Abstract
Background and Aims
Increasing soil salinity poses a major plant stress in agro-ecosystems worldwide. Surprisingly little is known about the quantitative effect of elevated salinity on secondary metabolism in many agricultural crops. Such salt-mediated changes in defence-associated compounds may significantly alter the quality of food and forage plants as well as their resistance against pests. In the present study, the effects of soil salinity on cyanogenesis in white clover (Trifolium repens), a forage crop of international importance, are analysed.
Methods
Experimental clonal plants were exposed to five levels of soil salinity, and cyanogenic potential (HCNp, total amount of accumulated cyanide in a given plant tissue), β-glucosidase activity, soluble protein concentration and biomass production were quantified. The attractiveness of plant material grown under the different salt treatments was tested using cafeteria-style feeding trials with a generalist (grey garden slug, Deroceras reticulatum) and a specialist (clover leaf weevil, Hypera punctata) herbivore.
Key Results
Salt treatment resulted in an upregulation of HCNp, whereas β-glucosidase activity and soluble protein concentration showed no significant variation among treatments. Leaf area consumption of both herbivore species was negatively correlated with HCNp, indicating bottom-up effects of salinity-mediated changes in HCNp on plant consumers.
Conclusions
The results suggest that soil salinity leads to an upregulation of cyanogenesis in white clover, which results in enhanced resistance against two different natural herbivores. The potential implications for such salinity-mediated changes in plant defence for livestock grazing remain to be tested.
Keywords: Bottom-up effects, β-glucosidase, cyanide, cyanogenesis, cyanogenic glucoside, HCNp, herbivore, forage crop, secondary metabolite, Trifolium repens, trophic interactions, white clover
INTRODUCTION
The increase in soil salinity levels worldwide poses a profound risk to the global food supply and to ecological interactions within zones of increased salinity. Salt stress compromises growth and causes declines in productivity in a considerable variety of plant species. Elevated soil salinity reduces water potential and causes shifts in ion homeostasis, thereby affecting all major plant processes including photosynthesis, growth and protein synthesis (Parida and Das, 2005). Compared with the detailed information available on the effects of soil salinity on plant primary metabolisms and plant productivity, there is a lack of information on the effects of salinity on defensive secondary plant compounds and the resulting bottom-up effects on plant consumers (Slocum et al., 2002; Ballhorn et al., 2011).
Cyanogenesis – the enzymatically enhanced release of toxic hydrogen cyanide (HCN) from pre-formed cyanide-containing precursors in response to cell damage – is one of the most widely distributed chemical defences in the plant kingdom. Overall, cyanogenesis is a trait observed in >3000 species of plants belonging to 110 families (Poulton, 1990), and belongs to the most common defensive traits in crop plants (Jones, 1988). Cyanogenic plants compartmentalize the cyanogenic precursors (mostly cyanogenic glycosides) in vacuoles and hydrolyse enzymes (β-glucosidases) in the apoplast to avoid autotoxicity in the undamaged plant (Kakes, 1985). When plant tissues are mechanically damaged by herbivores, the precursors and enzymes are brought into contact, resulting in formation of unstable α-hydroxy nitriles which decompose into cyanide and a corresponding carbonyl compound either spontaneously or through enzymatic acceleration by another enzyme (α-hydroxy nitrile lyase) (Selmar et al., 1987, 1989). Cyanogenesis is an efficient direct defence against a broad range of herbivores (Ballhorn et al., 2005, 2009a, 2010a, b). Cyanide inhibits the mitochondrial electron transport chain and cellular respiration (Zagrobelny et al., 2004; Ballhorn et al., 2009b). Furthermore, the bitterness of cyanogenic glycosides serves as a deterrent by decreasing the plant's palatability to generalist herbivores (Nahrstedt, 1985; Jones, 1998). Although cyanogenesis is widely distributed in agricultural plants, and increasing soil salinity represents an almost ubiquitous phenomenon in high productivity agricultural systems worldwide, the effects of increasing soil salinity on plant cyanogenesis are little understood. Such effects may have far-reaching impact on the function of agro-ecosystems, productivity and food safety.
In the present study, we used Trifolium repens (white clover) as an experimental plant to contribute to filling the gap in the understanding of the quantitative effects of soil salinity on plant cyanogenesis. White clover is a cyanogenic perennial legume of the family Fabaceae. It has been extensively researched for its polymorphism of cyanogenesis, which is controlled by alleles at two independent loci. The Ac locus controls the synthesis of the cyanogenic precursors, linamarin and lotaustralin, from hydrophobic proteinogenic amino acids. The Li locus controls the production of the enzyme linamarase, responsible for cleaving cyanogenic glycosides following mechanical damage to plant tissues (Hughes, 1991). Variable expression of alleles Ac and Li produces four cyanotypes (AcLi, Acli, acLi and acli), leading to a substantial range in effective cyanogenesis among white clover genotypes (Kakes, 1993).
White clover is a pasture crop of considerable international abundance, being highly nutritious to grazing animals while also maintaining soil fertility by providing nitrogen through its symbiotic interactions with rhizobia (Abberton and Thomas, 2010). It is used predominantly as livestock forage in the Mediterranean, Asia, Southern Africa, North and South America, Australia and New Zealand. Many of these temperate and semi-arid regions using irrigation face significant increases in soil salinity over the coming decades (Yeo, 1999). Beyond effects on livestock due to reduction of food quality, the effects of altered cyanogenesis on natural herbivores probably result in potentially far-reaching effects on non-vertebrate and vertebrate herbivore communities in agro-ecosystems (Bonte et al., 2010).
To elucidate the quantitative effects of soil salinity on plant cyanogenesis and plant–herbivore interactions, we compared plant responses on a whole-plant level, and on the level of individual leaves of a defined developmental stage. Using whole plants allowed for measuring the actual production of cyanogenic glycosides per plant individual, and individual leaves were used for cafeteria-style bioassays involving two common herbivores of T. repens: the clover weevil Hypera punctata (Cucurlionidae) and the grey garden slug Deroceras reticulatum (Agriolimacidae). In our experiments, we used clonal AcLi plants, and leaves of a defined developmental stage to reduce uncontrolled genotypic and ontogenetic variation of traits among plant individuals (Ballhorn et al., 2011). The present study is one of the first reporting quantitative effects of soil salinity on plant defence and different above-ground natural herbivores.
MATERIALS AND METHODS
Plant material
We screened 35 white clover plants (Trifolium repens, Fabaceae) growing at a coastal site near Newport (OR, USA) for cyanogenesis. All plants were cyanogenenic, thus producing both cyanogenic glycosides and a corresponding β-glucosidase (AcLi) enabling the rapid release of HCN. Nine of these 35 were selected randomly and clonally propagated using stem cuttings. The release of HCN was tested as described below for the quantification of the cyanogenic potential but without addition of external β-glucosidase. Plants were grown in the greenhouse adjusted to resemble natural outdoor conditions. Light in the greenhouse was provided by a combination (1 : 1) of HQI-BT 400 W (Osram) and RNP-T/LR 400 W (Radium) lamps with a light regime of 15 : 9 h light:dark under a photon flux density of 550–700 μmol photons m–2 s–1 at table height. The temperature was set at 24 °C during the light period and at 17 °C during the dark period. Relative air humidity was adjusted to 70–80 %. Plants were cultivated in plant containers of 10 × 10 × 11 cm (width, length, height; one plant per pot) in a 1 : 1 ratio of potting soil (Fox Farms, Arcata, CA, USA) and sand (grain size 0·5 mm). The substrate was autoclaved at 121 °C for 35 min at a pressure of 1260 mbar. To prepare soil for the salinity treatments, dry soil was enriched with salt (NaCl, p.a. grade; Merck) resulting in salt concentrations of 1·2 mg L–1 (level 1), 1·6 mg L–1 (level 2), 2·0 mg L–1 (level 3), 2·4 mg L–1 (level 4) and 2·8 mg L–1 (level 5). The ultimate salinity of the experimental soils was determined by measuring the electrical conductivity based on a soil-saturated paste extract (ECe) according to Rhoades et al. (1989). Salinity units are expressed as deci-Siemens per metre (dS m–1); n = 5 samples were analysed from the respective soil mixture. We recorded the following conductivities (dS m–1): control (0·2 ± 0·1), level 1 (1·7 ± 0·2), level 2 (2·3 ± 0·2), level 3 (2·9 ± 0·1), level 4 (3·4 ± 0·2) and level 5 (3·9 ± 0·2).
