Abstract
Background and Aims
Invasive clonal plants have two reproduction patterns, namely sexual and vegetative propagation. However, seedling recruitment of invasive clonal plants can decline as the invasion process proceeds. For example, although the invasive clonal Wedelia trilobata (Asteraceae) produces numerous seeds, few seedlings emerge under its dense population canopy in the field. In this study it is hypothesized that light limitation and the presence of a thick layer of its own litter may be the primary factors causing the failure of seedling recruitment for this invasive weed in the field.
Methods
A field survey was conducted to determine the allocation of resources to sexual reproduction and seedling recruitment in W. trilobata. Seed germination was also determined in the field. Effects of light and W. trilobata leaf extracts on seed germination and seedling growth were tested in the laboratory.
Key Results
Wedelia trilobata blooms profusely and produces copious viable seeds in the field. However, seedlings of W. trilobata were not detected under mother ramets and few emerged seedlings were found in the bare ground near to populations. In laboratory experiments, low light significantly inhibited seed germination. Leaf extracts also decreased seed germination and inhibited seedling growth, and significant interactions were found between low light and leaf extracts on seed germination. However, seeds were found to germinate in an invaded field after removal of the W. trilobata plant canopy.
Conclusions
The results indicate that lack of light and the presence of its own litter might be two major factors responsible for the low numbers of W. trilobata seedlings found in the field. New populations will establish from seeds once the limiting factors are eliminated, and seeds can be the agents of long-distance dispersal; therefore, prevention of seed production remains an important component in controlling the spread of this invasive clonal plant.
Keywords: Clonal plant ecology, leaf litter, biological invasion, invasive plant, light limitation, reproductive strategy, seedling recruitment inhibition, Wedelia trilobata, Asteraceae
INTRODUCTION
Many plants reproduce in two ways, namely sexual reproduction through seeds and clonal regeneration through vegetative propagation (Koivunen et al., 2004). Sexual reproduction occurs via seeds that are dispersed over long distances by wind, birds or mammals, whereas clonal propagation occurs via bulbils, corms or rhizomes for rapid colonization (Rautiainen et al., 2004; Liu et al., 2010). However, environmental resources are often limited to a certain range. More resources are allocated to sexual reproduction, while fewer resources might be allocated to rapid clonal propagation (Thompson and Eckert, 2004; Chu et al., 2011). The balance between these two reproduction modes is highly significant in the population expansion of a plant.
Various studies on the biological invasion mechanisms of the reproduction strategy of invasive plants have been reported. Invasive plants spread or recruit their population via sexual (e.g. Centaurea solstitialis) (Swope and Parker, 2010) or clonal reproduction (e.g. Oxalis pes-caprae) (Castro et al., 2007). Clonal growth is common and important for alien plant invasion (Otfinowski and Kenkel, 2008; Wang et al., 2008b); it facilitates population establishment and expansion in a new range (Yu et al., 2009; Roiloa et al., 2010). Clonal species often invest fewer resources in seedling recruitment than non-clonal species (Dorken and Eckert, 2001). Since many populations grow predominantly via vegetative reproduction, seedling recruitment has a minor significance for these populations, especially for invasive clonal plants, and may decline as the invasion process continues (Harald and Roldan, 1997). What are the reasons for the restriction of seedling recruitment of invasive clonal plants?
Plant regeneration is affected by numerous environmental parameters, such as light, temperature, water availability, nutrients and litter, among which light and litter may be two of the pivotal factors influencing seed germination and seedling establishment (Fuchs et al., 2000; Olson and Wallander, 2002; Sonohat et al., 2004; Wei et al., 2009). Although previous studies have reported that seeds of many Asteraceae plants are photosensitive (Schutz et al., 2002; Luo and Cardina, 2012), the light requirement still varies among different Asteraceae plants (Galindez et al., 2009; Sun et al., 2009; Li et al., 2012). The response of seed germination to light is significantly associated with seed characteristics such as seed weight and size (Schutz et al., 2002). Plant litter is another factor that affects seed germination and seedling establishment (Cavieres et al., 2007; Hata et al., 2010; Valera-Burgos et al., 2012). Wang et al. (2012) found that the litter of the invasive weed Wedelia trilobata inhibited seedling growth of radish (Raphanus sativus L.) and turnip (Brassica campestris L.).
Wedelia trilobata (Asteraceae), native to the tropics of South America (Weber et al., 2008), is a perennial evergreen creeping clonal herb (Supplementary Data Fig. S1). It has been listed as one of the 100 world's worst invasive alien species (IUCN, 2001). This noxious weed was introduced to South China on a large scale as a common groundcover plant in the 1970s, but it rapidly spread to the field (Li and Xie, 2003). Fast dispersal through vegetative propagation (clonal growth) is one of the pivotal factors for the successful invasion of W. trilobata (Wu et al., 2005; Song et al., 2010b). Once established in plantations, W. trilobata can overgrow into a dense groundcover and prevent the regeneration of other species (Song et al., 2010a). Abundant flowers of W. trilobata were found in the field (Supplementary Data Fig. S2A). Wedelia trilobata also produces seeds (Thaman, 1999). Most studies have focused on its vigorous clonal reproduction, whereas sexual reproduction has not received much attention. Also, seedling recruitment of W. trilobata has been poorly studied. In a previous investigation of vegetation in the field across the tropical Hainan Island in China (unpublished results), we found that invasion of a community of flora by a dense population of W. trilobata resulted in a microhabitat with low light and a thick layer of W. trilobata litter, and hardly any detectable W. trilobata seedlings.
In the field, W. trilobata produces numerous seeds; however, few seedlings were found under its population canopy in the field. We hypothesized that both the dense mother ramets and the thick litter layer caused light limitation for the seeds under W. trilobata populations. Darkness and litter might inhibit seed germination and seedling growth of W. trilobata. Therefore, lack of light and the presence of a layer of its own litter under ramets might be the primary reasons causing the failure of seedling recruitment for this invasive weed in the field. To test these hypotheses, we quantified both the seed production and seedling establishment of W. trilobata in the field, and assessed the effects of light and its own litter on seed germination and seedling growth in the greenhouse and in the field.
