Abstract
Substance abuse is a frequent comorbid condition among patients with Traumatic Brain Injury (TBI), but little is known about its potential additive or interactive effects on tissue injury or recovery from TBI. This study aims to evaluate changes in regional metabolism and cerebral perfusion in subjects who used methamphetamine(METH) prior to sustaining a TBI. We hypothesized that METH use would decrease pericontusional cerebral perfusion and markers of neuronal metabolism, in TBI patients compared to those without METH use.
Methods
This is a single center prospective observational study. Adults with moderate and severe TBI were included. MRI scanning was performed on a 3 Tesla scanner. MP-RAGE and FLAIR sequences as well as Metabolite spectra of NAA and lactate in pericontusional and contralateral voxels identified on the MP-RAGE scans. A spiral-based FAIR sequence was used for the acquisition of cerebral blood flow (CBF) maps. Regional CBF images were analyzed using Image J open source software. Pericontusional and contralateral CBF, NAA and lactate were assessed in the entire cohort and in the METH and non-METH groups.
Results
17 subjects completed the MR studies. Analysis of entire cohort: Pericontusional NAA concentrations (5.81 ± 2.0 mM/kg) were 12% lower compared to the contralateral NAA (6.98 ± 1.2 mM/kg; p=0.03). Lactate concentrations and CBF were not significantly different between the two regions, however, regional cerebral blood flow was equally reduced in the two regions. Subgroup analysis: 41% of subjects tested positive for METH. The mean age, Glasgow Coma Scale and time to scan did not differ between groups. The two subject groups also had similar regional NAA and lactate. Pericontusional CBF was 60% lower in the METH users than the non-users, p=0.04; contralateral CBF did not differ between the groups.
Conclusion
This small study demonstrates that tissue metabolism is regionally heterogeneous after TBI and pericontusional perfusion was significantly reduced in the METH subgroup.
Keywords: Adult brain injury, MRI, blood flow, metabolism, alcohol and drug abuse
Introduction
Traumatic brain injury (TBI) is a significant public health problem in the United States and around the world. Approximately, 1.5 million cases of TBI occur in the US each year. Fifty thousand of these victims die and 80,000 survive with moderate to severe cognitive and motor disability. 1,2 The Centers for Disease Control and Prevention (CDC) estimates that the annual cost of TBI per year in the United States is 56.3 billion dollars. 3 The occurrence of TBI is often linked to substance abuse, which increases risk taking behavior and leads to more severe TBI.4 As a consequence, 36–51% of victims of traumatic brain injury are found to be using illicit drugs.5,6,7 Worldwide 15 million people regularly use amphetamines8, and in particular methamphetamine (METH), a highly addictive drug of abuse that has been increasing in popularity in the US and around the world.9 METH is toxic to the dopaminergic neurotransmitter system 10,11 and has systemic hemodynamic effects.12
Since little is known about the effects of METH in patients with TBI, a study was initiated to assess for metabolic and perfusion changes in patients who used METH prior to sustaining a TBI.
Brain metabolism was evaluated non-invasively, using proton MR spectroscopy (1H MRS), an important tool in the evaluation of tissue metabolic state after TBI.13,14,15,16 1H MRS can non-invasively provide information about several important chemicals in the brain. Of particular interest in the context of TBI are N-acetyl-aspartate (NAA), a marker of neuronal cell function, and lactate, which may be elevated when aerobic metabolism is compromised.15 Additionally, cerebral blood flow is a quantifiable physiological parameter that can be measured by MRI techniques. Cerebral blood flow is altered after TBI, and pericontusional tissue seems to be at highest risk.17 Furthermore, METH significantly elevates synaptic dopamine levels. 10,18 Since dopaminergic nerve terminals on blood vessels can cause vasomotor changes of brain microvasculature19 patients who used methamphetamine prior to sustaining a TBI may have more profound alterations in regional tissue perfusion. Therefore, we hypothesized that METH use would further the decreases in pericontusional cerebral perfusion and NAA concentrations, as well as increases in lactate concentration in TBI patients compared to those without METH use.
