Abstract
Cochlear implants are highly successful neural prostheses for persons with severe or profound hearing loss who gain little benefit from hearing aid amplification. Although implants are capable of providing important spectral and temporal cues for speech perception, performance on speech tests is variable across listeners. Psychophysical measures obtained from individual implant subjects can also be highly variable across implant channels. This review discusses evidence that such variability reflects deviations in the electrode–neuron interface, which refers to an implant channel's ability to effectively stimulate the auditory nerve. It is proposed that focused electrical stimulation is ideally suited to assess channel-to-channel irregularities in the electrode–neuron interface. In implant listeners, it is demonstrated that channels with relatively high thresholds, as measured with the tripolar configuration, exhibit broader psychophysical tuning curves and smaller dynamic ranges than channels with relatively low thresholds. Broader tuning implies that frequency-specific information intended for one population of neurons in the cochlea may activate more distant neurons, and a compressed dynamic range could make it more difficult to resolve intensity-based information, particularly in the presence of competing noise. Degradation of both types of cues would negatively affect speech perception.
Keywords: cochlear implant, electrode configuration, neural survival, spectral resolution, psychophysics
Introduction
Many patients with severe to profound sensorineural hearing loss who do not receive substantial benefit from hearing aid amplification have improved listening capabilities after cochlear implantation. The average performance with cochlear implants has continued to improve as technology and sound processing techniques have progressed, and as people with more residual hearing have received implants. Nevertheless, the range of performance, especially on complex listening tasks, remains substantial. Several peripheral factors could be contributing to this variability, including degeneration of the spiral ganglion, electrode insertion trauma leading to bone and tissue growth, and placement of electrodes away from neural elements. More central factors, such as degeneration of neurons in the central auditory pathway and cognitive factors may also influence performance. However, the variability of psychophysical measures across channels within individual implant listeners suggests that peripheral factors are the primary contributors, because these often vary widely across the cochlea.
In this article, we describe the influence of peripheral factors on cochlear implant perception in the context of how individual implant electrodes interface with the auditory nerve: the “electrode–neuron interface.” Based on recent studies from our laboratory and others, we propose the use of focused electrical stimulation to evaluate and identify channels having poor electrode–neuron interfaces. Specifically, we show that channels exhibiting high thresholds with focused stimulation have relatively broad spatial selectivity and steeper growth of loudness, which leads to a smaller dynamic range. These properties are discussed in relation to their potential contribution to within- and across-subject variability on psychophysical measures and speech perception.
Spectral Encoding With Cochlear Implants
Before discussing the various factors that might cause poor cochlear implant performance, we will briefly review how an implant system works under ideal conditions. The implantable portion consists of a linear array of metal electrode contacts, numbering from 16 to 22 depending on the particular device, which connect via insulated leads to a processing unit. The array is surgically inserted into the scala tympani, one of the cochlea's three fluid-filled compartments, and typically spans a length over which basilar membrane motion would encode 3 to 4 octaves in frequency. The implant processor decomposes an incoming audio signal, detected by an externally worn microphone, into a discrete number of spectral bands and transmits current pulses to the multiple electrodes, with amplitudes that vary in time according to the energy in each band.
Spectral information in the original acoustic signal is encoded in the spatial pattern of stimulation along the implant array. Mimicking the tonotopic organization of the cochlea, each electrode carries information in a frequency range that, in normal hearing, would be transduced by hair cells and nerve fibers at the portion of the basilar membrane nearest the electrode: electrodes deeper in the cochlea (apical) carry lower frequency information, whereas those closer to the round window (basal) carry higher frequency information (Waltzman & Roland, 2006, chap. 4). With an implant, this tonotopic ordering is imposed by the clinical mapping procedure, which assigns each analyzed frequency band to one implant channel. The spectral content of a signal is thus transmitted as a frequency-place code, with each successive spectral band stimulating a progressively more basal section of the cochlea. As described later, the particular subset of electrodes that constitute a channel can greatly affect how the electrical current delivered by that channel ultimately activates the auditory nerve.
Numerous psychophysical studies have shown that both spectral and temporal cues contained in an acoustic stimulus are used by cochlear implant listeners (e.g., Xu & Pfingst, 2008). Temporal information is transmitted as pulse-to-pulse changes in current amplitude on individual channels (i.e., within each frequency pass band). In speech perception, for instance, temporal cues include the prosody, or rhythm, of spoken words, and transitions from one phoneme to the next. However, several lines of evidence indicate that implant listeners rely heavily on frequency-place coding, underscoring the critical nature of spectral cues for understanding speech and other sounds. For example, subjects better able to rank the pitches of individual electrodes (Donaldson & Nelson, 2000) or to use more channels of information (Friesen, Shannon, Baskent, & Wang, 2001) tend to perform better on speech recognition tasks. Pitch estimates for frequencies above 300 Hz appear to rely more on place cues than temporal cues (Zeng, 2002). Moreover, stretching, compressing, or shifting frequency allocations to implant channels can have a profound effect on speech perception (Baskent and Shannon, 2004), similar to manipulations of spectral cues presented to normal hearing listeners (e.g., Baskent & Shannon, 2004; Dorman & Loizou, 1997; Fu & Nogaki, 2005; Throckmorton & Collins, 2002). It is clear from these studies that the selectivity with which individual channels activate the cochlea has a profound impact on speech perception.
The Electrode–Neuron Interface
We refer to the process by which a stimulus applied to a given implant channel excites a population of auditory nerve fibers as the “electrode–neuron interface.” This term encompasses details about the electrode array itself, specifically how it is positioned within the cochlea and the relative locations of each electrode site, as well as patient-specific pathologies such as bone growth and the condition of the neural substrate. Our present understanding of the electrode–neuron interface is derived from anatomical and physiological studies, as well as from computer models. However, psychophysical studies of variability among subjects or among channels within the same subject can also provide important information about the interface. In this section, we will first review the basic process of electrical stimulation in the cochlea, especially in the context of spectral cues, then discuss the components of the electrode–neuron interface that influence the efficacy of that stimulation.
Current Flow and Channel Interaction
An implant channel generally consists of one active electrode along the cochlear array and one or more return electrodes, which may be inside or outside the cochlea. The active electrode delivers the intended current waveform (e.g., a train of biphasic pulses, cathodic phase first), and the return electrodes carry the opposite polarity of current to close the circuit loop. The flow of current establishes a potential field in the cochlea. Most of the current flows along the fluid-filled scala tympani, but some also flows into the less conductive bone adjacent to it, the osseous spiral lamina. Within this portion of the cochlea lie the spiral ganglion neurons, whose axons form the auditory nerve. At a high enough current, the resulting potential is sufficient to charge the membrane of some of the neurons, triggering a volley of action potentials in the auditory nerve (e.g., van den Honert & Stypulkowski, 1987a, 1987b).
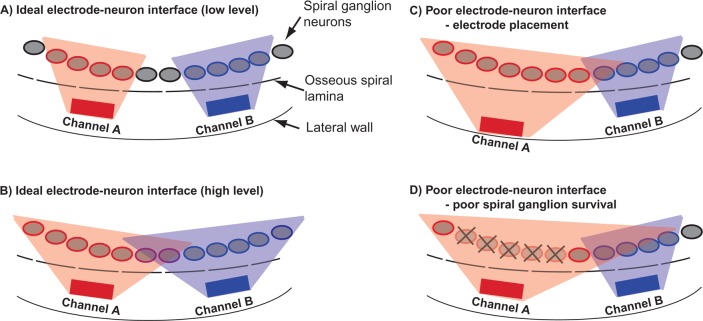
The ideal transmission of spectral information requires that different electrode channels stimulate spatially distinct, though possibly overlapping populations of auditory neurons. This concept of channel independence is illustrated in Figure 1A for two electrodes delivering relatively low levels of current simultaneously or near-simultaneously. The gray swaths emanating from each electrode represents the spread of electrical potential or current that is just sufficient to produce spiking activity in a small number of spiral ganglion neurons, depicted here as four similarly shaded ovals. (Note that the uniform shading and abrupt edges of the potential field do not reflect the expected attenuation of the field with distance from the current source.) Gray ovals with black outlines are not activated by either electrode, implying no overlap of the two activated neural populations. In Figure 1B, higher current levels produce wider potential fields and subsequently broader neural activation along the tonotopic extent of the cochlea. The larger number of active neurons would likely result in a louder percept than in Figure 1A, but it also causes partial overlap of the two neural populations. The excitation of common neural elements with dual-channel stimulation can ultimately affect perception of the separate stimulus components, a phenomenon referred to as channel interaction (Bierer, 2007; Bierer & Faulkner, 2010; Boëx, Kos, & Pelizzone, 2003; Chatterjee et al., 2006; de Balthasar et al., 2003; Kwon & van den Honert, 2006; McKay et al., 1996; Nelson et al., 2008; Stickney et al., 2006).
