Abstract
Background and Aims
Simultaneous formation of aerial and soil seed banks by a species provides a mechanism for population maintenance in unpredictable environments. Eolian activity greatly affects growth and regeneration of plants in a sand dune system, but we know little about the difference in the contributions of these two seed banks to population dynamics in sand dunes.
Methods
Seed release, germination, seedling emergence and survival of a desert annual, Agriophyllum squarrosum (Chenopodiaceae), inhabiting the Ordos Sandland in China, were determined in order to explore the different functions of the aerial and soil seed banks.
Key Results
The size of the aerial seed bank was higher than that of the soil seed bank throughout the growing season. Seed release was positively related to wind velocity. Compared with the soil seed bank, seed germination from the aerial seed bank was lower at low temperature (5/15 °C night/day) but higher in the light. Seedling emergence from the soil seed bank was earlier than that from the aerial seed bank. Early-emerged (15 April–15 May) seedlings died due to frost, but seedlings that emerged during the following months survived to reproduce successfully.
Conclusions
The timing of seed release and different germination behaviour resulted in a temporal heterogeneity of seedling emergence and establishment between the two seed banks. The study suggests that a bet-hedging strategy for the two seed banks enables A. squarrosum populations to cope successfully with the unpredictable desert environment.
Keywords: Aerial seed bank, soil seed bank, Agriophyllum squarrosum, Chenopodiaceae, seed germination, seedling establishment, fitness, sand dune ecology
INTRODUCTION
Asynchronous seed release and/or germination reduce the risk of offspring mortality and are considered effective strategies to cope with unpredictable environments (Narita and Wada, 1998; Harris and Pannell, 2010). Gradual seed release from the mother plants has long been known as a ‘bet-hedging’ strategy for many species (Cohen, 1966; Brown and Venable, 1986; Lamont and Enright, 2000; Venable, 2007; Peters et al., 2011), which results in two kinds of seed banks. One is an aerial seed bank, which is formed on the plant (Lamont, 1991); the other is a soil seed bank, which is formed in the soil (Thompson, 1987). Many studies have examined the ecological importance of the two seed banks in the persistence and recruitment of plants in populations (Gutterman and Ginott, 1994; Evans et al., 2007; Gutterman et al., 2007; Peters et al., 2011). However, we know little about the joint effect of these two seed banks on the population dynamics of species, especially for desert annuals.
For species with well-developed serotiny (having both aerial and soil seed banks), especially woody plants, there is no seed release until after specific dispersal or germination cues (fire or rain) (Lamont et al., 1991; Gutterman and Ginott, 1994). However, for annuals inhabiting sand dunes, such as Agriophyllum squarrosum, a small fraction of seeds falls onto the soil surface and forms a soil seed bank before the peak of seed release (Narita and Wada, 1998). A large fraction of seeds is retained on the mother plants (aerial seed bank). Seed retention is common in stressful habitats, such as those of fire-prone (Moya et al., 2013), nutrient-poor (Günster, 1992) and seasonal drought (Cowling and Lamont, 1985) conditions. The seeds are protected from biotic factors (e.g. predation) and/or abiotic factors (e.g. heat) before release from the mother plants (Gutterman and Ginott, 1994). Reproductive structures (scales, bracts or follicles) enclose the seeds, and are opened by fire (Clarke et al., 2013) or cycles of hydration–dehydration (Gutterman and Ginott, 1994) before seed release. For some desert plants, seed release is triggered by environmental cues (such as seasonal rain and wind) coinciding with the occurrence of favourable conditions for seed germination and seedling establishment (Gutterman and Ginott, 1994; Aguado et al., 2012).
Once environmental cues trigger seed release, the number of seeds retained on the mother plants will gradually decrease (Hamilton-Brown et al., 2008). On the other hand, the number of seeds in the soil will be not be directly related to seed release since the release cues (rain) may also trigger germination. At this time, a germinated seed can be from either the soil seed bank or an aerial seed bank. Therefore, the dynamics of the soil seed bank depend on both seed release from the aerial seed bank and germination from the soil. Although germination responses from soil seed banks have been well studied in different environments, few investigations have been carried out to determine germination responses of aerial seed banks to environmental factors. Moreover, studies of aerial seed banks are mainly focused on fire-prone habitats (Moya et al., 2013) and rarely on deserts (Peters et al., 2011), where water availability is a key environmental factor (Noy-Meir, 1973). For some desert plants, germination from an aerial seed bank can be rapid after release (Gutterman and Ginott, 1994), because seeds retained on the plants have already undergone hydration–dehydration cycles and have thus been primed (Santini and Martorell, 2013).
Although an aerial seed bank is a potential repository analogous to the soil seed bank and the two seed banks are from the same maternal plant, large differences may exist between them (Cowling and Lamont, 1987). The temporal variations in seed release depend on dispersal cues, which minimize the delay in germination and mediate fluctuations in the annual seedling establishment by ensuring that seeds arrive on the optimal substrates for germination and establishment (Lamont et al., 1991; Lamont and Enright, 2000). This has been proposed as a bet-hedging stategy for plants to cope with unpredictable environments (Venable, 2007). Soil seed banks, however, have an advantage in population maintenance after disturbances: even after all above-ground plants are destroyed, seeds in the soil still have the opportunity to germinate and establish when favourable conditions occur (Fenner and Thompson, 2005). Heretofore, the attributes and roles of an aerial vs. soil seed bank for the same species have not been elucidated.
Species that have both aerial and soil seed banks may have special adaptability in unpredictable environments. First, germination of seeds released from the aerial seed bank can result in higher seedling density than that only from the soil seed bank because prolonged seedling emergence resulting from gradual seed release can result in an escape from the intense competition among seedlings (Günster, 1994). Secondly, seeds from an aerial seed bank have been primed on the mother plant and thus can enhance seedling vigour and survival (Santini and Martorell, 2013). Thirdly, the different responses of germination of seeds from the two seed banks to environmental cues (temperature, light or soil moisture) can result in differentiation in the germination niche (phenology), which reduces the risk of mass germination when extreme conditions occur within a season. Finally, the contributions of the two seed banks to fitness may differ because unpredictable environmental conditions determine whether seedlings from an aerial or a soil seed bank can complete their life cycles. However, no study has been conducted to assess interactive effects of the two seed banks on population dynamics.
Agriophyllum squarrosum is a sand dune species that has both aerial and soil seed banks (Liu et al., 2006, 2007). This species slows wind speed and reduces sand movement with its lignified stems. Some seeds are retained on the dead plants for >1 year and are released by strong wind during the growing season and then buried deeply by active sand movement to become a part of the soil seed bank (Ma and Liu, 2008). Meanwhile, the wind dispersal of seeds results in the heterogeneous patterns of the soil seed bank and ultimatelly influences the establishment of seedlings and the distribution of plants in the sand dune (Bai et al., 2004; Liu and Wang, 2009; Bastida et al., 2010). Therefore, the seed bank dynamics of this species are complicated, and studying the functional difference between aerial and soil seed banks would help us to understand the adaptive mechanisms for population maintenance and plant regeneration involving the two seed banks.
