POLICY BACKGROUND
Earlier diagnosis of symptomatic cancer has become increasingly recognised as holding the key to better cancer outcomes. Many Western governments have prioritised actions to achieve earlier diagnosis of cancer. England revised its cancer policy in 2007 to address this, with a National Awareness and Early Diagnosis Initiative (NAEDI) to drive forward research, development, and service improvement. Actions to improve care in England were led by the National Cancer Action Team (NCAT) through Cancer Networks (now NHS Improving Quality and Strategic Clinical Networks), with GP cancer leads playing a key role to influence general practices. Public awareness of cancer has been raised through a sustained programme of media campaigns, currently led by Public Health England.
The purpose of this article is to show how research to improve diagnosis of symptomatic cancer undertaken by the authors, together and in collaboration with others, has influenced policy and practice. Close engagement with the research community has been a feature of NAEDI throughout, and the research of this collaborative group has been supported via a designated NAEDI funding stream, through an National Institute for Health Research funded programme grant (DISCOVERY), and through the Department of Health’s Policy Research Unit for Cancer Awareness, Screening and Early Diagnosis.
PUBLIC AWARENESS OF CANCER SYMPTOMS
The Cancer Reform Strategy identified public awareness of ‘cancer warning signs’ and attitudes to help seeking as key domains for promoting early diagnosis. This resulted in development of a validated instrument, the Cancer Awareness Measure (CAM),1 followed by the Awareness and Beliefs about Cancer scale (ABC), which added questions on beliefs.2 The first national CAM survey found low public awareness of warning signs, with average recall of 2.4 symptoms, and most responders recalling only ‘lump’. Awareness was lower in men, the younger and oldest age-groups, and people of lower socioeconomic status and from ethnic minority backgrounds, and also that lower awareness was associated with greater anticipated delay in help seeking.1 Fear of ‘wasting the doctor’s time’ was also identified as a barrier to prompt help seeking, with international comparisons showing it to be substantially higher in the UK.2
The CAM was used widely around the UK to benchmark awareness and barriers and to inform local public health initiatives, and has also been used internationally. The results led to an ongoing programme of campaigns to raise symptom awareness and encourage help seeking, under the ‘Be Clear on Cancer’ (BCOC) banner. Recent results from repeat CAM surveys have shown clear evidence of effects on awareness, although less on attitudes to visiting the GP. Campaign effects have also been seen on GP consultations, use of diagnostic tests, and urgent referrals for suspected cancer, and preliminary results also suggest an impact on stage of diagnosis for lung cancer.
An early NAEDI action was a programme of research to better understand the process of cancer diagnosis in primary care. Over 1100 practices participated in one of the largest cancer audits ever undertaken in primary care, contributing data on 19 000 patients. It found that 80% or more of patients were referred to a specialist after one or two appointments, contributing to the public debate on timeliness of cancer referral. There was considerable variation between cancers, in the length of time patients took before deciding to consult3 and in the number of consultations before referral.4 These findings informed government action to improve GP access to diagnostic tests for cancer in 2011 and have since influenced the choice of target cancers for BCOC.
Detailed insights into the diagnostic challenges faced by GPs were gained through the use of Significant Event Audit (SEA) in Cancer Network-wide studies of specified cancers. Using novel methods of analysis, key themes emerged of clinical complexity as a cause of delay and the importance of safety netting in the consultation.5
Finally, a General Practice Research Database study quantified for the first time the interval from the first presentation to diagnosis for 15 common cancers and showed that this had reduced between 2001 and 2007, probably as a result of National Institute of Health and Care Excellence (NICE) guidance on urgent referral for suspected cancer.6
Building on these findings, NCAT predicated funding to Cancer Networks to support primary care on the specific encouragement of safety netting, practice plans, and the use of our SEA and clinical audit tools. In Wales, SEA for lung and upper gastrointestinal cancer became part of the Quality and Productivity domain of the Quality and Outcomes Framework. A practice Cancer Toolkit was also commissioned by NCAT to support these practice improvement activities. By April 2013, 16% of practices in England had made cancer plans, 19% had used the SEA tool, 21% had done a cancer audit, and 9% had implemented safety netting. Practices taking up one or more of these initiatives referred more patients for suspected cancer and showed less variation in their referral practice.7
RISK OF CANCER
Over the past 8 years, the authors have systematically identified and quantified the risk of cancer when a patient describes symptoms to their GP.8 Risk Assessment Tools (RATs) are the main product of that work. They were first provided by NCAT to GPs in 152 pilot practices in 2010, in the form of mousemats and desktop flipcharts. In an evaluation at the time, their use was associated with increased urgent referrals; 47 additional lung and 10 additional colorectal cancers were identified, some of favourable staging.9 In 2012 they were provided to all practices in England and have now been adopted by NICE in the revision of their guidance for GPs. Alongside risk prediction algorithms developed by researchers in Nottingham, they lie behind an electronic cancer decision support tool for suspected cancer, promoted by Macmillan Cancer Support. RATs are also being disseminated in Denmark and underpin cluster randomised controlled trials in Western Australia and England.