MATERIALS AND METHODS
Field survey and sampling
A field survey was conducted in Hainan Province, China in June 2012. In order to examine biomass allocation in sexual reproduction of Wedelia trilobata (L.) Hitchc., 48 individuals were collected randomly from eight sites across eight cities in Hainan Province (six individuals from each site). Roots, stems, leaves and flowers from each individual were separated, washed, dried at 80 °C for 72 h, and weighed to calculate their biomass allocation ratios.
Many species of Asteraceae produce dimorphic achenes in a single capitulum (Brandel, 2004); this is the case for W. trilobata, which produces Type I and Type II achenes (Supplementary Data Fig. S2C). Type I seed is plump and fertilized, whereas Type II seed is shrivelled and sterile. A total of 267 mature capitulums were randomly selected to count the number of Type I and II seeds in one capitulum. Also, Type I seeds were collected from each sample site, mixed evenly and stored in a gauze bag at room temperature for seed germination experiments.
We also randomly established 117 quadrats (1 × 1 m) in the sites that were seriously invaded by W. trilobata both in Haikou and in Sanya city. Each quadrat was separated from the others by a distance of about 1 km. The number of W. trilobata seedlings (Supplementary Data Fig. S1) was counted in each quadrat. We also calculated light transmittance under each population canopy. Light intensity under the W. trilobata population canopy and that of the bare ground nearby for each quadrat were recorded using a CL-200 illumination photometer (Konica Minolta, Japan). Light transmittance (LT) of the W. trilobata population was calculated as the ratio of light intensity of the substrate under the W. trilobata population canopy (Lp) to the light intensity of the bare ground nearby (Lb), i.e. LT = Lp/Lb. The plant cover was estimated using a sub-divided quadrat method (Kolb et al., 2002; Karalius and Alpert, 2010).
Experiment 1: effects of light on seed germination
For all germination experiments, seeds of W. trilobata were surface-sterilized with 5 % sodium hypochloride solution for 10 min and washed thoroughly five times with sterilized distilled water. Seed germination was conducted in 9 cm Petri dishes with two layers of sterilized Whatman qualitative filter papers. To test the effects of light on seed germination of W. trilobata, five light intensity levels were created: full light (the light intensity of the incubator, 450 μmol m–2 s–1), 1/2, 1/4 and 1/8 of full light, and darkness. These five treatments were chosen to simulate light regimes in the field. The Petri dishes were wrapped in perforated aluminium foil with a different hole area for the 1/2, 1/4 and 1/8 light intensity treatments, intact aluminium foil without perforation for dark treatment, and no aluminium foil for full light treatment (Experiment 1 in Supplementary Data Fig. S3). Each light treatment was repeated five times (a total of 25 Petri dishes) and contained 50 surface-sterilized seeds (a total of 1250 seeds). All Petri dishes were placed in an incubator with a constant temperature of 24 °C and humidity of 70 % under light (450 μmol m–2 s–1; photoperiod: 16 h day and 8 h night). About 7 d after the study was set up, germinated seeds (radicle visible) were counted daily for about 7 weeks until no more germinated seeds were registered.
Experiment 2: effects of leaf extracts and light on seed germination
A factorial block design (Experiment 2 in Supplementary Data Fig. S3) was applied to study the effects of leaf extracts and light intensity. Due to the potential difference in chemical leaf constituents between fresh and dry leaves during decomposition (Gessner, 1991), we used both fresh and dry leaf extracts to test the effects on seed germination. The leaf extracts were obtained from fresh or dry leaves of W. trilobata. Fresh leaves were collected randomly from individuals in the field. Half of the samples were soaked in sterilized distilled water (0·2 g f. wt mL–1) for 72 h at room temperature, and then filtered. The other half of the fresh leaves were dried to constant mass at 80 °C. Fresh leaves (1 kg) were dried to yield 86 g of dry material. The dry leaves were soaked in sterilized distilled water (0·05 g d. wt ml–1) for 72 h at room temperature. The extracts were filtered and stored at 4 °C. The fresh leaf extracts were diluted to the final working concentrations of 0·05, 0·1 and 0·2 g f. wt mL–1 following Dorning and Cipollini (2006). The dry leaf extracts were diluted to 0·005, 0·025 and 0·05 g d. wt mL–1 (Hou et al., 2011), and sterilized distilled water was used as control treatment. Thus, a total of seven concentrations of leaf extract were used. Light intensity included high light (450 μmol m–2 s–1) and darkness (by covering with aluminium foil). All 14 treatments were arranged as one block and repeated five times.
Fifty seeds were placed on each Petri dish with filter paper. Each Petri dish was treated with 5 mL of prepared leaf extracts every 2 d, or treated with 5 mL of sterilized distilled water as control. Germination conditions were the same as those of expt 1. Germinated seeds (radicle visible) were counted daily for 7 weeks until germination stopped.
Experiment 3: effects of leaf extracts and light on seedling growth
To assess the effects of its own litter and light on seedling growth of W. trilobata, seven concentrations of leaf extract and two levels of light treatments were examined (Experiment 3 in Supplementary Data Fig. S3). The seven concentrations of leaf extract were consistent with those in expt 2. The two light levels were high light (450 μmol m–2 s–1) and shade treatment (approx. 22·5 μmol m–2 s–1, by covering with kraft paper).
