Abstract
Primary human papillomavirus (HPV) testing (without concurrent Pap tests) every 3 years is under consideration in the United States as an alternative to the two recommended cervical cancer screening strategies: primary Pap testing every 3 years, or concurrent Pap and HPV testing (“cotesting”) every 5 years. Using logistic regression and Weibull survival models, we estimated and compared risks of cancer and cervical intraepithelial neoplasia grade 3 or worse (CIN3+) for the three strategies among 1011092 women aged 30 to 64 years testing HPV-negative and/or Pap-negative in routine screening at Kaiser Permanente Northern California since 2003. All statistical tests were two sided. Three-year risks following an HPV-negative result were lower than 3-year risks following a Pap-negative result (CIN3+ = 0.069% vs 0.19%, P < .0001; Cancer = 0.011% vs 0.020%, P < .0001) and 5-year risks following an HPV-negative/Pap-negative cotest (CIN3+ = 0.069% vs 0.11%, P < .0001; Cancer = 0.011% vs 0.014%, P = .21). These findings suggest that primary HPV testing merits consideration as another alternative for cervical screening.
Recent trials have shown that human papillomavirus (HPV) testing provides greater protection against invasive cervical cancer compared to Pap-based screening (1,2). In the United States, concurrent HPV and Pap testing (“cotesting”) for primary screening every 5 years among women aged 30 to 65 is now recommended, with primary Pap testing using HPV tests for triage of equivocal results (atypical squamous cells of undetermined significance [ASC-US]) every 3 years as an alternative (3–5). Primary HPV testing (using Pap tests for triage of HPV-positive results) every 3 or more years is now under consideration by the US Food and Drug Administration and professional societies. If a negative HPV test can provide the same safety (ie, reassurance against future risk of precancer and cancer) as a negative Pap or negative cotest (currently recommended strategies), most of the Pap tests now conducted in screening would no longer be required.
Since 2003, women older than 30 years at Kaiser Permanente Northern California (KPNC), a large integrated health delivery system, have been screened with cotesting, allowing us to compare the long-term risks of precancer and cancer after negative screening results in a large, established screening program (6). These data represent the largest and longest experience with HPV testing in routine clinical practice, and risks from this population were used to develop current cervical screening and management guidelines (3,7). With newly available data through 2012, we were able to estimate risks among more than 1 million women.
The KPNC cervical screening program and our methods for risk calculation have been described previously (6,8). Briefly, in the period from 2003 to 2012, 1037021 women aged 30 to 64 years were screened at approximately 3-year intervals using cotesting with Pap and high-risk HPV testing (Hybrid Capture 2; Qiagen, Germantown, MD), and 1011 092 (97.5%) women in this analysis tested HPV-negative and/or Pap-negative at enrollment. Histologically-confirmed cases of cervical intraepithelial neoplasia grade 2 (CIN2), grade 3 (CIN3) and cancer were ascertained through December 31, 2012. Women testing HPV-positive/Pap-negative or HPV-negative/Pap-equivocal (ASC-US) returned in 1 year. Women testing HPV-negative with a low-grade or worse Pap were referred for colposcopy. Women testing HPV-negative/Pap-negative returned for repeat screening in 3 years. Approximately half of the women (n = 439212) did not have a second screen during follow-up because they either enrolled after 2009 and had not yet returned for a subsequent screen, had CIN2+ at enrollment, or left KPNC. For the 571880 women who were followed beyond enrollment, the mean follow-up time was 4.36 years (SD = 1.95 years, median = 3.71 years, IQR = 2.94 to 6.00 years, range = 0.022 to 9.97 years). The total follow-up time was 2495 946 person-years.
We estimated the reassurance against future risk of precancer and cancer of a negative screening result under 3 screening strategies: primary Pap testing, primary HPV testing and cotesting. For each woman, we considered as the enrollment screen the first available cotest (or in some instances only Pap or HPV test) in the study period. For each negative enrollment test result, 3- and 5-year cumulative risks of CIN2 or more severe (CIN2+), CIN3+, and cancer diagnoses were calculated using previously described methodology (8). Briefly, the cumulative risks were obtained by adding the risk at enrollment test (using logistic regression) to the risk after enrollment (using Weibull survival modeling). We considered each negative HPV and Pap test result combined and in the absence of the other. When risk was calculated for a negative Pap result without regard to HPV testing, we refer to those risks as “Pap-negative alone.” Similarly, when risk was calculated for a negative HPV result without regard to Pap testing, we refer to those risks as “HPV-negative alone.” A negative result on both tests is referred to as “cotest-negative.” All statistical tests were two-sided.