We hypothesized that aerial and soil seed banks of desert species have different roles in the process of seed germination, seedling establishment and plant fitness. To test this hypothesis, field observation and controlled experiments were used to explore: (1) relationships between seed release and environmental cues; (2) seasonal dynamics of the aerial and soil seed bank; (3) germination responses of seeds from the aerial and soil seed banks to temperature and light; (4) dynamics of seedling emergence from the two seed banks; and (5) fitness of plants that emerged from the two seed banks. Comparison of the functions of the two seed banks would provide insights into the ecological significance and adaptive strategies of seasonal aerial and soil seed banks in unpredictable environments.
MATERIALS AND METHODS
Study site
The study was carried out at the Ordos Sandland Ecological Research Station of the Chinese Academy of Sciences (39 °29′37·6′′N, 110 °11′29·4′′E, 1296 m a.s.l.), located in the north-eastern Mu Us Sandland in Inner Mongolia, China. The Sandland is semi-arid with a mean annual precipitation of 345·8 mm, most of which falls during the growing season (from June to August). Mean annual temperature is 6·8 °C with a minimum of –16·9 °C in January and maximum of 28·1 °C in July. In the growing season, mean wind velocity is 2·39 ± 0·14 m s–1, mean maximum velocity of the wind is 6·72 ± 0·34 m s–1 and the wind is strongest in early May. The landscape at the station is characterized by mobile and semi-fixed sand dunes dominated by the forbs Agriophyllum squarrosum, Corispermum declinatum and C. patelliforme, and by fixed sand dunes with the shrubs Artemisia ordosica, Hedysarum laeve, Salix psammophila and Sabina vulgaris. Seed germination of most species starts in late April, when temperature increases and snow melts away.
Study species
Agriophyllum squarrosum (L.) Moq. (Amaranthaceae) is an annual psammophyte, widely distributed in arid and semi-arid regions of northern China, Russia, Mongolia and Iran (Kong, 1996). Populations of this species form monospecific stands, with its stems slowing wind speed and reducing sand movement during the growing season as well as during the winter. The species is a heavily branched stiff forb with alternate, smooth and prickly leaves. The flowers are bisexual and grow in small capitate, axillary, spiny inflorescences, and the period of flowering is from July to September. The fruits are compressed with a narrow wing, and mature in September and October. The matured fruits are enclosed by stiff bracts with prickles, and seed release occurs due to wind when the bracts open. In natural habitats, many seedlings that emerge in early spring die due to spring frosts (pers. obs.).
Seed characteristics and germination behaviour of freshly harvested seeds
Fifty plants were randomly selected in a natural population of A. squarrosum near the station on 15 October 2011, and each plant was individually placed in a paper bag, and then taken to the laboratory. Seeds were removed from each plant by rubbing the infructescence by hand and counted to determine the average seed number per plant; these seeds from the different plants were then mixed and dried for 3 d at a temperature simulating natural conditions (5–15 °C). Ten groups of 1000 seeds were weighed using an electronic analytical balance (Sartorius BP 221 S, Sartorius, Germany) to obtain the average seed mass; the length and width of 100 seeds were measured by using a dissecting microscope (Nikon 80i, Nikon Corp., Tokyo, Japan).
To explore the germination characteristics of freshly matured seeds at different temperatures and in different light conditions, experiments were conducted at 15/5, 20/10, 25/15 and 30/20 °C (12/12 h) in alternating light and darkness (12 h photoperiod, approx. 400 mmol m–2 s–1, cool white fluorescent light) and in continuous darkness. The four temperature regimes represented mean minimum/maximum temperatures for late April and October, May and September, June and August, and July in the habitat, respectively. Four replicates of 25 seeds each were placed into 7 mm diameter Petri dishes on two layers of filter paper moistened with 3 mL of distilled water. Germination was checked every 24 h. For the dark treatment, germination was checked under green safe light in a dark room. Germinated seeds (radicle emerged) were removed, and the experiment was completed after 20 d when no additional seeds germinated for four successive days. The viability of ungerminated seeds was determined by prodding them with forceps to determine whether the embryo was firm, indicating a viable seed. Final germination percentage (GP) was calculated with the following equation:
where A is the number of germinated seeds and B is the number of ungerminated but viable seeds.
Establishment of plots for seed release, seed bank dynamics, seedling emergence and survival studies
Four plots were arrayed in natural populations of A. squarrosum near the station from April to August in 2012 to study the dynamics of seed release (seed release plot), of the seed bank (seed bank plot), of seedling emergence (seedling emergence plot) and of seedling survival (seedling survival plot). These plots were adjacent to each other and the density of plants was similar (7 ± 2·58 plants m–2). Climate data from March to September in 2012 were collected from a weather station at the Ecological Station, including daily maximum and minimum temperatures, amount of daily rainfall ( >5 mm) and daily maximum wind velocity.
Seed release
In order to determine the relationships between seed release dynamics and environmental cues, seed release was monitored in the seed release plot (20 × 15 m2) from 15 March to 15 September 2012. Thirty seed traps were randomly arrayed below the canopies of the species. The seed trap was made from a plastic pot (height, 18 cm; diameter, 14 cm) with holes at the bottom. A nylon bag (0·9 mm mesh size) was placed into the pot (covering the holes) and the open end was fixed on the edge of the pot by a length of elastic. Then the seed traps were buried to a 16 cm depth of sand and kept 2 cm above the sand surface to prevent shifting sand from falling into the pot. At 15 d intervals, the nylon bags in the pots were removed and any seeds that were present in the bags were collected. These bags were then put back into the pots. The density of seed release was calculated as the number of seeds per square metre.
Seasonal soil and aerial seed bank
Seed bank dynamics were determined in the seed bank plot (55 × 65 m2) in April and August 2012. To determine the dynamics of aerial and soil seed banks, 30 quadrats (2 × 2 m2) were set along five transects in lowlands between sand dunes. Each transect was 60 m in length, and the distance between neighbouring quadrats was 10 m. We considered the seeds that were released before germination (late April) as the soil seed bank and the seed that were still retained on the mother plant as the aerial seed bank. Aerial and soil seed banks were collected on 15 April (after the frozen soil thawed but before seed germination) and 27 August (before the freshly matured seeds were released). On each sampling date, a soil sample (10 × 10 × 5 cm3) and an aerial seed bank sample (all standing plants, 50 × 50 cm2) were randomly collected in each quadrat. The soil samples were taken to the laboratory and the seeds were sieved using a 0·9 mm mesh. Aerial seed bank samples were collected; the seeds of these plants were shed by rubbing and the number of seeds was counted. Finally, the density of both seed banks was calculated as the number of seeds per square metre.