Seedlings that were 2 d old after germination were transferred from the Petri dishes into pots (7 × 7 × 8 cm, one individual seedling per pot) with a 1:1:1 mixture of sterilized soil (total nitrogen, 0·88 ± 0·23 %; total phosphorus, 0·85 ± 0·33 %), perlite and vermiculite. Vigorous seedlings were chosen for further experiment after 7 d of culture. For each leaf extract concentration, 14–20 individual seedlings were planted under high light treatment (123 seedlings in total), and 15 individual seedlings were planted for shade treatment (105 seedlings in total). The seedlings were watered every 3 d with 10 mL of leaf extracts of each concentration or 10 mL of sterilized distilled water (control treatment) until harvest. All the pots of this experiment were randomly placed in an incubator with a diurnal rhythm of 16 h day and 8 h night. We harvested the seedlings after 6 weeks and weighed above- and below-ground mass. Their shoot and root lengths were also measured.
Experiment 4: seed germination in the field
The field seed germination experiment was conducted at a location in Haikou, China (110°20′24·2′′E, 20°00′16·4′′N) where the cover of the W. trilobata population was homogeneous and >90 %. Light transmission under the plant canopy was about 5 % of full sunlight. Twelve 1 × 1 m plots were randomly selected. The plant canopy was removed and litter was cleaned to make the ground bare in six plots for the full sunlight treatment, whereas that of the other six plots was left for shade treatment. In half of the plots of these two light treatments, we added 30 seeds of W. trilobata per plot, whereas no seeds were added to the remaining plots. Thus, four treatments were created (two light levels and two seed addition treatments) with three replications (Experiment 4 in Supplementary Data Fig. S3). Germinated seeds were counted every day for 2 months.
Data analysis
Data were analysed using SAS statistical software (version 9.1) (SAS Institute Inc., 2004). Experiments 1 and 3 were analysed using one-way analysis of variance (ANOVA) with light or leaf extract treatment as fixed factors. Duncan's multiple range test was used to compare pairwise least squares means of seed germination and seedling growth (shoot and root length, above- and below-ground biomass) of W. trilobata. Experiment 2 was analysed using two-way ANOVA with both light and leaf extract treatment as factors. Tukey's multiple comparison test was used to compare pairwise least squares means of seed germination of W. trilobata. Data are presented as the mean ± s.e. Statistical significance was set at P ≤ 0·05, and the results were adjusted by Bonferroni test. A quadratic non-linear regression model was used to detect the relationships between the canopy cover of W. trilobata and relative LT under the population canopy. The results of the regression analysis were visualized using SigmaPlot Version 11.
RESULTS
Sexual reproduction and seedling recruitment of W. trilobata in the field
Although biomass allocation to flowers was about 6 ± 1·9 % of the whole individual biomass (n = 48), W. trilobata produced flowers profusely year-around and a number of ramets with numerous stem nodes where a capitulum could possibly develop (Supplementary Data Fig. S2A, B). An average of 48 seeds was found in one capitulum based on 267 capitulums, and 12 (25·4 %) Type I seeds were counted in one capitulum. In the field, the stem length of W. trilobata was not constant, and the number of individuals per square metre varied. Thus, we used two scenarios to estimate seed production based on the number of stem nodes, capitulums and total seed number in a 1 × 1 m plot with 90 % cover of W. trilobata. In the first scenario, almost 1240 stem nodes (310 ramets × 4 nodes per ramet on average) were noted, and 1240 capitulums could exist in 1 m2. In the second scenario, there were approx. 30 individuals with about 30 nodes, i.e. 900 capitulums (30 individuals × 30 nodes × 1 capitulum per node) could exist in 1 m2. According to our survey in the field and the two scenarios of estimated capitulum number, approx. 10 800 (900 capitulums × 12 Type I seeds per capitulum on average) to 14 880 fertilized seeds (1240 capitulums × 12 Type I seeds) were present in 1 m2.
In the communities invaded by W. trilobata, light transmittance decreased to <20 % when the plant canopy cover was >50 %, and it was almost murky when the cover was >75 % (Fig. 1A). Thick litter layers were also frequently found under the ramets of W. trilobata. The frequency of seedling numbers in the sampled plots is shown in Fig. 1B. For the total of 117 plots, 91 % of the plots had no detectable seedlings (Fig. 1B) under the mother ramets with low LT and thick leaf litter layer. Only 21 seedlings, which were all distributed in the bare ground around the adjacent mother ramets (Supplementary Data Fig. S4), were found in 11 plots.
Fig. 1.
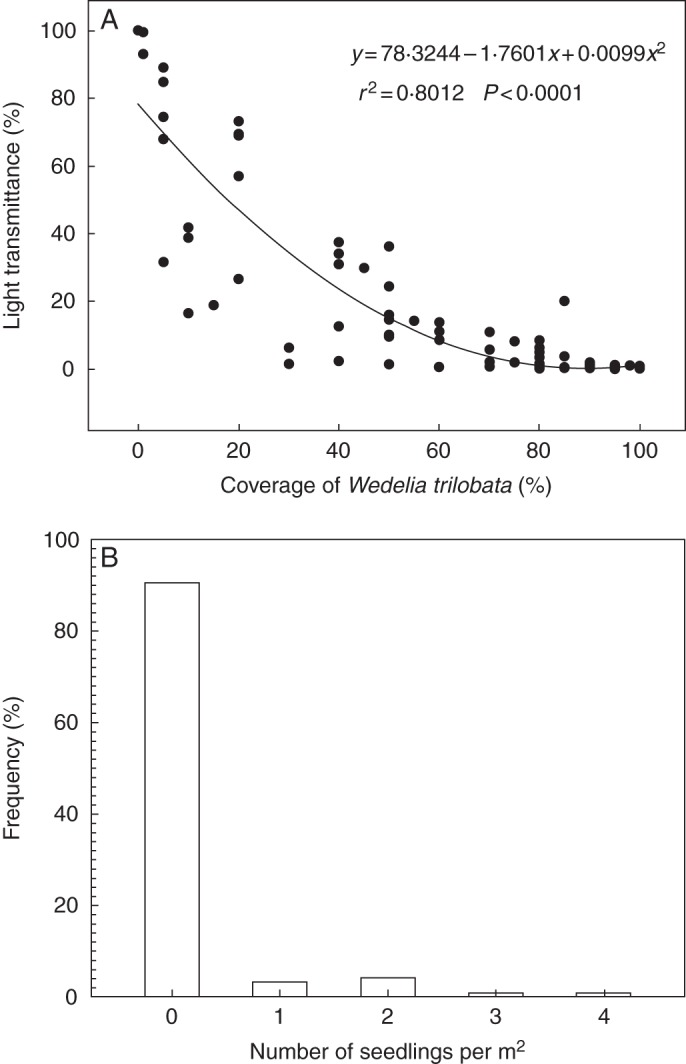
Relationship between cover of the W. trilobata population and light transmittance (A) and the frequency of the number of seedlings of W. trilobata per m2 which emerged in the surveyed plots (B).