Figure 1.
Schematic of the electrode–neuron interface
Two cochlear implant channels are represented by gray rectangles, spiral ganglion neurons by gray ovals, and the edge of the osseous spiral lamina by a dashed line. The spatial extent of currents required to activate neurons for each channel are indicated by the gray shaded areas, respectively. (A) An ideal electrode–neuron interface is shown such that each channel activates nearby and nonoverlapping populations of neurons. (B) Higher stimulation levels produce broader spatial extents of currents and overlapping neural populations. (C and D) A poor electrode–neuron interface is shown as a result of placement of channel A near the lateral wall of the scala tympani (C) and as a result of spiral ganglion cell loss (D). Both scenarios require a higher stimulus current for Channel A to stimulate the closest viable neurons, resulting in broader excitation and greater overlap with the neurons activated by Channel B.
Acoustic stimulation is generally “multichannel” in the sense that different frequency components, having different time-varying amplitudes, can fall within the passband of the same neuron. In this respect, channel interaction is an essential aspect of the normal hearing process. Interactions between spectral components have been well documented in studies presenting sounds simultaneously or successively (e,g., Moore, 2002; Oxenham & Shera, 2003). Likewise in electrical hearing, channel interactions have been observed in response to both simultaneous and nonsimultaneous pulse trains, the former presumably governed by the summation of current in the cochlea (Bierer 2007; de Balthasar et al., 2003; Stickney et al., 2006) and the latter by forward-masking effects in the auditory nerve and central pathways (Bierer & Faulkner, 2010; Boëx et al., 2003; Chatterjee et al., 2006; Kwon & van den Honert, 2006; Nelson et al., 2008). As in acoustic hearing, some degree of channel interaction is expected and probably desirable for discriminating complex sounds. Nevertheless, studies have shown that a higher degree of channel interaction is associated with poorer performance on speech recognition tests (Boëx et al., 2003; Throckmorton & Collins, 1999). In the remainder of this section, we describe how a poor electrode–neuron interface can reduce the spatial acuity of multichannel electrical stimulation, which can lead to undesirable channel interaction.
Within-Subject and Across-Subject Variability
Although the average performance with cochlear implants continues to improve with technological advances and expansion of candidacy criteria, there continue to be patients who perform excellently on speech-in-quiet tests, with scores near 100%, and patients who perform rather poorly, with scores as low as 0% (e.g., Koch et al., 2004). Only a small portion of this variability appears to be accounted for by etiology and duration of deafness and age at onset of hearing loss (Gantz et al., 1993; Gfeller et al., 2008). So a pertinent question is, what are the primary factors determining an individual's performance with a cochlear implant? One clue may be that there is non-negligible variability within an implant subject, from channel to channel. For example, our lab and others (Bierer, 2007; Mens & Berenstein, 2005; Pfingst & Xu, 2004) have recently shown that single channel thresholds across the array are highly variable for some subjects. The implication of these findings is that those electrodes with thresholds higher than their neighbors are not effectively stimulating the auditory nerve. Given that channel-to-channel threshold variability is highly correlated with speech scores (Bierer, 2007; Long et al., 2010, Pfingst et al., 2004), understanding the factors leading to a poor electrode–neuron interface should give better insight into limitations on overall performance.
Electrode Position
Charge disperses and voltage decreases with distance from a source electrode, the decrease in voltage requires that the applied current must be higher to activate a target neuron (Grill, 1999). For this reason, the location of the electrode array within the cochlea is an important aspect of the electrode–neuron interface (e.g., Shepherd et al., 1993). The schematic in Figure 1C illustrates the effect of radial electrode placement on auditory nerve activation near threshold. Compared with Channel B, Channel A is more distant from the inner wall, or osseous spiral lamina, where the spiral ganglion neurons and their processes reside. As supported by volume conduction models (Goldwyn et al., IN PRESS; Jolly et al., 1996; Litvak et al., 2007), the higher current level necessary to activate the neurons closest to Channel A produces a larger electrical field which activates additional neurons further along the cochlea. The result is a broader excitation pattern than that of the more closely positioned Channel B.
Imaging studies are consistent with this simplified relationship of electrode position, threshold, and spread of excitation. Using an x-ray technique to estimate the position of individual electrodes in the scala tympani, Cohen et al., (2006) demonstrated an inverse relationship between radial electrode–neuron distance and threshold and comfort levels. More recent techniques based on three-dimensional computed tomography (CT) have corroborated this finding (Finley et al., 2008; Long et al., 2010). In addition, thresholds in patients whose device was precurved (Cohen et al., 2006) or implanted with a positioner (Donaldson et al., 2001)—intended to hold the array close to the inner wall of the cochlea—were lower than in patients with traditional arrays. Forward-masking measures revealed more selective channel activation with precurved arrays, consistent with a closer electrode–neuron distance (Cohen et al., 2006; Hughes & Abbas, 2006; but see also Boëx et al., 2003).
Misplacement and Obstructed Current Paths
Histological (Wardrop et al., 2005) and imaging studies (Finley et al., 2008; Skinner et al., 2007) have documented cases where the array strayed out of the scala tympani during surgery or was inserted into the wrong scala. In these situations, the distance from the affected electrodes to the nearest region of the spiral ganglion is effectively greater. Indeed, recent CT analyses indicate poorer speech performance when a portion of the array has entered the the scala vestibuli (Finley et al., 2008).
Damage to the cochlea during surgery, including insertion trauma and effects of drilling, can cause growth of bone and fibrous tissue (Fayad et al., 2009; Li et al., 2007; Somdas et al., 2007). This, in turn, can form regions of high impedance around the implant array, altering the pathway that current must take to activate the auditory nerve (Hanekom, 2005). As in the case of displaced arrays, the effective electrode–neuron distance is increased, leading to elevated thresholds in some patients (Kawano et al., 1998).
Spiral Ganglion Loss
Patients with severe to profound hearing loss have widely varying degrees of spiral ganglion survival. Postmortem analyses of temporal bones reveal neuron counts of less than one third to almost 80% of those in age-matched healthy ears (Hinojosa & Marion 1983; Nadol, 1997; Ng et al., 2000). Neuron loss generally varies from segment to segment (Fayad & Linthicum, 2006; Khan et al., 2005), with electrodes near patches tending to have elevated thresholds and comfort levels (Khan et al., 2005). Figure 1D depicts the effect of a discrete spiral ganglion “dead region” (depicted as Xs) on neural activation. A higher current level on Channel A is required to activate neurons on either edge of the dead region, including those closer to, and normally targeted by, Channel B. As described previously, this condition can degrade spatial selectivity, limiting the transmission of spectral cues. Note that a degraded condition of otherwise intact spiral ganglion neurons, such as demyelination or other changes in membrane properties, can make individual neurons less or even more stimulable at typical current levels. Although the focus of this review is the increase in current level requirements in the presence of reduced spiral ganglion survival, as conveyed by Figure 1D, these other factors can also lead to localized increases in excitability.
Channel Independence
Evidence of degraded spectral selectivity in cochlear implant listeners can be found in studies manipulating the number of active stimulus channels. Even when all channels are available to an implant subject, the discrete number of tonotopic activation sites, usually covering only the first one-and-a-half turns of the cochlea, places an upper limit on spectral range and spectral resolution. However, a number of studies have demonstrated that most implant listeners perform as well with six to eight channels as with all channels on speech perception measured in quiet or background noise (Fishman et al., 1997; Friesen et al., 2001). Normal hearing subjects listening to cochlear implant simulations, on the other hand, continue to improve in speech perception as the number of spectral channels increases beyond eight (Dorman et al., 1998; Friesen et al., 2001; Shannon et al., 1995; Xu et al., 2002). As suggested by the schematics of Figures 1C and 1D, if poor electrode-to-neuron interfaces contribute to the reduced number of effective spectral channels, then the affected electrodes should have relatively elevated thresholds. In the next section, we describe recent findings in our laboratory that support a connection between elevated thresholds and degraded spectral resolution.
