Abstract
Purpose:
To report a novel application of a porous polyethylene implant for lid stabilization and management of eyelid retraction in a patient with an exposed Boston Keratoprosthesis Type II.
Methods:
A 54-year-old woman with a history of mucous membrane pemphigoid and failed penetrating keratoplasty of the left eye underwent implantation of a Boston Keratoprosthesis (KPro) Type II along with permanent surgical fusion of the upper and lower lids of the left eye in January 2010. At one month follow-up, significant retraction of the lower lid around the inferior margin of the optic was noted, resulting in partial exposure of the keratoprosthesis. The patient subsequently underwent left lower eyelid reconstruction with a porous polyethylene implant to ensure coverage and stability of the KPro.
Results:
Eyelid reconstruction using a porous polyethylene implant resulted in stable retention of the KPro Type II for over 2 years.
Conclusion:
In patients with Boston KPro Type II in the setting of severe cicatrizing ocular surface disease, the use of a porous polyethylene implant during eyelid reconstruction around the KPro optic may aid in maintaining eyelid integrity and improving KPro stability and longevity.
Keywords: Boston keratoprosthesis, Medpor, porous polyetheylene
The Boston Type II Keratoprosthesis (KPro) was developed for patients with severe cicatrizing ocular surface conditions and is designed with a telescoping optic that passes full-thickness through surgically fused eyelids.1,2 However, the systemic inflammatory diseases present in most patients requiring a Type II KPro (i.e. Stevens Johnson syndrome, mucous membrane pemphigoid) predispose to cicatricial lid changes;3 this may, in turn, be a driving factor in lid retraction and subsequent KPro exposure, which is a common postoperative problem.
Options for correction of lid retraction in patients with Type II KPro include skin revision, tunneled flaps, and buccal mucosal grafting.2,4,5 Herein we describe the novel use of a porous polyethylene eyelid spacer (Medpor®; Stryker Craniomaxillofacial, Portage, Michigan) for successful long term correction of eyelid retraction in a patient with Type II KPro.
CASE REPORT
A 48-year-old Hispanic female patient presented to our institution in September 2004 for management of her mucous membrane pemphigoid. After a prolonged course including a failed penetrating keratoplasty, she underwent placement of a Type II KPro with surgical eyelid fusion in the left eye in January 2010.
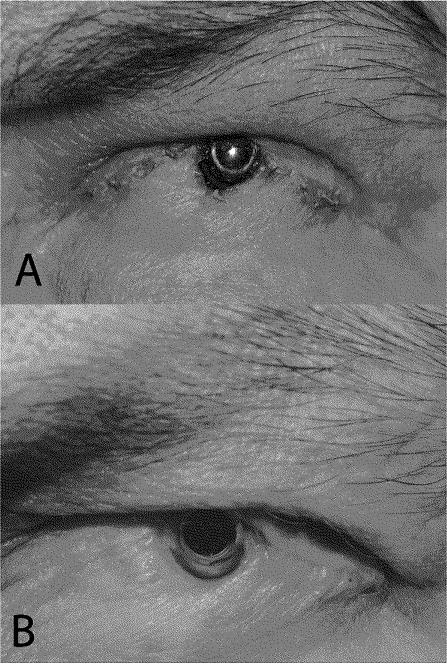
Within 1 week of surgery, eyelid retraction inferior to the optic was noted. This progressed until frank KPro exposure was noted on post-operative day 37 (Figure 1a).
FIGURE 1.
External photographs of the left eye in a patient with Boston Type II Keratoprosthesis taken (a) approximately 3 weeks after surgical lid fusion and Boston Type II Keratoprosthesis implantation. Significant skin retraction is evident at the inferonasal margin of the optic with frank exposure of the keratoprosthesis and underlying carrier cornea. (b) External photograph of patient’s left eye taken 24 months after eyelid revision with Medpor implant. A small area of skin retraction noted near the inferonasal optic margin has not progressed from 15 months prior and the KPro remains stable and in good position.
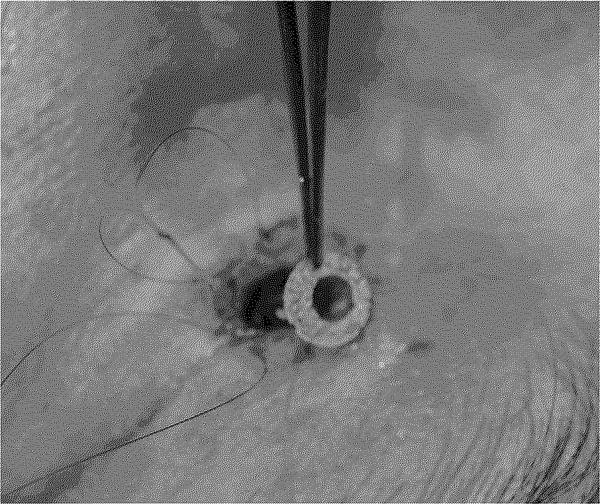
The patient subsequently underwent eyelid reconstruction on post-operative day 50. An incision was made adjacent to the telescoping portion of the KPro. The inferior portion of the eyelid was separated and the retraction corrected by recessing the posterior aspect of the lower eyelid. A discus was fashioned out of a 0.25-mm-thick sheet of Medpor using a 6-mm trephination blade to make an initial circle and a 3-mm skin biopsy punch to make a slightly eccentric hole (Figure 2). The discus was placed around the telescoping KPro optic and pushed deep underneath the surrounding tissue. Once the discus was in position, a 6-0 Prolene suture was passed in a purse-string manner for 360° along the eyelid margin and tied tightly to advance the tissue over the discus and along the cuff of the KPro.
