Abstract
Background and Aims
One of the most striking attributes of clonal plants is their capacity for physiological integration, which enables movement of essential resources between connected ramets. This study investigated the capacity of physiological integration to buffer differences in resource availability experienced by ramets of the clonal wild strawberry plant, Fragaria vesca. Specifically, a study was made of the responses of connected and severed offspring ramets growing in environments with different water availability conditions (well watered or water stressed) and nitrogen forms (nitrate or ammonium).
Methods
The experimental design consisted of three factors, ‘integration’ (connected, severed) ‘water status’ (well watered, water stressed) and ‘nitrogen form’ (nitrate, ammonium), applied in a pot experiment. The effects of physiological integration were studied by analysing photochemical efficiency, leaf spectral reflectance, photosynthesis and carbon and nitrogen isotope discrimination, the last of which has been neglected in previous studies.
Key Results
Physiological integration buffered the stress caused by water deprivation. As a consequence, survival was improved in water-stressed offspring ramets that remained connected to their parent plants. The nitrogen isotope composition (δ15N) values in the connected water-stressed ramets were similar to those in ramets in the ammonium treatment; however, δ15N values in connected well-watered ramets were similar to those in the nitrate treatment. The results also demonstrated the benefit of integration for offspring ramets in terms of photochemical activity and photosynthesis.
Conclusions
This is the first study in which carbon and nitrogen isotopic discrimination has been used to detect physiological integration in clonal plants. The results for nitrogen isotope composition represent the first evidence of preferential transport of a specific form of nitrogen to compensate for stressful conditions experienced by a member clone. Water consumption was lower in plants supplied with ammonium than in plants supplied with nitrate, and therefore preferential transport of ammonium from parents to water-stressed offspring could potentially optimize the water use of the whole clone.
Keywords: Clonal plant ecology, clonal integration, environmental heterogeneity, Fragaria vesca, wild strawberry, isotope discrimination, nitrogen forms, photosynthesis, water-use efficiency
INTRODUCTION
Natural habitats exhibit spatially heterogeneous distribution of essential resources (Lechowicz and Bell, 1991; Caldwell and Pearcy, 1994). This heterogeneity may be important in many plant processes, and may influence plant population structure and community dynamics (Hartgerink and Bazzaz, 1984; Price and Marshall, 1999; Day et al., 2003; Wijesinghe et al., 2005). Clonal reproduction results in extensive structures that occupy large areas and that are almost bound to experience spatial heterogeneity (Oborny and Cain, 1997; Hutchings et al., 2004). Thus, clonal plants in particular are suitable models for studying the responses of plants to spatial environmental heterogeneity.
One of the most striking attributes of clonal plants is their capacity for physiological integration. This enables the movement of essential resources between connected ramets (Pitelka and Ashmun, 1985), allowing clonal plants to colonize unfavourable territories that otherwise would remain unexploited (Hartnett and Bazzaz, 1983; Salzman and Parker, 1985; Jónsdóttir and Watson, 1997; Saitoh et al., 2002; Roiloa and Retuerto, 2006a).
Here we investigated the capacity of physiological integration to buffer differences in resource availability experienced by connected ramets of the clonal plant Fragaria vesca. We studied the response of connected (physiological integration allowed) and severed (physiological integration prevented) offspring ramets growing in environments with different water availability conditions (well watered or water stressed) and nitrogen forms (nitrate or ammonium). The responses of integrated ramets to heterogeneous distribution of nutrients have been studied extensively (Slade and Hutchings, 1987; Wijesinghe and Hutchings, 1999; Saitoh et al., 2006; Hutchings and Wijesinghe, 2008; D'Hertefeldt et al., 2011). However, to our knowledge only one study has attempted to determine the effects of physiological integration on ramets supplied with different forms of nitrogen (Jónsdóttir and Callaghan, 1990). The form of nitrogen absorbed by plants has been shown to affect plant growth (Claussen and Lenz, 1999; Tabatabaei et al., 2006; Guo et al., 2007). Nitrate and ammonium are the predominant inorganic nitrogen forms absorbed and metabolized by plant roots (Marschner, 1995). Ammonium uptake by roots appears to be a passive process, in contrast to both active and passive processes involved in the acquisition of nitrate (Higinbotham, 1973; Breteler and Hänisch ten Cate, 1980). Uptake of ammonium generally occurs at a higher rate than that of nitrate (Lee and Stewart, 1978; Marty et al., 2009; Mota et al., 2011; Gherardi et al., 2013; Lian et al., 2012; Yang et al., 2013). For most species, optimal growth is generally achieved when both nitrogen forms are available. However, the preferential form of nitrogen varies with species and environmental conditions (Errebhi and Wilcox, 1990). For example, although Fragaria ananassa grows better with nitrate than with ammonium as the sole nitrogen form (Darnell and Stutte, 2001), the growth rate is higher when both nitrate and ammonium are available (Ganmore-Neumann and Kafkafi, 1985). Previous studies have demonstrated low water consumption by plants supplied with ammonium compared with those supplied with nitrate (Guo et al., 2007). Therefore, we also aimed to determine how ammonium and nitrate affect plants growing under different conditions of water availability and how this may be modified by physiological integration.
We studied the effect of clonal integration at morphological and physiological levels. A number of studies have attempted to determine the effects of clonal integration on physiological traits, such as photosynthetic rates and photochemical efficiencies. Some studies have reported how clonal integration may allow, by a mechanism of feedback regulation, an enhancement of the photosynthetic efficiency of parents connected to daughter ramets growing in stressful conditions (Hartnett and Bazzaz, 1983; Roiloa and Retuerto, 2006a, b; Roiloa et al., 2007; Liu et al., 2008; Zhang et al., 2009). The present study aims to improve our understanding of the effects of clonal integration on photosynthetic process. Although previous studies have used single isotopes as labels or tracers to demonstrate resource translocation in clonal plants (Derner and Briske, 1998; D'Hertefeldt and Jonsdottir, 1999; D'Hertefeldt and Falkengren-Grerup, 2002; Hellström et al., 2006; Saitoh et al., 2006), our research is the first that uses stable isotope ratios to assess how physiological integration affects the way in which clonal plants use and acquire resources (water and nitrogen). Isotope discrimination techniques have been applied extensively in plant ecological research during the last few decades (Michener and Lajtha, 2007). However, no research has been carried out to determine how physiological integration affects isotope carbon discrimination and isotopic nitrogen composition in clonal plants. Due to its biochemical properties, the Rubisco enzyme discriminates against 13CO2 more than it does against 12CO2 (Farquhar et al., 1989). The lower the concentration of CO2 (Ci) at the active site of Rubisco, the lower the discrimination against the heavy isotope (Δ13C). The value of Ci reflects the balance between stomatal conductance and consequently the availability of water and the photosynthetic requirement for carbon dioxide. Thus, a low Ci and the consequent low Δ13C may be the result of either a high photosynthetic rate or, more commonly, low stomatal conductance, which would result in greater integrated water-use efficiency (Körner et al., 1988; Farquhar et al., 1989). The 15N/14N ratio (δ15N) has been proposed as an integrative trait to assess stress tolerance in plants (Robinson 2001). Plant and soil δ15N variation is primarily due to physiological and biogeochemical processes involved in the N cycle. Thus, the processes of N mineralization, nitrification, denitrification or NH3 volatilization discriminate against the heaviest isotope of nitrogen, so that that the different nitrogen forms have distinctive δ15N signatures (Handley and Raven, 1992; Robinson, 2001).
Movement of water and nitrogen between integrated ramets enables the clone to buffer environmental heterogeneity (Slade and Hutchings, 1987; Roiloa and Retuerto, 2007; Mao et al., 2009; D'Hertefeldt et al., 2011). Consequently, we expect similar carbon and nitrogen isotopic compositions (as indicators of water availability and nitrogen form, respectively) within the interconnected modules of the clone, despite differences in resource availability in the patches.
We tested the following specific hypotheses in the present study. (1) Physiological integration will buffer the stress induced by water deprivation. We predicted that water-stressed conditions in offspring ramets would be ameliorated by the movement of water from well-watered parents. As a result, we expected less restrictive water use (i.e. higher carbon discrimination, according to Farquhar et al., 1989) and higher rates of photosynthesis, growth and survival in connected than in severed water-stressed offspring ramets. (2) Physiological integration will allow the transport of N as nitrate or ammonium from parents to offspring ramets, depending on whether the offspring ramets are growing in soils lacking nitrate or ammonium, respectively. The form of nitrogen (ammonium or nitrate) taken up by plants will contribute to the determination of the nitrogen isotopic composition (δ15N) of their tissues. Given that nitrate-fed plants tend to be enriched in the heaviest N isotope, whereas ammonium fed plants are depleted (Evans, 2001; Kahmen et al., 2008; Ariz et al., 2011), we expected that integration would bring about changes in δ15N, leading to similar values in offspring ramets, regardless of whether the ramets were grown with ammonium or nitrate as the available nitrogen form. (3) Since water consumption is lower in plants supplied with ammonium than in plants supplied with nitrate (Høgh-Jensen and Schjoerring, 1997; Guo et al., 2007) and considering that ammonium uptake generally occurs at a higher rate than nitrate uptake (Lee and Stewart, 1978), we expected lower δ15N in integrated ramets than in disconnected ramets due to preferential transport of ammonium to offspring ramets under water-stressed conditions. As consequence, if, as reported in previous studies (Kahmen et al., 2008), ammonium uptake leads to a decrease in δ15N, we would expect a lower δ15N in connected water-stressed offspring than in connected well-watered offspring.