Identifying Poor Electrode–Neuron Interfaces
Channel-to-Channel Variability in Threshold
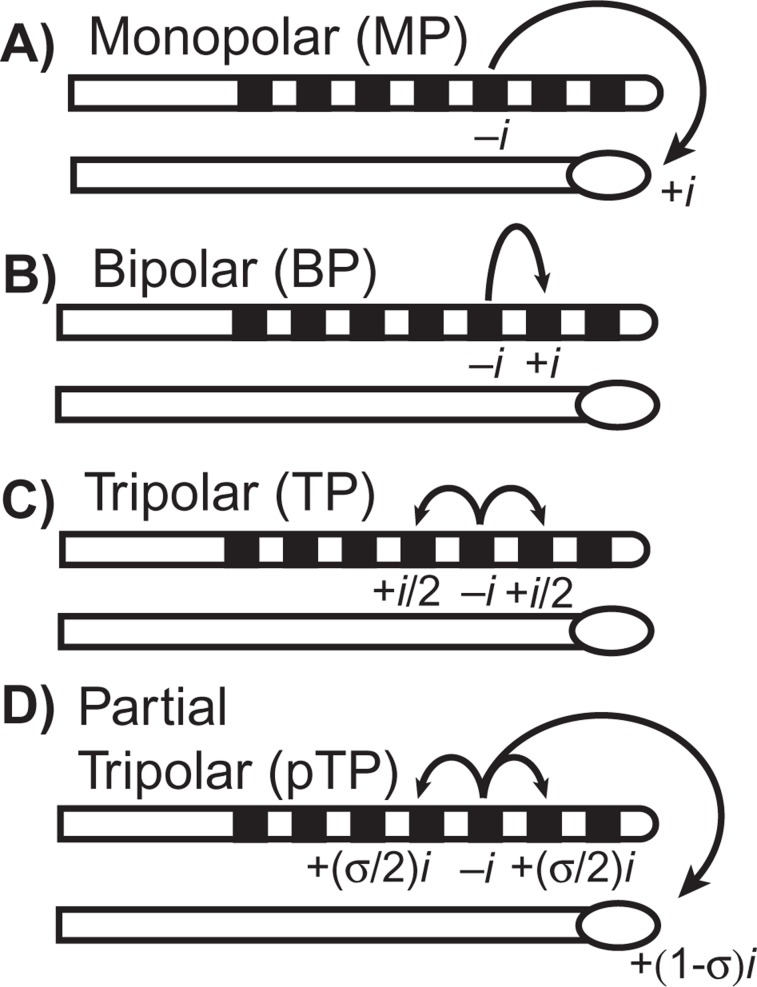
Intrasubject channel-to-channel variability in threshold and most comfortable level (MCL) has been reported since the first use of multichannel cochlear implants, and researchers have speculated that it is largely a result of variation in the electrode–neuron interface (Bierer, 2007; Bierer & Faulkner, 2010; Busby et al., 1994; Nelson et al., 2008; Pfingst et al., 2008; Pfingst & Xu, 2004). The degree of variability, however, is greater when a focused electrode configuration is used. Electrode configuration refers to the arrangement of active and return electrodes that defines a channel, and the resulting flow of current in the cochlea depends on the location of, and distance between, these electrodes. Most clinical devices today are programmed with the monopolar configuration, which uses one of the intrascalar electrodes as the active electrode and an extracochlear electrode as the return. As drawn in Figure 2A, the longitudinal spread of current with the monopolar configuration is relatively broad. More restricted current spread can be achieved when some or all of the return current is carried by intrascalar electrodes, as with the bipolar (Figure 2B), tripolar (Figure 2C; sometimes referred to as quadrupolar; e.g., Jolly et al., 1996) and partial tripolar (Figure 2D) configurations. Physiological studies in animal models have demonstrated that the focused electrical fields produced by bipolar and tripolar configurations elicit narrower patterns of neural activation in the central auditory system compared with the monopolar configuration (Bierer & Middlebrooks, 2002; Bonham & Litvak, 2008; Kral et al., 1998; Synder et al., 2004; Synder et al., 2008; van den Honert & Stypulkowski, 1987a). Figure 3, for example, shows progressively narrower tonotopic activation in the inferior colliculus for monopolar, partial tripolar and tripolar configurations, which differ by the proportion of return current flowing to two flanking electrodes in the scala tympani (0%, 70%, and 100% respectively). Focused electrode configurations presumably stimulate a more localized region of the spiral ganglion and should theoretically be more sensitive to local variations in electrode–neuron interface. Consistent with this hypothesis, Pfingst and Xu (2004) observed greater channel-to-channel variability in threshold for the bipolar configuration than the monopolar configuration.
Figure 2.
Schematic of electrode configurations
(A) The monopolar configuration uses one of the intrascalar electrodes as the active electrode and an extracochlear electrode as the return. (B) The bipolar configuration has both the active and return electrodes in the scala tympani, usually separated by one or more inactive electrodes. (C) The tripolar (sometimes referred to as quadrupolar) configuration uses an intrascalar active electrode and two adjacent electrodes that equally share the return current. (D) Partial tripolar is a hybrid between monopolar and tripolar configurations whereby a fraction (σ) of the return current is delivered to the flanking electrodes while the remainder flows to the distant extracochlear ground.
Figure 3.
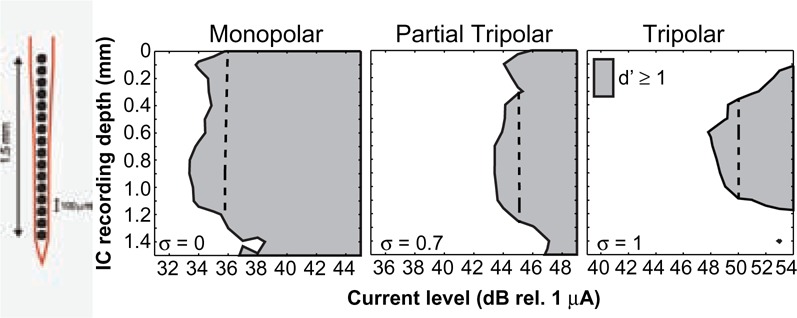
Neural activation patterns from the inferior colliculus of the anesthetized guinea pig for three electrode configurations
Responses were recorded across 16 sites covering approximately 3 octaves of the tonotopic axis of the inferior colliculus, shown on the ordinate (in mm). Stimuli were single, biphasic electrical pulses presented over a range of levels (abscissa) for three stimulus configurations, monopolar (left), partial tripolar with σ = 0.7 (middle), and tripolar (right). Neural responses are considered above threshold when the driven spike activity can be distinguished from a no-stimulus condition with a d' of at least 1. The dashed line in each panel represents 2 dB above the threshold of the most sensitive neuron in the inferior colliculus to that stimulus. For a more detailed description of the methods and additional examples of IC activation patterns see Snyder et al. (2004), Bonham and Litvak (2008), and Middlebrooks et al. (2008).
