Abstract
Cochlear Limited (Cochlear™) released the fourth-generation cochlear implant system, Nucleus® Freedom™, in 2005. Freedom is based on 25 years of experience in cochlear implant research and development and incorporates advances in medicine, implantable materials, electronic technology, and sound coding. This article presents the development of Cochlear's implant systems, with an overview of the first 3 generations, and details of the Freedom system: the CI24RE receiver-stimulator, the Contour Advance™ electrode, the modular Freedom processor, the available speech coding strategies, the input processing options of SmartSound™ to improve the signal before coding as electrical signals, and the programming software. Preliminary results from multicenter studies with the Freedom system are reported, demonstrating better levels of performance compared with the previous systems. The final section presents the most recent implant reliability data, with the early findings at 18 months showing improved reliability of the Freedom implant compared with the earlier Nucleus 3 System. Also reported are some of the findings of Cochlear's collaborative research programs to improve recipient outcomes. Included are studies showing the benefits from bilateral implants, electroacoustic stimulation using an ipsilateral and/or contralateral hearing aid, advanced speech coding, and streamlined speech processor programming.
Keywords: Cochlear implant, coding strategy, preprocessing, electroacoustic stimulation, combined stimulation, Nucleus Freedom
The cochlear implant is a remarkable combination of science, technology, and medicine that brings functional hearing to severe-profoundly deaf individuals. It has transformed the lives of many thousands of recipients and their families. In July 2006, more than 77,500 registered Nucleus® system implants were in use. Recent estimates indicate that 7 of every 10 implant candidates are implanted with the Nucleus system globally.1
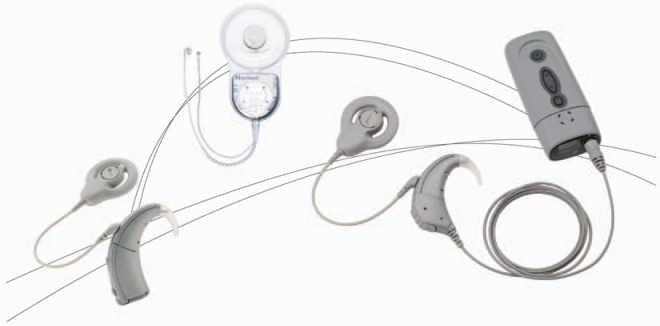
In 2005, Cochlear™ released Nucleus Freedom™ (Figure 1), the fourth-generation cochlear implant system. Since the release of Cochlear's first multichannel system in 1985, there have been many improvements in implant and speech processor technology, and in recipient outcomes. Cochlear implants are now being used in very young children and those with considerable amounts of residual hearing who gain only marginal benefit from hearing aids. Advances in Cochlear's technology have been driven by the desire to provide safe and reliable implant systems, improve recipient outcomes, and meet the needs of those for whom hearing aids are inadequate.
Figure 1.
Overview of the Nucleus® Freedom™ system, showing the CI24RE receiver-stimulator and the speech processor unit coupled to the behind-the-ear and the Bodyworn Controller. (Image by permission of Cochlear Limited)
Nucleus Freedom incorporates many new innovations, including:
Four SmartSound™ input processing options and unused processing capability in the behind-the-ear (BTE) processor that will allow further features to be added in the future.
Flexible speech processing options so that processing can be adjusted to meet individual needs.
Improved Neural Response Telemetry™ (NRT™) functionality to record the electrically evoked compound action potential (ECAP), which can be used to guide speech processor fitting, especially for young children.
Flexibility in the electronics of the implanted receiver-stimulator, so that future advances can be achieved by upgraded firmware and new generations of external hardware.
Streamlined fitting software with the flexibility to adjust parameters to maximize individual outcomes.
Design and manufacturing processes for industry-leading implant reliability.
The signal path, from input sound to auditory perception, is shown in Figure 2. There are 3 separate opportunities to improve recipient outcomes. First is with the microphone and audio preprocessing stage, where the effect of background noise is minimized so as to achieve higher quality signal input to the MAPping function. The second opportunity lies with the signal analysis and stimulus generator blocks. These translate the acoustic signal into an electrical representation for stimulating the auditory nerve. Freedom provides for both of these options via SmartSound at the input stage, and with flexible MAPping options. The third opportunity lies with advances in electrode design and methods of stimulus delivery. Positioning the electrode closer to the modiolus using the Contour Advance™ electrode is one instance of an improved design that has resulted in the benefit of lower stimulation levels.
Figure 2.
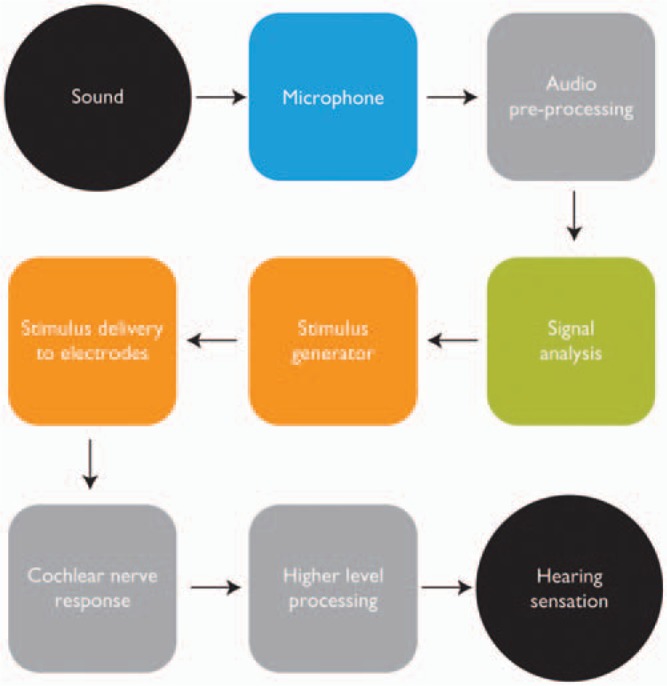
The signal path from sound to hearing sensation. The input stage consists of the microphone and audio preprocessing blocks. MAPping is represented by the signal analysis and stimulus generator blocks. Stimulus delivery is via the receiver-stimulator and electrodes, stimulating the auditory nerve fibers in the cochlea. Hearing sensations are then produced after additional higher level processing by the auditory system. (Image by permission of Cochlear Limited)
The first section of this article reviews the evolution of the first 3 generations of Nucleus implants. The second section presents the Freedom features and components, including implanted receiver-stimulator package, electrode array, speech processor technology, and input signal processing. The final section describes some of Cochlear's approaches to improving recipient outcomes.
The Evolution of the Nucleus Cochlear Implant System
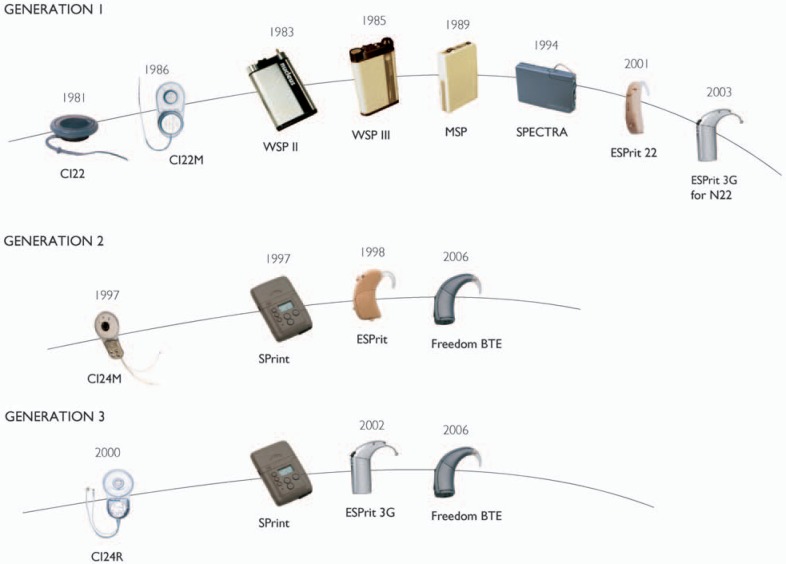
Cochlear has 25 years of experience in cochlear implants, beginning with the development of its first implant system in 1981 (Figure 3). Cochlear, a division of the Nucleus group of companies, was established following the research of Professor Graeme Clark and his team at the University of Melbourne, Australia. These early studies investigated the biocompatibility of implanted components, surgical approach to the cochlea, and the safe limits for electrical stimulation.2–5 The culmination of more than a decade of research was the prototype University of Melbourne multichannel implant, a fully implanted system with an intracochlear array of 10 electrodes that received power and data from an external speech processor system via radio frequency. Results from the 3 patients implanted in 1978 to 1979 confirmed the surgical approach and engineering design, and subjects gained some benefit from their devices when using a prototype speech processor.6,7 It was also found that with the simultaneous stimulation of 2 or more electrodes, the interactions between nearby electrodes resulted in uncontrolled loudness variations. Thus, much simpler stimulus patterns were adopted, where only a single electrode was stimulated at any time. This “sequential” stimulation technique is almost universally used today. Safety studies continue to be of central importance, with ongoing collaborations between Cochlear and independent research centers to verify new technologies and electrical stimulation regimes.
Figure 3.
The first 3 generations of Nucleus cochlear implants. (Image by permission of Cochlear Limited)
The Nucleus CI22M System
The first-generation Nucleus implant system, developed in 1981 and commercially released in 1985, consisted of the CI22 receiver-stimulator coupled to a 22-electrode array, and a body-worn speech processor known as a wearable speech processor (WSP) (Figure 3). A titanium case housed the receiver-stimulator electronics, and titanium casing has been used for all subsequent Nucleus implants. The mechanical components of the implant were redesigned, and the CI22M, also known as the Mini Implant, was released in 1986 with an internal magnet to hold the external transmitting coil in place. The electrode array was a straight array that lay against the lateral wall of the cochlea after insertion. Stimuli were delivered to pairs of nearby intracochlear electrodes in a bipolar configuration, or one electrode was stimulated against all others connected together (common ground stimulation). Four speech coding schemes were released during the lifetime of this system.
The initial speech coding strategy presented the fundamental or voicing frequency (F0) as pulse rate, and the second formant (F2) of speech determined the position of the stimulating electrode. F0 provides prosody, intonation, and voicing information, and F2 is the most important spectral component of speech. Input signal amplitude was coded as electric current level. Whereas the initial objective was that the device would assist lipreading, some open-set speech understanding was achieved. In the initial clinical trial in Melbourne in 1982, a mean score of 19% for Central Institute for the Deaf (CID) sentences was found for the group of 8 recipients when tested using the implant alone.8
The first formant (F1) and corresponding amplitude were added to the coding strategy in 1985, following psychophysics studies showing that electrical stimuli delivered to different regions of the cochlea by 2 interleaved pulse trains generated percepts having 2 independent dimensions.9 Further high frequency information was added in the Multipeak™ strategy (MPEAK™), with fricatives being represented by stimulation on fixed electrodes in the basal region of the cochlea using a new processor platform, known as mini speech processor (MSP), in 1989. Acoustic amplitude was coded using a combination of current amplitude and pulse width, known as stimulus level, in the MSP processor. The MSP also used 150 discrete steps for amplitude coding, whereas the WSP used only 31 steps; thus there was more accurate control of stimulation level and loudness. Speech perception performance improved with each of these advances in speech coding. The average Bamford-Kowal-Bench sentence score in quiet from a group of 5 subjects was 19% higher with MPEAK than with the earlier strategy coding only F1 and F2.10 In addition, the MSP processor used a custom designed digital signal processing circuit, and processor size and weight were markedly reduced.
All speech processors from Cochlear have used a directional microphone to help reduce the impact of background noise on speech understanding. A common communication situation is when the cochlear implant (CI) recipient faces the talker; thus the speech signal is from the front. By using a directional microphone, the intensity of sounds from other directions is reduced and the signal-noise ratio (SNR) improves. This is a single-element dual-port microphone with 7 mm between the 2 ports (see also Figure 8). Note that the microphone provides 6 dB/octave emphasis up to 4 kHz and then falls at about 12 dB/octave for the higher frequencies. This is very close to the inverse of the free-field hearing thresholds for normally hearing listeners. Thus the frequency response of the microphone is relatively flat in dB HL.
Figure 8.
Schematic of the Nucleus Freedom BTE processor and controller showing the major system components. Image by permission of Cochlear Limited)
Feedback from recipients indicated that many were adjusting the microphone sensitivity control to reduce the audibility of the noise. The sensitivity control adjusts the sound level that triggers the automatic gain control (AGC) circuit at the input stage of the processor (as further described in “Instantaneous Input Dynamic Range”). The MSP therefore included an automatic sensitivity control (ASC), a selectable input signal processing scheme designed to reduce the impact of background noise. At the default settings, ASC aims to ensure that the noise floor remains 15 dB below the AGC knee-point. The noise floor is the level to which sound decreases during breaks in speech. The ASC circuit is triggered using a programmable “autosensitivity breakpoint.” When the noise floor is above the breakpoint, the ASC gradually reduces the input signal gain; thus the noise will be lower within the instantaneous input dynamic range (IIDR). When the noise floor drops below the breakpoint, the input gain gradually increases back toward the default gain. The time taken for the ASC to adapt is in the order of seconds. The overall perceptual effect of ASC is a reduction in the loudness of background noise. Automatic sensitivity control has been available in all subsequent speech processors.
In June 1990, the Nucleus CI22M system was approved by the US Food and Drug Administration (FDA) for use in children aged 2 years and older. This was the first multichannel cochlear implant to obtain FDA approval for children.
In 1994, the SPEAK™ strategy was released for the CI22M with a new body-worn processor, Spectra. This strategy was a major change in speech coding direction, taking advantage of new technology and speech coding research that showed improved speech understanding by implant recipients using the spectral maxima sound processor (SMSP) strategy developed at the University of Melbourne.11,12 In the SMSP strategy, 6 frequency bands with the largest amplitudes (maxima) were usually selected from a 16-channel digital bandpass filterbank to stimulate the electrodes using a frequency-to-electrode MAPping function consistent with the tonotopic organization of the cochlea. The entire estimated spectrum after the filterbank was scanned to select the maxima. Several of the maxima could originate from a single spectral peak in the original input signal, providing some information about the bandwidths of the larger spectral peaks. The center frequencies of the 6th order bandpass filters were between 250 Hz and 5.4 kHz.11
The SPEAK strategy in the Spectra continually sampled the outputs of 20 filter bands spanning the frequency range and presented between 5 and 10 of the largest maxima, depending on the spectral composition of the input signal, with an average of 6 maxima. The filters were linearly spaced up to approximately 1850 Hz and then logarithmically spaced to the maximum frequency. The minimum (lower limit of 75 Hz) and maximum (upper limit of 10,823 Hz) frequencies were selectable by the clinician using a set of frequency allocation tables.13 This flexibility is very useful in cases where some electrodes could not be stimulated; for example, in cases of partial insertion. Cochlear's fitting software continues to support this flexibility in frequency allocation. The average stimulation rate was 250 Hz per electrode, but this could vary depending on spectral composition and intensity of the input sound. The amplitude of the selected maxima was usually coded using stimulus level, as described previously.
