Abstract
Cochlear implantation is an accepted treatment method for adults and children with severe to profound hearing loss. Confidence in technology has led to changes in individuals who can receive a cochlear implant and changes in expected benefit with a cochlear implant. This article describes the research and development activities at MED-EL, which make possible the implementation of new speech-coding strategies as well as the application of acoustic and electric stimulation via a combined speech processor in MED-EL devices. Research on benefits from bilateral cochlear implantation and electric-acoustic stimulation are also reviewed. Finally, the potential of drug delivery systems is considered as a way to improve cochlear implant outcomes, and results from preliminary evaluations of a hybrid cochlear implant system with drug delivery capabilities are reported.
Keywords: cochlear implant, MAESTRO, fine structure processing, bilateral, electric-acoustic stimulation, drug delivery
Cochlear implants (CIs) are a well-known and accepted treatment method for adults and children with severe to profound hearing loss. There has been much progress in the CI field since the article detailing trends in cochlear implantation.1 Advances in technology, increased confidence in experience, and changes in candidacy have led to CIs being made available to a larger population.
Recent CI recipients perform much better than those who received implants many years ago. Back then, CI performance focused on differentiating between the presence and absence of sound or female versus male voice. Expectations for recent CI recipients, however, have changed. Their performances have been tested under increasingly difficult listening conditions. The general experiences in the CI community are that CI users usually perform very well in quiet but worse in noise, and not all CI users are able to enjoy music. For further improvements in these areas, MED-EL's research focuses on providing additional pitch information. One means to achieve this could be to increase the information per time unit (information rate) delivered to the cochlea; this can be done by increasing the stimulation rate. Another way would be to add additional information into the stimulation patterns such as temporal as well as spatial fine structure information. This will be further discussed in the development of speech-coding strategies section.
In addition, this article provides an overview of the new technology incorporated in the MED-EL MAESTRO CI system, reviews recent research on bilateral cochlear implantation and electric-acoustic stimulation, and describes current research efforts on developing a CI device with drug delivery capability.
The MAESTRO CI System
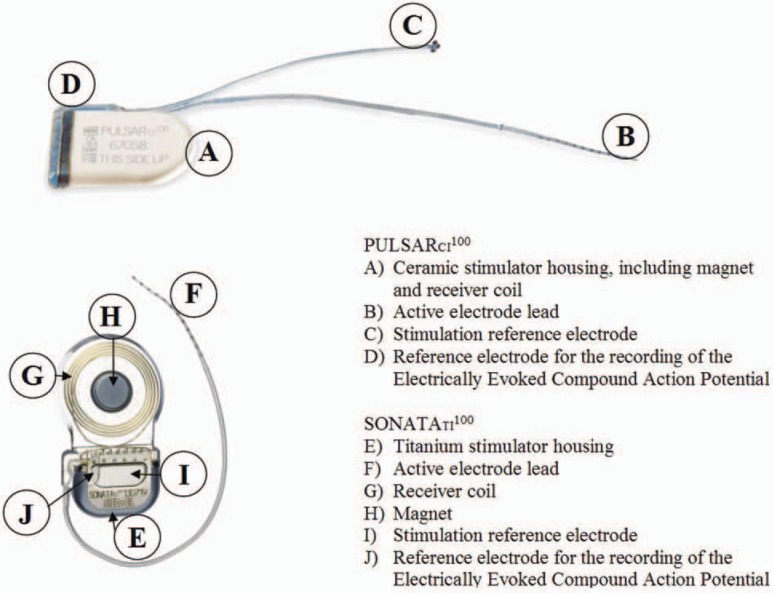
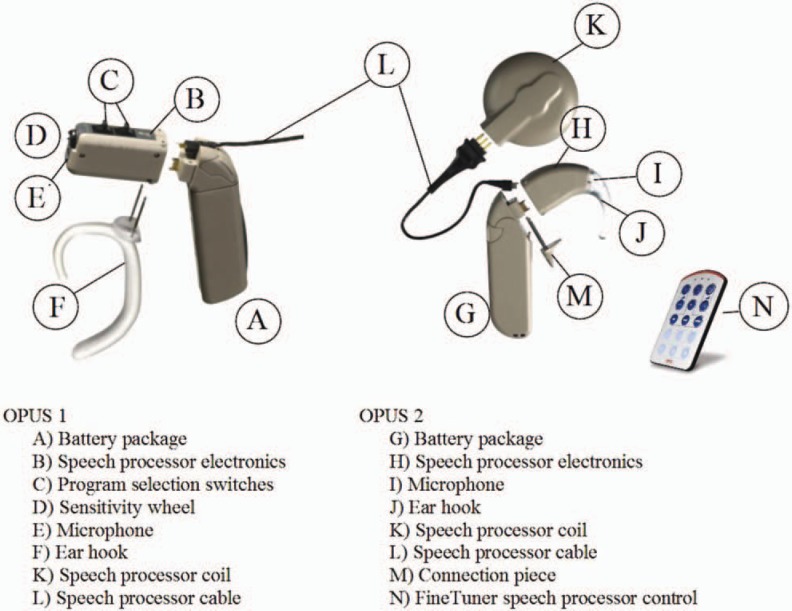
The MED-EL MAESTRO CI system comprises the PULSARci100 and SONATAti100 CIs, which incorporate the I100 electronics platform; the speech processors and the MAESTRO system software, together with the Diagnostic Interface Box II, is the link between software and speech processors or implants. The MAESTRO CI system provides fine structure information for CI users (refer to the section on speech-coding strategies). In addition, audiologists have access to telemetry features for research and biological and technical assessments. The telemetry also allows the audiologist to simplify the fitting procedure in young children and in complicated cases. A major characteristic of the system is that the PULSARci100 and SONATAti100 cis incorporate the same electronics package (see Figure 1); thus, users have access to the same features with regard to speech-coding strategies. The difference between the 2 implants is the housing design. The choice of which implant to use depends on the client's or surgeon's preference. This would be a choice between an integrated coil (PULSARci100) and an external coil (SONATAti100). The OPUS 1 and the OPUS 2 speech processors use the same microchip, and the differences are in the mechanical design of the housing (see Figure 2), allowing the user a choice of speech processor design. With the OPUS processors, the patient has a choice between a standard behind-the-ear (BTE) with on-board controls or a switchless BTE controlled by a FineTuner device (see Figure 2). The following section describes all components of the MAESTRO CI system. The design philosophy, the features, and the differences between the components are explained.
Figure 1.
The PULSARci100 and SONATAti100 cochlear implants.
Figure 2.
Modular concept of the OPUS 1 and OPUS 2 speech processors.
The Implant
Design Philosophy
In a CI system, the implantable part should remain implanted for decades, whereas the external speech processors can be considered as an upgradeable variable that activates implant features by providing different speech-coding strategies. There are several objectives when developing new CIs such as the PULSARci100 and the SONATAti100. On one hand, patients should benefit from new research and developments of technological innovations as well as speech-coding strategies. On the other hand, proven concepts of the earlier CI systems should be retained. In addition, the implant has to be compatible with existing components of the C40+ system, and there should not be any limitations with regard to power consumption. With regard to the electrodes, MED-EL's design philosophy is to provide atraumatic insertion and extensive cochlear coverage, which goes hand in hand with the concept of providing fine structure information for CI users.
PULSARci100 and SONATAti100 Features
Proven concepts such as the principle of monopolar stimulation, as implemented in the C40+ system, were prerequisites for designing power-efficient CIs. The PULSARci100 uses the ceramic housing of the C40+ device, and the SONATAti100 is based on new titanium housing technology (Figure 1). Both housing types provide magnetic resonance imaging safety without magnet removal surgery for 0.2T, 1.0T, and 1.5T. In addition, both incorporate the I100 electronics platform, which provides new features such as 100% on-chip design, built-in safety design, backward compatibility, high stimulation rates up to 50 700 pulses per second (pps), individual current sources for each channel, and extensive telemetry capabilities (status telemetry, precision impedance and electrical field telemetry, and auditory nerve response telemetry). In the future fitting software, there is the possibility for sequential and parallel stimulation as well as the possibility of creating various pulse shapes and variable interphase gaps.
The Speech Processor
Design Philosophy
External speech processors should be backward compatible to make it possible for recipients of earlier CI generations to benefit from the latest outcomes in CI research. Processors have to be upgradeable by simply activating new hardware features via software. With regard to the speech-coding strategies implemented, it would be ideal to have an optimum strategy that gives the best performance with all patients. Although a large variability exists among users because of neural survival and other factors, the CIS+ strategy has been shown to provide excellent speech understanding,2 and thus it has been implemented as the strategy of choice. A new strategy, fine structure processing (FSP), is also included. It is meant to address difficulties of CI users in understanding speech in noise and in being able to appreciate music. The hardware thus includes application-specific circuits for FSP. This type of hardware is the most efficient way to realize very complex and power-efficient applications. Last but not least, special attention is paid to the mechanical design, which has to be convenient and easy to use. In this regard, the TEMPO+ speech processor is modular, allowing for different battery pack options as well as different wearing options.
