Abstract
Background and Aims
Several studies show apparently contradictory findings about the functional convergence within the Mediterranean woody flora. In this context, this study evaluates the variability of functional traits within holm oak (Quercus ilex) to elucidate whether provenances corresponding to different morphotypes represent different ecotypes locally adapted to the prevaling stress levels.
Methods
Several morphological and physiological traits were measured at leaf and shoot levels in 9-year-old seedlings of seven Q. ilex provenances including all recognized morphotypes. Plants were grown in a common garden for 9 years under the same environmental conditions to avoid possible biases due to site-specific characteristics.
Key Results
Leaf morphometry clearly separates holm oak provenances into ‘ilex’ (more elongated leaves with low vein density) and ‘rotundifolia’ (short and rounded leaves with high vein density) morphotypes. Moreover, these morphotypes represent two consistent and very contrasting functional types in response to dry climates, mainly in terms of leaf area, major vein density, leaf specific conductivity, resistance to drought-induced cavitation and turgor loss point.
Conclusions
The ‘ilex’ and ‘rotundifolia’ morphotypes correspond to different ecotypes as inferred from their contrasting functional traits. To the best of our knowledge, this is the first time that the combined use of morphological and physiological traits has provided support for the concept of these two holm oak morphotypes being regarded as two different species.
Keywords: Mediterranean vegetation, Quercus ilex, holm oak, ‘ilex’ morphotype, ‘rotundifolia’ morphotype, functional traits, palaeobotany, ecotype
INTRODUCTION
The growth and distribution of plant species subjected to Mediterranean climate conditions are mainly constrained by summer drought (Corcuera et al., 2002, 2004, 2005; Mediavilla and Escudero, 2003) in adddition to other limiting factors (Nardini et al., 1998). The Mediterranean climate and the existence of evergreen vegetation dominated by woody plant species with small, thickened and narrow leaves, i.e. sclerophyllous species (Traiser et al., 2004), have been historically bound together by botanists (Braun-Blanquet and Bolòs, 1957; Di Castri, 1981; Breckle, 2002). These leaf features define a physiognomic group (Traiser et al., 2004), which has been also considered a homogeneous functional group as the consequence of an evolutionary convergence in response to a common climate (Kummerov, 1973; Cody and Mooney, 1978).
The morphological and physiological traits of Mediterranean vegetation have been interpreted by some authors as a way to withstand summer drought (Mediavilla and Escudero, 2003; Corcuera et al., 2005). For instance, a reduced leaf area has been proposed as one of the key traits that allow Mediterranean oaks to withstand water deficit (Baldocchi and Xu, 2007). Others report common functional traits among Mediterranean woody plants, in terms of both cell wall elasticity (Corcuera et al., 2002) and xylem resistance to drought-induced embolism (Maherali et al., 2004).
However, many researchers have not found functional convergence within this vegetation type (Lo Gullo and Salleo, 1988; Salleo and Lo Gullo, 1990; Martínez-Vilalta et al., 2003; Peguero-Pina et al., 2009). Thus, no clear association was observed between increased sclerophylly and drier conditions (Salleo and Nardini, 2000; Ogaya and Peñuelas, 2006). In fact, sclerophylly originated in the tropical vegetation of the Tertiary, prior to the origin of Mediterranean climate and vegetation in the late Pliocene (Palamarev, 1989; Herrera, 1992; Verdú et al., 2003). Therefore, the geological age of traits is relevant here since plant species which evolved under Quaternary Mediterranean conditions show higher sclerophylly and drought resistance at the leaf level than Tertiary pre-Mediterranean taxa (Paula and Pausas, 2006; Hernández et al., 2011).
These apparently contradictory findings observed in the evergreen sclerophyllous Mediterranean woody flora may be partially explained by considering the evolution of taxa and their process of adaptation to the environment. Here, we use this framework to evaluate the morphological and physiological variability of holm oak (Quercus ilex) populations encompassing the distribution of the species across the Western Mediterranean Basin. Holm oak is a keystone Circum-Mediterranean tree species which displays a huge morphological (Gratani, 1996; Ogaya and Peñuelas, 2007) and ecophysiological variability, at least in terms of xylem resistance to drought-induced embolism (Lo Gullo and Salleo, 1993; Tyree and Cochard, 1996; Corcuera et al., 2004; Limousin et al., 2010).
Morphological variability within holm oak provenances has been reflected by some botanists who consider the existence of two subspecies (Amaral-Franco, 1990) or even two different species (Govaerts and Frodin, 1998). Michaud et al. (1995) and Lumaret et al. (2002) defined three different morphotypes for holm oak: (1) the ‘ilex’ morphotype, corresponding to those populations located from Greece to the French Riviera and also found along the Atlantic coast of France, showing elongated and large leaves and being restricted to humid or sub-humid sites mainly in mild coastal areas; (2) the ‘rotundifolia’ morphotype, characterized by individuals showing small and rounded thick leaves and dominating inland areas under more continental and dry variations of the Mediterranean climate (in northern Africa and the Iberian Peninsula); and (3) an ‘intermediate’ morphotype for individuals with intermediate characteristics and dominating coastal areas of eastern Spain and south-eastern France. The current ecological conditions in the main distribution areas for the first two morphotypes could explain the existence of different functional types in response to drought stress: the less tolerant ‘ilex’ morphotype and the more tolerant ‘rotundifolia’ morphotype (Corcuera et al., 2004).
Genetic variability among Q. ilex populations could explain this morphological variability at a large biogeographic scale where diverse climatic conditions occur (Michaud et al., 1995; Lumaret et al., 2002). However, the question remains open of whether Mediterranean holm oak provenances corresponding to different morphotypes represent true functionally different ecotypes. We aim to answer this question by quantifying several morphological and physiological traits in seedlings of seven Q. ilex provenances including different morphotypes. To avoid possible biases due to site-specific environmental characteristics, plants were grown in a common garden under the same environmental conditions.
MATERIALS AND METHODS
Plant material and provenances
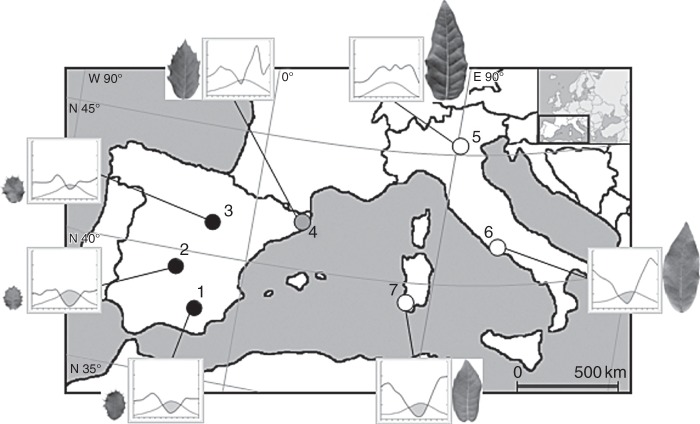
Holm oak (Quercus ilex L.) seeds from seven provenances located in Spain and Italy (Fig. 1, Table 1) were sown and cultivated in 2003 under the same conditions (mixture of 80 % sub-stratum and 20 % perlite in 500 mL containers) inside a greenhouse. After the first growth cycle, the seedlings were transplanted to 25 L containers and after 2 years they were again transplanted to a field plot located at CITA de Aragón (41°39′N, 0°52′W, Zaragoza, Spain; mean annual temperature 15·4 °C, total annual precipitation 298 mm). All plants were grown under the same environmental conditions and drip irrigated every 2 d. Morphological and physiological measurements were performed during 2012 in 9-year-old seedlings. A previous classification of the provenances was made according to the morphotypes defined by Lumaret et al. (2002): ‘ilex’ morphotype for the Italian Veneto, Lazio and Sardinia provenances; ‘rotundifolia’ morphotype for the Spanish Cazorla, Ciudad Real and Soria provenances; and ‘intermediate’ morphotype for the Spanish Gerona provenance.
Fig. 1.
Geographical location in the Western Mediterranean Basin and climate diagrams (grey areas indicate dry periods; left and right y-axes correspond to mean temperature and total precipitation, respectively) of the seven studied Q. ilex provenances (provenance codes are as in Table 1). A photograph of a representative leaf of each studied provenance has also been included.
Table 1.
Geographical and climatic characteristics of each Q. ilex provenance
| Provenance (code) | Country | Morphotype | Latitude | Longitude | Elevation (m a.s.l.) | P (mm) | Ps (mm) | T (°C) | Ts (°C) | MAI |
|---|---|---|---|---|---|---|---|---|---|---|
| Cazorla (1) | Spain | rotundifolia | 38°06′N | 02°33′W | 1236 | 532 | 61 | 12·2 | 21·1 | 24 |
| Ciudad Real (2) | Spain | rotundifolia | 39°27′N | 04°24′W | 724 | 443 | 42 | 14·0 | 23·3 | 18 |
| Soria (3) | Spain | rotundifolia | 41°46′N | 02°29′W | 1074 | 570 | 63 | 10·4 | 18·5 | 28 |
| Gerona (4) | Spain | intermediate | 42°03′N | 02°46′E | 177 | 936 | 168 | 14·9 | 22·4 | 38 |
| Veneto (5) | Italy | ilex | 45°44′N | 10°48′E | 617 | 833 | 250 | 10·1 | 19·1 | 41 |
| Lazio (6) | Italy | ilex | 41°13′N | 13°03′E | 29 | 904 | 97 | 15·5 | 22·2 | 36 |
| Sardinia (7) | Italy | ilex | 39°21′N | 08°34′E | 627 | 784 | 65 | 13·5 | 20·7 | 33 |
P, annual precipitation; Ps, summer precipitation; T, mean annual temperature; Ts, mean summer temperature; MAI, Martonne aridity index.