In the multicenter trial of SPEAK with 63 adult subjects, Skinner et al13 reported significant improvements in speech perception scores in quiet and noise when compared with MPEAK. The average score for City University of New York (CUNY), or speech intelligibility test (SIT) for deaf children,14 sentences in quiet using SPEAK was 77.5%, and for a subgroup of 58 subjects who could be tested in noise, the mean score for sentences at +10 dB SNR was 61.5%. Sentence scores with the MPEAK strategy were 67.4% in quiet and 37.1% in noise at +10 dB SNR. Note that for testing in quiet, ceiling effects influenced these average scores. For testing in noise, there was a 24.4% improvement in performance when using SPEAK.
Within 9 years of the release of the first Nucleus implant system, the majority of recipients were now obtaining significant levels of speech understanding in quiet and noise. Figure 4 shows improvements in scores for consonant-vowel nucleus-consonant (CNC) words in quiet and CID sentences in noise over the 4 speech processing schemes for different groups of unselected recipients in Melbourne.3 Loizou15 and Clark3–5 provide more-detailed reviews of the CI22M system, speech coding strategies, and clinical results. Cochlear continues to support CI22M recipients with later generations of BTE speech processors that incorporate some of the advances in input processing (Figure 3).
Figure 4.
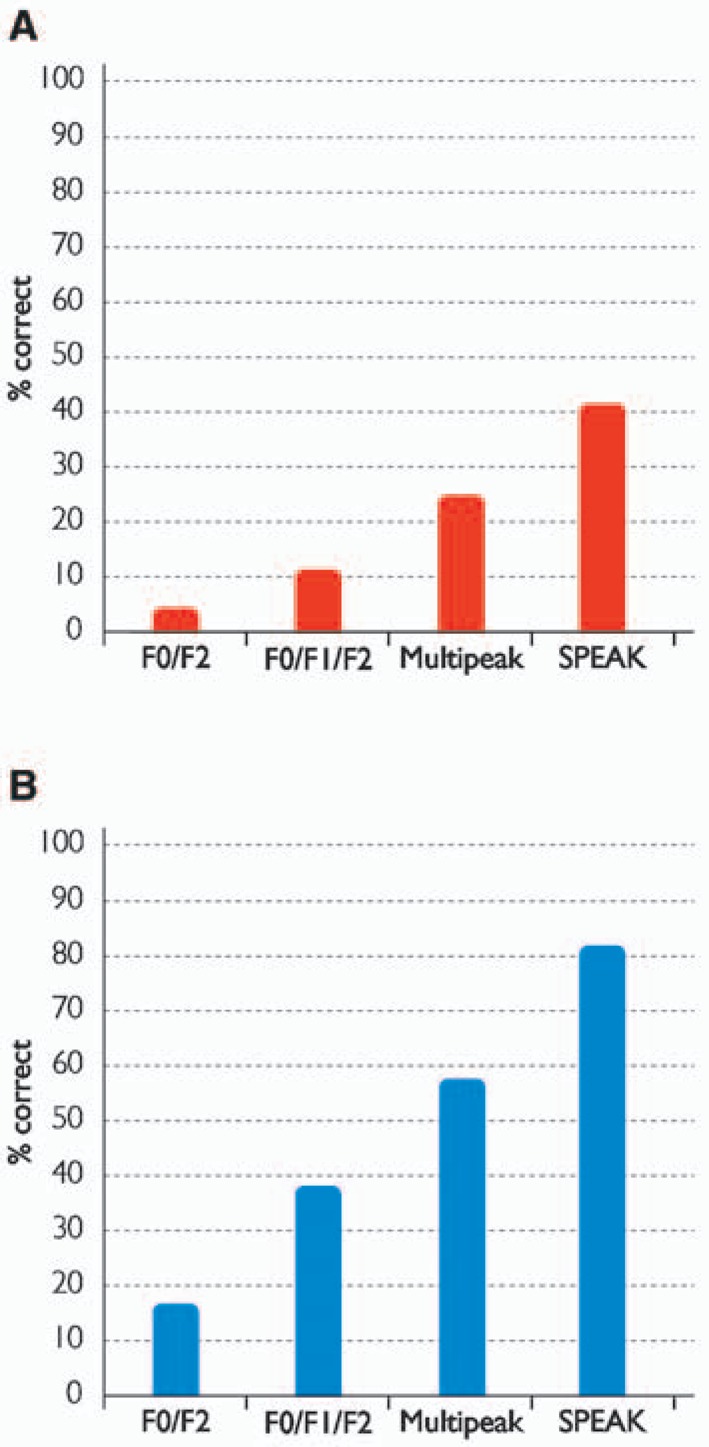
Percent correct scores for (A) CNC words in quiet and (B) CID sentences in noise over the first 4 speech processing schemes used with the first-generation CI22M system: F0/F2 (n = 16), F0/F1/F2 (n = 45), Multipeak (n = 32), and SPEAK (n = 22). Scores are from different groups of unselected recipients in Melbourne. (Image by permission of Cochlear Limited)
The Nucleus CI24M System
Cochlear's second-generation implant system released in 1997 consisted of the CI24M receiver-stimulator, a 22-electrode straight array, and the SPrint™ body-worn digital speech processor (Figure 3) supporting 3 speech coding strategies: SPEAK, advanced combination encoder (ACE™), and continuous interleaved sampling (CIS). The CI24M introduced new stimulation capability by addition of a plate electrode on the package and an additional lead wire connected to a ball electrode intended to be placed under the temporalis muscle. The plate and ball electrodes can be programmed as the return for monopolar stimulation, separately or in parallel. This provides greater flexibility in stimulation and can help in programming in cases of unwanted sensations, such as from vestibular or facial nerve stimulation. One of the advantages of monopolar stimulation is a reduction in current levels to achieve hearing thresholds and comfortable listening levels when compared with bipolar stimulation16; thus power consumption was reduced.
One of the many innovations implemented in the CI24M was an increase in available pulse rates to 14.4 kHz. During development of the system, safety studies were conducted to determine whether long-term stimulation using high pulse rates might have a detrimental effect on neural responses and auditory nerve fiber integrity, in particular the spiral ganglion cells. Although there were some initial concerns with stimulation at 1000 Hz using a very high current amplitude and bipolar stimulation,17 subsequent studies using even higher rates, up to 2000 Hz, and amplitude modulated current within the dynamic range of hearing showed no evidence of neural fatigue or abnormal cochlear histopathology for both bipolar and monopolar stimulation.18,19 These outcomes were confirmed in a later study investigating the long-term physiologic and histologic effects of a higher stimulation rate, 4831 Hz, using constant current amplitude within the dynamic range of hearing with monopolar stimulation.20
Another leading innovation in the CI24M was the inclusion of telemetry to measure electrode voltage compliance and impedance, to diagnose implant and electrode function. Telemetry also supported the world's first recording of the ECAP using the intracochlear electrodes via NRT.21 Neural response telemetry can be used to assist in device fitting22–24 and can be very helpful in fitting young children25 and others who do not give reliable behavioral responses. It has been shown that NRT-based fitting procedures are quicker than behaviorally based methods in young children, with similar aided thresholds and speech detection scores for the 2 methods.26 Research on the capabilities and clinical use of NRT continues with the advanced NRT features of Nucleus Freedom.
The ACE strategy is an extension of SPEAK, taking advantage of the higher stimulation rates available in the CI24M. Higher rate stimulation has the capability to better represent the temporal changes in the output amplitudes of the analysis filters. In addition, up to 20 maxima could be selected in ACE. The CIS strategy was developed by Wilson et al27 as a 4-to-6 channel system and was implemented in the Nucleus system using between 4 and 12 channels. The CIS strategy sequentially stimulates a fixed set of electrodes, differing from SPEAK and ACE which dynamically select the electrodes for stimulation. In addition, the filterbank bandwidths are usually larger with CIS because fewer channels are used to divide the input frequency spectrum.
A multicenter study with 62 experienced adult subjects was conducted to determine strategy and stimulation rate preference with this second-generation Nucleus system.28 The study compared SPEAK at 250 Hz with ACE and CIS at rates between 500 Hz and 2400 Hz, using speech perception and subjective preference measures. The most significant finding was that scores were highest when subjects used their preferred strategy, which was predominantly SPEAK or ACE at 720 Hz or 1800 Hz. A similar finding was reported in a study with 12 newly implanted adult subjects.29 In addition, a study by Holden et al30 with 8 adults using the ACE strategy showed that recipients have individual preferences for stimulation rate, and the provision of more than one rate during initial take-home experience lets recipients select from among the different rates for various listening environments. Therefore, the CI24M system gave clinicians a wider range of speech coding strategies and stimulation rates than previously available to optimize the fitting to suit individual needs. It was also clear from these studies that the preferred rate was almost always not the highest stimulation rate available.
The biggest physical limitation of the early implant systems was the body-worn processor and accompanying cable. In 1998, Cochlear released the first BTE multichannel speech processor for cochlear implants, the ESPrit™ for the CI24M system (Figure 3). Initially, the ESPrit used the SPEAK strategy with support for ACE provided in 2000. The ESPrit 22 processor for CI22M recipients supporting the SPEAK strategy was released in 2001, giving recipients of the first-generation system access to a BTE processor.
The Nucleus 3 System
The third-generation Nucleus system introduced in 2000 was the Nucleus 24 Contour™, which consisted of the CI24R receiver-stimulator and the Contour™ self-curling perimodiolar electrode array (Figure 3). The Nucleus 24 Contour was compatible with the existing body-worn SPrint and BTE ESPrit speech processors. Generally, the SPrint was the processor choice for very young children. The BTE ESPrit™ 3G processor followed in 2002. The improvements in receiver-stimulator design with the CI24R and the development of the Contour electrode are more fully described in the sections titled “The CI24RE Receiver-Stimulator” and “Contour Advance” as they are particularly relevant to the current Nucleus Freedom system.
The dimensions of the CI24R receiver-stimulator were considerably smaller than those of the CI24M and the package was designed with a low profile (see “The CI24RE Receiver-Stimulator” and Figure 5). Very young children were being considered for implantation, and thus it was important to provide an implant appropriate for this age group. Suitability of the CI24R for pediatric use was investigated in a multicenter trial in 46 North American clinics.31 The 256 children implanted with the CI24R were aged between 1 and 17 years at the time of surgery. The study showed that the smaller CI24R was preferred over the CI24M by 97% of the 29 surgeons who responded to a surgical questionnaire, particularly for use in infants.
Figure 5.
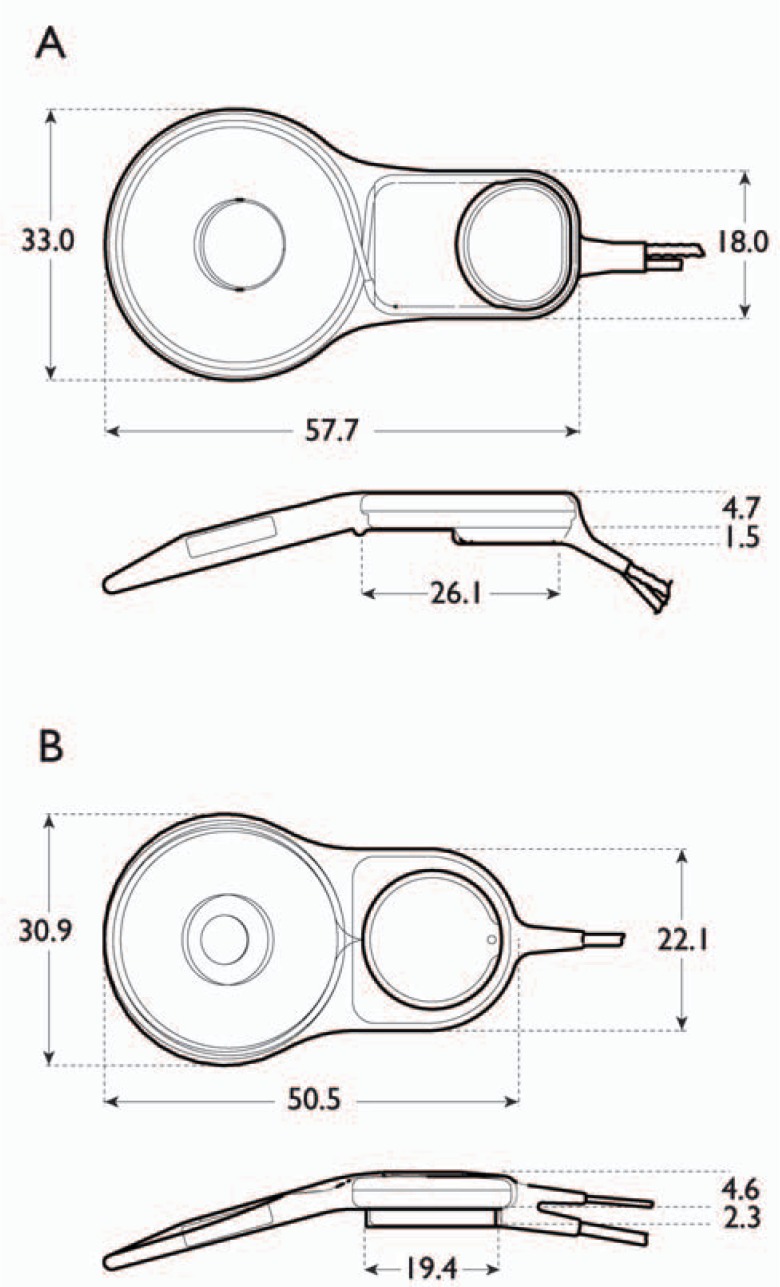
Comparison of the (A) CI24M and the (B) CI24R and CI24RE receiver-stimulators showing the changes in implant package dimensions. The CI24R and CI24RE have the same dimensions. Units are in millimeters. (Image by permission of Cochlear Limited)
The CI24R is particularly well suited to minimal access surgery, in which a small and relatively straight incision is used, close to the hairline behind the pinna. The reduced size of the surgical site has improved cosmesis, reduced the incidence of flap-related complications, and minimized postoperative complication rates.32–37 The success of minimal access surgery has been shown in reports on 52 adults and children,32 23 children,34 100 children,35 and 25 infants between 7 and 12 months of age.36
The Contour electrode used in the Nucleus 3 System is a precurved array with 22 half-banded electrodes (see “Contour Advance”). An internal malleable stylet holds the electrode array in a straightened shape for insertion into the cochlea, and the stylet is withdrawn after insertion. The array then curls to its precurved shape, with the electrodes positioned close to the modiolus. The smooth outer surface of the half-banded array reduces the possibility of damage to the outer wall of the scala tympani during insertion. In addition, the half-banded electrodes face the inner wall, directing stimulation toward the spiral ganglion cells in the modiolus.