For patients with residual hearing, MED-EL provides another speech processor type that combines a hearing aid (HA) and an OPUS 1 speech processor. This is known as the DUET EAS hearing system (refer to the section on the combined speech processor in the section on monaural electric acoustic stimulation).
Features of the Latest Processor Developments
The OPUS 1 and OPUS 2 speech processors (Figure 2) are based on the newest microchip technology, which allows future upgrades in speech-coding strategies, for example, FSP. The OPUS 1 offers these features in the same housing as the TEMPO+ speech processor. The OPUS 2 offers these features in a new housing design while maintaining the same modularity as the TEMPO+ and OPUS 1 speech processors. Independent of the speech processor type (OPUS 1, OPUS 2), each speech processor features a compact and lightweight design with interchangeable wearing options for the user's comfort and backward and forward compatibility with MED-EL CIs to ensure that all users have access to the latest technology. In addition, the OPUS 2 speech processor features a switch-free design with a FineTuner remote control to manipulate the speech processor, an integrated telecoil, and a wireless FM system connection.
Development of New Coding Strategies
Background
The mathematician David Hilbert demonstrated that a (band-pass) signal can be decomposed into a slowly varying envelope (ie, amplitude modulation) and a high frequency carrier of constant amplitude, which is referred to as the fine structure of the signal.3 In speech and other acoustic signals, the fine structure varies continuously and carries important information such as pitch and timbre. It is well known that in normal hearing individuals, the neural response to low frequencies reflects both the envelope and the fine structure elements of the signal, whereas only the envelope is represented in the neural response to high frequencies, with the highest frequency being about 5 kHz.4
Recent research in normal hearing subjects has shown that for the number of independent processing channels as found in CIs today, the primary information carrier for speech signals is the envelope, whereas for music, it is the fine structure.5 In the same experiment, it was also found that interaural time delays coded in the fine structure determine where a sound is heard from rather than interaural time delays coded in the envelope, although it is still the speech signal coded in the envelope that is perceived.
Prior to signal processing per the selected speech-coding strategy, the microphone signal is passed through a dual-loop AGC6 designed to minimize the perceptual effects of changes in acoustical level and of loud transient sounds and a preemphasis filter with a slope of 2.5 dB/octave greater than 1 kHz designed to attenuate low-frequency components to avoid masking of high-frequency components.
Envelope-Based Processing
All CIS and n-of-m–based coding strategies that have been in use for the past 15 to 20 years rely mainly on envelope information.7 In general, users of these coding strategies show good to very good speech perception in quiet, moderate speech perception in noise, and poor to moderate music appreciation.1 Specifically, the transmission of tonal speech information, such as prosodic contour or speaker gender, as well as music perception and appraisal, is poor in CI users compared to normal hearing listeners.8–10
Thus, the principal performance characteristics of these coding strategies are in agreement with the results by Smith et al5 as described above, namely, that envelope information is suitable for supporting moderate to very good degrees of speech perception but is unsuitable for allowing the average user a satisfactory degree of music appreciation. In short, these strategies lack fine structure information for speech in noise and music appreciation.
FSP
With the introduction of the I100 electronics platform and the OPUS speech processors, MED-EL has developed a new concept in coding strategies that is designed to overcome the limitations of envelope-based coding strategies. The goal of the FSP strategy currently implemented in the OPUS speech processors is to improve both the temporal and tonotopic coding of sounds in CIs.
In contrast to fixed-rate envelope-based coding strategies in which the timing of stimulation is not an information carrier, FSP works in both time and place. The timing of stimulation is used to code the temporal structure of the sound signal particularly in the low- to mid-frequency range. This is achieved by using channel-specific sampling sequences (CSSSs).11 A CSSS is a series of stimulation pulses that is started at each positive-going zero crossing in a channel's band-pass filter output. In FSP, the length of these sequences is related to the band-pass filter's upper corner frequency. Thus, the instantaneous repetition rate of these sequences equals the instantaneous fine structure frequency of the signal in the respective frequency range. In the FSP strategy, CSSS is typically used on the lower (ie, apical) 2 to 3 channels, which means that depending on the bandpass filters’ arrangement, CSSS is used for frequencies up to 300 to 500 Hz.
On the remaining channels, tonotopic fine structure coding is achieved by creating pitch percepts that are intermediate to the pitch percepts created by stimulating single electrodes in isolation using so-called virtual channels.12 Similar to CIS+ as implemented in the TEMPO+ speech processor, the OPUS speech processors use band-pass filters with a bell-shaped frequency response. This allows a smooth transition of stimulation from 1 electrode to the adjacent apical or basal electrode as frequency decreases or increases. As an example, stimulation amplitude on the more apical electrode will decrease and stimulation amplitude on the more basal electrode will increase as input frequency increases. It was found in previous studies13 that even when these channels are stimulated sequentially, the perceived pitch is intermediate to the single-electrode pitches. Thus, although the concept of virtual channels as originally published used parallel stimulation, virtual channels can be created using both parallel and sequential stimulation.
In summary, FineHearing technology is designed to better model normal hearing than purely envelope-based coding strategies. Similar to frequency coding in normal hearing, the FSP strategy codes the fine structure both in time (via CSSS) and place (via virtual channels).
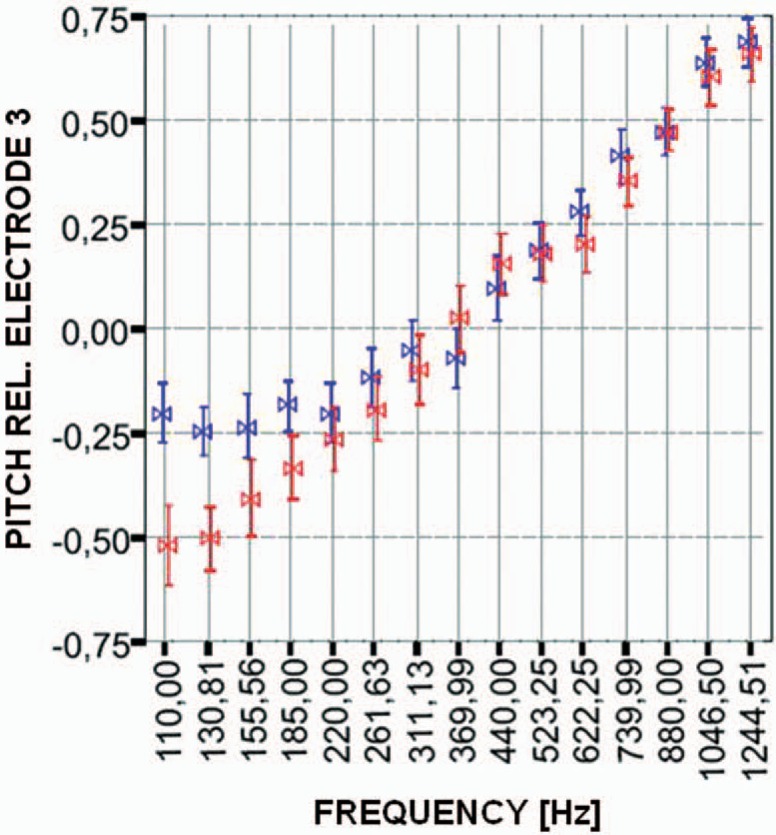
Preliminary results with the FSP coding strategy have been encouraging. Mitterbacher et al14 measured pitch discrimination and pitch perception in CI users using the FSP coding strategy and the envelope-based CIS+ strategy, as implemented in the MED-EL TEMPO+ speech processor. Pitch discrimination was tested using synthetic signals such as sawtooth and triangle waves. Better pitch discrimination was obtained with the FSP strategy than the CIS+ strategy, particularly in subjects who were poor performers. On average, just noticeable differences in pitch were 10 percentage points smaller with FSP than with CIS+ in these subjects. Mitterbacher et al15 presented pitch-scaling data obtained with the FSP strategy and with a CIS+ strategy. In contrast to the clinical version of the CIS+ strategy, CIS+ as well as FSP used an analysis frequency range of 100 to 8500 Hz in this experiment. In the FSP strategy, CSSS was used for frequencies up to 300 Hz. For pure-tone signals (ie, sinusoids), the researchers found that in the frequency range greater than 300 Hz, where both strategies presented the envelope only, performance does not depend on the strategy. Specifically, both CIS+ and FSP produce decreasing pitch percepts with decreasing acoustic frequency, as desired (Figure 3). However, for frequencies less than 300 Hz, in contrast to CIS+, FSP presented the temporal code of the fine structure. For the CIS+ strategy, pitch saturates for frequencies less than 200 Hz, whereas for the FSP strategy, pitch further decreases, with a decrease in acoustic frequency. This is most probably due to place coding being impeded by the fact that the most apical electrode does not have a more apical neighbour producing lower pitch. In contrast, for complex tones (wide-band signals), the researchers found similar results for FSP and CIS+ across the whole frequency range tested. This suggests that providing additional temporal information in FSP does not compromise the transmission of spectral cues when compared to CIS+.