All plants were initially fertilized with 50 mL of a 0·2 % aqueous solution of Flory-3 fertilizer [NPK plus magnesium (%); 15, 10, 15, +2; EUFLOR, Munich, Germany]. To replicate natural growing conditions further, experimental plants were inoculated with a nodulating rhizobia strain isolated from white clover plants derived from the natural site in Newport, OR, USA. When harvested for chemical analyses and feeding trials, all plants were extensively nodulated.
All potted plants were placed in individual plastic trays. During watering, overflow was prevented to avoid dilution of salt concentration in the soil. The position of plants in the greenhouse was changed every 3 d to control for position effects. Feeding experiments, chemical analyses of leaf material and leaf number counts were conducted after a cultivation period of 5 weeks. Above-ground biomass was weighed on a balance (Mettler Toledo, XA 204 Delta Range, Zurich, Switzerland) to the nearest 0·001 g. We did not include below-ground material in our analyses as clover roots were not used as a food source by either herbivore species and did not show measurable levels of cyanogenic glycosides and β-glucosidase activity.
Herbivores
Clover leaf weevils (Hypera punctata) and slugs (Deroceras reticulatum) were collected in the wild from the same location as the white clover stock material and rhizobia used to produce the experimental plants. Beetles were randomly collected from white clover host plants to represent natural ratios of sexes and ages. To promote experimental handling of slugs, we collected juvenile individuals of a similar size (about 2 cm in length). The insects and slugs were collected no longer than 4 d before the experiments and were maintained individually in transparent 250 mL plastic cups (water supplied on cotton) at a temperature regime of 18 °C (day, 14 h) and 14 °C (night, 10 h) to resemble natural outdoor conditions (climatic chamber; Conviron BDW 160-R walk-in CE chamber; Conviron, Winnipeg, Canada). To ensure a similar physiological state, before the feeding trials the insects were kept for 24 h with ad libitum access to white clover leaf material but they were food deprived for 2 h prior to the bioassays. The slugs were treated identically but were deprived of food for 24 h prior the experiment.
Cyanogenic potential (HCNp)
The HCNp of white clover (whole plants and individual leaves of a defined developmental stage) was analysed by complete enzymatic degradation of cyanogenic glycosides, and HCN released from cyanogenic precursors was spectrophotometrically quantified (at 585 nm) using the Spectroquant® cyanide test (Merck, Darmstadt, Germany) following the method of Ballhorn et al. (2005). External β-glucosidase isolated from lima bean (Fabaceae: Phaseolus lunatus L.) was added in excess (100 μL, 20 nkat), to ensure complete degradation of cyanogenic precursors. This enzyme, isolated following Ballhorn et al. (2006), showed high affinity for cyanogenic glyosides in white clover as both plants produce the same two glycosides, linamarin and lotaustralin. In short, to obtain β-glucosidase for quantification of HCNp, whole young lima bean leaves were weighed and homogenized in a 4-fold volume of 67 mmol L–1 phosphate buffer adjusted to pH 6·4. The extract was filtered through cotton fabric and centrifuged at 20 000 g and 4 °C (RC5C, Sorvall). The protein-containing supernatant was concentrated by ammonium sulfate fractionation and filtered through membrane caps with a pore size <10 000 kDa (Schleicher & Schuell BioScience GmbH, Dassel, Germany). As the β-glucosidase solution used for HCNp quantification in this study was prepared from β-glucosidase derived from cyanogenic lima bean, we tested the enzyme solution for contamination with cyanide. No detectable levels of cyanide were found in β-glucosidase enzyme solution prepared from lima bean leaves.
Activity of β-glucosidase
The activity of β-glucosidase was measured according to Ballhorn et al. (2006) using p-NP-glucoside (Merck) as the chromogenic artificial substrate (2 mmol L–1). The p-nitrophenol released was quantified spectrophotometrically at 400 nm (Ultraspec 3000; Pharmacia Biotech, Nümbrecht, Germany). The β-glucosidase activity was calculated per gram of leaf fresh weight as katal (kat). An enzyme activity of 1 kat is defined as a substrate conversion rate of 1 mol of substrate per second under standard temperature and pressure. Enzyme activity was calculated using a coefficient of extinction for p-nitrophenol (400 nm = 16 159 L mol–1 cm; Voß, 2001). The same extract used for the determination of HCNp was also used for measuring β-glucosidase activity in leaves. To remove cyanogenic precursors from the β-glucosidase extract, the leaf homogenate was incubated in a water bath for 10 min at 30 °C prior to analysis. This incubation period proved to be sufficient to remove cyanide from the preparation.
Soluble protein concentration
Covariation of cyanogenic and nutritive plant traits, particularly soluble protein (Ganzhorn, 1992), may strongly determine the overall attractiveness or resistance of plants to herbivores. The soluble protein concentration in clover leaves was quantified according to Bradford (1976). Leaf material was homogenized in ice-cold sodium acetate buffer (pH 5·0). Leaf extracts were centrifuged at 13 000 g in a cooled (4 °C) centrifuge (Eppendorf 5810 R) and the supernatant was filtered over NAP™ columns containing Sephadex™ G-25 DNA-Grade (GE Healthcare, Munich, Germany). Subsequently, 5 μL of the eluate were pipetted on microplates (96-well Microplates, F-bottom; Greiner Bio-One, Frickenhausen, Germany), and 250 μL of Bradford reagent (diluted with deionized water in the ratio 1 : 4) were added. The protein concentration of samples was spectrophotometrically quantified at 595 nm. Bovine serum albumin solutions (Merck) in the range between 10 and 600 μg mL–1 served as standard.
Feeding experiments with clover leaf weevils (Hypera punctata)
Single beetles were placed in 9 cm Petri dishes lined with moist filter paper to avoid wilting of leaf material. Feeding experiments were conducted under controlled conditions (24 °C, photon flux density of 200 μmol m−2 s−1). In each Petri dish, leaf material from plants grown under each of the different salt treatments was offered simultaneously to the insects in a cafeteria-style experiment for 6 h. For the feeding trials, leaves of a defined developmental stage were selected to reduce potential effects of leaf age on herbivore choice behaviour. Leaves used for the experiments were fully developed and inserted four leaf insertion positions down the apex. To exclude further the effects of leaf morphology, in particular leaf size, on herbivore choice behaviour, we used leaf discs (7 mm in diameter) rather than intact leaves. After the feeding experiments (6 h), leaf discs were digitally photographed (Camedia C-4000 Zoom; Olympus, Hamburg, Germany) on a scale to calculate missing leaf area using the analySIS software (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
We used field-collected weevils in natural ratios of sexes in the experiments. Before the feeding trials, the insects were kept for 24 h with ad libitum access to white clover leaf material but they were food deprived for 2 h prior to the bioassays to guarantee a similar physiological state of the insects.