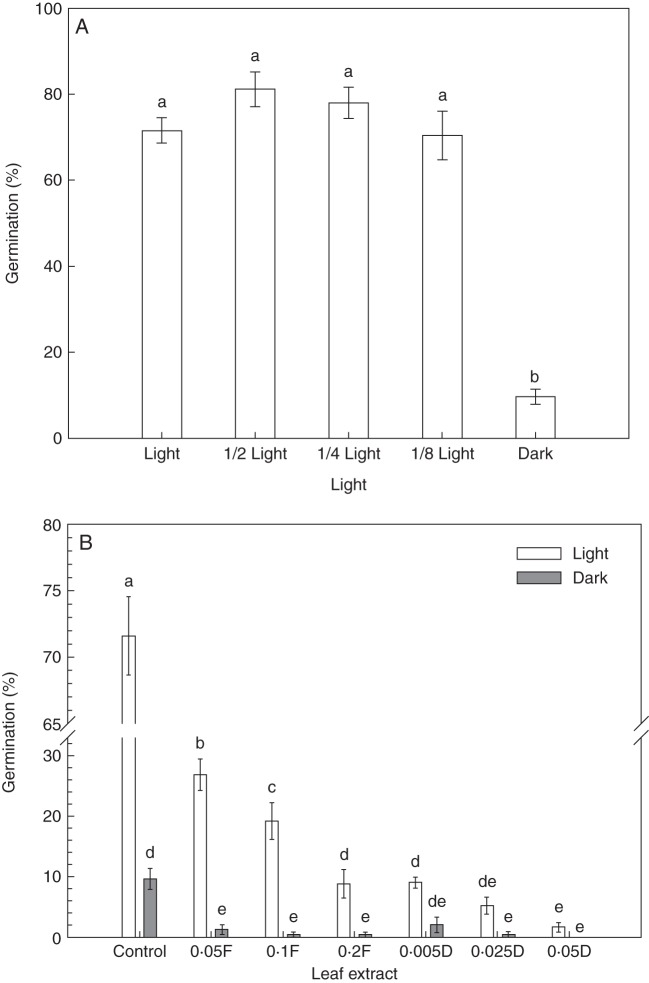
Effects of light intensity on seed germination in the laboratory (expt 1)
Darkness significantly limited seed germination (P < 0·001, Table 1; Fig. 2A). The seed germination rates were between 70 and 80 % under the four levels of light intensity, but the germination rate was only 9·6 % under the dark condition (Fig. 2A).
Table 1.
One-way ANOVA for the effects of light on seed germination of W. trilobata (expt 1)
| Factor | d.f. | Mean square | F | P |
|---|---|---|---|---|
| Light | 4 | 4363·04 | 63·64 | < 0·001 |
| Error | 20 | 68·56 |
Fig. 2.
(A) Effects of different light intensity on seed germination of W. trilobata. (B) Germination (%) of W. trilobata seeds treated with different concentrations (g mL–1) of fresh (F) or dry (D) leaf extract under high light (450 μmol m–2 s–1) or dark conditions (as indicated in the key). Error bars are the s.e. (n = 5). Different letters indicate a significant difference at P < 0·05.
Effects of leaf extracts and light on seed germination in the laboratory (expt 2)
Seed germination was greatly influenced by both leaf extracts and light (P < 0·001, Table 2; Fig. 2B). The interaction effects between leaf extracts and light on seed germination were also significant (P < 0·001, Table 2).
2.
Two-way ANOVA for the effects of both light and leaf extract on seed germination of W. trilobata (expt 2)
| Factor | d.f. | Mean square | F | P |
|---|---|---|---|---|
| Light (L) | 1 | 5870·18 | 424·45 | < 0·001 |
| Leaf extract (E) | 6 | 1881·48 | 136·04 | < 0·001 |
| L × E | 6 | 1104·15 | 79·84 | < 0·001 |
| Error | 56 | 13·83 |
In high light treatment, seed germination was significantly inhibited by the presence of both fresh and dry leaf extracts. Moreover, the aqueous leaf extract presented concentration-dependent effects on seed germination (Fig. 2B).
The seed germination rate without leaf extract (control treatment) declined from approx. 71 % in high light treatment to approx. 10 % in dark treatment. Germination of the seeds that were simultaneously exposed to leaf extracts (especially for high concentrations) and dark conditions was drastically decreased; more extremely, seeds treated with the highest concentration of dry leaf extracts (0·05 g d. wt mL–1) did not germinate under dark conditions (Fig. 2B).