MATERIALS AND METHODS
Plant material
The study was carried out with the clonal wild strawberry plant Fragaria vesca, which is a perennial, evergreen herb of the Rosaceae family and is widely distributed in Europe, North America and Asia, where it inhabits a broad range of habitats. The habitat range of F. vesca includes sites such as stabilized scree, quarry and mine spoils, wastelands and pastures. It is frequent in open woodland and scrub and present in hedgerows, but it is absent from wetlands and arable land. This wide-ranging species is associated with vegetation characteristics of relatively infertile conditions. It is absent from highly disturbed or productive communities (Grime et al., 1996). Fragaria vesca has a high capacity for clonal reproduction by stolons that produce above-ground ramets at every other node. Clonal reproduction enables F. vesca to form fairly large clonal systems that are likely to experience small-scale environmental heterogeneity. A single genet can spread over more than 2 m2 in 1 year (S. R. Roiloa, pers. observations). Clones of F. vesca are integrated physiologically through vascular connections that allow translocation of substances between the connected ramets.
Plant material for the study was collected from three different sites in a forest understorey located 10 km south of Santiago de Compostela (north-west Spain) and vegetatively propagated in an open-end greenhouse for several months. The sites were at least 50 m from one another, so that it was assumed that each set of plants was derived from different genotypes. Previous studies with the congener stoloniferous Fragaria chiloensis have shown that plants more than 10 m apart are almost certain to belong to different clones (Alpert et al., 1993). To avoid possible confounding effects of genotypes, the plants were assigned to the treatments at random.
Experimental design
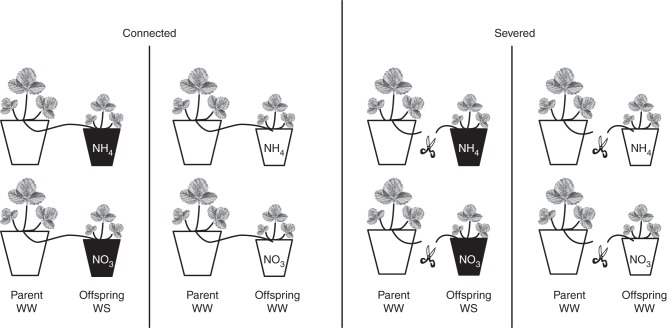
The experimental design consisted of three crossed factors: ‘integration’ (connected, severed), ‘water status’ (well watered, water stressed) and ‘nitrogen form’ (nitrate, ammonium) (Fig. 1). Eighty pairs of ramets (parent and offspring ramets) were selected for uniformity of size, from the plant stock. The ramets of each pair were rooted individually in 0·75-L plastic pots, filled with potting compost for the parent ramet and with washed sand for the offspring ramet. Washed sand was used to enable close control of nutrient availability.
Fig. 1.
Schematic representation of the experimental treatments showing the N form supplied (nitrate, NO3; ammonium, NH4) and the water treatments (white pots for well watered, WW; black pots for water stressed, WS) applied to pairs of connected and severed ramets. Each treatment was replicated ten times.
Offspring ramets were subjected to each of two different water availability conditions: well watered and water stressed. Soil water content was monitored with a soil moisture meter every 3 days throughout the experiment (Moisture Meter HH2; Delta-T Devices, Cambridge, UK). The volumetric soil moisture content was determined as the ratio between the volume of water present and the total volume of the sample. This is a dimensionless parameter, expressed as a percentage (% vol). Well-watered offspring ramets were maintained at field capacity by watering them with as much water as necessary to maintain an average volumetric soil water content of 20·7 % vol. We have previously determined that the water capacity for the pots used corresponds to a volumetric soil moisture content of 22 %. Water-stressed offspring ramets were subjected throughout the experiment to consecutive cycles of water deprivation by withholding water, but preventing the soil water content from decreasing below 8 %, to avoid wilting of the water-stressed plants. During the water deprivation cycles, water-stressed plants received only the amount of water required to maintain an average volumetric soil moisture content of 8·8 %vol. At the end of each 2-week cycle of water deprivation we brought water-stressed plants to field capacity. At the end of the experiment, water-stressed plants had received 43 % of the water added to well-watered ramets. The duration of water deprivation cycles was 2 weeks. Soil moisture was significantly higher in the well-watered than in the water-stressed treatment (ANOVA: F1,78 = 432·38, P < 0·001). Parent ramets were maintained at field capacity during the experiment.
The nitrogen form treatment was applied by adding a nutrient solution containing nitrate or ammonium to the offspring ramets as the sole nitrogen form (modified from Thomas et al., 1979) (Table 1). Replacement of nitrate (anion) by ammonium (cation) in a nutrient solution leads to important changes in the cation/anion balance. To maintain the cation/anion balance as equilibrated as possible we slightly modified the concentration of additional ions (PO43−, K+, Ca2+ and SO42−) (Table 1). The nitrate and ammonium solutions contained identical amounts of N and provided an adequate nutrient mixture for plant growth. A nutrient solution was applied to the plants every 2 weeks. To avoid nutrient build-up, each ramet was always watered with 200 mL of solution, which was sufficient to ensure that some solution drained freely from the pot. Parent ramets were grown in a potting compost substrate (CompoSana, Compo Iberia, Barcelona, Spain) containing all main nutrients and trace elements required for optimal growth of plants. Nitrate and ammonium contents in the potting compost of the parents were determined using the Kjeldahl method (Kjeldahl Distiller Pro-Nitro M, JP Selecta, Barcelona, Spain), and were found to be 1523 p.p.m. of NO3 and 141 p.p.m. of NH4. The presence of nitrate and ammonium in the potting compost allows the potential transport of the two nitrogen forms from the parents to their connected offspring ramets.
Table 1.
Salts (g L–1) and ions (mm) in the nitrate and ammonium solutions
| NO3− solution |
NH4+ solution |
||||||
|---|---|---|---|---|---|---|---|
| Salt | g L–1 | Ion | mm | Salt | g L–1 | Ion | mm |
| Ca(NO3)2.4H2O | 0·8 | NH4+ | 0 | (NH4)2SO4 | 0·5 | NH4+ | 7·46 |
| KH2PO4 | 0·8 | NO3– | 6·78 | K2HPO4 | 0·3 | NO3– | 0 |
| MgSO4.7H2O | 0·75 | PO43– | 5·88 | KH2PO4 | 0·46 | PO43– | 5·1 |
| CaSO4.2H2O | 0·645 | K+ | 5·88 | MgSO4.7H2O | 0·75 | K+ | 6·82 |
| Fe-EDTA | 0·042 | Ca2+ | 7·14 | CaSO4.2H2O | 0·89 | Ca2+ | 5·17 |
| Trace elements | 0·5 | Mg2+ | 3·04 | Fe-EDTA | 0·042 | Mg2+ | 3·04 |
| SO42– | 6·79 | Trace elements | 0·5 | SO42– | 11·94 | ||
At the beginning of the experiment, in order to determine the effect of physiological integration, the stolon connections were severed in half of the ramet pairs, selected at random. The new ramets produced during the experiment remained connected to their parents but were prevented from rooting.
The experiment was performed in a greenhouse under a natural day/night light cycle. After 2 weeks to allow plant establishment, the treatments began on 23 April 2010 and continued for 60 days. Each treatment was replicated ten times.
Measurements
Chlorophyll fluorescence
Chlorophyll fluorescence parameters were measured by the saturation pulse method (Schreiber et al., 1998) with a portable fluorometer (MINI-PAM photosynthesis yield analyser; Walz, Effeltrich, Germany) 15, 30, 45 and 60 days after treatment application. A pulse of saturating light (>4000 μmol photons m−2 s−1, 0·8 s pulse length, actinic white light) was applied through an optical fibre at an angle of 60° relative to the sample and a distance of 12 mm from the leaf. Measurements were made on the upper surface of the youngest fully expanded leaf of each offspring ramet. The maximum quantum yield of photosystem II (PSII) and the effective quantum yield of PSII (ΦPSII) were determined. We calculated the maximum quantum yield of photosystem II PSII as the ratio Fv/Fm = (Fm – F0)/Fm (Bolhàr-Nordenkampf et al., 1989), where F0 and Fm are the minimal and maximal fluorescence yields of a dark-adapted sample, respectively, with all PSII reaction centres fully open (i.e. all primary acceptors oxidized). This parameter was measured after dark adaptation for 30 min, which is considered sufficient time to enable opening of all PSII reaction centres in F. vesca plants (Roiloa and Retuerto, 2005). The Fv/Fm ratio provides an estimate of the efficiency of excitation energy capture by open PSII reaction centres (Butler and Kitajima, 1975). We calculated ΦPSII as (F′m – Ft)/F′m (Genty et al., 1989), where F′m is the maximal fluorescence yield reached in a pulse of saturating light with an illuminated sample and Ft is the fluorescence yield of the leaf at a given photosynthetic photon flux density. This parameter was measured under artificial light conditions of 692·6 ± 113·0 μmol m–2 s–1 (mean ± s.e., n = 320) provided by an auxiliary lamp (External Halogen Lamp 2050-HB; Walz). ΦPSII is a measure of the fraction of the light absorbed by chlorophyll that is photochemically converted into PSII (Maxwell and Johnson, 2000).