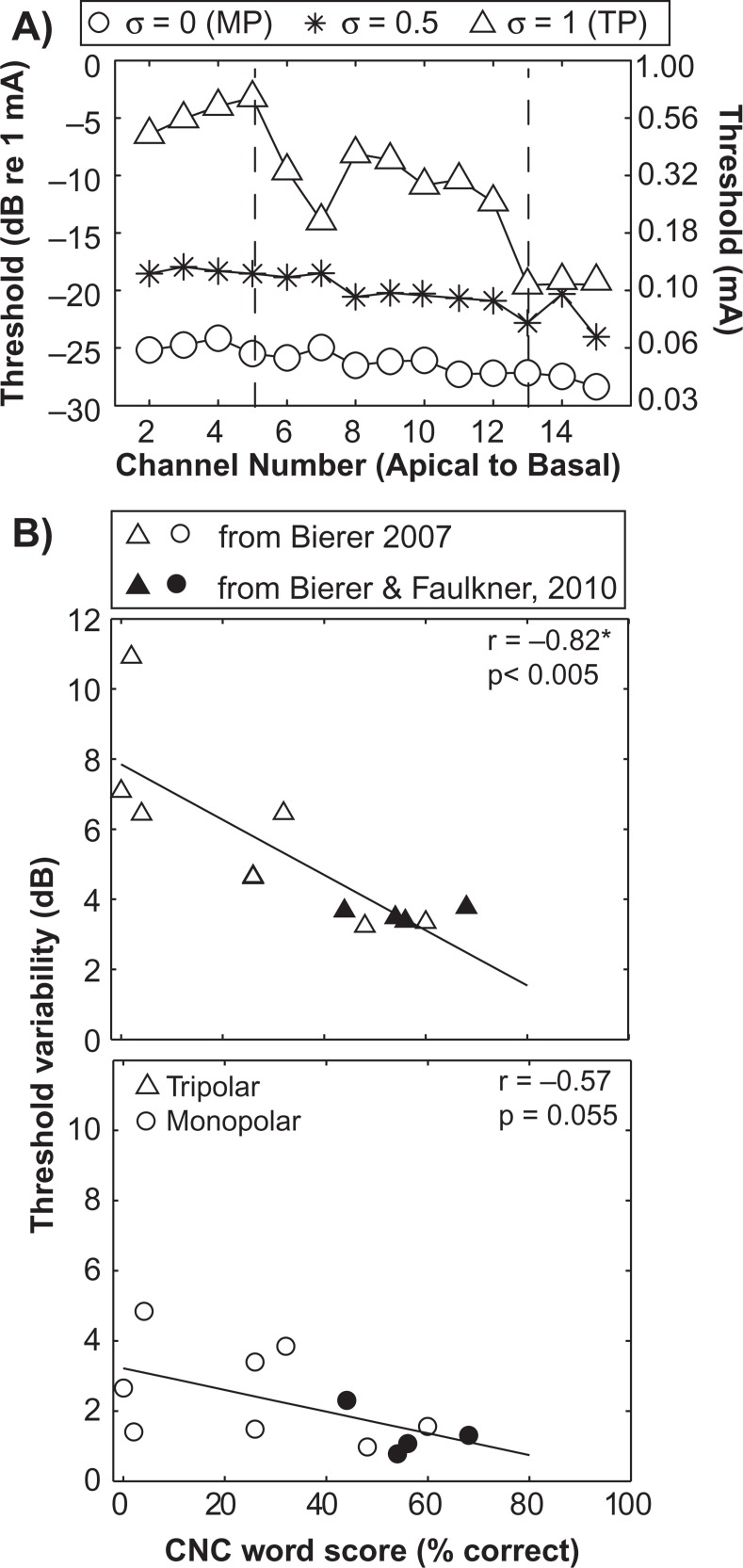
We have extended the findings of Pfingst and Xu (2004) by demonstrating even greater cross-channel variability in threshold with the tripolar configuration, which produces more restricted tonotopic activation than bipolar (Bierer & Middlebrooks, 2002; Kral et al., 1998; Snyder et al., 2004). Figure 4A plots behavioral thresholds measured in one cochlear implant listener using the monopolar (circles) and tripolar (triangles) configurations across channels. It is clear that thresholds across the array are higher for the more focused tripolar condition, which follows from physical principles of electrical conduction (Jolly et al., 1996). (For the partial tripolar configuration [asterixes], which is a hybrid of the monopolar and tripolar modes, thresholds fall between those in monopolar and tripolar modes.) More important, the channel-to-channel variability, quantified as the standard deviation of the differences between all neighboring channels' thresholds (Bierer, 2007), increases from 0.78 dB for the monopolar to 3.48 dB for the tripolar configuration. One explanation for this finding is that channels having a high tripolar threshold reflect some type of degraded electrode–neuron interface (Figures 1C and 1D), such that higher current is required to activate the nearest viable auditory neurons. The relatively flat threshold profile with the monopolar configuration, on the other hand, might reflect its broader electrical field, requiring only a little additional current to activate viable neurons.
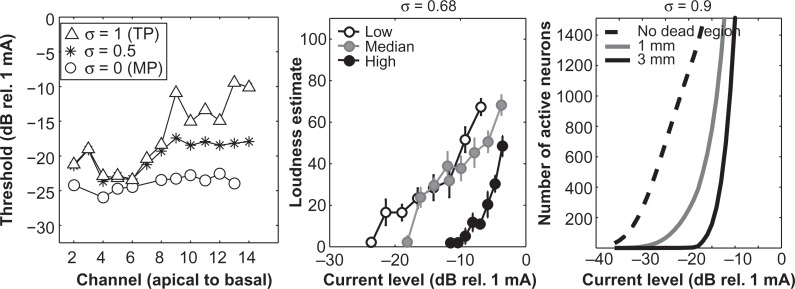
Figure 4.
Behavioral thresholds and speech perception
(A) Behavioral thresholds (ordinate) for one cochlear implant listener for monopolar, partial tripolar and tripolar electrode configurations across channels from apical to basal (abscissa). (B) Intrasubject variability in threshold measures (ordinate) as a function of speech scores on consonant–nucleus–consonant (CNC) words (abscissa). Variability was determined by calculating the standard deviation of the unsigned difference between thresholds of adjacent tested channels. Each triangle represents data from an individual subject. Open symbols are from Bierer (2007) and filled symbols are from Bierer and Faulkner (2010). The Pearson correlation coefficient (r) and significance level (p) are shown in each panel. The best-fitting line by least-squares error criterion is plotted.
We also find a negative correlation between speech perception and channel-to-channel threshold variability (Bierer, 2007; Bierer & Faulkner, 2010). An example of this relation is shown in Figure 4B, where variability in threshold for tripolar stimulation is plotted as a function of the percent correct scores for consonant–nucleus–consonant (CNC) words (e.g., “goose,” “name,” “jar”; Peterson and Lehiste, 1962). Similar correlations have been observed for bipolar (Pfingst et al., 2004) and another type of focused configuration known as “phased array” (Long et al., 2010). In contrast, results with the monopolar mode have been less consistent, with channel-to-channel variability exhibiting a significant correlation with speech scores in one study (Pfingst et al., 2004) and no correlation in two other studies (Bierer, 2007; Long et al., 2010). The negative results underscore the monopolar configuration's insensitivity to local irregularities in the electrode–neuron interface.
Tuning Properties
Speech measures provide only a global indication of potential deficits in spectral/spatial resolution and are also dependent on temporal information. A more specific estimate of the spatial resolution associated with individual channels might inform clinicians how to better create a patient-tailored cochlear implant program. The literature on hearing aids, for which amplification is tailored to patients with variable acoustic thresholds across frequency—and presumably the cochlea—suggest an approach to the problem. Recently, Moore and colleagues developed a technique based on psychophysical tuning curves (PTCs) to identify cochlear “dead regions,” localized segments of inner hair cell and/or spiral ganglion cell loss (Markessis et al., 2006; Moore & Alcantara, 2001). A PTC describes the minimum level of one sound (the masker) needed to suppress the detection of a subsequent sound (the probe), as a function of frequency. The shape of the function approximates the cochlear activation pattern in response to the probe, and generally its minimum or “tip” is close to the probe frequency. Moore determined that PTCs are a sensitive diagnostic tool to detect locations of discrete dead regions. For probe frequencies normally encoded at the location of a putative dead region, the tip may be shifted to the basal or apical edge of the dead region. In other cases, the dead region may cause widening of the PTC. In cochlear implantees, we predicted that the PTC technique would also be a sensitive indicator of channels affected by a poor electrode–neuron interface.
In a recent study, we obtained PTCs in five subjects for whom the putative status of the electrode–neuron interface was first determined by measuring thresholds with the tripolar configuration (Bierer & Faulkner, 2010). We then chose the channels with the highest and lowest thresholds with the tripolar configuration for tuning curve analysis. For the PTC, the desired probe level could often not be applied with the tripolar configuration, because it would have exceeded the voltage compliance limit of the implant system (see also Mens & Berenstein, 2005). For that reason, the PTC stimuli were delivered in the partial tripolar configuration, a hybrid between monopolar and tripolar configurations. In this stimulation mode, a fraction of the return current (σ in Figure 2D) is delivered to two flanking electrodes while the remainder flows to the distant extracochlear ground. Thus, a fraction of zero is equivalent to the monopolar mode (no return current is directed to the intracochlear neighboring electrodes); while a fraction of one is the tripolar mode (all return current is directed to the intracochlear electrodes). We chose the highest partial tripolar fraction for each subject that allowed both probe channels to be stimulated with the same fraction. We then constructed a set of forward-masked PTCs, each based on a fixed probe and variable masker channel and current level. The probe was a 10-ms pulse train at one of the tested channels, at a low level 3 dB above threshold and at one of two current fractions (monopolar, σ = 0 or partial tripolar, σ ≥ 0.55); the masker was a 200-ms pulse train at a fraction of σ = 0.5 applied to each channel (one at a time) at various current levels.