Each provenance region has contrasting climatic conditions. The Italian regions and Gerona have annual and summer precipitation values (P and Ps, respectively) higher than the other regions (Table 1). In contrast, the mean annual and summer temperatures (T and Ts, respectively) are very similar among all provenances (Table 1). As a consequence, the Martonne aridity index [MAI = P/(T + 10)] is also higher in the Italian and Gerona provenances (Table 1). In addition, all regions except Veneto are characterized by a summer drought period and a humid winter, which is typical of the Mediterranean climate (see climate diagrams in Fig. 1). Climatic information was obtained using the WorldClim database (http://www.worldclim.org/).
Morphological variables
Quantitative and qualitative morphological traits were analysed in 30 leaves sampled from ten individuals per provenance, i.e. three leaves were randomly taken from each individual. Specifically, the lamina length and width and the length of petioles were measured. Length–width ratios were obtained to characterize the leaf shape (elliptic, ovate or orbiculate) (Hickey, 1973). In addition, the patterns of leaf apexes, margin, teeth, venation and course of secondary veins were described following the categories of leaf architecture of dicotyledonous angiosperms proposed by Ash et al. (1999) and adapted to our plant material. Leaf apexes were classified into sharply pointed, pointed–acute and rounded–truncate. Leaf margin was classified into entire (smooth, without teeth), serrate (teeth pointed with their axes inclined to the trend of the leaf margin) and partially serrate (teeth not present in the whole margin). Teeth were classified into sharply pointed, not sharply pointed and no teeth, according to the shape of the tooth apex as previously described for the leaf apex. Leaf venation was classified as weak brochidodromous, craspedodromous and mixed craspedodromous. The course of secondary veins was classified into straight, curved and mixed, according to their trajectory from the origin in the midrib to the end.
Leaf thickness (LT), individual leaf area (LA) and leaf mass area (LMA) were measured in 30 mature leaves (again three leaves were taken per individual) per provenance. The LT was determined using a GTH10L digital contact sensor coupled to a GT-75AP amplifier (GT Series, Keyence Co., Japan) that applies a clamp pressure of 7 kPa to the leaf surface (Sancho-Knapik et al., 2011). The LA was measured by digitalizing the leaves and using the ImageJ image analysis software (http://rsb.info.nih.gov/nih-image/). Leaves were then oven dried at 70 °C for 3 d, to determine their dry weight. The LMA was calculated as the ratio of the foliage dry weight to foliage area, and was used as an estimator of sclerophylly (Corcuera et al., 2002). Major vein density (MVD) was determined in another set of ten mature leaves per provenance following the method described in Scoffoni et al. (2011) with some modifications. Leaves were chemically cleared with 5 % NaOH in aqueous solution, washed with bleach solution, dehydrated in an ethanol dilution series (70, 90, 95 and 100 %) and stained with safranin. Then, leaves were scanned at 1200 dpi resolution and the leaf area and lengths of first-, second- and third-order veins were measured using the ImageJ software. Vein densities for each order were calculated as the vein length/leaf area ratio. The MVD was then obtained as the sum of the first-, second- and third-order vein densities. Finally, the leaf area ratio (LAR) was calculated in ten current-year shoots per provenance by dividing the total leaf area (measured as described above) by the dry weight of the shoot.
Pressure–volume analysis
Pressure–volume relationships were determined in six leaves per provenance following the free transpiration method described in previous studies (Corcuera et al., 2002; Brodribb and Holbrook, 2003). The water relations parameters analysed were the leaf water potential at the turgor loss point (ΨTLP), the relative water content at the turgor loss point (RWCTLP), the osmotic potential at full turgor (Πo) and the maximum bulk modulus of elasticity (ϵmax).
Xylem embolism and hydraulic conductivity
Vulnerability to embolism was measured in current-year twigs by constructing vulnerability curves using the dehydration method (Tyree and Sperry, 1989). First, we collected 10–12 large branches (>1 m long) from 10–12 different individuals of each provenance and they were allowed to dehydrate in the lab. Secondly, to measure xylem conductivity and vulnerability to cavitation, five current-year twigs in each branch were collected in which water potential had previously been measured. Previously, each branch was enclosed in a black bag for water potential stabilization. The twigs were cut underwater into segments of 30–50 mm in length, and both ends were shaved with a razor blade. The segments were placed in a tubing manifold and immediately connected to a Xyl'em apparatus (Bronkhorst, Montigny-les-Cormeilles, France). This device measures the percentage loss of hydraulic conductance due to the presence of air-filled conduits in stem segments (Cochard et al., 2002). The manifold with the twigs was immersed in distilled water to prevent desiccation and to maintain a near constant temperature. The segments were perfused with degassed water with 10 mm KCl and 1 mm CaCl2 filtered to 0·2 mm (see Cochard et al., 2010). Sample percentage loss of conductivity (PLC) was computed as:
| (1) |
where K is the initial hydraulic conductivity of the twig measured at low pressure and Kmax is the maximum hydraulic conductivity determined after removing the air embolisms. The Kmax was achieved after short perfusions at 0·15 MPa for 60–90 s. The PLC was plotted as a function of water potential for each species to obtain a vulnerability curve. Pairs of data were adjusted by using a sigmoidal function according to Pammenter and Van der Willigen (1998):
| (2) |
where Ψ is the water potential and the a and b coefficients are the slope of the regression and the 50 % loss of hydraulic conductivity (PLC50), respectively.
The same twig segments were measured in length, diameter without bark, and leaf surface area supplied, to compute the main hydraulic architecture parameters, namely specific conductivity (Ks) as the hydraulic conductivity on a sapwood area basis, and leaf specific conductivity (LSC) as the hydraulic conductivity on a leaf area basis.
Statistical analyses
To compare leaf functional traits among provenances, one-way analyses of variance (ANOVAs) were performed after log-transforming the variables to reach normality. Multiple comparisons were carried out among provenances for the morphological and physiological variables using the post-hoc Tukey's honestly significant difference test. To compare vulnerability to cavitation among provenances, an analysis of covariance of vulnerability curves was performed (Underwood, 1997).
To summarize the multivariate relationships among morphometric variables and physiological traits of Q. ilex provenances, we performed a two principal components analysis (PCA). Based on previous analyses, we selected those leaf shape variables which: (1) accounted for more variance in the first two principal components of the PCA and (2) were not redundant (multicolinear) between them. Then, we calculated the PCA based on a correlation matrix using the Spearman rank correlation coefficient and the selected variables. Categorical variables related to leaf shape (form, teeth types, etc.) were transformed into dummy variables. The statistical analyses were performed with the R statistical suite version 3.0 (R Development Core Team, 2013).
RESULTS
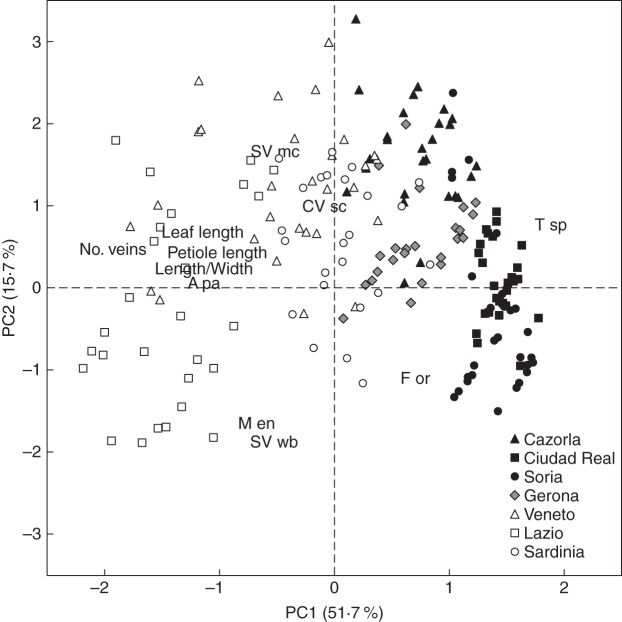
The study of leaf morphometry revealed the existence of significant differences among the studied holm oak provenances, in both quantitative and qualitative terms (Table 2). A PCA biplot based on leaf morphometry indicated that the first and second axes accounted for 52 and 16 % of the total variation, respectively (Fig. 2). We detected segregation between the ‘rotundifolia’ and the ‘ilex’ morphotypes in terms of leaf morphometric parameters. Provenances of the ‘rotundifolia’ morphotype (e.g. Soria, Ciudad Real and Cazorla) showed significantly shorter leaves than ‘ilex’ morphotypes (e.g. Veneto and Lazio provenances). The large differences in leaf length that can occur between the extremes studied here must be highlighted; the leaves of Lazio plants, for instance, are more than three times longer than those of Soria plants. Leaves of the ‘ilex’ morphotypes are not only longer but also clearly more lengthened (higher length/width ratio) and with a higher number of pairs of secondary veins than the leaves of the ‘rotundifolia’ morphotype. Finally, the petiole was also longer in the ‘ilex’ than in the ‘rotundifolia’ morphotype. The Gerona provenance, representing the ‘intermediate’ morphotype, showed quantitative variables closer to the ‘rotundifolia’ morphotype, except for the leaf length, with values more similar to those measured in the provenances included within the ‘ilex’ morphotype. It should be noted that the petiole length of the Gerona provenance was particularly short with respect to its relatively long leaf.