Figure 3.
Pitch judgments (normalized) as a function of frequency, as measured in 5 subjects. Pitch was judged relative to a constant-amplitude (MCL) constant rate (1515 pps) burst on electrode 3. Input signals were sinusoids presented acoustically via the speech processor. Results are shown for fine structure processing (red) and CIS+ (blue). The same analysis range was used for both strategies (100–8500 Hz). Bowties = average values; bars = standard deviation.15
Similar results were found by Schatzer et al.16 With the FSP strategy and in comparison to a CIS+ strategy, they also found large improvements in pitch discrimination for low frequencies and a constant increase in pitch over a logarithmic frequency axis while pitch saturates for low frequencies for CIS+.
These results show that the FSP strategy allows better pitch perception than a traditional envelope-based CIS-type strategy, at least for narrow-band signals such as sinusoids.15 The results also indicate both time (via CSSS) and place (via virtual channels) coding complement each other in that that if 1 of the 2 codes fails, such as the place code for the low frequencies in CIS, then patients can effectively use the other code to extract pitch information. Furthermore, as discussed above, the results suggest that providing additional temporal information in FSP does not compromise the transmission of spectral information.
Providing both the time and the place code should make frequency coding more robust in CIs. The literature suggests that each code should have its own specific advantages and weaknesses. In a large body of literature, the time code was shown to be very reliable; however, it is restricted to frequencies less than 300 to 1000 Hz, depending on the subject.14,15,17,18 In contrast, the place code works across a wider frequency range (basically the complete cochlear region covered by the electrode); however, the number of intermediate pitches in a certain cochlear region should depend on subject-specific parameters such as, for example, neural survival and was shown to vary largely across subjects and across the cochlea even within subjects.19
Summary
In summary, the I100 platform and the OPUS speech processors’ support can provide additional temporal acoustic information to CI users that has previously been omitted in purely envelope-based coding strategies. The FSP strategy allows better pitch perception (ie, better pitch discrimination and a wider pitch range, as mentioned in the review above) than the standard envelope-based CIS strategy. This new information is expected to yield better speech discrimination in noise, improved localization, and provide users with enhanced music appreciation.
Bilateral Cochlear Implantation
Background
Until recently, CIs have in most cases been supplied monolaterally. Many users show high levels of speech understanding after monolateral cochlear implantation (eg, Helms et al2). However, with monolateral implantation, there comes the loss of binaural hearing presumably resulting in deterioration in speech understanding and in the ability to localize sounds. It is well known that listening with 2 ears allows subjects with normal hearing to understand speech better in background noise or in reverberant environments.20–25 The overall gain is usually attributed to 3 effects: the head shadow effect (the benefit from the head acting as an acoustic barrier between 1 of the ears and the noise source), the squelch effect (the benefit arising from a spatial separation between the speech and the noise source), and the binaural (or diotic) summation effect (the benefit from providing identical acoustic inputs to the 2 ears).26 However, there are more advantages to binaural hearing. Binaural hearing also is an essential requirement for spatial hearing and sound localization.27 In general, most noise reduction and acoustical orientation abilities of the human auditory system crucially depend on the subject's having access to time, level, and spectral differences between the sound signals sensed by the 2 ears.
There is overwhelming evidence in hearing-impaired listeners that 2 HAs provide better performance for most users than using only 1 aid (eg, Byrne28). A recent study again documents the advantage of bilateral HAs under a wide variety of conditions.29 Sound localization with bilateral HAs has been investigated for decades, and it is also now well accepted that binaural HA fitting can restore sound localization,30 at least in moderately to severely impaired listeners.31
Until approximately 1995, bilateral cochlear implantation was mostly either the result of a technology upgrade, in which an older (mostly singlechannel) device was still functioning so that, rather than replacing the old device, the second ear was implanted with the newer (multichannel) device, or a result of insufficient functioning of, or inadequate performance with, the device in the first ear.32 Thus, prior to 1995, bilateral cochlear implantation was mainly not intended as a treatment for restoring binaural hearing abilities. In tests on these early patients,32–35 it was nonetheless found that the auditory system has the potential to integrate information provided by 2 different devices. However, apart from Balkany et al33 and van Hoesel et al,35 no improvement in speech understanding had been reported.
In 1996, the ENT clinic at the University of Würzburg started to provide bilateral MED-EL CIs in an attempt to restore binaural hearing.36 Since then, bilateral cochlear implantation has gained increased momentum, and the principal benefits are now well established, as discussed below. As of June 2006, there are more than 1000 bilateral MED-EL users worldwide, two thirds of which are children.
Performance of Bilateral CIs
Generally speaking, the benefits of bilateral cochlear implantation are now well proven, although benefits sometimes differ from person to person. As far as speech reception is concerned, it was shown that bilateral CI users on average benefit from all binaural effects that are known from normal hearing listeners.36–38 For sentences at a signal-to-noise ratio of 10 dB, Müller et al36 found a head shadow effect of 20.4% and a squelch effect of 10.7%. For monosyllables in quiet, a summation effect of 18.7% was found. All effects were statistically significant. Using an adaptive sentence test, Schleich et al38 demonstrated that bilateral CI users show a significant head shadow effect of 6.8 dB, a significant squelch effect of 0.9 dB, and a significant summation effect of 2.1 dB. Gains in speech reception with bilateral CIs have also been demonstrated for tonal languages.39,40
It is also been shown that bilateral CIs can promote sound localization, at least in the frontal horizontal plane.41,42 Nopp et al41 showed that when using both implants, adult postlingually deafened subjects are able to localize sound sources with an average error of 16.6°, while the error (53.7°) was at chance when using 1 implant only. An alternative measure for spatial hearing is the minimum audible angle (MAA), that is, the minimum shift in space of a sound source somebody can detect. Senn et al43 showed that bilateral CI users show near-normal MAAs of 3° to 8° in the front and the back of the head, while at the sides, relatively poor MAAs of 30° to 45° (normal hearing: 7° to 10°) were found. These data again confirm that at least in certain sections of the 3-dimensional space, bilateral CI users do exhibit some spatial hearing to some extent.
A study investigating bilateral cochlear implantation in children showed that with bilateral CIs, children's communicative behavior improved, particularly in complex listening conditions.44 The children showed significantly better speech reception in noise when using both implants. In addition, the integration of the second implant and the use of binaural information were observed to be faster and easier with children with a short time lag between implantations. In contrast, in postlingual adults, Nopp et al41 did not find a significant correlation between sound localization and the duration of unilateral implant use, indicating that the time lag between implantations is not critical in this group.
The debate about which binaural cues bilateral CI users are able to perceive and use is still ongoing. It seems well established today that bilateral users have moderate to good access to interaural-level differences (ILDs) and poor to moderate access to interaural time differences (ITDs).45–47 It is normally argued that when localizing sound sources, CI users almost solely rely on ILDs. However, at least 1 study found a significant correlation between a subject's ability to localize sound sources and his sensitivity to ITDs.42 In addition, the fact that subjects show a significant squelch effect38 might also be indicative of the fact that ITDs play a greater role than currently thought. The discussion might gain some additional momentum with the introduction of fine structure coding such as the FSP strategy currently implemented in the OPUS speech processors. Laback et al48 and Majdak et al49 investigated the effect of interaural time delays in the envelope and the fine structure, respectively, on subjects’ lateralization. They presented pulse trains of the same rate to both ears. The temporal offset between stimulation pulses across sides represented the fine structure delay. The offset between the start and the end of the trains represented the envelope delay. The experiment was conducted using different stimulation rates, that is, fine structure frequencies. They found that the fine structure ITDs had the strongest impact on lateralization—for fine structure frequencies (ie, stimulation rates), up to 800 Hz. This might indicate that fine structure strategies might make interaural time delays better accessible to CI users and that improved bilateral performance might result from the use of coding strategies such as the FSP strategy.
