Abstract
The level of interest in aural rehabilitation has increased recently, both in clinical use and in research presentations and publications. Advances in aural rehabilitation have seen previous techniques such as speech tracking and analytic auditory training reappear in computerized forms. These new delivery methods allow for a consistent, cost-effective, and convenient training program. Several computerized aural rehabilitation programs for hearing aid wearers and cochlear implant recipients have recently been developed and were reported on at the 2006 State of the Science Conference of the Rehabilitation Engineering Research Center on Hearing Enhancement at Gallaudet University. This article reviews these programs and outlines the similarities and differences in their design. Another promising area of aural rehabilitation research is the use of pharmaceuticals in the rehabilitation process. The results from a study of the effect of d-amphetamine in conjunction with intensive aural rehabilitation with cochlear implant patients are also described.
Keywords: adult hearing loss, psychology, aural rehabilitation, humans, treatment outcome
There has been a noticeable, and welcome, resurgence of interest in aural rehabilitation in the past few years. It is noticeable from the growing number of audiologists engaging in at least some form of rehabilitation designed to augment the benefit from hearing aids or cochlear implantation, as well as in the increasing number of scientific papers and presentations addressing this topic. It is welcome because neither wearable amplification nor cochlear implantation can restore to the wearer the frequency and temporal resolution required to provide a listening experience that allows for comfortable and successful communication in all adverse listening environments. This renewed attention is also welcome because it appears that the use of aural rehabilitation had been on the decline for the past 2 decades.
In the 1940s, when the profession of audiology was founded, aural rehabilitation was considered the centerpiece of care for hearing-impaired individuals. Over time, however, the focus of research shifted to improving wearable amplification. As manufacturers promoted and marketed advances in hearing aids, the number of audiologists engaging in the delivery of aural rehabilitation decreased.1 This may have been the result of the misplaced belief that once audibility was established, adequate communication would automatically follow, and was almost certainly affected by the poor or nonexistent reimbursement available to audiologists for aural rehabilitation services.
Individualized auditory training is not very common, but there is some evidence that the approach can provide benefit to hearing-impaired people. Sweetow and Palmer2 systematically reviewed the research related to individual adult auditory training and found some evidence suggesting that at least with synthetic training, improvements in communication strategies can be expected. But in the past, it has been difficult to perform individualized auditory training and to study the effect of such training in a systematic manner. Sweetow and Palmer reported that of 213 articles published on individual auditory training, only 6 met the rigorous criteria required to qualify as evidence-based.
Such training on a one-to-one basis can be both time-intensive and cost-intensive. Fortunately, alternatives are available in our high-tech world through computerized auditory training. Indeed, since the Sweetow-Palmer article appeared in print, a number of authors have presented evidence of the efficacy of computerized auditory training procedures.3–5
The need for additional therapy beyond that provided by devices alone is underscored by the fact that individuals presenting similar audiometric patterns frequently report a wide range in satisfaction and benefit from amplification. Perhaps it was the recognition that most cochlear implant recipients could not function adequately on the basis of the new auditory input alone that inspired the renewed interest in aural rehabilitation. Indeed, many scientific publications and presentations now describe not only the benefits provided by new technologies but also the limitations created by cochlear distortion, the inability of some devices to replicate fine structure of incoming signals, and the restrictions imposed by central nervous system deficiencies arising from aging.6–9
Although audiologists appear to recognize the need for assistance beyond that which can be provided by hearing aids or cochlear implants, questions remain on how additional aural rehabilitation can be delivered in an efficient and cost-effective manner. Moreover, there remains the overriding question of how audiologists can be motivated to recommend and then deliver these therapies and how patients can be motivated to participate.
In this article, we will discuss some of the common principles and underlying assumptions characteristic of the new genre of computerized individual aural rehabilitation procedures currently under investigation and development. We will then describe 4 recently developed approaches to aural rehabilitation reported on at the 2006 State of the Science Conference on Hearing Enhancement at Gallaudet University.
The focus of these procedures is on individual rather than group therapies. Some of these are enhancements of earlier programs that showed promise in their initial stage but never enjoyed widespread popularity, perhaps as a result of limitations in the delivery mode. But now, with the pervasive use of computers, individualized aural rehabilitation can be brought into the homes of hearing-impaired patients so that therapy can be conducted in the privacy of the individual's home and at the correct pace for that person. Each program will be described and components and objectives will be compared. The question of whether aural rehabilitation outcomes can be improved by pharmacologic enhancement will also be considered. We will then describe unresolved questions and significant remaining barriers that must be overcome to make aural rehabilitation a standard part of patient care.
Common Principles and Underlying Assumptions
In a previous publication,10 we listed a number of principles and assumptions that might be considered in creating an individualized, self-administered aural rehabilitation program. The following factors were considered as being important:
The training should be interactive. It is important for the patient or trainee to assume an active rather than a passive role. This is not to say, however, that all learning requires active listening. Moore and Amitay11 have shown a certain amount of incidental learning also can occur from passive listening.