Leaf spectral reflectance
Immediately after the chlorophyll fluorescence measurements, the spectral reflectance parameters were determined in offspring ramets with a portable spectrometer (UniSpec Spectral Analysis System; PP Systems, Haverhill, MA, USA). We measured the photochemical reflectance index (PRI) and the chlorophyll content index (CHL) in the same leaves as those used to measure chlorophyll fluorescence parameters. The PRI was calculated as (R531 – R570)/(R531 + R570), where R refers to reflectance and the subscripts to a specific spectral wavelength in nanometres. The 531 nm wavelength is sensitive to changes in the epoxidation state of the xanthophyll cycle, and 570 nm is a reference wavelength unaffected by xanthophyll activity (Guo and Trotter, 2004). The PRI is significantly correlated with both net CO2 uptake and the photosynthetic radiation use efficiency (mol CO2 mol–1 photons) (Peñuelas et al., 1995; Gamon et al., 1997). It is also thought to be correlated with the levels of de-epoxidized (photoprotective) xanthophyll cycle pigments (Sims and Gamon, 2002; Stylinski et al., 2002). The CHL, calculated as R750/R700, is correlated with the chlorophyll content of leaves (Wood et al., 1993; Lichtenthaler et al., 1996).
Gas exchange
At the end of the experiment, leaf gas exchange measurements were made in offspring ramets, with a portable photosynthesis system (LI-6400XT; Li-Cor, Lincoln, NE, USA) at a leaf temperature of 27 °C and at the natural irradiance at midday (photosynthetically active radiation 605·9 ± 1·8 μmol m–2 s–1, mean ± s.e., n = 58). Photosynthetic carbon assimilation, stomatal conductance and the intercellular CO2 concentration were also determined. The small size of the leaves prevented us from recording gas exchange parameters in four offspring ramets.
Carbon and nitrogen isotope discrimination and leaf content
At the end of the experiment, two new leaves that had grown during the experiment were harvested from each treated offspring ramet and bulked for determination of carbon (δ13C) and nitrogen (δ15N) isotopic compositions. Sampled leaves were cleaned of organic debris, oven-dried at 70 °C for 48 h, then ball-milled to a fine homogenized powder to pass through a 40-mesh screen. Determinations of δ13C and δ15N for each offspring ramet were made with an isotope ratio mass spectrometer (Delta Plus; Finnigan MAT, San Jose, CA, USA). The δ13C and δ15N values of samples were calculated as δ(‰) = [(Rsample/Rstandard) – 1] × 1000, where R is the ratio of heavy to light isotope, Rsample is the ratio in the sample and Rstandard is the ratio in the Pee Dee Belemnite carbonate standard. We calculated 13C/12C discrimination (Δ13C) as Δ13C (‰) = [(δ13Cair – δ13Csample)/(1000 + δ13Csample)] × 1000. Under the well-mixed air conditions of the study, we assumed a δ13C value for atmospheric CO2 of –8·05 ‰ (Ferrio et al., 2005). Δ13C has been used as an indicator of plant ecophysiological processes such as leaf conductance, water-use efficiency and photosynthetic capacity (Körner et al., 1988; Farquhar et al., 1989; Ehleringer et al., 1993). The plant δ15N value provides information about the source, absorption and assimilation of nitrogen in plants (Evans, 2001). Carbon and nitrogen contents (percentage dry mass) were determined in the same samples as those in which isotopic composition was measured, with an elemental analyser (EA 1108 CHNS/O; Carlo Erba Instruments, Milan, Italy).
Growth and survival
At the end of the experiment, offspring ramets were harvested separately, divided into above- and below-ground structures and dried to constant weight at 60 °C to determine root dry mass, total dry mass and the proportional biomass allocated to roots (root dry mass ratio = root dry mass/total dry mass). The newly produced stolons and new ramets were assigned to the above-ground biomass of the ramets producing them. Mortality rates of offspring ramets were determined every week.
Statistical analyses
Total mass and root mass were log10-transformed and root mass ratio was square root-transformed to meet the requirements of normality and homoscedasticity for ANOVA.
Differences between treatments for growth, isotopic composition, carbon and nitrogen contents and gas exchange were evaluated by three-way ANOVA, with ‘integration’, ‘nitrogen form’ and ‘water status’ as the main factors. Two additional two-way ANOVAs were conducted separately for connected and severed ramets in order to detect the effects of ‘nitrogen form’ and ‘water status’ on isotopic composition. Differences in chlorophyll fluorescence and spectral reflectance parameters were analysed by repeated measures three-way analysis of variance (ANOVAR), with ‘integration’, ‘nitrogen form’ and ‘water status’ as between-subject effects and ‘time’ as the within-subject effect. Survivorship during the experiment was analysed using Kaplan–Meier analysis with the log rank (Mantel–Cox) test (Kaplan and Meier, 1958).
Mortality reduced the number of replicates used in the different analyses, as indicated by the error degrees of freedom. Differences were considered significant at P < 0·05. Statistical tests were performed with SPSS 18·0 (SPSS, Chicago, IL, USA).
RESULTS
Chlorophyll fluorescence and leaf spectral reflectance
In water-stressed ramets there were significant decreases, relative to well-watered ramets, in maximum quantum yield of PSII (Fv/Fm) and ΦPSII, PRI and (CHL, regardless of whether the ramets were connected to the parent ramet or not (Table 2, Fig. 2). Integration significantly affected chlorophyll fluorescence and leaf reflectance parameters. The ΦPSII, PRI and CHL values were significantly higher in connected offspring ramets than in severed offspring ramets (Table 2, Fig. 2). No significant effects of the form of nitrogen or of the interactions of the studied factors were detected for any of the chlorophyll fluorescence and spectral reflectance variables (Table 2).
Table 2.
Results of three-way repeated-measures analysis of variance, with ‘integration’, ‘nitrogen form’ and ‘water status’ as between-subject effects, for differences in chlorophyll fluorescence parameters [maximum (Fv/Fm) and effective (ΦPSII) quantum yield of photosystem II] and spectral reflectance [photochemical reflectance index (PRI) and chlorophyll content index(CHL)] between offspring ramets
| df |
Fv/Fm |
ΦPSII |
PRI |
CHL |
|||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | ||
| Between-subject effect | |||||||||
| Integration | 1 | 0·284 | 0·596 | 21·169 | <0.001 | 23·283 | <0.001 | 23·143 | <0.001 |
| Nitrogen | 1 | 0·210 | 0·649 | 1·212 | 0·276 | 1·075 | 0·305 | 1·060 | 0·308 |
| Water | 1 | 6·563 | 0.013 | 4·943 | 0.030 | 4·076 | 0.048 | 5·035 | 0.029 |
| Integration × nitrogen | 1 | 1·597 | 0·212 | 0·331 | 0·568 | 0·951 | 0·334 | 0·978 | 0·327 |
| Integration × water | 1 | 2·042 | 0·159 | 0·199 | 0·657 | 1·168 | 0·285 | 1·019 | 0·317 |
| Nitrogen × water | 1 | 0·033 | 0·856 | 0·075 | 0·785 | 0·603 | 0·441 | 1·113 | 0·296 |
| Integration × nitrogen × water | 1 | 0·619 | 0·435 | 0·263 | 0·610 | 0·004 | 0·947 | 0·058 | 0·810 |
| Error | 54 | ||||||||
| Within-subject effect | |||||||||
| Time | 3 | 9·616 | <0.001 | 27·183 | <0.001 | 21·113 | <0.001 | 42·895 | <0.001 |
| Integration × time | 3 | 2·069 | 0·106 | 3·161 | 0.026 | 0·346 | 0·792 | 0·740 | 0·530 |
| Nitrogen × time | 3 | 0·385 | 0·764 | 0·417 | 0·741 | 0·213 | 0·888 | 0·448 | 0·719 |
| Water × time | 3 | 1·339 | 0·264 | 1·725 | 0·164 | 5·044 | 0.002 | 10·872 | <0.001 |
| Integration × nitrogen × time | 3 | 0·222 | 0·881 | 1·826 | 0·144 | 0·278 | 0·841 | 0·539 | 0·656 |
| Integration × water × time | 3 | 0·999 | 0·395 | 0·947 | 0·419 | 1·736 | 0·162 | 0·535 | 0·659 |
| Nitrogen × water × time | 3 | 0·498 | 0·684 | 0·371 | 0·774 | 2·236 | 0·086 | 0·287 | 0·835 |
| Integration × nitrogen × water × time | 3 | 0·304 | 0·822 | 0·215 | 0·886 | 0·886 | 0·450 | 0·960 | 0·413 |
| Error | 162 | ||||||||
See Fig. 2 for data.
Significant effects (P < 0·05) are shown in bold type.
Fig. 2.