Methods
This protocol was reviewed and approved by the institutional research review committee at The Queen's Medical Center. The study is a single center prospective observational study evaluating the effects of METH on cerebral metabolism and perfusion in patients with moderate or severe TBI. Subjects were screened between October, 2007 and May, 2009. Subjects were screened by the neurointensivist on call and informed consents were obtained from the surrogate decision makers. Inclusion criteria include: age between 18 and 65 years, traumatic brain injury with post resuscitation Glasgow Coma Scale < 13, informed consent provided and signed by a surrogate decision maker. Exclusion criteria include: profound hypoxia (apnea in field or PaO2 < 60mmHg), sustained hypotension with systolic blood pressure (SBP) < 90 mmHg, MRI incompatible device or implant, any preexisting neurologic, psychiatric or systemic disorder that might alter CBF or metabolism, or pregnancy. Incarcerated patients were not included and neither were those with penetrating brain injury. All patients received standard clinical care in accordance with the Brain Trauma Foundation.20 Fifty eight patients were screened, 20 subjects were enrolled and 17 underwent MRI imaging. Reasons for screen failure included the following: surrogate decision maker unavailable or unwilling to consent to study, GCS improved to > 13, Uncontrolled hypoxia or hypotension documented, previous traumatic brain injury, pacemaker or other MRI incompatible device.
MRI scanning
Subjects who were clinically stable for MRI scanning within the first 30 days post injury were transported to the University of Hawaii/QMC MR Research Center when medically stable enough to tolerate the supine head position without increased intracranial pressure. Intracranial and hemodynamic monitoring was continued while the subject was in the MR scanner if necessary. ICP monitoring during MRI scanning was performed using a standard tunneled ventriculostomy catheter with a fluid coupled pressure transducer. Subjects with intracranial monitors inserted through a cranial bolt system underwent MRI scanning after the monitor was removed. An ICU nurse and a respiratory therapist, as well as a research nurse and an investigator accompanied each subject. All these personnel could continue to monitor and treat cerebral and hemodynamic abnormalities if they arose during the scan. MRI scanning was performed on the research-dedicated Siemens MAGNETOM Trio, a 3 Tesla Tim system scanner (Erlangen, Germany), using an 8-channel array head coil. The total MR acquisition time was less than one hour. Low dose midazolam and opiate analgesia were used as needed, but subjects did not receive propofol during scanning.
Structural MRI
Following a localizer scan, a high-resolution sagittal 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence with whole-brain coverage was acquired (TE/TR/TI 4.9ms/2.2s/1s, 12 degrees flip angle, image matrix 256×256×144, isotropic 1mm resolution. Next, a fluid-attenuated inversion recovery (FLAIR) sequence with T2 contrast was acquired for the detection of cerebral contusions (axial, TE/TR/TI 85ms/10s/2.5s; image matrix 320×256; FOV 220×176 mm2; 3–4mm slices without gap; in-plane-resolution 0.7mm).
1H MRS
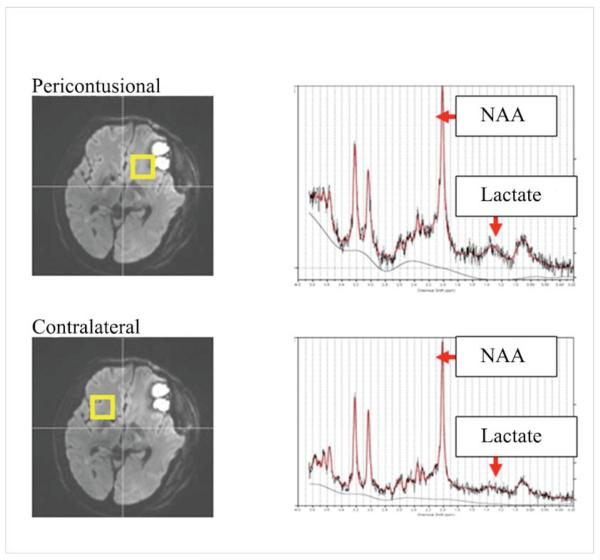
A short echo-time Point RESolved Spectroscopy (PRESS) sequence was used to localize 2 voxels (4cc each) on the MP-RAGE scans which were in areas of white matter pericontusional and contralateral to cerebral contusions. Spectra were acquired at short echo time (TE = 30ms, TR = 3s, 64 averages, 2kHz bandwidth, 2K data points), followed by the acquisition of fully-relaxed (TR = 10s) unsuppressed water FIDs at 8 different echo times (TE = 30ms to 1s).These data allowed calculation of metabolite concentrations corrected for the partial volume of cerebral spinal fluid within the voxels.15 Spectra were fitted with a customized version of the LC model program21, which models each in vivo spectrum as a linear superposition of ideal individual metabolite spectra, including those of NAA and lactate.