The training should be practical and easily accessible. Therapy conducted at home has the advantages of being more cost-effective than repeated clinic visits with a professional and can allow the patient to practice in a nonthreatening atmosphere. It also allows for training to proceed at the patient's optimal pace.
Training should be difficult enough to maintain the patient's interest and attention while being easy enough so that it minimizes fatigue and frustration. To effectively accomplish this, training should be adaptive so that exercises are conducted near the patient's performance threshold.
Because studies have demonstrated benefits from either analytic (bottom-up) or synthetic (top-down) training, it would be useful to integrate listening training with the presentation of communication strategies.
Elderly patients, those comprising the bulk of individuals wearing hearing aids, have additional skills and deficits to consider when implementing a rehabilitation protocol. At the University of California, San Francisco Audiology Clinic, the mean age of the patients engaging in computerized or group-based aural rehabilitation is 70 years. In addition, many elderly patients are good candidates for cochlear implants.12 Healthy older listeners have a strong linguistic knowledge base. In fact, vocabulary and language skills remain stable or continue to improve in these later decades.13,14 Other skills important for interpreting speech decline with age, however, and cognitive speed of processing, working memory, and executive function are areas where declines are seen.14,15 Older listeners generally have more trouble in difficult listening situations than younger equivalents with similar hearing thresholds.16
Performance must be measurable and patients should be given feedback on their progress or lack of progress. Feedback should be provided on a trial-by-trial basis as well as at the conclusion of each training session.
If training is conducted away from the clinical site, performance should be verifiable by remote “data logging.”
We have divided new therapies into those that focus primarily on auditory training, those that combine auditory training with visual (speechreading) enhancement, and those that concentrate on overall aural rehabilitation, incorporating behavioral and lifestyle changes, as well as cognitive training in an effort to maximize everyday performance and quality of life of the hearing-impaired patient. Some of these approaches have been designed specifically for cochlear implant recipients, but all of the therapies are intended to exploit the flexibility and plasticity of the central nervous system. The 4 programs reviewed are Computer-Assisted Speech-Perception Testing and Training at the Sentence Level (CASPERSent), Computer Assisted Tracking Simulation (CATS), Computer-Assisted Speech Training (CAST), and Listening and Communication Enhancement (LACE).
Computer-Assisted Speech-Perception Testing and Training at the Sentence Level
CASPERSent is a multimedia program that is an expansion of the original CASPER program first reported in 1987 by Arthur Boothroyd.17 The software design (created under the Gallaudet Rehabilitation Engineering Research Center) is based on a model of speech perception that focuses on 4 components: sensory evidence, contextual evidence, knowledge, and skill. The primary training target is perceptual skill. Perceptual skill is assumed to include attention, use, and balance of sensory and contextual evidence, balance of speed and accuracy, confidence, risk tolerance, and error handling. The principle target of testing is performance in a conversational context. Secondary testing targets analyze the effects of talker, perceptual modality, topic knowledge, sentence length, and sentence type. Testing and training can be self-administered, administered with the aid of another nonprofessional (ie, the patient's significant other), or clinician controlled.
When self-administered, the basic process is as follows: the trainee hears and/or sees a spoken sentence, repeats as much of the sentence as possible, views the text, clicks on the words correctly identified, hears and/or sees the sentence again, and then continues on to the next sentence. There also can be semi-automated tracking; that is, feedback is given only on the words correctly identified, and then the trainee is given another attempt at identification of the remainder of the sentence.
The stimuli consist of 60 sets of City University of New York (CUNY) sentences representing 12 topics and 3 sentence types (statements, questions, and commands) that are presented by lipreading only, hearing only, or a combination of the two. The sentences are 3 to 14 words long. Scores are based on sets of 12 sentences and are automatically recorded for later data analysis. The presentation modality, talker, viewing angle, feedback, and topic-knowledge are under software control. Learning effects have been demonstrated in subjects with hearing loss. The effects of feedback type on learning rate and of topic-knowledge on performance have been demonstrated in subjects with normal hearing. The CASPERSent program can be used for training, research, or for teaching and demonstration.
Computer Assisted Tracking Simulation and Computer-Assisted Speech Training
Harry Levitt, working with colleagues Claire Bernstein and Matt Bakke, reported on an updated approach to the Continuous Discourse Tracking method originally developed at Central Institute for the Deaf by DeFilippo and Scott in 1978.18 Continuous discourse speech tracking was initially based on a classroom training method and was used in evaluating tactile aids as well as cochlear implants. It can be used for both training and evaluation of communication skills. The key aspect of this approach is that the training involves interaction (a vital component of real-life communication) between the trainee and the tester/trainer. The advantages of the tracking approach are that it can cover a wide range of communication skills, it can be used with different modes of communication (ie, auditory, visual, auditory/visual), and the training process can be tailored specifically to the patient.