Feeding experiments with grey garden slugs (Deroceras reticulatum)
In feeding trials with slugs, the same type of cafeteria experiment was conducted as described for clover weevils, offering leaf material from plants grown at different soil salinity for 6 h. The missing leaf area was again quantified using the photoimaging approach mentioned above. However, in feeding trials with slugs, Petri dishes were not lined with filter paper (as slugs used filter paper as an additional food source in preliminary experiments) and experiments were conducted at a lower temperature (18 °C) and in the dark to account for the fact that slugs are nocturnal. To guarantee high air humidity in the feeding arenas, Petri dishes were placed in a large plastic box containing wet cotton tissue.
Statistical analysis
To test for significant differences in plant parameters (HCNp, β-glucosidase activity and soluble protein) among salt treatments, we applied post-hoc analyses [Tukey's least significant difference (l.s.d.); P < 0·05] after one-way analysis of variance (ANOVA) using the respective plant feature as variable and ‘salt treatment’ as factor. Analyses of plant traits were carried out separately for both leaves of a specific stage and entire plants. In feeding trials, herbivore leaf area consumption among salt-treated plants was quantified and compared over the various treatments using post-hoc analyses (Tukey's l.s.d.; P < 0·05) after one-way ANOVA. Correlations between cyanogenic potential in leaves derived from differently salt-treated plants and the observed variation in herbivore feeding behaviour were analysed with Pearson's correlations. All statistical analyses were conducted using SPSS (IBM SPSS Statistics 21).
RESULTS
Plant biomass and leaf number
Production of above-ground plant biomass was only marginally affected by salt treatments. White clover plants grown at the highest salt concentration (level 5; 3·9 dS m–1) developed less above-ground biomass (by a factor of 0·9) compared with the controls. However, the differences in biomass production among the different treatment groups were not significant (Fig. 1A). In line with the limited effects on overall plant biomass, leaf number of experimental plants did not change significantly in response to salt treatments (Fig. 1B). The number of leaves of plants grown at the highest level of soil salinity (level 5) was slightly reduced compared with the controls (by a factor of 0·9).
Fig. 1.
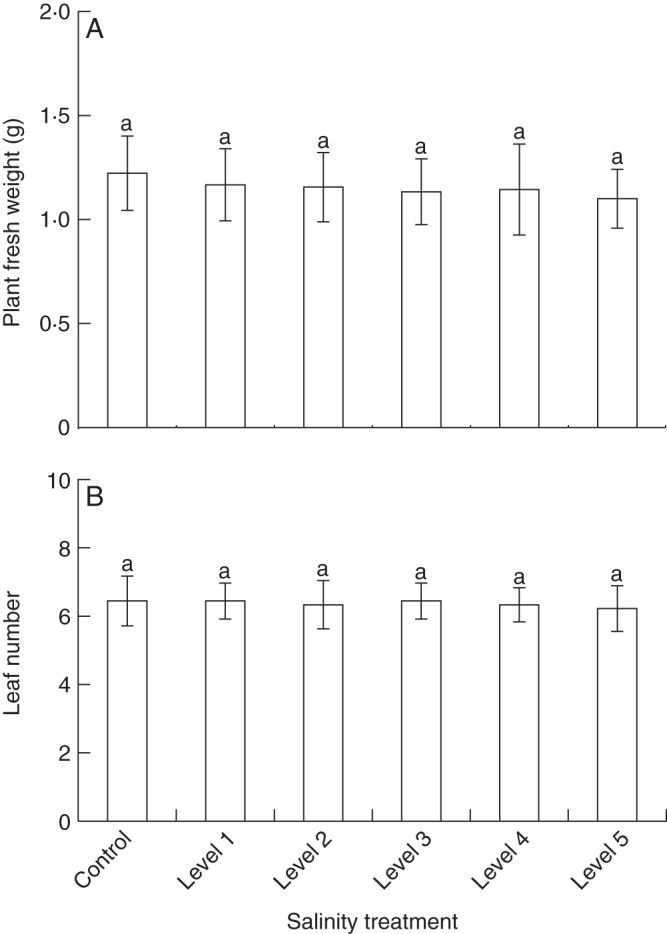
Growth parameters of white clover plants under varying soil salinity. White clover plants (n = 9 plants per group) were cultivated under six salt (NaCl, sodium chloride) treatment conditions. Soil salinity was measured as electrical conductivity (dS m–1) in soil-saturated paste extracts; control plants were grown in soil without added salt (control, 0·2 ± 0·1; level 1, 1·7 ± 0·2; level 2, 2·3 ± 0·2; level 3, 2·9 ± 0·1; level 4, 3·4 ± 0·2; level 5, 3·9 ± 0·2). Above-ground plant fresh weight (A) and leaf number (B) were determined. Bars are means ± s.d. Significant differences among treatments are indicated by lower case letters [according to post-hoc analysis (l.s.d.; P < 0·05) after one-way ANOVA].
Chemical traits of whole plants
In extracts prepared from entire plants (above-ground material), we observed significant differences in HCNp among experimental groups (Table 1; Fig. 2A). Compared with the controls, plants exposed to the lowest level of soil salinity (level 1; 1·7 dS m–1) showed a non-significantly increased HCNp by a factor of 1·1, whereas in all other experimental groups (levels 2–5; 2·3–3·9 dS m–1) we observed significantly elevated HCNp which increased with applied salt concentrations (Fig. 2A). In plants growing at soil salinity levels 2–5, the HCNp was increased by factors of 1·2, 1·4, 1·5 and 1·6 compared with non-salt-treated controls. In contrast to the quantitative effects of soil salinity on HCNp, neither β-glucosidase activity nor the amounts of soluble protein were significantly affected by the experimental treatments (Fig. 2B, C).
Table 1.
Chemical traits and growth parameters of plants
| Plant traits | F5,48 | P |
|---|---|---|
| HCNp | 28·697 | <0·001 |
| Soluble protein | 0·131 | 0·984 |
| β-Glucosidase activity | 0·618 | 0·686 |
| Leaf number | 0·488 | 0·783 |
| Plant fresh weight | 0·195 | 0·963 |
Individual leaves of a defined developmental stage were quantitatively analysed for cyanogenic potential (HCNp; μmol HCN g–1 f. wt), soluble protein (mg g–1 f. wt) and β-glucosidase activity (μkat g–1 f. wt).
Number of leaves per plant as well as total fresh weight (g) were determined.
Differences in traits among soil salinity treatment groups were tested for significance with one-way ANOVAs (P < 0·05), n = 9 plants per group.
Fig. 2.