Table 2.
Leaf morphological traits for the seven studied Q. ilex provenances (the numbers in parentheses correspond to the site codes used in Table 1 and Fig. 1)
| Variable | Categories* | Cazorla (1) | Ciudad Real (2) | Soria (3) | Gerona (4) | Veneto (5) | Lazio (6) | Sardinia (7) |
|---|---|---|---|---|---|---|---|---|
| Leaf length (cm) | – | 2·5 ± 0·1a | 1·9 ± 0·0b | 1·7 ± 0·1b | 4·0 ± 0·1c | 5·1 ± 0·3d | 6·1 ± 0·1e | 4·3 ± 0·1c |
| Leaf length/width ratio | – | 1·6 ± 0·1a | 1·6 ± 0·1a | 1·2 ± 0·1b | 1·7 ± 0·1a | 2·1 ± 0·1c | 2·3 ± 0·0d | 2·1 ± 0·1c |
| No. of pairs of secondary veins | – | 8·2 ± 0·2a | 5·2 ± 0·1b | 5·0 ± 0·2b | 6·6 ± 0·1c | 9·6 ± 0·2d | 10·1 ± 0·2d | 8·0 ± 0·2a |
| Petiole length (mm) | – | 3·7 ± 0·2ab | 2·6 ± 0·1c | 3·1 ± 0·2ac | 3·8 ± 0·2b | 6·6 ± 0·3d | 10·0 ± 0·3e | 5·6 ± 0·2f |
| Shape (%) | F e | 79 | 67 | 31 | 56 | 69 | 96 | 76 |
| F ov | 18 | 7 | 3 | 40 | 31 | 4 | 13 | |
| F or | 4 | 27 | 66 | 4 | 0 | 0 | 12 | |
| Margin (%) | M en | 14 | 40 | 37 | 44 | 79 | 43 | 76 |
| M pse | 0 | 20 | 7 | 0 | 7 | 36 | 24 | |
| M se | 86 | 40 | 57 | 56 | 14 | 21 | 0 | |
| Teeth (%) | T sp | 86 | 53 | 87 | 100 | 14 | 0 | 44 |
| T nsp | 14 | 27 | 7 | 0 | 79 | 64 | 32 | |
| T nt | 0 | 20 | 7 | 0 | 7 | 36 | 24 | |
| Apex (%) | A sp | 82 | 47 | 20 | 100 | 0 | 0 | 36 |
| A pa | 0 | 33 | 53 | 0 | 100 | 100 | 60 | |
| A rt | 18 | 20 | 27 | 0 | 0 | 0 | 4 | |
| Course of secondary veins (%) | CV s | 18 | 33 | 17 | 40 | 38 | 43 | 40 |
| CV c | 82 | 50 | 77 | 60 | 59 | 21 | 56 | |
| CV sc | 0 | 17 | 7 | 0 | 3 | 36 | 4 | |
| Secondary venation (%) | SV wb | 0 | 20 | 7 | 0 | 7 | 36 | 24 |
| SV mc | 43 | 40 | 37 | 44 | 76 | 64 | 76 | |
| SV cr | 57 | 40 | 57 | 56 | 17 | 0 | 0 |
Values of quantitative traits are shown as means ± s.e. Different letters indicate significant differences among provenances (Tukey test, P < 0·05).
Qualitative traits are expressed as percentage of leaves included in each category. Variables corresponding to leaf shape: elliptic (F e), ovate (F ov) and orbiculate (F or) leaf; entire (M en), partially serrate (M pse) and serrate (M se) margin types; sharply pointed (T sp) or not sharply pointed (T nsp) teeth and no teeth (T nt); sharply pointed (A sp), pointed–acute (A pa) and rounded–truncate (A rt) apex shape; straight (CV s), curved (CV c) and straight–curved (CV sc) secondary veins; weak brochidodromous (SV wb), mixed craspedodromous (SV mc) and craspedodromous (SV cr) secondary venation.
Categories are shown only in the case of categorical variables (qualitative traits).
Fig. 2.
Relationships between the first two principal components (PC1 vs. PC2) of the principal component analysis (PCA) computed on leaf morphometric variables of the seven Q. ilex provenances. Selected leaf variables were the most robust to separate provenances: leaf length, petiole length, length/width ratio, number of secondary veins (No. veins), apex pointed (A pa), margin entire (M en), teeth sharply pointed (T sp), secondary venation mixed craspedodromous and weak brochidodromous (SV mc and SV wb), course of secondary veins straight–curved (CV sc) and form orbiculate (F or). The symbols correspond to each plant and are colour-coded according to each morphotype: black, ‘rotundifolia’; grey, ‘intermediate’; white, ‘ilex’.
On the other hand, regarding qualitative parameters, the most discriminating variables were the types of margin, teeth and secondary venation (Table 2). A serrate margin, sharply pointed teeth and craspedodromous secondary venation were predominant in ‘rotundifolia’ and ‘intermediate’ morphotypes. In contrast, ‘ilex’ morphotypes showed a higher proportion of leaves with an entire margin, with teeth that were not sharply pointed, and weak brochidodromous or mixed craspedodromous secondary venations. An elliptic shape was more common, but not exclusive, among leaves of the ‘ilex’ morphotype, while orbiculate leaves were mostly present in Ciudad Real and Soria provenances of the ‘rotundifolia’ morphotype.
The LA, total leaf area per shoot and LAR of Q. ilex provenances included in the ‘rotundifolia’ morphotype were lower than those found for the ‘intermediate’ and ‘ilex’ morphotypes (Figs 3A, 4B and C, respectively). In contrast, MVD was significantly higher for the ‘rotundifolia’ morphotype (Fig. 3B). The number of leaves per shoot was slightly higher for the ‘rotundifolia’ morphotype than for the others (Fig. 4A). All provenances showed similar values of LT and LMA (data not shown).
Fig. 3.
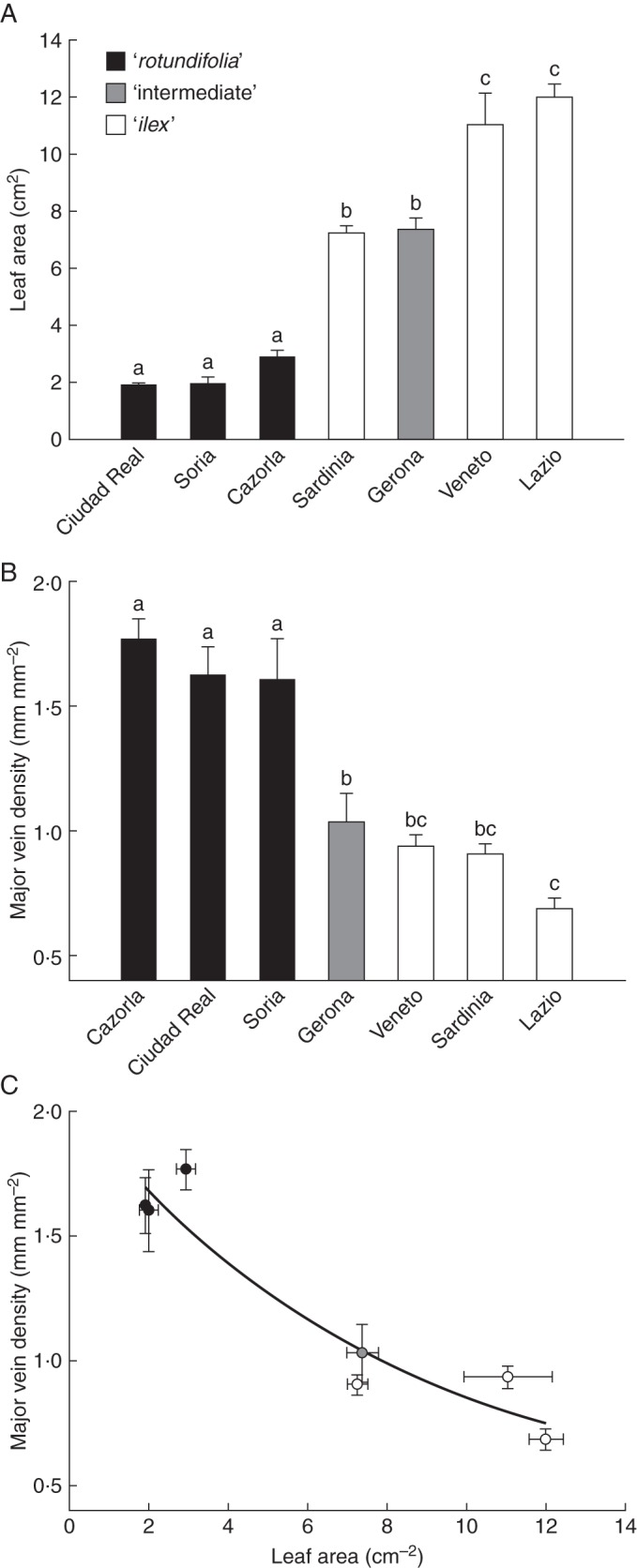
Leaf area (A), major vein density (B) and the relationship observed between both parameters (C) for the seven studied Q. ilex provenances. Data are means ± s.e. The ‘rotundifolia’, ‘intermediate’ and ‘ilex’ morphotypes are indicated in the key. Different letters indicate significant differences among provenances (Tukey test, P < 0·05).