Summary
The principal benefits of bilateral CIs in speech perception and sound localization are well proven. Bilateral users benefit from all binaural effects known from normal hearing and basically regain the ability to localize sounds at least in the frontal horizontal plane. Bilateral CI users seem to be sensitive to fine structure ITDs so that fine structure coding might result in a further improvement with bilateral CIs.
Monaural Electric Acoustic Stimulation
The Concept
In recent years, a new type of therapy (electric-acoustic stimulation [EAS]) has been developed with the aim of treating individuals with a ski-slope–type hearing loss, that is, a mild to moderate hearing loss in the low-frequency area less than 1 kHz and a severe to profound hearing loss in the higher frequencies. These individuals normally gain only little benefit from HA amplification. The idea behind EAS is to combine a CI and an HA in the same ear. In EAS, users are implanted with a CI, which stimulates the mid- to high-frequency range. The low frequencies are amplified with an HA or, in cases with mild low-frequency hearing losses, using natural (nonamplified) hearing.
A pioneering first report on EAS was published in 1999 by Christoph von Ilberg's team in Frankfurt, Germany,50 demonstrating several points: first, that the preservation of low-frequency hearing after cochlear implantation is possible. Second, that the central auditory system is able to combine the signal from acoustic hearing at low frequencies with direct electric stimulation of the cochlear nerve without disturbing interferences. Third, the combined EAS results in improvements in speech perception and a more natural sound perception.
Hearing Preservation
Preservation of low-frequency hearing after cochlear implantation is possible with careful surgical technique and with careful attention to electrode design. Based on the soft surgery concept introduced by Lehnhardt,51 Kiefer et al52 developed a surgical procedure aimed at achieving atraumatic cochlear implantation with conservation of low-frequency hearing. Other surgical approaches with the same aim have been introduced since. Skarzynski et al53–55 suggested inserting the CI electrode through the round window membrane rather than using a cochleostomy, and Roland et al56 proposed a cochleostomy adjacent to the round window. All these surgical techniques aim at preserving the remaining hearing capacity by minimizing trauma to inner ear structures and the avoidance of inflammatory and excessive fibrotic reactions.
One important aspect of hearing preservation is the depth of CI electrode insertion. While a deep insertion into the functioning low-frequency region of the cochlea bears a higher risk of damage to the delicate inner ear structures, a too shallow insertion would lead to a reduced CI function. Most authors therefore recommend a 360° insertion with insertion depths of about 18 to 20 mm for a hearing-preserving CI implantation.50,52,54,56,57
To facilitate hearing preservation in CI surgery for EAS, MED-EL has developed the FLEXeas electrode. The FLEXeas electrode is a medium-length electrode (20.9 mm contact extent) with a highly flexible tip suitable for both cochleostomy and round window insertions. Measurements showed that with the design of this electrode, it was possible to significantly reduce the force necessary for insertion by more than 40% compared to the standard electrode array.58 Histological studies in human temporal bones showed no substantial trauma to cochlear structures.58
While it is not possible to preserve low-frequency hearing in all cases, it was possible in a high percentage of them. Gstoettner et al57 reported on 18 of 21 cases (86%) in which a full or partial preservation was achieved. Skarzynski et al54 reported on 9 of 10 cases (90%) of hearing preservation. With regard to long-term preservation, Gstoettner et al59 presented data on 23 subjects and concluded that long-term preservation of ipsilateral hearing after CI implantation can be achieved in about 70% of cases.
Gstoettner et al60 reported on the results of a European multicenter study on EAS using the MED-EL system. In this study, a longer-term hearing preservation rate of 83.2% was achieved. It was reported that in these cases, hearing was preserved not only on the basis of the pure-tone audiogram but also with respect to speech perception: the mean score for open sentence testing in quiet in the HA-only condition in the implanted ear was 23% 12 months after the first fitting versus 24% preoperatively.
It is the expectation that with growing experience in hearing preserving surgical techniques, with the described advances in electrode designs, with an increasing knowledge on the mechanisms of cochlear trauma, and with advances in inner ear drug treatment, the percentage of hearing preservation will further increase in the future.
Performance
In the first case report on EAS,50 a large benefit was observed when using CI and HA together, compared to each device alone. In the most extreme of several testing conditions in a sentence test, HA-only scores and CI-only scores were at 0%, whereas the combination of CI and HA (EAS condition) added up to a score of 58.3%. At the time, it was questioned whether these results were a singularity or if they represented a general pattern.
In the meantime, additive or synergistic effects between electric and acoustic stimulation have been confirmed in larger populations. Kiefer et al61 presented data on a study with 13 subjects, demonstrating large synergistic effects in EAS, especially in conditions with interfering background noise. In all patients, performance with the CI alone was already significantly above the results obtained preoperatively with hearing aids. Results in the CI-only condition were comparable with results in standard CI users using a similar device. In sentence tests in quiet, mean scores in the EAS condition were 8% higher (P < .05) than those in the CI-only condition. In sentence tests in noise, using a signal-to-noise ratio of 10 dB, results in the EAS condition were on average 23% higher (P < .01) than in the CI-only condition.
With regard to qualitative perception, Kiefer et al61 reported that patients were able to integrate both the acoustic and electric stimuli to obtain 1 impression of sound, even though they were able to distinguish the characteristics of EAS when presented separately. They also noted that subjects found the sound to be natural and helpful in identifying melodies compared to using a CI alone. Wilson et al62 presented data on 5 subjects and concluded that the biggest difference in data could be seen in the increase in EAS benefit with an increase in noise. There also appeared to be no evidence of any negative interaction between the 2 modes of stimulation. Brockmeier et al63 presented data on music perception using the Mu.S.I.C. Test, comparing unilateral CI users, EAS users, and normal hearing subjects. EAS users scored the same as normal hearing subjects and better than unilateral CI users in pitch, chord, and melody differentiation tasks.
As a general pattern, 2 effects can be observed in EAS users: speech in noise is—in many cases considerably—increased, and subjects report an improved sound quality and music appreciation.
Combined Speech Processor
While a substantial benefit was observed in the laboratory, and EAS users reported positively on the sound quality, another challenge emerged on a more practical level. To obtain combined stimulation, EAS patients were provided with a BTE CI speech processor and an in-the-ear (ITE) HA. After being provided with this combination, a portion of EAS users chose not to wear the HA. As a reason for abandoning the HA, they reported that handling 2 devices was too cumbersome. In addition, the devices needed different numbers of different types of hearing aid batteries with a different battery lifetime. In a few EAS subjects with relatively high pure-tone thresholds, the ITE HAs did not provide sufficient amplification in the low-frequency range. Soon after the start of the EAS project, it was clear that a combination device was necessary for the acceptance by EAS users.
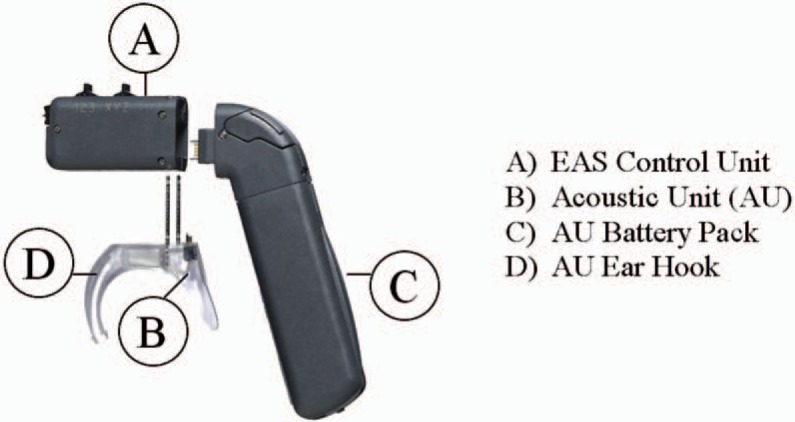
In November 2005, MED-EL introduced the first EAS combination device, the DUET EAS Hearing System, after 4 years of research and development (Figure 4). It combines the technology of the MED-EL TEMPO+ speech processor with a 2-channel digital hearing aid circuitry. The DUET features a common microphone but independent compression circuits to provide for the different compression requirements in electric and acoustic stimulation. The crossover frequency is adjustable for people with different degrees of low-frequency hearing loss.
Figure 4.
The DUET EAS Hearing System.