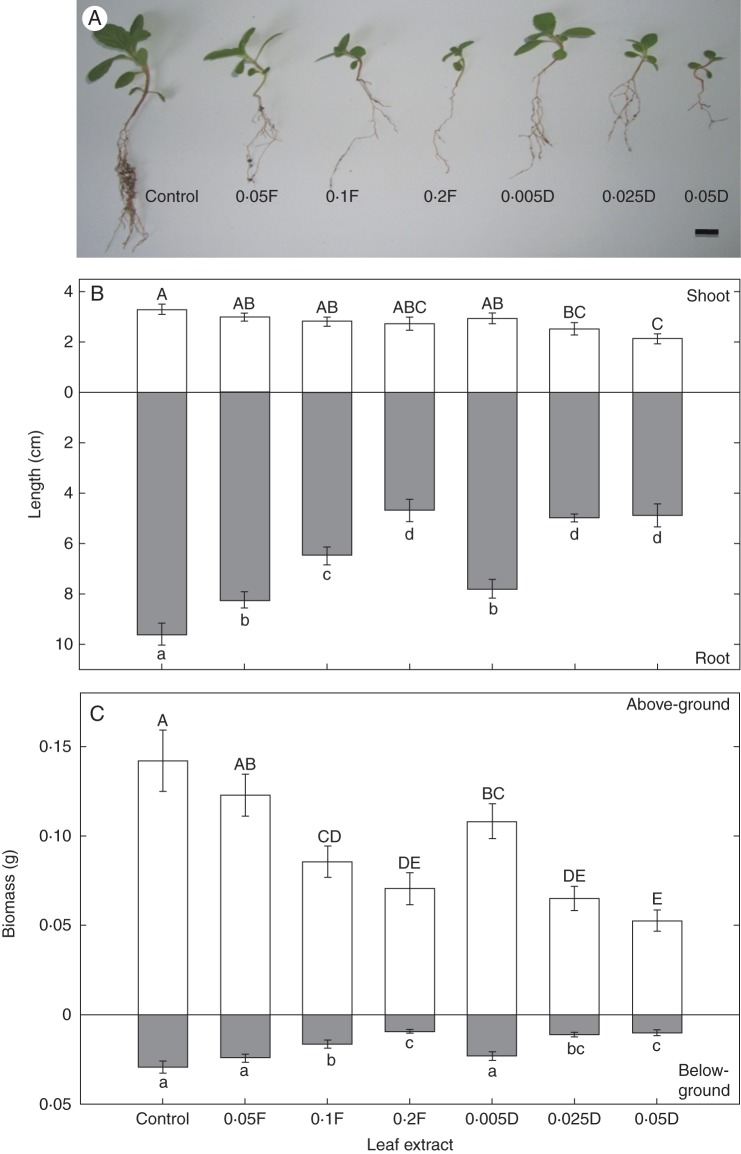
Effects of leaf extracts and light on seedling growth (expt 3)
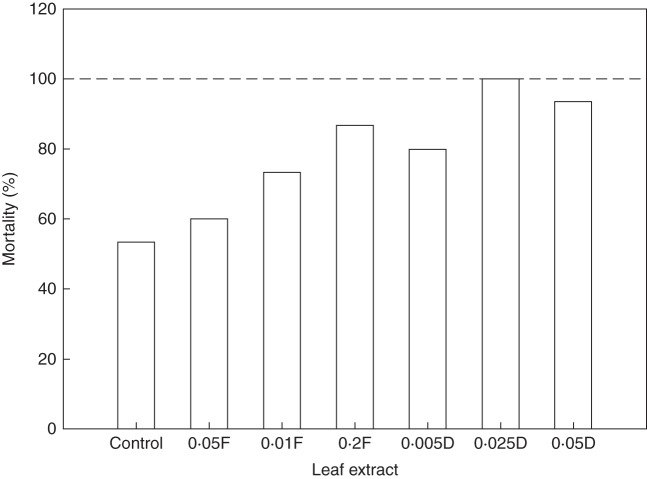
In the high light treatment, the presence of fresh or dry leaf extracts (especially moderate and high concentrations) significantly suppressed W. trilobata seedling growth (shoot and root length, above- and below-ground biomass) (Table 3; Fig. 3), but did not cause any seedling death. More notably, under the shade treatment, almost all the seedlings treated with dry leaf extracts died, and the surviving seedlings hardly grew at all and eventually died. There were insufficient data for the shade treatment to conduct statistical analysis; instead, the high mortality is shown in Fig. 4.
3.
One-way ANOVA for the effects of leaf extracts on seedling growth of W. trilobata (expt 3)
| Traits | Source | d.f. | Mean square | F | P |
|---|---|---|---|---|---|
| Shoot length | Leaf extracts | 6 | 2·44 | 3·16 | 0·007 |
| Error | 116 | 0·77 | |||
| Root length | Leaf extracts | 6 | 63·31 | 24·15 | <0·001 |
| Error | 116 | 2·62 | |||
| Above-ground biomass | Leaf extracts | 6 | 0·019 | 9·91 | <0·001 |
| Error | 116 | 0·0019 | |||
| Below-ground biomass | Leaf extracts | 6 | 0·0011 | 14·48 | <0·001 |
| Error | 116 | 0·0008 |
Fig. 3.
(A) Seedling growth (scale bar = 1 cm), (B) seedling shoot and root length, and (C) above- and below-ground seedling biomass of W. trilobata treated with different concentrations (g mL–1) of fresh (F) or dry (D) leaf extracts under high light (450 μmol m–2 s–1) for 6 weeks. Error bars are the s.e. Different letters indicate a significant difference at P < 0·05.
Fig. 4.
Mortality of W. trilobata seedlings treated with different concentrations (g mL–1) of fresh (F) or dry (D) leaf extracts under shade treatment (22·5 μmol m–2 s–1) for 6 weeks. There were 15 seedlings for each treatment.
Seed germination in the field (expt 4)
Seeds did not germinate well in the field germination experiment. Seedlings were only detected in the plots where the plant canopy was removed and seeds were added (the germination ratio was about 12·22 ± 3·9 %). No seedlings were found in any of the plots where seeds were not added, or in the plots where the plant canopy was not removed but seeds were added. Therefore, no statistical analysis was possible for the field germination.
DISCUSSION
In this study, we showed that the resources of the invasive clonal weed W. trilobata were also allocated to its sexual reproduction. In the field, W. trilobata produced fecund vigorous seeds every season, but few seedlings were found and population recruitment via seedlings was obstructed. The simulated experiments in the laboratory and the field germination experiment showed that seed germination of W. trilobata was constrained by shade and its own aqueous leaf extracts. Thus, we considered light limitation and its own leaf litter to be two of the crucial factors causing scarcely detectable seedling recruitment for this invasive clonal weed.
