Abstract
During the last decade, cochlear implantation has evolved into a well-established treatment of deafness, predominantly because of many improvements in speech processing and the controlled excitation of the auditory nerve. Cochlear implants now also feature telemetry, which is highly useful to monitor the proper functioning of the implanted electronics and electrode contacts. Telemetry can also support the clinical management in young children and difficult cases where neural unresponsiveness is suspected. This article will review recent advances in the telemetry of the electrically evoked compound action potential that have made these measurements simple and routine procedures in most cases. The distribution of the electrical stimulus itself sampled by “electrical field imaging” reveals general patterns of current flow in the normal cochlea and gross abnormalities in individual patients; models have been developed to derive more subtle insights from an individual electrical field imaging. Finally, some thoughts are given to the extended application of telemetry, for example, in monitoring the neural responses or in combination with other treatments of the deaf ear.
Keywords: cochlear implant, deafness, telemetry, evoked potentials, integrity
Introduction
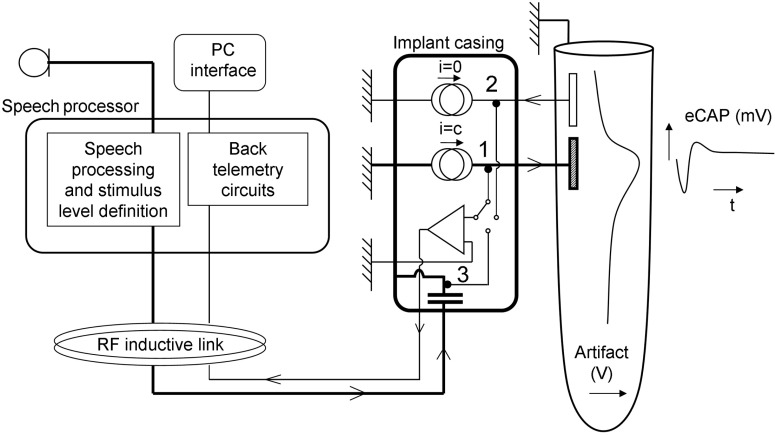
More than 20 years ago, electrical stimulation of the auditory nerve in deaf subjects advanced from acute tests inside a laboratory to a clinical treatment with the introduction of the 3M-House cochlear implant system.1 Some of the early cochlear implants already utilized the transcutaneous approach that featured an inductive coupling between the external wearable signal processor and the internal electronics. Today, all commercially available systems use the transcutaneous approach and all implants are able to send information back to the speech processor conveying the status of the implanted electronics (see Figure 1). The latter is generally referred to as telemetry, although back-telemetry is technically the more appropriate term.
Figure 1.
Block diagram of a cochlear implant system. The microphone signal is sent to the external speech processor. Through an inductive radio frequency link, energy and instructions are coupled to the implant. The bold lines indicate the power and signal path. The implanted electronics are capable of sensing voltages at different points of the circuitry and signal the status of the system to the speech processor and, if connected, the fitting station. Voltages sensed within the active output circuits (1) show open circuits. Voltages on nonstimulated electrodes (2) show short circuits, how the electrical field generated by the stimulus itself spreads across scala tympani, and the electrically elicited compound action potential (eCAP). The adjustment of the energy on the RF link is carried out by monitoring the supply voltage available to the current sources (3). In actual implant systems, the number of current sources varies between 1 and 16 and the number of intracochlear electrodes between 12 and 22.
Telemetry has become increasingly important as a means to monitor the correct functioning of the external and internal hardware, to assess the electrical fields induced in the cochlea, as well as to assess neural responsiveness. Furthermore, scientific investigations use telemetry to test hypotheses about the effect of different types of stimulation on the auditory system. In this article, we will review these applications and available evidence of their effectiveness. Similar to other active implantable devices such as heart pacemakers, cochlear implants are expected to evolve into systems that know about their effect on the organism and that not only can report that knowledge to the outside world but also use it to automatically optimize its own actions.
Telemetry of the Compound Action Potential
Excitation of the auditory nerve results in a cascade of measurable potentials ranging from early receptor potentials to cortical responses. Clinically, any objective response guiding the fitting of a cochlear implant in young children is highly welcome, especially because children as young as 1 year or less can receive implants. Early responses have the advantage of being less dependent on the arousal state and cooperation of the subject and can be recorded during surgery. In response to acoustic stimuli, three early potentials can readily be recorded with an electrode placed close to the cochlea as in electrocochleography.2 Hair cells generate receptor potentials known as cochlear microphonics closely locked to the stimulus waveform. A second potential called the summating potential is also generated as a result of the movements of hear cells in response to vibrations. In a deaf cochlea, only the third potential remains, which is the action potential of the auditory nerve. Most of the recent cochlear implant systems can measure the electrically elicited compound action potential (eCAP) using the same electrode array as is used to stimulate the nerve. Manufacturers refer to this technique as neural response imaging (Advanced Bionics Corporation), neural response telemetry (Cochlear Corporation), or auditory nerve response telemetry (MED-EL). Each of these implementations allows for an estimation of an eCAP response threshold using only standard fitting hardware. Recording an eCAP input–output series during surgery on some or all electrodes is more or less standard clinical practice in pediatric implantation. Typically, a biphasic electrical pulse is presented on one intracochlear electrode using the remote implant case as reference electrode (so-called monopolar stimulation—although an intracochlear ground is also possible). The resulting neural response is measured on another electrode close but not directly adjacent to the stimulating electrode to optimize the response amplitude while minimizing stimulus artifacts. The recording is sent to a computer via the externally worn speech processor and a programming interface (see Figure 1). By varying the stimulus amplitude, a response growth function is obtained from which the eCAP threshold can be estimated.
Action potentials are initiated within the depolarizing spiral ganglion cells. If present and myelinated, the dendritic sections possibly contribute as well.3 The action potentials predominantly propagate toward the brainstem along the cell axon, jumping across myelinated sections of the axon from one node of Ranvier to another. As the recording electrode is not in the near field of any of the individual nerve fibers and many nerve fibers will respond to stimulation of even a single intracochlear electrode because of current spread, a compound action potential (CAP) is measured instead of a single-fiber recording. This CAP is the complex time-varying geometrical product of the response of many fibers as well as the placement of the recording electrode within the volume-conducting medium relative to the generators.
The eCAP is an indicator of the peripheral excitability of the auditory nerve. In hearing impaired subjects, the threshold of the acoustically evoked CAP shows a moderate correlation with audiometric thresholds.4 The error of estimate, however, is considerable, for example, a CAP threshold of 40 dB indicates a hearing loss of anywhere between 10 and 70 dB. As the recording electrodes are located inside the cochlea in eCAP telemetry, the effect of the complex geometry on the recorded waveform is much larger than if a recording electrode in the middle ear is used, as in conventional electrocochleography. Therefore, considerable variability of eCAP measurements is to be expected, especially when different implant systems and recording procedures are used. Below, we will review the resulting discrepancy between the electrically evoked CAP threshold and the behavioral levels used for device fitting. However, we will first consider the different techniques to arrive at a clear recording of the response.
Recording Techniques
The electrically evoked action potential typically contains a negative peak N1 and a positive peak P1. Compared with the auditory brainstem response (ABR) recorded with surface electrodes, the eCAP is a robust and large response because the recording electrodes are very close to the auditory nerve. The N1–P1 amplitude difference varies between 50 and 1500 μV, at least an order of magnitude larger than the ABR. In addition, noise created by muscle activity is much reduced inside the cochlea compared with using surface potentials. As a result, the number of averages needed to obtain a reliable response can be as little as 50, even at near threshold stimulation levels.