In addition, we used enrollment cotest results to project under the 3 screening strategies in a hypothetical population of 1 million women: 1) the guidelines-based management outcomes after 1 round of screening, and 2) a simple approximation of the number of HPV and Pap tests for screening or triage over 15 years of routine screening without consideration of abnormal results and associated follow-up.
Figure 1 shows that the 3-year risks following an HPV-negative alone result were lower than the 3-year risks following a Pap-negative alone result, both for CIN3+ (0.069% vs 0.19%, P < .0001) and for cancer (0.011% vs 0.020%, P < .0001). Furthermore, the 3-year risks following an HPV-negative alone result were lower than the 5-year risks following a cotest-negative result, both for CIN3+ (0.069% vs 0.11%, P < .0001) and for cancer (0.011% vs 0.014%, P = .21). Similar trends were also observed in stratified analyses across 5-year age groups (data not shown). Comparisons of CIN2+ risks had the same statistical significance as CIN3+ endpoints (P < .0001).
Figure 1.
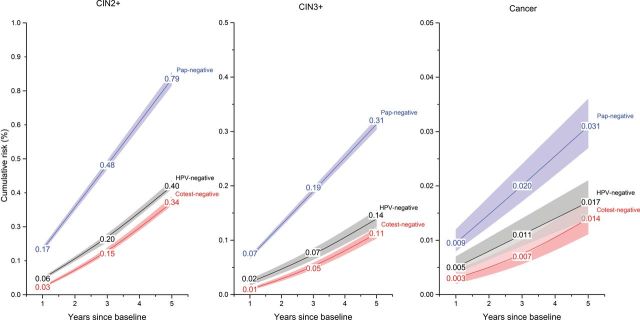
Cumulative risks of cervical intraepithelial neoplasia grade 2 or more severe (left panel), grade 3 or more severe (center panel) and cancer (right panel) among women aged 30 to 64 years at Kaiser Permanente Northern California by enrollment Pap and human papillomavirus (HPV) test result, 2003 to 2012. Women are HPV-negative if they tested negative by Hybrid Capture 2 (hc2; Qiagen, Germantown, MD), an assay that tests for 13 high-risk HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). Among 1011092 (97.5%) women testing HPV-negative and/or Pap-negative, 980268 tested Pap-negative alone (regardless of HPV result), 923706 women tested HPV-negative alone (regardless of Pap result) and 892882 women tested cotest-negative. Briefly, the cumulative risks were obtained by adding the risk at enrollment test (using logistic regression) to the risk after enrollment (using Weibull survival modeling). Note that the y axes have different scales for different panels. Risk bands are 95% confidence intervals. All statistical tests were two sided. CIN2 = cervical intraepithelial neoplasia grade 2; CIN3 = cervical intraepithelial neoplasia grade 3; HPV = human papillomavirus.
Within 5 years of enrollment, 405 women were diagnosed with cancer, and 155 screened either HPV-negative and/or Pap-negative at enrollment: 76 (18.8%) HPV-negative, 129 (31.9%) Pap-negative and 50 (12.3%) cotest-negative. Compared to Pap testing, HPV testing missed fewer cervical adenocarcinoma (47.4% vs 11.4%, P < .0001) and adenocarcinoma in situ (51.1% vs 18.7%, P < .0001).
Table 1 presents the projected guidelines-based management outcomes after 1 round of screening for the 3 strategies (primary Pap testing with HPV triage of ASC-US results, primary HPV testing with Pap triage of HPV-positive results, and cotesting) per 1 million women. Both cotesting and primary HPV testing would require that some women (3.7% and 3.5% respectively at KPNC) return for repeat screening in 1 year because of HPV-positive/Pap-negative or HPV-negative/LSIL results that indicate an intermediate risk of precancer and cancer (9,10). At KPNC, approximately half of these women still had a screening abnormality 1 year later and would require colposcopy, likely resulting in higher colposcopy referral over time for primary HPV testing and cotesting compared to primary Pap.
Table 1.