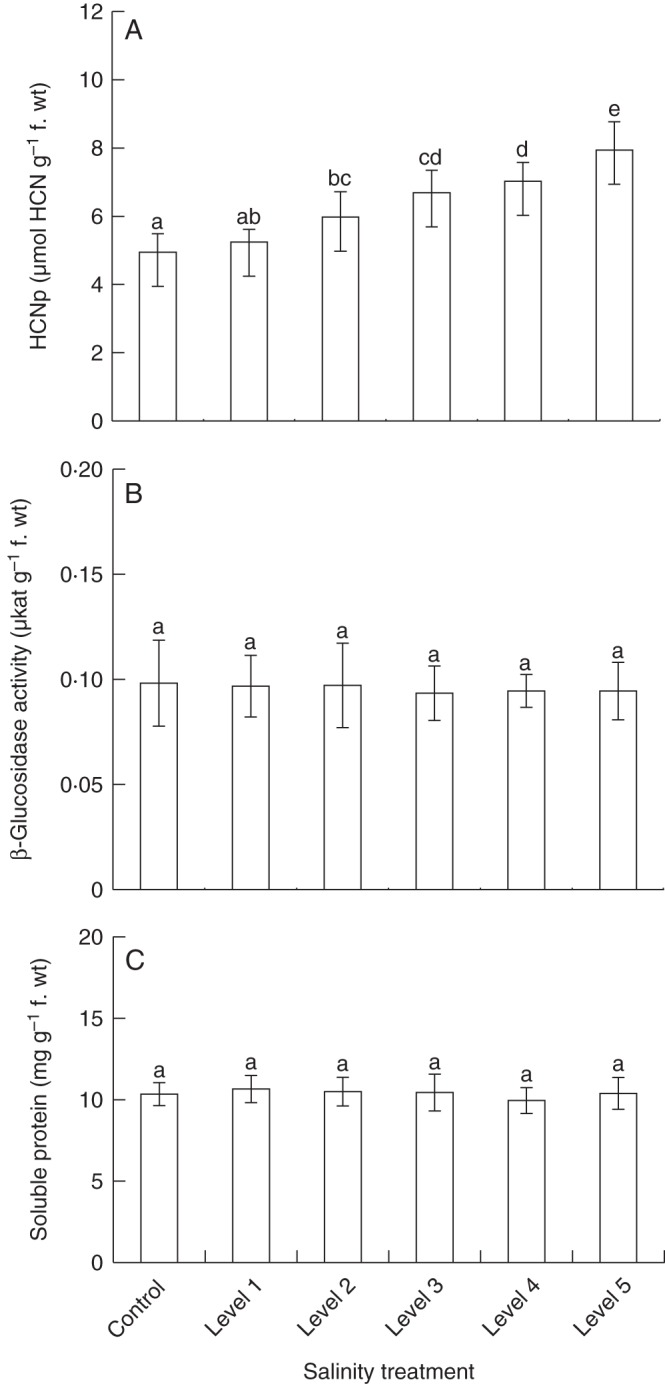
Responses of whole plants to variation in soil salinity. Six groups (n = 9 plants per group) of clonal white clover plants were cultivated under six salt (NaCl, sodium chloride) treatment conditions. Soil salinity was measured as electrical conductivity (dS m–1) in soil-saturated paste extracts; control plants were grown in soil without added salt (control, 0·2 ± 0·1; level 1, 1·7 ± 0·2; level 2, 2·3 ± 0·2; level 3, 2·9 ± 0·1; level 4, 3·4 ± 0·2; level 5, 3·9 ± 0·2). Whole plants were analysed for cyanogenic potential (HCNp, amount of cyanogenic precursors) (A), β-glucosidase activity (B) and soluble protein concentration (C). Bars are means ± s.d. Significant differences among treatments are indicated by lower case letters [according to post-hoc analysis (l.s.d.; P < 0·05) after one-way ANOVA].
Chemical traits of leaves used in feeding trials
Corresponding to the changes in the overall cyanide production per plant in response to salt treatment, the HCNp of individual leaves of a defined developmental stage was significantly affected by soil salinity (Table 2; Fig 3). Compared with whole plants, the HCNp of these leaves was 3·3–3·7 times higher, due to the fact that for the analyses of entire plants, nearly non-cyanogenic stems and low cyanogenic older leaves were also included. The HCNp of leaves from plants that have been grown at the lowest level of soil salinity (level 1; 1·7 dS m–1) as well as leaves derived from plants growing under the next highest salt regime (level 2; 2·3 dS m–1) was non-significantly elevated compared with the controls (Fig. 3A). However, leaves from plants growing at the higher salinity levels (levels 3–5; 2·9–3·9 dS m–1) showed significantly elevated HCNp values (by factors of 1·2, 1·3 and 1·4). As for entire plants, β-glucosidase activity and soluble protein levels in individual leaves were not significantly affected by salt treatment (Fig. 3B, C).
Table 2.
Chemical traits of leaves used in feeding trials
| Leaf traits | F5,48 | P |
|---|---|---|
| HCNp | 27·249 | <0·001 |
| Soluble protein | 0·007 | 1·000 |
| β-Glucosidase activity | 0·105 | 0·991 |
Individual leaves of a defined developmental stage were quantitatively analysed for cyanogenic potential (HCNp; μmol HCN g–1 f. wt), soluble protein (mg g–1 f. wt) and β-glucosidase activity (μkat g–1 f. wt).
Differences in traits among soil salinity treatment groups were tested for significance with one-way ANOVAs (P < 0·05), n = 9 leaves per group.
Fig. 3.
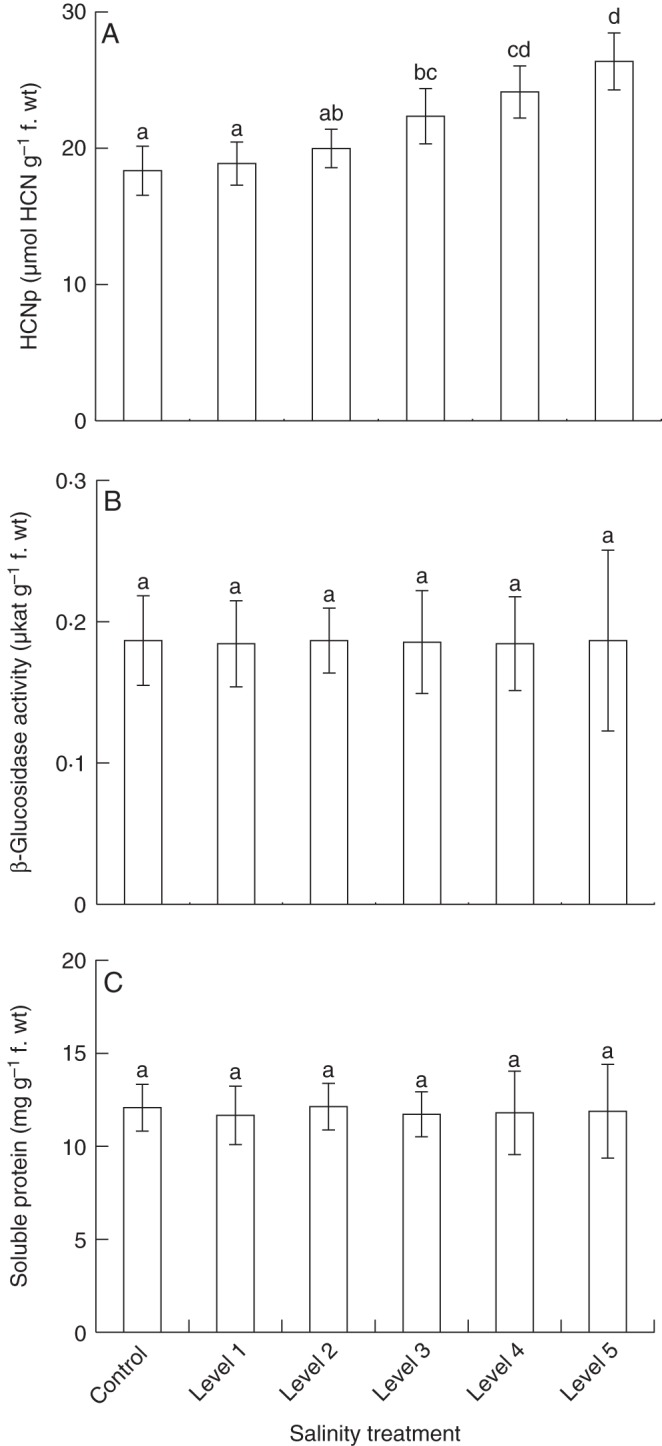
Variation of chemical traits of individual leaves under different soil salinity. Six groups (n = 9 plants per group) of clonal white clover plants were cultivated under six salt (NaCl, sodium chloride) treatment conditions. Soil salinity was measured as electrical conductivity (dS m–1) in soil-saturated paste extracts; control plants were grown in soil without added salt (control, 0·2 ± 0·1; level 1, 1·7 ± 0·2; level 2, 2·3 ± 0·2; level 3, 2·9 ± 0·1; level 4, 3·4 ± 0·2; level 5, 3·9 ± 0·2). Leaves of a defined developmental stage (fully unfolded, four leaf insertion positions down the apex) were analysed for cyanogenic potential (HCNp, amount of cyanogenic precursors) (A), β-glucosidase activity (B) and soluble protein concentration (C). Bars are means ± s.d. Significant differences among treatments are indicated by lower case letters [according to least significant difference post-hoc analysis (l.s.d.; P < 0·05) after one-way ANOVA].