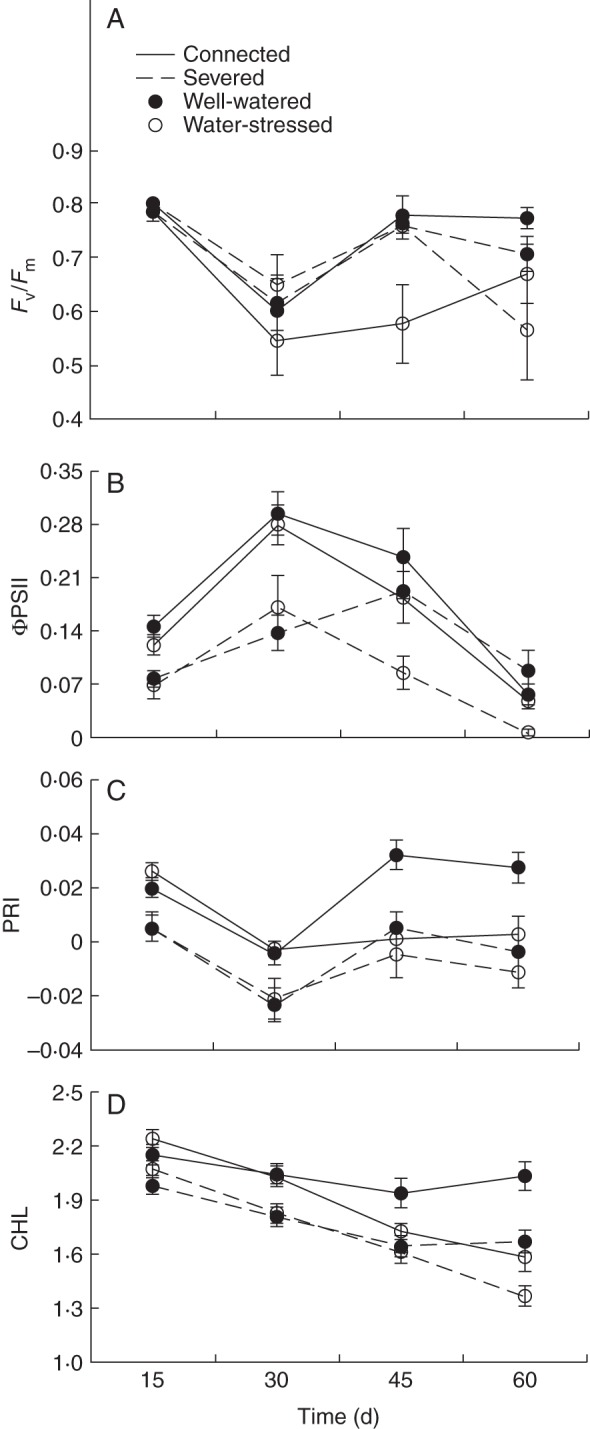
Time course of mean values (± s.e.) of chlorophyll fluorescence parameters Fv/Fm (A) and ΦPSII (B) and the spectral reflectance parameters photochemical reflectance index (PRI) (C) and chlorophyll content index (CHL) (D) for connected (solid lines) and severed (dashed lines) offspring ramets in well-watered (closed symbols) and water-stressed (open symbols) treatments. See Table 2 for ANOVA parameters.
Gas exchange
Severed and water-stressed offspring ramets experienced a significant reduction in photosynthetic carbon assimilation and stomatal conductance relative to connected and well-watered ramets, respectively (Table 3, Fig. 3). No significant effects were detected for the nitrogen form or for the interactions of the studied factors (Table 3).
Table 3.
Three-way ANOVA for analyses of differences in photosynthetic carbon assimilation, stomatal conductance and intercellular CO2 concentration (Ci) of offspring ramets with ‘integration’, ‘nitrogen form’ and ‘water status’ as main factors
| Effect | df | Photosynthesis |
Conductance |
Ci |
|||
|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | ||
| Integration | 1 | 6·731 | 0.012 | 10·831 | 0.002 | 0·335 | 0·566 |
| Nitrogen | 1 | 1·981 | 0·165 | 1·500 | 0·226 | 0·134 | 0·716 |
| Water | 1 | 15·469 | <0.001 | 21·077 | <0.001 | 0·302 | 0·585 |
| Integration × nitrogen | 1 | 0·179 | 0·674 | 0·204 | 0·653 | 0·055 | 0·815 |
| Integration × water | 1 | 3·211 | 0·079 | 2·772 | 0·102 | 2·277 | 0·138 |
| Nitrogen × water | 1 | 0·910 | 0·345 | 1·150 | 0·289 | 1·409 | 0·241 |
| Integration × nitrogen × water | 1 | 0·010 | 0·919 | 0·002 | 0·966 | 0·272 | 0·605 |
| Error | 50 | ||||||
See Fig. 3 for data.
Significant effects (P < 0·05) are shown in bold type.
Fig. 3.
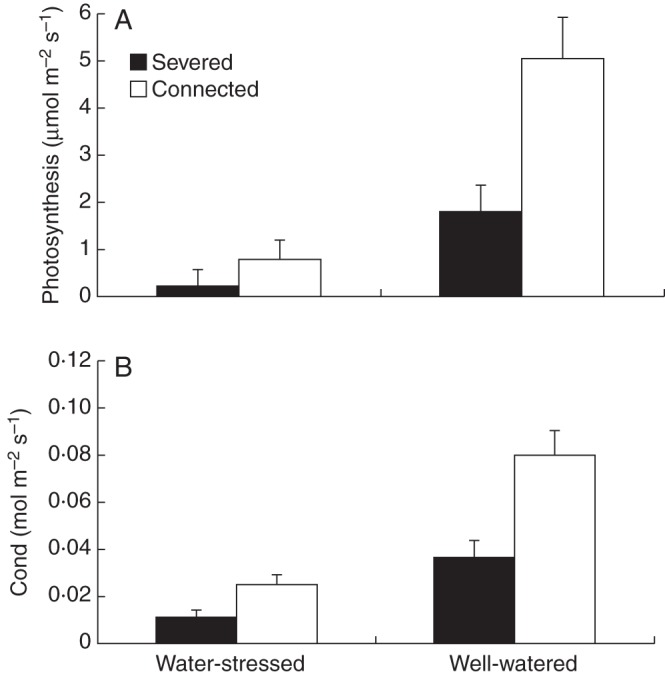
Mean (± s.e.) photosynthetic carbon assimilation (A) and stomatal conductance (B) of severed and connected offspring ramets (as indicated in the key) in the water-stressed and well-watered treatments. See Table 3 for ANOVA parameters.
Carbon and nitrogen isotope discrimination and leaf content
Nitrogen isotopic composition (δ15N) was significantly affected by the nitrogen form. The value of δ15N was significantly lower in offspring ramets supplied with ammonium than in offspring ramets supplied with nitrate (Table 4, Fig. 4A). The interaction between integration and water was significant for δ15N. The δ15N values of severed ramets were similar in the well-watered and water-stressed treatments. However, for connected ramets, δ15N was lower in the water-stressed treatment than in the well-watered treatment (Table 4, Fig. 4B). This result was supported by the separate two-way ANOVA conducted for connected and severed ramets. In this way, we detected a significant effect of the interaction between nitrogen form and water for the connected treatment (ANOVA, F1, 33 = 4·225, P = 0·048; Fig. 4C). For connected ramets, we found a significant reduction in δ15N with water stress, and this reduction was especially strong for ramets growing in the nitrate treatment. However, this effect was not detected in the severed treatment (ANOVA, F1, 33 = 0·003, P = 0·960; Fig. 4D). The δ15N values in the connected ramets in the water-stressed treatment were similar to those in ramets in the ammonium treatment. However, δ15N values in connected well-watered ramets were similar to those in the nitrate treatment (Fig. 4).
Table 4.
Three-way ANOVA for analyses of differences in nitrogen isotopic composition (δ15N), 13C/12C discrimination (Δ13C), leaf nitrogen content (%N) and leaf carbon content (%C) of offspring ramets with ‘integration’, ‘nitrogen form’ and ‘water status’ as main factors
| Effect | df | δ15N |
Δ13C |
%N |
%C |
||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | ||
| Integration | 1 | 0·668 | 0·417 | 3·338 | 0·073 | 2·498 | 0·120 | 0·259 | 0·613 |
| Nitrogen | 1 | 46·831 | <0.001 | 0·179 | 0·674 | 0·009 | 0·924 | 0·165 | 0·686 |
| Water | 1 | 3·615 | 0·063 | 4·690 | 0.035 | 4·016 | 0.050 | 14·700 | <0.001 |
| Integration × nitrogen | 1 | 3·305 | 0·075 | 0·126 | 0·724 | 0·376 | 0·542 | 1·284 | 0·262 |
| Integration × water | 1 | 7·740 | 0.007 | 0·535 | 0·468 | 5·796 | 0.020 | 5·030 | 0.029 |
| Nitrogen × water | 1 | 1·373 | 0·246 | 0·021 | 0·886 | 0·789 | 0·378 | 0·906 | 0·345 |
| Integration × nitrogen × water | 1 | 1·696 | 0·198 | 1·637 | 0·206 | 2·252 | 0·139 | 0·243 | 0·624 |
| Error | 54 | ||||||||
See Figs 4 and 5 for data.
Significant effects (P < 0·05) are shown in bold type.
df, degrees of freedom.
Fig. 4.