Sexual reproduction of W. trilobata
A review of seedling dynamics and life histories found that >40 % of clonal plants establish populations by seedling recruitment, especially for plants growing in grasslands (Eriksson, 1989). Wedelia trilobata also spreads rapidly through very vigorous clonal ramets (Wu et al., 2005; Song et al., 2010b) and a greater ratio of resources was allocated to its clonal reproduction. In the field survey, W. trilobata flowered profusely in almost all seasons. Thus, the number of seeds was considerable because at least one capitulum which possessed 10–20 fertilized seeds could be produced in every stem node. However, few seedlings of W. trilobata were found beneath the canopy of mother ramets. Also seedlings seldom emerged in the bare ground near the populations. Our findings were similar to those reported by Singh and Singh (2002) in the invasive plant Centella asiatica, i.e. the population is mainly maintained by ramets in spite of many emerging seeds and seedlings, which experience high mortality.
The inhibition of seedling recruitment will save more resources (e.g. light, water and nutrients) to promote the spread of clonal reproduction in a short period, because sexual and clonal reproduction may directly compete for resources (Thompson and Eckert, 2004). Thus, a balance between these two reproduction modes could contribute to the rapid population expansion and colonization of W. trilobata by inhibiting its seedling recruitment.
Potential mechanisms regulating seedling recruitment of W. trilobata
Critical effects of light on seed germination have been found in various species (Milberg et al., 2000). Only very few seedlings of W. trilobata in the field were found on the bare ground near to the population, and no seedlings were detected beneath W. trilobata adult ramets. The light intensity under the W. trilobata population in the field was frequently poor because of its dense and reticular branches. Moreover, seeds that fell onto the substrate of the population were often covered by the thick litter layer. Moreover, our controlled experiments showed that seeds of W. trilobata germinated better in light than in darkness, and seeds could germinate after removing the canopy in the field. Thus, lack of light might be one of the main factors causing few seeds of W. trilobata to germinate in the field. However, the response of seeds to light varies in different plant species (Ahmed and Khan, 2010). Exposure to light reduces the germination of pyrethrum (Tanacetum cinerariifolium) seed and inhibits its seedling growth (Li et al., 2011). The germination rate of larger seeds is higher in the dark than in the light, whereas smaller seeds require more light (Bell, 1999; Milberg et al., 2000). Light may act as a depth-sensing cue to small seeds that are buried too deep in the soil or for seeds to germinate on the soil surface with decreasing humidity (Schutz et al., 2002).
The presence of W. trilobata litter might be another important factor that explains the low number of seedlings detected in the field. In our chamber experiments, the application of leaf extracts of W. trilobata significantly decreased seed germination and prevented seedling growth, and seedlings even died in the presence of leaf extract and absence of light. Allelopathy of the chemicals produced from leaf litter may explain this phenomenon (Wu et al., 2009; Inderjit et al., 2011). However, numerous mechanisms may also explain the effects of litter on seedling recruitment. For instance, accumulation of a massive amount of litter may reduce the emergence of seedlings by mechanical damage (Hovstad and Ohlson, 2008), or by the osmotic potential of chemicals released from the litter (Wardle et al., 1992). Also, plant litter may affect seedling growth by altering the microbial community (Elgersma et al., 2012), or changing the light conditions of the seedling habitat (Koyama and Tsuyuzaki, 2012). After all, litter layers can affect seed germination and seedling development (Holmgren et al., 1997). In addition, thick litter layers (including fresh leaves and dry leaves) were frequently found under the mother ramets of W. trilobata in the field. Therefore, the changes in the microhabitat of dropped seeds caused by thick litter might affect seed recruitment of W. trilobata.
The results of our field investigation and laboratory experiments show that both light limitation and a layer of its own litter resulting in an unfavourable microhabitat might be the factors preventing seedling recruitment of the invasive clonal weed W. trilobata. These factors are considered to be the potential mechanisms regulating seedling recruitment of W. trilobata, and they are thought to be the potential mechanisms that regulate the balance of clonal and sexual reproduction (seedling recruitment) of clonal invasive plants. However, clonal reproduction is also affected by light conditions (Yu and Dong, 2003; Wang et al., 2008a), and the effects of litter feedback on plant growth differ among plant species (Dorrepaal et al., 2007). Further, more research on the effects of light and litter on the clonal growth of W. trilobata should be conducted in the future.
Implications for biological invasion control
For the prevention and control of alien invasive plants which rapidly expand by clonal reproduction with maintenance of sexual reproduction, such as W. trilobata, their seeds must be taken into consideration. Although seedling recruitment is limited by the presence of litter and the absence of light, W. trilobata still allocates resources for sexual reproduction and produces fertilized seeds. This is significant for population dynamics of invasive plants in new sites by long-distance seed dispersal or in the very bare ground where there is no possibility for their vegetative propagation. Seeds will establish new populations once the limiting factors are eliminated. Seeds and seedlings might be the pioneer in new habitats. Therefore, to prevent seedling recruitment, invasive plants should be eradicated before they yield seeds.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (NSFC-30970556, 31170386), the Doctoral Program of Higher Education of China (20093227110004), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Jiangsu University Research Foundation (09JDG020, 14JDG010), and the Research and Innovation Project for College Graduates of Jiangsu Province of China (CXZZ12_0697). We would like to thank Si-Chong Chen (Evolution and Ecology Research Centre, The University of New South Wales, Australia) for her valuable comments on and modifications to the early draft of the manuscript. We also appreciate the editors and anonymous reviewers of Annals of Botany for their critical comments on the manuscript.