Choice behaviour of weevils and slugs
Among all treatments, we observed reduced leaf consumption of both herbivore species, slugs (D. reticulatum) and weevils (H. punctata), in response to elevated HCNp of salt-treated plants (Fig. 4). Leaf area consumption and HCNp were significantly negatively correlated for both herbivores (slugs, r = –0·668; weevils, r = –0·581 according to Pearson's correlation). In cafeteria-style choice experiments with slugs and weevils, the consumption of leaf material varied significantly among treatments (Fig. 5) and decreased with increasing salt exposure of plants. However, reduction of leaf area consumption was significant only for leaf material from plants grown at higher salt concentrations (levels 4 and 5; 3·4 and 3·9 dS m–1) (Fig. 5). The rate of slug herbivory declined at a greater rate than thre rate of weevil herbivory in response to elevated levels of cyanogenic precursors. While slugs consumed approximately twice the amount of leaf area as beetles among controls and plants growing at lower salt concentrations (levels 1–4), the leaf area consumed from level 5 plants was similar for both herbivore species.
Fig. 4.
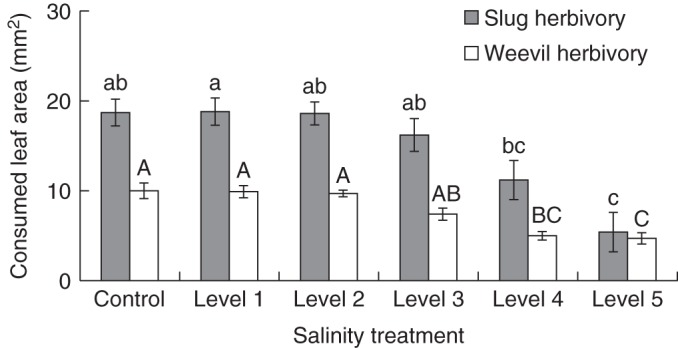
Slug and weevil herbivory on plants growing under different soil salinity. Six groups (n = 9 plants per group) of clonal white clover plants were cultivated under six salt (NaCl, sodium chloride) treatment conditions. Soil salinity was measured as electrical conductivity (dS m–1) in soil-saturated paste extracts; control plants were grown in soil without added salt (control, 0·2 ± 0·1; level 1, 1·7 ± 0·2; level 2, 2·3 ± 0·2; level 3, 2·9 ± 0·1; level 4, 3·4 ± 0·2; level 5, 3·9 ± 0·2). In cafeteria-style feeding trials, weevil (clover leaf weevil, Hypera punctata) and slug (grey garden slug, Deroceras reticulatum) were offered leaf material from all six salt treatment groups simultaneously and herbivory was measured as removed leaf area (mm2) per time (6 h). Bars represent means ± s.d. Significant differences are indicated by lower case and upper case letters [according to post-hoc analysis (l.s.d.; P < 0·05) after one-way ANOVA].
Fig. 5.
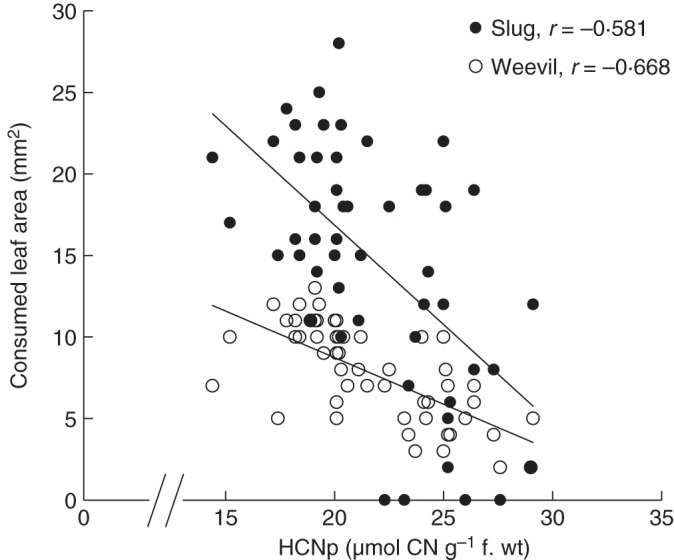
Correlation between cyanogenic potential (HCNp) and leaf consumption by slug and insect herbivores. In cafeteria-style feeding trials, individual weevils (clover leaf weevil, Hypera punctata) and slugs (grey garden slug, Deroceras reticulatum) were offered leaf material from all six salt treatment groups simultaneously. After an experimental period of 6 h, herbivory was measured as removed leaf area (mm2) per time. Data from all feeding trials (n = 9 per herbivore species) were pooled, and correlations between HCNp and leaf area consumption were calculated (two-tailed Pearson's correlation).
DISCUSSION
Soil salinity belongs to the most common and dramatic stress factors in agricultural ecosystems. Surprisingly, the quantitative effects of increasing soil salinity on plant defensive traits and subsequently on plant consumers are still little understood. In this study, we showed that elevated salinity led to significantly increased cyanogenic potential (HCNp; amount of cyanogenic precursors in a given tissue) in white clover (Trifolium repens), resulting in enhanced plant defence against mollusc and insect herbivores. In contrast to the significantly increased HCNp in our experimental plants, the activity of β-glycosidases – enzymes that critically determine the kinetics of cyanide release from cyanogenic precursors from damaged cells – as well as nutritive traits (soluble protein concentration) remained unaffected.
In a gradient of salt treatments, we observed a distinct increase in HCNp not only on the level of individual leaves but also for whole plants. Thus, we suggest a de novo synthesis rather than re-distribution of cyanogenic precursors in salt-stressed plants to be responsible for the observed effects. In other plant systems, concentration effects of secondary compounds have been demonstrated in stressed plants due to reduced biomass production under the respective stress conditions (Koricheva, 1999). In our study, the strong increase in HCNp per whole plant together with the non-significantly altered plant biomass among the plants subjected to salt treatments rules out concentration effects as the mechanism responsible for increased HCNp. However, while de novo synthesis of cyanogenic glycosides represents a plausible explanation, reduced rates of turnover of cyanogenic precursors may be another mechanism affecting variation in HCNp in salt-stressed plants (reviewed in Gleadow and Møller, 2014). Studies on gene expression will be necessary to determine functionally the mechanism resulting in increased HCNp in response to elevated salt concentration in the soil.
Although we observed no significant effects of salt treatments on plant biomass production, in other studies white clover has been demonstrated to respond to soil salinity with decreased growth. Specifically, increased soil salinity has been shown to reduce dry matter production, photosynthesis, growth rates and leaf expansion in T. repens (Rogers et al., 1993). This apparent variation in the effects of soil salinity on biomass production in white clover might have several reasons. First, as we were ultimately interested in the effects of salt-mediated plant quality on herbivores, we used exclusively fresh plant material for feeding trials in our study, as this is the form of plant material herbivores are exposed to in nature. As water comprises the major proportion of fresh weight, we may not have detected variation in dry weight in response to salt treatment. Another possibility explaining the relatively small observed response in terms of biomass accumulation may be the fact that our experimental plants originated from a pasture near the Oregon Pacific coast (approx. 2 km) and may have been pre-selected to some extent for salinity tolerance. Data showing the existence of white clover ecotypes from seawater flooding zones demonstrating enhanced resistance to soil salinity have recently been published (White et al., 2014). Flooding, however, has not occurred in recent times at our site, but the input of salt through wind over time and concentration effects via evaporation from the soil may have been sufficient as selective factors.