Germination of seeds from the aerial and soil seed bank
Seeds from the 30 samples of the seed bank plot were mixed according to seed bank types collected on 15 April; seeds from the soil were put in an opaque bag to reduce the influence of light on seed germination. To explore the differences in seed germination responses between aerial and soil seed banks, experiments were conducted simultaneously on 15 April as described above in ‘Seed characteristics and germination behaviour of freshly harvested seeds’.
Seedling emergence
Ninety quadrats (15 × 15 cm2) were used in this experiment and each quadrat was randomly arrayed in the seedling emergence plot (30 × 30 m2) to monitor the dynamics of seedling emergence. Seeds from the aerial seed bank were allowed to be released into these quadrats, and thus these quadrats were named ‘open quadrats’ which were randomly divided into three groups. (1) Early-emerged seedling group: 30 quadrats were used to monitor seedling emergence from 16 April to 15 May. (2) Intermediate-emerged seedling group: 30 quadrats were used to monitor seedling emergence from 16 May to 15 June, with seedlings emerging before 16 May being removed and not considered. (3) Late-emerged seedling group: 30 quadrats were used to monitor seedling emergence from 16 June to 15 July, with seedlings emerging before 16 June being removed and not considered. For each of the above-mentioned groups, the number of seedlings was recorded every 5 d and the experiments were terminated on 15 May, 15 June and 15 July, respectively.
To monitor seedling emergence only from the soil seed bank, another 90 quadrats (15 × 15 cm2) were covered with a nylon net (0·9 mm mesh size, see above) and arrayed corresponding to the three groups of ‘open quadrats’. The nylon net prevented the incorporation of dispersed seeds from the aerial bank into the soil seed bank. If there were Agriophyllum plants in the plots, the net was cut and rewoven at the bottom of the stem to exclude seed release from the plants onto the soil surface. These quadrats were named ‘covered quadrats’ because seeds from the aerial seed bank could not fall onto the soil surface, and therefore seedling emergence from these quadrats originated only from the soil seed bank. Each of the ‘covered quadrats’ was enclosed by four white PVC pipes, on which the edges of the nylon nets were fixed by eight iron nails to 10 cm above the sand surface. The nets were not close to the sand surface due to the strut of the PVC pipes, and thus had no effect on seedling emergence. The numbers of seedlings emerging from the three groups of ‘covered quadrats’ were checked at 5 d intervals, as was previously described for the ‘open quadrats’.
Therefore, seedlings from ‘open quadrats’ originated from seeds of both soil and aerial seed banks, while the seedlings from ‘covered quadrats’ came only from seeds in the soil seed bank.
Seedling survival
To assess the seedling survival under natural conditions, 90 open quadrats were arrayed in the seedling survival plot (30 × 30 m2), in the same way as for the seedling emergence experiment. Seedlings that emerged in these quadrats within a given month were retained to the end of the growing season so that they had the possibility to complete their life cycle. A plant was considered to have survived when it completed its life cycle and produced seeds. Finally, the number of seeds produced by the surviving plants was determined in each quadrat on 28 September and the seed number per plant and seed yield per square metre were calculated.
Data analyses
Statistical analyses were conducted with SPSS 13·0 (SPSS Inc., Chicago, IL, USA). To assess whether seed release was related to key environmental factors, correlation analyses were carried out between seed release, mean maximum wind velocity, maximum and minimum temperature, amount of rainfall and number of rainfall events. The mean densities of the seed bank and seedlings in the quadrats were standardized to yield the mean number of seeds and seedlings per square metre and transformed by normal logarithm to enhance normality and homogeneity of variance. An analysis of variance (ANOVA) was then carried out to test for the differences in seed density between the two seed banks and in seedling density among different times during the growing season. Germination data were transformed by arcsin square root. ANOVAs were carried out to identify the effects of temperature, light and/or seed bank type on germination percentages. If ANOVAs showed significant effects, the test of least significant difference (l.s.d.) was used to determine differences between treatments.
RESULTS
Seed characteristics and germination behaviour of fresh seed
Average seed number per plant was 1837 ± 452 which varied greatly according to plant size, and the yellow, flat, ellipsoid seed was 1·267 ± 0·025 g per 1000 seeds. The lengths of the long and short axes were 1·88 ± 0·02 and 1·46 ± 0·02 mm, respectively.
A two-way ANOVA showed that temperature regimes, light and their interaction had significant impacts on the germination of fresh seeds (Table 1). After 20 d of incubation, fresh seeds germinated to significantly higher percentages at high (15/25 and 20/30 °C) than at low (5/15 and 10/20 °C) temperatures in continuous darkness. When comparing the responses of seed germination in the light regimes, alternating light/dark (12/12 h) conditions significantly inhibited germination under all temperature regimes (Fig. 1), indicating that fresh seeds were sensitive to light.
Table 1.
Results from ANOVAs testing for the effects of various factors on germination of freshly harvested seeds and of seeds from seed banks for Agriophyllum squarrosum
| Variance source | Type III sum of squares | d.f. | Mean square | F | P |
|---|---|---|---|---|---|
| Freshly harvested seeds | |||||
| Temperature | 2·640 | 3 | 0·880 | 42·711 | < 0·001 |
| Light | 2·500 | 1 | 2·500 | 121·380 | < 0·001 |
| Temperature × light | 0·862 | 3 | 0·287 | 13·950 | < 0·001 |
| Seeds from the seed bank | |||||
| Temperature | 20 559·272 | 3 | 6853·091 | 57·526 | < 0·001 |
| Light | 69 673·428 | 1 | 69 673·428 | 584·850 | < 0·001 |
| Seed bank type | 502·458 | 1 | 502·458 | 4·218 | 0·045 |
| Temperature × light | 7502·363 | 3 | 2500·788 | 20·992 | < 0·001 |
| Temperature × seed bank type | 3145·927 | 3 | 1048·642 | 8·802 | < 0·001 |
| Light × seed bank type | 641·723 | 1 | 641·723 | 5·387 | 0·025 |
| Temperature × light × seed bank type | 1603·825 | 3 | 534·608 | 4·488 | 0·007 |
Fig. 1.
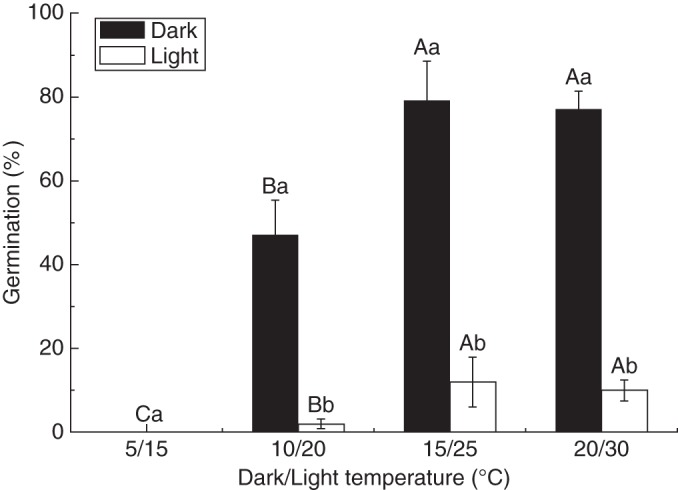
Germination percentage (mean ± s.e.) of fresh seeds of Agriophyllum squarrosum under different temperature regimes under full darkness and alternating light/dark conditions. Bars with different upper case letters indicate significant differences among temperatures within light/dark or dark treatment, and those with different lower case letters show significant differences between light/dark and dark treatment within each temperature (P < 0·05), according to the l.s.d. test.