The process is as follows: The talker (tester/trainer) articulates a sentence or phrase, and the task of the listener is to repeat back verbatim the sentence or phrase. If the repetition is correct, the talker produces the next utterance. If the repetition is incorrect, the talker repeats the utterance or a portion of it, or may use other repair strategies, until the utterance is correctly repeated in its entirety. The measure of performance is the speed of tracking in words per minute. A major drawback of the traditional tracking technique, however, is its high variability resulting from interspeaker differences and the manner in which the listener responds to this source of variability.
In addition, a variety of different correction strategies are used by individuals acting as the speaker, results may depend on the complexity of the material as well as on the listener's motivation and alertness, and the tracking procedure requires a substantial amount of the clinician's time. To combat these shortcomings, methods for reducing the variability of the technique have been devised. For example, Dempsey et al19 described a computer-automated version of the continuous discourse tracking procedure, but it could only be used with a computer/video laser disc system developed specifically for that purpose.
Levitt, Bernstein, and Bakke (at the 2006 conference) have developed a new software platform for face-to-face speech tracking–training using a conventional personal computer. The software is based on the Kungliga Tekniska högskolan (KTH) speech tracking–protocol developed at the Royal Institute of Technology in Sweden and refined by Gnosspelius and Spens.20 This protocol provides strict rules for the tracking procedure, correction strategies are limited and predetermined, the difficulty of the material is controlled, and the client's performance is monitored and analyzed by a computer. A key component of this approach is to maintain the inherent interactive nature of the communication process.
The computer-based tracking procedure works as follows: First, the clinician/trainer's display shows the text to be used, and the text is then read to the subject (listener) using a conversational manner of speech. The listener then repeats as much of the text as he/she can. The KTH protocol uses verbal repetition. When a word is incorrectly identified, the tester enters the information into the computer and then repeats the sentence from the incorrect word onward. Up to 2 repetitions are provided to the listener. If the word is still not correctly identified, the incorrect word is shown as text on the listener's computer screen. The results of each session are saved in a data log. Tracking rate, ceiling rate, and a list of blocked words are saved for later analysis.
This method is being evaluated experimentally as a training technique to improve communication skills for adult cochlear implant users. It is also being adapted for self-training applications using prerecorded materials. Although it has only been piloted on a few patients at the time of this writing, the program is interesting, results are promising, and larger studies are currently underway to determine its efficacy.
Computer-Assisted Speech Training
Although some cochlear implant users have very dramatic improvements in auditory function, even in the first months of using their device, others may use the cochlear implant for years with little benefit.21 Variability in the outcomes of cochlear implant users is reflected not only in differences in their ability to understand speech but also in the time course of adaptation to speech that is processed and delivered through a cochlear implant. Fu et al4 reported on an auditory rehabilitation tool for cochlear implant recipients, the CAST software developed at the House Ear Institute. The CAST program is used by cochlear implant patients at home, monitors progress, and saves results of the sessions to share with the audiologist or speech pathologist. Versions of CAST have been produced commercially for 2 of the cochlear implant companies: “Sound and Beyond” for Cochlear Corporation, and “Hearing Your Life” for Advanced Bionic.
The CAST program provides auditory training that is specific to the needs of cochlear implant users. It focuses on the acoustic contrasts that are especially problematic for this population, rather than more synthetic, global exercises. Training materials include pure tones, environmental sounds, monosyllabic words, consonant stimuli (in vowel-consonant, vowel-consonant-vowel, and consonant-vowel contexts), familiar words, familiar sentences, simple melodic sequences, and familiar melodies. To train targeted phonetic contrasts, CAST uses more than 1000 novel monosyllabic and nonsense words (for initial, medial, and final vowel and consonant training) spoken by 4 different talkers. The program is adaptive in that the level of difficulty is automatically adjusted according to individual patient performance by increasing the number of response choices or reducing the acoustic differences between response choices, or both.
Auditory and visual feedback is provided during training, which allows users to repeatedly compare incorrect responses with correct responses. At the end of each testing and training session, the program offers training guidance. For example, based on testing or training results, the program may suggest that the user move to an easier or more difficult training level. Should the patient require additional training materials or training modules, these can be added. The CAST program can also simulate a difficult listening environment by incorporating noise or competing speech.
Several studies have been conducted using auditory training with the CAST program for recognition of phonemes, melodic sequences, and Chinese tones. Auditory training resulted in improved performance in the targeted listening task. In addition, improvement occasionally generalized to tasks that were not explicitly trained, such as improved sentence recognition after training with phonetic contrasts, or improved familiar melody identification after training with simple melodic sequences.4 Results are shown in Figure 1. Performance on measured tasks did not revert to baseline levels even at 1 to 2 months after training. Because nearly all of the subjects had at least 1 year of experience with their cochlear implant device before training (ie, at least 1 year of relatively “passive” learning), the results suggest that this “active” learning could be used to improve speech performance.22
Figure 1.