While the functional basis underlying the upregulation of cyanogenesis in plants exposed to soil salinity within this system remains to be studied, distinct effects of soil salinity on the production of secondary compounds have also been demonstrated in other plants. The root alkaloid of a medicinal plant, Catharanthus roseus (Apocynaceae), was reported to have increased concomitantly with saline treatment (Jaleel et al., 2008). In a comparative study of two varieties of Lactuca sativa (Asteraceae) under salt stress, Mahmoudi et al. (2010) found that the more salt-tolerant ‘Verte’ variety exhibited enhanced production of carotenoids, phenolics and antioxidative enzymes relative to the salt-sensitive ‘Romaine’ variety. Similarly, a salt-tolerant clone of Saccharum officinarum (Poaceae) exhibited significantly greater increases in phenolics, anthocyanins and flavones relative to a salt-sensitive clone of the same species under salt stress (Wahid and Ghazanfar, 2006). Such examples show that plants frequently respond to enhanced soil salinity with changes in their secondary metabolism. In general, these secondary metabolites may perform a multitude of functions relative to salinity tolerance beyond providing defence against herbivores or pathogens, conferring an adaptive advantage in response to abiotic stress (Mansour, 2000).
Multiple functions have also been reported for cyanogenic glycosides. In many plants, cyanide-containing precursors are not only a chemical defence against herbivores but can also serve as nitrogen storage (Møller, 2010). In addition, the synthesis and turnover of such precursors may be a way of dissipating excess energy and reducing power, thereby mitigating stress (Nielsen et al., 2013; Selmar and Kleinwachter, 2013; Gleadow and Møller, 2014). Furthermore, cyanogenic precursors can affect the osmotic potential of plant cells as they are accumulated in the form of glycosides in vacuoles (Møller, 2010). Plants frequently employ nitrogen-containing compounds as well as sugars and inorganic ions as a means of osmotic adjustment when faced with salt stress (Parida and Das, 2005). The increased concentration of solutes reduces cell water potential and allows for absorption of water despite low soil water potential (Mansour, 2000; Ashraf and Harris, 2004). In this line, in a recent study on the responses of white clover to flooding by seawater, the authors found increased solute accumulation (sucrose and proline) in plants growing in saline soils (White et al., 2014). The increased levels of cyanogenic glycosides we report here for salt-exposed white clover plants potentially serve this purpose as well. However, more work needs to be conducted to determine whether genotypes of T. repens demonstrating greater salt tolerance accumulate different amounts of cyanogenic glycosides than more salt-sensitive conspecifics. Whether or not the enhanced production of cyanogenic glycosides in salt-stressed plants is primarily a mechanism to reduce water potential in plant cells, we showed that the increase in HCNp resulted in elevated defence against two natural herbivores of white clover – slugs and weevils. While in our study significant reduction of leaf consumption by both herbivores was observed only at higher treatment levels (levels 4 and 5, corresponding to soil conductivities of 3·4 and 3·9 dS m–1; Fig. 5) it should be noted that these levels of soil salinity are only considered ‘moderate’. The salt concentration in agricultural areas is frequently much higher (Abrol et al., 1988). Furthermore, we observed a significant correlation of HCNp and reduced herbivory among all salt treatments, indicating effects of increased HCNp on herbivore leaf consumption at even lower soil salt concentrations (Fig. 4). Our findings indicate that changes in plant biochemistry in response to soil salinity are likely to affect above-ground food webs in a broad range of environments affected to various degrees by elevated salt concentrations in the soil. Nevertheless, it should be taken into account that despite the strong correlation between increased HCNp and decreased herbivory, other salinity-related factors such as Cl– or Na+ accumulation in leaves (White et al., 2014) may have contributed to reduced herbivory in salt-stressed white clover plants.
In general, plant cyanogenesis is an efficient defence against a wide range of herbivores, including both generalist and specialist insect herbivores (Ballhorn et al., 2005, 2010b). For example, the Mexican bean beetle (Coccinellidae: Epilachna varivestis) is a specialist herbivore feeding on foliage of a small range of legume host plants including cyanogenic lima bean (Phaseolus lunatus). While the insects show a distinct preference for this host, in long-term studies bean beetle fitness was reduced on high cyanogenic lima bean genotypes compared with lower cyanogenic conspecifics (Ballhorn et al., 2007). However, in other plant–herbivore systems cyanogenesis did not provide an efficient defence even against generalist herbivores, which are usually considered more sensitive to chemical plant defence compounds. These differences in efficacy of cyanogenesis as a defence against various herbivore species are often due to the mode of herbivore feeding (Slocum et al., 2002). For example, piercing–sucking herbivores often cause only minimal tissue disruption, thus avoiding efficient release of toxic HCN from cyanogenic precursors in the course of plant cyanogenesis (Gleadow and Woodrow, 2002).
While both invertebrate and vertebrate herbivores possess enzymes for cyanide detoxification (β-cyano-alanine synthase or rhodanese) (Ballhorn et al., 2007, 2009b), constant exposure to sub-lethal doses of cyanide reduces the fitness of these organisms. Furthermore, many herbivores (including vertebrate livestock) have endogenous β-glucosidases capable of degrading cyanogenic precursors and releasing toxic quantities of HCN, effectively increasing levels of free cyanide during digestion (Kakes, 1993; Jones, 1998). Herbivore preference for low cyanogenic plants, however, often occurs pre-digestion via detection of free cyanide during test feeding (Ballhorn et al., 2005, 2010b). The increase in constitutive precursors without enhanced β-glucosidase activity, as we report in the present study, may pose a dual threat to herbivores as the high levels of bound cyanide potentially are not detected by the herbivores (Kakes, 1989; Ballhorn, 2005). In livestock, HCN released via β-glucosidase activity in the gastrointestinal tract upon ingestion can lead to malnourishment or intoxication if no other food source is available (Webber et al., 1985; Hopkins, 1995; Caradus and Forde, 1996). The latter outcome may be particularly significant when white clover β-glucosidase is not upregulated as drastically as HCNp, which could result in decreased cyanide release during feeding but an increased and more harmful internal release of cyanide from ingested cyanogenic glycosides.
Beyond plant–herbivore interactions, an increased HCNp in plants growing under salt stress may further impact plant resistance to fungal pathogens and consequently reduce crop production. While cyanide is generally toxic to all eukaryotes due to inhibition of the mitochondrial respiration pathway through blocking the cytochrome a/a3-dependent oxidase (Solomonson, 1981), no consistent functional association between cyanogenesis and resistance of plants to pathogenic fungi has been found so far (Rissler and Millar, 1977; Fry and Myers, 1981; Lieberei, 1988; Lieberei et al., 1989, 1996; Nielsen et al., 2006). This is in part due to the effective HCN detoxification in some pathogenic fungi, e.g. by cyanide hydratases (Osbourn, 1996). Several studies suggest that highly cyanogenic plants are even more susceptible to fungal pathogens than conspecifics with lower cyanogenic properties (Dirzo and Harper, 1982; Lieberei et al., 1989, 1996; Osbourn, 1996). This can be explained by negative effects of cyanide on plant enzymes involved in anti-pathogen defence (Solomonson, 1981; Lieberei, 2007). Hydrogen cyanide quantitatively affects many enzymatic activities, most of all those of metal-containing enzymes. In addition to catalases and peroxidases, polyphenol oxidases represent an important group of plant enzymes involved in plant defences against pathogens and herbivores (Ballhorn, 2011; Ballhorn et al., 2010a, 2011). These enzymes oxidize phenolic compounds to reactive quinones (Felton et al., 1992; Bi et al., 1995; Stout et al., 1996; Thaler et al., 2001; Karban et al., 2002; Kranthi et al., 2003) centrally involved in plant defence responses. Thus, higher susceptibility to fungal pathogens in salt-stressed plants in the course of enhanced production of cyanide may add a further dimension to the ecological and agricultural impact of increasing soil salinity.