Guidelines-based management outcomes and test utilization under 3 screening strategies (primary Pap testing, primary human papillomavirus testing, and cotesting) among women aged 30 to 64 years in a hypothetical population of 1000000 women
| Category | Primary Pap screening with HPV triage of ASC-US results | Primary HPV screening with Pap triage of HPV-positive results | Cotest screening using HPV and Pap | |
|---|---|---|---|---|
| Guidelines-based management outcome after 1 round of screening | ||||
| Routine screening† | 971731‡ | 940435 | 936261‡ | |
| 1-year return | 0 | 35470§ | 37 411§ | |
| Colposcopy referral | 28269 | 24095 | 26 327 | |
| Approximate number of tests for screening or triage over 15 years of routine screening†‖ | 3-year return | 3-year return | 5-year return | 5-year return |
| Pap tests | 5000000 | 297825 | 178695 | 3000000 |
| HPV tests | 145310 | 5000000 | 3000000 | 3000000 |
| Total number of screening tests | 5145310 | 5297825 | 3178695 | 6000000 |
* Women are HPV-positive if they tested positive by Hybrid Capture 2 (hc2; Qiagen, Germantown, MD), an assay that targets 13 high-risk HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). The guidelines-based management outcome after screening is determined by the distribution of enrollment cotest results among women aged 30 to 64 at Kaiser Permanente Northern California 2003 to 2012 and US screening and management recommendations (3-5,7). “Routine screening” is recommended for women testing Pap-negative and women testing HPV-negative/atypical squamous cells of undetermined significance (ASC-US) for the primary Pap screening strategy, women testing HPV-negative (regardless of Pap result) for the primary HPV screening strategy, and women testing HPV-negative/Pap-negative and women testing HPV-negative/ASC-US for the cotest screening strategy. “One-year return” is recommended for women testing HPV-positive/Pap-negative for the primary HPV screening strategy and women testing HPV-positive/Pap-negative and women testing HPV-negative/low grade squamous intraepithelial lesion (LSIL) for the cotest screening strategy. “Colposcopy referral” is recommended for women with an LSIL or worse Pap (regardless of HPV result) and women testing HPV-positive/ASC-US for the primary Pap screening strategy, women testing HPV-positive with ASC-US or worse Pap for the primary HPV screening strategy, and women testing HPV-positive/ASC-US, women testing HPV-positive with LSIL or worse Pap, and women testing HPV-negative with a Pap worse than LSIL for the cotesting screening strategy. ASC-US = atypical squamous cells of undetermined significance; HPV = human papillomavirus; LSIL = low grade squamous intraepithelial lesion.
† U.S. practice guidelines recommend a 3-year return after a negative Pap screen and a 5-year return after a negative cotest [3–5].
‡ Includes 17 686 women testing HPV-negative/ASC-US for whom routine screening is recommended [3,4,7].
§ Among 35 470 women testing HPV-positive/Pap-negative and 1 941 women testing HPV-negative/Pap-LSIL, 18 591 (52.4%) and 527 (27.2%) respectively would test HPV-positive and/or ASC-US or worse Pap at their second screening visit in 1 year and would therefore be referred to colposcopy at that time under U.S. guidelines [7].
‖ Calculations are a simple approximation of the number of HPV and Pap tests for screening or triage over 15 years of routine screening without consideration of abnormal results and associated follow-up.
Table 1 also presents a simple approximation of the number of HPV and Pap tests for screening or triage over 15 years of routine screening without consideration of abnormal results and associated follow-up. To examine the impact of varying screening interval, we considered both 3 and 5-year returns for primary HPV testing. Under primary HPV screening, many fewer Pap tests are conducted compared to primary Pap and cotesting. A 5-year screening interval for primary HPV screening could reduce the total number of screening tests by one-third to one-half of screening tests, compared to primary Pap every 3 years or cotesting every 5 years.
Our analysis of over 1 million women undergoing cotesting confirms the very low risk of cervical precancer and cancer after a negative HPV test; ie, it is mainly the HPV test component that provides the negative predictive value of cotesting. The 3-year safety (ie, reassurance against future risk of precancer and cancer) conferred by a negative HPV test exceeded the 3-year safety conferred by a negative Pap test as well as the 5-year safety conferred by a negative cotest. Recently, US practice guidelines have used comparisons of risk as a basis for deciding management of abnormal and normal screening results (7,8). Since both cotesting every 5 years and primary Pap testing every 3 years are recommended for primary screening, and given that primary HPV testing every 3 years might provide as much, if not more, reassurance than these two established screening methods, primary HPV testing merits consideration as another alternative for cervical screening.
The women enrolled in KPNC represent a generally well-screened population. We note that the relative patterns of risk between negative screening test results have also been observed in other cohort studies: a negative Pap test provides less reassurance against high grade cervical lesions than a negative HPV test or negative cotest (1,2,11–13).