In a multicenter North American study with 56 adult recipients,38 32 of the 37 surgeons (86%) who responded to a surgical questionnaire on electrode insertion reported that insertion of the Contour was easier than the previous straight array. A similar finding was found in the multicenter North American pediatric study, where 86% of the 37 surgeons who responded to the questionnaire about insertion also reported that the Contour was easier to insert than the straight array.31
Speech perception results from the 56 subjects in the North American multicenter study showed that at 3 months after fitting, the mean score for monosyllabic CNC words in quiet was 38.4%, and for CUNY sentences in noise at +10 dB SNR the mean score was 59.4%.38 Subjects used their preferred strategy, SPEAK, ACE, or CIS, and stimulation rate, between 250 Hz and 1800 Hz. Almost all subjects preferred SPEAK or ACE, 25% and 71% of subjects respectively, and only 2 subjects (4%) preferred CIS. Further data were collected after the clinical trial at 6 months from 25 subjects using their preferred strategy. For CUNY sentences in noise the average score was 74.0%, which was a 14.6% improvement between 3 and 6 months. For CNC words in quiet the average six month score was 47.0%, which was an improvement of 8.6%.39
In 2002, two new input signal processing options were introduced in the Nucleus 3 system to improve recipient outcomes: Adaptive dynamic range optimization™ (ADRO™) in the SPrint and Whisper™ in the ESPrit 3G BTE processor.
Adaptive dynamic range optimization is a preprocessing scheme that continually adjusts the gain on each bandpass filter channel to position the signal in the hearing dynamic range, ensuring that speech components are always presented at a comfortable listening level within each frequency band. The gain adjustment rules use percentile estimates of the long-term output level of each frequency band, with slow-acting changes in channel gains. Adaptive dynamic range optimization was developed at the Cooperative Research Centre for Cochlear Implant and Hearing Aid Innovation, Melbourne.40,41 In the initial study in Melbourne with 9 adults, scores were significantly higher using ADRO for CNC words in quiet at soft and normal levels, 40 dB and 60 dB, with improvements of 20% and 9.5%, respectively.41 For CUNY sentences in noise at +10 dB and +15 dB SNR, scores for ADRO were not significantly different from scores without ADRO. More than half of the subjects preferred ADRO for everyday use, assessed by use of a rating questionnaire for everyday listening situations. Improvements in speech perception in quiet and noise when using ADRO were reported in a study with pediatric implant recipients.42
Whisper is a fast-acting compression circuit at the input stage of the processor that gives recipients better access to soft or more distant sounds. With Whisper activated, input signals above the 52 dB SPL knee-point are compressed at a 2:1 ratio. For input signal levels below 52 dB SPL, there is no compression; thus the 1:1 relationship between input and output dB is maintained. Whisper was developed at the University of Melbourne, and in an evaluation of the prototype Whisper, a 14% improvement in CNC word scores at a low-level input of 55 dB was found.43 However, there was a decrement in performance for sentences in noise for some subjects. Thus, Whisper provides benefit when listening to soft speech in quiet, but might not be beneficial to recipients in noisy environments.
An enhancement of the Contour electrode was released in 2003/2004 with the Contour Advance™ electrode and the Advance Off-Stylet™ (AOS™) insertion technique. These are described more fully in “Contour Advance”. Following Cochlear's established practice of providing upgraded speech processors for people with earlier implant generations, the ESPrit 3G for the CI22M system was released in 2003. This gave recipients of all the commercial generations of implants access to innovations such as Whisper and the convenience of a BTE speech processor.
Nucleus Freedom
This fourth-generation Nucleus cochlear implant system (Figure 1) was released in 2005. Nucleus Freedom consists of the CI24RE receiver-stimulator coupled to the Contour Advance electrode and the digital Freedom BTE processing unit. Freedom uses a modular design and supports a BTE or body-worn controller/battery holder. The BTE processor also provides 4 SmartSound™ input processing options on the one processing platform: Beam™, Whisper, ADRO, and ASC. The design strategy was to provide flexibility in the receiver-stimulator electronics, with the stimulation-specific algorithms in the external processor where they can be upgraded by new firmware or by new generations of hardware. For example, the receiver-stimulator electronics were designed with an asynchronous architecture, allowing the timing of each stimulation pulse to be individually adjusted in 200-ns increments. This fine-timing capability has the potential for improved recipient outcomes in the future. As new coding schemes that use such fine-timing control become available, Nucleus Freedom recipients will be able to take advantage of these strategies without the need to replace the implanted device.
Each of the major system components has been improved over that used in previous systems, with the improvements being based on research, technologic advances, and feedback from surgeons, clinicians, and recipients. The following 5 sections describe the new system.
The CI24RE Receiver-Stimulator
Over the 4 generations of Nucleus cochlear implants, the receiver-stimulator has undergone significant changes in design for reliability and ease of surgical placement. The CI24RE receiver-stimulator has the same external size and shape characteristics as the CI24R, thus it continues to meet the minimal access surgery requirements but has improvements in internal mechanical design, package strength, and electronic capabilities, including improved telemetry with a new NRT amplifier, the ability to stimulate at rates up to 32 kHz, and the capability of dual-electrode stimulation.
Changes in the implant package from the CI24M to the CI24R and CI24RE included modifications to the shape of the package and reductions in length and width (Figure 5). The CI24RE implant pedestal has a round base and vertical sides; thus drilling is simpler and the implant can be rotated in the bed to optimize placement on the skull. The depth of the pedestal was also increased, as requested by surgeons, for more secure positioning in the mastoid. The total length of the CI24RE is less than that of the CI24M, 50.5 mm compared with 57.7 mm, respectively. By reducing the length of the most inflexible part of the implant from 26.1 mm (CI24M) to 19.4 mm (CI24RE), it is easier to match skull curvature, especially for small children. The diameter of the antenna coil on the CI24RE is 30.9 mm, reduced from 33.0 mm on the CI24M.
Internally, the electronic components in the CI24RE receiver-stimulator have a lower profile than in the CI24R. The extra internal space has been used to strengthen the implant against external impact. The use of a titanium case to house the electronics also helps reduce the effects of impact, as the metal case is less likely to crack or shatter on direct impact than other housing materials, such as ceramics.44 This is particularly relevant for pediatric implantation, as children are more prone to falls. Impact toleration is evaluated using vertical and horizontal hammer tests in accordance with the standard Environmental Testing Part 2: Tests–Test Eh: Hammer Tests (EH 60068-2-75). The CI24RE is more resistant to impact damage than the CI24R, and this is of clinical importance as shown in the most recent cumulative failure rates for the CI24RE implant in children (see “Improved Implant Reliability”).
Nucleus Freedom can stimulate at pulse rates up to 32 kHz. To verify the safety of high rate stimulation, acute and chronic physiologic and histologic studies were conducted at the Cooperative Research Centre for Cochlear Implant and Hearing Aid Innovation, Melbourne.45 These studies showed that a high rate-stimulation paradigm did not have a detrimental effect on auditory nerve activity or function. In the acute animal studies, the effects of high rates and worst-case high current levels on neural fatige were investigated by measuring the recovery of the electrically evoked auditory brainstem response (EABR) after 2 hours of stimulation. A total stimulation rate of 32 kHz was distributed over 3 apical electrodes on the array, and monopolar stimulation was used. There was no significant reduction in poststimulus EABR amplitude following stimulation at 1.8 mA, which is just above the maximum output current amplitude of the Nucleus Freedom system (1.75 mA). In the chronic safety study, deafened animals were stimulated in one ear for approximately 16 hours per day for 3 to 4 months. Monopolar stimulation at a total rate of 32 kHz was delivered over 3 electrodes. The stimulating current levels were individually adjusted to be within the dynamic range of hearing. The other ear was implanted with a dummy device to serve as the control. Histologic examination of the cochleae showed no evidence of spiral ganglion cell damage on the side with high rate stimulation when compared with the opposite control.
The improved telemetry of Nucleus Freedom allows for more accurate measurement of electrode voltage compliance and impedance to assist in diagnosing any problems with the implanted device. The new NRT amplifier has improved linearity, sampling rate, sensitivity, signal-noise ratio, and artifact recovery compared with the previous NRT system in the CI24M and CI24R. The noise floor of the CI24RE amplifier is up to 10 times below the noise floor of the Nucleus CI24M and CI24R, 2 μV compared with 20 μV respectively, at the same amplifier gain of 60 dB. It is therefore possible to now detect ECAP responses in the order of 2–5 μV, compared with about 20–25 μV with the Nucleus 3 system. Integrated NRT support is provided in the Freedom clinical software, Custom Sound™ and Custom Sound EP™, the speech processor fitting and evoked potential measurement applications, respectively (see “Custom Sound Suite”).
Initial investigations in a multicenter study exploring the enhanced NRT capabilities of Nucleus Freedom verified the better signal-noise ratio of the system and the ability to detect low-voltage ECAP responses46,47 It was also found that optimization of the recording parameters to reduce the effect of stimulus artifact on the response, typically amplifier gain and measurement delay, is usually not required with Nucleus Freedom. This substantially reduces NRT recording time intraoperatively, when it is used to verify that electrical stimulation produces auditory responses, and postoperatively, when using NRT to assist in speech processor fitting. In addition, a fully automatic ECAP measuring algorithm, AutoNRT™,48 is included in the Nucleus Freedom clinical software. AutoNRT is a quick procedure for obtaining the ECAP thresholds and can be used by less experienced clinicians intraoperatively and postoperatively.49,50
Dual-electrode stimulation is produced in Nucleus Freedom by electrically coupling 2 adjacent intracochlear electrodes as the active electrode. There is no additional processing overhead or system bandwidth requirement for dual-electrode stimulation. Thus, the array has the potential of 43 distinct electrodes for stimulation, 22 single and 21 dual electrodes. A recent study51 with 8 subjects investigated whether electrode impedances were lower for dual electrodes, because of the increased surface area, and whether subjects were able to hear pitch differences between stimulation on dual electrodes and the adjacent single electrodes. The average electrode impedance was 39% lower for dual-electrode stimulation compared with single-electrode stimulation, using monopolar stimulation with the 2 external electrodes as the return. Dual-electrode stimulation therefore has the potential to reduce power requirements for stimulation. Pitch ranking results showed that most subjects were able to rank the pitch of the dual electrode as being between the pitches of the 2 adjacent electrodes at several positions on the array. Thus the tonotopic pitch order for electrical stimulation was generally maintained. The potential uses of dual electrodes in combination with single electrodes, or as the sole mode of stimulation, are being investigated.
Contour Advance
One of the important innovations of the Nucleus 3 system was the Contour electrode array, a perimodiolar array with 22 half-banded electrodes. The design goals of the Contour were: to safely position 22 electrodes close to the modiolus, to provide consistent insertion depth across recipients, to ensure an absence of static force on cochlear structures, and to provide ease of surgical placement with minimal insertion force. These objectives have been achieved through the development of the Contour with an enhanced design, the Contour Advance, used in Nucleus Freedom.
Positioning electrodes closer to the modiolus has several potential advantages. Previous physiologic and modeling studies have suggested that a reduced distance between the electrodes and stimulated spiral ganglion cells would reduce the amount of current required for stimulation, longitudinal current spread in the scala, and spread of neural excitation.52–54 Initial research in Melbourne with a prototype perimodiolar array in 3 recipients showed lower current levels for hearing thresholds and comfortable listening levels with decreased distance of the electrode from the modiolus.55 This outcome was verified in a multicenter study with 21 adults implanted with the Contour electrode, which found significant positive correlations between radial distance from the modiolus and the current levels for hearing thresholds and comfortable listening levels for most subjects.56 The Nucleus 24 Contour multicenter study in North American clinics38 also found that threshold and comfortable listening levels were significantly lower for the Contour (n = 40) when compared with previous data using the straight array (n = 56). These studies confirmed the hypothesis that by placing electrodes closer to the modiolus, lower stimulating current levels are required for effective stimulation.
Surgical insertion and histologic studies in human temporal bones before the release of the Contour array demonstrated minimal insertion trauma.57–59 Continuing research identified 2 significant improvements for the Contour that were implemented in the Contour Advance in 2003/2004: the Softip™ modification of the tip of the array and the AOS insertion technique. The primary goal of these design changes was to minimize insertion force on the lateral wall of the cochlea and so minimize trauma to cochlear structures. Many researchers, including Shepherd et al60 and Kennedy,61 measured and described the mechanisms of trauma in the cochlea with early electrode designs. Kennedy, for instance, identified that one of the primary causes of trauma was the pressure point where the tip of the electrode first impacts on the lateral wall of the cochlea. Temporal bone studies have demonstrated tears in the spiral ligament and, in some instances, perforation of the basilar membrane and migration of the array into the scala vestibula.59–62
The Softip on the Contour Advance works in conjunction with the AOS insertion technique to create an insertion dynamic that essentially prevents contact of the tip of the electrode with the lateral wall of the cochlea, thus reducing the force on the lateral wall of the cochlea to near zero. In the AOS technique, the electrode is initially inserted for a distance of 8.5 mm, as indicated when a white marker on the array reaches the site of the cochleostomy. The array is then advanced off the stylet while the stylet is held stationary. This takes advantage of the properties of the array to curve itself around the modiolus. Figure 6 shows the perimodiolar position of the Contour Advance inserted using AOS. The upper image shows a cross-sectional histologic view of a human temporal bone, and the lower image shows a high-definition radiograph from a collaborative electrode insertion study at the Cooperative Research Centre for Cochlear Implant and Hearing Aid Innovation, Melbourne. The Softip also has the benefit of reducing the potential for tip foldover, which was seen in a very small number of cases in the Contour clinical population. In a recent temporal bone study with the Contour Advance, all specimens (n = 16) showed atraumatic insertion properties and good perimodiolar electrode positioning.63
Figure 6.