Fig. 4.
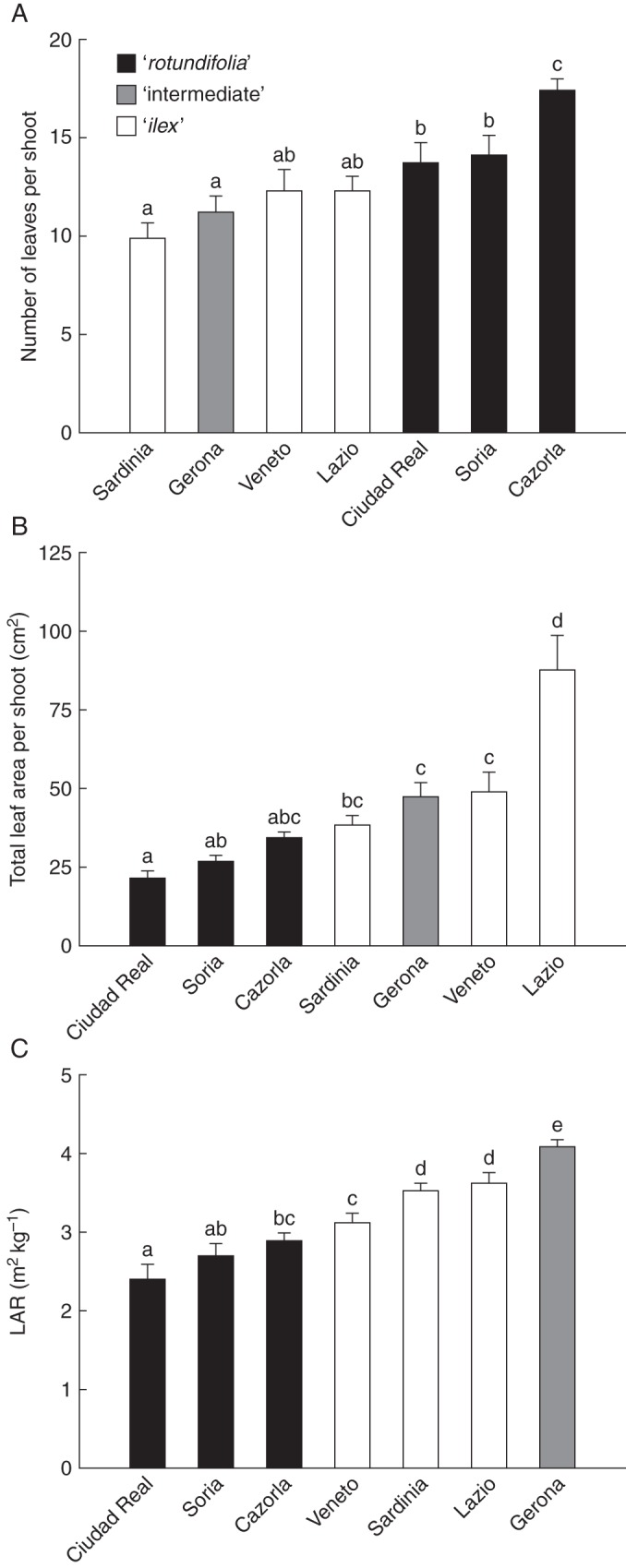
(A) Number of leaves per shoot, (B) total leaf area per shoot and (C) leaf area ratio (LAR) for the seven studied Q. ilex provenances. Data are means ± s.e. Morphotypes are as indicated in the key. Different letters indicate significant differences among provenances (Tukey test, P < 0·05).
In relation to parameters derived from the pressure–volume curves, provenances included on the ‘rotundifolia’ morphotype had higher values of ΨTLP (Fig. 5B), Π0 (Fig. 5C) and εmax (Fig. 5D), whereas RWCTLP did not differ among morphotypes (Fig. 5A).
Fig. 5.
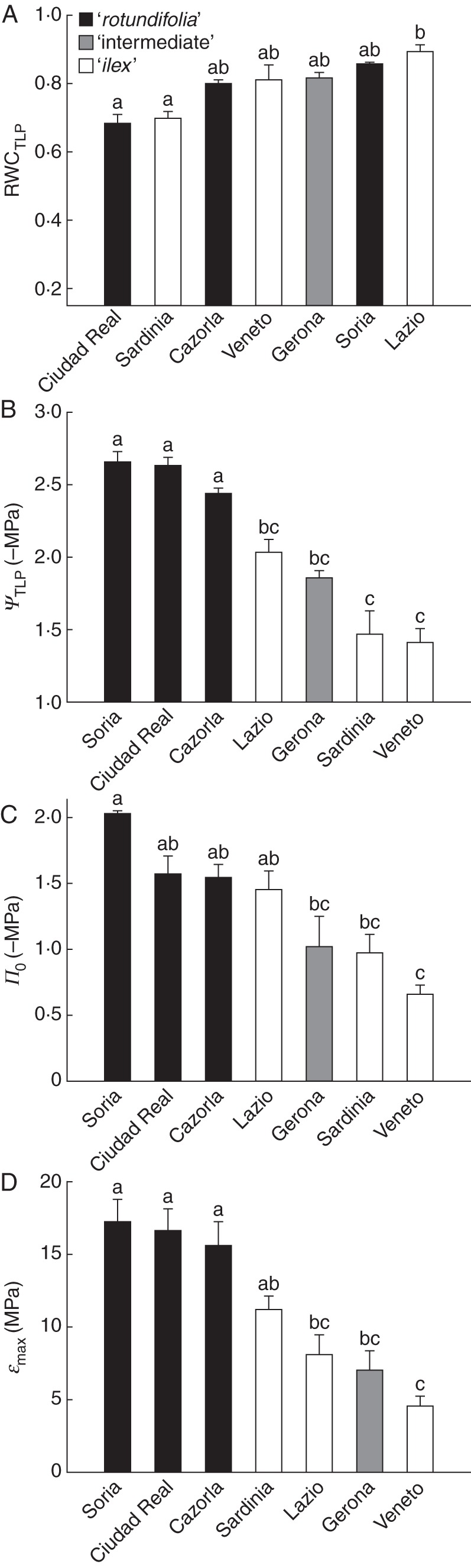
Parameters derived from the pressure–volume curves for the seven studied Q. ilex provenances: (A) relative water content at the turgor loss point (RWCTLP), (B) water potential at the turgor loss point (ΨTLP), (C) osmotic potential at full turgor (Π0) and (D) the maximum bulk modulus of elasticity (εmax). Data are means ± s.e. Morphotypes are as indicated in the key. Different letters indicate significant differences among provenances (Tukey test, P < 0·05).
The hydraulic parameters of current-year twigs for the studied Q. ilex provenances show that the higher Ks values were found in Sardinia and Lazio provenances, whereas Gerona and Ciudad Real provenances showed the lower Ks values (Fig. 6A). In spite of this, a clear segregation among morphotypes was not observed for Ks. However, LSC clearly presented different values among the holm oak morphotypes (Fig. 6B). Thus, ‘rotundifolia’ morphotypes showed significantly higher LSC values that those found for the ‘ilex’ morphotype, which can be explained by the higher values of total leaf area supported by plants of the latter morphotype.
Fig. 6.