Current thresholds for referral or investigation range from a 2% risk upwards, depending on cancer site. They are largely implicit and determined by clinicians and policymakers. On the other hand, patient views elicited through their responses to clinical vignettes indicate that, for lung, colorectal, and pancreatic cancer, more than 80% would want to be investigated for suspected cancer even when the risk was as low as 1%.10
RESEARCH METHODOLOGY
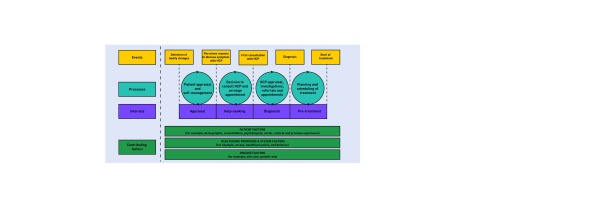
Studies of cancer diagnostic pathways are best conducted if they apply explicit methodological frameworks and report their findings consistently. Based on a systematic review of cancer studies that had applied the Andersen Model of Total Patient Delay, we developed the Model of Pathways to Treatment (Figure 1). This model is now widely used. It identifies key intervals and processes along the cancer diagnostic and treatment pathway and recognises the importance of patient, health system, and tumour factors in time to diagnosis and treatment.11
Figure 1.
Model of pathways to treatment. HCP = healthcare practitioner. Reproduced with permission of SAGE Publications Ltd.©Walter, 2012.11
In addition, the Department of Health and Cancer Research UK commissioned a landmark consensus statement on the conduct and reporting of studies of symptomatic diagnostic journeys.12 Its recommendations regarding methods and definitions are being widely adopted and should improve the quality of the evidence base in this area, making findings more transparent and comparable.
DIAGNOSTIC TECHNOLOGIES
There is significant interest in the potential of a range of diagnostic technologies to support early cancer diagnosis in primary care. Our work in melanoma diagnosis has trialled various diagnostic aids. The MoleMate Trial compared the use of a new imaging device, based on a technique called SIAscopy, against systematic application of the 7-point checklist (7-PCL). The authors found no difference between these two approaches in terms of appropriate referrals to urgent skin cancer clinics or in detection of melanoma.13 The authors were also the first to validate the 7-PCL in a primary care setting, suggesting that a cut-off score of 4 rather than 3 may be more accurate. The 7-PCL has been added to the Macmillan electronic cancer decision support tool for suspected cancer, and its effect on GP management of suspicious pigmented lesions is being studied using thickness of melanoma at diagnosis (a key determinant of prognosis) as the outcome measure.
INTERNATIONAL COMPARISONS
Cancer policymakers in the UK have always eyed mainland Europe with envy. It is now well accepted that our comparatively poor cancer outcomes are real, and, at least in part, based on an greater willingness on the part of primary care physicians in those countries to investigate for possible cancer. An ecological study showed a clear inverse relationship between 1-year relative survival after cancer diagnosis and three principles of gatekeeping systems, including the GP as the first point of contact.14 Denmark, a country with a closely comparable primary care system to the UK, has dramatically addressed this, liberalising access to cancer diagnostics, both directly and by referral, with guidance that roughly equates to ‘if the GP suspects cancer, refer’: a stance very different to that of NICE and Scottish Intercollegiate Guidelines Network guidance in the UK. Results are eagerly awaited from this policy decision, with early indicators suggesting that diagnostic intervals have fallen modestly (comparable to the reductions seen in the UK when the urgent referral pathways were introduced).6 Also imminent are the findings of a survey of GPs in 11 jurisdictions in the UK, Scandinavia, Canada and Australia. This has mapped out what cancer diagnostic facilities those GPs have access to, and has elicited their readiness to proceed to test or referral using clinical vignettes: the hypothesis being that jurisdictions with better 1 and 5 year cancer survival rates will also have GPs who would investigate for cancer sooner.