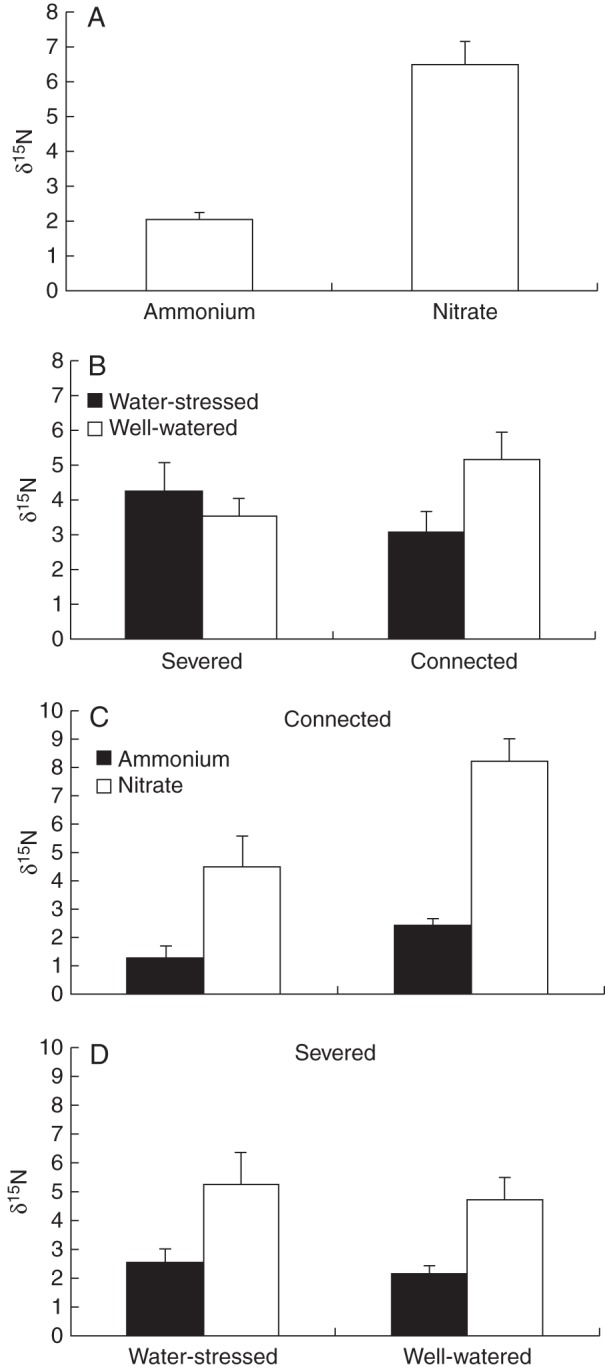
Mean (±s.e.) nitrogen isotopic composition (δ15N) in offspring ramets in ammonium and nitrate treatments (A) and in connected and severed offspring ramets in water-stressed and well-watered treatments (B). (C, D) Mean (±s.e.) nitrogen isotopic composition (δ15N) in offspring ramets in water-stressed and well-watered treatments and growing in nitrate or ammonium nitrogen for connected ramets (C) and severed offspring ramets (D). See Table 4 and text for ANOVA parameters.
Water status had a significant effect on 13C/12C discrimination (Δ13C), leaf nitrogen content (%N) and leaf carbon content (%C). The value of Δ13C was lower in well-watered ramets than in water-stressed ramets (Table 4, Fig. 5A). Both %N and %C were significantly higher in well-watered ramets than in water-stressed ramets (Table 4, Fig. 5B, C). The effect of integration on %N and %C depended on the water treatment (Table 4). Thus, integration significantly increased %N and decreased %C in well-watered offspring ramets but not in the water-stressed ramets (Table 4, Fig. 5B, C).
Fig. 5.
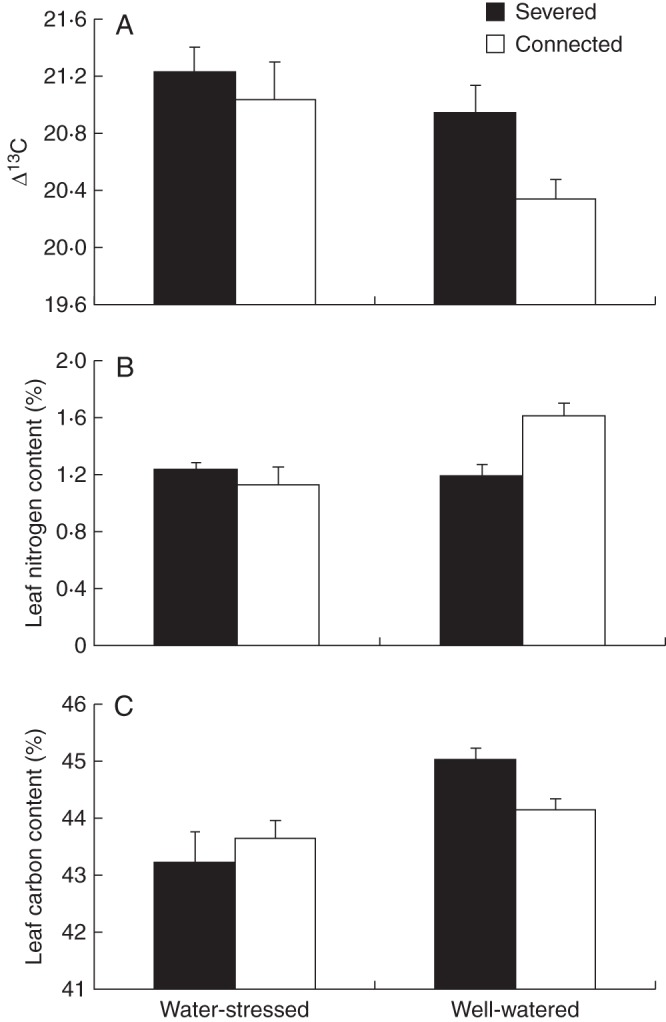
Mean 13C/12C discrimination (Δ13C) (A), leaf nitrogen content (B) and leaf carbon content (C) of severed and connected offspring ramets (as indicated in the key) in the water-stressed and well-watered treatments. See Table 4 for ANOVA parameters.
Growth and survival
Root mass, root mass ratio and total dry mass of water-stressed offspring ramets were significantly reduced relative to well-watered offspring ramets (Table 5, Fig. 6). Connection increased the survival of offspring ramets growing in water-stressed conditions (χ2 = 23·955, P < 0·001) (Fig. 7).
Table 5.
Three-way ANOVA for analyses of differences in root biomass, root mass ratio and total biomass of offspring ramets with ‘integration’, ‘nitrogen form’ and ‘water status’ as main factors
| Effect | df | Root biomass |
Root mass ratio |
Total biomass |
|||
|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | ||
| Integration | 1 | 2·002 | 0·163 | 0·177 | 0·675 | 3·187 | 0·080 |
| Nitrogen | 1 | 3·752 | 0·058 | 1·322 | 0·255 | 2·255 | 0·139 |
| Water | 1 | 10·787 | 0.002 | 4·955 | 0.033 | 6·956 | 0.011 |
| Integration × nitrogen | 1 | 0·744 | 0·392 | 0·207 | 0·651 | 0·106 | 0·746 |
| Integration × water | 1 | 0·229 | 0·634 | 0·287 | 0·594 | 0·495 | 0·485 |
| Nitrogen × water | 1 | 0·071 | 0·791 | 0·444 | 0·508 | 0·600 | 0·442 |
| Integration × nitrogen × water | 1 | 0·137 | 0·713 | 2·789 | 0·100 | 2·848 | 0·097 |
| Error | 54 | ||||||
Fig. 6.
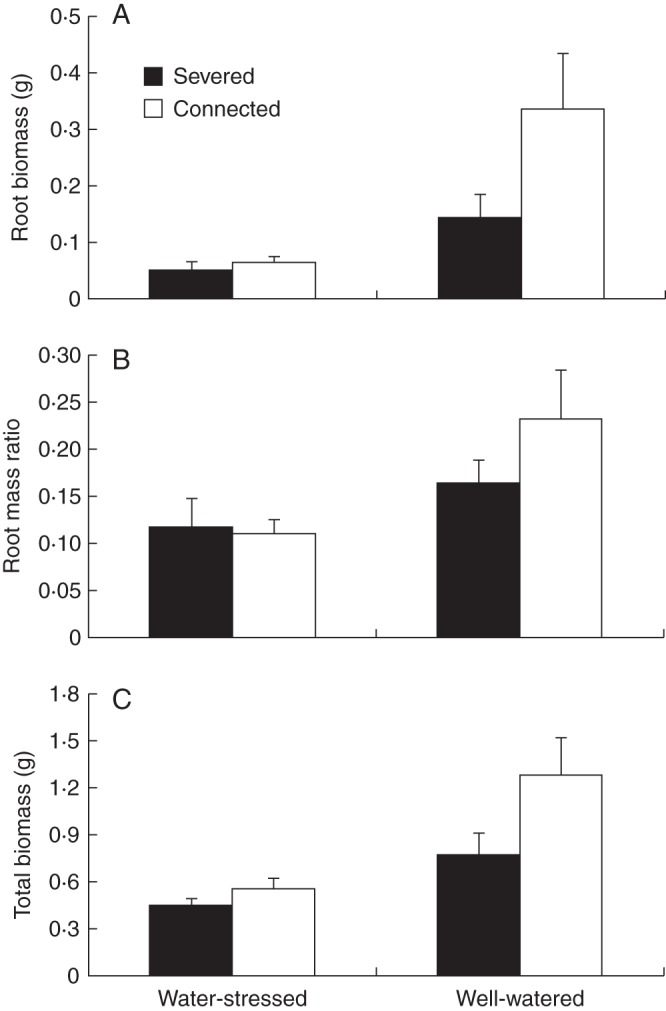
Mean (±s.e) root dry mass (A), root mass ratio (B) and total dry mass (C) of severed and connected offspring ramets (as indicated in the key) in the water-stressed and well-watered treatments. See Table 5 for ANOVA parameters.
Fig. 7.