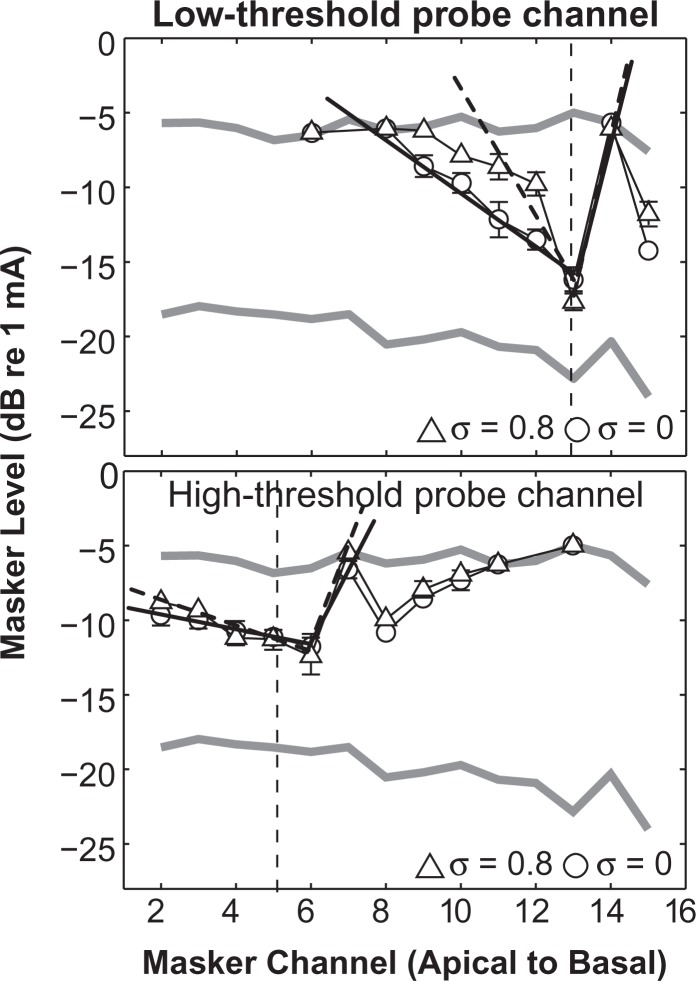
Figure 5 displays examples of PTCs measured in the same subject for probes delivered to the low-threshold channel (Channel 13, top) and to the high-threshold channel (Channel 5, bottom) with monopolar (circles) and partial tripolar (triangles) probe configurations. The curves indicate the level of masking required to just suppress detection of the probe. With the partial tripolar configuration, the PTC of the low-threshold channel was narrower than that of the high-threshold channel. For some subjects, in addition to broader tuning for high-threshold channels, there was also a tip-shift in the PTC, such that the greatest degree of masking occurred on, for example, Channel 6 rather than the on-probe Channel 5. The monopolar probe condition gave the same result: The high-threshold channel had a broader and tip-shifted PTC. All five subjects tested in this manner showed broader tuning for the high-threshold channel, and two of the five subjects showed a tip shift (Bierer & Faulkner, 2010).
Figure 5.
Forward-masked psychophysical tuning curves (PTCs).
PTCs were measured in the same subject as plotted in Figure 4. The masker level (ordinate) needed to just mask the detection of a fixed level probe is plotted as a function of masker channel (abscissa). The thick gray lines indicate the masker-only threshold and most comfortable levels, that is, the range of masker levels possible. The vertical dashed line indicates the location of the probe channel. The symbol indicates the probe configuration, circles for monopolar and triangles for partial tripolar. The probe stimulus level was fixed to 3 dB above the threshold for the probe-only threshold. The top and bottom panels represent PTCs for the probe channels with the lowest and highest tripolar thresholds, respectively. The bold lines were used to estimate the apical and basal slopes of the PTCs (dashed for partial tripolar probe and solid for monopolar probe). Apical and basal slopes of the PTCs were calculated based on a least-square error line between the tip of the tuning curve (i.e., the lowest masker level required to mask the probe identified using normalized masker levels) and the point at which the curve crossed 80% of masker dynamic range in either direction. The best-fit line was calculated from the raw masker levels in units of decibels per millimeter. Error bars represent one standard deviation of the mean for each data point and are shown when the error is greater than the size of the symbols.
An effect of probe configuration was also evident in our study. The PTCs of the subject in Figure 5 were sharper for the partial tripolar configuration, especially for the low-threshold channel (top). Across channels and subjects the sharpness of tuning, quantified by apical slope and the width at half height (see figure caption for details), was significantly narrower for the partial tripolar than for the monopolar configuration probes (see figures 6 and 7 in Bierer & Faulkner, 2010). Even with the less selective monopolar probe configuration, however, higher tripolar configuration thresholds were predictive of broader tuning. Importantly, the data with the monopolar configuration suggest that probe level does not greatly contribute to the tuning differences because, for some subjects, the monopolar probe levels were similar for the (tripolar) low- and high-threshold channels.
Figure 6.
Growth of loudness and neural activation
(Left) As in Figure 4, behavioral thresholds (ordinate) for one cochlear implant listener for monopolar, partial tripolar, and tripolar electrode configurations across channels from apical to basal (abscissa). (Middle) Loudness estimations measured in a single subject using the partial tripolar electrode configuration (σ = 0.68) for the channels with the lowest (open), median (gray), and highest (filled) tripolar threshold. (Right) Model simulation of auditory neuron recruitment as a function of current for three conditions of neural survival: 0 mm or no dead region (dotted line), 1 mm dead region centered on the active electrode (gray line), 3 mm dead region (black line). Configuration was partial tripolar at σ = 0.9 and the electrode-to-neuron distance was 0.8 mm. The cochlea was modeled as a cylinder with an abrupt fluid/bone conductance boundary; 33,000 neurons were distributed along the cochlea in the outer compartment in clusters of 100. The potential field due to three-point current sources spaced longitudinally inside the cylinder (modeling the active and return tripolar electrodes) was calculated analytically and subsequent neural activation was based on a second spatial derivative of the potential. See Goldwyn et al., IN PRESS for details.
These findings support the concept that implant channels with high thresholds, when tested with a focused electrode configuration, are indicative of a poor electrode–neuron interface. The broader tuning curves for these channels indicate a greater degree of channel interaction (forward masking in this case), leading to degraded resolution of spatial information conveyed to the auditory system. It should be pointed out that the thresholds with the monopolar mode in these subjects showed relatively less variation across channels, so that high- and low-threshold channels identified using the monopolar configuration would not be expected to exhibit a systematic relationship with spatial resolution.
A consistent effect of electrode configuration on forward-masked tuning properties is not evident in previous psychophysical studies. Nelson et al. (2008) also measured sharper PTCs with bipolar compared with monopolar stimuli. Other studies, however, have shown no significant effect of configuration on tuning (Boëx et al., 2003; Chatterjee et al., 2006; Cohen et al., 2003; Kwon & van den Honert, 2006; Shannon, 1990). Several methodological differences may explain the discrepancy across these studies. First, the latter studies used a fixed masker and variable probes, an approach which generally requires higher probe levels. The broader spread of activation at higher levels could have obscured a configuration effect. Second, the other studies compared monopolar with bipolar stimulation, which volume conduction and animal models have shown is not as restricted as the tripolar configuration (Bierer & Middlebrooks, 2002; Jolly et al., 1996; Kral et al., 1998; Snyder et al., 2004). Third, in the studies showing no effect of configuration, both the masker and probe were set to the same configuration (i.e., monopolar/monopolar and bipolar/bipolar). In our study, we used the same masker configuration, partial tripolar (σ = 0.5), for both the monopolar and partial tripolar probe configurations (σ = 0.55), to better isolate the effect of probe configuration on spatial tuning properties. Last, the choice of test channels may have contributed to the variable outcomes. In the previous studies, either one channel was chosen in the middle of the array, or several channels distributed along the cochlea were chosen (e.g., apical, middle, or basal). Our recent data suggest that channels with a high tripolar configuration threshold exhibit a smaller effect of configuration (e.g., Figure 5, bottom panel). Thus, some of the variability in the previous studies may have been the result of differences in the electrode–neuron interfaces of the chosen channels. Further experiments should be conducted to determine the interplay of electrode configuration with the underlying electrode–neuron interface.