Seed release
More than half of the seeds (57·6 ± 4·7 %) were released from the total dispersed seeds (monitored from 15 March to 15 September) of mother plants before 1 May, after which the seed release decreased with the decrease in wind velocity (Fig. 2). Seed release was positively related to wind velocity (Pearson correlation coefficient = 0·911, P < 0·001) but was not related to the maximum and minimum temperature, amount of rainfall and the number of rainfall events (all P > 0·05). The peak of seed release (1359 ± 133 seeds m–2) was in late April, when the mean maximum wind velocity was highest (9·1 m s–1); seed release sharply declined at the end of July when the wind velocity decreased to 5·7 m s–1. After July, a small number of seeds (12–15 seeds m–2) were released from the plants (Fig. 2).
Fig. 2.
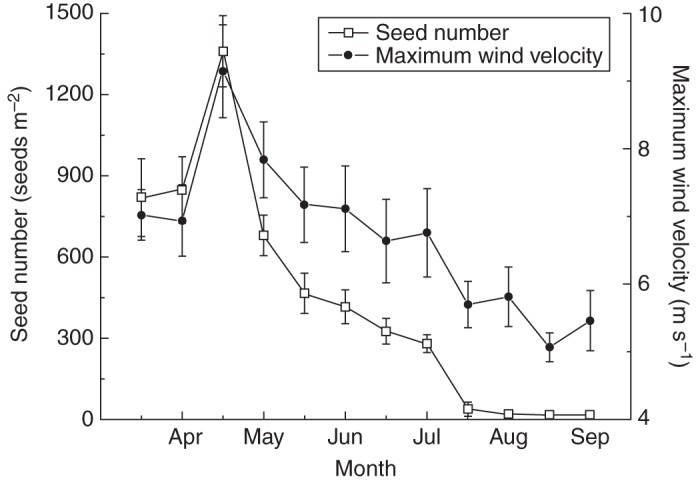
The relationships between seed release of Agriophyllum squarrosum and mean maximum wind velocity (mean ± s.e.).
Seasonal soil and aerial seed bank
The number of seeds in the aerial seed bank was significantly higher than that in the soil seed bank in both April and August (all P < 0·001). The density of seeds in the two seed banks decreased significantly from April to August (all P < 0·001) (Fig. 3). At the end of the germination season in August, most of the seeds from both seed banks were exhausted, but there was still a small portion remaining in the aerial (13·2 ± 1·75 %) and soil (9·3 ± 2·43 %) seed banks.
Fig. 3.
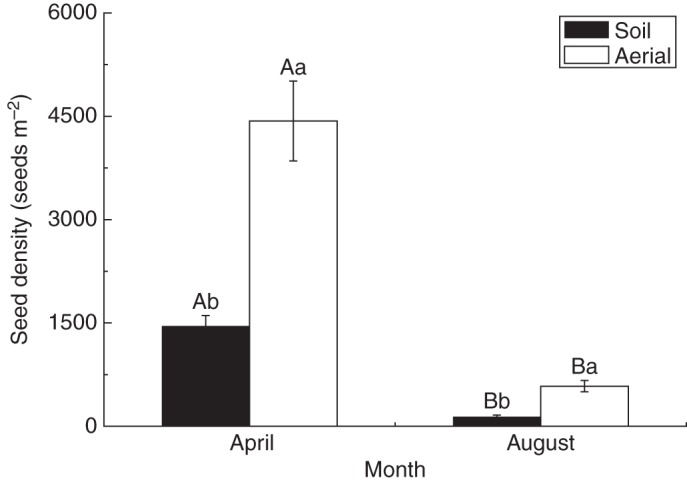
The number of seeds (mean ± s.e.) in aerial and soil seed banks of Agriophyllum squarrosum in April and August 2012. Bars with different upper case letters indicate significant differences between April and August within each seed bank type, and those with different lower case letters show significant differences between the soil and aerial seed banks within each month (P < 0·05), according to the l.s.d. test.
Seed germination in aerial and soil seed bank
A three-way ANOVA showed that germination responses to the temperature regimes were highly dependent on the light condition (Table 1). Moreover, germination responses to temperature regimes and light conditions varied significantly between the two types of seed banks.
Seeds from the two seed bank types had different germination responses to seasonal temperature regimes and light conditions. Under both full darkness and alternating light/dark conditions, germination percentages from soil and aerial seed banks increased with the increase in temperature, but those of seeds from the aerial seed bank increased more rapidly than those of seeds from the soil seed bank (Fig. 4A, B).
Fig. 4.
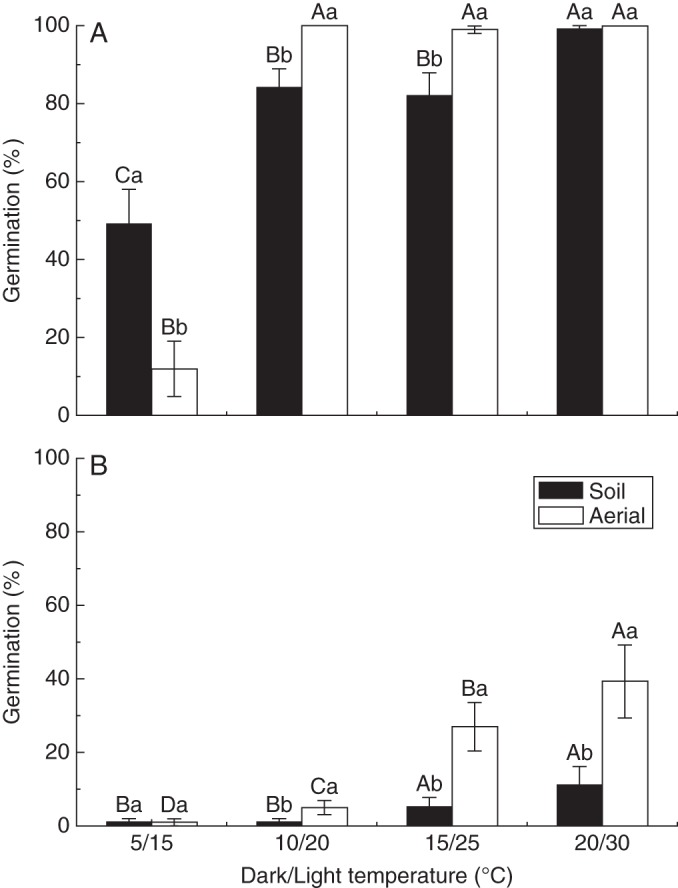
Germination percentages (mean ± s.e.) of seeds from the soil and aerial seed banks of Agriophyllum squarrosum under different temperature regimes under full darkness (A) and alternating light/dark conditions (B). Bars with different upper case letters indicate significant differences among temperatures within each seed bank, and those with different lower case letters show significant differences between the soil and aerial seed bank within each temperature (P < 0·05), according to the l.s.d. test.