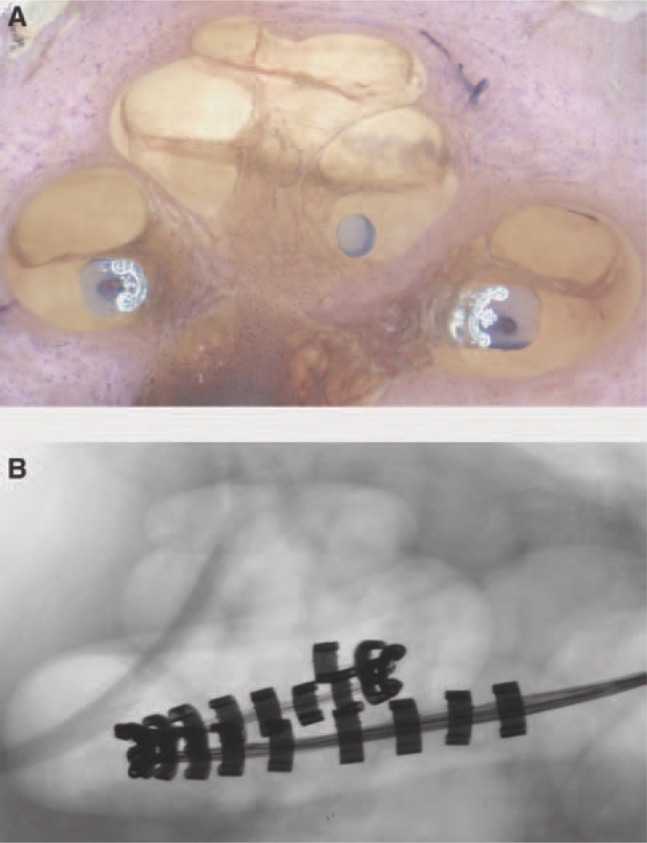
Human temporal bone showing (A) the perimodiolar position of the Contour Advance inserted using AOS; (B) a cross-sectional, high-resolution radiograph of the human temporal bone. (Images by permission of the Cooperative Research Centre for Cochlear Implant and Hearing Aid Innovation, Melbourne, Australia.)
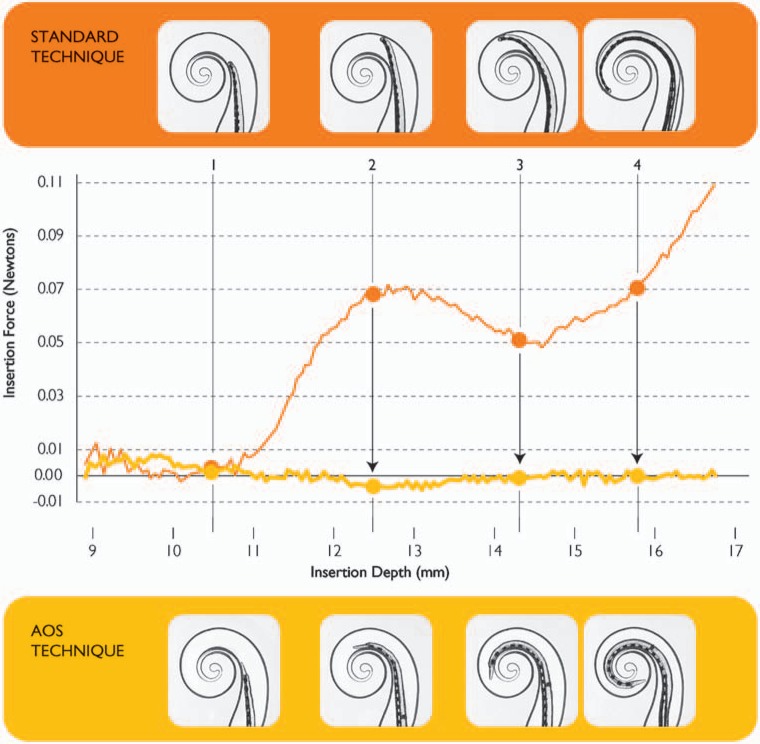
In a further study of electrode insertion,64 it was found that the Contour Advance provided a more reliable and less traumatic insertion than was achieved with the Contour electrode inserted without AOS (Figure 7). This was primarily because of a marked reduction in insertion force along the outer wall. Without AOS, when the array tip first contacts the lateral wall, insertion force increases because of the pressure of the tip on the lateral wall. With AOS, there is essentially no pressure on the lateral wall, with the measured force in the order of 0.002 N.
Figure 7.
Comparison of insertion forces when the Contour Advance is inserted using a standard and AOS technique (adapted from Roland64). Insertion forces are considerably lower using the AOS technique. In the standard technique, the array is fully inserted and the stylet is then withdrawn. In the AOS technique, the array is inserted for 8.5 mm and then advanced off the stylet while the stylet is held stationary. (Image by permission of Cochlear Limited)
Speech Processor and Speech Coding
The Nucleus Freedom speech processor consists of the transmitting coil and the BTE processing unit and controller (Figure 1). The modular design of the speech processor allows recipients to use a BTE or Bodyworn controller. The main BTE processing unit contains all the relevant speech processing and MAP functions and is therefore unaffected by changing the controller. The controller contains the batteries, user-adjustable controls, and an LCD screen. The BTE controller uses 3 Zinc Air disposable batteries or a Lithium-ion rechargeable pack. A 2-battery BTE controller is also available. The Bodyworn controller uses 2 AAA Alkaline or rechargeable batteries.
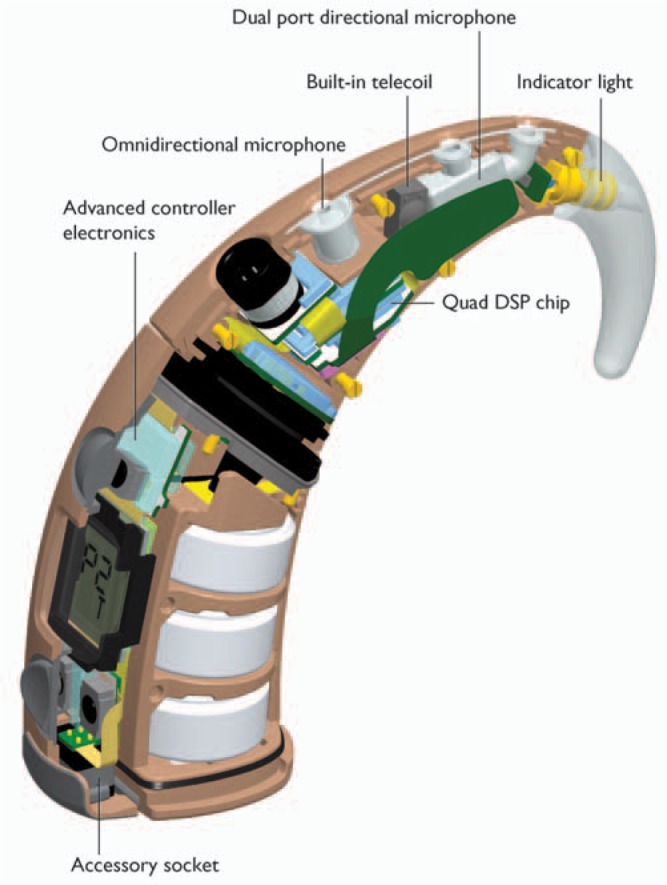
The BTE processing unit contains a custom digital integrated circuit containing 4 parallel digital signal processing (DSP) units, a microcontroller, and memory (Figure 8). The ultralow power custom DSP architecture is capable of performing more than 180 million operations per second, allowing for future input processing and speech coding upgrades. The parallel processing architecture uses much less power than would be required if a single processing unit was used. Up to 4 MAPs can be stored in the processor. Each MAP is independent of the others, thus can differ in T-SPL and C-SPL levels, SmartSound options, and other MAP functions. The unit also contains 2 microphones, an omnidirectional and a directional. In normal operation the directional microphone is used. The new SmartSound beamformer option, Beam, uses both microphones.
The BTE precessing unit includes an integrated telecoil for use with telephone and induction loop assistive listening devices (Figure 8). The BTE controller unit also contains an integrated accessory connector. An extensive set of accessories is available for Nucleus Freedom to connect with common audio transmitters such as televisions, stereo systems, FM receivers, personal audio and audio-visual systems, and gaming consoles.
The Freedom processor can be programmed with any of the speech coding strategies: SPEAK, ACE, and CIS. The stimulation rate for SPEAK is 250 Hz per channel. Stimulation rates for ACE and CIS are between 250 Hz and 3.5 KHz per channel. The North American multicenter study of Nucleus Freedom is investigating subjective preference for stimulation rate. Subjects were assigned to a low-rate ACE group (A) or high-rate ACE (RE) group (B) at initial fitting. The group A rates were 500, 900, and 1200 Hz, and the group B rates were 1.8, 2.4, and 3.5 kHz. Rate group assignment was changed using an ABAB experimental design, giving subjects experience with the rates in both groups over time. Subjects selected their preferred group A and group B rates, and then made a final single rate selection between these 2 rates. Preliminary results of the final preference from 60 subjects are shown in Table 1. There was a tendency for subjects to select the lowest pulse rate in each of the 2 groups of rates: 500 Hz for group A and 1.8 kHz for group B. There was, however, considerable spread across rates except for the 2 highest rates, 2.4 and 3.5 kHz, which were selected by only 5 subjects. Because these are preliminary findings, no conclusion can be made until study completion. The findings are, however, consistent with earlier studies with the previous Nucleus systems, which showed individual preference for stimulation rate,28,30,38 as described previously in this article.
Table 1.
Preliminary Results from the North American Freedom Trial (n = 60)
| Pulse Rate per Electrode | Percentage of Subjects |
|---|---|
| Group A | |
| 500 | 33.33 |
| 900 | 18.33 |
| 1200 | 15 |
| Group B | |
| 1800 | 25 |
| 2400 | 6.67 |
| 3500 | 1.67 |
Preliminary results from the North American multicenter trial of Nucleus Freedom showing the preferred pulse rate at 4 months (n = 60). Subjects were assigned to group A or B, selected their preference within that group after experience, and then selected their preference with the other group after experience, using an ABAB experimental design. Subjects then selected a single rate between their preferred group A and group B rate.
In a recent study by Galvin and Fu,65 it was found that modulation detection thresholds were better for a low carrier pulse rate (250 Hz) than for a high carrier rate (2000 Hz) over a range of current levels within the dynamic range of hearing. In speech coding strategies, the acoustic amplitude envelope is coded by modulation of the stimulating current. The psychophysical results suggest that a low carrier rate might provide better access to the modulated amplitude envelope cues of speech. Thus, the preference for the lower stimulation rates found in the Nucleus Freedom and in previous studies would be consistent with this finding.
The preliminary speech perception scores at 3 months from the North American Nucleus Freedom study are shown in Figure 9, and for comparison, scores from the North American Nucleus 24 Contour study.38 Freedom subjects used their preferred stimulation rate and SmartSound input processing option. At 3 months, the average CNC word score for the Freedom subjects in quiet, 51% (n = 63), was significantly higher than the average CNC word score from the Nucleus 24 Contour study, 38% (n = 56). In addition, the 3-month Freedom score was marginally higher than the 6-month Nucleus 24 Contour study score, 47%.39 These findings indicate that when using Nucleus Freedom with preferred processing strategy and SmartSound option, recipients obtain an equivalent level of performance at 3 months as was previously achieved with the Nucleus 3 system at 6 months. For CUNY sentences in noise at 3 months, scores were also higher in the Nucleus Freedom study than in the Nucleus 24 Contour study, 64% (n = 54) and 59% (n =56), respectively, but this difference was not significant. Although these Freedom findings are encouraging, no final conclusion can be made until study completion.
Figure 9.
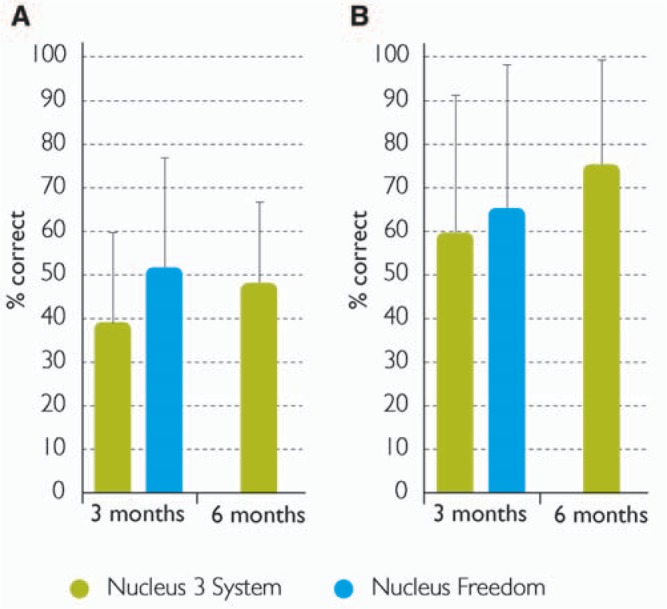
Percentage correct scores for (A) CNC words in quiet and (B) CUNY sentences in noise from the North American multicenter trials of the Nucleus 3 system and the Nucleus Freedom. Error bars show the standard deviation. Image by permission of Cochlear Limited)
The Freedom processor in BTE or body-worn configuration has been designed to protect the internal components from the external environment. Both processor configurations have been certified to level IP44 of the international standard IEC 60529, which indicates that they are protected against splashing water.66 Microphones are particularly vulnerable to moisture, which could invade through the openings required to receive sound. A protective membrane that repels moisture but passes sound waves is used to protect the microphones. All push-button controls are molded directly onto the plastic housing, providing a firm seal against liquid ingress and durability to withstand thousands of button presses. Connectors for cables and modules include exposed metal that is prone to corrosion if subjected to sweat and moisture over a long period. Rubber compression seals and O-rings are used to seal between connecting components to protect the exposed metal conductors. Thus, the Freedom processor is resistant to high humidity, excessive perspiration, and rain, and will therefore be more reliable in these environments.