Percent correct on vowel identification (n = 10), consonant identification (n = 7), gender identification (n = 7), and Hearing-in-Noise Test sentence identification (n = 3) for subjects before (gray bars) and after (black bars) training on the Computer-Assisted Speech Training program. Significance (paired Student t test): ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Figure was redrawn from data presented in Fu et al.4
Results from these recent training studies with the CAST program have shown successful training outcomes with cochlear implant patients; however, previous studies of similar programs showed lesser gains.23,24 The current CAST program task and training protocol is different from those used in the previous studies. In particular, the frequency and intensity of the training program may be of some importance. Subjects in the CAST program trained for 30 to 60 minutes per day, 5 days per week for at least a month. The other studies trained subjects only once a week for 50 minutes for 10 weeks. The CAST program required more consistent training and resulted in approximately twice as much training time.
Fu et al also reported preliminary data on the effects of training electrode discrimination. Initial findings suggest that electrode discrimination and speech recognition abilities improved significantly after “moderately” intensive daily training on electrode discrimination. Improvements in electrode discrimination were not restricted to the trained contrasts but also appeared to generalize to speech perception. In addition, Fu et al described future versions of CAST that may further enhance training by fully integrating the standardized testing and training features of the CAST program into the clinical mapping system.
Listening and Communication Enhancement
The LACE software-based program is designed for home use by patients, although it also can be used within the clinic setting.10 The LACE program provides a variety of interactive and adaptive tasks that are divided into the 3 main categories of degraded speech, cognitive skills, and communication strategies. For degraded speech exercises, speech is presented with background babble noise or a single competing speaker or is time compressed. The rationale for this is that rapid speech stresses the temporal constraints of the auditory system and makes demands on cognitive speed of processing. On each trial of the degraded and competing speech tasks, the patient listens to and repeats the target stimuli, then sees the correct response on the screen. If the stimuli are correctly comprehended, the next presentation will be slightly more difficult. If it is incorrectly understood, the next presentation will be slightly easier. The difficulty level of the current trial is thus based on the accuracy of the person's response to the previous trial. It also gives stimulating training exercises to enhance auditory memory and speed of processing, 2 elements of listening that are particularly important in difficult listening environments. The LACE program provides more than 150 interactive communication strategies.
In addition to the immediate feedback given for each task, LACE presents to the patient a graph depicting cumulative daily improvement and progress at the end of each training session. The results of the training are also tracked and electronically transmitted to a secure Web site that is compliant with the Health Insurance Portability and Accountability Act (HIPPA) and is accessible by the audiologist so that the patient's progress can be monitored. Future versions will allow for modifications in training to be implemented remotely.
Although LACE is appropriate for listeners of all hearing levels and hearing aid experience, the program was designed as a complement to the hearing aid fitting process. Hearing aid technology is constantly improving. In contrast to the rapid advances in amplification in the past decades, however, satisfaction with hearing aids is only recently starting to change for the better.25 This disparity is likely due to the components of optimal listening that modern hearing aids cannot improve, such as rectifying impaired frequency and temporal resolution due to a damaged cochlea, improving ingrained maladaptive listening and communication strategies, reversing plastic or degenerative changes in the central auditory system, or correcting for declines in cognitive function.
Cognitive function is an area typically not addressed in aural rehabilitation programs; however, the average new hearing aid user is about 70 years old.25 Studies have shown that older listeners require a greater signal-to-noise ratio to perform as well as younger listeners with similar thresholds.26–28 Declines in areas of cognitive processing speed and auditory working memory also are observed in this population,14,29 but studies have shown that training can improve skills in these areas.30,31 Because of these cognitive changes and the effects they may have on listening,32 exercises based on improving response time and auditory memory are included in the LACE training.
The efficacy of individualized aural rehabilitation using the LACE training system was assessed in a preliminary study that enrolled 65 subjects at 5 sites across the United States. A randomized crossover control design was used. Subjects completed 20 half-hour sessions during a period of about 1 month. They were asked to complete 5 training sessions per week. At every session, the patient response from each trial was saved. The baseline measure for the training tasks was the entire first quarter of training (approximately 1 week of training), to minimize the influences of procedural learning.
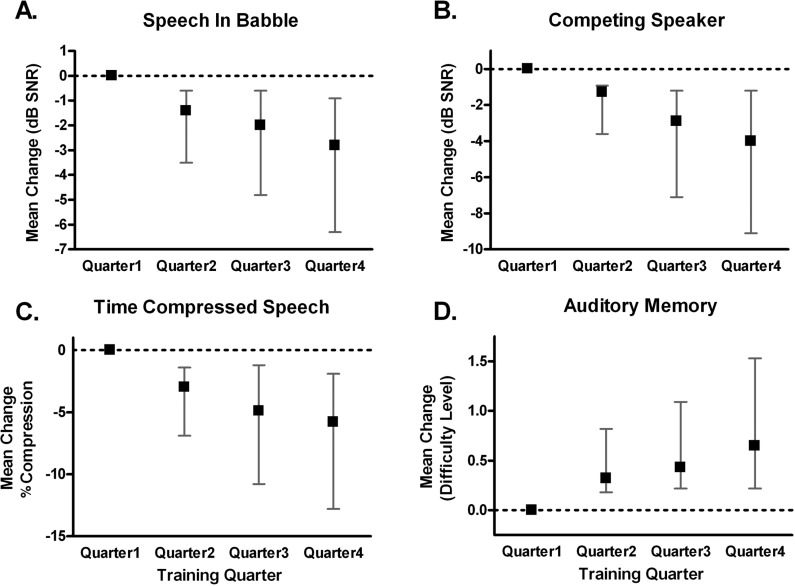
Group mean task performance changes for each quarter of the training protocol are shown in Figure 2. Quarter 2 mean scores were significantly improved from quarter 1 scores on all tasks but the auditory memory task, which did not improve significantly until quarter 3 (analysis of variance, P < .05). Most subjects’ scores continued to improve, often significantly, through the last quarter of training. Subjects were tested at baseline using the Quick Speech-in-Noise (QuickSIN) test and Hearing-in-Noise Test (HINT), subjective questionnaires (Communication Scale for Older Adult and Hearing Handicap Inventory for Elderly), and cognitive tests (Stroop color word test, and listening span). These tests were repeated at the end of the 20 training sessions and again at 4 weeks after the completion of training. Improvement on 4 of the 5 measured training tasks occurred in 83% of patients.