Perfusion MRI
A spiral-based flow-sensitive alternating inversion recovery (FAIR) sequence was used for the acquisition of cerebral blood flow (CBF) maps.22,23 The sequence parameters were TI = 1200 ms, TE = 3 ms, TR = 2 sec, 22 cm FOV, 128×128 matrix, 8 interleaves; 10 repeats; 10 5 mm slice pairs, and a 5:20 scan time. A T1 map for CBF quantification was also acquired using the spiral sequence with the same parameters except TI = 50, 100, 500, 1000, 3000, and 5000 ms; 4 interleaves; TR = 7 sec; no repeats; and a 2:48 scan time. We estimated CBF (ml/g tissue/s) using the method previously reported by.24 Regional CBF images from the FAIR studies were analyzed using Image J open source software.25 Two control subjects were scanned initially to establish a range of normal CBF. For the TBI subjects regional CBF was determined in regions of interest that were manually drawn to match the voxels from the 1H MRS scans. Pericontusional areas were assigned by drawing a 2 cm rim around the area of cerebral contusion as previously described by Steiner et al.26 (Figure 1)
Figure 1.
voxel location
All subjects underwent urine toxicology testing on admission as part of routine clinical care. This urine test detected recent use of cocaine, phencyclidine, methamphetamine, benzodiazeopines and opiates, Marijuana and Alcohol. The cutoff level of methamphetamine detected is 500 ng/ml. This typically indicates use within the past 24 to 72 hours depending on factors such as amount taken, fluid intake, and urinary pH. An effort to obtain further history regarding drug from subject's friends and family was made. However, this was not consistently available to allow stratification of subjects by severity of substance abuse. Unfortunately, it was not possible to obtain an accurate drug use history in most subjects because the subjects were severely injured and unable to communicate. Additionally, most friends and family did not know for certain the exact extent of the subject's drug use history.
Pericontusional and contralateral CBF, NAA and lactate were assessed in the entire cohort and in the METH and non-METH groups. Independent sample t-tests were used to compare group differences; statistical significance was met with p < 0.05. Statistical analysis was performed using SSPS Version 19.
Results
Twenty subjects were enrolled in the study over a period of two years, and 17 successfully completed the MR studies. The other 3 subjects remained medically unstable throughout the scanning window and did not complete the MRI protocol. Blood flow data were not available on four subjects due to technical problems, and pericontusional spectra in one subject were unacceptable due to an artifact from blood in the nearby contusion. Data are shown in Table 1
Table 1.
MRI data for all TBI subjects
| Pt | Age years | G | MET H | GCS | TTS days | pNA A mM/kg | cNA A mM/kg | pLac mM/kg | cLac mM/kg | pCBF cc/10 0g/min | cCBF cc/10 0g/min |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | Yes | 9 | 7 | 6.025 | 5.824 | 1.669 | 0.207 | - | - |