One of the challenges for measuring the eCAP is the electrical stimulus. A very large electrical stimulus with an onset asynchrony as small as 0.2 milliseconds precedes the N1 peak. This makes it nontrivial to separate a genuine neural response from recording artifacts. Possible causes of the artifact have been suggested, such as residual charge stored on the recording electrode,5 on the membrane at the nodes of Ranvier, and at the unmyelinated cell body.3 However, a major cause of the artifact is saturation of the amplifier. Recovery from amplifier saturation may take longer than the duration of the neural response. Different techniques have been proposed and implemented to reduce recording artifacts: alternating stimulus polarity, template subtraction, and forward masking (see Figure 2 and Brown6 for details).
Figure 2.
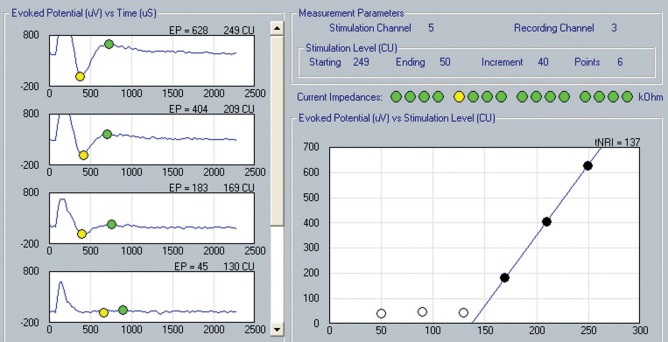
Left panel: Actual recordings of the electrically evoked compound action potential (eCAP). Right panel: the N1–P1 amplitude growth curve (Y-axis) as a function of stimulus amplitude once threshold is exceeded. Through linear extrapolation, the eCAP threshold is estimated to be at 137 current units in this case (Soundwave™ software). Reproduced with permission from Advanced Bionics Corporation.
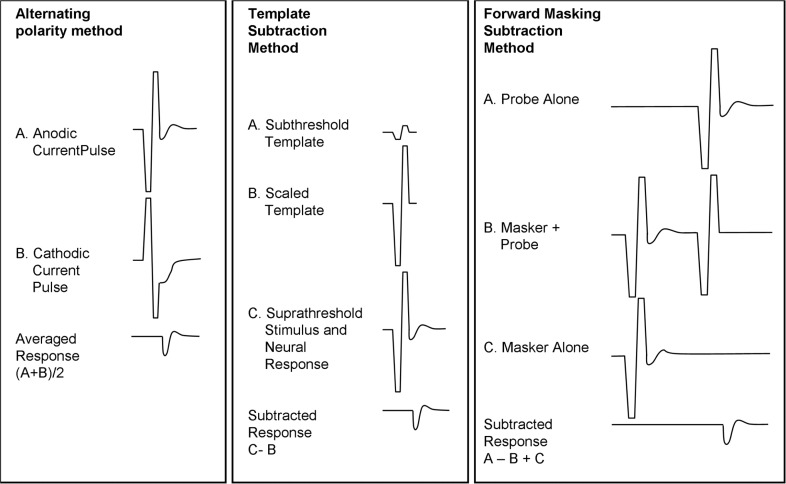
Alternating stimulus polarity: This technique takes advantage of the fact that only the electrical stimulus changes polarity but the neural response in principle does not. By alternating stimulus polarity in consecutive stimuli, the electrical stimulus, which is assumed to have identical amplitude for both polarities, cancels out in the averaging process. The problem with this method is that stimulus polarity has been shown to affect the threshold, the amplitude, and the latency of the response. As a result, the averaged response is smeared in time and smaller in amplitude compared with the true response. The alternating stimulus polarity technique has been made available as a standard option in clinical software of the Advanced Bionics Clarion C-II and 90K device (see Figure 3) and of the recent Cochlear Nucleus Freedom device.
Template subtraction: A recording is made using a low, presumably subthreshold level stimulus (ie, only the electrical stimulus and no neural response is present in the recording). This trace is then scaled up to the amplitude of the electrical stimulus recorded at higher intensities. Neural responses, if present, are obtained by subtracting the scaled template from the suprathreshold recording. In principle, this technique requires an unsaturated recording of the electrical stimulus in both conditions, which is generally difficult to achieve.
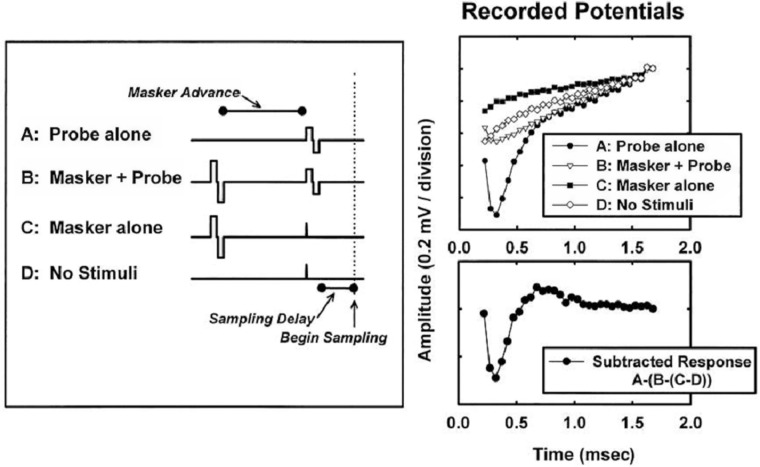
Forward masking: This technique was first used by Charlet de Sauvage7 and further developed by the Iowa group.8 The goal, as in template subtraction, is to make a recording with electric stimulus only and no neural response A high-amplitude “masker” pulse followed by a “probe” pulse are presented with a small delay (eg, 0.5 ms) that is much shorter than the refractory period of the auditory neurons (see Figure 4). The assumption is that if the amplitude of the masker is high enough to bring all neurons within the excitation area of the second pulse completely in refraction, the recording of the second pulse (the probe) should not contain any neural response. A probe-alone condition as well as a masker-alone condition is recorded. If the recordings are properly time-shifted and added, the electrical stimulus is removed and the resultant response is the neural response to the probe-alone condition. Ideally, one would use a fixed high-level masker. In practice, to avoid overstimulation, the amplitude of the masker is set at a fixed number of current units above the probe amplitude and the amplitude of both stimuli is increased.
Figure 3.
Different techniques to separate the electrical stimulus from the much smaller electrically evoked compound action potential (eCAP) neural response. By alternating the polarity of the stimulus and averaging, the stimulus will cancel out while the eCAP remains. This technique is used in the Advanced Bionics system. The template subtraction method collects a template of the stimulus at a subthreshold level and subtracts it from the recording after scaling. The forward masking subtraction method is used in the Cochlear device and uses a 2-pulse stimulus. The probe in (B) will not produce a physiological response because the nerves are assumed to be in the refractory period after the presentation of the masker. From Brown.6 Copyright John Wiley & Sons Limited. Reproduced with permission.
Figure 4.
Example showing intermediate (upper right panel) and final (lower right panel) results from the forward masking subtraction technique. Figure 2 in Brown et al.9 Reprinted with permission from Lippincot, Williams & Wilkins.
The forward masking technique has been implemented in clinical software for the first cochlear implant system to offer eCAP (ie, Cochlear Nucleus 24M device) after a clinical trial with an eCAP research platform which showed that it successfully produced responses in contrast to the other two methods.10 During clinical use of the Nucleus 24M system, it became apparent that several parameters (delay between stimulus and onset of recording, and amplifier gain) need to be optimized to avoid aberrant recordings, even after the reduction of recording artifacts made possible by the forward masking subtraction technique. Therefore, tools were developed to free the clinician from the task of parameter optimization, and from the need to identify genuine responses from no-response recordings. In principle, such tools would eliminate any subjective bias and would reduce recording time, which can be essential during surgery.