Cochlear will soon release the Freedom processor for recipients implanted with the CI24M and CI24R receiver-stimulators. These recipients will have access to most of the features of Freedom, with the exception being the high stimulation rates that are available only with the CI24RE receiver-stimulator. Support for recipients implanted with the CI22M receiver-stimulator is also planned.
Instantaneous Input Dynamic Range
Normally hearing listeners have a usable hearing dynamic range of up to 120 dB. However, the electrical hearing dynamic range of implant recipients is much smaller, in the order of 10 dB to 30 dB.67–70 To ensure the most relevant range of input intensities is presented within the recipient's smaller dynamic range, so that soft sounds are audible and high intensity sounds are not uncomfortably loud, all Nucleus speech processors use an AGC and a microphone sensitivity control to adjust the input signal gain. The AGC is a fast-acting infinite compression circuit, and the microphone sensitivity control sets the AGC knee-point. Microphone sensitivity can be adjusted by the recipient for different listening environments. The maximum output level of the AGC translates into stimulation at the comfortable listening level (C-SPL). The IIDR is the selected intensity range that is coded between C-SPL and hearing threshold (T-SPL) at any instant in time. Nucleus Freedom will support IIDRs up to 75 dB, although an IIDR above approximately 45 dB is not likely to be beneficial because it will increase the audibility of background noise within the small dynamic range of hearing.
Recent studies by Zeng et al71 and Dawson et al72 have shown that IIDRs in the order of 45 dB are likely to be beneficial to CI recipients. Zeng et al71 showed that the acoustic dynamic range was 46 dB for vowels and 47 dB for consonants. When vowel and consonant perception in CI recipients was tested in quiet, an IIDR of approximately 50 dB gave the optimum vowel and consonant scores. For consonants in noise, the decrement in scores was greater at the higher IIDRs, more than 50 dB. For vowels in noise, the decrement in performance was fairly uniform over the range of IIDRs tested.
A recent study compared speech perception and subjective preference for IIDRs of 31, 46, and 56 dB in a group of 9 adult subjects using the Nucleus implant.72 For CNC words in quiet at soft (45 dB SPL) and moderate (55 dB SPL) levels, scores were significantly higher for IIDRs of 46 dB and 56 dB compared with 30 dB. This was consistent with the results from the previous study.71 For sentences in noise, there was no significant difference in scores for the 3 IIDRs. However, subjective preferences showed that some subjects found the 56-dB IIDR uncomfortable in everyday listening environments. Thus, an IIDR of 46 dB was preferred.
SmartSound Input Processing
Nucleus Freedom provides 4 selectable SmartSound technologies at the input stage of the speech processor that can be used to optimize performance in different listening environments: Beam, ASC, Whisper, and ADRO. Beam, a new input signal processing scheme, is an adaptive beamformer that reduces background noise from the surrounding environment. ASC, Whisper, and ADRO were released in earlier generations of the Nucleus system, as previously described in this article. These have been implemented digitally in the BTE processing unit.
Beam is a 2-stage adaptive beamformer designed to improve the signal-noise ratio of speech coming from in front of the CI recipient in a noisy environment and was developed at the Laboratory for Experimental Otorhinolaryngology, Katholieke Universiteit Leuven, Belgium, in collaboration with Cochlear. Figure 10 shows the directional sensitivity plots comparing Beam and the directional microphone for noise from different directions. Noise reduction from the direction of the noise source is greater with Beam than with the directional microphone.
Figure 10.
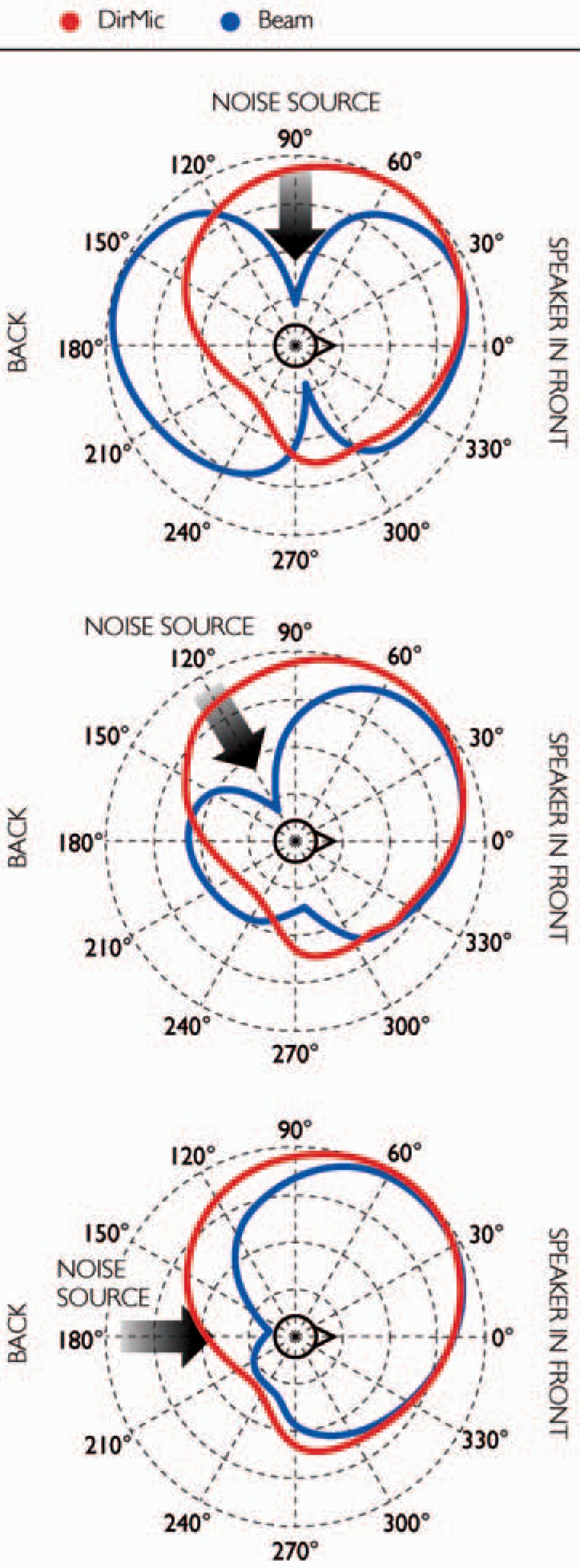
Directional sensitivity plots comparing Beam and the directional microphone, showing the additional attenuation of noise using Beam. The source signal was at 0° and noise at 90°, 120°, or 180°. The concentric circles are in units of 5 dB. (Image by permission of Cochlear Limited)
Beam consists of a fixed spatial preprocessor and an adaptive noise cancelation stage.73–75 The first stage is effectively a fixed end-fire beamformer. This is optimized to have maximum sensitivity to the front and maximum suppression of signals arriving from 90° to 270°. Outputs of the first stage are a speech reference and a noise reference. The second stage is an adaptive noise canceler that attenuates the residual noise. It operates on the noise reference and is allowed to adapt only during noise-only time periods, that is, when no speech is detected in the speech reference signal. A voice activity detector73 is used to detect speech and thus identify the noise periods with absent speech that controls the adaptation process.
An evaluative study of Beam was recently conducted with 5 recipients.76 Speech perception was tested with a single noise source at 90°, on the same side as the speech processor, and with 3 uncorrelated noise sources of the same intensity at 90°, 180°, and 270°. The speech signal was delivered at 0°. The speech and noise signal sources were 1 meter from the subject. Significant improvements were found using Beam compared with the directional microphone in the Freedom BTE processor for speech reception thresholds for sentences in noise, with improvements of 5 dB to 16 dB, and for percent correct phoneme scores for monosyllabic words in noise, with improvements of 10% to 41%. In addition, subjective spatial hearing questionnaires showed a preference for Beam in noisy environments.
The North American multicenter clinical trial of Nucleus Freedom is assessing recipient preference for SmartSound options when listening in quiet and noise. Sixty-two percent of subjects preferred to use an input processing option in quiet and noise (Figure 11). Shown are preliminary results of the subjects’ preferred SmartSound input processing option at 6 months, selected from among no input processing, ADRO, Whisper, and ASC (n = 47). Beam was not assessed as part of this study. Thirty-two percent preferred ADRO in quiet, and almost 50% preferred ASC in noisy environments. Smaller numbers of subjects preferred Whisper in quiet and noise and ADRO in noise. These findings also indicate that recipients have individual preferences for SmartSound options, and these preferences can vary according to the listening environment. Note that no conclusion about SmartSound preferences can be drawn until study completion.
Figure 11.
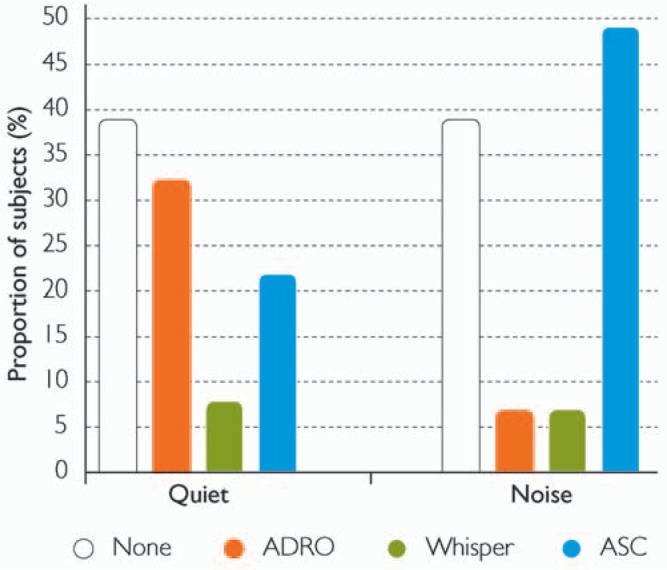
Preliminary results from the North American multicenter trial of Nucleus Freedom (n = 47) showing the preferred SmartSound input processing options for listening in quiet and in noise. Subjects selected from among no input processing, ADRO, Whisper, and ASC, at 6 months. The percentage of subjects preferring the SmartSound option in quiet and in noisy environments is shown. (Image by permission of Cochlear Limited)
Custom Sound Suite™
The Custom Sound Suite™ comprises Custom Sound and Custom Sound EP, the 2 Nucleus Freedom software products for programming the processor and for advanced NRT and electrophysiologic functions, respectively. They share a common database; and data collected using one program are available in the other. For example, AutoNRT measures from Custom Sound EP are available for fitting NRT-based MAPs in Custom Sound. Recipient data can also be imported and exported, making it easy to transfer information between computer systems, such as from a notebook computer used in the operating theater to the main database on a system server.
Custom Sound provides simplified programming for the Freedom processor, with an intuitive work flow from initial measurement of hearing thresholds and comfortable listening levels through to final MAP fitting. Simplified navigation is provided for routine fitting, with access to all adjustable parameters when needed for advanced programming. The software can be customized by the clinician for individual preference and it also has a training mode.
Custom Sound has automated a number of programming functions:
Electrode impedance and voltage compliance measurement, to ensure the electrodes can deliver the required current.
AutoNRT for automatically measuring ECAP. Thresholds from these ECAP measures or other electrophysiologic tests are integrated in the fitting software, providing a reference for MAP fitting.
Streamlined programming methods for generating initial and progressive MAPs and full support for interpolation of stimulation levels.
Optimizing speech processor power usage is provided by the AutoPower function and battery life estimation given.
Studies on streamlined fitting methods have concluded that they can be successfully used in clinical practice without compromising recipient performance.77 They can also significantly reduce clinical fitting time.78 Custom Sound is designed on streamlined fitting principles, providing an efficient and effective method to fit cochlear implant recipients, especially during initial activation. As the recipient gains more listening experience, the speech processor can be further optimized using the advanced features of Custom Sound.
Custom Sound EP provides advanced NRT functionality, and support for measuring the electrically evoked auditory brainstem response (EABR), stapedius reflex threshold (ESRT), and the cortical evoked response (CEP). The NRT functionality includes AutoNRT and stimulation templates to measure ECAP amplitude growth functions, recovery functions, and spread of excitation. The EABR, ESRT, and CEP functions require the appropriate external recording equipment. A trigger pulse is provided and is accessed via the Programming Pod. Custom Sound EP also provides user-adjustable electric stimulation parameters to measure the evoked potentials and the facility to store the measured thresholds. These thresholds can then be used in speech processor fitting via Custom Sound.
Connection between the Freedom speech processor and programming computer is by the Programming Pod. This small interface connects via USB and is self-powered by the computer. It has indicator lights to show power and data transmission. Custom Sound is compatible with previous body-worn and BTE speech processors, from Spectra to Freedom, although additional hardware is required for communication with other speech processor models. At present, Custom Sound EP supports only the CI24RE receiver-stimulator, with support for CI24M and CI24R receiver-stimulators in the near future. This is because of the different NRT capabilities in these systems.
Advancing Recipient Outcomes
Cochlear implant systems have been highly successful in providing benefit to severe-profoundly deaf adults and children, resulting in expanding indications for implantation. Implant reliability and safety are 2 of the most important factors in meeting the expanding candidature and recipient expectations. Continuing research is also exploring new methods to improve outcomes for recipients, and Cochlear actively explores new developments in collaboration with research and clinical centers worldwide.
Improved Implant Reliability
There have been significant changes in implant technology over the 4 generations of Nucleus cochlear implants. Reliability and safety are critical factors in implant design and manufacture, as very young recipients might be reliant on the technology for 70 or more years. Cochlear has published reliability reports on the implanted device for more than 15 years, currently every 6 months. These reports are prepared in accordance with ISO 5841-2,79 the most relevant standard available. More recently, the European Consensus Statement on Cochlear Implant Failures and Explantations, 200580 was released. Cochlear also complies with this new statement.
The cumulative failure percentage (CFP) is the appropriate method of reporting reliability, as it demonstrates the percentage of devices that are no longer functioning over a given period of time. As the index is cumulative, a CFP that does not increase over time indicates no new failures. Cochlear includes all failures in the calculation, including those caused by external impact, for all implant models, adults and children separately. The cumulative survival percentage (CSP) is the inverse of the failure percentage and shows the cumulative number of functioning implants over time.