Helbig et al64 conducted a study of 10 EAS subjects, of whom 7 had rejected the HA for the above-mentioned reasons and wore CI only. The remaining 3 subjects used EAS. The subject's performance was measured before and after the fitting of the DUET and then after 2 months of DUET use. The subjects’ mean scores showed a significant increase in monosyllabic word test scores in quiet and in sentence test scores in noise at 10 dB, 5 dB, and 0 dB signal-to-noise ratio. The subgroup of subjects who had worn both a CI and HA showed equivalent speech recognition with the DUET and the CI plus HA. These users reported that the DUET allowed easier handling, better telephone use, more natural sound quality, and increased wearing comfort. Most important, the DUET was accepted by those users who had not used the HA before.
Summary
EAS has proven effective in candidates with considerable low-frequency hearing but no hearing in the high frequencies. Results show improved speech perception, especially in background noise, and better sound quality and music perception. A number of ongoing research projects look into further improving hearing preserving CI implantation, into the physiological and psychophysical mechanisms of EAS to determine what makes EAS so effective, and into aspects of optimally fitting CIs and HAs for combined use.
Progress in the Development of Bioactive CIs
MED-EL is concerned with future developments in cochlear implantation using drug delivery to further support the auditory system. Drug delivery might be a way of preventing further degeneration of the auditory system in individuals with hearing impairment, in addition to electrical stimulation. Drug delivery may also help to prevent tissue growth within the cochlea postoperatively to keep electrode impedances and thus power requirements low. This could be an important step toward further reducing the size and weight of CI systems.
In our research and development efforts, we have investigated a variety of techniques, with the aim of being able to provide solutions for the following 2 approaches:
specifically tailor a delivery system to drug/biologic and pathology requirements and
development of a multipurpose CI design incorporating a drug delivery option.
An approach to the latter are described as follows.
The Drug Delivery System
The cochlea is a delicate and highly structured organ offering its own unique risks and challenges. The development of a delivery system requires extensive multidisciplinary collaboration, with a full knowledge of cochlear anatomy and solute movement, and the following are essential criteria for the therapy: (1) there should be negligible risk of toxicity, allergic reaction, or infection; (2) there should be negligible risk to residual hearing or to balance function; (3) the delivery system should not impede or endanger the normal function of the implant; (4) the system must be acceptable to the patient; (5) the extended surgical procedure should pose negligible additional risk; (6) it must be possible to terminate the delivery of the drug quickly in the event of unacceptable side effects, without explanation of the electrode array; (7) the benefits of the treatment must be measurable and must outweigh all additional risks.
Such a delivery system requires careful preclinical evaluation to evaluate safety, tolerability, and performance. Garnham et al65 considered risks associated with a fluid-based delivery system in detail previously.
Previous experience with drug delivery to the inner ear is limited. Intraoperative deposition of corticosteroids in fluidic or crystal form at the cochleostomy have been used during cochlear implantation. One CI electrode design was modified to allow theoretical drug delivery. The system, however, may not provide a tested safe connection to an infusion pump.66 An earlier middle ear complete delivery system activated by the patient through subcutaneous pressure was also built and tested.67 The volume of the bolus in this delivery system is too high for inner ear application (25 μL). Round window delivery has been used in clinical practice but does not allow accurate dosage to the inner ear and tends to restrict treatment to the base of the cochlea.68 Furthermore, the round window permeability is poor for large molecules and variable for all drugs. It is not possible using this technique to provide further applications of drug at a later time. A subsequent delivery of drug postoperatively would require a surgical operation that would imperil the implanted electrode and the cochlea. Biodegradeable coatings may offer certain advantages (such as ease of use and spatially uniform delivery), but products of breakdown may be toxic, delivery rate is not precisely defined, and drug application cannot be terminated without explantation. Furthermore, each new drug approaching clinical evaluation requires full development of the coating in full collaboration with the CI manufacturer.
Prieskorn et al69 have demonstrated the feasibility of delivery of drugs, including proteins in solution, to the cochlea and modiolus through scala tympani application, using an implantable pump. The CI electrode array is inserted deep into the perilymph of the scala tympani. If used as a conduit, it offers the potential for safe delivery of an accurate and controllable dose of drug to the cochlea. Delivery of a drug in fluid form over a predefined time period allows for slow exposure of the cochlea to the drug, termination of delivery in the event of unacceptable side effects, and the possibility of a multipurpose delivery system designed to be suitable for a variety of drug treatments.
Design and Testing of a Hybrid CI With Perilymphatic Drug Delivery
The greatest difficulty in providing a CI electrode with a channel that can supply drugs to the perilymphatic space is ensuring safety for the patient. The inner ear communicates directly with the cerebrospinal fluid. Any bacterial infection around the implant or in the middle ear could be transmitted to the brain with serious consequences, such as meningitis. This section presents the design and testing of a CI able to receive fluid intra- and postoperatively, from a syringe or from a micro infusion pump.
Material and Methods
The CI for drug delivery consists of a modified commercial CI (MED-EL PULSARci100) and a custom made catheter linking the electrode channel to an infusion pump (not described here). The infusion pump could be an implantable device or an external body worn device.
The MED-EL PULSARci100 CI was converted to a dual system (neural stimulation + drug delivery) by modifying the internal aspect of the electrode and by adding a titanium micro port with a septum membrane at the header of the implant (Figure 5). The internal aspect of the electrode was modified by forming a 200 to 300 μm–diameter channel within the electrode lead and shaping 2 double outlets on the intracochlear portion of the electrode array. The 4 outlets are 50 μm in diameter and are designed to allow diffusion of medication in the whole basal turn of the cochlear. Apical regions of the cochlear spiral are expected to receive benefits of the medication though cross-turn diffusion. Cross-turn diffusion has been shown to take place in animal studies using neurotrophic factors.
Figure 5.
PULSARci100 with titanium port extending on the upper right corner of the implant.
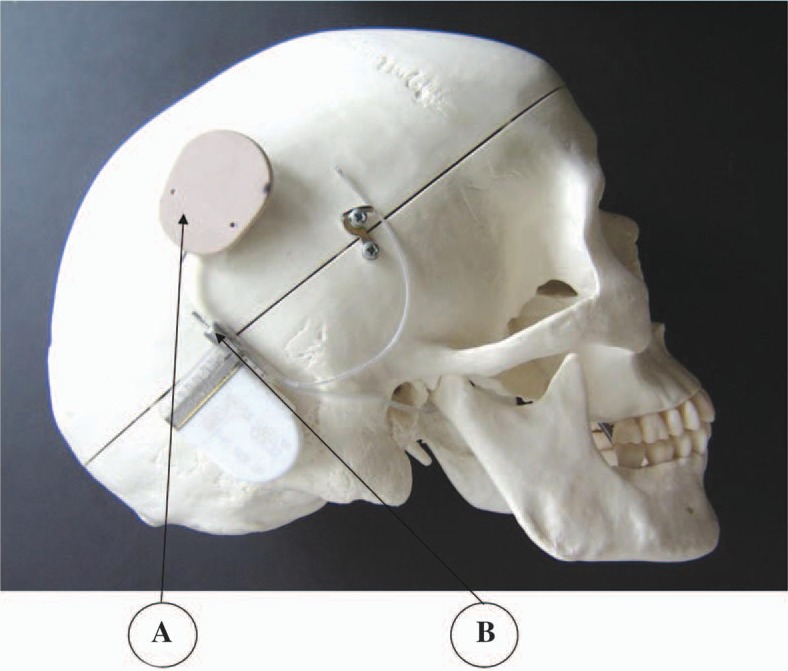
The micro port is a small metallic cavity made of biocompatible titanium 6 mm in length and 4 mm in diameter. The port is terminated on the superior and external side by a septum made of silicone. The septum silicone is compressed by 10%. Compression provides a self-sealing mechanism when a needle is inserted and removed from the septum. The inferior aspect of the port is connected to the electrode lead channel within the implant header. The CI, once assembled with the micro port, forms a single, standalone unit. The implant can be used without drug delivery or can be connected to an infusion pump at a later time through reopening of the skin. The implant can also be connected to a pump intraoperatively.
The connection to a putative infusion pump is made by penetrating the port's septum with a noncoring 30 G needle, which is assembled to a 2 mm outer diameter catheter. The internal diameter of the catheter is 0.5 mm. These 2 diameter dimensions ensure that the catheter will not collapse under skin pressure and block drug delivery. The needle is noncoring so that no material is taken from the septum silicone during penetration. The other end of the catheter is designed to fit snugly into the snout of the infusion pump outlet. The catheter provides the channel link between the electrode channel and the pump outlet while the micro port with septum provides the interface between the electrode channel and the pump (Figure 6). The interface can be connected, disconnected, and reconnected.
Figure 6.
Cochlear implant with port and septum connected to the model of an implantable pump. (A) Pump. (B) Port.