In addition to affecting biotic interactions such as the interactions with natural herbivore communities, livestock and pathogens, an increased expression of HCNp in salt-stressed white clover plants may have a far-reaching impact on the resistance of this important forage crop to abiotic stresses. Research on white clover cyanogenesis shows that high cyanogenic plants are more susceptible to frost and drought (Daday, 1965; Foulds and Grime, 1965). In this line, Olsen and Ungerer (2008) observed decreased numbers of high cyanogenic plants in cold climates, whereas in warm climates, high cyanide morphs tended to prevail. Thus, the sensitivity to cold temperatures may increase in white clover populations exposed to salt or drought stress. On the other hand, an additional upregulation of the HCNp of high cyanogenic salt-stressed plants may further enhance negative effects of HCNp on herbivores in these locations. If cyanide production increases as a result of increasing salinity and is selected for under warmer conditions, we are likely to see an increase in the proportion of high cyanogenic morphs, particularly in regions experiencing increasing temperatures and secondary soil salinization due to irrigation. These effects would be potentially compounded by drought (as increased evapotranspiration increases solute concentrations in shoots) and elevated CO2, both of which have been demonstrated to increase the HCN/soluble protein ratio significantly in white clover (Gleadow et al., 2009).
Conclusions
The increased production of cyanide-containing glycosides under elevated soil salinity reported here could have major implications concerning food security, agricultural economics and also the function of natural and agricultural ecosystems. Increasing soil salinity is a globally widespread problem affecting many densely populated regions utilizing agricultural systems heavily reliant on irrigation. Extensive research has been conducted to increase salt tolerance in plants through transgenic approaches (Yamaguchi and Blumwald, 2005) and on novel irrigation strategies to diminish salt accumulation (Yeo, 1999). However, the quantitative chemical responses of plants to soil salinity and the resulting bottom-up effects on plant consumers are still little understood. The present study provides new insights into these interactions using a natural plant–herbivore system.
ACKNOWLEDGEMENTS
We thank Adrienne Godschalx, Rachael Workman and Dr Stefanie Kautz for helpful comments and discussion. Funding by Portland State University is gratefully acknowledged.
LITERATURE CITED
- Abberton MT, Thomas I. Genetic resources in Trifolium and their utilization in plant breeding. Plant Genetic Resources. 2010;9:38–44. [Google Scholar]
- Abrol IP, Yadav JSP, Massoud F. Soils Bulletin. Vol. 39. Rome: Food and Agricultural Organization of the United Nations (FAO); 1988. Salt affected soils and their management. [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Science. 2004;166:3–16. [Google Scholar]
- Ballhorn DJ. Constraints of simultaneous resistance to a fungal pathogen and an insect herbivore in lima bean (Phaseolus lunatus L.) Journal of Chemical Ecology. 2011;37:141–144. doi: 10.1007/s10886-010-9905-0. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Lieberei R, Ganzhorn JU. Plant cyanogenesis of Phaseolus lunatus and its relevance for herbivore–plant interaction: the importance of quantitative data. Journal of Chemical Ecology. 2005;31:1445–1473. doi: 10.1007/s10886-005-5791-2. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Heil M, Lieberei R. Phenotypic plasticity of cyanogenesis in lima bean Phaseolus lunatus – activity and activation of beta-glucosidase. Journal of Chemical Ecology. 2006;32:261–275. doi: 10.1007/s10886-005-9001-z. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Pietrowski A, Heil M, Lieberei R. Quantitative effects of cyanogenesis on an adapted herbivore. Journal of Chemical Ecology. 2007;33:2195–2208. doi: 10.1007/s10886-007-9380-4. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Kautz S, Heil M, Hegeman AD. Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient and direct defense in nature. PLoS One. 2009a;4:e5. doi: 10.1371/journal.pone.0005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballhorn DJ, Kautz S, Rakotoarivelo FP. Quantitative variability of cyanogenesis in Cathariostachys madagascariensis – the main food plant of bamboo lemurs in southeastern Madagascar. American Journal of Primatology. 2009b;71:305–315. doi: 10.1002/ajp.20653. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Pietrowski A, Lieberei R. Direct trade-off between cyanogenesis and resistance to a fungal pathogen in lima bean (Phaseolus lunatus L.) Journal of Ecology. 2010a;98:226–236. [Google Scholar]
- Ballhorn DJ, Kautz S, Lieberei R. How generalist and specialist herbivores respond to various cyanogenic plant features. Entomologia Experimentalis et Applicata. 2010b;134:245–259. [Google Scholar]
- Ballhorn DJ, Kautz S, Jensen M, Schmitt I, Heil M, Hegeman AD. Genetic and environmental interactions determine plant defences against herbivores. Journal of Ecology. 2011;99:313–326. [Google Scholar]
- Bi YM, Kenton P, Mur L, Darby R, Draper J. Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. The Plant Journal. 1995;8:235–245. doi: 10.1046/j.1365-313x.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- Bonte D, De Roissart A, Vandegehuchte ML, Ballhorn DJ, Van Leeuwen T, de la Peña E. Local adaptation of aboveground herbivores towards plant phenotypes induced by soil biota. PLoS One. 2010;5:e11174. doi: 10.1371/journal.pone.0011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caradus JR, Forde MB. Characterisation of white clover populations collected from the Caucuses and high altitude regions of eastern Turkey. Genetic Resources and Crop Evolution. 1996;43:143–155. [Google Scholar]
- Daday H. Gene frequencies in wild populations of Trifolium repens L.: mechanism of natural selection. Heredity. 1965;20:355–365. [Google Scholar]
- Dirzo R, Harper J. Experimental studies on slug–plant interactions: the performance of cyanogenic and acyanogenic morphs of Trifolium repens in the field. Journal of Ecology. 1982;70:119–138. [Google Scholar]
- Felton W, Donato KK, Duffey SS. Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. Journal of Insect Physiology. 1992;38:277–285. [Google Scholar]
- Foulds W, Grime JP. The response of cyanogenic and acyanogenic phenotypes of Trifolium repens to soil moisture supply. Heredity. 1965;28:181–187. [Google Scholar]
- Fry W, Myers D. Hydrogen cyanide metabolism by fungal pathogens of cyanogenic plants. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F, editors. Cyanide in biology. London: Academic Press; 1981. pp. 321–334. [Google Scholar]
- Ganzhorn JU. Leaf chemistry and the biomass of folivorous primates in tropical forests. Test of a hypothesis. Oecologia. 1992;91:540–547. doi: 10.1007/BF00650329. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Møller BL. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annual Review of Plant Biology. 2014;65:155–185. doi: 10.1146/annurev-arplant-050213-040027. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. Journal of Chemical Ecology. 2002;28:1301–1313. doi: 10.1023/a:1016298100201. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Edwards EJ, Evans JR. Changes in nutritional value of cyanogenic Trifolium repens grown at elevated atmospheric CO2. Journal of Chemical Ecology. 2009;35:476–478. doi: 10.1007/s10886-009-9617-5. [DOI] [PubMed] [Google Scholar]
- Hopkins A. Factors influencing cattle bracken-poisoning in Great Britain. In: Thornton Smith R, Taylor JA, editors. Bracken: an environmental issue. Bracken 94 Conference, Aberystwyth, Wales. International Bracken Group Special Publication. Vol. 2. 1995. pp. 120–123. [Google Scholar]