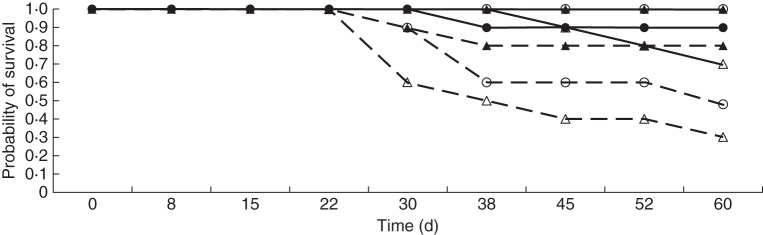
Time course of the Kaplan–Meier unconditional survival probability for connected (solid lines) and severed (dashed lines) offspring ramets growing in the well-watered (closed symbols) and water-stressed (open symbols) treatments and receiving nitrate (circles) or ammonium (triangles) as the only form of nitrogen. See text for log rank (Mantel–Cox) test.
DISCUSSION
The increased survival of water-stressed offspring ramets connected to parents supports our first hypothesis that physiological integration would buffer the stress caused by water deprivation. Previous studies have shown that offspring ramets growing in unfavourable conditions may be supported by the translocation of resources from parent ramets established in favourable patches, thus increasing their growth and survival (Hartnett and Bazzaz, 1983; Slade and Hutchings, 1987; Jónsdóttir and Watson, 1997; Roiloa and Retuerto, 2006a; Saitoh et al., 2006).
Although most of the literature on clonal plant ecology has focused on morphological responses (growth, biomass allocation ratios, stolon length, etc.), the present study demonstrates that the effects of integration can also be detected at a physiological level. The findings of the present study demonstrate the benefit of integration for the offspring ramets in terms of photochemical activity and photosynthesis, regardless of water status and the nitrogen form supplied. Similar benefits in photosynthetic traits have also been reported for offspring of F. vesca growing under unfavourable conditions (Roiloa and Retuerto, 2006a; Roiloa and Retuerto, 2007). However, the benefits of integration in terms of photochemical activity and photosynthesis were not translated into benefits in growth. Although connected offspring produced more total dry mass than severed offspring, the differences were not significant. Considering that growth in term of dry mass is the result of the balance between photosynthesis and respiration, our results strongly suggest that integration also increased respiration at a rate that cancelled out the benefits of an increased photosynthetic rate.
Contrary to our prediction, the Δ13C of water-stressed ramets was not affected by integration. The Δ13C value reflects the ratio of leaf internal to external CO2 concentration and has been used as a suitable integrator of stomatal conductance, water-use efficiency and photosynthetic rate of plants (Körner et al., 1988; Farquhar et al., 1989; Ehleringer et al., 1993). High Δ13C values are associated with high stomatal conductance and consequently with high photosynthetic activity and growth. An increase in Δ13C would be expected when the water availability was high (Ehleringer and Cooper, 1988; Farquhar et al., 1989). Well-watered conditions will enlarge stomatal apertures and increase Δ13C (Farquhar et al., 1989). However, the present results showed a significant increase in Δ13C in the water-stressed ramets relative to the well-watered ramets. In our study, the higher Δ13C in water-stressed plants occurred together with a significant decrease in photochemical activity, photosynthetic rate, stomatal conductance and growth experienced by the water-stressed plants in the present study. According to Virgona (1992), a negative relationship between growth and Δ13C may occur when constraints in photosynthetic capacity prevail over stomatal limitation. The Δ13C value provides a very good indication of the leaf intercellular concentration of CO2 (Ci). High Ci is explained either by a low photosynthetic rate or, more commonly, by high stomatal conductance (Larcher, 2003). The higher Δ13C in water-stressed plants may be explained by an increase in Ci as a consequence of the reduction in photosynthetic activity. This explanation is supported by the higher Ci found in the water-stressed ramets than in the well-watered ramets, although the difference was not significant. Increased Δ13C in water-stressed plants may be related to a significant reduction in leaf nitrogen content (Guehl et al., 1995; Sparks and Ehleringer, 1997; Retuerto et al., 2000), as we observed in the water-stressed ramets. Most of the leaf nitrogen is bound in photosynthetic enzymes, and therefore the reduction in leaf nitrogen content reported in water-stressed ramets is consistent with the reduction in photosynthesis and growth observed in these plants. In short, the present results show that water stress reduced leaf nitrogen content and consequently photosynthetic activity and growth. However, Δ13C increased because photosynthetic constraints prevailed over stomatal conductance in determining Ci. Similar results have been reported by Sparks and Ehleringer (1997), who found decreased leaf nitrogen and photosynthesis but increased Δ13C values in woody species. In addition, Aranda et al. (2010) reported that, within some species, populations from more xeric sites were not those with the lowest Δ13C values, suggesting that other species-specific traits, such as specific leaf area and nitrogen content, may modulate the isotopic signal.
The nitrogen form (ammonium or nitrate) on which the offspring ramets grew determined the nitrogen isotopic composition (δ15N) of their tissues. The δ15N of offspring ramets growing in ammonium was significantly lower than that in offspring ramets growing in nitrate, for both connected and severed ramets. Similarly, other studies have found that an increase in ammonium uptake leads to a decrease in δ15N (Evans, 2001; Kahmen et al., 2008). Thus, our results support the use of the nitrogen isotopic signature (δ15N) as a useful tool for determining the nitrogen form used by plants. However, we found no evidence to support our second hypothesis. In contrast to our expectations, there was no preferential transport of N from ammonium or nitrate sources from parents to offspring ramets, to supply these ramets with the nitrogen form in which they were deficient. Rather, our results (Fig. 4C, D) showed that connection, in water-stressed ramets, decreased δ15N values, suggesting that offspring mainly received N from ammonium sources, whereas connection in well-watered ramets increased δ15N values, which suggests that these ramets could be obtaining mainly nitrates from their parents. As a result, δ15N values in connected water-stressed ramets were within the range of values found for plants grown in ammonium, whereas δ15N values in connected well-watered ramets resembled the values in plants grown with nitrate as the only nitrogen form. However, δ15N of severed offspring was similar regardless of the water availability conditions. These results represent a novel finding that provide compelling evidence of preferential transport of N from the ammonium source (parents) to water-stressed offspring, giving support for our third hypothesis. Since ammonium is toxic to plants and typically not found in the xylem, it is likely that, after uptake in the roots, the ammonium is incorporated immediately into glutamate, which is the main transport agent of reduced N in plants. One plausible reason for the preferential transport of ammonium from parents to water-stressed offspring is that water consumption in plants may be affected by the form of nitrogen that they receive (Guo et al., 2007). Previous studies have shown that plants supplied with ammonium consume less water than those supplied with nitrate. Høgh-Jensen and Schjoerring (1997) found that the water-use efficiency of the clonal Trifolium repens was higher in plants supplied with ammonium than in those supplied with nitrate. Similar results have been reported for wheat (Yin and Raven, 1998). We interpret the preferential transport of N from the ammonium source as a mechanism for reducing water consumption in water-stressed ramets. Higher aquaporin activity in plants with ammonium nutrition than in plants with nitrate nutrition (Gao et al., 2010) is consistent with our interpretation. This is a novel result that represents a potential benefit of clonal integration in terms of water consumption that has not been reported previously, and therefore this research extends the concept of physiological integration in clonal plants to include preferential transport of nutrients.
Previous studies have found both physiological and morphological compensatory responses of parents in response to demands from offspring ramets growing in unfavourable conditions. Experiments with F. vesca showed that photosynthetic efficiency in parent ramets increased in response to the assimilate demand made by drought-stressed offspring ramets (Roiloa and Retuerto, 2007). Similarly, parent ramets of F. vesca increased the biomass allocated to roots as a non-local compensatory response to the copper-polluted soil in which the offspring ramets were placed (Roiloa and Retuerto, 2012). However, this is the first study suggesting preferential transport of a specific form of nitrogen in order to optimize water use by a member of the clone. The preferential transport of N from the ammonium source (parents) to water-stressed offspring ramets is consistent with three well-known facts: (1) ammonium uptake generally occurs at a higher rate than nitrate uptake for a wide diversity of species of different functional types (Lee and Stewart, 1978; Marty et al., 2009; Mota et al., 2011; Gherardi et al., 2013; Lian et al., 2012; Yang et al., 2013); (2) a water potential gradient facilitates the movement of resources between connected ramets (Stuefer and Hutchings, 1994; Jónsdóttir and Watson, 1997); and (3) in clonal plants acropetal transport (from the parent to the offspring ramet) of resources exceeds basipetal transport (from offspring to the parent ramet) (Alpert and Mooney, 1986; Price and Hutchings, 1992; Alpert, 1996). Although it is clear that our results suggest a potential benefit of clonal integration in terms of water consumption for plants that are water-stressed, we should not necessarily expect an increase in biomass in plants growing in ammonium. In fact, previous studies have demonstrated that, compared with nitrate as an N source, ammonium results in small root and small leaf area, which may contribute to a low carbon gain and inhibition of growth (Guo et al., 2007). Substantially higher losses of carbon through root and leaf respiration have been found with ammonium than with nitrate (Bloom et al., 1992; Peuke and Jeschke, 1993; Cramer and Lewis, 1993).
Finally, the probable preferential transport of N from the ammonium source to the water-stressed ramets is also consistent with the unexpected higher Δ13C values we found in these ramets. Previous studies have detected higher Δ13C values in ammonium-supplied plants than in nitrate-supplied plants (Raven and Farquhar, 1990). Therefore, the increased Δ13C in water-stressed offspring ramets could also be explained by the preferential transport of N from the ammonium source to the offspring ramets.