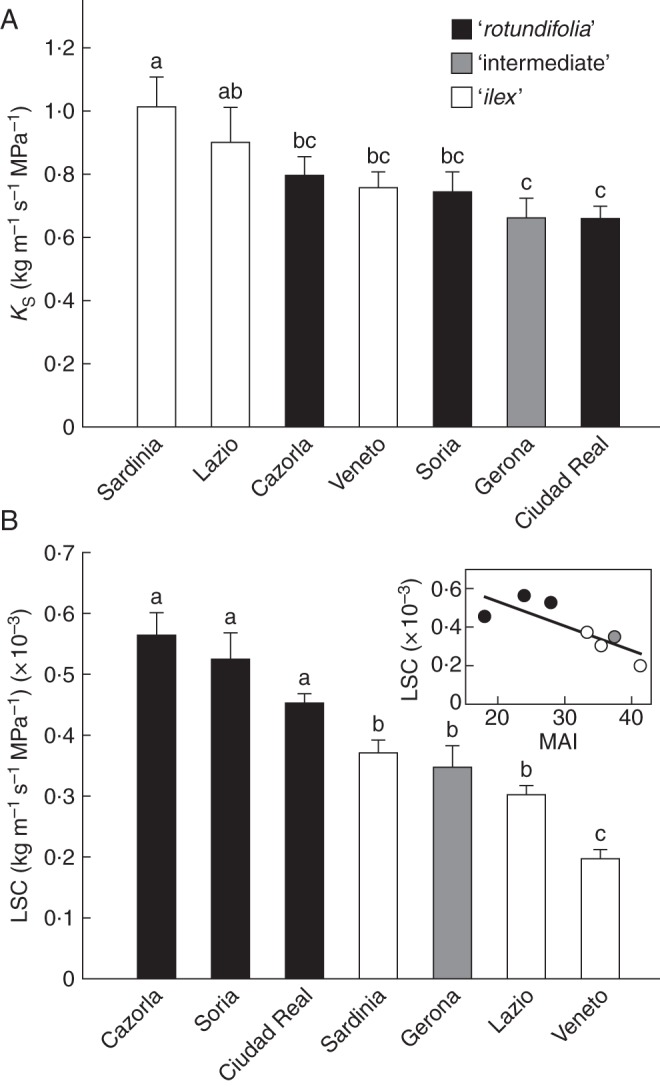
Specific hydraulic conductivity (KS) (A) and leaf specific conductivity (LSC) (B) for the seven studied Q. ilex provenances. Data are means ± s.e. Morphotypes are as indicated in the key. Different letters indicate significant differences among provenances (Tukey test, P < 0·05). The inset in (B) shows the linear relationship between the annual Martonne aridity index (MAI) and the LSC for the studied provenances (R2 = 0·66, P = 0·0255).
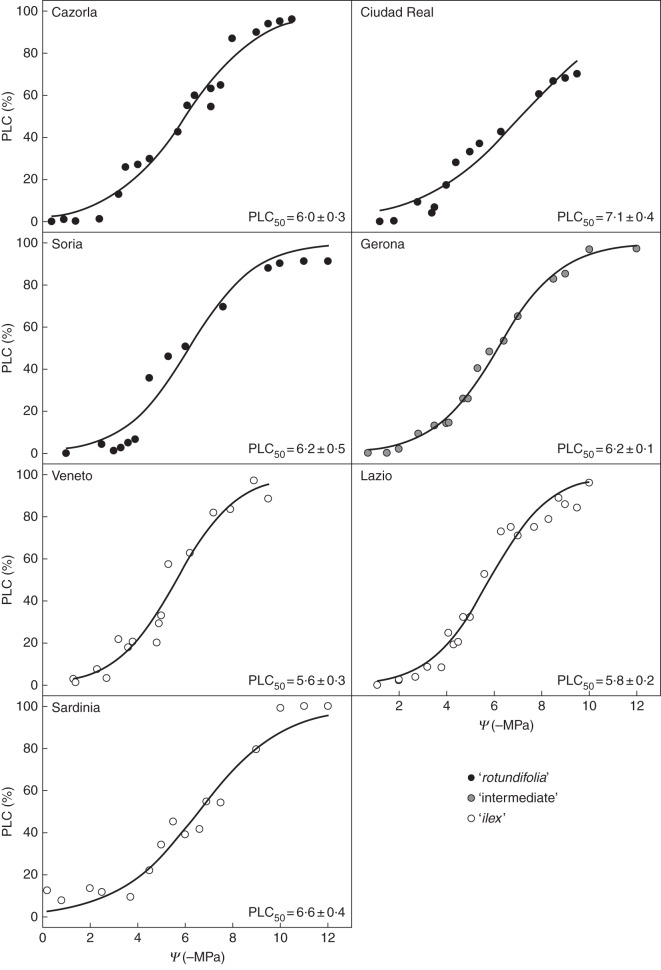
The Veneto and Lazio ‘ilex’-type provenances were the most vulnerable to xylem cavitation, with PLC50 mean values in the range –5·6 to –5·8 MPa (Fig. 7). Both provenances showed statistically (P < 0·05) different PLC50 values from the most resistant provenance to cavitation, namely the Ciudad Real ‘rotundifolia’-type provenance (–7·1 MPa).
Fig. 7.
Curves of vulnerability to cavitation for the seven studied Q. ilex provenances. Pairs of points were adjusted by using a sigmoidal function to estimate the potential at which the 50 % loss of hydraulic conductivity occurs (PLC50; mean PLC50 ± s.e. values in MPa for each provenance are shown in each graph). Morphotypes are as indicated in the key.
The PCA based on the morphological and physiological variables indicated that the first and second principal components accounted for 62 and 18 % of the total variation, respectively (Fig. 8). The scores of the studied Q. ilex provenances in the PCA biplot reflected that the functional traits analysed here established the existence of a clear differentiation between the ‘rotundifolia’ and the ‘ilex’ morphotypes. Regarding the ‘intermediate’ morphotype plants (Gerona provenance), and contrary to what leaf morphological data suggested, they were functionally close to the ‘ilex’ morphotype.
Fig. 8.
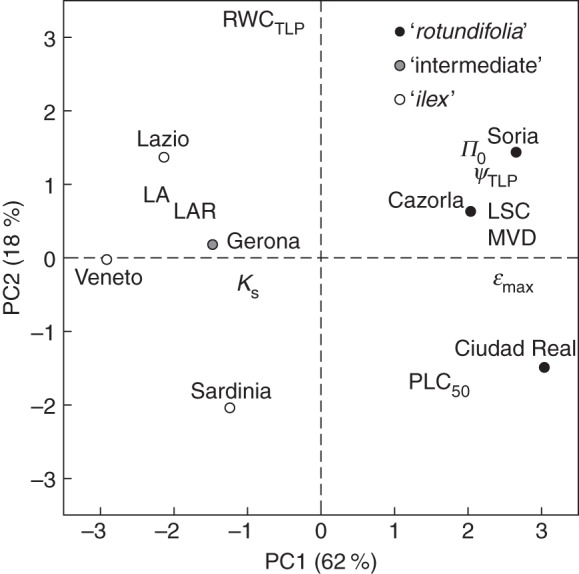
Relationship between the first two principal components (PC1 vs. PC2) of the principal component analysis (PCA) computed on the functional traits obtained for the seven studied Q. ilex provenances. Variables: leaf area (LA), major vein density (MVD), leaf area ratio (LAR), relative water content at the turgor loss point (RWCTLP), water potential at the turgor loss point (ΨTLP), osmotic potential at full turgor (Π0), the maximum bulk modulus of elasticity (εmax), specific hydraulic conductivity (KS), leaf specific conductivity (LSC) and the 50 % loss of hydraulic conductivity (PLC50). Morphotypes are as indicated in the key.
DISCUSSION
The high phenological, morphological and ecological variability exhibited by Q. ilex across its Circum-Mediterranean distribution area (Gratani, 1996; Crescente et al., 2002; Gratani et al., 2003) suggests that it hardly constitutes a homogeneous species. Michaud et al. (1995) and Lumaret et al. (2002) found clear genetic differences that support the existence of two main morphotypes for holm oak (‘ilex’ vs. ‘rotundifolia’ morphotypes). These morphotypes have been considered by botanists either as different species (Sáenz de Rivas, 1967; Rafii et al., 1991; Govaerts and Frodin, 1998) or as subspecies (Amaral-Franco, 1990; Schwarz, 1993). Here, we provide evidence that these morphotypes showing different leaf morphological features do correspond to different ecotypes as inferred from their contrasting functional traits.
The ‘ilex’ morphotype exhibits long leaves whereas the ‘rotundifolia’ morphotype presents shorter and more rounded leaves (orbicular, sub-orbicular or broadly ovate shapes), with fewer lateral veins than the former type. Such morphological characterization allows the separation of Italian ‘ilex’-type (Veneto, Lazio and Sardinia) from Spanish ‘rotundifolia’-type (Cazorla, Ciudad Real and Soria) provenances (Table 2). Beyond leaf shape, both morphotypes show distinctive qualitative leaf features. For instance, the ‘rotundifolia’ morphotype mainly corresponds to leaves with a serrate margin, sharply pointed teeth and craspedodromous secondary venation, while leaves with an entire and serrate margin with teeth that are not sharply pointed and weak brochidodromous or mixed craspedodromous secondary venation are more common among the ‘ilex’ morphotype (Table 2). The ‘intermediate’ morphotype, as previously suggested by Lumaret et al. (2002) and here represented by the Gerona provenance, can be considered morphologically closer to the ‘rotundifolia’ morphotype in most of the studied parameters (Fig. 2), except that leaves were longer than in other Iberian ‘rotundifolia’-type provenances.