The CI24RE receiver-stimulator has been implanted in more than 10,000 recipients as of July 2006. Reliability data for the CI24RE have been included in the latest report, dated June 30, 2006 (Figure 12). These data cover the entire life of each device and all registered recipients worldwide. With each new generation of receiver-stimulators, there has been a lower CFP. For example, the CFP at 6 years for the second- and third-generation implants, Nucleus CI24M and CI24R (shown in Table 2), indicates a lower proportion of failures in children for the CI24R. The 6-year cumulative failure rate per 1000 children is 31 devices for the CI24M and only 13 devices for the CI24R. The results for the CI24RE at 1.5 years show no failures for adults (n = 5232) and 6 failures for children (n = 4767). The CFP for Nucleus Freedom at 18 months is only 0.2% for children, which is much lower than for the CI24R receiver-stimulator at 12 and 24 months, 0.4% and 0.7% respectively. These encouraging findings suggest that the internal design modifications in Nucleus Freedom have achieved their goal of improved reliability.
Figure 12.
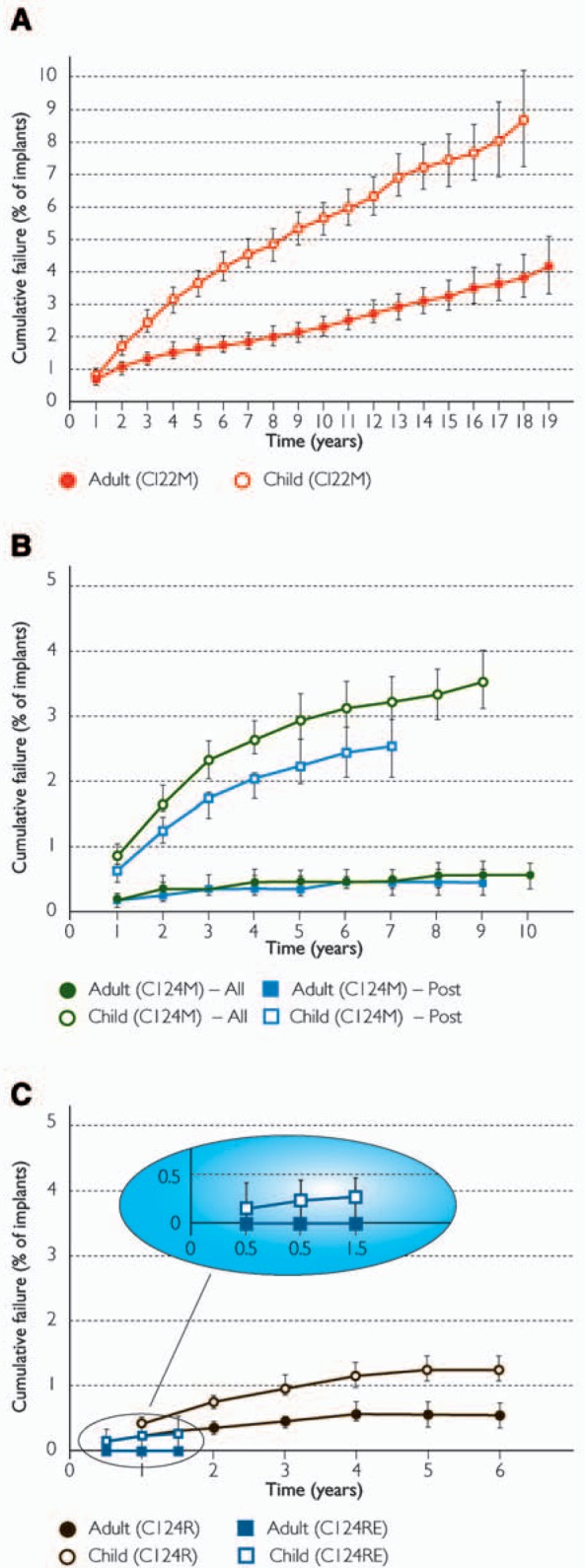
Cumulative failure percentages for the 4 generations of Nucleus receiver-stimulators, all recipients worldwide as of June 30, 2006, adults and children separately: (A) CI22M, (B) CI24M (all models and after modification), and (C) CI24R and CI24RE. Error bars show the 95% confidence intervals. Note the y-axis for (A) is twice that of (B) and (C).(Image by permission of Cochlear Limited)
Table 2.
Cochlear Implant Reliability at Six Years
| CI24M (All) | CI24R | |
|---|---|---|
| Cumulative failure (%) | ||
| CFP adults | 0.4 | 0.5 |
| CFP children | 3.1 | 1.3 |
| Cumulative survival (%) | ||
| CSP adults | 99.6 | 99.5 |
| CSP children | 96.9 | 98.7 |
Cumulative failure percentage (CFP) and cumulative survival percentage (CSP) at 6 years for the second-generation (CI24M all models) and third-generation (CI24R) systems, adults and children separately, as of June 30, 2006.
A recent study presented the surgeon-reported incidence of device failure in 26 European clinics.81 Across all device models, the failure rate for the Nucleus device was much lower than that of the other manufacturers: 1.97% (n = 8581) for the Nucleus device, 9.01% (n = 1987) for the MedEl device, and 6.98% (n = 1761) for the Advanced Bionics device.
Electrode Array Options
Cochlear also supports a range of electrode arrays to meet expanding indications for cochlear implantation. The Contour array was released in the Nucleus 3 System in 2000. The CI24R receiver-stimulator connected to the straight array was also available as an option for those cases where the surgeon felt that a straight array was preferable to the Contour. Similar support for the straight array is provided with the CI24RE receiver-stimulator.
The auditory brainstem implant (ABI24M) was released in 2000, consisting of the CI24M receiver-stimulator coupled to an electrode pad for placement on the cochlear nucleus. A double electrode array was released in 2002, with 2 arrays of 11 electrodes each.82 This array might be suitable in cases where normal insertion of the standard array is not possible but 2 shallow insertions, one in the basal turn of the cochlea and one in the second turn, is feasible.
Bilateral Cochlear Implants
Binaural hearing provides better speech understanding in noise and sound localization for normally hearing listeners and those wearing bilateral hearing aids. Cochlear recently completed a series of multicenter research studies in the United States, United Kingdom, and German-speaking clinics in Germany and Switzerland to measure the benefits of bilateral Nucleus cochlear implants in adults. The US study investigated the benefits of simultaneous bilateral implants,83–85 the UK study measured the incremental benefit of sequential bilateral implants,86 and the studies in German-speaking clinics investigated simultaneous and sequential bilateral implants.87
Probably one of the most important benefits for speech perception comes from the headshadow effect, when speech and noise are from different directions, producing a difference in the signal-noise ratio at each ear. The collaborative studies showed that bilaterally implanted adults were typically able to take advantage of the headshadow effect and use the ear with the better signal-noise ratio. When speech and noise are from the same location, there is an improvement from binaural redundancy, where binaural performance is better than performance using the better unilateral ear. Across studies, a number of bilaterally implanted subjects were able to obtain a binaural redundancy advantage. However, performance was variable across subjects, suggesting that some bilateral implant recipients were not able to use the additional information when the same signal was presented to both ears. In all studies, there were clear benefits of bilateral implants over a unilateral implant for sound localization in the horizontal plane.88
Bilateral cochlear implantation also ensures that the better ear is always implanted, ie, the ear with the better postoperative speech perception outcome. Preoperatively, it can be difficult to accurately predict which ear will give the better postoperative monaural performance. Bilateral implantation ensures that the recipient will always be able to take advantage of the better performing ear. The average bilateral word score (n = 33) for CNC words in quiet at 3 months was 51% in the US study, primarily the contribution of the better ear. This was significantly higher than the average score of 38% for monaurally implanted adults (n = 56) in the clinical trial of the Nucleus 24 Contour.38 Subjects were implanted with the same Nucleus system in both studies.
Cochlear is collaborating in research studies investigating the benefits of bilateral implants in children and innovative speech coding schemes that might improve bilateral performance. Multicenter studies are in progress in Europe and North America to measure the benefits of pediatric bilateral implants. There are several compelling reasons for bilateral implantation in children. Bilateral hearing aid fitting in children is typically the standard clinical practice of the binaural hearing benefits.89 It is therefore possible that bilateral cochlear implants will provide a similar degree of benefit. In addition, binaural stimulation is probably important for the development of the biological mechanisms that are used for binaural hearing. Without the development of these mechanisms in children, it is possible that bilateral implantation at a later age might not provide the same benefits in speech perception and sound localization that have been found with adults. Recent findings in children who received sequential bilateral implants suggest that the time between surgeries and the amount of bilateral experience might be important factors in the development of sound localization abilities.90 In adults, binaural hearing mechanisms would have developed normally before onset of deafness; thus maturation and experience will likely have less impact on outcomes.
There are also research studies investigating fundamental binaural hearing mechanisms with electric stimulation and advanced binaural speech coding schemes.91,92 For example, a collaborative study with Medizinische Hoschschule Hannover, Germany, and the Cooperative Research Centre for Cochlear Implant and Hearing Aid Innovation, Melbourne, is investigating the benefit of a peak derived timing bilateral strategy on binaural unmasking for speech perception. This strategy is designed to better preserve the fine-timing characteristics of the signal.
Electroacoustic Stimulation
Some candidates present with a severe high-frequency hearing loss but only a mild to moderate loss in the low frequencies. These candidates also have some open-set speech understanding when using hearing aids. It is therefore important to preserve residual hearing in the implanted ear and to complement hearing via electrical stimulation with acoustic stimulation in the contralateral ear (bimodal stimulation), the ipsilateral ear when residual hearing is preserved after implantation (hybrid stimulation), or in both ears (combined stimulation).
Bimodal stimulation can provide significant benefit to adult and pediatric implant recipients when the contralateral ear has sufficient residual hearing for successful hearing aid fitting.93–97 Benefits include improved speech perception in noise and sound localization. However, these benefits have not been found for all subjects,97 and further research is needed to improve the integration of electric and acoustic signals delivered to opposite ears. For example, Kong et al96 suggest that additional low-frequency fine-timing information delivered to the nonimplanted ear via a hearing aid might improve music appreciation and speech recognition in noise. Cochlear is currently investigating new approaches to further improve bimodal benefits in collaborative studies, such as different prescriptive methods for fitting the contralateral hearing aid, and to also identify factors that influence outcomes.
Cochlear has collaborated with the University of Iowa in the development of a short, 10-mm Hybrid™ electrode with 6 contacts designed to preserve the residual hearing of candidates with considerable low-frequency hearing.98 High-frequency information is presented electrically via the Hybrid implant and low-frequency information via an ipsilateral hearing aid. Preliminary results from a Cochlear-supported multicenter clinical trial in the United States and Europe indicate that the residual low-frequency hearing is preserved in the majority of cases, with a median hearing loss of 11 dB for the frequencies 125 Hz to 1 kHz, 12 months after surgery (n = 29). Hybrid stimulation (electric plus ipsilateral acoustic) also provides significant benefit for speech perception in quiet and in noise when compared with preoperative performance in the same ear using a hearing aid. Performance in the combined condition, using bilateral hearing aids plus electrical stimulation, has been significantly better than in the preoperative bilateral hearing aid condition.99 These results show that electrical stimulation using a short array in the basal portion of the cochlea can provide significant benefit for recipients while preserving low-frequency residual hearing in the same ear.
Key factors for the success of hearing preservation are the surgical approach to the entry point to the cochlea and minimal insertion trauma. For the 10-mm Hybrid electrode, the location of the cochleostomy, the technique for creating the opening, and the orientation of the electrode array during insertion have been shown to be important for hearing preservation.98,99
A separate multicenter European study is currently investigating whether hearing can be preserved in candidates with residual hearing when the Contour Advance is inserted using AOS and “soft-surgery” techniques to create the cochleostomy.100,101 As described previously herein, the Contour Advance was designed to minimize insertion forces and intracochlear trauma. Preliminary results in 12 recipients showed a median hearing loss of 28 dB for the frequencies 125 Hz to 500 Hz. Hearing was preserved in 10 subjects, and 6 of these subjects retained sufficient residual hearing for the successful fitting of a hearing aid in the implanted ear. The other 2 subjects lost all residual hearing immediately after surgery, but in both cases the soft-surgery technique was not followed as planned. These preliminary findings suggest that it was possible to preserve some residual hearing with careful insertion of the Contour Advance. The results also showed that changes in low-frequency thresholds have been greater in subjects implanted with the Contour Advance than with the 10-mm Hybrid electrode. This could be related to the different depths of insertion of the 2 arrays, although this is only one of the several differences between these studies.
Cochlear has also been developing a longer Hybrid electrode array in conjunction with surgeons at Medizinische Hochschule Hannover, Germany, and the Cooperative Research Centre for Cochlear Implant and Hearing Aid Innovation, Melbourne. The 16-mm Hybrid array has 22 half-banded contacts on a thin and flexible carrier and is designed for insertion through the round window using surgical techniques that minimize insertion trauma.102 A small number of adults have been implanted, and residual low-frequency hearing has been successfully preserved after surgery.103
The 10-mm and 16-mm Hybrid electrode arrays are designed to be positioned with minimal trauma in the basal portion of the cochlea. Thus, these arrays meet expanded indications for cochlear implantation in those candidates with residual hearing. Further research is also needed to understand how the consequences of trauma arising from the surgical approach and electrode insertion can be minimized.
Advanced Speech Coding
Cochlear has an active speech coding research program, with several studies in its collaborative research program. Research is driven by the desire to improve recipient performance and deliver better performance more efficiently.
Psychoacoustic Masking Models
All speech coding techniques currently available attempt to translate the major components of the acoustic signal into a directly equivalent electrical stimulus: frequency, intensity, and the temporally changing patterns of these 2 components. For normally hearing listeners, many of the low-level spectral components are inaudible because they are masked by other, louder components in the signal.
At the Medizinische Hochschule Hannover, Germany, a new method for selecting spectral peaks has been developed in conjunction with Cochlear. This psychoacoustic strategy selects the largest spectral peaks to be delivered using a psychoacoustic masking model.104 This model is designed to extract the normally audible components from the input signal, leaving out components that are masked by the louder components. This approach is similar to that used in audio compression algorithms such as the MP3 scheme, which is able to reduce the data to approximately 10% of the original size without noticeable loss of sound quality.
Initial results of a pilot study with 8 subjects showed equivalent levels of performance comparing a standard 8-channel ACE strategy with a 4-channel prototype psychoacoustic strategy.105 Although there was a reduction in the number of stimulating channels, performance was not compromised, which verified the application of the model. Further studies are continuing to confirm these initial findings and to develop better masking models for electrical stimulation.
Another advantage of reduced data throughput when using psychoacoustic masking model strategies is the potential to reduce power requirements, which could increase speech processor battery life. In addition, for recipients of the first-generation CI22M system there is the potential to increase stimulation rate when using a psychoacoustic strategy, as fewer electrodes will need to be stimulated than for the SPEAK strategy. These possibilities will be explored in future research studies to ensure that recipients of all generations of Nucleus implants receive the benefits of advances in speech coding technology.