Automated Detection
To reduce the complexity of the measurement techniques, attempts have been made to automate response detection and threshold definition. At least two approaches have been used: similarity-based approaches and expert systems containing explicit decision rules. One of the similarity-based approaches is to train artificial neural networks to respond to the similarity of a recording with sets of traces that do, or do not, contain a true response according to human experts. Charasse and colleagues11 developed a neural network which, after training, found eCAP thresholds in new traces which on average differed from those identified by human experts by 3.6 clinical units—a smaller difference than that among the experts themselves. Another similarity method is to calculate the cross-correlation product between the recording and templates that contain genuine responses. Comparing the two similarity approaches, the authors concluded that cross-correlation yielded a better sensitivity and specificity.
In expert systems, decision trees are formulated using the knowledge collected from available scientific data. Botros et al12 developed the “Auto-NRT™” expert system which is integrated in the Cochlear clinical fitting software for the Freedom device and has been subjected to several clinical evaluations. Prior to two response detection trees, a classification tree processes each individual recording. The classification tree optimizes recording parameters if the amplifier is still in saturation within the period that a response is expected. If necessary, an attempt is made to reduce the effects of the electrical stimulus by adding a very short third phase to the biphasic stimulus with polarity opposite to that of the recovery slope of the biphasic stimulus. This technique has been described earlier for improving the electrically evoked auditory brainstem response (eABR).13 Once an acceptable trace is obtained, a peak-picker algorithm searches for N1 and P1. The trace will then be classified as a neural response if constraints are met which are set for the N1 and P1 latency and the interpeak interval, among others. The algorithm also weighs the noise level, the N1–P1 amplitude relative to the noise, and the correlation between the trace and a template chosen by the human expert that is a typical example of a recording containing a response plus recording artifact.
If classified as noise-only, an ascending decision tree will increase the stimulus level with a large step size until a response is found with high confidence (ie, to minimize the rate of false positives). After that, a descending decision tree is activated using less strict criteria for response detection and a smaller step size to approach threshold accurately (see Figure 5). The criteria used by the decision trees were derived using machine-learning software from more than 3500 eCAP measurements collected in 18 patients, about half of which were judged to contain a response by two human experts. These criteria were tested on a set of new traces that were judged by human experts. The resulting descending series procedure showed a sensitivity of 91% and a specificity of 92% per trace.12 The authors conclude that the sensitivity and specificity of Auto-NRT are higher than that of a neural network approach, autocorrelation, and a combination of the two.
Figure 5.
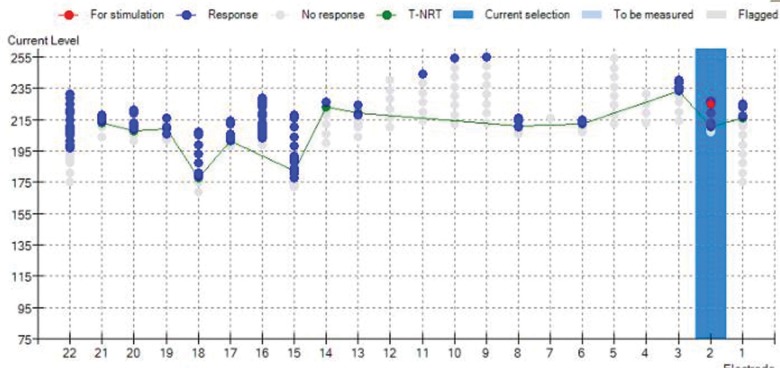
Intraoperative measurements immediately after a difficult insertion of the electrode array because of obliterations. Screenshot of the Auto-NRT software (Cochlear Corporation). gray dots indicate that no eCAP was found; blue dots indicate series of positive eCAP responses in which the software has decreased stimulation amplitude gradually until the response disappears. For several electrodes, no replicable response has been found, possibly because of excessive noise or because the current source reached its compliance limit because of high impedance. Reproduced with permission from Cochlear Benelux NV.
An automated procedure can establish thresholds on all electrodes in less time than a manual one. Recording can start at high stimulus levels intraoperatively with the result that Auto-NRT needs less than 23 seconds per electrode, which compares favorably with several minutes in a manual procedure. The result is that no extension of surgery time is required to obtain thresholds on all electrodes. Since its introduction, Auto-NRT has changed eCAP measurements from a time-consuming and elective procedure into a short routine task that can be performed in every surgery without extra time. Furthermore, it has eliminated variability between clinicians from threshold estimation. If a response is present, it assures the surgeon that the electrodes are in a position to evoke a neural response and provides the clinician a baseline against which to compare repeated measures in case of complications later.14
eCAP-Guided Threshold Prediction for Device Fitting
Primary measures of the clinical efficacy of the eCAP telemetry are how often a response can be measured in patients (the success rate), and how predictive eCAP thresholds are for the stimulus levels required for fitting the speech processor, that is, how well the eCAP predicts the threshold (“T”) as well as the most (or maximum) comfortable loudness (MCL) level. If sufficiently predictive, eCAP would support or even replace behavioral tests, making the eCAP measurements valuable in fitting young children and difficult to test adults. In numerous studies the predictive power has been assessed by calculating the correlation coefficient across subjects between the eCAP threshold and T or the MCL level measured per electrode using conventional fitting procedures. The absolute error of prediction is less relevant. This error, for instance, is partly unavoidable given the observation that subjective thresholds are much lower with the high pulse rates used during fitting (eg, 1000 pps) than with the low pulse rates used for eCAP recording (eg 80 pps),15 probably because of central temporal integration. On average, behavioral thresholds and MCL levels decrease with increasing pulse rate.16 Such a phenomenon is likely to be because of temporal integration, although neural refractoriness and decay may also lead to increased levels.17 Unfortunately, there are large individual differences in the ratio between the low-rate eCAP thresholds and the high-rate behavioral levels (particularly at the MCL level).15
Cafarelli-Dees and colleagues attempted to establish a normative description of eCAPs in 147 young adults recorded after the cochlear implants are turned on for at least 1 month.18 All subjects used a Nucleus CI24M device. A reproducible and clear eCAP could be recorded in 96% or 87% of subjects with one or all five test electrodes, respectively. Nevertheless, the correlation between eCAP threshold and T and MCL levels, did not exceed 0.44 and 0.58, even when excluding the T and MCL measurements made with stimulus rates above 250 pps.
To improve the correlation, several studies have attempted to reduce the individual differences by shifting the eCAP thresholds across electrodes so that the eCAP threshold on one anchor electrode is equal to the T or MCL levels for the same electrode measured at a high stimulus rate using the conventional fitting procedure.9,19–22 The anchor electrode is normally in the middle of the array. Without shifting individual eCAP threshold profiles, Brown and colleagues9 found correlations of 0.55 and 0.56 across subjects between eCAP thresholds and the T and MCL level, respectively. These correlations are not high enough for mapping the cochlear implants based on eCAPs. Subsequently, Brown and colleagues increased or decreased each subject's eCAP thresholds by the same amount across electrodes until the eCAP threshold and the T level of the anchor electrode was equal and found a more satisfactory correlation with the T levels, 0.83. The same procedure was applied to the MCL of the anchor electrode and yielded a correlation of 0.77 with the MCL levels.