Under full darkness, the final germination percentages of seeds from the soil seed bank were significantly higher than those from the aerial seed bank at 5/15 °C (P < 0·05). In contrast, germination was significantly higher for seeds from the aerial seed bank than those from the soil seed bank at 10/20 and 15/25 °C (all P < 0·05). Seed germination was almost 100 % at 20/30 °C for both seed banks (Fig. 4A). Compared with the full darkness treatment, germination under alternating light/dark conditions under the same temperature regimes was very low. The lowest seed germination (1·0 ± 1·0 %) occurred at 5/15 °C for the two seed banks. Seed germination of the aerial seed bank was higher than that of the soil seed bank at the other temperatures (all P < 0·05), especially at 15/25 and 20/30 °C (Fig. 4B).
Seedling emergence and survival
Seedling emergence from ‘open quadrats’ (containing seeds from both aerial and soil seed banks) lasted for 3 months, while that from ‘covered quadrats’ (containing seeds from only the soil seed bank) only occurred in the first month and no seedlings appeared in the next 2 months (Fig. 5). There was no significant difference in early seedling emergence between open and covered quadrats (P = 0·880). In the open quadrats, seedling emergence was not significantly different among times of seedling emergence (P > 0·05) (Fig. 5).
Fig. 5.
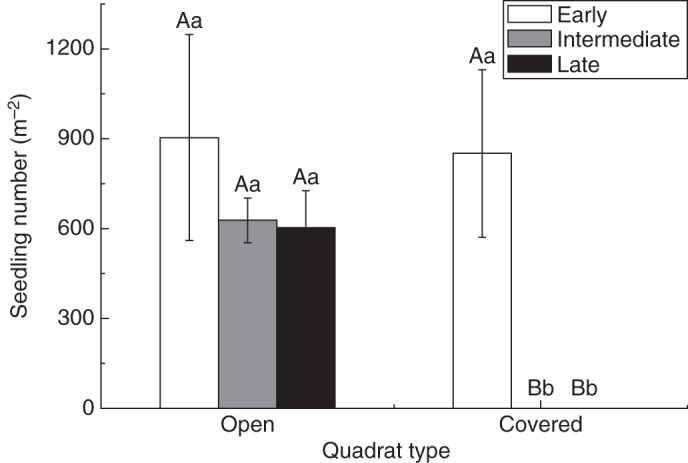
The densities of Agriophyllum squarrosum seedlings in open and covered quadrats. Bars with different upper case letters indicate significant differences between quadrat types within a given time of seedling emergence (early, intermediate, late), and those with different lower case letters show significant differences among times of seedling emergence within each quadrat type (P < 0·05), according to the l.s.d. test.
The timing of seedling emergence had a large impact on seedling survival. The seedling survival percentages of the early-emerged seedlings sharply decreased to 33·4 ± 7·3 % within the first month due to the occurrence of the frost events (Figs 6, 7). At the end of the growing season, all the early-emerged seedlings died, while the survival percentages for intermediate- and late-emerged seedlings were 62·4 ± 6·4 % and 52·2 ± 8·1 %, respectively (Fig. 6).
Fig. 6.
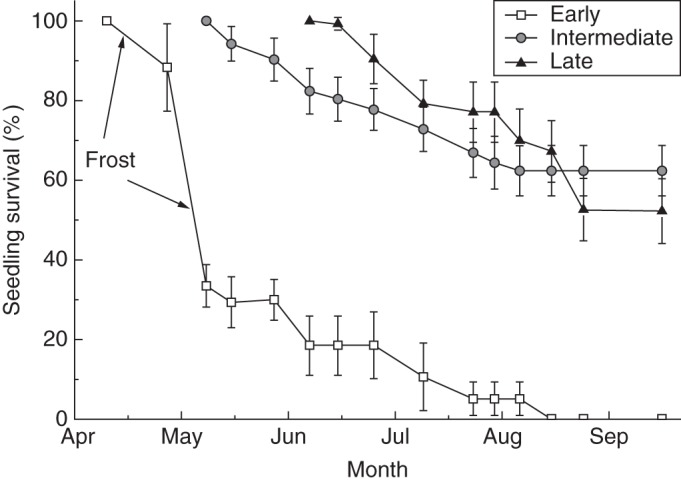
The survival of Agriophyllum squarrosum seedlings that emerged at different times (early, intermediate, late) from 16 April to 28 September 2012. Arrows indicate the times of frost occurrence.
Fig. 7.
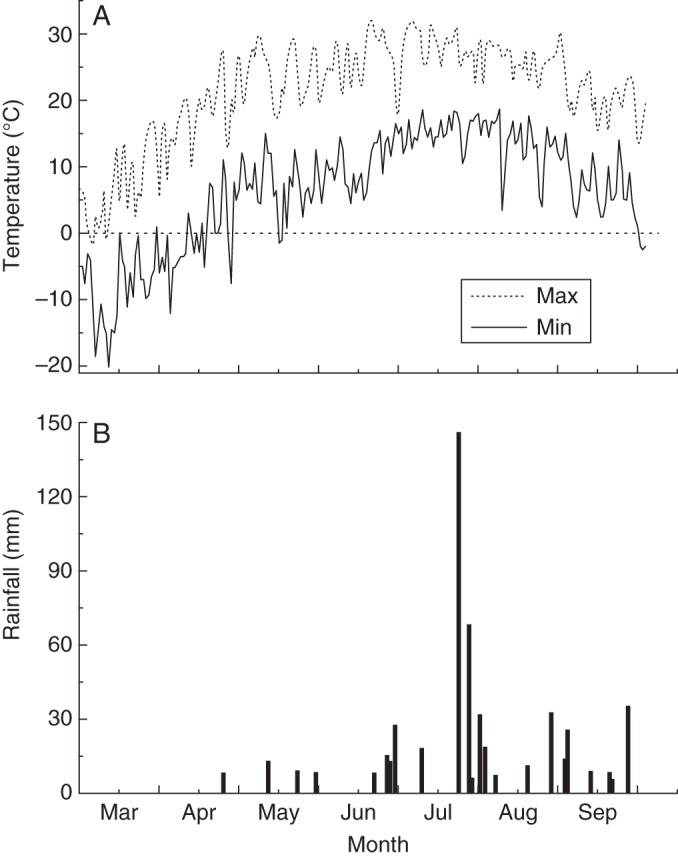
Daily temperature (A) and amount of rainfall (B) from 1 March to 30 September in 2012. Max and Min indicate the daily maximum and minimum temperatures, respectively. Amounts of rainfall are only shown for rainfall events >5 mm.