Testing
To evaluate the functionality and safety of the device, several tests were performed. One test included fluid flow and visualization, effect of air bubble, and fluid travel down to the small outlets. A second test evaluated the leak-proofness of the septum under accelerated life test conditions. The electrode was also evaluated histologically for insertion properties and trauma to cochlea structures.
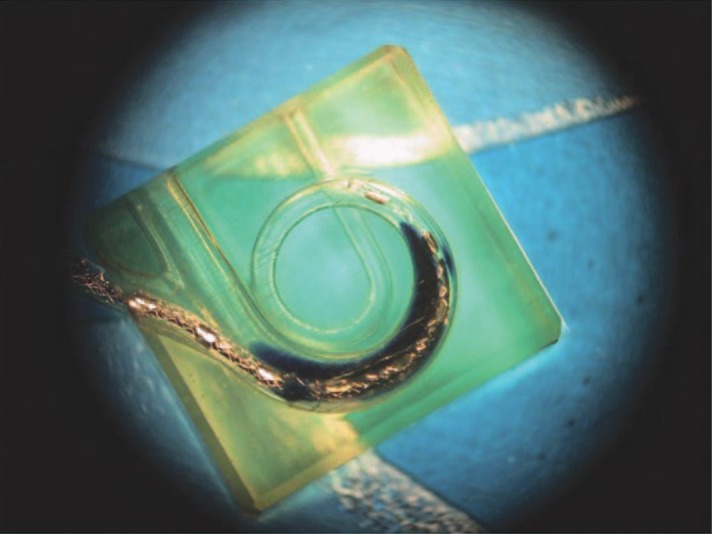
Fluid flow evaluation. Fluid flow was evaluated by connecting a laboratory pump (UltraMicroPump II; World Precision Instruments, Inc, Sarasota, Fla) with the CI. The catheter and pump were primed with an ink solution. The ink solution was infused into a saline bath and into a saline-filled 3-dimensional model of the scala tympani. Injection took place at 5 μL per hour. The main fluid delivery channel within the electrode and the 50 μ diameter outlets were visualized to assess diffusion of the ink solution (Figure 7). Some air bubbles were usually present within the catheter after connection and start of the pump. The air bubbles in the catheter, septum, and electrode channel dissolved within a few hours and did not prevent infusion. Diffusion of the ink solution took place slowly in the scala tympani model. The ink marker was more concentrated in the apex of the scala tympani model, which is narrower. The marker was more diluted in the basal region where the volume is larger. After stopping infusion, the solution in the scala tympani model was clear in less than 24 hours.
Figure 7.
Fluid flow into a scala tympani model.
Septum leak test. A major concern with drug delivery to the inner ear is the prevention of extracellular fluid infiltration into the channels of the delivery system. The drug delivery system should be leak proof to ensure the preservation of the sterile condition of the inner ear. Leakage should not take place during the drug delivery phases while the needle is going though the septum. Furthermore, no leakage of the septum should take place after removal of the needle. Leak-proofness should be guaranteed for the lifetime of the implanted device. A leak at any time, no matter how small, could facilitate the transport of bacteria to the inner ear. The septum was tested for a leak every month after immersion in saline for 8 months at 100°C, without agitation. Elevated temperature was applied to accelerate aging of the septums. Leakage was evaluated by applying an air pressure of up to 2.5 bar against the back end of the port (electrode side). The pressure was held for 2 minutes, and the presence of air bubbles on the septum side of the port was evaluated with a microscope focused on the septum. Pressures of 60 mb and 300 mb were also used to track transient leak behavior after septum needle removal. Leakage was defined as air bubbles coming out of the septum under the conditions mentioned above. In all test conditions, applying a pressure of 2.5 bar and below, there was no leakage of the septum through the perforation traces (Figure 8).
Figure 8.

Septum after 30 perforations and needle in place (right). After needle removal 3 weeks later, a small depression is visible on the septum. The device is leak proof at 2.5 bar pressure.
Histology
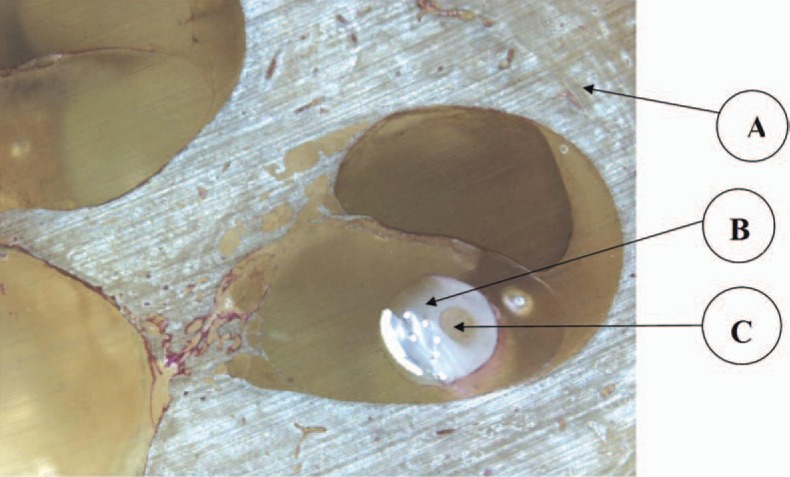
The electrode used in this study is a modification of a deep insertion electrode used with CI patients. The number of wires and contact has been reduced, but the number of auditory channels has been kept constant at 12. To keep the electrode as atraumatic as possible, the front end electrode is ultra thin. It is therefore not possible to build a drug delivery channel in the ultra thin portion of the electrode.
Five electrodes were inserted in human temporal bones and processed according to standard techniques (MED-EL Surgical Guide COMBI 40+ Cochlear Implant System, C40+ and PULSARci100 Implants, Rev 4.0, 2005) to evaluate the electrode in situ (Figure 9). The electrode was free fitting against the lateral wall. Damage to the cochlear structure was limited to elevation of the basilar membrane in some samples. Perforation and rupture of the basilar membrane in the base took place when the electrode was pushed beyond the point of the first resistance. The channel within the electrode was clearly visible in the histology sections. The electrode mechanical properties involved less force of insertion than a normal electrode used in cochlear implantation. This is due to the decreased surface of platinum used as well as the lesser number of wires. The profile of the electrode, however, remains the same. Trauma related to the force of electrode insertion is expected to be less since the force of insertion is expected to be lower. Other tests were performed. One test evaluated, in vivo, the proper opening of the drug delivery channel after a period of flow and flow interruption inside the guinea pig scala tympani. Flow was able to be reestablished. Another test evaluated biofilm formation around the depressions caused by the septum needle and into a chamber below the septum. Results are reported elsewhere.70 No biofilm formation took place when the septum was perforated with a 30 G needle and challenged with bacteria.
Figure 9.
Histology of a temporal bone showing the electrode with channel under the basilar membrane. This photography was provided by MED-EL and is used with permission from Dr Stöver and Professor Lenarz, Hannover, Germany. (A) Temporal bone. (B) Electrode. (C) Microchannel.
Advantages
The advantage of the micro port and septum fused to the implant is that the implant remains small and yet provides the necessary safety against infection. The implant stays small since only 5% of the volume has increased. The volume increase is absorbed in the form of a micro port protruding in the back of the implant. No Y branching is necessary to fuse the drug delivery option with the stimulating electrode. A Y branching adds additional risk for placement on the skull and does not solve the safe connection and disconnection between the drug delivery channel and the extracellular fluid.
Mode of Operation
The design of a CI with drug delivery presented in this article is designed for several modes of operation:
Single intraoperative bolus slowly injected in the cochlea. The volume should not exceed 20% to 30% of the scala tympani volume (about 10 μL), and injection speed should not cause additional damage to surviving hair cells.
Immediate connection to an infusion pump. The pump may be implantable and refillable through the skin. The pump could also be external, requiring additional precautions for septic preservation of catheter through the wound.
Connection to an infusion pump at a later date when powerful drugs are available for clinical trial. Connection postimplantation requires reopening the skin over the implant and placing a connecting catheter into the septum of the port. The other end of the catheter may be connected to an implantable or to an external pump.
The capability to connect, disconnect, and reconnect the CI to the infusion pump postoperatively is desirable. Reconnection of an infusion pump may be required for periodic treatment. The ability to access the inner ear when new and powerful drugs are approved for human use without having to change the implant or electrode is advantageous. Anticipated new treatments include gene therapy, stem cell therapy, and the addition of neural precursor cells; permanent and functional hair cell regeneration is the ultimate goal. The greatest challenge with connection, disconnection, and reconnection of the infusion pump to the implant is the safeguarding of a tight seal between the extracellular fluid and the perilymph.