In addition to using sparse behavioral data from individuals, statistical trends have been identified in group data to improve the prediction. Thai-Van and colleagues23 established that the offset between eCAP threshold and T (but not MCL level) systematically differed between apical, medial, and basal electrode locations, which could be used to improve the prediction of individual T levels. Gordon and colleagues24 suggested correcting eCAP thresholds for age of implantation and the time after initial activation. Cafarelli-Dees and colleagues18 conducted a multicenter trial including 147 adult subjects and reported that the individual eCAP profiles could be rather well described by two factors representing the overall level and the slope across the electrode array, as were T and MCL level profiles, which reflects earlier findings of Smoorenburg et al.25 However, the correlations between the eCAP level factor and the behavioral level factors were only moderate (r = 0.6 or less); the slope factor performed slightly worse (r = 0.5 or less). In the same study, the eCAP shift-to-anchor level was used. Although the variability was reduced, the remaining error of prediction (rms) was 15 and 14 current units, deemed by the authors unacceptable for clinical use (the mean dynamic range across electrodes was about 40 current units).
A third approach to correct eCAP thresholds for a better prediction of behavioral levels was used by McKay et al.26 It was hypothesized that the effect of rate on loudness (and thus threshold) varies between subjects because of differences in neural refractoriness. In this study, refractoriness was measured by varying the duration of a gap (from 8 to 45 μs) between the two phases of the biphasic eCAP stimulus and registering the eCAP amplitude. However, the effect of the interphase gap on the eCAP amplitude did not correlate with the behavioral-eCAP threshold discrepancy for a high rate stimulus (900 pps), and so it did not contribute to accuracy of predicting the behavioral thresholds.
Surprisingly, doubts have been raised whether much is to be gained by correcting individual eCAP profiles using sparse behavioral data. Setting all T and MCL level of all electrodes to the behavioral levels found for the anchor electrode (creating a “flat” map) resulted in a better approximation of the actual T and MCL levels than if the profile of eCAP thresholds were used to estimate T and MCL levels.27 Similar results were found in another study if the group average T and MCL profiles were used instead of eCAP thresholds.18
In a limited number of studies, speech perception performance using the conventional behavioral fittings and the eCAP-based fittings have been compared, either without any behavioral data28–30 or with limited behavioral data (the “anchor” approach mentioned earlier).25,28–30 Some studies have included children as well as adults, either experienced or first users. None of these studies found large effects of fitting method on performance; the conventional fitting method was slightly superior but the difference was not significant25 at some but not all presentation levels.28 Although encouraging, larger studies including more subjects are needed to examine whether eCAP-based fittings, in particular without using behavioral data, are effective for the average patient and safe in every individual case.
In sum, eCAP thresholds can be found in a high percentage of subjects (96%) and electrodes (87%) and limited experience with cochlear implant fitting using eCAP thresholds thus far seems promising. The large error in predicting behavioral levels based on eCAP thresholds, however, cautions against their use as the sole basis of device fitting. Careful behavioral observation remains paramount, if only to avoid overstimulation. It is possible that the eCAP thresholds will turn out to be more informative if stimulation strategies other than standard monopolar stimulation are used. Other stimulation strategies may result in more differences in the T (and MCL) levels between electrodes and thus may render objective measures per electrode less redundant.
Quality of the eCAP and Performance
Clinicians are confronted with many programming parameters that can be optimized for the individual patient. One of the parameters is the stimulus repetition rate. Kiefer31 reported that performance was correlated to the time needed for recovery from refraction measured with the forward masking eCAP paradigm. Recovery, however, did not predict which strategy or which repetition rate was best for an individual subject. In contrast, in a study testing subjective preference after 2 weeks of cochlear implant use, 6 subjects who preferred a relatively slow rate strategy (1200 pps per electrode or less) showed a recovery period of at least 3.5 milliseconds, and 5 subjects who preferred a faster strategy (1800 pps) showed a shorter recovery period.32 Unfortunately, their speech recognition performance was not measured. More studies are needed to determine the relationship between eCAP measurement data and cochlear implant parameter settings.
Recovery from refractoriness is one indication of neural viability. The eCAP amplitude as a function of stimulus level is another. A steep response growth and large N1–P1 amplitude may indicate the presence of many surviving neurons near the stimulating electrode and thus a positive relationship between the slope of the amplitude growth function and performance can be assumed. However, this relation is not as strong as one may expect. A modest relationship was found in postlingually deafened subjects, but congenitally deafened individuals on average show steeper slopes than postlingually deafened individuals.33 Although related to the electrical dynamic range, the amplitude growth function slope did not predict speech recognition performance of postlingually deafened adults in another study.34
Variants of the forward masking paradigm have been proposed to measure the spread of excitation along the cochlea. Excessive spread of excitation is considered a major factor limiting the number of independent channels through which spectral information can be transmitted, and therefore reducing speech recognition performance, particularly in noise and for music appreciation. To measure the spread of excitation in the cochlea, masker and probe stimuli were initially presented on the same electrode while the recording electrode was varied along the array, which typically results in a smaller response amplitude with larger electrode distances. Cohen35 pointed out that this method resulted in unrealistically broad spread profiles (for instance compared with the width of psychophysical tuning curves), which is thought to be the result of the additional spatial spread of the neural response before arriving at the recording electrode. By separating the locations of the masker and the probe electrodes, a deliberate incomplete overlap is introduced to the excitation regions “seen” by masker and probe electrodes. The degree of spatial spread dictates the effective overlap between the two regions and thus the response amplitude measured at the recording electrode. As the position of the recording electrode is held constant, current spread from the excited neural elements to the recording electrode does not affect the measured width of neural excitation. In a small set of subjects, this method showed good agreement with psychophysical forward masking spread functions. In another study including subjects implanted with the Cochlear and Advanced Bionics devices, longitudinal spread of excitation was found to increase with stimulus intensity and to be smaller in the base of the cochlea.36 Neither electrode pitch ranking nor speech recognition performance was found to correlate with the eCAP spread of excitation measure.37 In a within-subject analysis, less spread of excitation has been shown for electrodes that were placed closer to the modiolus.38 Similarly, a between-subject comparison between straight and modiolus-hugging electrode designs showed less spread in the latter, although, surprisingly, the difference between electrode designs was less at the basal end.36
Telemetry of the eCAP provides several indications of the quality of neural excitation. Rate of recovery and spread of excitation measures have been found to agree with some psychophysical measures but not with the differences in performance. It has been argued, after the fact, that this lack of agreement is not surprising as the eCAP is a peripheral response and subjects may differ in many aspects of central auditory processing capabilities. Previous and recent findings indicated that the number of surviving spiral ganglion cells found in postmortem samples does not explain the large performance differences between subjects. On the contrary, performance was significantly better in ears with fewer spiral ganglion cells.39 Obviously, a better understanding of the electrode–neural interface is needed and eCAP telemetry will continue to be explored to find peripheral constraints limiting cochlear implant performance.
Telemetry of the Intracochlear Electrical Field
Recordings of the eCAP are complicated by the large electrical stimulus as the current spreads from the active to the sensing electrode. Being much larger than the eCAP, the electrical stimulus itself can be readily recorded even without averaging. Although referred to as “electrical field imaging” (EFI) by the manufacturer of the Advanced Bionics device, it obviously has the limitation that only the voltage gradient at the location of the electrode array is known. The voltage gradient along the nerve, which essentially is tangential to the electrode array, can only be inferred. Therefore, the term “imaging” is not fully justified and the potentials from the electrical stimulus would better be referred to as intracochlear electrode voltages, analogous to the surface potentials known as averaged electrode voltages (AEVs).