Conclusions
Summarizing, the present findings suggest preferential transport of N from the ammonium source to offspring ramets of F. vesca growing under water-stressed conditions. In the light of the lower water consumption due to ammonium use, we interpret this result as a compensatory response in order to improve water use by the clone. This novel finding deepens and extends the concept of physiological integration in clonal plants to include preferential transport of nutrients. Our study contributes a novel perspective to the understanding of the complementary use of different forms of nitrogen by the different components of a clonal system. Integration improved survival of offspring ramets growing in water-stressed conditions, providing evidence of the importance of physiological integration in maintaining the presence of ramets in unfavourable conditions. We also demonstrate the benefit of integration, regardless of water availability or nitrogen form, in terms of photochemical activity and photosynthesis, which has scarcely been studied. Finally, this is the first study in which carbon and nitrogen isotopic composition has been used to detect physiological integration in clonal plants. Physiological integration enables clonal plants to buffer stressful conditions, and therefore this clonal trait should considered as a key contribution to the success of clonal growth in plant communities.
ACKNOWLEDGEMENTS
We thank R. Bermúdez-Villanueva and X. Santiso for assistance in the greenhouse. We are grateful to M. Lema of SAI-UTIA (University of A Coruña) for carbon and nitrogen isotope analyses of leaves, to M. Santiso of the Department of Edaphology (University of Santiago de Compostela) for ammonium and nitrate soil content analyses, and Christine Francis for English correction. We are grateful to two anonymous referees and to the editor Ming Dong for their valuable comments on an earlier version of this paper.
LITERATURE CITED
- Alpert P. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. Journal of Ecology. 1996;84:395–406. [Google Scholar]
- Alpert P, Mooney HA. Resource sharing among ramets in the clonal herb, Fragaria chiloensis. Oecologia. 1986;70:227–233. doi: 10.1007/BF00379244. [DOI] [PubMed] [Google Scholar]
- Alpert P, Lumaret R, Di Giusto F. Population structure inferred from allozyme analysis in the clonal herb Fragaria chiloensis (Rosaceae) American Journal of Botany. 1993;80:1002–1006. [Google Scholar]
- Aranda I, Alía R, Ortega U, Dantas AK, Majada J. Intra-specific variability in biomass partitioning and carbon isotopic discrimination under moderate drought stress in seedlings from four Pinus pinaster populations. Tree Genetics and Genomes. 2010;6:169–178. [Google Scholar]
- Ariz I, Cruz C, Moran JF, et al. Depletion of the heaviest stable N isotope is associated with NH4+/NH3 toxicity in NH4+-fed plants. BMC Plant Biology. 2011;11:83. doi: 10.1186/1471-2229-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiology. 1992;99:1294–1301. doi: 10.1104/pp.99.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhàr-Nordenkampf HR, Long SP, Baker NR, Öquist G, Scheiber U, Lechner EG. Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Functional Ecology. 1989;3:497–514. [Google Scholar]
- Breteler H, Hänisch ten Cate CH. Fate of nitrate during initial nitrate utilization by nitrogen-depleted dwarf bean. Physiologia Plantarum. 1980;48:292–296. [Google Scholar]
- Butler W, Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochimica et Biophysica Acta. 1975;376:116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Pearcy RW. Exploitation of environmental heterogeneity by plants. Ecophysiological processes above and belowground. San Diego: Academic Press; 1994. [Google Scholar]
- Claussen W, Lenz F. Effect of ammonium and nitrate nutrition on net photosynthesis, growth, and activity of the enzymes nitrate reductase and glutamine synthetase in blueberry, raspberry and strawberry. Plant and Soil. 1999;208:95–102. [Google Scholar]
- Cramer MD, Lewis OAM. The influence of NO3– and NH4+ nutrition on the gas exchange characteristics of the root of wheat (Triticum aestivum) and maize (Zea mays) plants. Annals of Botany. 1993;72:37–46. [Google Scholar]
- D'Hertefeldt T, Jonsdottir IS. Extensive physiological integration in intact clonal systems of Carex arenaria. Journal of Ecology. 1999;87:258–264. [Google Scholar]
- D'Hertefeldt T, Falkengren-Grerup U. Extensive physiological integration in Carex arenaria and Carex disticha in relation to potassium and water availability. New Phytologist. 2002;156:469–477. doi: 10.1046/j.1469-8137.2002.00529.x. [DOI] [PubMed] [Google Scholar]
- D'Hertefeldt T, Falkengren-Grerup U, Jónsdóttir IS. Responses to mineral nutrient availability and heterogeneity in physiologically integrated sedges from contrasting habitats. Plant Biology. 2011;13:483–492. doi: 10.1111/j.1438-8677.2010.00393.x. [DOI] [PubMed] [Google Scholar]
- Darnell RL, Stutte GW. Nitrate concentration effects on NO3-N uptake and reduction, growth, and fruit yield in strawberry. Journal of the American Society for Horticultural Science. 2001;125:560–563. [PubMed] [Google Scholar]
- Day KJ, John EA, Hutchings MJ. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Functional Ecology. 2003;17:454–463. [Google Scholar]
- Derner JD, Briske DD. An isotopic (15N) assessment of intraclonal regulation in C4 perennial grasses: ramet interdependence, independence or both? Journal of Ecology. 1998;86:305–314. [Google Scholar]
- Ehleringer JR, Cooper TA. Correlations between isotope ratio and microhabitat in desert plants. Oecologia. 1988;76:562–566. doi: 10.1007/BF00397870. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Hall EA, Farquhar GD. Stable isotopes and plant carbon/water relations. San Diego: Academic Press; 1993. [Google Scholar]
- Errebhi M, Wilcox GE. Plant species responses to ammonium-nitrate concentrations ratios. Journal of Plant Nutrition. 1990;13:1017–1029. [Google Scholar]
- Evans RD. Physiological mechanisms influencing plant nitrogen isotope composition. Trends in Plant Science. 2001;6:121–126. doi: 10.1016/s1360-1385(01)01889-1. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Ferrio JP, Araus JL, Buxó R, Voltas J, Bort J. Water management practices and climate in ancient agriculture: inference from the stable isotope composition of archaeobotanical remains. Vegetation History and Archaeobotany. 2005;14:510–517. [Google Scholar]
- Gamon JA, Serrano I, Surfus JS. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia. 1997;112:492–501. doi: 10.1007/s004420050337. [DOI] [PubMed] [Google Scholar]
- Ganmore-Neumann R, Kafkafi U. The effect of root temperature and nitrate/ammonium ratio on strawberry plants. II. Nitrogen uptake, mineral ions, and carboxylate concentration. Agronomy Journal. 1985;77:835–840. [Google Scholar]
- Gao YX, Li Y, Yang XX, Li HJ, Shen QR, Guo SW. Ammonium nutrition increases water absorption in rice seedlings (Oryza sativa L.) under water stress. Plant and Soil. 2010;331:193–201. [Google Scholar]
- Genty BE, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gherardi LA, Sala OE, Yahdjian L. Preference for different inorganic nitrogen forms among plant functional types and species of the Patagonian steppe. Oecologia. 2013;173:1075–1081. doi: 10.1007/s00442-013-2687-7. [DOI] [PubMed] [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. Comparative plant ecology: a functional approach to common British species. London: Chapman and Hall; 1996. [Google Scholar]
- Guehl JM, Fort C, Ferhi A. Differential response of leaf conductance, carbon isotope discrimination and water-use efficiency to nitrogen deficiency in maritime pine and pedunculate oak plants. New Phytologist. 1995;131:149–157. [Google Scholar]
- Guo J, Trotter CM. Estimating photosynthetic light-use efficiency using the photochemical reflectance index: variations among species. Functional Plant Biology. 2004;31:255–265. doi: 10.1071/FP03185. [DOI] [PubMed] [Google Scholar]
- Guo S, Zhou Y, Shen Q, Zhang F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants – growth, photosynthesis, photorespiration, and water relations. Plant Biology. 2007;9:21–29. doi: 10.1055/s-2006-924541. [DOI] [PubMed] [Google Scholar]
- Handley LL, Raven JA. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell and Environment. 1992;15:965–985. [Google Scholar]
- Hartgerink AP, Bazzaz FA. Seedling-scale environmental heterogeneity influences individual fitness and population structure. Ecology. 1984;65:198–206. [Google Scholar]
- Hartnett DC, Bazzaz FA. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology. 1983;64:779–788. [Google Scholar]
- Hellström K, Kytöviita MM, Tuomi J, Rautio P. Plasticity of clonal integration in the perennial herb Linaria vulgaris after damage. Functional Ecology. 2006;20:413–420. [Google Scholar]
- Higinbotham NB. The mineral absorption process in plants. Botanical Review. 1973;39:15–69. [Google Scholar]
- Høgh-Jensen H, Schjoerring JK. Effects of drought and inorganic N form on nitrogen fixation and carbon isotope discrimination in Trifolium repens. Plant Physiology and Biochemistry. 1997;35:55–62. [Google Scholar]
- Hutchings MJ, Wijesinghe DK. Performance of a clonal species in patchy environments: effects of environmental context on yield at local and whole-plant scales. Evolutionary Ecology. 2008;22:313–324. [Google Scholar]
- Hutchings MJ, John EA, Wijesinghe DK. Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology. 2004;84:2322–2334. [Google Scholar]