Figure 2.
Mean improvement on training task scores for each quarter of the training relative to the first quarter of training, for all subjects completing Listening and Communication Enhancement (LACE) training. (A) Group mean change on speech in babble performance. A decrease in decibels of signal-to-noise ratio (dB SNR) score indicates improvement. (B) Group mean improvement on speech with a competing speaker performance. A decrease in dB SNR score indicates improvement. (C) Group mean improvement on time-compressed speech performance. A decrease in score indicates improvement. (D) Group mean improvement on auditory memory performance. An increase in score indicates improvement. Error bars indicate 95% confidence interval of the mean.
Group mean improvements in the outcome measures for the control subjects and the trained subjects are shown in Figure 3. Scores were significantly improved in the trained group (Wilcoxon matched pairs, P < .05) and were not in the control group on all measures except the HINT. Scores on outcome measures assessed one month later were not statistically different from the posttraining session (P < .05) at 1-month posttraining.
Figure 3.
Mean change in scores for all outcome measures. Circles indicate changes in control group scores, and squares indicate changes in the trained group scores. Increase in score indicates improvement on all measures. Error bars indicate the standard error of the mean. HHIE = Hearing Handicap Inventory for the Elderly; CSOA-S = Communication Scale for Older Adults; CSOA-A = Communication Scale for Older Adults attitudes subscale; SPAN = Listening Span; STROOP = Stroop Color Word Test; Quick SIN 45 = Quick Speech-in-noise at 45 dB HL; Quick SIN 70 = Quick Speech-in-noise at 70 dB HL; HINT = Hearing-in-Noise Test.
Differences and Similarities of Individual Aural Rehabilitation Programs
The programs outlined share numerous similarities even though they were designed for somewhat different purposes and for different populations. For example, the CAST and CATS training programs are being looked at specifically for the cochlear implant population. The LACE and CASPERSent programs also can be used by cochlear implant recipients but may require changes in the content of the training material. At the time of this writing, new content reflecting the wider range of auditory language skills of cochlear implant subjects is currently being contemplated for addition to LACE. Some of the programs (LACE and CAST) incorporate adaptive difficulty levels, and some (CATS and CASPERSent) include both auditory and visual training.
The frequency and duration of training differ. Some programs are clearly designed for more bottom-up training using phonemes and single words, whereas others (LACE) use a more combined bottom-up and top-down approach by including sentence length training materials, communication strategies, and cognitive exercises. Another difference is in the training stimuli. The CATS program can use sentences and even longer phrases, whereas LACE and CASPERSent use sentences. The CAST program includes pure tones, nonsense syllables, words, sentences, and even melodies.
Some programs are specifically designed for training in noise or with other degraded signals (LACE and CAST), although all can be adapted so that noise is included. Some programs can be used both for training and testing, others just for training. Some use a form of tracking (CATS and CASPERSent). All include feedback, although at different intervals, and all allow for data retrieval and later analysis. Some, like LACE, allow for remote access through a HIPAA-compliant server. Table 1 compares some of the program features.
Table 1.
Components of the 4 Training Programs
| Programa | Bottom-up AT | Top-down AT | Feedback | Communication Strategies | Adaptive | Video | Data Retrieval | Remote Access |
|---|---|---|---|---|---|---|---|---|
| LACE | • | • | • | • | • | • | ||
| CASPERSent | • | • | • | • | ||||
| CATS | • | • | • | • | • | |||
| CAST | • | • | • | • |
Note: AT = auditory training; LACE = Listening and Communication Enhancement; CASPERSent = Computer-Assisted Speech-Perception Testing and Training at the Sentence Level; CATS = Computer Assisted Tracking Simulation; CAST = Computer-Assisted Speech Training
Filled circles indicate that the training program includes this aspect of aural rehabilitation.