Loudness
In addition to identifying channels with broad spatial excitation patterns, our preliminary data indicate that channels with high thresholds measured with the tripolar configuration also have relatively small dynamic ranges and steep growth of loudness. The left panel of Figure 6 displays threshold data for the tripolar, monopolar, and partial tripolar (σ = 0.5) configurations. Growth of loudness measured for the lowest, median, and highest tripolar configuration threshold channels are shown in the middle panel of Figure 6. The functions for the high-threshold channel were steeper than for the other channels and have a narrower dynamic range. Such variability in the slope of loudness functions has also been observed by Cohen (2009), including functions obtained on different channels from the same subject. High thresholds with the tripolar configuration and steep growth of loudness were significantly correlated across eight subjects (Nye & Bierer, 2010). Additionally, steep growth of loudness functions and narrow dynamic ranges have been associated with poor speech perception (Fu & Shannon, 2000; Loizou et al., 2000).
Some insight into the relationship between the electrode–neuron interface and growth of loudness can be obtained from computer modeling. We have recently developed a model based on volume conduction in a simplified cochlear geometry with deterministic activation of nerve fibers (Goldwyn et al., IN PRESS). Controllable elements of the model include electrode configuration, electrode-to-neuron radial distance, and local spiral ganglion survival. The right panel of Figure 6 plots a series of model solutions as a function of current level on one channel stimulated with a focused partial tripolar configuration (σ = 0.9). The model output in this case is the number of activated neurons across the cochlea, which we take as a proxy for loudness (e.g., Litvak et al., 2007; McKay et al., 2001). The figure shows three conditions of the electrode–neuron interface, obtained by creating dead regions of different widths centered on the active electrode. The electrode position is fixed at a radial distance of 0.8 mm to the inner wall of the cochlea, which is relatively close to the osseous spiral lamina. The 0 mm (no dead region) condition has the lowest threshold and shallowest growth function, whereas the 1-mm and 3-mm conditions have progressively higher thresholds and steeper functions. Qualitatively, these curves are similar to the loudness data of Figure 6. An inverse relationship between threshold and steepness of neural recruitment was also modeled by Briaire and Frijns (2006), but manipulations of the electrode–neuron interface were made via electrode position rather than the simulation of discrete dead regions. Alhough the exact nature of the underlying neural survival patterns for the subject in Figure 6 is unknown, these models present plausible mechanisms by which the electrode–neuron interface can influence perception.
Conclusions
The evidence presented in this review, that cochlear implant perception can be adversely affected by poor electrode–neuron interfaces, has potential implications for clinical mapping procedures. Current guidelines suggest that a channel be deactivated if it is believed to be detrimental to perception. Deactivations occur primarily for physical issues, such as known or suspected placement outside the cochlea or evidence of electrode shorting or open circuits. A channel can also be deactivated to avoid nonauditory percepts, such as those caused by facial nerve activation, if there is abnormal growth of loudness, or if the pitch sequence is not tonotopically ordered. However, according to one analysis of a large number of patients, channel deactivations occur infrequently in the clinic (Zeitler et al., 2009). Considering the psychophysical evidence that implant listeners generally do not make full use of their available channels to extract spectral information (Fishman et al., 1997; Friesen et al., 2001), prudent deactivation of channels affected by a poor electrode–neuron interface—and the subsequent remapping of frequency bands to the remaining channels—has the potential to improve the perception of speech and other spectrally complex stimuli. With the growing application of current focusing and current steering strategies (Koch et al., 2007; Mens & Berenstein, 2005; van den Honert & Kelsall, 2007), optimizing the allocation of spectral information becomes increasingly important. (For further discussion of channel reprogramming, see Pfingst et al., 2008.)
Here we have shown that tripolar configuration threshold is correlated with both psychophysical tuning curve width and growth of loudness. The observed channel-to-channel effects may be a significant source of the variability observed in other studies, both within and across subjects. The condition of the electrode–neuron interface has been offered as an explanation for variability in threshold (e.g., Bierer, 2007; Pfingst & Xu, 2004), broad tuning (Bierer & Faulkner, 2010; Nelson et al., 2008), indiscriminable electrodes (Collins et al., 1997; Henry et al., 2000; McKay et al., 1999; Pfingst et al., 1999; Zwolan et al., 1997) steep growth of loudness or small dynamic ranges (Bierer, 2007; Pfingst & Xu, 2004), abnormal growth of evoked potential amplitudes (Prado-Guitierrez et al., 2006), abnormal pitch scaling/ranking (e.g., Collins et al., 1997; Mens & Berenstein, 2005; Throckmorton & Collins, 2000), and temporal modulation detection (Chatterjee & Yu, 2010; Pfingst et al., 2008). Evaluating the local effects that a poor electrode–neuron interface has on these diverse measures should lead to a better understanding of overall implant performance.
Acknowledgments
I would like to thank Steven Bierer, John Middlebrooks, Joshua Goldwyn, Kathleen Faulkner, and Amberly Nye for their contributions to this work and the research subjects for their many hours of participation.
Author's Note
Portions of this work were presented at the Conference on Implantable Auditory Prostheses in Lake Tahoe, California, 2007 and 2009 and the Midwinter meetings of the Association for Research in Otolaryngology in 2007–2010.
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article:
This work was supported by NIH R03 DC8883, the University of Washington, a Virginia Merrill Bloedel Hearing Research Center Mini-grant, and a University of Washington Royalty Research Fund grant (#3652).
References
- Baskent D., Shannon R. V. (2004). Frequency-place compression and expansion in cochlear implant listeners. Journal of the Acoustical Society of America, 116, 3130–3140 [DOI] [PubMed] [Google Scholar]
- Bierer J. A. (2007). Threshold and channel interaction in cochlear implant users: Evaluation of the tripolar electrode configuration. Journal of the Acoustical Society of America, 121, 1642–1653 [DOI] [PubMed] [Google Scholar]
- Bierer J. A., Faulkner K. F. (2010). Identifying cochlear implant channels with poor electrode-neuron interface: Partial tripolar, single-channel thresholds, and psychophysical tuning curves. Ear and Hearing, 31, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer J. A., Middlebrooks J. C. (2002). Auditory cortical images of cochlear-implant stimuli: Dependence on electrode configuration. Journal of Neurophysiology, 87, 478–492 [DOI] [PubMed] [Google Scholar]
- Busby P. A., Whitford L. A., Blamey P. J., Richardson L. M., Clarke G. M. (1994). Pitch perception for different modes of stimulation using the cochlear multiple-electrode prosthesis. Journal of the Acoustical Society of America, 95, 2658–2669 [DOI] [PubMed] [Google Scholar]
- Boëx C., Kos M. I., Pelizzone M. (2003). Forward masking in different cochlear implant systems. Journal of the Acoustical Society of America, 114(4 Pt. 1), 2058–2065 [DOI] [PubMed] [Google Scholar]
- Bonham B. H., Litvak L. M. (2008). Current focusing and steering: Modeling, physiology, and psychophysics. Hearing Research, 242, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briaire J. J., Frijns J. H. (2006). The consequences of neural degeneration regarding optimal cochlear implant position in scala tympani: A model approach. Hearing Research, 214, 17–27 [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Galvin J. J., III, Fu Q.-J., Shannon R. V. (2006). Effects of stimulation mode, level and location on forward-masked excitation patterns in cochlear implant patients. Journal of the Association for Research in Otolaryngology, 7, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M., Yu J. (2010). A relation between electrode discrimination and amplitude modulation detection by cochlear implant listeners. Journal of the Acoustical Society of America, 127, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. T. (2009). Practical model description of peripheral neural excitation in cochlear implant recipients: 1. Growth of loudness and ECAP amplitude with current. Hearing Research, 247, 87–99 [DOI] [PubMed] [Google Scholar]