Another major challenge is the long-term preservation of the functional channel within the electrode. The channel could become unusable if blockage took place at the fluid exit in the cochlea. Preliminary tests show that the channel outlets remain functional even after several weeks without flow. Blockage at the outlets by fibrous tissue growth can be overcome by the pump pressure. A strategy to preserve the greatest portion of the electrode channel for future use is necessary. Preservation entails the maintenance of a neutral liquid phase in the channel. Minimum protein adhesion to the walls of the lumen should take place. Anestomosis should not take place. It is conceivable to replace the drug delivery fluid occupying the lumen of the electrode with a neutral solution such as sterile saline or artificial perilymph. The neutral solution could incorporate antiemulsion/precipitation agents. The fluid replacement from the intracochlea outlets to the port chamber does not completely substitute drug residual with another fluid. However, a significant dilution takes place.
With the micro port and septum link, the connection to an infusion pump is greatly facilitated. The surgeon needs only to align the noncoring needle with the port and pierce through the septum silicone for a few millimeters. The titanium port prevents the needle from deviating. A simple suturing knot between the catheter and protruding port can prevent the noncoring needle from drifting away from the port. Disconnection takes place by cutting the suture and simply moving the needle catheter away from the implant. No plugging of a drug delivery channel is necessary since the septum is self-sealing immediately or shortly after needle removal. In addition, reconnection of the drug delivery channel situated inside the electrode array is possible anytime the back of the implant is exposed to show the port and septum.
Summary
A CI with drug delivery through a pump, catheter, port, and septum and electrode channel has been designed. The design was built and assembled, and several samples were fabricated. Testing indicates that clogging of the cannula is not an issue when pump pressure is high enough to disrupt encapsulation of the outlets. Leak-proofness is established.
Conclusion
In this article, we have described some of the research and development activities leading to the development of a new class of coding strategies aimed at providing more temporal fine structure information to CI users (FSP strategy) and to the development of a single device providing both electric and acoustic stimulation (DUET hearing system). Preliminary results of studies of the FSP strategy, available in the MED-EL MAESTRO, indicate better pitch perception when compared to the standard CIS+ strategy. The DUET hearing system is the first commercially available system to provide electric-acoustic stimulation via a single device. In the future, CIs might include drug delivery systems for further supporting the auditory system, in addition to electrical stimulation. We have described our ongoing research efforts in developing a CI with a drug delivery system.
References
- 1.Zeng FG. Trends in cochlear implants. Trends Amplif. 2004;8: 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Muller J, Schon F, et al. Comparison of the TEMPO+ ear-level speech processor and the CIS PRO+ body-worn processor in adult MED-EL cochlear implant users. ORL J Otorhinolaryngol Relat Spec. 2001;63: 31–40 [DOI] [PubMed] [Google Scholar]
- 3.Hilbert D. Grundzüge einer allgemeinen Theorie der linearen Integralgleichungen. Leipzig, Germany: Teubner; 1912 [Google Scholar]
- 4.Pickles JO. An Introduction to the Physiology of Hearing. 2nd ed. London, UK: Academic Press; 1988 [Google Scholar]
- 5.Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416: 87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stöbich B, Zierhofer C, Hochmair E. Influence of automatic gain control parameter settings on speech understanding of cochlear implant users employing the continuous interleaved sampling strategy. Ear Hear. 1999;20: 104–116 [DOI] [PubMed] [Google Scholar]
- 7.Wilson BS. Strategies for representing speech information with cochlear implants. In: Niparko JK, Kirk KI, Mellon NK, et al. eds. Cochlear Implants: Principles & Practices. Philadelphia, Pa: Lippincott Williams & Wilkins; 2000: 5–46 [Google Scholar]
- 8.Wong AO, Wong LL. Tone perception of Cantonese-speaking prelingually hearing-impaired children with cochlear implants. Otolaryngol Head Neck Surg. 2004;130: 751–758 [DOI] [PubMed] [Google Scholar]
- 9.Fu QJ, Chinchilla S, Galvin JJ. The role of spectral and temporal cues in voice gender discrimination by normal-hearing listeners and cochlear implant users. J Assoc Res Otolaryngol. 2004;5: 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott HJ. Music perception with cochlear implants: a review. Trends Amplif. 2004;8: 49–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zierhofer C. Electrical nerve stimulation based on channel-specific sequences. World patent WO 01/13991 A1. 2001 [Google Scholar]
- 12.Wilson BS, Lawson DT, Zerbi M, Finley CC. Virtual channels interleaved sampling (VCIS) processor. First Quarterly Progress Report. NIH Contract N01-DC-2-2401, Neural Prosthesis Program. Bethesda, Md: National Institutes of Health; 1992 [Google Scholar]
- 13.McDermott HJ, McKay CM. Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea. J Acoust Soc Am. 1994;96: 155–162 [DOI] [PubMed] [Google Scholar]
- 14.Mitterbacher A, Zierhofer C, Schatzer R, Kals M. Encoding fine time structure with channel specific sampling sequences. Paper presented at: Conference on Implantable Auditory Prosthesis; August 4, 2005; Pacific Grove, Calif. [Google Scholar]
- 15.Mitterbacher A, Zierhofer C, Schatzer R, et al. Pitch, fine structure and CSSS—results from patient tests. Paper presented at: British Cochlear Implant Group Academic Meeting; April 18–19, 2005; Birmingham, UK. [Google Scholar]
- 16.Schatzer R, Kals M, Zierhofer C. Encoding fine time structure with CSSS: concept and first results. Wien Med Wochenschr. 2006;156(suppl 119): 93–94 [Google Scholar]
- 17.Wilson B, Zerbi M, Finely C, Lawson D, van den Honert C. Relationships between temporal patterns of nerve activity and pitch judgments for cochlear implant patients. Eighth Quarterly Progress Report. NIH Contract N01-DC-5-2103, Neural Prosthesis Program. Bethesda, Md: National Institutes of Health; 1997 [Google Scholar]
- 18.Zeng FG. Temporal pitch in electric hearing. Hear Res. 2002;174: 101–106 [DOI] [PubMed] [Google Scholar]
- 19.Donaldson GS, Kreft HA. Place pitch discrimination of single versus dual electrode stimuli by cochlear implant users. J Acoust Soc Am. 2005;118: 623–626 [DOI] [PubMed] [Google Scholar]
- 20.Arsenault MD, Punch JT. Nonsense-syllable recognition in noise using monaural and binaural listening strategies. J Acoust Soc Am. 1999;105: 1821–1830 [DOI] [PubMed] [Google Scholar]
- 21.Bronkhorst AW, Plomp R. The effect of head-induced interaural time and level differences on speech intelligibility in noise. J Acoust Soc Am. 1988;83: 1508–1516 [DOI] [PubMed] [Google Scholar]
- 22.Bronkhorst AW, Plomp R. Binaural speech intelligibility in noise for hearing-impaired listeners. J Acoust Soc Am. 1989;86: 1374–1383 [DOI] [PubMed] [Google Scholar]
- 23.Carhart R. Monaural and binaural discrimination against competing sentences. Int Audiol. 1965;4: 5–10 [Google Scholar]
- 24.Cox RM, DeChicchis AR, Wark DJ. Demonstration of binaural advantage in audiometric test rooms. Ear Hear. 1981;2: 194–201 [DOI] [PubMed] [Google Scholar]
- 25.MacKeith NW, Coles RRA. Binaural advantages in hearing of speech. J Laryngol Otol. 1971;85: 213–232 [DOI] [PubMed] [Google Scholar]
- 26.Dillon H. Hearing Aids. Norwood, Australia: Boomerang Press; 2002 [Google Scholar]
- 27.Durlach NI, Colburn HS. Binaural phenomena. In: Carterette EC, Friedman MP. ed. Handbook of Perception. Vol 4. New York, NY: Academic Press; 1978: 365–466 [Google Scholar]
- 28.Byrne D. Clinical issues and options in binaural hearing aid fitting. Ear Hear. 1981;2: 187–193 [DOI] [PubMed] [Google Scholar]
- 29.Ricketts T, Lindley G, Henry P. Impact of compression and hearing aid style on directional hearing aid benefit and performance. Ear Hear. 2001;22: 348–361 [DOI] [PubMed] [Google Scholar]