Previously EFI measurements have been performed in animal and temporal bone studies using temporary probes,40–42 the implanted electrode array itself,43 and intraoperatively using temporary probes.44 Early EFI measurements through telemetry were made using the Med-El system45 and using an experimental hardware platform.46 EFI now is available for the Advanced Bionics system as a stand-alone software.47
Although EFI only samples the longitudinal voltages with considerable coarseness, it does present information about the volume conduction in the cochlea. Basically, EFI measures have shown a voltage gradient that is about three times larger toward the base (with apical stimulation) than toward the apex (with basal stimulation). This suggests a preferential current pathway through the basal openings (the internal auditory canal, the vestibular system, the cochlear and vestibular aqueduct, and the oval and round windows,48 also in agreement with AEV recordings in patients with a normal cochlear bone density.49 In these patients, the AEVs increase monotonically with increasing distance between intracochlear current source and sink electrodes best modeled with an electrode array positioned inside an almost insulating cochlear tube open to the middle ear,50 in contrast to the AEVs from otosclerotic ears, which do reflect the spiraling structure of the cochlea.
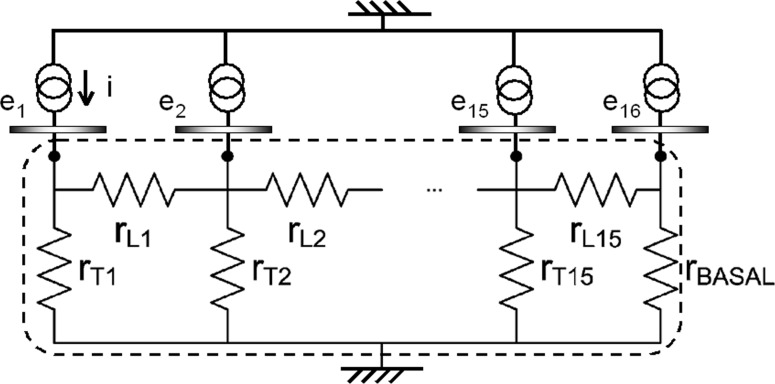
To infer the magnitude of the current flow toward the nerve from the longitudinal voltage gradient picked up by the telemetry of the electrical field in individual subjects, simplified electrical models of the cochlea have been developed.42,51,52 In these models, the geometry of the cochlea is reduced into longitudinal impedances (because of scala tympani perilymph) from one electrode to the next, and transversal impedances from each scala tympani section to a corresponding spiral ganglion section. In the model by Vanpoucke and colleagues,48 these sections are combined in a ladder network (see Figure 6) complemented by one impedance representing the volume conduction from the cochlea to the extracochlear reference electrode. The model impedances can be changed until an optimal fit is found between the predicted and the actual field strengths from an individual subject. Applying actual measures from monopolar stimulation in limited numbers of subjects, the modeled current through transversal sections was found to be much smaller than that through longitudinal sections.52 In some subjects, the transversal current was highly limited to a location at approximately 270° of the basal turn of the cochlea. This finding seems to be at odds with all indications that monopolar electrical stimulation does achieve some degree of channel specificity. Further studies are needed to replicate these results.53
Figure 6.
Electrical impedance ladder network of the cochlear tissues (lower compartment enclosed in dashed lines) connected to 16 current sources through electrodes ei. The impedances between the active electrodes and the ground electrode created by the tissues in and around scala tympani are lumped together in longitudinal resistances rLi and transversal resistances rTi. Resistor rBASAL represents the total conductance of the base of the cochlea and the reference electrode. Actual EFI measurements produce voltages on the nodes which can be fitted by varying rLi and rTi. Note that no current can flow from one cochlear compartment to another except to a neighboring one, which implies that a good fit with actual measurements is possible to the extent that the spiraling cochlea is equivalent to a straight semi-insulating tube. A fit of more than 90% was reported,48 which agrees with earlier studies (see text). Adapted from Vanpoucke et al.48 Copyright 2004 IEEE.
EFI and AEVs can be used for diagnostic purposes (eg, to detect technical failures, see the next paragraph). Even if no hardware failure is present, the EFI pattern recorded from an individual patient and its development over time can potentially be helpful to shed light on problematic fittings. For example, a patient in our clinic complained about an unpleasant echo from stimulating several electrodes after a device failure and reimplantation. Before reimplantation, a normal field pattern was recorded in this patient. Figure 7A shows the abnormal EFI pattern recorded after reimplantation, in particular for electrodes 10 to 14. Vanpoucke and colleagues52 conclude, “EFI can provide clinically relevant information, especially in problematic cases of cochlear malformations, postoperative fibrosis/ossification, implanted otosclerotic cochleae, postoperative facial nerve stimulation, increased stimulation thresholds, and so on.”
Figure 7.
(A) Electrical field measurements in a patient after reimplantation. Intracochlear electrode voltages are measured on all nonstimulating electrodes showing the longitudinal intracochlear potential gradient along the electrode array. Increased current spread is visible for most of the basal electrodes (in particular 10, 11, 13, and 14—bold lines). The voltage peaks on stimulated electrodes do not show double peaks, which indicates that no electrode shorts are present. (B) Threshold and maximum comfortable loudness levels for the same patient. Current levels were heavily clipped to avoid unpleasant booming sounds, in particular for medial and basal electrodes (higher electrodes numbers); this agrees somewhat with the increased current spread in this region shown in (A). Open symbols represent current levels for electrodes inactivated in the clinical map.
Technical Integrity of the External and Internal Hardware
The output of a conventional hearing aid can be checked in a number of ways, some of which requiring little or no extra hardware, such as listening to it while generating feedback or through a simple stethoscope. Overall, cochlear implants are very reliable devices allowing for stable patient performance over many years. In the case of device failures, however the implications for the subject are far greater than in case of a conventional hearing aid, and cochlear implants offer fewer opportunities for integrity checks. Recently, telemetry has brought important advancements in integrity testing and it has become an essential part of cochlear implants.
Consider two of the most common failures of a cochlear implant system: an unreliable power supply and a broken cable for the microphone or transmitter coil. If the failure is intermittent, it may be difficult even for adult users to distinguish these problems from a weak RF link because of misalignment of the transmitter and receiver coil or even an implant failure. These comparatively simple problems are difficult to diagnose for adult users, let alone for pediatric users. Only some speech processors feature a display showing the instantaneous operational status and RF lock to the implant (for details, see Mens54). To diagnose intermittency, a continuous and time-stamped logging of the operational status inside the speech processor made available for comprehensive offline analysis would be needed.
Other examples of device failure are fluid leakages in the implant casing and “soft failures.” Leakage may result in an abrupt and total loss of communication with the implanted electronics, which is easily verified by exchanging all external parts. A soft failure is an instance in which a device failure is suspected but cannot be proven through integrity testing. Symptoms may be a performance decrement, large shifts in programming levels and the occurrence of atypical noises or painful sensations over the implant. In young children, unwillingness to wear the device or behavioral problems may be primary clues in the absence of neurological, radiological, and telemetric explanations.55 These cases do occur and are highly disturbing for patients, parents, and clinicians. It is extremely important that better tools become available to detect more subtle failure modes.
One attempt at a more comprehensive test of soft failures is the AEV Crystal system developed by Cochlear Corporation. It records surface potential measures of the AEV and checks stimulus morphology and continuity while varying the pulse width, transmission range and electrode coupling among others.56 In principle, the same tests can be run on the intracochlear electrical stimulus if the telemetry circuits are intact (obviating the need of dedicated hardware). Prototype software called “Bionic Ear Integrity Test” performing part of these functions has been used for the Advanced Bionics device. If such a test would be available to the clinician in a practical format, a periodic integrity check—more thorough then currently possible—would become standard practice. Ideally, parents would be able to initiate such a test when in doubt and the result would be sent to the clinic for offline analysis.