FIGURE 2.
Intraoperative photograph of eyelid revision in a patient with recent Boston Type II Keratoprosthesis placement complicated by eyelid retraction and keratoprosthesis exposure. A discus was fashioned out of a 0.25-mm-thick sheet of porous polyethylene (Medpor) using a 6-mm trephination blade to make an initial circle and a 3-mm skin biopsy punch to make a slightly eccentric hole. The discus was then placed in the operative site around the telescoping keratoprosthesis optic and pushed deep into the surrounding tissue prior to closure.
On postoperative day 3, the KPro was properly positioned without lid retraction or exposure. Although minimal inferior eyelid retraction was noted at post-op week 3, this did not progress and the KPro remained stable over the next 24 months without the need for any further procedures (Figure 1b).
COMMENT
Porous polyethylene (Medpor) is a versatile synthetic material previously described for use as an eyelid spacer.6,7 To our knowledge, this is the first report of the use of Medpor in conjunction with a keratoprosthesis for management of eyelid retraction in this setting. Medpor has the advantage of achieving rapid biointegration much like autologous tissue, but without the need for a harvesting procedure.8
The largest report to date on Type II KPro did not specifically report on eyelid retraction, however, 10% of patients in this series had early implant extrusion, which would be mechanically unlikely in the absence of lid retraction.2 Although 34.5% of patients in this study required skin revision, the proportion of these revisions that were due to lid retraction and the success rate of simple skin revision is not reported.2 In our experience, skin revision alone is often inadequate to achieve long-lasting keratoprosthesis coverage.
Buccal grafting over the optic of the Type II KPro is another option when faced with implant exposure due to lid retraction. This graft is ideally left in place for several months and therefore has the disadvantages of obscuring the visual axis and requiring another procedure to re-create the optic opening.5 Nanavaty et al. described using a tunneled pericranial flap to provide tissue coverage in two Type II KPro patients with recurrent skin retraction.4 However, without follow-up information, the long-term efficacy of this procedure is unknown.
Although we report on only a single case of successful correction of eyelid retraction with Medpor in a patient with Type II KPro, we believe this represents a promising option for treatment of recurrent eyelid retraction. There is no risk of donor site complications as seen with autologous grafts, but if needed Medpor may be combined with an overlying autologous graft.6 However, the use of Medpor is not without risk, as there are a number of prior reports of infection and inflammation associated with Medpor.8,9,10 Further studies are therefore needed to fully describe the advantages and complications of Medpor use with Type II KPro.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Dahlman CH, Schneider HA, Doane MG. Prosthokeratoplasty. Am J Ophthalmol. 1974;77(5):694–700. doi: 10.1016/0002-9394(74)90534-0. [DOI] [PubMed] [Google Scholar]
- 2.Pujari S, Siddique SS, Dahlman CH, et al. The Boston Keratoprosthesis type II: the Massachusetts eye and ear infirmary experience. Cornea. 2011;30:1298–1303. doi: 10.1097/ICO.0b013e318215207c. [DOI] [PubMed] [Google Scholar]
- 3.Kompella VB, Sangwan VS, Bansal AK, et al. Ophthalmic complications and management of Stevens-Johnson syndrome at a tertiary care centre in South India. Ind J Ophthalmol. 2002;50:283–286. [PubMed] [Google Scholar]
- 4.Nanavaty MA, Avisar I, Lake DB, et al. Management of skin retraction associated with Boston type II keratoprosthesis [published online ahead of print August 3 2012] Eye. 2012 doi: 10.1038/eye.2012.144. http://www.nature.com/eye/journal/vaop/ncurrent/full/eye2012144a.html [last accessed 15 Sept 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MS, Krakauer M, Gupta S, et al. Eyelid procedures in patients who have undergone Boston Keratoprosthesis surgery. Ophthal Plast Reconstr Surg. 2012;28:286–288. doi: 10.1097/IOP.0b013e31825b648e. [DOI] [PubMed] [Google Scholar]
- 6.Wong JF, Soparkar CN, Patrinely JR. Correction of lower eyelid retraction with high density porous polyethylene: the Medpor Lower Eyelid Spacer. Orbit. 2001;20:217–225. doi: 10.1076/orbi.20.3.217.2623. [DOI] [PubMed] [Google Scholar]
- 7.Tan J, Olver J, Wright M, et al. The use of porous polyethylene (Medpor) lower eyelid spacers in lid heightening and stabilization. Br J Ophthalmol. 2004;88:1197–1200. doi: 10.1136/bjo.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavrikakis I, Francis N, Poitelea C, et al. Medpor lower eyelid spacer: does it biointegrate? Orbit. 2009;28:58–62. doi: 10.1080/01676830802414855. [DOI] [PubMed] [Google Scholar]
- 9.Cenzi J, Farina A, Zuccarino L, et al. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J Craniofac Surg. 2005;16:526–530. doi: 10.1097/01.scs.0000168761.46700.dc. [DOI] [PubMed] [Google Scholar]
- 10.Musadiq M, Bhatt R, Mudhar HS, et al. Abscessed porous polyethylene (Medpor) orbital implant: a case report. Orbit. 2008;27:127–129. doi: 10.1080/01676830701376163. [DOI] [PubMed] [Google Scholar]