- 30.Dermody P, Byrne D. Auditory localization by hearing-impaired persons using binaural in-the-ear hearing aids. Br J Audiol. 1975;9: 93–101 [Google Scholar]
- 31.Byrne D, Noble W, LePage B. Effects of long-term bilateral and unilateral fitting of different hearing aid types on the ability to locate sounds. J Acoust Soc Am. 1992;3: 369–382 [PubMed] [Google Scholar]
- 32.Green JD, Mills DM, Bell BA, Luxford WM, Tonokawa LL. Binaural cochlear implants. Am J Otol. 1992;6: 502–506 [PubMed] [Google Scholar]
- 33.Balkany T, Boggess W, Dinner B. Binaural cochlear implantation: comparison of 3M/House and Nucleus devices with evidence of sensory integration. Laryngoscope. 1988;98: 1040–1043 [DOI] [PubMed] [Google Scholar]
- 34.Pijl S. Single-channel versus bilateral multichannel cochlear implant results: a case report. Ear Hear. 1991;12: 431–433 [DOI] [PubMed] [Google Scholar]
- 35.van Hoesel RJM, Tong YC, Hollow RD, Clark GM. Psychophysical and speech perception studies: a case report on a binaural cochlear implant subject. J Acoust Soc Am. 1993;94: 3178–3189 [DOI] [PubMed] [Google Scholar]
- 36.Müller J, Schön F, Helms J. Speech understanding in quiet and noise in bilateral users of the MED-EL COMBI 40/40+ cochlear implant system. Ear Hear. 2002;23: 198–206 [DOI] [PubMed] [Google Scholar]
- 37.Schön F, Müller J, Helms J. Speech reception thresholds obtained in a symmetrical four-loudspeaker arrangement from bilateral users of MED-EL cochlear implants. Otol Neurotol. 2002;23: 710–714 [DOI] [PubMed] [Google Scholar]
- 38.Schleich P, Nopp P, D'Haese P. Head shadow, squelch and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant. Ear Hear. 2004;25: 197–204 [DOI] [PubMed] [Google Scholar]
- 39.Au D, Hui Y, Wei W. Superiority of bilateral cochlear implantation over unilateral cochlear implantation in tone discrimination in Chinese patients. Am J Otolaryngol. 2003;24: 19–23 [DOI] [PubMed] [Google Scholar]
- 40.Kong W, Yu L, Xu Y, et al. Benefit of bilateral cochlear implantation in congenital prelingually deafened Chinese-speaking children [in Chinese]. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2003;17: 577–579 [PubMed] [Google Scholar]
- 41.Nopp P, Schleich P, D'Haese P. Sound localization in bilateral users of MED-EL COMBI 40/40+ cochlear implants. Ear Hear. 2004;25: 205–214 [DOI] [PubMed] [Google Scholar]
- 42.Schoen F, Mueller J, Helms J, Nopp P. Sound localization and sensitivity to inter-aural cues in bilateral users of the MED-EL COMBI 40/40+ cochlear implant system. Otol Neurotol. 2005;26: 429–437 [DOI] [PubMed] [Google Scholar]
- 43.Senn P, Kompis M, Vischer M, Häusler R. Minimum audible angle, just noticeable interaural differences and speech intelligibility with bilateral cochlear implants using clinical speech processors. Audiol Neurootol. 2005;10: 342–352 [DOI] [PubMed] [Google Scholar]
- 44.Kühn-Inacker H, Shehata-Dieler W, Müller J, Helms J. Bilateral cochlear implants: a way to optimize auditory perception abilities in deaf children? Int J Ped Otorhinolaryngol. 2004;68: 1257–1266 [DOI] [PubMed] [Google Scholar]
- 45.Lawson D, Wilson B, Zerbi M, Finley C. Speech processors for auditory prostheses. Fifth Quarterly Progress Report. NIH Project N01-DC-5-2103. Bethesda, Md: Neural Prosthesis Program, National Institutes of Health; 1996 [Google Scholar]
- 46.Lawson D, Zerbi M, Wilson B. Speech processors for auditory prostheses. First Quarterly Progress Report. NIH Project N01-DC-8-2105. Bethesda, Md: Neural Prosthesis Program, National Institutes of Health; 1998 [Google Scholar]
- 47.Laback B, Pok M, Baumgartner W, Deutsch W, Schmid K. Sensitivity to interaural level and envelope time differences of two bilateral cochlear implant listeners using clinical sound processors. Ear Hear. 2004;25: 488–500 [DOI] [PubMed] [Google Scholar]
- 48.Laback B, Majdak P, Baumgartner WD. Fine structure and gating interuaral time differences in electrical and acoustical hearing: effect of stimulus duration. Paper presented at: Conference on Implantable Auditory Prosthesis; August 4, 2005; Pacific Grove, Calif. [Google Scholar]
- 49.Majdak P, Laback B, Baumgartner WD. Interaural time differences in fine structure and envelope in bilateral electrical and acoustical hearing. Paper presented at: Conference on Implantable Auditory Prosthesis; August 4, 2005; Pacific Grove, Calif. [Google Scholar]
- 50.von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system. ORL J Otorhinolaryngol Relat Spec. 1999;61: 334–340 [DOI] [PubMed] [Google Scholar]
- 51.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41: 356–359 [PubMed] [Google Scholar]
- 52.Kiefer J, Gstoettner W, Baumgartner W, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124: 272–280 [DOI] [PubMed] [Google Scholar]
- 53.Skarzynski H, Lorens A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9(4): CS20–CS24 [PubMed] [Google Scholar]
- 54.Skarzynski H, Lorens A, Piotrowska A, Anderson I. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol. In press. [DOI] [PubMed] [Google Scholar]
- 55.Adunka O, Unkelbach MC, Hambeck M, Gstöttner W, Kiefer J. Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: a histologically controlled study. Acta Otolaryngol. 2004;124: 1–6 [DOI] [PubMed] [Google Scholar]
- 56.Roland PS, Gstöttner W, Adunka O. Method for hearing preservation in cochlear implant surgery. Oper Tech Otolaryngol. 2005;16: 93–100 [Google Scholar]
- 57.Gstoettner W, Kiefer J, Baumgartner W, Pok SM, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124: 348–352 [DOI] [PubMed] [Google Scholar]
- 58.Adunka O, Kiefer J, Unkelbach MH, Lehnert T, Gstoettner W. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope. 2004;114: 1237–1241 [DOI] [PubMed] [Google Scholar]
- 59.Gstoettner W, Helbig S, Maier N, Kiefer J, Radeloff A, Adunka O. Ipsilateral electric acoustic stimulation of the auditory system: long-term preservation of acoustic hearing. Audiol Neurootol. In press. [DOI] [PubMed] [Google Scholar]
- 60.Gstoettner W, Van de Heyning P, O'Connor AF, Morera C, Sainz M. Results from a multi-centre EAS clinical investigation. Wien Med Wochenschr. 2006;156(suppl 119): 121 [Google Scholar]
- 61.Kiefer J, Pok M, Adunka O, et al. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol. 2005;10: 134–144 [DOI] [PubMed] [Google Scholar]
- 62.Wilson B, Wolfort R, Lawson D, Schatzer R. Additional perspectives on speech reception with combined electric and acoustic stimulation, speech processors for auditory prostheses. Third Quarterly Progress Report. NIH Project N01-DC-2-1002. Bethesda, Md: National Institutes of Health; 2002 [Google Scholar]
- 63.Brockmeier SJ, Vermeire K, Grasmeder M, et al. Music perception of different CI users and comparison to normal hearing subjects as assessed in the Mu.S.I.C. Test. Wien Med Wochenschr. 2006;156(suppl 119): 10216699941 [Google Scholar]
- 64.Helbig S, Unkelbach M, Maier N, et al. Preliminary results with the new DUET in EAS users. Wien Med Wochenschr. 2006;156(suppl 119): 126 [Google Scholar]
- 65.Garnham C, Reetz G, Jolly C, Miller J, Salt A, Beal F. Drug delivery to the cochlea after implantation: consideration of the risk factors. Cochlear Implants International. 2005;6(suppl 1): 12–14 [DOI] [PubMed] [Google Scholar]
- 66.Paasche G, Bogel L, Leinung M, Lenarz Y, Stover T. Substance distribution in a cochlea model using different pump rates for cochlear implant drug delivery electrode prototypes. Hear Res. 2006;212: 74–82 [DOI] [PubMed] [Google Scholar]
- 67.Praetorius M, Limberger A, Muller M, et al. A novel microperfusion system for the long-term local supply of drugs to the inner ear: implantation and function in the rat model. Audiol Neurootol. 2001;6: 250–258 [DOI] [PubMed] [Google Scholar]
- 68.Salt A, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154: 88–97 [DOI] [PubMed] [Google Scholar]
- 69.Prieskorn D, Miller J. Technical report: chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140: 212–215 [DOI] [PubMed] [Google Scholar]
- 70.Johnson T, Loeffler K, Jolly CN, Burne RA, Antonelli PJ. Biofilm formation in cochlear implants with cochlear drug delivery channels. Wien Med Wochenshr. 2006;156(suppl 119): 86–87 [Google Scholar]