and chronic use of opioids in cancer patients. The physiologic observations we have made are consistent with the initial findings in breast and lung cancer patients.5,6 The only prospective study in this area failed to validate that the type of anaesthesia had a radically different effect in colon cancer patients,8 and the proof of a direct effect of anaesthetic techniques in cancer surgery awaits results of prospective trials. In conclusion, our study extends our observations that opioids and the opioid receptor affect tumour progression in humans and suggests a potential use of MOR antagonists as a therapeutic option in tumour treatment.
Authors’ contributions
P.A.S., R.S., and J.M. conceived of the strategies and supervised the project. P.A.S. and T.M. designed and performed experiments and analysed data. P.A.S. and J.M. wrote the manuscript. R.H. designed and performed experiments.
Declaration of interest
MNTX was developed at the University of Chicago and licensed to Progenics Pharmaceuticals, subsequently sub-licensed to Salix Pharmaceuticals. J.M. was a paid consultant for Progenics Pharmaceuticals and currently is a paid consultant for Salix Pharmaceuticals. He receives royalties through the University of Chicago.
Funding
Support was provided from institutional, departmental sources, or both, and National Institutes of Health grant CTSA UL1 TR000430. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bovill JG. Surgery for cancer: does anesthesia matter? Anesth Analg. 2010;110:1524–6. doi: 10.1213/ANE.0b013e3181d8d183. [DOI] [PubMed] [Google Scholar]
- 2.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–15. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 3.Yeager MP, Rosenkranz KM. Cancer recurrence after surgery: a role for regional anesthesia? Reg Anesth Pain Med. 2010;35:483–4. doi: 10.1097/AAP.0b013e3181fa11a9. [DOI] [PubMed] [Google Scholar]
- 4.Lennon FE, Moss J, Singleton PA. The mu-opioid receptor in cancer progression: is there a direct effect? Anesthesiology. 2012;116:940–5. doi: 10.1097/ALN.0b013e31824b9512. [DOI] [PubMed] [Google Scholar]
- 5.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–7. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 6.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–32. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 8.Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. Br Med J. 2011;342:d1491. doi: 10.1136/bmj.d1491. [DOI] [PubMed] [Google Scholar]
- 9.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–50. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 10.Afsharimani B, Cabot PJ, Parat MO. Morphine use in cancer surgery. Front Pharmacol. 2011;2:46. doi: 10.3389/fphar.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–38. doi: 10.1007/s10555-011-9285-0. [DOI] [PubMed] [Google Scholar]
- 12.Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the mu-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857–67. doi: 10.1097/ALN.0b013e31824babe2. [DOI] [PubMed] [Google Scholar]
- 13.Mathew B, Lennon FE, Siegler J, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–67. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiro SG, Tanner NT, Silvestri GA, et al. Lung cancer: progress in diagnosis, staging and therapy. Respirology. 2010;15:44–50. doi: 10.1111/j.1440-1843.2009.01674.x. [DOI] [PubMed] [Google Scholar]
- 15.Stinchcombe TE, Bogart J, Veeramachaneni NK, Kratzke R, Govindan R. Annual review of advances in non-small cell lung cancer research: a report for the year 2010. J Thorac Oncol. 2011;6:1443–50. doi: 10.1097/JTO.0b013e3182246413. [DOI] [PubMed] [Google Scholar]
- 16.Carlson JJ. Erlotinib in non-small-cell lung cancer: a review of the clinical and economic evidence. Expert Rev Pharmacoecon Outcomes Res. 2009;9:409–16. doi: 10.1586/erp.09.49. [DOI] [PubMed] [Google Scholar]
- 17.Choi EJ, Ryu YK, Kim SY, et al. Targeting epidermal growth factor receptor-associated signaling pathways in non-small cell lung cancer cells: implication in radiation response. Mol Cancer Res. 2010;8:1027–36. doi: 10.1158/1541-7786.MCR-09-0507. [DOI] [PubMed] [Google Scholar]
- 18.Ganjoo KN, Wakelee H. Review of erlotinib in the treatment of advanced non-small cell lung cancer. Biologics. 2007;1:335–46. [PMC free article] [PubMed] [Google Scholar]