In addition, telemetry has enabled a fast and accurate monitoring of several other components of most cochlear implant devices. One of these is the integrity of the lead wires to the electrodes and the connections between the lead wires and the electrode contacts. The lead wires and their connections are relatively frequent causes of malfunction, being outside the rigid and hermetically sealed receiver case. The clinician can usually work around electrode failures by not stimulating the affected electrodes. Open circuit or short-circuited electrodes should be deactivated because they may create erratic electrical fields with potentially negative effects on the behavioral thresholds and pitch perception.
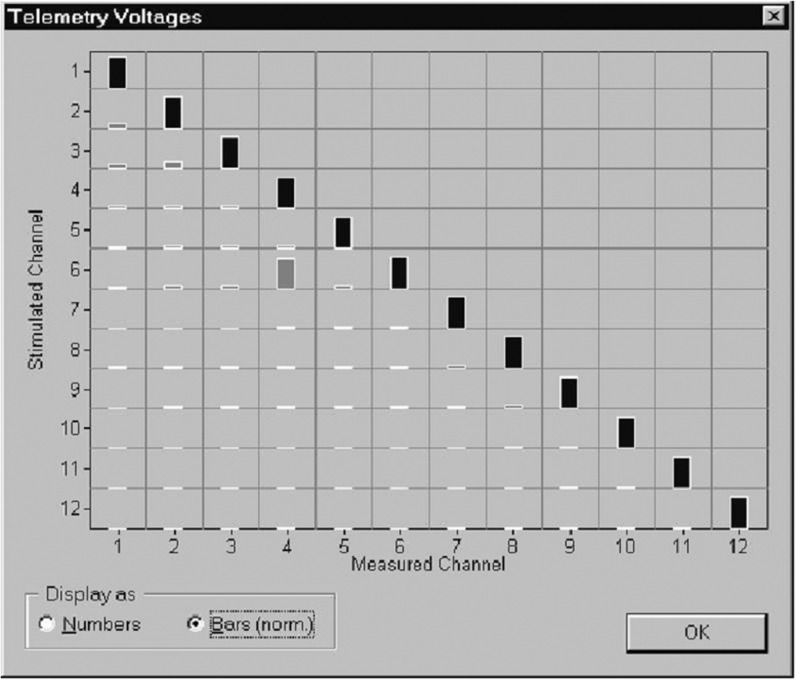
All recent cochlear implants are equipped with telemetry modes to detect malfunctioning electrodes. The voltage drop across the stimulating electrodes is measured at a particular point of the stimulus waveform that is chosen to be representative of the overall amplitude, or at the end of the second phase where the output capacitor, if present, will be more or less discharged and has no effect. This momentary voltage divided by the current amplitude yields a measure of the impedance. To distinguish a genuine open circuit from a larger-than-average impedance with a biological cause, a criterion needs to be set above which the electrode is flagged. This criterion will depend on, among other things, the effective electrode surface. For example, the criterion was set at 20 kohm for the Nucleus 24 system with its relatively large electrodes, and 150 kohm in the case of the Clarion preformed array with small ball-shaped electrodes. In the default impedance test condition, each intracochlear electrode is tested separately in monopolar coupling against the extracochlear ground. However, a short-circuited electrode will only produce a near zero impedance if the affected nonstimulating electrode is connected to ground during the measurement. Therefore, some devices not only test in the monopolar stimulation mode but also in “common ground” in which all nonstimulating electrodes are connected to another.57 Another option is to stimulate each electrode one by one in monopolar mode, and collect the resulting voltages on nonstimulated electrodes, as in EFI. A short circuit will result in a voltage that is as high as the one on the stimulated electrode, as it effectively is stimulating as well (see Figure 8).
Figure 8.
The voltage table produced by the CI.STUDIO+ software (MED-EL GmbH) in graphical format. This result suggests a short circuit between electrodes 4 and 6. The height of the bars is proportional to the voltage measured in the stimulated electrodes (on the diagonal) and the nonstimulated electrodes (off-diagonal). Note that the off-diagonal measures are identical to those collected for EFI. Reprinted with permission from MED-EL GmbH.
Another component monitored by telemetry in most systems is the coupling between the transmitter and receiver coil. To save battery life, the RF power spent by the processor must be balanced against the supply voltage available in the implant. The supply voltage cannot be less than a certain value depending on the particular stimulation strategy and sound input level to avoid the implant cutting out. Other factors affecting the required RF power are the current requirement of the subject, the electrode impedances, skin thickness over the implant, the type of transmitter coil, the particular processor and even the cable length (because each cable has a slightly different impedance). Given a particular configuration, the processor can set the RF power to a minimum while continuously monitoring that the power is just sufficient to uphold the necessary supply voltage. Telemetry will also detect whether the supply voltage is sufficient for the implanted current sources to deliver the required currents. A current source is considered out of compliance if the combination of the required current and the actual impedance load exceeds the maximum supply voltage inside the implant. Some systems can detect if the implant has reached this compliance voltage while stimulating, other systems estimate the maximum current output based on previously stored impedance values. In either case the clinician can take advantage of the information by knowing that setting the upper limit of the dynamic range above this point will not result in a further increase of loudness but instead the compression of louder sounds.
Caution must be taken not to assume that telemetry can detect all kinds of device failure. The microphones may deteriorate gradually over time and the analog input components in cochlear implants may cause degradation in the signal. At present, users and parents of young users can only marginally check the output of the microphone by watching a visual indication of the overall input level as a function of the sensitivity setting. Clinicians and parents can check the microphone of some systems by listening to it through earphones. What is needed is a comprehensive test similar to the standardized hearing aid testing procedures to check the frequency characteristic at several sound input levels, distortions, and compression characteristics. In addition, tests of the transmitted signals from assistive listening devices such as an FM system are lacking for cochlear implants.
Extended Applications
Telemetry circuits have already increased the quality of modern cochlear implant systems in several ways. They have removed part of the uncertainty about the technical integrity of both external and internal components, during surgery, during clinical troubleshooting, as well as during everyday use. Telemetry has begun to contribute to the fitting of young children by providing preliminary data on the responsiveness of the peripheral auditory system. Currently available systems obviously differ in the type of telemetry-based features, and not all features are equally necessary. Most vital is the possibility of evaluating the technical integrity of the implanted receiver during surgery and during postoperative fitting sessions; all systems provide this feature.
What is not consistently offered to the clinician is the possibility of ruling out more trivial but frequent causes of an absent response of the implanted electronics. These include a broken cable, defective transmitter coil or a weak coupling to the implant. Ideally, these different modes of failure would be logged in the processor and accessed by the user and by the clinician in a direct consultation or through a remote connection. Although telemetry may become quite advanced at detecting failures, it seems important for a clinical centre to be able to collect surface potentials, as these are device independent and may show intermittent failures and deviant stimulation patterns missed by telemetry.
As objective measures become increasingly important in fitting young children, telemetry can be expected to provide a convenient access to a number of neural responses. The intracochlear registration of the compound action potential will possibly be complemented with that of potentials from remaining hair cells, the stapedius muscle, the brainstem and the auditory cortex, either transient or steady state. One can envisage extra electrodes being placed for this purpose. Possibly, these would be very long unipolar electrodes attached to the receiver/stimulator, similar to the separate ground electrode already used in some systems but placed further away. Attempts at recording the eABR through telemetry of the voltage on the ball-shaped reference electrode of the Nucleus system are underway. If successful, telemetry may be useful in adjusting several parameters, such as the MCL levels (stapedius reflex threshold), relative timing of pulses to increase neural synchrony in case of bilateral implantation (eABR amplitude), and signal processing parameters and electrical stimulus configurations to enhance the discriminability of complex stimuli (cortical response amplitude and specificity). Implanted electrodes are less susceptible to external noise than surface electrodes and, if close to the targeted areas (such as the auditory cortex), will provide higher quality recordings.