- Hughes MA. The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity. 1991;66:105–115. [Google Scholar]
- Jaleel CA, Sankar B, Sridharan R, Panneerselvam R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turkish Journal of Biology. 2008;32:79–83. [Google Scholar]
- Jones DA. Cyanogenesis in animal–plant interactions. In: Evered D, Harnett S, editors. Cyanide compounds in biology. Chichester, UK: John Wiley & Sons; 1988. pp. 151–165. [Google Scholar]
- Jones DA. Why are so many food plants cyanogenic? Phytochemistry. 1998;47:155–162. doi: 10.1016/s0031-9422(97)00425-1. [DOI] [PubMed] [Google Scholar]
- Kakes P. Linamarase and other β-glucosidases are present in the cell walls of Trifolium repens L. leaves. Planta. 1985;166:156–160. doi: 10.1007/BF00397342. [DOI] [PubMed] [Google Scholar]
- Kakes P. An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theoretical and Applied Genetics. 1989;77:111–118. doi: 10.1007/BF00292324. [DOI] [PubMed] [Google Scholar]
- Kakes P. Function and variation of the beta-glucosidase linamarase in natural populations of Trifolium repens. In: Esen A, editor. β-Glucosidases. Biochemical and molecular biology. ACS Symposium Series. Vol. 533. Washington, DC: American Chemical Society; 1993. pp. 145–152. [Google Scholar]
- Karban R, Ullman D, Thaler J, Boege K, Bostock R. Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia. 2002;131:227–235. doi: 10.1007/s00442-002-0885-9. [DOI] [PubMed] [Google Scholar]
- Koricheva J. Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia. 1999;119:467–473. doi: 10.1007/s004420050809. [DOI] [PubMed] [Google Scholar]
- Kranthi S, Kranthi KR, Wanjari RR. Influence of semilooper damage on cotton host-plant resistance to Helicoverpa armigera (Hub) Plant Science. 2003;164:157–163. [Google Scholar]
- Lieberei R. Relationship of cyanogenic capacity (HCN-c) of the rubber tree Hevea brasliensis to susceptibility to Microcydus ulei, the agent causing South American leaf blight. Journal of Phytopathology. 1988;122:54–67. [Google Scholar]
- Lieberei R. South American leaf blight of the rubber tree (Hevea spp.): new steps in plant domestication using physiological features and molecular markers. Annals of Botany. 2007;100:1125–1142. doi: 10.1093/aob/mcm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberei R, Biehl B, Giesemann A, Junqueira NT. Cyanogenesis inhibits active defense reactions in plants. Plant Physiology. 1989;90:33–36. doi: 10.1104/pp.90.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberei R, Fock H, Biehl B. Cyanogenesis inhibits active pathogen defence in plants: inhibition by gaseous HCN of photosynthetic CO2 fixation and respiration in intact leaves. Angewandte Botanik. 1996;70:230–238. [Google Scholar]
- Mahmoudi H, Huang J, Gruber MY, et al. The impact of genotype and salinity on physiological function, secondary metabolite accumulation, and antioxidative responses in lettuce. Journal of Agricultural and Food Chemistry. 2010;58:5122–5130. doi: 10.1021/jf904274v. [DOI] [PubMed] [Google Scholar]
- Mansour MMF. Nitrogen containing compounds and adaptations of plants to salinity stress. Biologia Plantarum. 2000;43:491–500. [Google Scholar]
- Møller BL. Functional diversifications of cyanogenic glucosides. Current Opinion in Plant Biology. 2010;13:338–347. doi: 10.1016/j.pbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Nahrstedt A. Cyanogenic compounds as protecting agents for organisms. Plant Systematics and Evolution. 1985;150:35–47. [Google Scholar]
- Nielsen AZ, Ziersen B, Jensen K, et al. Redirecting photosynthetic reducing power toward bioactive natural product synthesis. ACS Synthetic Biology. 2013;2:308–315. doi: 10.1021/sb300128r. [DOI] [PubMed] [Google Scholar]
- Nielsen KA, Hrmova M, Nielsen JN, et al. Reconstitution of cyanogenesis in barley (Hordeum vulgare L.) and its implications for resistance against the barley powdery mildew fungus. Planta. 2006;223:1010–1023. doi: 10.1007/s00425-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Olsen KM, Ungerer MC. Freezing tolerance and cyanogenesis in white clover (Trifolium repens L. Fabaceae) International Journal of Plant Sciences. 2008;169:1141–1147. [Google Scholar]
- Osbourn AE. Preformed antimicrobial compounds and plant defense against fungal attack. The Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Poulton JE. Cyanogenesis in plants. Plant Physiology. 1990;94:401–405. doi: 10.1104/pp.94.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades JD, Manteghi NA, Shouse PJ, Alves WJ. Estimating soil salinity from saturated soil-paste electrical conductivity. Soil Science Society of America Journal. 1989;53:248–433. [Google Scholar]
- Rissler JF, Millar RL. Biochemical evidence for the histochemical localization of linamarase activity in Lotus corniculatus infected with Stemphylium loti. Protoplasma. 1977;92:57–70. [Google Scholar]
- Rogers ME, Noble CL, Nicolas ME. Variation in yield potential and salt tolerance of selected cultivars and natural populations of Trifolium repens L. Australian Journal of Agriculture. 1993;44:785–798. [Google Scholar]
- Selmar D, Lieberei R, Biehl B, Voigt J. Hevea linamarase, a non-specific β-glycosidase. Physiologia Plantarum. 1987;83:557–563. doi: 10.1104/pp.83.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Conn EE, Biehl B. α-Hydroxynitrile lyase in Hevea brasiliensis and its significance for rapid cyanogenesis. Physiologia Plantarum. 1989;75:97–101. [Google Scholar]
- Selmar D, Kleinwächter M. Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant and Cell Physiology. 2013;54:817–826. doi: 10.1093/pcp/pct054. [DOI] [PubMed] [Google Scholar]
- Slocum DJ, Woodrow IE, Gleadow RM. Influence of water stress on cyanogenic capacity in Eucalyptus cladocalyx. Functional Plant Biology. 2002;29:103–110. doi: 10.1071/PP01116. [DOI] [PubMed] [Google Scholar]
- Solomonson L. Cyanide as a metabolic inhibitor. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F, editors. Cyanide in biology. London: Academic Press; 1981. pp. 11–28. [Google Scholar]
- Stout MJ, Workman KV, Duffey SS. Identity, spatiality and variability of induced chemical responses in tomato plants. Entomologia Experimentalis et Applicata. 1996;79:225–271. [Google Scholar]
- Thaler JS, Stout MJ, Karban R, Duffey SS. Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecological Entomology. 2001;26:312–324. [Google Scholar]
- Voß K. Germany: University of Hamburg; 2001. Biologische Bedeutung und Aktivierbarkeit der β-d-Glycosidase in Blättern von Hevea brasiliensis (Willd.) Muell. Arg. (1865) PhD thesis. [Google Scholar]
- Wahid A, Ghazanfar A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. Journal of Plant Physiology. 2006;163:723–730. doi: 10.1016/j.jplph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Webber JJ, Rofcroft CR, Callinan JD. Cyanide poisoning of goats from sugar gums (Eucalyptus cladocalyx) Australian Veterinary Journal. 1985;62:28–30. doi: 10.1111/j.1751-0813.1985.tb06041.x. [DOI] [PubMed] [Google Scholar]
- White AC, Colmer TD, Cawthray GR, Hanley ME. Variable response of three Trifolium repens ecotypes to soil flooding by seawater. Annals of Botany. 2014;114:347–355. doi: 10.1093/aob/mcu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends in Plant Science. 2005;10:615–620. doi: 10.1016/j.tplants.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yeo A. Predicting the interaction between the effects of salinity and climate change on crop plants. Scientia Horticulturae. 1999;78:159–174. [Google Scholar]
- Zagrobelny M, Bak S, Rasmussen AV, Jørgensen B, Naumann CM, Lindberg Møller B. Cyanogenic glucosides and plant–insect interactions. Phytochemistry. 2004;65:293–306. doi: 10.1016/j.phytochem.2003.10.016. [DOI] [PubMed] [Google Scholar]