Number of Channels
Nucleus Freedom has the capability to stimulate up to 43 electrodes: 22 single electrodes and 21 dual electrodes. As described previously, dual-electrode stimulation is produced by electrically coupling 2 adjacent intracochlear electrodes. In addition, all Nucleus implant systems have been able to produce “virtual” channels. Virtual channels are the intermediate pitch percepts produced by stimulation on 2 closely spaced electrodes.106 Stimulation can be sequential,107,108 as used in Nucleus processors, or simultaneous.109,110 Intermediate pitch percepts can be produced by varying the ratio of current delivered to the 2 electrodes. The underlying mechanism is probably related to the extent of current field overlap and neural integration.
Nucleus speech processing strategies incorporate virtual channels by default because of the overlap in the widths of the analysis bandpass filters. For example, when 2 adjacent electrodes are being stimulated, the relative current levels delivered to each electrode will vary depending on the energy in each filter band, with changes in spectral energy distribution causing shifts in perceived pitch. However, several studies have shown that increasing the number of effective channels above approximately 8 to 10 electrodes does not result in marked changes in speech understanding in quiet.111–114 Thus, it is not clear whether the explicit use of virtual channels in speech coding will lead to marked improvements in speech understanding. On the other hand, it has been hypothesized that to obtain better performance in noisy environments and for music appreciation, the number of effective channels might need to be increased substantially in order to convey more spectral information.115 Cochlear is currently investigating methods to increase the number of effective channels by increasing the number of electrodes on the array and by current focusing using phased-array stimulation.116
Streamlined and Self-Programming
Recent studies have shown that streamlined fitting methods, where a limited amount of information about threshold and comfortable levels is used to fit a speech processor, can be successfully used without compromising recipient outcomes. Plant et al77 showed that 2 streamlined techniques could be successfully used in the clinical setting. The first technique was interpolation across 3 measured threshold levels and 3 measured comfortable listening levels, while the other was interpolation across 5 measured threshold or comfortable levels and the interpolated profile was used for fitting the alternative profile. A multicenter North American study78 also found that streamlined fitting methods provided outcomes similar to traditional methods, and that there was considerable time saving when using streamlined methods. Fitting methods based on objective measures, such as ECAP, ESRT, and EABR, combined with limited behavioral measures have also been successfully used.22,25,117,118 The Custom Sound Suite supports streamlined fitting using behavioral and electrophysiologic measures.
More recently, research studies have investigated the application of genetic algorithms in recipient-controlled MAP optimization.119 In this adaptive procedure, the recipient selects from among several MAP choices that vary along a number of parametric dimensions such as pulse rate and number of maxima. The procedure converges to the recipient-preferred MAP. Preliminary results suggest that a large number of dimensions can be efficiently optimized by the recipient using user-controlled software.
In addition, Cochlear is exploring the application of Internet-based service systems for recipient support, where the recipient does not need to attend the clinic, reducing the time and trouble necessary for ongoing maintenance. It might also be possible to establish satellite services through other clinical service providers for noncomplex cases using similar technology, freeing up clinician time.
Conclusion
The cochlear implant has come a very long way since the early single-channel implants. Multichannel implants typically provide significant benefits to severe-profoundly deaf adults and children. Cochlear's fourth-generation system, Nucleus Freedom, builds on 25 years of experience and, since the release in 2005, has demonstrated better levels of speech perception than the previous system and improved implant reliability. The Nucleus Freedom system is designed to give access to further advances in signal processing, as well as being backward compatible to earlier generations of the Nucleus implant. Cochlear continues to work with the implant research community in a wide range of studies to further improve recipient outcomes.
References
- 1.Goldman Sachs JBWere Pty Ltd Cochlear Report; March 10, 2006 [Google Scholar]
- 2.Clark GM, Tong YC, Patrick JF. eds. Cochlear Prostheses. Edinburgh: Churchill Livingstone; 1990 [Google Scholar]
- 3.Clark GM. Research advances for cochlear implants. Auris Nasus Larynx. 1998;25: 73–87 [DOI] [PubMed] [Google Scholar]
- 4.Clark G. Cochlear Implants: fundamentals and applications. New York: Springer; 2003 [Google Scholar]
- 5.Clark GM. The multiple-channel cochlear implant: the interface between sound and the central nervous system for hearing, speech, and language in deaf people—a personal perspective. Philosophical Transactions of Royal Society B: Biological Sciences. 2006;361: 791–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong YC, Black RC, Clark GM, et al. A preliminary report on a multiple-channel cochlear implant operation. J Laryngol Otol. 1979;93: 679–695 [DOI] [PubMed] [Google Scholar]
- 7.Tong YC, Millar JB, Clark GM, Martin LFA, Busby PA, Patrick JF. Psychophysical and speech perception studies on two multiple channel cochlear implant patients. J Laryngol Otol. 1980;94: 1241–1256 [DOI] [PubMed] [Google Scholar]
- 8.Dowell RC, Martin LFA, Clark GM, Brown AM. Results of a preliminary clinical trial on a multiple channel cochlear prosthesis. Ann Otol Rhinol Laryngol. 1985;94: 244–250 [PubMed] [Google Scholar]
- 9.Tong YC, Dowell RC, Blamey PJ, Clark GM. Two-component hearing sensations produced by two-electrode stimulation in the cochlea of a deaf person. Science. 1983;219: 993–994 [DOI] [PubMed] [Google Scholar]
- 10.Skinner MW, Holden LK, Holden TA, et al. Performance of postlinguistically deaf adults with the wearable speech processor (WSP III) and mini speech processor (MSP) of the Nucleus multi-electrode cochlear implant. Ear Hear. 1991;12: 3–22 [DOI] [PubMed] [Google Scholar]
- 11.McDermott HJ, McKay CM, Vandali AE. A new portable sound processor for the University of Melbourne/Nucleus Limited multielectrode cochlear implant. J Acoust Soc Am. 1992;91: 3367–3371 [DOI] [PubMed] [Google Scholar]
- 12.McKay C, McDermott H, Vandali A, Clark G. Preliminary results with a six spectral maxima sound processor for the University of Melbourne/Nucleus multiple-electrode cochlear implant. J Otolaryngol Soc Australia. 1991;6: 354–359 [Google Scholar]
- 13.Skinner MW, Clark GM, Whitford LA, et al. Evaluation of a new spectral peak coding strategy for the Nucleus 22 channel cochlear implant system. Am J Otolaryngol. 1994;15(Suppl 2): 15–27 [PubMed] [Google Scholar]
- 14.Magner ME. A speech intelligibility test for deaf children. Northampton, Mass: Clark School for the Deaf; 1972 [Google Scholar]
- 15.Loizou PC. Mimicking the human ear. IEEE Signal Processing Magazine. September 1998: 101–130 [Google Scholar]
- 16.Zwolan TA, Kileny P, Ashbaugh C, Telian SA. Patient performance with the Cochlear Corporation ‘20+2’ implant: bipolar versus monopolar activation. Am J Otol. 1996;17: 717–723 [PubMed] [Google Scholar]
- 17.Tykocinski M, Shepherd RK, Clark GM. Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates. Hear Res. 1995;88: 124–142 [DOI] [PubMed] [Google Scholar]
- 18.Tykocinski M, Shepherd RK, Clark GM. Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates II. Comparison of fixed amplitude with amplitude modulated stimuli. Hear Res. 1997;112: 147–157 [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105: 1–29 [DOI] [PubMed] [Google Scholar]
- 20.Tykocinski M, Linahan N, Shepherd RK, Clark GM. Chronic monopolar high rate stimulation of the auditory nerve: physiological and histopathological effects. In: Kubo T, Takahashi Y, Iwaki T. eds. Cochlear Implants: An Update. The Hague: Kugler Publications; 2002: 3–9 [Google Scholar]
- 21.Abbas PJ, Brown CJ, Shallop JK, et al. Summary of results using the Nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear. 1999;20: 45–59 [DOI] [PubMed] [Google Scholar]
- 22.Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver AA, Gervais JP. The relationship between EAP and EABR thresholds and levels used to program the Nucleus 24 speech processor: data from adults. Ear Hear. 2000;21: 151–163 [DOI] [PubMed] [Google Scholar]
- 23.Lai WK, Aksit M, Akdas F, Dillier N. Longitudinal behaviour of Neural Response Telemetry (NRT) data and clinical implications. Int J Audiol. 2004;43: 252–263 [DOI] [PubMed] [Google Scholar]
- 24.Cafarelli Dees D, Dillier N, Lai WK, et al. Normative findings of electrically evoked compound action potential measurements using the neural response telemetry of the Nucleus CI24M cochlear implant system. Audiol Neurootol. 2005;10: 105–116 [DOI] [PubMed] [Google Scholar]
- 25.Hughes ML, Brown CJ, Abbas PJ, Wolaver AA, Gervais JP. Comparison of ECAP thresholds with MAP levels in the Nucleus 24 cochlear implant: data from children. Ear Hear. 2000;21: 164–174 [DOI] [PubMed] [Google Scholar]
- 26.Ramos Macias A, Maggs J, Castillo C, Cafarelli Dees D, Geenham P. Use of intraoperative NRT in the initial fitting of very young children: preliminary findings. Presented at: the VIII International Cochlear Implant Conference; Indianpolis, In; 2004 [Google Scholar]
- 27.Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352: 236–238 [DOI] [PubMed] [Google Scholar]
- 28.Skinner MW, Arndt PL, Staller SJ. Nucleus 24 advanced encoder conversion study: performance versus preference. Ear Hear. 2002;23(suppl): 2S–17S [DOI] [PubMed] [Google Scholar]
- 29.Skinner MW, Holden LK, Whitford LA, Plant KL, Psarros C, Holden TA. Speech recognition with the Nucleus 24 SPEAK, ACE, and CIS speech coding strategies in newly implanted adults. Ear Hear. 2002;23: 207–223 [DOI] [PubMed] [Google Scholar]
- 30.Holden LK, Skinner MW, Holden TA, Demorest ME. Effects of stimulation rate with the Nucleus 24 ACE speech coding strategy. Ear Hear. 2002;23: 463–476 [DOI] [PubMed] [Google Scholar]
- 31.Staller SJ, Parkinson AJ, Arcaroli J, Arndt P. Pediatric outcomes with the Nucleus 24 Contour: North American clinical trial. Ann Otol Rhinol Laryngol. 2002;111(Suppl 189): 56–61 [DOI] [PubMed] [Google Scholar]
- 32.Gibson WPR, Harrison HC, Prowse C. A new incision for placement of cochlear implant. J Laryngol Otol. 1995;109: 821–825 [DOI] [PubMed] [Google Scholar]
- 33.Gibson WPR, Harrison HC. Further experience with a straight, vertical incision for placement of cochlear implants. J Laryngol Otol. 1997;111: 924–927 [DOI] [PubMed] [Google Scholar]
- 34.O'Donoghue GM, Nikolopoulos TP. Minimal access surgery for pediatric cochlear implantation. Otol Neurotol. 2002;23: 891–894 [DOI] [PubMed] [Google Scholar]
- 35.James AL, Papsin BC. Device fixation and small incision access for pediatric cochlear implants. Int J Pediatr Otorhinolaryngol. 2004;68: 1017–1022 [DOI] [PubMed] [Google Scholar]
- 36.James AL, Papsin BC. Cochlear implant surgery at 12 months of age or younger. Laryngoscope. 2004;114: 2191–2195 [DOI] [PubMed] [Google Scholar]
- 37.Roberson JB. Cochlear implant surgery: minimally invasive technique. Operat Tech Otolaryngol Head Neck Surg. 2005;16: 74–77 [Google Scholar]
- 38.Parkinson AJ, Arcaroli J, Staller SJ, Arndt PL, Cosgriff A, Ebinger K. The Nucleus 24 Contour cochlear implant system: adult clinical trial results. Ear Hear. 2002;23(suppl): 41S–48S [DOI] [PubMed] [Google Scholar]
- 39.Cochlear Limited . 2005. Nucleus Report—February/March. [Google Scholar]
- 40.Blamey PJ. Adaptive dynamic range optimization (ADRO): a digital amplification strategy for hearing aids and cochlear implants. Trends Amplif. 2005;9: 77–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James CJ, Blamey PJ, Martin L, Swanson B, Just Y, MacFarlane D. Adaptive dynamic range optimization for cochlear implants: a preliminary study. Ear Hear. 2002;23(suppl): 49S–58S [DOI] [PubMed] [Google Scholar]
- 42.Dawson PW, Decker JA, Psarros CE. Optimizing dynamic range in children using the Nucleus cochlear implant. Ear Hear. 2004;25: 230–241 [DOI] [PubMed] [Google Scholar]
- 43.McDermott HJ, Henshall KR, McKay CM. Benefits of syllabic input compression for users of cochlear implants. J Am Acad Audiol. 2001;13: 14–24 [PubMed] [Google Scholar]
- 44.Maurer J, Marangos N, Ziegler E. Reliability of cochlear implants. Otolaryngol Head Neck Surg. 2005;132: 746–750 [DOI] [PubMed] [Google Scholar]
- 45.Huang CQ, Stathopoulos D, Tykocinski M, Cowan R. Histopathological effects of electrical stimulation on the auditory nerve using very high rates. Proc 25th Aust Neurosci Soc. 2005;16: 96 [Google Scholar]
- 46.Battmer RD, Dillier N, Lai WK, et al. Evaluation of the neural response telemetry (NRT) capabilities of the nucleus research platform 8: initial results from the NRT trial. Int J Audiol. 2004;43(suppl): S10–S15 [PubMed] [Google Scholar]
- 47.Killian M, Cafarelli Dees D, von Wallenberg E. Enhanced NRT research using the Nucleus electrophysiology software: Custom Sound EP. Paper presented at: the Fourth International Symposium and Workshops: Objective Measures in Cochlear Implants; Hannover, Germany; 2005 [Google Scholar]