Cochlear implant systems are being developed that can deliver drugs for neural protection or even enhancement of the responsiveness.58,59 Accurate dosage according to prescription obviously is paramount and requires mechanical precision. Telemetry may make it possible to go beyond a prescriptive procedure and implement a continuous monitoring of the result. This may range from sensing the concentration of the drug (or its byproducts) across the cochlea to more complex functional measures of the effect on the tissues. Possibly, some of the neural response measures already under investigation and discussed in this article will be part of such an integrated procedure. These and other developments will show telemetry to continuously increase the quality of cochlear implants in individual patients as well as over generations of increasingly refined systems.
Acknowledgments
The author wants to thank King Chung, Assistant Professor at the University of Purdue and editor of this journal and two anonymous reviewers for their comments and many efforts to enhance this article. Sections of this article regarding the telemetry of the technical integrity (Chapter 4) have appeared before in Cochlear Implants: Objective Measures and are used with permission of John Wiley & Sons Limited.
References
- 1.Berliner KI, House WF. The cochlear implant program: an overview. Ann Otol Rhinol Laryngol Suppl. 1982;91(2 Pt 3): 11–14 [PubMed] [Google Scholar]
- 2.Hall JW. Handbook of Auditory Evoked Responses. Needham, MA: Allyn & Bacon; 1991 [Google Scholar]
- 3.Briaire JJ, Frijns JHM. Unraveling the electrically evoked compound action potential. Hear Res. 2005;205: 143–156 [DOI] [PubMed] [Google Scholar]
- 4.Schoonhoven R, Lamore PJ, de Laat JA, Grote JJ. The prognostic value of electrocochleography in severely hearing-impaired infants. Audiology. 1999;38: 141–154 [DOI] [PubMed] [Google Scholar]
- 5.Klop WMC, Hartlooper A, Briare JJ, Frijns JHM. A new method for dealing with the stimulus artefact in electrically evoked compound action potential measurements. Acta Otolaryngol. 2004;124: 137–143 [DOI] [PubMed] [Google Scholar]
- 6.Brown C. The electrically evoked whole nerve action potential. In: Cullington HE. ed. Cochlear Implants: Objective Measures. London, England: Whurr; 2003: 96–129 [Google Scholar]
- 7.Charlet de Sauvage R, Cazals Y, Erre JP, Aran JM. Acoustically derived auditory nerve action potential evoked by electrical stimulation: an estimation of the waveform of single unit contribution. J Acoust Soc Am. 1983;73: 616–627 [DOI] [PubMed] [Google Scholar]
- 8.Brown CJ, Abbas PJ, Gantz B. Electrically evoked whole-nerve action potentials: data from human cochlear implant users. J Acoust Soc Am. 1990;88: 1385–1391 [DOI] [PubMed] [Google Scholar]
- 9.Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver A, Gervais J. The relationship between EAP and EABR thresholds and levels used to program the nucleus 24 speech processor: data from adults. Ear Hear. 2000;21: 151–163 [DOI] [PubMed] [Google Scholar]
- 10.Battmer RD, Dillier N, Lai WK, et al. Evaluation of the neural response telemetry (NRT) capabilities of the nucleus research platform 8: initial results from the NRT trial. Int J Audiol. 2004;43: S10–S15 [PubMed] [Google Scholar]
- 11.Charasse B, Thai-Van H, Chanal JM, Berger-Vachon C, Collet L. Automatic analysis of auditory nerve electrically evoked compound action potential with an artificial neural network. Artif Intell Med. 2004;31: 221–229 [DOI] [PubMed] [Google Scholar]
- 12.Botros A, van Dijk B, Killian M. AutoNRT™: an automated system that measures ECAP thresholds with the Nucleus(r) Freedom™ cochlear implant via machine intelligence. Artif Intell Med. 2007;40: 15–28 Epub 2006 Aug 22. [DOI] [PubMed] [Google Scholar]
- 13.Marsh A new stimulus for promonoty E-ABR. Paper presented at: 1993 Conference on implantable auditory prostheses; July 11–15, 1993; Smithfield, RI. [Google Scholar]
- 14.Mason S. Electrophysiologic and objective monitoring of the cochlear implant during surgery: implementation, audit and outcomes. Int J Audiol. 2004;43: S33–S38 [PubMed] [Google Scholar]
- 15.Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate on threshold and dynamic range in Clarion cochlear-implant users. J Acoust Soc Am. 2004;115(5 Pt 1): 1885–1888 [DOI] [PubMed] [Google Scholar]
- 16.Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18: 135–143 [DOI] [PubMed] [Google Scholar]
- 17.Vischer M, Haenggeli A, Zhang J, Pelizzone M, Hausler R, Rouiller EM. Effect of high-frequency electrical stimulation of the auditory nerve in an animal model of cochlear implants. Am J Otol. 1997;18(6 Suppl.): S27–S29 [PubMed] [Google Scholar]
- 18.Cafarelli-Dees D, Dillier N, Lai WK, et al. Normative findings of electrically evoked compound action potential measurements using the neural response telemetry of the Nucleus CI24M cochlear implant system. Audiol Neurootol. 2005;10: 105–116 [DOI] [PubMed] [Google Scholar]
- 19.Hughes ML, Brown CJ, Abbas PJ, Wolaver AA, Gervais JP. Comparison of EAP thresholds with MAP levels in the nucleus 24 cochlear implant: data from children. Ear Hear. 2000;21: 164–174 [DOI] [PubMed] [Google Scholar]
- 20.Mason SM, Cope Y, Garnham J, O'Donoghue GM, Gibbin K P. Intra-operative recordings of electrically evoked auditory nerve action potentials in young children by use of neural response telemetry with the Nucleus CI24M cochlear implant. Br J Audiol. 2001;35: 225–235 [DOI] [PubMed] [Google Scholar]
- 21.Franck KH. A model of a nucleus 24 cochlear implant fitting protocol based on the electrically evoked whole nerve action potential. Ear Hear. 2002;23(1 Suppl): 67S–71S [DOI] [PubMed] [Google Scholar]
- 22.Zimmerling MJ, Hochmair ES. EAP recordings in ineraid patients—correlations with psychophysical measures and possible implications for patient fitting. Ear Hear. 2002;23: 81–91 [DOI] [PubMed] [Google Scholar]
- 23.Thai-Van H, Truy E, Charasse B, et al. Modeling the relationship between psychophysical perception and electrically evoked compound action potential threshold in young cochlear implant recipients: clinical implications for implant fitting. Clin Neurophysiol. 2004;115: 2811–2824 [DOI] [PubMed] [Google Scholar]
- 24.Gordon KA, Papsin BC, Harrison RV. Toward a battery of behavioral and objective measures to achieve optimal cochlear implant stimulation levels in children. Ear Hear. 2004;25: 447–463 [DOI] [PubMed] [Google Scholar]
- 25.Smoorenburg GF, Willeboer C, van Dijk JE. Speech perception in nucleus C124M cochlear implant users with processor settings based on electrically evoked compound action potential thresholds. Audiol Neurootol. 2002;7: 335–347 [DOI] [PubMed] [Google Scholar]
- 26.McKay CM, Fewster L, Dawson P. A different approach to using neural response telemetry for automated cochlear implant processor programming. Ear Hear. 2005;26(4 Suppl.): 38S–44S [DOI] [PubMed] [Google Scholar]