Seed yield
Among the plants that emerged at different times, seed number per plant was highest for seedlings that emerged at an intermediate time; no seed was produced from early-emerged plants (Fig. 8A). Seed yield of plants that emerged at an intermediate time was highest and significantly different from that at the other two emergence times (all P < 0·05); no seed was produced by plants that emerged during the early time period (Fig. 8B).
Fig. 8.
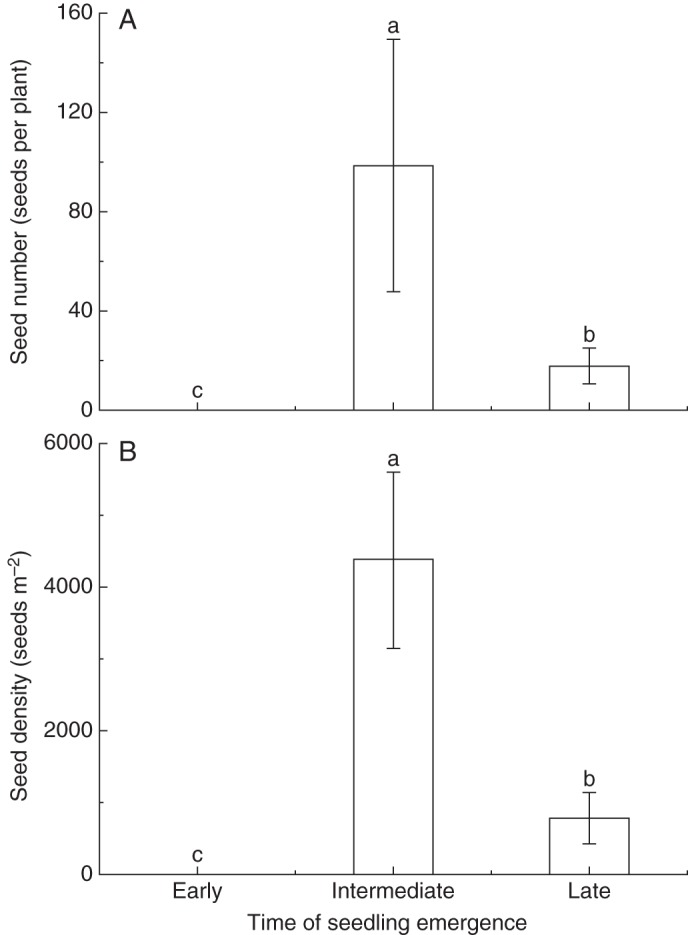
Seed number per plant (A) and the density of seeds (B) of plants that emerged at different times and survived until the end of the growing season. Values are mean ± s.e. Bars with different lower case letters show significant differences in seed yield among different times (P < 0·05), according to the l.s.d. test.
DISCUSSION
Gradual seed release can be a strategy to reduce the seasonal risk in germination and seedling establishment (Bastida et al., 2010). Seed release of A. squarrosum was triggered by wind and significantly related to wind velocity (Fig. 2). This indicates that wind may provide the seeds with the mechanical force to detach from the mother plants. Moreover, the strongest wind occurred in late April and might be an environmental cue for the subsequent germination season, with rising (spring-time) temperature and increasing precipitation (Figs 2, 7B). Although deep sand burial caused by strong wind might be disadvantageous for germination (Bai et al., 2004; Liu et al., 2006), gradual release might ensure that some seeds fall in shallow sand and have the opportunity for germination and establishment with the stochastic rainfall events. In this way, gradual release cannot only spread the arrival of seed in the soil over a longer time period, but can also affect the spatial distribution of seeds in the soil. This might be a bet-hedging strategy (Evans et al., 2007), which is adaptive for A. squarrosum in its unpredictable environment.
Throughout the growing season, the number of seeds in the aerial seed bank of A. squarrosum was far more than that in the soil seed bank (Fig. 3). Prolonged seed release might be a strategy of maternal care. A large number of A. squarrosum seeds were retained on the mother plants with prickly leaves and fruits (Fig. 3), which may protect the seeds from grazing and from sand burial. In sand dune systems, eolian activity causes sand burial and greatly affects growth and regeneration of plants. Retaining seeds in an aerial seed bank could not only prevent seeds from deep sand burial (Liu and Wang, 2009), but could also enable seeds to be released from mother plants by specific environmental cues (wind or rain) predicting favourable conditions. Therefore, an aerial seed bank could provide A. squarrosum with a great opportunity for successful population maintenance in the sand dune system.
However, the soil seed bank lacks such protection and might be more at risk on the shifting sand dune than the aerial seed bank. Seeds in the soil seed bank of A. squarrosum sharply decreased from April to August (Fig. 3) possibly for several reasons. First, germination of seeds from the soil seed bank can be a major cause of the loss of seeds from the soil seed bank. In fact, a massive number of seeds in the soil seed bank would germinate once germination requirements are met. Secondly, the loss of a small number of seeds has been attributed to sand burial (Liu et al., 2006; Ma and Liu, 2008). The vertical distribution of the seed bank (0–1 m) of A. squarrosum in soils suggests that seeds are buried deeply by active sand movement very soon after release. These deeply buried seeds can be exposed to the shallow sand by disturbances (wind, grazing and engineering) and germinate (Liu et al., 2007; Ma and Liu, 2008). Finally, the decrease of seeds in the soil seed bank may result from seed loss due to predators and pathogens, which are typically present in desert environments (Gutterman, 1993).
Seed germination of A. squarrosum under full darkness was higher than that under alternating light/dark conditions, regardless of whether seeds were from the soil or aerial seed bank (Table 1, Fig. 4A, B). This shows that seeds of this species can germinate in darkness, and a persistent soil seed bank is unlikely to develop. In other words, the soil seed bank in this species is short lived – seeds are only 1 year old. The importance of photo-inhibited germination and soil seed bank formation has been found in other studies, e.g. several species in Cactaceae (Flores et al., 2006).
Under dark conditions, responses of germination to temperature regimes differed between seeds from the soil and aerial seed banks: higher germination occurred for seeds from the aerial seed bank than for those from the soil seed bank at high temperatures (Fig. 4A). However, germination of seeds from the aerial seed bank was lower than that from seeds of the soil seed bank at 5/15 °C (Fig. 4A). These results suggest that seed germination of the aerial seed bank is sensitive to low temperature, i.e. the seeds could not germinate in early spring (from 16 April to 15 May) while seeds from the soil seed bank could do so. On the other hand, the germination niche is wider for the aerial seed bank than for the soil seed bank, with aerial seeds gaining the ability to germinate during the following 2 months (from 16 May to 15 July). Thus, the germination of aerial seed and soil seed showed temporal heterogeneity, with seeds from the soil seed bank germinating earlier than those from the aerial seed bank in the habitat.