Michaud et al. (1995) proposed that the ‘ilex’ and ‘rotundifolia’ morphotypes are associated with mild (coastal sites) and continental (inland sites) variants of the Mediterranean climate, respectively. Our findings confirm such an assumption because the Iberian inland provenances (Cazorla, Ciudad Real and Soria) come from drier sites than those of the coastal Iberian (Gerona) or Italian (Sardinia, Lazio and Veneto) provenances (Table 1, Fig. 1). In fact, the Mediterranean climate, which is characterized by mild wet winters and warm dry summers, shows many sub-regional variations (Lionello et al., 2006). Consequently, the large Q. ilex distribution area, extending 6000 km longitudinally and 1500 km latitudinally mainly across the Western Mediterranean Basin (Gratani et al., 2003), encompasses very different degrees of aridity and continentality (Martin-StPaul et al., 2012, 2013).
Previous works have reported meaningful variations in several functional traits among Q. ilex populations, indicating a differential response to drought (Tyree and Cochard, 1996; Pesoli et al., 2003; Corcuera et al., 2004; Sánchez-Vilas and Retuerto, 2007). In our study, the different holm oak provenances can be clearly grouped according to several functional traits (Fig. 8). Hence, the ‘rotundifolia’ and ‘ilex’ morphotypes seem to represent contrasting ‘functional types’ according to their hydraulic traits.
Among all the studied traits, the differences found in leaf size can be proposed as the most outstanding divergence between the morphotypes (Fig. 3A). The extreme reduction in leaf size found in the ‘rotundifolia’ morphotype is a common trait of many Mediterranean woody plants (Ackerly, 2004). Three main physiological advantages have been attributed to a reduced leaf size under Mediterranean dry-summer conditions. First, the development of small leaves has been associated with high levels of radiation and high temperatures (Fonseca et al., 2000; Ackerly et al., 2002). Yates et al. (2010) proposed that a thinner boundary layer associated with a smaller leaf facilitates a sensible heat loss in summer through ‘free convection’ (Roth-Nebelsick, 2001). Secondly, smaller leaf sizes are associated with a higher major vein density that, according to Scoffoni et al. (2011), would increase the resistance of the leaf to hydraulic conductivity losses, quantified as the water potential at 80 % loss of conductivity (PLC80). Our results confirm that the relationship between leaf size and major vein density for the studied holm oak provenances (Fig. 3C) also fits an exponential decay model, indicating that major vein density has a great dependence on leaf size, even when the range of small-leaved species is considered. Moreover, if we apply the linear model that relates the major vein density and the PLC80 provided by Scoffoni et al. (2011), our provenances would show clear differences in terms of leaf hydraulic vulnerability. Thus, provenances included on the ‘ilex’ morphotype would have PLC80 values ranging from –2 to –3 MPa, whereas mean PLC80 values for the ‘rotundifolia’ morphotype would be –6 MPa. Thus, leaves of the ‘ilex’ morphotype could not withstand very low water potentials due to a high risk of cavitation. Hydraulic dysfunction in leaves drives key aspects of whole-plant responses (Blackman et al., 2010), determining the climatic limits in a wide range of species (Blackman et al., 2012). Further studies are needed to confirm the differences in the resistance of the leaf to hydraulic conductivity losses among holm oak populations, which may be hypothetically considered as one of the main functional traits supporting different responses to drought of ‘ilex’ and ‘rotundifolia’ morphotypes.
Finally, the third benefit provided by small leaves is the improvement in the LSC in the ‘rotundifolia’ morphotypes (Fig. 6B), which previously has been related to climate dryness as a way of increasing the capacity to supply water to the transpiring leaves (Martínez-Vilalta et al., 2009), in particular when the atmospheric evaporative demand is very high (Peguero-Pina et al., 2011). According to our results, the lower whole leaf area per shoot (Fig. 4B), more than an increase in sapwood area or the existence of a more efficient xylem (e.g. through an increase in Ks, Fig. 6A), is responsible for the higher LSC values found in the ‘rotundifolia’ morphotypes. It should be noted that the reduction in the whole leaf area per shoot was due to the reduction in leaf size (Fig. 3A) more than to the number of leaves per shoot, which was even higher in the ‘rotundifolia’ morphotype (Fig. 4A). In spite of the benefits of a reduced leaf size, it has negative impacts by reducing the LAR (Fig. 4C), which is considered a major driver of the relative growth rate (Poorter and Remkes, 1990). Therefore, it can be suggested that ‘rotundifolia’ morphotypes sacrifice their growth ability in order to achieve a better hydraulic performance under drier conditions.
The results derived from the study of vulnerability curves indicate that all provenances showed shoots with a high resistance to cavitation, which has been previously recognized as a common feature of woody plants growing in habitats with water limitations (Hacke et al., 2000; Vilagrosa et al., 2010), including species living in Mediterranean-type ecosystems (Maherali et al., 2004). Some studies have addressed the resistance to cavitation in Q. ilex, with contrasting results. Although the most recent PLC50 value proposed for this species (–5·5 MPa, see Urli et al., 2013) is quite close to the values found in our study, different values have been reported previously (ranging from –2·0 to –3·6 MPa, see Pinto et al., 2012, and references therein). Among the factors which may be responsible for these differences, the methods used for establishing the vulnerability curves may be of great importance (Choat et al., 2010; Cochard et al., 2010; Sperry et al., 2012). In general, there is a reasonable agreement among techniques but, in some angiosperms with large xylem vessels, some methods reported anomalous vulnerability curves. This may be the case for Q. ilex for which several techniques have been used to establish vulnerability curves. Here we used the method of benchtop natural dehydration which is considered as the most reliable technique because the embolism is induced naturally by transpiration (Sperry et al., 2012). In this sense, the reported curves of xylem cavitation and PLC50 values for the different provenances in this study would reflect variations in xylem resistance among populations ranging from –5·6 to –7·1 MPa for PLC50. Although xylem vulnerability to cavitation was initially considered a species-specific trait which was relatively stable across sites, it must be highlighted that several studies recently reported relevant intraspecific variations among sites of different aridity (Corcuera et al., 2012). Thus, the relationship observed between PLC50 and MAI found in our study (R2 = 0·50, P = 0·08; data not shown) confirms this idea.
Another group of functional traits that are related to the tolerance of a plant to water stress are those derived from the pressure–volume curves (Corcuera et al., 2002). Recently, Bartlett et al. (2012b) reviewed studies of 317 plant species living in biomes with very different degrees of aridity and concluded that the water potential at the turgor loss point (ΨTLP) is a reliable indicator of drought tolerance within and across biomes, reflecting the ability of the bulk leaf tissue to maintain function during drought. In our study, ΨTLP values in the provenances included on the ‘ilex’ morphotype were significantly different from those of the ‘rotundifolia’ populations (Fig. 5B), and similar to those reported by Bartlett et al. (2012b) for tropical species. On the other hand, the higher values for ΨTLP in the ‘rotundifolia’ provenances coincided with those reported for woody Mediterranean species (Bartlett et al., 2012b). The osmotic potential at full turgor (Π0), as the major driver of ΨTLP (Bartlett et al., 2012a), points in the same direction, although statistical differences among populations are not as clear as they are in the case of ΨTLP (Fig. 5C). The lower ΨTLP in ‘ilex’ morphotypes may indicate an early stomatal regulation of the water losses (Brodribb and Hoolbrook, 2003), which is in accordance with the lower PLC80 values inferred from their major vein density values. In contrast, the maintenance of cell turgor at more negative water potentials would imply a greater ability to keep the net carbon assimilation in ‘rotundifolia’ morphotypes even when low soil and tissue water potentials occur (Baltzer et al., 2008). A clear segregation of ‘ilex’ and ‘rotundifolia’ morphotypes is also observed when considering the maximum bulk modulus of elasticity (εmax) (Fig. 5d). Although a higher εmax cannot be considered exclusive for species from dry climates (Bartlett et al., 2012b), Niinemets (2001) suggested that the elastic adjustment may be a common mechanism for plants to adapt to environments where water is scarce. In this sense, Corcuera et al. (2002) found that εmax values in Mediterranean oak species were significantly higher than those observed in temperate oak species, even when grown under common environmental conditions.
The differential morphology and contrasting physiological performance at leaf level found in this study could be related to the paleontological history of holm oak. According to different studies (see Ackerly, 2009, and references therein), those evergreen and sclerophyllous Quercus species that have been associated with genuine Mediterranean-type climates have a pre-Mediterranean origin. In this sense, several Neogene fossil species with leaf morphological characteristics very similar to those found in the ‘ilex’ morphotype have been described in Europe. Thus, the late Miocene species Quercus praeilex Saporta is considered a xeromesophytic to xerophytic taxon (Palamarev, 1989; Palamarev and Ivanov, 2003; Palamarev and Tzenov, 2004). Other species such as Quercus praecursor Saporta and Marion and Quercus drymeja Unger seem to be associated with sub-tropical broad-leaved evergreen vegetation, being an element of sub-humid warm climates with not much of a pronounced drier period during the summer (Kvaček et al., 2002). However, until now, no Neogene fossils with morphological characteristics that resemble the ‘rotundifolia’ morphotype have been found. At the end of the Neogene (approx. 3·1–3·2 million years ago), a progressive reduction in summer rainfall coinciding with a temperature increase probably triggered the replacement of the sub-tropical vegetation by Mediterranean species that exist today (Barrón et al., 2010). How did this change affect holm oak or its predecessors? The two current main morphotypes with contrasting functional performance suggest different evolutionary pathways. On the one hand, the ‘ilex’ morphotype shows nothophylous leaves, which indicate an affinity for Neogene sub-tropical environments, and it is dominant in coastal habitats subjected to mild climatic conditions. On the other hand, the ‘rotundifolia’ morphotype, which is only found in inland sites of the Iberian Peninsula and Northern African mountains, may be a Quaternary taxon that manifests more recent adaptations to stressful dry and cold conditions, showing small leaves with a low number of secondary veins.