Pharmacologically Enhanced Aural Habilitation
These programs demonstrate that modern computer technology has afforded the capability of providing unprecedented flexibility, adaptability, control, and data management for aural rehabilitation. As with other modes of training (eg, physical and mental), it is logical to question whether performance also can be improved with pharmacologic enhancement. In patients with aphasia, for example, it has been shown that d-amphetamine stimulates the cortical region surrounding the damaged area and the contralateral homologous regions to promote their receptiveness to therapeutic interventions. Although the mechanism is unclear, peripherally administered amphetamine likely increases levels of dopamine, serotonin, and noradrenaline in the central nervous system.
Tobey et al33 conducted a series of experiments designed to determine whether d-amphetamine administered in conjunction with behavioral therapy could alter cortical responses to electric hearing. The outcomes used were behavioral performance and functional measures of brain activity. They assessed regional cerebral blood flow (rCBF) responses to cochlear implant stimulation and investigated the potential benefit of pharmacologically enhanced aural rehabilitation therapy as a means of increasing speech-tracking skills in adult cochlear implant users. Using single photon emission tomography to conduct rCBF imaging, they studied 26 normal hearing control subjects and 18 cochlear implant subjects listening to a videotaped story. Adult cochlear implant participants received either d-amphetamine or a placebo 60 minutes before a 1.5-hour aural rehabilitation session occurring twice a week for 2 months. Treatment consisted of a multi-step rehabilitation program individualized for each participant for development of speech-tracking skills.
Results showed that individuals with minimal open-set speech perception scores demonstrated unilateral cortical activation of the hemisphere contralateral to the implanted ear. Speech-tracking scores for the placebo and treatment groups were similar before intervention. In the placebo group, speech-tracking performance increased by 13.5% for visual plus auditory and auditory-only presentations as a function of aural therapy alone. The subjects in the d-amphetamine–facilitated program yielded small increases in visual plus auditory tracking scores (2%) but showed a 43% increase for auditory-only speech tracking.
Preliminary conclusions were that cortical imaging reveals important aspects of responsiveness in cochlear implant users. For example, cortical responses are less robust than in normal hearing users, and cortical responses in placebo participants demonstrate changes in plasticity. In the group receiving treatment, however, plasticity responses appeared to be greater. The optimal dosage and frequency of d-amphetamine remains to be determined, as does the ideal type and duration of aural rehabilitation intervention combined with a pharmacologic approach.
Unresolved Questions
A variety of unresolved questions relevant to the design and evaluation of aural rehabilitation programs remain. Certain types of auditory learning are likely to occur regardless of the presence of formal training. This is called incidental learning and it transpires merely because the individual is exposed to everyday speech and other acoustic stimuli. In other words, there is no specific intent to learn, it simply happens as a result of passive exposure to repeated stimuli. This differs from formalized training in which learning is specifically intended.
All of the training programs described here use some type of stimulus-response interaction with feedback provided to reinforce correct responses. Some of the training may produce task-related learning, in other words, learning specific to the actual training task. Thus, there would be no carryover to real life. This would not be beneficial to the aural rehabilitation of the patient. The objective of any aural rehabilitation protocol is to produce generalized learning that carries over to real life situations. The question that must be answered is: Do clinician-directed or self-administered computer-assisted training therapies add to the incidental learning that occurs in every day communication? The results from some of the programs described here suggest that they can, because both trained and control subjects were exposed to passive learning, whereas only the trained subjects were given formal training.
In addition, a number of other important questions about how to structure individualized aural rehabilitation programs remain unresolved. For example, what are the best training parameters (duration, frequency, stimulus type)? Learning seems to take place using a wide range of training parameters. Previous studies,30,34 however, have indicated that parameter-dependent differences exist in the rate and magnitude of learning.
Will the improvement in performance due to training last over an extended period of time? Some studies indicate that subjects engaging in aural rehabilitation programs show improved performance relative to control subjects, but that after 1 year, the untrained subjects’ performances are equal to those of the experimental subjects.35 Of course, this does not negate the importance of training. Indeed, the ability to demonstrate rapid improvement may encourage patients to keep and wear hearing aids. Some of the programs described have generated follow-up data indicating perceptual learning for at least 2 months after treatment, but there are no long-term data reflecting whether additional training is required.
Studies of the efficacy of aural rehabilitation programs are difficult to conduct. When conducting research on the efficacy of aural rehabilitation programs, who should be recruited as “typical” subjects? Many subjects recruited for research are not representative of the general hearing-impaired public. Individuals engaging in aural rehabilitation research may represent the “motivated” wing of the general hearing-impaired population. It is likely, for example, that cochlear implant recipients recognize the need for additional aural rehabilitation more than the average hearing aid user. Furthermore, one might question whether training effects shown by cochlear implant patients generalize to hearing aid patients and vice versa. Cochlear implant patients receive an electrical signal characterized primarily by amplitude envelope reproduction. This is not the case with the acoustic signal received by hearing aid wearers.
What are the best outcome measures to assess benefit? Certainly subjective scales such as the Hearing Handicap Inventory for the Elderly (HHIE) or Communication Scale for Older Adults (CSOA) can be useful, but are they sensitive enough to accurately represent changes occurring from therapeutic intervention? The use of sentence-length tests in noise, such as the HINT or QuickSIN, represent an improvement over monosyllabic word testing in quiet in terms of face validity, but they still do not incorporate the interactive nature of conversation and the use of conversational repair strategies.