- 27.Brown CJ. Clinical uses of electrically evoked auditory nerve and brainstem responses. Curr Opin Otolaryngol Head Neck Surg. 2003;11: 383–387 [DOI] [PubMed] [Google Scholar]
- 28.Seyle K, Brown CJ. Speech perception using maps based on neural response telemetry measures. Ear Hear. 2002;23(1 Suppl.): 72S–79S [DOI] [PubMed] [Google Scholar]
- 29.Sun YS, Wu CM, Liu TC. Mandarin speech perception in nucleus CI 24 implantees using MAPs based on neural response telemetry. ORL J Otorhinolaryngol Relat Spec. 2004;66: 255–261 [DOI] [PubMed] [Google Scholar]
- 30.Kaplan-Neeman R, Henkin Y, Yakir Z, et al. NRT-based versus behavioral-based MAP: a comparison of parameters and speech perception in young children. J Basic Clin Physiol Pharmacol. 2004;15: 57–69 [DOI] [PubMed] [Google Scholar]
- 31.Kiefer J, Hohl S, Sturzebecher E, Pfennigdorff T, Gstoettner W. Comparison of speech recognition with different speech coding strategies (SPEAK, CIS, and ACE) and their relationship to telemetric measures of compound action potentials in the nucleus CI 24M cochlear implant system. Audiology. 2001;40: 32–42 [DOI] [PubMed] [Google Scholar]
- 32.Shpak T, Berlin M, Luntz M. Objective measurements of auditory nerve recovery function in nucleus CI 24 implantees in relation to subjective preference of stimulation rate. Acta Otolaryngol. 2004;124: 679–683 [DOI] [PubMed] [Google Scholar]
- 33.Gantz BJ, Brown CJ, Abbas PJ. Intraoperative measures of electrically evoked auditory nerve compound action potential. Am J Otol. 1994;15: 137–144 [PubMed] [Google Scholar]
- 34.Franck KH, Norton SJ. Estimation of psychophysical levels using the electrically evoked compound action potential measured with the neural response telemetry capabilities of Cochlear Corporation's CI24M device. Ear Hear. 2001;22: 289–299 [DOI] [PubMed] [Google Scholar]
- 35.Cohen LT, Saunders E, Richardson LM. Spatial spread of neural excitation: comparison of compound action potential and forward-masking data in cochlear implant recipients. Int J Audiol. 2004;43: 346–355 [DOI] [PubMed] [Google Scholar]
- 36.Eisen MD, Franck KH. Electrode interaction in pediatric cochlear implant subjects. J Assoc Res Otolaryngol. 2005;6: 160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes ML, Abbas PJ. The relation between electrophysiologic channel interaction and electrode pitch ranking in cochlear implant recipients. J Acoust Soc Am. 2006;119: 1527–1537 [DOI] [PubMed] [Google Scholar]
- 38.Cohen LT, Richardson LM, Saunders E, Cowan RSC. Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hear Res. 2003;179: 72–87 [DOI] [PubMed] [Google Scholar]
- 39.Fayad JN, Linthicum FH. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116: 1310–1320 [DOI] [PubMed] [Google Scholar]
- 40.Ifukube T, White RL. Current distributions produced inside and outside the cochlea from a scala tympani electrode array. IEEE Trans Biomed Eng. 1987;34: 883–890 [DOI] [PubMed] [Google Scholar]
- 41.Jolly CN, Spelman FA, Clopton BM. Quadrupolar stimulation for cochlear prostheses: modeling and experimental data. IEEE Trans Biomed Eng. 1996;43: 857–865 [DOI] [PubMed] [Google Scholar]
- 42.Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hear Res. 1998;121: 11–28 [DOI] [PubMed] [Google Scholar]
- 43.O'Leary SJ, Black RC, Clark GM. Current distributions in the cat cochlea: a modelling and electrophysiological study. Hear Res. 1985;18: 273–281 [DOI] [PubMed] [Google Scholar]
- 44.Kasper A, Pelizzone M, Montandon P. Intracochlear potential distribution with intracochlear and extracochlear electrical stimulation in humans. Ann Otol Rhinol Laryngol. 1991;100: 812–816 [DOI] [PubMed] [Google Scholar]
- 45.Mens LHM, Van den Broek P. Telemetry of longitudinal current spread: does it show spatial selectivity?. Paper presented at: 1997 Conference on Implantable Auditory Prostheses; August 12–21, 1997; Pacific Grove, CA. [Google Scholar]
- 46.Mens LHM, Boyle PJ, Mulder JJ. The Clarion electrode positioner: approximation to the medial wall and current focussing? Audiol Neurotol. 2003;8: 166–175 [DOI] [PubMed] [Google Scholar]
- 47.Faltys M, Griffith G, Segel P, et al. Electrical field imaging methodology. Paper presented at: Conference on Implantable Auditory Prostheses; August 19–24, 2001; Asilomar, Pacific Grove, CA. [Google Scholar]
- 48.Vanpoucke FJ, Zarowski AJ, Peeters SA. Identification of the impedance model of an implanted cochlear prosthesis from intracochlear potential measurements. IEEE Trans Biomed Eng. 2004;51: 2174–2183 [DOI] [PubMed] [Google Scholar]
- 49.Mens LHM, Huiskamp G, Broek Pvd, Oostendorp TF. Modelling surface potentials from intra-cochlear electrical stimulation. Scand Audiol. 1999;28: 249–255 [DOI] [PubMed] [Google Scholar]
- 50.Mens LHM, Oostendorp TF, Van den Broek P. Cochlear implant generated surface potentials: current spread and side effects. Ear Hear. 1994;15: 339–345 [DOI] [PubMed] [Google Scholar]
- 51.Suesserman MF, Spelman FA. Lumped-parameter model for in vivo cochlear stimulation. IEEE Trans Biomed Eng. 1993;40: 237–245 [DOI] [PubMed] [Google Scholar]
- 52.Vanpoucke F, Zarowski A, Casselman J, Frijns J, Peeters S. The facial nerve canal: an important cochlear conduction path revealed by Clarion electrical field imaging. Otol Neurotol. 2004;25: 282–289 [DOI] [PubMed] [Google Scholar]
- 53.Sprögel J. Untersuchungen der elektrsichen Feldaus-breitung in de Cochlea unter Berücksichtigung des Elektrodensystems. Hannover, Germany: Medizinischen Hochschule Hannover; 2006 [Google Scholar]
- 54.Mens LHM. Telemetry: features and applications. In: Cullington HE. ed. Cochlear Implants: Objective Measures. London, England: Whurr; 2003: 23–39 [Google Scholar]
- 55.Balkany TJ, Hodges AV, Buchman CA, et al. Cochlear implant soft failures consensus development conference statement. Otol Neurotol. 2005;26: 815–818 [DOI] [PubMed] [Google Scholar]
- 56.Mauch H, Möller C, Nevison B. Diagnosing implant failures with the nucleus integrity test system. Paper presented at: Fourth International Symposium and Workshops: Objective Measures in Cochlear Implants; June 1–4, 2005; Hannover, Germany. [Google Scholar]
- 57.Carter P. Determining electrode faults using different stimulation modes. Paper presented at: XVI World Congress of ORL—Head and Neck Surgery, 1997; Sydney, Australia. [Google Scholar]
- 58.Marzella PL, Clark GM. Growth factors, auditory neurones and cochlear implants: a review. Acta Otolaryngol. 1999;119: 407–412 [DOI] [PubMed] [Google Scholar]
- 59.Miller AL, Prieskorn DM, Altschuler RA, Miller JM. Mechanism of electrical stimulation-induced neuroprotection: effects of verapamil on protection of primary auditory afferents. Brain Res. 2003;966: 218–230 [DOI] [PubMed] [Google Scholar]