In conclusion, this study has confirmed that leaf morphometric parameters allow holm oak provenances to be separated into two main morphotypes, as proposed by Lumaret et al. (2002) using genetic markers. Moreover, we have found that these ‘morphotypes’ are reflected in two consistent and very contrasting functional types that would establish a differential physiological performance, especially in response to climate dryness. Therefore, we conclude that these morphotypes correspond to different ecotypes locally adapted to the stress level as inferred from their contrasting functional traits. To the best of our knowledge, this is the first time that the combination of morphological and physiological traits provides evidence supporting the consideration of the two main holm oak morphotypes as two different species of the genuine Mediterranean evergreen woody vegetation.
ACKNOWLEDGEMENTS
We thank Juli G. Pausas for his assistance with the statistical analyses of the cavitation curves. This work was supported by Gobierno de Aragón (A54 research group), the projects AGL2010–21153-C02-02, CGL-2011–30531-CO2-02 and CSD2007-00067 funded by the Spanish government, and GA243888 funded by the EC. CEAM is supported by the Generalitat Valenciana. The work of J.J.P.P. is supported by a ‘Juan de la Cierva’-MINECO post-doctoral contract. J.J.C. acknowledges the support of ARAID.
LITERATURE CITED
- Ackerly DD. Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California chaparral. American Naturalist. 2004;163:654–671. doi: 10.1086/383062. [DOI] [PubMed] [Google Scholar]
- Ackerly DD. Evolution, origin and age of lineages in the Californian and Mediterranean floras. Journal of Biogeography. 2009;36:1221–1233. [Google Scholar]
- Ackerly DD, Knight CA, Weiss SB, Barton K, Starmer KP. Leaf size, specific leaf area and microhabitat distribution of woody plants in a California chaparral: contrasting patterns in species level and community level analyses. Oecologia. 2002;130:449–457. doi: 10.1007/s004420100805. [DOI] [PubMed] [Google Scholar]
- Amaral-Franco J. Quercus L. In: Castroviejo S, Laínz M, López-Gónzalez G, et al., editors. Flora Ibérica 2. Madrid: Real Jardín Botánico, CSIC; 1990. pp. 15–36. [Google Scholar]
- Ash A, Ellis B, Hickey LJ, Johnson K, Wilf P, Wing S. Manual of leaf architecture – morphological description and categorization of dicotyledonous and net-veined monocotyledonous angiosperms. Washington, DC: Smithsonian Institution, 1–65; 1999. [Google Scholar]
- Baldocchi DD, Xu L. What limits evaporation from Mediterranean oak woodlands – the supply of moisture in the soil, physiological control by plants or the demand by the atmosphere? Advances in Water Resources. 2007;30:2113–2122. [Google Scholar]
- Baltzer JL, Davies SJ, Bunyavejchewin S, Noor NSM. The role of desiccation tolerance in determining tree species distributions along the Malay–Thai Peninsula. Functional Ecology. 2008;22:221–231. [Google Scholar]
- Barrón E, Rivas-Carballo R, Postigo-Mijarra JM, et al. The Cenozoic vegetation of the Iberian Peninsula: a synthesis. Review of Palaeobotany and Palynology. 2010;162:382–402. [Google Scholar]
- Bartlett MK, Scoffoni C, Ardy R, et al. Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods in Ecology and Evolution. 2012a;3:880–888. [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters. 2012b;15:393–405. doi: 10.1111/j.1461-0248.2012.01751.x. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist. 2010;188:1113–1123. doi: 10.1111/j.1469-8137.2010.03439.x. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulic vulnerability influences species' bioclimatic limits in a diverse group of woody angiosperms. Oecologia. 2012;168:1–10. doi: 10.1007/s00442-011-2064-3. [DOI] [PubMed] [Google Scholar]
- Braun-Blanquet J, Bolòs O. Les groupements végétaux du Bassin Moyen de l'Ebre et leur dynamisme. Anales de la Estación Experimental de Aula Dei. 1957;5:1–266. [Google Scholar]
- Breckle SW. Walter's vegetation of the earth. Berlin: Springer; 2002. [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology. 2003;132:2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Drayton WM, Brodersen C, et al. Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species. Plant, Cell and Environment. 2010;33:1502–1512. doi: 10.1111/j.1365-3040.2010.02160.x. [DOI] [PubMed] [Google Scholar]
- Cochard H, Coll L, Le Roux X, Améglio T. Unraveling the effects of plant hydraulics on stomatal conductance during water stress in walnut. Plant Physiology. 2002;128:282–290. [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Herbette S, Barigah T, Badel E, Ennajeh M, Vilagrosa A. Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique. Plant, Cell and Environment. 2010;33:1543–1552. doi: 10.1111/j.1365-3040.2010.02163.x. [DOI] [PubMed] [Google Scholar]
- Cody ML, Mooney HA. Convergence versus nonconvergence in Mediterranean-type ecosystems. Annual Review of Ecology and Systematics. 1978;9:265–321. [Google Scholar]
- Corcuera L, Camarero JJ, Gil-Pelegrín E. Functional groups in Quercus species derived from the analysis of pressure–volume curves. Trees - Structure and Function. 2002;16:465–472. [Google Scholar]
- Corcuera L, Camarero JJ, Gil-Pelegrín E. Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees - Structure and Function. 2004;18:83–92. [Google Scholar]
- Corcuera L, Morales F, Abadía A, Gil-Pelegrín E. Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. Tree Physiology. 2005;25:599–608. doi: 10.1093/treephys/25.5.599. [DOI] [PubMed] [Google Scholar]
- Corcuera L, Gil-Pelegrín E, Notivol E. Differences in hydraulic architecture between mesic and xeric Pinus pinaster populations at the seedling stage. Tree Physiology. 2012;32:1442–1457. doi: 10.1093/treephys/tps103. [DOI] [PubMed] [Google Scholar]
- Crescente MF, Gratani L, Larcher W. Shoot growth efficiency and production of Quercus ilex L. in different climates. Flora. 2002;197:2–9. [Google Scholar]
- Di Castri F. Mediterranean-type shrublands of the world. In: Di Castri F, Goodall DW, Specht RL, editors. Mediterranean-type shrublands. Amsterdam: Elsevier; 1981. pp. 1–52. [Google Scholar]
- Fonseca CR, Overton JM, Collins B, Westoby M. Shifts in trait combinations along rainfall and phosphorus gradients. Journal of Ecology. 2000;88:964–977. [Google Scholar]
- Govaerts R, Frodin DG. World checklist and bibliography of Fagales. Kew: Royal Botanic Gardens, Kew; 1998. [Google Scholar]
- Gratani L. Leaf and shoot growth dynamics of Quercus ilex L. Acta Oecologica. 1996;17:17–27. [Google Scholar]
- Gratani L, Meneghini M, Pesoli P, Crescente MF. Structural and functional plasticity of Quercus ilex seedlings of different provenances in Italy. Trees - Structure and Function. 2003;17:515–521. [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic and Applied Ecology. 2000;1:31–41. [Google Scholar]
- Hernández EI, Pausas JG, Vilagrosa A. Leaf physiological traits in relation to resprouter ability in the Mediterranean Basin. Plant Ecology. 2011;212:1959–1966. [Google Scholar]
- Herrera CM. Historical effects and sorting processes as explanations for contemporary ecological patterns; character syndromes in Mediterranean woody plants. American Naturalist. 1992;140:421–446. [Google Scholar]
- Hickey LJ. Classification of the architecture of dicotyledonous leaves. American Journal of Botany. 1973;60:17–33. [Google Scholar]
- Kummerov J. Comparative anatomy of sclerophylls of Mediterranean climate areas. In: di Castri F, Mooney HA, editors. Mediterranean type ecosystems. Origin and structure. Berlin: Springer; 1973. pp. 213–224. [Google Scholar]
- Kvaček Z, Velitzelos D, Velitzelos E. Late Miocene flora of Vegora Macedonia N. Greece. Athens: University of Athens; 2002. [Google Scholar]
- Limousin JM, Longepierre D, Huc R, Rambal S. Change in hydraulic traits of Mediterranean Quercus ilex subjected to long-term throughfall exclusion. Tree Physiology. 2010;30:1026–1036. doi: 10.1093/treephys/tpq062. [DOI] [PubMed] [Google Scholar]
- Lionello P, Malanotte-Rizzoli P, Boscolo R, et al. The Mediterranean climate: an overview of the main characteristics and issues. In: Lionello P, Malanotte-Rizzoli P, Boscolo R, editors. Mediterranean climate variability. Amsterdam: Elsevier; 2006. pp. 1–26. [Google Scholar]