Although perhaps lacking scientific validity, in that it is not a direct measure of communication or successful use of amplification, a metric that may be considered is the percentage of hearing aids returned for credit. If those who participate in aural rehabilitation programs were to also keep their hearing aids with greater frequency than those who do not receive aural rehabilitation, this might motivate audiologists to recommend aural rehabilitation, as well as encourage third-party insurers to reimburse for aural rehabilitation.
Perhaps the most important practical questions relate to whether patients will comply with the recommendation of aural rehabilitation therapy and whether audiologists and other professionals will recommend it. A number of considerations are relevant. For example, what is the cost to the patient? Money is not the only issue. Some patients will reject adjunctive therapies to amplification because of the cost related to their personal time and effort. It is important to consider that patients will, however, abide by recommendations for physical therapy. It may be fruitful for audiologists to consider why patients obey these recommendations yet seem reluctant to engage in aural therapy. Perhaps it is because the recommendation for physical therapy is provided by a medical doctor. On the other hand, long-term advocates of aural rehabilitation such as Donna Wayner have reported high rates of participation in aural rehabilitation. Wayner36 has averaged a commendable 87% participation for group aural rehabilitation for the past 14 years. And, not coincidentally, her return for credit rate is an enviable 3%.
Conclusions
The interventions discussed in this article are representative of the renewed interest in aural rehabilitation. We hope that these will be merely a starting point for newer and better programs as more data are published and more audiologists recognize the need for therapy. Given the powerful and untapped resources of computers, it seems probable that future programs will include training in a virtual reality environment that encourages individuals to use auditory scene analysis and more closely resembles the real world. Enhanced graphics can make the training more attractive and the ability to continually adapt the stimuli and training to the communicative needs and interests of the individual will render compliance more likely.
It is important to establish through research that specific auditory training programs provide substantial benefit to hearing aid and cochlear implant users to convince audiologists to recommend aural rehabilitation in conjunction with hearing technology. The future for aural rehabilitation is bright, but will depend on convincing professionals and patients that it is an important, and perhaps an absolutely integral, component to the fitting and use of prosthetic hearing devices.
Acknowledgment
Much of the information in this manuscript was presented orally at the State of the Science Conference of the Rehabilitation Engineering Research Center on Hearing Enhancement, Gallaudet University, Sep 18–19, 2006.
References
- 1.Schow R, Balsara N, Smedley T, Whitcomb C. Aural rehabilitation by ASHA audiologists. Am J Audiol. 1993;2: 28–37 [DOI] [PubMed] [Google Scholar]
- 2.Sweetow R, Palmer CV. Efficacy of individual auditory training in adults: a systematic review of the evidence. J Am Acad Audiol. 2005;16: 494–504 [DOI] [PubMed] [Google Scholar]
- 3.Moore DR, Amitay S, Vavasour E, Irwin A. Optimising auditory learning for variable training stimuli. Abstract presented at the 29th Midwinter Meeting Association of Research in Otolaryngology. Baltimore, Md; 2006 [Google Scholar]
- 4.Fu QJ, Galvin J, Wang X, Nogaki G. Moderate auditory training can improve speech performance of adult cochlear implant patients. Acoust Res Lett Online. 2005;6: 106–111 [Google Scholar]
- 5.Stacey PC, Summerfield AQ. Auditory-perceptual training using a simulation of a cochlear implant system: a controlled study. In: Hazan V, Iverson P. eds. ISCA Workshop on Plasticity in Speech Perception (PSP2005). Senate House, London, UK; 2005: 143–145 [Google Scholar]
- 6.Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42(4 suppl 2): 9–24 [DOI] [PubMed] [Google Scholar]
- 7.Chisolm TH, Willott JF, Lister JJ. The aging auditory system: anatomic and physiologic changes and implications for rehabilitation. Int J Audiol. 2003;42(suppl 2): 3–10 [PubMed] [Google Scholar]
- 8.Schneider BA, Daneman M, Murphy DR. Speech comprehension difficulties in older adults: cognitive slowing or age-related changes in hearing? Psychol Aging. 2005;20: 261–271 [DOI] [PubMed] [Google Scholar]