WHAT’S NEXT?
Our findings, particularly on patient preference and differences in clinical practice, have informed the recent announcement by England’s National Director of Cancer Services of a programme that will accelerate and coordinate NHS initiatives to improve diagnostic pathways, and be rigorously evaluated. It is likely that England will adopt some features of the Danish model, but with more emphasis on local solutions.
Novel biomarkers may assist in diagnosing symptomatic cancer earlier by adding value to other diagnostic tests and symptoms profiles. In due course they may also have value in case-finding in higher risk but pre-symptomatic individuals to find early stage disease that can be treated more effectively. They may also be used to risk-stratify ‘higher-risk’ groups for which specific risk reduction and primary prevention strategies are appropriate.
Clinical decision support (CDS) may evolve from its current narrow focus on specific cancers. Two members of this group are now collaborating with German experts in artificial intelligence and complex thinking, and with London Cancer, on the development of a next-generation electronic CDS tool that utilises a sophisticated artificial intelligence approach.
Funding
Richard Neal receives funding from Public Health Wales and Betsi Cadwaladr University Health Board.
Provenance
Commissioned; not externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.Robb K, Stubbings S, Ramirez A, et al. Public awareness of cancer in Britain: a population based survey of adults. Br J Cancer. 2009;101(Suppl 2):S18–S23. doi: 10.1038/sj.bjc.6605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes LJL, Simon AE, Warburton F, et al. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br J Cancer. 2013;108(2):292–300. doi: 10.1038/bjc.2012.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keeble S, Abel GA, Saunders CL. Variation in promptness of presentation among 10,297 patients subsequently diagnosed with one of 18 cancers: evidence from a national audit of cancer diagnosis in primary care. Int J Cancer. 2014;135(5):1220–1228. doi: 10.1002/ijc.28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyratzopoulos G, Abel GA, McPhail S, et al. Measures of promptness of cancer diagnosis in primary care: Secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. 2013;108(3):686–690. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell E, Rubin G, Macleod U. Understanding diagnosis of lung cancer in primary care: qualitative synthesis of significant event audit reports. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X660760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal RD, Din NU, Hamilton W, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2013;110(3):584–592. doi: 10.1038/bjc.2013.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ablett Spence I, Howse J, Gildea C, Rubin G. The NAEDI / Cancer Networks Supporting Primary Care programme 2012 to 2013. https://www.dur.ac.uk/resources/school.health/erdu/SupportingPrimaryCare2012to2103.pdf (accessed 8 Jul 2014) [Google Scholar]
- 8.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101(Suppl 2):S80–S86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton W, Green T, Martins T, et al. Evaluation of risk assessment tools for suspected cancer in general practice. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X660751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks J, Hollinghurst S, Bigwood L, et al. Preferences for cancer investigation: a vignette-based study of primary-care attendees. Lancet Oncol. 2014;15(2):232–240. doi: 10.1016/S1470-2045(13)70588-6. [DOI] [PubMed] [Google Scholar]
- 11.Walter F, Scott S, Webster A, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17(2):110–118. doi: 10.1258/jhsrp.2011.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller D, Vedsted P, Rubin G, et al. The Aarhus Statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter FM, Morris HC, Humphrys E, et al. Effect of adding a diagnostic aid to best practice to manage suspicious pigmented lesions in primary care: randomised controlled trial. BMJ. 2012;345:e4110. doi: 10.1136/bmj.e4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vedsted P, Olesen F. Are the serious problems in cancer survival partly rooted in gatekeeper principles? An ecologic study. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X588484. [DOI] [PMC free article] [PubMed] [Google Scholar]