- 19.Gridelli C, Morabito A, Gebbia V, et al. Cetuximab and gemcitabine in elderly or adult PS2 patients with advanced non-small-cell lung cancer: the cetuximab in advanced lung cancer (CALC1-E and CALC1-PS2) randomized phase II trials. Lung Cancer. 2010;67:86–92. doi: 10.1016/j.lungcan.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 20.De Greve J, Decoster L, Van Meerbeek J, Vermeij J, Teugels E, Schallier D. [Targeted therapies in the treatment of non-small cell lung cancer] Bull Cancer. 2008;95:358–64. doi: 10.1684/bdc.2008.0591. [DOI] [PubMed] [Google Scholar]
- 21.Ng R, Loreto M, Lee R, Leighl NB. Brief report: retrospective review of efficacy of erlotinib or gefitinib compared to docetaxel as subsequent line therapy in advanced non-small cell lung cancer (NSCLC) following failure of platinum-based chemotherapy. Lung Cancer. 2008;61:262–5. doi: 10.1016/j.lungcan.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian J, Madadi AR, Dandona M, Williams K, Morgensztern D, Govindan R. Review of ongoing clinical trials in non-small cell lung cancer: a status report for 2009 from the ClinicalTrials.gov website. J Thorac Oncol. 2010;5:1116–9. doi: 10.1097/JTO.0b013e3181e76159. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka N, Nguyen J, Chen C, et al. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg. 2011;113:1353–64. doi: 10.1213/ANE.0b013e318232b35a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zylla D, Gourley BL, Vang D, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–10. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada I, Hasina R, Lennon FE, et al. Paxillin mutations affect focal adhesions and lead to altered mitochondrial dynamics: relevance to lung cancer. Cancer Biol Ther. 2013;14:679–91. doi: 10.4161/cbt.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faoro L, Singleton PA, Cervantes GM, et al. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J Biol Chem. 2010;285:18575–85. doi: 10.1074/jbc.M109.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cembrowski GS, Westgard JO, Conover WJ, Toren EC., Jr Statistical analysis of method comparison data. Testing normality. Am J Clin Pathol. 1979;72:21–6. doi: 10.1093/ajcp/72.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- 29.Singleton PA, Mambetsariev N, Lennon FE, et al. Methylnaltrexone potentiates the anti-angiogenic effects of mTOR inhibitors. J Angiogenesis Res. 2010;2:5. doi: 10.1186/2040-2384-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singleton PA, Moss J. Effect of perioperative opioids on cancer recurrence: a hypothesis. Future Oncol. 2010;6:1237–42. doi: 10.2217/fon.10.99. [DOI] [PubMed] [Google Scholar]
- 31.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Singleton PA, Garcia JG, Moss J. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol Cancer Ther. 2008;7:1669–1679. doi: 10.1158/1535-7163.MCT-07-2217. [DOI] [PubMed] [Google Scholar]
- 33.Bortsov AV, Millikan RC, Belfer I, Boortz-Marx RL, Arora H, McLean SA. mu-Opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. Anesthesiology. 2012;116:896–902. doi: 10.1097/ALN.0b013e31824b96a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Li Y, Liu XD, Zhao CX, Yang KQ. Polymorphism of A118G in mu-opioid receptor gene is associated with risk of esophageal squamous cell carcinoma in a Chinese population. Int J Clin Oncol. 2013;18:666–9. doi: 10.1007/s10147-012-0441-5. [DOI] [PubMed] [Google Scholar]
- 35.Rastogi B, Gupta K, Rastogi A, Gupta PK, Singhal AB, Singh I. Hemiarthroplasty in high risk elderly patient under epidural anesthesia with 0.75% ropivacaine-fentanyl versus 0.5% bupivacaine-fentanyl: clinical trial. Saudi J Anaesth. 2013;7:142–5. doi: 10.4103/1658-354X.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donahue RN, McLaughlin PJ, Zagon IS. Low-dose naltrexone targets the opioid growth factor-opioid growth factor receptor pathway to inhibit cell proliferation: mechanistic evidence from a tissue culture model. Exp Biol Med (Maywood) 2011;236:1036–50. doi: 10.1258/ebm.2011.011121. [DOI] [PubMed] [Google Scholar]
- 37.Boehncke S, Hardt K, Schadendorf D, Henschler R, Boehncke WH, Duthey B. Endogenous mu-opioid peptides modulate immune response towards malignant melanoma. Exp Dermatol. 2011;20:24–8. doi: 10.1111/j.1600-0625.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 38.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am J Resp Cell Mol Biol. 2007;37:222–31. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 39.Lennon FE, Mirzapoiazova T, Mambetsariev B, et al. The mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS One. 2014;9:e91577. doi: 10.1371/journal.pone.0091577. [DOI] [PMC free article] [PubMed] [Google Scholar]