This analysis focused on 3-year risks after a negative HPV test, because a 3-year screening interval is under consideration for initial introduction of primary HPV testing in the U.S. Yet, the optimal screening interval for primary HPV testing has not yet been established and might exceed 3 years, as has been advocated in Europe. European screening trialists suggest that HPV screening can be safely implemented with at least a 5-year interval, and countries are implementing extended screening intervals (1,12,14). At KPNC, the low CIN3+ and cancer risk among women testing HPV-negative extends for at least 4–5 years. In particular, the 5-year risks among women testing HPV-negative were lower than or statistically similar to the 3-year risks among women testing Pap-negative alone (CIN3+ = 0.14% vs 0.19%, P < .0001; cancer = 0.17% vs 0.20%, P = .24) and higher than or statistically similar to the 5-year risks among women testing cotest negative (CIN3+ = 0.14% vs 0.11%, P < .0001; cancer = 0.017% vs 0.014%, P = .112). Further consideration is required to define the optimal screening interval within the context of patient benefits (ie, cancer prevention) and harms (eg, increased screening visits, colposcopy and treatment). Analyses should also incorporate risks and resource utilization after multiple screening rounds across screening strategies.
This study also had some limitations. Although the KPNC cohort represents experience with cotesting in routine clinical practice, it does not directly characterize experience with primary HPV or primary Pap testing. We have estimated the impact of testing HPV-negative alone and Pap-negative alone, irrespective of the other cotest result, by ignoring the Pap result for women testing HPV-negative and ignoring the HPV result for women testing Pap-negative. Yet, a small proportion of women testing either Pap-negative or HPV-negative did not return to routine screening because their management was based upon the entire cotest result (both Pap and HPV tests). Specifically, 3.7% of women testing Pap-negative were concurrently HPV-positive and had a repeat screen in 1 year, with colposcopy referral if their screen was positive, while 0.44% of women testing HPV-negative had a concurrent Pap result of low-grade or worse, triggering immediate colposcopy referral. This more aggressive management would find more cases of CIN2/3, many of which might regress to normal histology within 3 years before being diagnosed in a primary HPV or Pap screening program. However, the aggressive management would also remove the few extra CIN2/3 that would have rapidly progressed to cancer within 3 years. Thus, compared to estimates from a real-life primary HPV or Pap testing program, our estimates for HPV-negative alone results and Pap-negative alone results likely somewhat overestimate risks of CIN2+ and CIN3+, and slightly underestimate cancer risk.
In conclusion, we find that primary HPV testing every 3 years might provide as much, if not more, reassurance against precancer and cancer, compared to primary Pap testing every 3 years and cotesting every 5 years. Health decision analyses are now imperative to identify the optimal screening interval and preferred screening strategy.
Dr. Schiffman and Dr. Gage have received HPV testing for research at no cost from Roche and BD. Dr. Castle has received compensation for serving as a member of a Data and Safety Monitoring Board for HPV vaccines for Merck. Dr. Castle has received HPV tests and testing for research at a reduced or no cost from Qiagen, Roche, MTM, and Norchip. Dr. Castle is a paid consultant for BD, GE Healthcare, Roche, Gen-Probe/Hologic, and Cepheid, and has received a speaker honorarium from Roche. No other authors report any conflicts of interest.
The Kaiser Permanente Northern California institutional review board (IRB) approved use of the data, and the National Institutes of Health Office of Human Subjects Research deemed this study exempt from IRB review.
References
- 1. Ronco G, Dillner J, Elfstrom KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. [DOI] [PubMed] [Google Scholar]
- 2. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385–1394. [DOI] [PubMed] [Google Scholar]
- 3. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–1238. [DOI] [PubMed] [Google Scholar]
- 5. Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891, W312. [DOI] [PubMed] [Google Scholar]
- 6. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1-–S27. [DOI] [PubMed] [Google Scholar]
- 8. Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S28-–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S56-–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 2+ and CIN 3+ among women with HPV-positive and HPV-negative LSIL Pap results. J Low Genit Tract Dis 2013;17(5 Suppl 1):S43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cox JT, Castle PE, Behrens CM, et al. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208(3):184 e1–184 e11. [DOI] [PubMed] [Google Scholar]
- 12. Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao FH, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol. 2010;11(12):1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Netherlands HCot. Population screening for cervical cancer. In: The Hague: Health Council of the Netherlands; 2011. [Google Scholar]