Under the alternating light/dark condition, seed germination increased with the increase in temperature, and germination of seed from the aerial seed bank was higher than that from the soil seed bank (Fig. 4B). The relatively weak sensitivity to light of the aerial seed bank suggests that seed germination can be high when seeds fall on the sand surface after release during the intermediate and late germination seasons. This photo-response may be attributed to the fact that dormancy break occurred in the seeds during exposure to low winter temperatures and during hydration–dehydration cycles when they are retained on the mother plants (Figs 1, 4B) (Baskin and Baskin, 2001). The differences in the sensitivity of seed germination to light and temperature between the two seed banks may regulate the germination phenology and temporal patterns of seedling emergence in the habitat.
Seedling emergence of A. squarrosum is related to seasonal sand moisture content. Seedlings emerged during the early germination season (16 April to 15 May) from the soil seed bank in ‘covered quadrats’ (Fig. 5) soon after snowmelt. The temperature (5/15 °C) at this time is suitable for germination of seeds from the soil seed bank. The absence of seedling emergence during the intermediate and late germination seasons in ‘covered quadrats’ (Fig. 5) may be due to the lack of shallow buried seeds (since many had already germinated); the remaining seeds are buried deeply in the soil. However, seedlings occurred in all months in the ‘open quadrats’. Thus, late-emerged seedlings are mainly from the aerial seed bank.
Seedling establishment of some plants is affected by extreme climate events that occurred after seedling emergence, which was also observed in A. squarrosum. Most of the early-emerged A. squarrosum seedlings died (Fig. 6). The survival of seedlings that emerged in the early germination season was lowest; this resulted from the four frost events and drought stress that occurred before the rainy season began (Fig. 7). Although a small proportion of early seedlings survived after the extreme events, they grew slowly with unhealthy leaves and gradually died during the late growth period, and only a few individuals produced seeds (pers. obs.). Therefore, early seedling emergence from the soil seed bank is risky, because the first rainfall is not predictive of subsequent favourable conditions (Petrů and Tielbörger, 2008). In contrast, the intermediate- and late-emerged seedlings had high survival because seedling emergence was followed by favourable temperature regimes and rainfall events.
The fitness of a plant has often been estimated by seed number and yield (Nicotra et al., 2010). In deserts, the timing of seedling emergence is important for reproduction and fitness (Donohue et al., 2010). In favourable years, early-emerged seedlings may be larger, have stronger competitiveness and higher seed yield than late-emerged seedlings (Günster, 1994; Levine et al., 2005; Donohue et al., 2010). In unfavourable years, early-emerged seedlings are at risk because unpredictable climatic events (e.g. freezing and drought) can occur after seedling emergence. For A. squarrosum, the differences in seedling emergence time between the aerial and soil seed bank might spread the risk in the unpredictable environment.
The different contributions of the two seed banks to plant fitness remain unclear, especially in an unpredictable environment (Donohue et al., 2010). In Mammillaria hernandezii (Cactaceae), late-released seeds had higher fitness than seeds from the soil seed bank. Seeds retained on the mother plants may undergo hydration–dehydration cycles, which promote changes in seed physiology and further enhance seed germination by a priming effect (Santini and Martorell, 2013). We suggest that priming by hydration–dehydration cycles may be the reason for the high seed germinability and seedling vigour, survival and fitness for the aerial seed bank of A. squarrosum. Our results also showed that few early-emerged seedlings of A. squarrosum produced seeds; intermediate- and late-emerged seedlings not only had high survival but also successfully produced seeds (Fig. 8). Although a small number of seeds per plant were produced due to a short life cycle and small plant size, the aerial seed bank apparently increases plant fitness after the occurrence of extreme environmental events following the first seedling emergence period.
Summary
In harsh drought habitats, such as in the Ordos Sandland of China, with unpredictable amounts and distribution of precipitation throughout the growing season, the regulation of germination and seedling establishment is critical to plant survival and reproduction. The regulation mechanism is related mainly to the germination process that ensures germination of fractions of the seed banks at the appropriate time and in a suitable place (Gutterman, 1993; Huang et al., 2004; Cao et al., 2012). Thus, plants in deserts such as A. squarrosum have evolved complementary adaptations and survival strategies throughout the stages of their life cycles, i.e. the aerial and soil seed banks. Formation of these two types of seed banks may be recognized as a mechanism for plant population maintenance in the face of unpredictable environments.
Our study identified the different roles of the two seed banks in regulating seed germination, seedling emergence and establishment, and plant fitness. Our results suggest that aerial and soil seed banks may be a bet-hedging strategy for population maintenance in unpredictable environments: early-emerging plants from the soil seed bank may have a relatively long life cycle and therefore may be relatively large and may yield seed. However, they also have a high risk of death due to drastic fluctuations in environmental conditions (such as low temperatures in early spring and periods of drought). On the other hand, intermediate- and late-emerging plants from the aerial seed bank are small and have low seed yield because of a short life cycle. However, they have low risk of death due to favourable growing conditions.
Considering the projected future climate change, the increase in the minimum temperature and in rainfall during early spring may enhance high recruitment for plant populations (Ibanez et al., 2007). However, the predicted increase of extreme climate events may also result in the occurrence of increased seedling mortality. In such a case, desert annuals with two seed banks, such as A. squarrosum, have a strong capacity to cope with unpredictable events and maintain the regeneration of populations. Our study provides a new insight for understanding the functional significance and adaptive strategies of the seasonal aerial and soil seed banks in population maintenance of sand dune plants.
ACKNOWLEDGEMENTS
This work was supported by the Key Basic Research and Development Plan of China (2010CB951304), the National Key Technology R&D Program (2012BAD16B03) and the National Natural Science Foundation (31370705).