- Lo Gullo MA, Salleo S. Different strategies of drought resistance in three Mediterranean sclerophyllous trees growing in the same environmental conditions. New Phytologist. 1988;108:267–276. doi: 10.1111/j.1469-8137.1988.tb04162.x. [DOI] [PubMed] [Google Scholar]
- Lo Gullo MA, Salleo S. Different vulnerabilities of Quercus ilex L. to freeze and summer drought-induced xylem embolism: an ecological interpretation. Plant, Cell and Environment. 1993;16:511–519. [Google Scholar]
- Lumaret R, Mir C, Michaud H, Raynal V. Phylogeographical variation of chloroplast DNA in holm oak (Quercus ilex L.) Molecular Ecology. 2002;11:2327–2336. doi: 10.1046/j.1365-294x.2002.01611.x. [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman W, Jackson R. Adaptative variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Martínez-Vilalta J, Mangirón M, Ogaya R, et al. Sap flow of three co-occurring Mediterranean woody species under varying atmospheric and soil water conditions. Tree Physiology. 2003;23:747–758. doi: 10.1093/treephys/23.11.747. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Cochard H, Mencuccini M, et al. Hydraulic adjustments of Scots pine across Europe. New Phytologist. 2009;184:353–364. doi: 10.1111/j.1469-8137.2009.02954.x. [DOI] [PubMed] [Google Scholar]
- Martin-StPaul NK, Limousin JM, Rodríguez-Calcerrada J, et al. Photosynthetic sensitivity to drought varies among populations of Quercus ilex along a rainfall gradient. Functional Plant Biology. 2012;39:25–37. doi: 10.1071/FP11090. [DOI] [PubMed] [Google Scholar]
- Martin-StPaul NK, Limousin JM, Vogt-Schilb H, et al. The temporal response to drought in a Mediterranean evergreen tree: comparing a regional precipitation gradient and a throughfall exclusion experiment. Global Change Biology. 2013;19:2413–2426. doi: 10.1111/gcb.12215. [DOI] [PubMed] [Google Scholar]
- Mediavilla S, Escudero A. Stomatal responses to drought at a Mediterranean site: a comparative study of co-occurring woody species differing in leaf longevity. Tree Physiology. 2003;23:987–996. doi: 10.1093/treephys/23.14.987. [DOI] [PubMed] [Google Scholar]
- Michaud H, Toumi L, Lumaret R, Li TX, Romane F, Di Giusto F. Effect of geographical discontinuity on genetic variation in Quercus ilex L. (holm-oak). Evidence from enzyme polymorphism. Heredity. 1995;74:590–606. [Google Scholar]
- Nardini A, Ghirardelli L, Salleo S. Vulnerability to freeze stress of seedlings of Quercus ilex L.: an ecological interpretation. Annals of Forest Science. 1998;55:553–565. [Google Scholar]
- Niinemets U. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82:453–469. [Google Scholar]
- Ogaya R, Peñuelas J. Contrasting foliar responses to drought in Quercus ilex and Phillyrea latifolia. Biologia Plantarum. 2006;50:373–382. [Google Scholar]
- Ogaya R, Peñuelas J. Leaf mass per area ratio in Quercus ilex leaves under a wide range of climatic conditions. The importance of low temperatures. Acta Oecologica. 2007;31:168–173. [Google Scholar]
- Palamarev E. Paleobotanical evidences of the Tertiary history and origin of the Mediterranean sclerophyll dendroflora. Plant Systematics and Evolution. 1989;162:93–107. [Google Scholar]
- Palamarev E, Ivanov D. A contribution to the Neogene history of Fagaceae in the Central Balkan area. Acta Palaeobotanica. 2003;43:51–59. [Google Scholar]
- Palamarev E, Tzenov B. Genus Quercus in the late Miocene flora of Baldevo Formation (Southwest Bulgaria): taxonomical composition and palaeoecology. Phytologia Balcanica. 2004;10:147–156. [Google Scholar]
- Pammenter NW, Van der Willigen C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Paula S, Pausas JG. Leaf traits and resprouting ability in the Mediterranean basin. Functional Ecology. 2006;20:941–947. [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Cochard H, Barredo G, Villarroya D, Gil-Pelegrín E. Hydraulic traits are associated with the distribution range of two closely related Mediterranean firs, Abies alba Mill. and Abies pinsapo Boiss. Tree Physiology. 2011;31:1067–1075. doi: 10.1093/treephys/tpr092. [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Morales F, Flexas J, Gil-Pelegrín E. Differential photosynthetic performance and photoprotection mechanisms of three Mediterranean evergreen oaks under severe drought stress. Functional Plant Biology. 2009;36:453–462. doi: 10.1071/FP08297. [DOI] [PubMed] [Google Scholar]
- Pesoli P, Gratani L, Larcher W. Responses of Quercus ilex from different provenances to experimentally imposed water stress. Biologia Plantarum. 2003;46:577–581. [Google Scholar]
- Pinto CA, David JS, Cochard H, et al. Drought-induced embolism in current-year shoots of two Mediterranean evergreen oaks. Forest Ecology and Management. 2012;285:1–10. [Google Scholar]
- Poorter H, Remkes C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- Rafii ZA, Zavarin E, Pelleau Y. Chemosystematic differentiation of Quercus ilex and Q. rotundifolia based on acorn fatty acids. Biochemical Systematics and Ecology. 1991;19:163–166. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: 2013. [Google Scholar]
- Roth-Nebelsick A. Computer-based analysis of steady-state and transient heat transfer of small-sized leaves by free and mixed convection. Plant, Cell and Environment. 2001;24:631–640. [Google Scholar]
- Sáenz de Rivas C. Estudios sobre Quercus ilex L. y Quercus rotundifolia Lamk. Anales del Instituto Botánico A. J. Cavanilles. 1967;2:243–262. [Google Scholar]
- Salleo S, Lo Gullo M. Sclerophylly and plant water relations in three Mediterranean Quercus species. Annals of Botany. 1990;65:259–270. [Google Scholar]
- Salleo S, Nardini A. Sclerophylly: evolutionary advantage or mere epiphenomenon? Plant Biosystems. 2000;134:247–259. [Google Scholar]
- Sánchez-Vilas J, Retuerto R. Quercus ilex shows significant among-population variability in functional and growth traits but maintains invariant scaling relations in biomass allocation. International Journal of Plant Sciences. 2007;168:973–983. [Google Scholar]
- Sancho-Knapik D, Gómez Álvarez-Arenas T, Peguero-Pina JJ, Fernández V, Gil-Pelegrín E. Relationship between ultrasonic properties and structural changes in the mesophyll during leaf dehydration. Journal of Experimental Botany. 2011;62:3637–3645. doi: 10.1093/jxb/err065. [DOI] [PubMed] [Google Scholar]
- Schwarz O. Quercus L. In: Tutin TG, Burges NA, Chater AO, editors. Flora Europaea, vol. 1 revised: Lycopodiaceae to Platanaceae. Cambridge: Cambridge University Press; 1993. pp. 61–64. [Google Scholar]
- Scoffoni C, Rawls M, McKown A, Cochard H, Sack L. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiology. 2011;156:832–843. doi: 10.1104/pp.111.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Christman M, Torres-Ruiz JM, Taneda H, Smith DD. Vulnerability curves by centrifugation: is there an open vessel artefact, and are ‘r’ shaped curves necessarily invalid? Plant, Cell and Environment. 2012;35:601–610. doi: 10.1111/j.1365-3040.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- Traiser C, Klotz S, Uhl D, Mosbrugger V. Environmental signals from leaves – a physiognomic analysis of European vegetation. New Phytologist. 2004;166:465–484. doi: 10.1111/j.1469-8137.2005.01316.x. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Cochard H. Summer and winter embolism in oak: impact on water relations. Annals of Forest Science. 1996;53:173–180. [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:19–38. [Google Scholar]
- Underwood AJ. Experiments in ecology. Their logical design and interpretation using analysis of variance. Sydney: University of Sydney; 1997. [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology. 2013;33:672–683. doi: 10.1093/treephys/tpt030. [DOI] [PubMed] [Google Scholar]
- Verdú M, Dávila P, García-Fayos P, Flores-Hernández N, Valiente-Banuet A. ‘Convergent’ traits of mediterranean woody plants belong to pre-mediterranean lineages. Biological Journal of the Linnean Society. 2003;78:415–427. [Google Scholar]
- Vilagrosa A, Morales F, Abadía A, Bellot J, Cochard H, Gil-Pelegrín E. Are symplast tolerance to intense drought conditions and xylem vulnerability to cavitation coordinated? An integrated analysis of photosynthetic, hydraulic and leaf level processes in two Mediterranean drought-resistant species. Environmental and Experimental Botany. 2010;69:233–242. [Google Scholar]
- Yates MJ, Verboom GA, Rebelo AG, Cramer MD. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Functional Ecology. 2010;24:485–492. [Google Scholar]