LITERATURE CITED
- Ahmed MZ, Khan MA. Tolerance and recovery responses of playa halophytes to light, salinity and temperature stresses during seed germination. Flora. 2010;205:764–771. [Google Scholar]
- Bell D. The process of germination in Australian species. Australian Journal of Botany. 1999;47:475–517. [Google Scholar]
- Brandel M. Dormancy and germination of heteromorphic achenes of Bidens frondosa. Flora. 2004;199:228–233. [Google Scholar]
- Castro S, Loureiro J, Santos C, Ater M, Ayensa G, Navarro L. Distribution of flower morphs, ploidy level and sexual reproduction of the invasive weed Oxalis pes-caprae in the western area of the Mediterranean region. Annals of Botany. 2007;99:507–517. doi: 10.1093/aob/mcl273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavieres LA, Chacon P, Penaloza A, Molina-Montenegro MA, Arroyo MTK. Leaf litter of Kageneckia angustifolia D. Don (Rosaceae) inhibits seed germination in sclerophyllous montane woodlands of central Chile . Plant Ecology. 2007;190:13–22. [Google Scholar]
- Chu SH, Zhang QS, Liu SK, et al. Trade-off between vegetative regeneration and sexual reproduction of Sargassum thunbergii. Hydrobiologia. 2011;678:127–135. [Google Scholar]
- Dorken ME, Eckert CG. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae) Journal of Ecology. 2001;89:339–350. [Google Scholar]
- Dorning M, Cipollini D. Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecology. 2006;184:287–296. [Google Scholar]
- Dorrepaal E, Cornelissen JHC, Aerts R. Changing leaf litter feedbacks on plant production across contrasting sub-arctic peatland species and growth forms. Oecologia. 2007;151:251–261. doi: 10.1007/s00442-006-0580-3. [DOI] [PubMed] [Google Scholar]
- Elgersma KJ, Yu S, Vor T, Ehrenfeld JG. Microbial-mediated feedbacks of leaf litter on invasive plant growth and interspecific competition. Plant and Soil. 2012;356:341–355. [Google Scholar]
- Eriksson O. Seedling dynamics and life histories in clonal plants. Oikos. 1989;55:231–238. [Google Scholar]
- Fuchs MA, Krannitz PG, Harestad AS. Factors affecting emergence and first-year survival of seedlings of Carry oaks (Quercus garryana) in British Columbia, Canada. Forest Ecology and Management. 2000;137:209–219. [Google Scholar]
- Galindez G, Ortega-Baes P, Daws MI, Suhring S, Scopel AL, Pritchard HW. Seed mass and germination in Asteraceae species of Argentina. Seed Science and Technology. 2009;37:786–790. [Google Scholar]
- Gessner MO. Differences in processing dynamics of fresh and dried leaf litter in a stream ecosystem. Freshwater Biology. 1991;26:387–398. [Google Scholar]
- Harald A, Roldan B. Seedling recruitment in the invasive clonal shrub, Mahonia aquifolium Pursh (Nutt.) Oecologia. 1997;110:205–211. doi: 10.1007/s004420050151. [DOI] [PubMed] [Google Scholar]
- Hata K, Kato H, Kachi N. Litter of an alien tree, Casuarina equisetifolia, inhibits seed germination and initial growth of a native tree on the Ogasawara Islands (subtropical oceanic islands) Journal of Forest Research. 2010;15:384–390. [Google Scholar]
- Holmgren M, Scheffer M, Huston MA. The interplay of facilitation and competition in plant communites. Ecology. 1997;78:1966–1975. [Google Scholar]
- Hou YP, Peng SL, Chen BM, Ni GY. Inhibition of an invasive plant (Mikania micrantha H.B.K.) by soils of three different forests in lower subtropical China. Biological Invasions. 2011;13:381–391. [Google Scholar]
- Hovstad KA, Ohlson M. Physical and chemical effects of litter on plant establishment in semi-natural grasslands. Plant Ecology. 2008;196:251–260. [Google Scholar]
- Inderjit, Evans H, Crocoll C, et al. Volatile chemicals from leaf litter are associated with invasiveness of a Neotropical weed in Asia. Ecology. 2011;92:316–324. doi: 10.1890/10-0400.1. [DOI] [PubMed] [Google Scholar]
- IUCN. Auckland: Invasive Species Specialist Group; 2001. 100 of the world's worst invasive alien species. [Google Scholar]
- Karalius T, Alpert P. High abundance of introduced plants on ancient Native American middens. Biological Invasions. 2010;12:1125–1132. [Google Scholar]
- Koivunen S, Saikkonen K, Vuorisalo T, Mutikainen P. Heavy metals modify costs of reproduction and clonal growth in the stoloniferous herb Potentilla anserina. Evolutionary Ecology. 2004;18:541–561. [Google Scholar]
- Kolb A, Alpert P, Enters D, Holzapfel C. Patterns of invasion within a grassland community. Journal of Ecology. 2002;90:871–881. [Google Scholar]
- Koyama A, Tsuyuzaki S. Mechanism of facilitation by sedge and cotton-grass tussocks on seedling establishment in a post-mined peatland. Plant Ecology. 2012;213:1729–1737. [Google Scholar]
- Li J, Yin LY, Jongsma MA, Wang CY. Effects of light, hydropriming and abiotic stress on seed germination, and shoot and root growth of pyrethrum (Tanacetum cinerariifolium) Industrial Crops and Products. 2011;34:1543–1549. [Google Scholar]
- Li XH, Jiang DM, Alannusa, Zhou QL, Oshida T. Comparison of seed germination of four Artemisia species (Asteraceae) in northeastern Inner Mongolia, China. Journal of Arid Land. 2012;4:36–42. [Google Scholar]
- Li ZY, Xie Y. China Forest Publishing House; 2003. Invasive species in China (in Chinese) [Google Scholar]
- Liu F, Liao YY, Li W, Chen JM, Wang QF, Motley TJ. The effect of pollination on resource allocation among sexual reproduction, clonal reproduction, and vegetative growth in Sagittaria potamogetifolia (Alismataceae) Ecological Research. 2010;25:495–499. [Google Scholar]
- Luo J, Cardina J. Germination patterns and implications for invasiveness in three Taraxacum (Asteraceae) species. Weed Research. 2012;52:112–121. [Google Scholar]