LITERATURE CITED
- Aguado M, Vicente MJ, Miralles J, Franco JA, Martinez-Sanchez JJ. Aerial seed bank and dispersal traits in Anthemis chrysantha (Asteraceae), a critically endangered species. Flora. 2012;207:275–282. [Google Scholar]
- Bai WM, Bao XM, Li LH. Effects of Agriophyllum squarrosum seed banks on its colonization in a moving sand dune in Hunshandake Sand Land of China. Journal of Arid Environments. 2004;59:151–157. [Google Scholar]
- Bastida F, Gonzalez-Andujar JL, Monteagudo FJ, Menendez J. Aerial seed bank dynamics and seedling emergence patterns in two annual Mediterranean Asteraceae. Journal of Vegetation Science. 2010;21:541–550. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biologeography, and evolution of dormancy and germination. San Diego: Academic Press; 2001. pp. 14–17. [Google Scholar]
- Brown JS, Venable DL. Evolutionary ecology of seed-bank annuals in temporally varying environments. American Naturalist. 1986;127:31–47. [Google Scholar]
- Cao DC, Baskin CC, Baskin JM, Yang F, Huang ZY. Comparison of germination and seed bank dynamics of dimorphic seeds of the cold desert halophyte Suaeda corniculata subsp. mongolica. Annals of Botany. 2012;110:1545–1558. doi: 10.1093/aob/mcs205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Knox KEJ, Butler D. Fire, soil fertility and delayed seed release: a community analysis of the degree of serotiny. Evolutionary Ecology. 2013;27:429–443. [Google Scholar]
- Cohen D. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology. 1966;12:119–129. doi: 10.1016/0022-5193(66)90188-3. [DOI] [PubMed] [Google Scholar]
- Cowling RM, Lamont BB. Variation in serotiny of 3 banksia species along a climatic gradient. Australian Journal of Ecology. 1985;10:345–350. [Google Scholar]
- Cowling RM, Lamont BB. Postfire recruitment of 4 cooccurring banksia species. Journal of Applied Ecology. 1987;24:645–658. [Google Scholar]
- Donohue K, Casas RR, Burghardt L, Kovach K, Willis CG. Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics. 2010;41:293–319. [Google Scholar]
- Evans MEK, Ferriere R, Kane MJ, Venable DL. Bet hedging via seed banking in desert evening primroses (Oenothera, Onagraceae): demographic evidence from natural populations. American Naturalist. 2007;169:184–194. doi: 10.1086/510599. [DOI] [PubMed] [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press, 89–96; 2005. [Google Scholar]
- Flores J, Jurado E, Arredondo A. Effect of light on germination of seeds of Cactaceae from the Chihuahuan Desert, Mexico. Seed Science Research. 2006;16:149–155. [Google Scholar]
- Günster A. Aerial seed banks in the central namib – distribution of serotinous plants in relation to climate and habitat. Journal of Biogeography. 1992;19:563–572. [Google Scholar]
- Günster A. Variability in life history parameters of four serotinous plants in the Namib Desert. Vegetatio. 1994;114:149–160. [Google Scholar]
- Gutterman Y. Seed germination in desert plants. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Gutterman Y, Ginott S. Long-term protected seed bank in dry inflorescences of Asteriscus pygmaeus: achene dispersal mechanism and germination. Journal of Arid Environments. 1994;26:149–163. [Google Scholar]
- Gutterman Y, Gendler T, Huang ZY. Plant dispersal strategies, seed bank distribution and germination of Negev Desert species. In: Adkins SW, Ashmore SE, Navie SC, editors. Seeds: biology, development and ecology. Trowbridge, UK: Cromwell Press; 2007. pp. 407–415. [Google Scholar]
- Hamilton-Brown S, Boon PI, Raulings E, Morris K, Robinson R. Aerial seed storage in Melaleuca ericifolia Sm. (Swamp Paperbark): environmental triggers for seed release. Hydrobiologia. 2008;620:121–133. [Google Scholar]
- Harris MS, Pannell JR. Canopy seed storage is associated with sexual dimorphism in the woody dioecious genus Leucadendron. Journal of Ecology. 2010;98:509–515. [Google Scholar]
- Huang ZY, Dong M, Gutterman Y. Factors influencing seed dormancy and germination in sand, and seedling survival under desiccation, of Psammochloa villosa (Poaceae), inhabiting the moving sand dunes of Ordos, China. Plant and Soil. 2004;259:231–241. [Google Scholar]
- Ibanez I, Clark JS, LaDeau S, Hille Ris Lambers J Exploiting temporal variability to understand tree recruitment response to climate change. Ecological Monographs. 2007;77:163–177. [Google Scholar]
- Kong XW. Flora of China. Vol. 25, part 2. Beijing: Science Press; 1996. Chenopodiaceae; pp. 48–49. Flora Editorial Board. [Google Scholar]
- Lamont BB. Canopy seed storage and release – what's in a name. Oikos. 1991;60:266–268. [Google Scholar]
- Lamont BB, Enright N. Adaptive advantages of aerial seed banks. Plant Species Biology. 2000;15:157–166. [Google Scholar]
- Lamont BB, Lemaitre DC, Cowling RM, Enright NJ. Canopy seed storage in woody-plants. Botany Reviews. 1991;57:277–317. [Google Scholar]
- Levine JM, McEachern AK, Cowan C. Rainfall effects on rare annual plants. Journal of Ecology. 2008;96:795–806. [Google Scholar]
- Liu ZM, Yan QL, Baskin CC, Ma JL. Burial of canopy-stored seeds in the annual psammophyte Agriophyllum squarrosum Moq. (Chenopodiaceae) and its ecological significance. Plant and Soil. 2006;288:71–80. [Google Scholar]
- Liu ZM, Yan QL, Liu B, Ma JL, Luo Y. Persistent soil seed bank in Agriophyllum squarrosum (Chenopodiaceae) in a deep sand profile: variation along a transect of an active sand dune. Journal of Arid Environments. 2007;71:236–242. [Google Scholar]
- Liu ZM, Wang XM. Functions of canopy-stored seeds in the dune ecosystem: conclusions from Agriophyllum squarrosum and Artemisia wudanica. Frontiers of Biology in China. 2009;4:486–490. [Google Scholar]
- Ma JL, Liu ZM. Spatiotemporal pattern of seed bank in the annual psammophyte Agriophyllum squarrosum Moq. (Chenopodiaceae) on the active sand dunes of northeastern Inner Mongolia, China. Plant and Soil. 2008;311:97–107. [Google Scholar]
- Moya D, De las Heras J, Salvatore R, Valero E, Leone V. Fire intensity and serotiny: response of germination and enzymatic activity in seeds of Pinus halepensis Mill. from southern Italy. Annals of Forest Science. 2013;70:49–59. [Google Scholar]
- Narita K, Wada N. Ecological significance of the aerial seed pool of a desert lignified annual, Blepharis sindica (Acanthaceae) Plant Ecology. 1998;135:177–184. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. Plant phenotypic plasticity in a changing climate. Trends in Plant Science. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Noy-Meir I. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics. 1973;4:25–51. [Google Scholar]
- Peters EM, Martorell C, Ezcurra E. The effects of serotiny and rainfall-cued dispersal on fitness: bet-hedging in the threatened cactus Mammillaria pectinifera. Population Ecology. 2011;53:383–392. [Google Scholar]
- Petrů M, Tielbörger K. Germination behaviour of annual plants under changing climatic conditions: separating local and regional environmental effects. Oecologia. 2008;155:717–728. doi: 10.1007/s00442-007-0955-0. [DOI] [PubMed] [Google Scholar]
- Santini BA, Martorell C. Does retained-seed priming drive the evolution of serotiny in drylands? An assessment using the cactus Mammillaria hernandezii. American Journal of Botany. 2013;100:365–373. doi: 10.3732/ajb.1200106. [DOI] [PubMed] [Google Scholar]
- Thompson K. Seeds and seed banks. New Phytologist. 1987;106:23–34. [Google Scholar]
- Venable DL. Bet hedging in a guild of desert annuals. Ecology. 2007;88:1086–1090. doi: 10.1890/06-1495. [DOI] [PubMed] [Google Scholar]