- Cohen L. T., Richardson L. M., Saunders E., Cowan R. S. (2003) Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hear Res., 179(1–2):72–87 [DOI] [PubMed] [Google Scholar]
- Cohen L. T., Saunders E., Knight M. R., Cowan R. S. (2006). Psychophysical measures in patients fitted with Contour and straight Nucleus electrode arrays. Hearing Research, 212, 160–175 [DOI] [PubMed] [Google Scholar]
- Collins L. M., Zwolan T. A., Wakefield G. H. (1997). Comparison of electrode discrimination, pitch ranking, and pitch scaling data in postlingually deafened adult cochlear implant subjects. Journal of the Acoustical Society of America, 101, 440–455 [DOI] [PubMed] [Google Scholar]
- de Balthasar C., Boëx C., Cosendai G., Valentini G., Sigrist A., Pelizzone M. (2003). Channel interactions with high-rate biphasic electrical stimulation in cochlear implant subjects. Hearing Research, 182, 77–87 [DOI] [PubMed] [Google Scholar]
- Donaldson G. S., Nelson D. A. (2000). Place-pitch sensitivity and its relation to consonant recognition by cochlear implant listeners using the MPEAK and SPEAK speech processing strategies. Journal of the Acoustical Society of America, 107, 1645–1658 [DOI] [PubMed] [Google Scholar]
- Donaldson G. S., Peters M. D., Ellis M. R., Friedman B. J., Levine S. C., Rimell F. L. (2001). Effects of the Clarion Electrode Positioning System on auditory thresholds and comfortable loudness levels in pediatric patients with cochlear implants. Archives of Otolaryngology–Head & Neck Surgery, 127, 956–960 [DOI] [PubMed] [Google Scholar]
- Dorman M. F., Loizou P. C. (1997). Speech intelligibility as a function of the number of channels of stimulation for normal-hearing listeners and patients with cochlear implants. American Journal of Otology, 18(6 Suppl.), S113–S114 [PubMed] [Google Scholar]
- Dorman M. F., Loizou P. C., Fitzke J., Tu Z. (1998). The recognition of sentences in noise by normal-hearing listeners using simulations of cochlear-implant signal processors with 6–20 channels. Journal of the Acoustical Society of America, 104, 3583–3585 [DOI] [PubMed] [Google Scholar]
- Fayad J. N., Linthicum F. H., Jr (2006) Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 116(8):1310–20 [DOI] [PubMed] [Google Scholar]
- Fayad J., Markarem A., Linthicum F., Jr. (2009). Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngology–Head and Neck Surgery, 141, 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley C. C., Holden T. A., Holden L. K., Whiting B. R., Chole R. A., Neely G. J., Skinner M. W. (2008). Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otology & Neurotology, 29, 920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman K. E., Shannon R. V., Slattery W. H. (1997). Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. Journal of Speech, Language, and Hearing Research, 40, 1201–1215 [DOI] [PubMed] [Google Scholar]
- Friesen L. M., Shannon R. V., Baskent D., Wang X. (2001). Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants. Journal of the Acoustical Society of America, 110, 1150–1163 [DOI] [PubMed] [Google Scholar]
- Fu Q.-J., Nogaki G. (2005). Noise susceptibility of cochlear implant users: The role of spectral resolution and smearing. Journal of the Association for Research in Otolaryngology, 6, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q.-J., Shannon R. V. (2000). Effects of dynamic range and amplitude mapping on phoneme recognition in Nucleus-22 cochlear implant users. Ear and Hearing, 21, 227–235 [DOI] [PubMed] [Google Scholar]
- Gantz B. J., Woodworth G. G., Knutson J. F., Abbas P. J., Tyler R. S. (1993) Multivariate predictors of success with cochlear implants. Adv Otorhinolaryngol. 48: 153–67 [DOI] [PubMed] [Google Scholar]
- Gfeller K., Oleson J., Knutson J. F., Breheny P., Driscoll V., Olszewski C. (2008). Multivariate predictors of music perception and appraisal by adult cochlear implant users. Journal of the American Academy of Audiology, 19, 120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwyn J. H., Bierer S. M., Bierer J. A. (IN PRESS). Modeling the electrode-neuron interface of cochlear implants: Effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res. [DOI] [PMC free article] [PubMed]
- Grill W. M. (1999). Modeling the effects of electric fields on nerve fibers: Influence of tissue electrical properties. IEEE Transactions on Biomedical Engineering, 46, 918–928 [DOI] [PubMed] [Google Scholar]
- Hanekom T. (2005). Modelling encapsulation tissue around cochlear implant electrodes. Medical & Biological Engineering & Computing, 43, 47–55 [DOI] [PubMed] [Google Scholar]
- Henry B. A., McKay C. M., McDermott H. J., Clark G. M. (2000). The relationship between speech perception and electrode discrimination in cochlear implantees. Journal of the Acoustical Society of America, 108(3 Pt. 1), 1269–1280 [DOI] [PubMed] [Google Scholar]
- Hinojosa R., Marion M. (1983). Histopathology of profound sensorineural deafness. Annals of the New York Academy of Sciences, 405, 459–484 [DOI] [PubMed] [Google Scholar]
- Hughes M. L., Abbas P. J. (2006). Electrophysiologic channel interaction, electrode pitch ranking, and behavioral threshold in straight versus perimodiolar cochlear implant electrode arrays. Journal of the Acoustical Society of America, 119, 1538–1547 [DOI] [PubMed] [Google Scholar]
- Jolly C. N., Spelman F. A., Clopton B. M. (1996). Quadrupolar stimulation for cochlear prostheses: Modeling and experimental data. IEEE Transactions on Biomedical Engineering, 43, 857–865 [DOI] [PubMed] [Google Scholar]
- Kawano A., Seldon H. L., Clark G. M., Ramsden R. T., Raine C. H. (1998). Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngologica, 118, 313–326 [DOI] [PubMed] [Google Scholar]
- Khan A. M., Whiten D. M., Nadol J. B., Jr., Eddington D. K. (2005). Histopathology of human cochlear implants: Correlation of psychophysical and anatomical measures. Hearing Research, 205, 83–93 [DOI] [PubMed] [Google Scholar]
- Koch D. B., Downing M., Osberger M. J., Litvak L. (2007). Using current steering to increase spectral resolution in CII and HiRes 90K users. Ear and Hearing, 228, 38S–41S [DOI] [PubMed] [Google Scholar]
- Koch D. B., Osberger M. J., Segel P., Kessler D. (2004). HiResolution and conventional sound processing in the HiResolution bionic ear: Using appropriate outcome measures to assess speech recognition ability. Audiology & Neurotology, 9, 214–223 [DOI] [PubMed] [Google Scholar]
- Kral A., Hartmann R., Mortazavi D., Klinke R. (1998). Spatial resolution of cochlear implants: The electrical field and excitation of auditory afferents. Hearing Research, 121, 11–28 [DOI] [PubMed] [Google Scholar]
- Kwon B. J., van den Honert C. (2006). Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners. Journal of the Acoustical Society of America, 119(5 Pt. 1), 2994–3002 [DOI] [PubMed] [Google Scholar]
- Li P. M., Somdas M. A., Eddington D. K., Nadol J. B., Jr. (2007). Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Annals of Otology, Rhinology, & Laryngology, 116, 731–738 [DOI] [PubMed] [Google Scholar]
- Litvak L. M., Spahr A. J., Emadi G. (2007). Loudness growth observed under partially tripolar stimulation: Model and data from cochlear implant listeners. Journal of the Acoustical Society of America, 122, 967–981 [DOI] [PubMed] [Google Scholar]
- Loizou P., Dorman M., Poroy O., Spahr T. (2000). Speech recognition by normal-hearing and cochlear implant listeners as a function of intensity resolution. Journal of the Acoustical Society of America, 108, 2377–2387 [DOI] [PubMed] [Google Scholar]
- Long C., Holden T., Parkinson W., Smith Z., van den Honert C. (2010, February). Towards a measure of neural survival in recipients of cochlear implants: Focused stimulation thresholds, speech understanding and electrode locations. Paper presented at the 33rd annual midwinter research meeting of the Association for Research in Otolaryngology, Anaheim, CA.