- Jónsdóttir IS, Callaghan TV. Intraclonal translocation of ammonium and nitrate nitrogen in Carex bigelowii Torr. ex Schwein. using 15N and nitrate reductase assays. New Phytologist. 1990;114:419–428. doi: 10.1111/j.1469-8137.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Jónsdóttir IS, Watson MA. Extensive physiological integration: an adaptive trait in resource-poor environments? In: de Kroon H, van Groenendael J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. pp. 109–136. [Google Scholar]
- Kahmen A, Wanek W, Buchmann N. Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia. 2008;156:861–870. doi: 10.1007/s00442-008-1028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Körner C, Farquhar GD, Roksandic Z. A global survey of carbon isotope discrimination in plants from high altitude. Oecologia. 1988;74:623–632. doi: 10.1007/BF00380063. [DOI] [PubMed] [Google Scholar]
- Larcher W. Physiological plant ecology. New York: Springer; 2003. [Google Scholar]
- Lechowicz MJ, Bell G. The ecology and genetics of fitness in forest plants. II. Microspatial heterogeneity of the edaphic environment. Journal of Ecology. 1991;79:687–696. [Google Scholar]
- Lee JA, Stewart GR. Ecological aspect of nitrogen assimilation. In: Woolhouse HW, editor. Advances in botanical research. London: Academic Press; 1978. pp. 1–43. [Google Scholar]
- Lian Y, Chen MX, Shah F, et al. Difference between NH4+ and NO3– uptake kinetics of different rice (Oryza sativa L.) grown hydroponically. Journal of Food Agriculture and Environment. 2012;10:437–442. [Google Scholar]
- Lichtenthaler HK, Gitelson A, Lang M. Non-destructive determination of chlorophyll content of leaves of a green and an Aurea mutant of tobacco by reflectance measurements. Journal of Plant Physiology. 1996;148:483–493. [Google Scholar]
- Liu J, He WM, Zhang SM, Liu FH, Dong M, Wang RQ. Effects of clonal integration on photosynthesis of the invasive clonal plant Alternanthera philoxeroides. Photosynthetica. 2008;46:299–302. [Google Scholar]
- Mao SY, Jiang CD, Zhang WH, et al. Water translocation between ramets of strawberry during soil drying and its effects on photosynthetic performance. Physiologia Plantarum. 2009;3:225–234. doi: 10.1111/j.1399-3054.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. London: Academic Press; 1995. [Google Scholar]
- Marty C, Pornon A, Lamaze T. High NH4+ efflux from roots of the common alpine grass, Festuca nigrescens, at field-relevant concentrations restricts net uptake. Environmental and Experimental Botany. 2009;67:84–86. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Michener R, Lajtha K. Stable isotopes in ecology and environmental science. Oxford: Blackwell Publishing; 2007. [Google Scholar]
- Mota M, Neto CB, Monteiro AA, Oliveira CM. Preferential ammonium uptake during growth cycle and identification of ammonium transporter genes in young pear trees. Journal of Plant Nutrition. 2011;34:798–814. [Google Scholar]
- Oborny B, Cain ML. Models of spatial spread and foraging in clonal plants. In: de Kroon H, van Groenendael J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. pp. 155–183. [Google Scholar]
- Peñuelas J, Filella I, Gamon JA. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytologist. 1995;131:291–296. [Google Scholar]
- Peuke AD, Jeschke WD. The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. 1. Growth with ammonium or nitrate as nitrogen source. Journal of Experimental Botany. 1993;44:1167–1176. [Google Scholar]
- Pitelka LF, Ashmun JW. Physiology and integration of ramets in clonal plants. In: Jackson JBC, Buss LW, Cook RR, editors. Population biology and evolution of clonal organisms. New Haven: Yale University Press; 1985. pp. 339–437. [Google Scholar]
- Price EAC, Hutchings MJ. The causes and developmental effects of integration and independence between different parts of Glechoma hederacea clones. Oikos. 1992;63:376–386. [Google Scholar]
- Price EAC, Marshall C. Clonal plants and environmental heterogeneity. Plant Ecology. 1999;141:3–7. [Google Scholar]
- Raven JA, Farquhar GD. The influence of N metabolism and organic acid synthesis on the natural abundance of isotopes of carbon in plants. New Phytologist. 1990;116:505–529. doi: 10.1111/j.1469-8137.1990.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Retuerto R, Fernandez-Lema B, Rodriguez-Roiloa S, Obeso JR. Gender, light and water effects in carbon isotope discrimination, and growth rates in the dioecious tree Ilex aquifolium. Functional Ecology. 2000;14:529–537. [Google Scholar]
- Robinson D. δ15N as an integrator of the nitrogen cycle. Trends in Ecology and Evolution. 2001;16:153–162. doi: 10.1016/s0169-5347(00)02098-x. [DOI] [PubMed] [Google Scholar]
- Roiloa SR, Retuerto R. Presence of developing ramets of Fragaria vesca increase photochemical efficiency in parent ramets. International Journal of Plant Science. 2005;166:795–803. [Google Scholar]
- Roiloa SR, Retuerto R. Physiological integration ameliorates effects of serpentine soils in the clonal herb Fragaria vesca. Physiologia Plantarum. 2006a;128:662–676. [Google Scholar]
- Roiloa SR, Retuerto R. Development, photosynthetic activity and habitat selection of the clonal plant Fragaria vesca growing in copper polluted soil. Functional Plant Biology. 2006b;33:961–971. doi: 10.1071/FP06018. [DOI] [PubMed] [Google Scholar]
- Roiloa SR, Retuerto R. Responses of the clonal Fragaria vesca to microtopographic heterogeneity under different water and light conditions. Environmental and Experimental Botany. 2007;61:1–9. [Google Scholar]
- Roiloa SR, Retuerto R. Clonal integration in Fragaria vesca growing in metal-polluted soils: parents face penalties for establishing their offspring in unsuitable environments. Ecological Research. 2012;27:95–106. [Google Scholar]
- Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC. Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology. 2007;95:397–405. [Google Scholar]
- Saitoh T, Seiwa K, Nishiwaki A. Importance of physiological integration of dwarf bamboo to persistence in forest understorey: a field experiment. Journal of Ecology. 2002;90:78–85. [Google Scholar]
- Saitoh T, Seiwa K, Nishiwaki A. Effects of resource heterogeneity on nitrogen translocation within clonal fragments of Sasa palmata: an isotopic (N-15) assessment. Annals of Botany. 2006;98:657–663. doi: 10.1093/aob/mcl147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman AG, Parker MA. Neighbors ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia. 1985;65:273–277. doi: 10.1007/BF00379229. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Bilger W, Hormann H, Neubauer C. Chlorophyll fluorescence as a diagnostic tool: basics and some aspects of practical relevance. In: Raghavendra AS, editor. Photosynthesis. A comprehensive treatise. Cambridge: Cambridge University Press; 1998. pp. 320–336. [Google Scholar]
- Sims DA, Gamon JA. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sensing of Environment. 2002;81:337–354. [Google Scholar]
- Slade AJ, Hutchings MJ. An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea. Oecologia. 1987;73:425–431. doi: 10.1007/BF00385260. [DOI] [PubMed] [Google Scholar]
- Sparks JP, Ehleringer JR. Leaf carbon isotope discrimination and nitrogen content for riparian trees along elevational transects. Oecologia. 1997;109:362–367. doi: 10.1007/s004420050094. [DOI] [PubMed] [Google Scholar]
- Stuefer JF, Hutchings MJ. Environmental heterogeneity and clonal growth: a study of the capacity for reciprocal translocation in Glechoma hederacea. Oecologia. 1994;100:302–308. doi: 10.1007/BF00316958. [DOI] [PubMed] [Google Scholar]
- Stylinski CD, Gamon JA, Oechel WC. Seasonal patterns of reflectance indices, carotenoid pigments and photosynthesis of evergreen chaparral species. Oecologia. 2002;131:366–374. doi: 10.1007/s00442-002-0905-9. [DOI] [PubMed] [Google Scholar]
- Tabatabfaei SJ, Fatemi L, Fallahi E. Effect of ammonium:nitrate ratio on yield, calcium concentration, and photosynthesis rate in strawberry. Journal of Plant Nutrition. 2006;29:1273–1285. [Google Scholar]
- Thomas RJ, Feller U, Erismann KH. The effect of different inorganic nitrogen sources and plant age on the composition of bleeding sap of Phaseolus vulgaris. New Phytologist. 1979;82:657–669. doi: 10.1111/j.1469-8137.1979.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Virgona JM. Genotypic variation in growth and carbon isotope discrimination. Australia: PhD Thesis, Australian National University; 1992. [Google Scholar]
- Wijesinghe DK, Hutchings MJ. The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale. Journal of Ecology. 1999;87:860–872. [Google Scholar]
- Wijesinghe DK, John EA, Hutchings MJ. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. Journal of Ecology. 2005;93:99–112. [Google Scholar]
- Wood CW, Reeves DW, Himelrick DG. Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: a review; Proceedings of the Agronomy Society of New Zealand; 1993. pp. 1–9. [Google Scholar]
- Yang YY, Li XH, Ratcliffe RG, Ruan JY. Characterization of ammonium and nitrate uptake and assimilation in roots of tea plants. Russian Journal of Plant Physiology. 2013;60:91–99. [Google Scholar]
- Yin ZH, Raven JA. Influences of different nitrogen sources on nitrogen- and water-use efficiency, and carbon isotope discrimination, in C3 Triticum aestivum L. and C4 Zea mays L. plants. Planta. 1998;205:574–580. [Google Scholar]
- Zhang YC, Zhang QY, Luo P, Wu N. Photosynthetic response of Fragaria orientalis in different water contrast clonal integration. Ecological Research. 2009;24:617–625. [Google Scholar]