- 48.Botros A, van Dijk B, Killian M. AutoNRT™: an automated system that measures ECAP thresholds with the Nucleus® Freedom™ cochlear implant via machine intelligence. Artif Intell Med. 2006. Aug 18 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49.Battmer RD. AutoNRT, first clinical results of a completely automatic ECAP recording system. Paper presented at: 5th Asia Pacific Symposium on Cochlear Implant and Related Sciences; Hong Kong; 2005 [Google Scholar]
- 50.Gärtner L, Pesch J, Büchner A, Battmer RD, Lenarz T. ECAP measurements with the new Nucleus CI24RE implant and the Custom Sound EP software: comparison of automatically and manually obtained results. Paper presented at: Fourth International Symposium and Workshops: Objective Measures in Cochlear Implants; Hannover, Germany; 2005 [Google Scholar]
- 51.Busby PA, Plant KL. Dual electrode stimulation using the Nucleus CI24RE cochlear implant: electrode impedance and pitch ranking studies. Ear Hear. 2005;26: 504–511 [DOI] [PubMed] [Google Scholar]
- 52.Finley CC, Wilson BS, White MW. Models of neural responsiveness to electrical stimulation. In: Miller JM, Spelman FA. eds. Cochlear Implants: Models of the Electrically Stimulated Ear. New York: Springer-Verlag; 1990: 55–96 [Google Scholar]
- 53.Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res. 1993;66: 108–120 [DOI] [PubMed] [Google Scholar]
- 54.Frijns JHM, de Snoo SL, ten Kate JH. Spatial selectivity in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res. 1996;95: 33–48 [DOI] [PubMed] [Google Scholar]
- 55.Cohen LT, Saunders E, Clark GM. Psychophysics of a prototype peri-modiolar cochlear implant electrode array. Hear Res. 2001;155: 63–81 [DOI] [PubMed] [Google Scholar]
- 56.Saunders E, Cohen L, Aschendorff A, et al. Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance. Ear Hear. 2002;23(suppl): 28S–40S [DOI] [PubMed] [Google Scholar]
- 57.Roland JT, Fishman AJ, Alexiades G, Cohen N. Electrode to modiolus proximity: a fluoroscopic and histologic analysis. Am J Otol. 2000;21: 218–225 [DOI] [PubMed] [Google Scholar]
- 58.Richter B, Aschendorff A, Lohnstein P, Husstedt H, Nagursky H, Laszig R. The Nucleus 24 Contour perimodiolar electrode array: a radiological and histological study in human temporal bones. Laryngoscope. 2001;111: 508–513 [DOI] [PubMed] [Google Scholar]
- 59.Tykocinski M, Cohen LT, Pyman BC, et al. Comparison of electrode position in the human cochlea using various perimodiolar electrode arrays. Am J Otol. 2001;21: 205–211 [DOI] [PubMed] [Google Scholar]
- 60.Shepherd RK, Clark GM, Pyman BC, Webb RL. Banded intracochlear electrode array: evaluation of insertion trauma in human temporal bones. Ann Otol Rhinol Laryngol. 1985;94: 55–59 [DOI] [PubMed] [Google Scholar]
- 61.Kennedy DW. Multichannel intracochlear electrodes: mechanism of insertion trauma. Laryngoscope. 1987;97: 42–49 [DOI] [PubMed] [Google Scholar]
- 62.Wardrop P, Whinney D, Rebscher SJ, Roland JT, Luxford W, Leake P. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: comparison of Nucleus banded and Nucleus Contour electrodes. Hear Res. 2005;203: 54–67 [DOI] [PubMed] [Google Scholar]
- 63.Adunka O, Pillsbury HC, Kiefer J. Combining perimodiolar electrode placement and atraumatic insertion properties in cochlear implantation—fact or fantasy? Acta Otolaryngol. 2006;126: 475–482 [DOI] [PubMed] [Google Scholar]
- 64.Roland JT. A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115: 1325–1339 [DOI] [PubMed] [Google Scholar]
- 65.Galvin JJ, Fu QJ. Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J Assoc Res Otolaryngol. 2005;6: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibson P, Capcelea E, Darley I, Leavens J, Parker J. A water-resistant speech processor. Cochlear Implants Int. 2006;7: 159–166 [DOI] [PubMed] [Google Scholar]
- 67.Kalnins LAE. Threshold and comfort levels and impedance values for the US clinical trial of the Nucleus 24. Unpublished Cochlear Limited Technical Memo. 1998 [Google Scholar]
- 68.Kalnins LAE. Threshold and comfort levels for the US paediatric clinical trial of the Nucleus 24. Unpublished Cochlear Limited Technical Memo. 1998 [Google Scholar]
- 69.Zeng FG, Shannon RV. Loudness-coding mechanisms inferred from electric stimulation of the human auditory system. Science. 1994;264: 564–566 [DOI] [PubMed] [Google Scholar]
- 70.Zeng FG, Shannon RV. Psychophysical laws revealed by electric hearing. Neuroreport. 1999;10: 1931–1935 [DOI] [PubMed] [Google Scholar]
- 71.Zeng FG, Grant G, Niparko J, et al. Segel P. Speech dynamic range and its effect on cochlear implant performance. J Acoust Soc Am. 2002;111: 377–386 [DOI] [PubMed] [Google Scholar]
- 72.Dawson P, Vandali AE, Knight MR, Heasman JM. Clinical evaluation of expanded input dynamic range in Nucleus cochlear implants. Ear Hear. In press. [DOI] [PubMed] [Google Scholar]
- 73.van den Berghe J, Wouters J. An adaptive noise canceller for hearing aids using two nearby microphones. J Acoust Soc Am. 1998;103: 3621–3626 [DOI] [PubMed] [Google Scholar]
- 74.Wouters J, Van den Berghe J. Speech recognition in noise for cochlear implantees with a two-microphone monaural adaptive noise reduction system. Ear Hear. 2001;22: 420–430 [DOI] [PubMed] [Google Scholar]
- 75.Maj JB, Wouters J, Moonen M. Noise reduction results of an adaptive filtering technique for dual-microphone behind-the-ear hearing aids. Ear Hear. 2004;25: 215–229 [DOI] [PubMed] [Google Scholar]
- 76.Spriet A, Van Deun L, Eftaxiadis K, et al. Speech understanding in background noise with the two-microphone adaptive beamformer BEAM in the Nucleus Freedom cochlear implant system. Ear Hear. In press. [DOI] [PubMed] [Google Scholar]
- 77.Plant K, Law MA, Whitford L, et al. Evaluation of streamlined programming procedures for the Nucleus cochlear implant with the Contour electrode array. Ear Hear. 2005;26: 651–668 [DOI] [PubMed] [Google Scholar]
- 78.Arcaroli A, Plant N, Chute P, Popp A, Hose B. Streamlined programming study in newly implanted subjects. Poster presented at: 7th European Symposium on Peadiatric Cochlear Implantation; Geneva, Switzerland; 2004 [Google Scholar]
- 79.International Organization for Standardization International Standard ISO 5841-2 Implants for Surgery—Cardiac pacemakers—Part 2: Reporting of clinical performance of populations of pulse generators or leads. 2nd ed. 2000 [Google Scholar]
- 80.European Consensus Statement on Cochlear Implant Failures and Explantations Otol Neurotol. 2005;26: 1097–1099 [PubMed] [Google Scholar]
- 81.Battmer RD, O'Donoghue GM, Lenarz T. A multi-centre study of device failure in European cochlear implant centres. Ear Hear. In press. [DOI] [PubMed] [Google Scholar]
- 82.Lenarz T, Büchner A, Tasche C, et al. The results in patients implanted with the Nucleus double array cochlear implant: pitch discrimination and auditory performance. Ear Hear. 2002;23(suppl): 90S–101S [DOI] [PubMed] [Google Scholar]
- 83.Staller S, Arcaroli J, Parkinson A, Menapace C. US Nucleus adult bilateral cochlear implantation study results. Paper presented at: 4th International Symposium on Electronic Implants in Otology and Conventional Hearing Aids; Toulouse, France; 2003 [Google Scholar]
- 84.Litovsky RY, Parkinson A, Arcaroli J, et al. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130: 648–655 [DOI] [PubMed] [Google Scholar]
- 85.Litovsky R, Parkinson A, Arcaroli Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear. 2006;27: 714–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramsden R, Greenham P, O'Driscoll M, et al. Evaluation of bilaterally implanted adult subjects with the Nucleus 24 cochlear implant system. Otol Neurotol. 2005;26: 988–998 [DOI] [PubMed] [Google Scholar]
- 87.Laszig R, Aschendorff A, Stecker M, et al. Benefits of bilateral electrical stimulation with the Nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol. 2004;25: 958–968 [DOI] [PubMed] [Google Scholar]
- 88.Verschuur CA, Lutman ME, Ramsden R, Greenham P, O'Driscoll M. Auditory localization abilities in bilateral cochlear implant recipients. Otol Neurotol. 2005;26: 965–971 [DOI] [PubMed] [Google Scholar]
- 89.Dillon H. Hearing Aids. New York: Thieme; Medical; 2000 [Google Scholar]
- 90.Litovsky RY, Johnstone PM, Godar S, et al. Bilateral cochlear implants in children: localization acuity measured with minimum audible angle. Ear Hear. 2006;27: 43–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Audiol Neurootol. 2004;9: 234–246 [DOI] [PubMed] [Google Scholar]
- 92.van Hoesel R, Bohm M, Battmer RD, Beckschebe J, Lenarz T. Amplitude-mapping effects on speech intelligibility with unilateral and bilateral cochlear implants. Ear Hear. 2005;26: 381–388 [DOI] [PubMed] [Google Scholar]
- 93.Ching TYC, Psarros C, Hill M, Dillon H, Incerti P. Should children who use cochlear implants wear hearing aids in the opposite ear? Ear Hear. 2001;22: 365–380 [DOI] [PubMed] [Google Scholar]
- 94.Ching TYC, Incerti P, Hill M, Priolo S. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25: 9–21 [DOI] [PubMed] [Google Scholar]
- 95.Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J Speech Lang Hear Res. 2005;48: 668–680 [DOI] [PubMed] [Google Scholar]
- 96.Kong YY, Stickney GS, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005;117: 1351–1361 [DOI] [PubMed] [Google Scholar]
- 97.Mok M, Grayden D, Dowell RC, Lawrence D. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J Speech Lang Hear Res. 2006;49: 338–351 [DOI] [PubMed] [Google Scholar]
- 98.Gantz BJ, Turner CW, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;15: 796–802 [DOI] [PubMed] [Google Scholar]
- 99.Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurotol. 2006;11: 63–68 [DOI] [PubMed] [Google Scholar]
- 100.James C, Albegger K, Battmer R, et al. Preservation of residual hearing with cochlear implantation: how and why. Acta Otolaryngol. 2005;125: 481–491 [DOI] [PubMed] [Google Scholar]
- 101.Fraysse B, James C. Residual hearing conservation and electro-acoustic stimulation with the Nucleus 24 Contour Advance. Paper presented at: 1st International Electro-Acoustic Workshop; Toulouse, France; 2005 [Google Scholar]
- 102.Briggs RJS, Tykocinski M, Xu J, et al. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurotol. 2006;11: 42–48 [DOI] [PubMed] [Google Scholar]
- 103.Lenarz T, Stöver T, Buechner A, et al. Temporal bone results and hearing preservation with a new straight electrode. Audiol Neurotol. 2006;11: 34–41 [DOI] [PubMed] [Google Scholar]
- 104.Nogueira W, Büchner A, Lenarz T, Elder B. A psychoacoustic “NofM”-type speech coding strategy for cochlear implants. EURASIP J App Signal Processing. 2005;18: 3044–3059 [Google Scholar]
- 105.Büchner A, Nogueira W, Elder B, Battmer RD, Lenarz T. Results of a psychoacoustic-model-based ACE strategy (PACE) for the Nucleus-24 device. Paper presented at: 2005. Conference on Implantable Auditory Prostheses; Asilomar, Calif; 2005. [Google Scholar]
- 106.Wilson BS, Zerbi M, Lawson D. Speech processors for auditory prostheses. NIH Contract N01-DC-2-2401, 3rd Quarterly Progress Report, February 1-April 30, 1993 [Google Scholar]
- 107.McDermott HJ, McKay CM. Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea. J Acoust Soc Am. 1994;96: 155–162 [DOI] [PubMed] [Google Scholar]
- 108.Kwon BJ, van den Honert C. Dual-electrode pitch discrimination with sequential interleaved stimulation by cochlear implant users. J Acoust Soc Am. 2006;120: EL1–EL6 [DOI] [PubMed] [Google Scholar]
- 109.Townshend B, Cotter N, van Compernolle D, White RL. Pitch perception by cochlear implant subjects. J Acoust Soc Am. 1987;82: 106–115 [DOI] [PubMed] [Google Scholar]
- 110.Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single- versus dual-electrode stimuli by cochlear implant users. J Acoust Soc Am. 2005;118: 623–626 [DOI] [PubMed] [Google Scholar]
- 111.Dorman MF, Loizou PC, Fitzke J, Tu Z. The recognition of sentences in noise by normal-hearing listeners using simulations of cochlear-implant signal processors with 6–20 channels. J Acoust Soc Am. 1998;104: 3583–3585 [DOI] [PubMed] [Google Scholar]
- 112.Fishman K, Shannon RV, Slattery WH. Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. J Speech Lang Hear Res. 1997;40: 1201–1215 [DOI] [PubMed] [Google Scholar]
- 113.Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110: 1150–1163 [DOI] [PubMed] [Google Scholar]
- 114.Loizou PC, Dorman MF, Tu Z. On the number of channels needed to understand speech. J Acoust Soc Am. 1999;106: 2097–2103 [DOI] [PubMed] [Google Scholar]
- 115.Shannon RV, Fu QJ, Galvin J., 3rd. The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol. 2004; (suppl 522): 50–54 [DOI] [PubMed] [Google Scholar]
- 116.van den Honert C, Kelsall DC. Focused intracochlear electric stimulation with phased array channels. J Am Acoust Soc. Submitted. [DOI] [PubMed] [Google Scholar]
- 117.Gordon K, Papsin BC, Harrison RV. Programming cochlear implant stimulation levels in infants and children with a combination of objective measures. Int J Audiol. 2004;43 (suppl): S28–S32 [PubMed] [Google Scholar]
- 118.Smoorenburg GF, Willeboer C, van Dijk JE. Speech perception in Nucleus CI24M cochlear implant users with processor settings based on electrically evoked compound action potential thresholds. Audiol Neurootol. 2002;7: 335–347 [DOI] [PubMed] [Google Scholar]
- 119.Wakefield GH, van den Honert C, Parkinson W, Lineaweaver S. Genetic algorithms for adaptive psychophysical procedures: recipient-directed design of speech-processor MAPs. Ear Hear. 2005;26 (suppl). 57S–72S [DOI] [PubMed] [Google Scholar]