- Markessis E., Kapadia S., Munro K., Moore B. C. (2006). Modification of the threshold equalising noise (TEN) test for cochlear dead regions for use with steeply sloping high-frequency hearing loss. International Journal of Audiology, 45, 91–98 [DOI] [PubMed] [Google Scholar]
- McKay C. M., McDermott H. J., Clark G. M. (1996). The perceptual dimensions of single-electrode and nonsimultaneous dual-electrode stimuli in cochlear implantees. Journal of the Acoustical Society of America, 99, 1079–1090 [DOI] [PubMed] [Google Scholar]
- McKay C. M., O'Brien A., James C. J. (1999). Effect of current level on electrode discrimination in electrical stimulation. Hearing Research, 136, 159–164 [DOI] [PubMed] [Google Scholar]
- McKay C. M., Remine M. D., McDermott H. J. (2001). Loudness summation for pulsatile electrical stimulation of the cochlea: Effects of rate, electrode separation, level, and mode of stimulation. Journal of the Acoustical Society of America, 110, 1514–1524 [DOI] [PubMed] [Google Scholar]
- Mens L. H., Berenstein C. K. (2005). Speech perception with mono- and quadrupolar electrode configurations: A crossover study. Otology & Neurotology, 26, 957–964 [DOI] [PubMed] [Google Scholar]
- Middlebrooks J. C., Bierer J. A., Bierer S. M. The partial tripolar cochlear implant configuration assessed by forward masking in the inferior colliculus. Midwinter meeting of the Association for Research in Otolaryngology, Feb. 16–21, 2008, Phoenix, AZ, USA.
- Moore B. C. (2002). Psychoacoustics of normal and impaired hearing. British Medical Bulletin, 63, 121–134 [DOI] [PubMed] [Google Scholar]
- Moore B. C., Alcantara J. I. (2001). The use of psychophysical tuning curves to explore dead regions in the cochlea. Ear and Hearing, 22, 268–278 [DOI] [PubMed] [Google Scholar]
- Nadol J. B., Jr. (1997). Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngology–Head and Neck Surgery, 117(3 Pt. 1), 220–228 [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Donaldson G. S., Kreft H. (2008). Forward-masked spatial tuning curves in cochlear implant users. Journal of the Acoustical Society of America, 123, 1522–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M., Niparko J. K., Nager G. T. (2000). Inner ear pathology in severe to profound sensorineural hearing loss. In Niparko J. K., Kirk K. I., Mellon N. K., McConkey Robbins A., Tucci D. L., Wilson B. S. (Eds.), Cochlear implants: Principles and practices (pp. 57–92). Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Nye A. D., Bierer J. A. (2010, February). Poor electrode-neuron interface demonstrated by steep growth of loudness with the partial tripolar configuration. Paper presented at the 33rd annual midwinter research meeting of the Association for Research in Otolaryngology, Anaheim, CA.
- Oxenham A. J., Shera C. A. (2003). Estimates of human cochlear tuning at low levels using forward and simultaneous masking. Journal of the Association for Research in Otolaryngology, 4, 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. E., Lehiste I. (1962) Revised CNC lists for auditory test. J Speech Hear Disord, 27, 62–70 [DOI] [PubMed] [Google Scholar]
- Pfingst B. E., Burkholder-Juhasz R. A., Xu L., Thompson C. S. (2008). Across-site patterns of modulation detection in listeners with cochlear implants. Journal of the Acoustical Society of America, 123, 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst B. E., Burkholder-Juhasz R. A., Zwolan T. A., Xu L. (2008). Psychophysical assessment of stimulation sites in auditory prosthesis electrode arrays. Hearing Research, 242, 172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst B. E., Holloway L. A., Zwolan T. A., Collins L. M. (1999). Effects of stimulus level on electrode-place discrimination in human subjects with cochlear implants. Hearing Research, 134, 105–115 [DOI] [PubMed] [Google Scholar]
- Pfingst B. E., Xu L. (2004). Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. Journal of the Association for Research in Otolaryngology, 5, 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst B. E., Xu L., Thompson C. S. (2004). Across-site threshold variation in cochlear implants: Relation to speech recognition. Audiology & Neurotology, 9, 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Guitierrez P., Fewster L. M., Heasman J. M., McKay C. M., Shepherd R. K. (2006). Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hearing Research, 215, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R. V. (1990) Forward masking in patients with cochlear implants. J Acoust Soc Am. 88(2):741–4 [DOI] [PubMed] [Google Scholar]
- Shannon R. V., Zeng F. G., Kamath V., Wygonski J., Ekelid M. (1995). Speech recognition with primarily temporal cues. Science, 270, 303–304 [DOI] [PubMed] [Google Scholar]
- Shepherd R. K., Hatsushika S., Clark G. M. (1993). Electrical stimulation of the auditory nerve: The effect of electrode position on neural excitation. Hearing Research, 66, 108–120 [DOI] [PubMed] [Google Scholar]
- Skinner M. W., Holden T. A., Whiting B. R., Voie A. H., Brunsden B., Neely J. G., Finley C. C. (2007). In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Annals of Otology, Rhinology, & Laryngology Supplement, 197, 2–24 [PubMed] [Google Scholar]
- Snyder R. L., Bierer J. A., Middlebrooks J. C. (2004). Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. Journal of the Association for Research in Otolaryngology, 5, 305–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. L., Middlebrooks J. C., Bonham B. H. (2008). Cochlear implant electrode configuration effects on activation threshold and tonotopic selectivity. Hearing Research, 235, 23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somdas M. A., Li P. M., Whiten D. M., Eddington D. K., Nadol J. B., Jr. (2007). Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiology & Neurotology, 12, 277–284 [DOI] [PubMed] [Google Scholar]
- Stickney G., Loizou P., Mishra L., Assmann P., Shannon R., Opie J. (2006). Effects of electrode design and configuration on channel interactions. Hearing Research, 1, 33–45 [DOI] [PubMed] [Google Scholar]
- Throckmorton C. S., Collins L. M. (1999). Investigation of the effects of temporal and spatial interactions on speech-recognition skills in cochlear-implant subjects. Journal of the Acoustical Society of America, 105(2 Pt. 1), 861–873 [DOI] [PubMed] [Google Scholar]
- Throckmorton C. S., Collins L. M. (2000). Investigating perceptual features of electrode stimulation via a multidimensional scaling paradigm. Journal of the Acoustical Society of America, 108, 2353–2365 [DOI] [PubMed] [Google Scholar]
- Throckmorton C. S., Collins L. M. (2002). The effect of channel interactions on speech recognition in cochlear implant subjects: Predictions from an acoustic model. Journal of the Acoustical Society of America, 112, 285–296 [DOI] [PubMed] [Google Scholar]
- van den Honert C., Kelsall D. C. (2007). Focused intracochlear electric stimulation with phased array channels. Journal of the Acoustical Society of America, 121, 3703–3716 [DOI] [PubMed] [Google Scholar]
- van den Honert C., Stypulkowski P. H. (1987a). Single fiber mapping of spatial excitation patterns in the electrically stimulated auditory nerve. Hearing Research, 29, 195–206 [DOI] [PubMed] [Google Scholar]
- van den Honert C., Stypulkowski P. H. (1987b). Temporal response patterns of single auditory nerve fibers elicited by periodic electrical stimuli. Hearing Research, 29, 207–222 [DOI] [PubMed] [Google Scholar]
- Waltzman S. B., Roland J. T. (2006). Cochlear implants. New York, NY: Thieme [Google Scholar]
- Wardrop P., Whinney D., Rebscher S. J., Luxford W., Leake P. (2005). A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. II: Comparison of Spiral Clarion and HiFocus II electrodes. Hearing Research, 203, 68–79 [DOI] [PubMed] [Google Scholar]
- Wardrop P., Whinney D., Rebscher S. J., Roland J. T., Jr., Luxford W., Leake P. A. (2005). A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus Contour electrodes. Hearing Research, 203, 54–67 [DOI] [PubMed] [Google Scholar]
- Xu L., Pfingst B. E. (2008). Spectral and temporal cues for speech recognition: Implications for auditory prostheses. Hearing Research, 242, 132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Tsai Y., Pfingst B. E. (2002). Features of stimulation affecting tonal-speech perception: Implications for cochlear prostheses. Journal of the Acoustical Society of America, 112, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler D. M., Lalwani A. K., Roland J. T., Jr., Habib M. G., Gudis D., Waltzman S. B. (2009). The effects of cochlear implant electrode deactivation on speech perception and in predicting device failure. Otology & Neurotology, 30, 7–13 [DOI] [PubMed] [Google Scholar]
- Zeng F. G. (2002). Temporal pitch in electric hearing. Hearing Research, 174, 101–106 [DOI] [PubMed] [Google Scholar]
- Zwolan T. A., Collins L. M., Wakefield G. H. (1997). Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. Journal of the Acoustical Society of America, 102, 3673–3685 [DOI] [PubMed] [Google Scholar]