- 9.Divenyi PL, Stark PB, Haupt KM. Decline of speech understanding and auditory thresholds in the elderly. J Acoust Soc Am. 2005;118: 1089–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweetow RW, Sabes JH. The need for and development of an adaptive Listening and Communication Enhancement (LACE) Program. J Am Acad Audiol. 2006;17: 538–558 [DOI] [PubMed] [Google Scholar]
- 11.Moore DR, Amitay S. Auditory training: rules and applications. Semin Hear. 2007;28: 99–109 [Google Scholar]
- 12.Orabi AA, Mawman D, Al-Zoubi F, Saeed SR, Ramsden RT. Cochlear implant outcomes and quality of life in the elderly: Manchester experience over 13 years. Clin Otolaryngol. 2006;31: 116–122 [DOI] [PubMed] [Google Scholar]
- 13.Schum RL, Sivan AB. Verbal abilities in healthy elderly adults. Appl Neuropsychol 1997;4: 130–134 [DOI] [PubMed] [Google Scholar]
- 14.Wingfield A. Cognitive factors in auditory performance: context, speed of processing, and constraints of memory. J Am Acad Audiol 1996;7: 175–182 [PubMed] [Google Scholar]
- 15.Pichora-Fuller MK. Cognitive aging and auditory information processing. Int J Audiol. 2003;42(suppl 2): 26–32 [PubMed] [Google Scholar]
- 16.Gordon-Salant S. Hearing loss and aging: New research findings and clinical implications. J Rehabil Res Dev. 2005;42(4 suppl 2): 9–24 [DOI] [PubMed] [Google Scholar]
- 17.Boothroyd A. CASPER, computer-assisted speech-perception evaluation and training. Proceeding of the 10th Annual Conference of the Rehabilitation Society of North America. Washington, DC: Association for Advancement of Rehabilitation Technology; 1987: 734–736 [Google Scholar]
- 18.De Filippo CL, Scott BL. A method for training and evaluating the reception of ongoing speech. J Acoust Soc Am. 1978;63: 1186–1192 [DOI] [PubMed] [Google Scholar]
- 19.Dempsey JJ, Levitt H, Josephson J, Porrazzo J. Computer-assisted tracking simulation (CATS). J Acoust Soc Am. 1992;92: 701–710 [DOI] [PubMed] [Google Scholar]
- 20.Gnosspelius J, Spens K-E. A computer-based speech tracking procedure. STL-QPRS. 1992;1: 131–137 [Google Scholar]
- 21.Tyler RS, Gantz BJ, Parkinson AJ, Woodworth GG, Lowder MW, Parkinson WS. Initial comparison between the Clarion, Nucleus and Ineraid cochlear implants. Adv Otorhinolaryngol. 1997;52: 274–278 [DOI] [PubMed] [Google Scholar]
- 22.Fu QJ, Shannon RV, Galvin JJ., 3rd. Perceptual learning following changes in the frequency-to-electrode assignment with the Nucleus-22 cochlear implant. J Acoust Soc Am. 2002;112: 1664–1674 [DOI] [PubMed] [Google Scholar]
- 23.Dawson PW, Clark GM. Changes in synthetic and natural vowel perception after specific training for congenitally deafened patients using a multichannel cochlear implant. Ear Hear. 1997;18: 488–501 [DOI] [PubMed] [Google Scholar]
- 24.Busby PA, Roberts SA, Tong YC, Clark GM. Results of speech perception and speech production training for three prelingually deaf patients using a multiple-electrode cochlear implant. Br J Audiol. 1991;25: 291–302 [DOI] [PubMed] [Google Scholar]
- 25.Kochkin S. Hearing loss population tops 31 million people. Hear Rev. 2005;12: 16–29 [Google Scholar]
- 26.Gordon-Salant S, Fitzgibbons PJ. Comparing recognition of distorted speech using an equivalent signal-to-noise ratio index. J Speech Hear Res. 1995;38: 706–713 [DOI] [PubMed] [Google Scholar]
- 27.Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am. 1995;97: 593–608 [DOI] [PubMed] [Google Scholar]
- 28.Dubno JR, Dirks DD, Morgan DE. Effects of age and mild hearing loss on speech recognition in noise. J Acoust Soc Am. 1984;76: 87–96 [DOI] [PubMed] [Google Scholar]
- 29.Murphy DR, Daneman M, Schneider BA. Why do older adults have difficulty following conversations? Psychol Aging. 2006;21: 49–61 [DOI] [PubMed] [Google Scholar]
- 30.Mahncke HW, Connor BB, Appelman J, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103: 12523–12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288: 2271–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tun PA, Wingfield A. One voice too many: adult age differences in language processing with different types of distracting sounds. J Gerontol B Psychol Sci Soc Sci. 1999;54: P317–P327 [DOI] [PubMed] [Google Scholar]
- 33.Tobey EA, Devous MD, Buckley K, Overson G, Harris T, Ringe W, Martinez-Verhoff J. Pharmacological enhancement of aural habilitation in adult cochlear implant users. Ear Hear. 2005;26(4 suppl): 45S–56S [DOI] [PubMed] [Google Scholar]
- 34.Watson CS. Time course of auditory perceptual learning. Ann Otol Rhinol Laryngol Suppl. 1980;89: 96–102 [PubMed] [Google Scholar]
- 35.Chisolm TH, Abrams HB, McArdle R. Short- and long-term outcomes of adult audiological rehabilitation. Ear Hear. 2004;25: 464–477 [DOI] [PubMed] [Google Scholar]
- 36.Wayner DS. Aural rehabilitation adds value, lifts satisfaction, cuts returns. Hear J. 2005;12: 30–38 [Google Scholar]