- Milberg P, Andersson L, Thompson K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Science Research. 2000;10:99–104. [Google Scholar]
- Olson BE, Wallander RT. Effects of invasive forb litter on seed germination, seedling growth and survival. Basic and Applied Ecology. 2002;3:309–317. [Google Scholar]
- Otfinowski R, Kenkel NC. Clonal integration facilitates the proliferation of smooth brome clones invading northern fescue prairies. Plant Ecology. 2008;199:235–242. [Google Scholar]
- Rautiainen P, Koivula K, Hyvarinen M. The effect of within-genet and between-genet competition on sexual reproduction and vegetative spread in Potentilla anserina ssp. egedii. Journal of Ecology. 2004;92:505–511. [Google Scholar]
- Roiloa SR, Rodriguez-Echeverria S, de la Pena E, Freitas H. Physiological integration increases the survival and growth of the clonal invader Carpobrotus edulis. Biological Invasions. 2010;12:1815–1823. [Google Scholar]
- SAS Institute Inc. Cary, NC, USA: SAS Institute Inc.; 2004. SAS/STAT user's guide.Version 9.1. [Google Scholar]
- Schutz W, Milbert P, Lamont BB. Seed dormancy, after-ripening and light requirements of four annual Asteraceae in south-western Australia. Annals of Botany. 2002;90:707–714. doi: 10.1093/aob/mcf250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Singh JS. Recruitment and competitive interaction between ramets and seedlings in a perennial medicinal herb, Centella asiatica. Basic and Applied Ecology. 2002;3:65–76. [Google Scholar]
- Song LY, Li CH, Peng SL. Elevated CO2 increases energy-use efficiency of invasive Wedelia trilobata over its indigenous congener. Biological Invasions. 2010a;12:1221–1230. [Google Scholar]
- Song LY, Chow WS, Sun LL, Li CH, Peng CL. Acclimation of photosystem II to high temperature in two Wedelia species from different geographical origins: implications for biological invasions upon global warming. Journal of Experimental Botany. 2010b;61:4087–4096. doi: 10.1093/jxb/erq220. [DOI] [PubMed] [Google Scholar]
- Sonohat G, Balandier P, Ruchaud F. Predicting solar radiation transmittance in the understory of even-aged coniferous stands in temperate forests. Annals of Forest Science. 2004;61:629–641. [Google Scholar]
- Sun HZ, Lu JJ, Tan DY, Baskin JM, Baskin CC. Dormancy and germination characteristics of the trimorphic achenes of Garhadiolus papposus (Asteraceae), an annual ephemeral from the Junggar Desert, China. South African Journal of Botany. 2009;75:537–545. [Google Scholar]
- Swope SM, Parker IM. Widespread seed limitation affects plant density but not population trajectory in the invasive plant Centaurea solstitialis. Oecologia. 2010;164:117–128. doi: 10.1007/s00442-010-1641-1. [DOI] [PubMed] [Google Scholar]
- Thaman RR. IAS Technical Report. Suva, Fiji Islands: Institute of Applied Science, University of the South Pacific; 1999. Wedelia trilobata: daisy invader of the Pacific islands. [Google Scholar]
- Thompson FL, Eckert CG. Trade-offs between sexual and clonal reproduction in an aquatic plant: experimental manipulations vs. phenotypic correlations. Journal of Evolutionary Biology. 2004;17:581–592. doi: 10.1111/j.1420-9101.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- Valera-Burgos J, Diaz-Barradas MC, Zunzunegui M. Effects of Pinus pinea litter on seed germination and seedling performance of three Mediterranean shrub species. Plant Growth Regulation. 2012;66:285–292. [Google Scholar]
- Wang MT, Zhao ZG, Du GZ, He YL. Effects of light on the growth and clonal reproduction of Ligularia virgaurea. Journal of Integrative Plant Biology. 2008a;50:1015–1023. doi: 10.1111/j.1744-7909.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Wang N, Yu FH, Li PX, et al. Clonal integration affects growth, photosynthetic efficiency and biomass allocation, but not the competitive ability, of the alien invasive Alternanthera philoxeroides under severe stress. Annals of Botany. 2008b;101:671–678. doi: 10.1093/aob/mcn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RL, Staehelin C, Dayan FE, Song YY, Su YJ, Zeng RS. Simulated acid rain accelerates litter decomposition and enhances the allelopathic potential of the invasive plant Wedelia trilobata (creeping daisy) Weed Science. 2012;60:462–467. [Google Scholar]
- Wardle DA, Nicholson KS, Ahmed M. Comparison of osmotic and allelopathic effects of grass leaf extracts on grass seed germination and radicle elongation. Plant and Soil. 1992;140:315–319. [Google Scholar]
- Weber E, Sun SG, Li B. Invasive alien plants in China: diversity and ecological insights. Biological Invasions. 2008;10:1411–1429. [Google Scholar]
- Wei SH, Zhang CX, Li XJ, et al. Factors affecting buffalobur (Solanum rostratum) seed germination and seedling emergence. Weed Science. 2009;57:521–525. [Google Scholar]
- Wu AP, Yu H, Gao SQ, et al. Differential belowground allelopathic effects of leaf and root of Mikania micrantha. Trees-Structure and Function. 2009;23:11–17. [Google Scholar]
- Wu YQ, Hu YJ, Cheng JN. Reproductive characteristics of alien plant Wedelia trilobata. Acta Scientiarum Naturalium Universitatis Sunyatseni. 2005;44:93–96. [Google Scholar]
- Yu FH, Dong M. Effect of light intensity and nutrient availability on clonal growth and clonal morphology of the stoloniferous herb Halerpestes ruthenica. Acta Botanica Sinica. 2003;45:408–416. [Google Scholar]
- Yu FH, Wang N, Alpert P, He WM, Dong M. Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. American Journal of Botany. 2009;96:1983–1989. doi: 10.3732/ajb.0800426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.