| 2 | 33 | M | Yes | 5 | 4 | 6.083 | 8.253 | 0.571 | 0.342 | - | - |
| 3 | 37 | M | No | 3 | 2 | 7.607 | 8.17 | 0.069 | 1.037 | 41 | 28 |
| 4 | 42 | M | No | 5 | 3 | 7.675 | 8.763 | 0.911 | 0.748 | 20.7 | 27.3 |
| 5 | 21 | F | Yes | 7 | 10 | 7.96 | 7.014 | 0.3 | 0.935 | 20.3 | 38 |
| 6 | 56 | F | No | 6 | 11 | - | 6.353 | - | 0.156 | 25.4 | 27.7 |
| 7 | 48 | M | Yes | 7 | 17 | 5.848 | 5.259 | 2.466 | 0.274 | 12.4 | 15.6 |
| 8 | 21 | M | No | 7 | 17 | 5.805 | 6.527 | 0.649 | 0.449 | 10.3 | 12.4 |
| 9 | 48 | M | No | 6 | 4 | 6.708 | 7.342 | 0.576 | 0.881 | 16.3 | 15.5 |
| 10 | 46 | M | No | 8 | 18 | 6.84 | 6.73 | 1.322 | 0.482 | 23.0 | 24.1 |
| 11 | 30 | F | Yes | 8 | 27 | 6.656 | 4.85 | 0.068 | 0.604 | 13.3 | 17.2 |
| 12 | 49 | F | Yes | 5 | 4 | 6.701 | 9.469 | 0.055 | 1.135 | - | - |
| 13 | 49 | F | No | 7 | 10 | 4.859 | 7.486 | 2.488 | 0.563 | - | - |
| 14 | 18 | M | No | 7 | 11 | 5.855 | 6.223 | 0 | 0.921 | 31.8 | 18.3 |
| 15 | 37 | M | No | 5 | 10 | 5.548 | 6.659 | 3.346 | 1.702 | 16.2 | 12.4 |
| 16 | 21 | M | No | 7 | 15 | 2.002 | 5.987 | 2.832 | 0.769 | 11.3 | 14 |
| 17 | 54 | M | Yes | 7 | 8 | 6.589 | 7.66 | 0.532 | 0.118 | 10.7 | 12.4 |
| Summary data | Mean 39 | M:F 12:5 | Y:N 7:10 | Mean 6.4 | Mean 10 | 5.81±2.0, 6.89±1.2 p=0.03 | 1.02±1.1, 0.67±0.42 p=0.17 | 15±12, 15±11 p= 0.72 |
GCS- Glasgow Coma Scale, G- gender, TTS – time to scan in days, p- pericontusional c- contralateral, NAA- N- acetyl-aspartate, Lac- lactate, NA –not available, p- pericontusional c- contralateral, CBF- cerebral blood flow measures in 13 subjects only, METH- positive urine toxicology for methamphetamine on admission
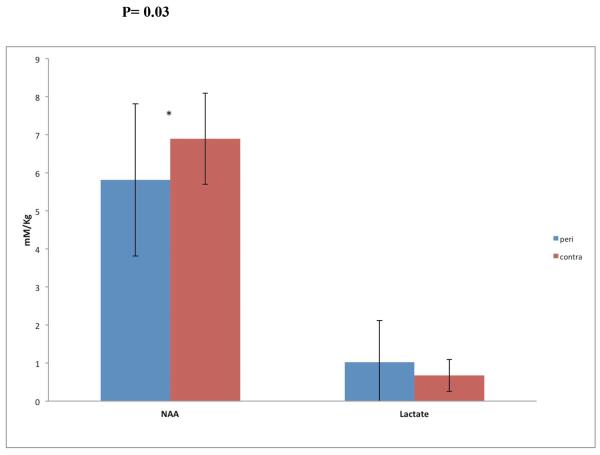
Analysis of the entire cohort of TBI patients (Table 1, Figure 2)
Figure 2.
MR spectroscopy for all TBI subjects
Pericontusional NAA concentrations (5.81 ± 2.0 mM/kg) were 12% lower compared to the contralateral NAA (6.98 ± 1.2 mM/kg; p=0.03, two-tailed t-test, d = 1.22, large effect size). Lactate concentrations were not significantly different between the two regions (d = 0.71. moderate effect size). However, a moderate effect size (d = 0.71) indicates that this non-significant result may be attributed to small sample size. Regional cerebral blood flow (rCBF) was equally reduced in the two regions compared to two control subjects who were scanned twice with 30 minutes in between studies. We found rCBF of 50 (+/− 10) cc/100g/min and 57 (+/− 8) cc/100g/min in control subject 01 and CBF of 52 (+/− 12) cc/100g/min and 54 (+/− 12) cc/100g/min in control subject 02.
Analysis of subgroups based on METH status (Table 2, Figures 3 and 4)
Table 2.
TBI patients who had METH positive versus METH negative urine tests
| All subjects | p-value | METH positive | METH negative | p-value | |
|---|---|---|---|---|---|
| Sample size (N) | 17 | 7 | 10 | ||
| Mean Age (years) | 39 (18–58) | 42 ± 14 | 38 ± 13 | 0.52 | |
| Glasgow Coma Scale | 6.4 ±1.46 | 6.8±1.5 | 6.1±1.5 | 0.31 | |
| M:F | 12:5 | 4:3 | 8:2 | ||
| Time to scan (mean) | 10 days | 11 days | 10 days | 0.79 | |
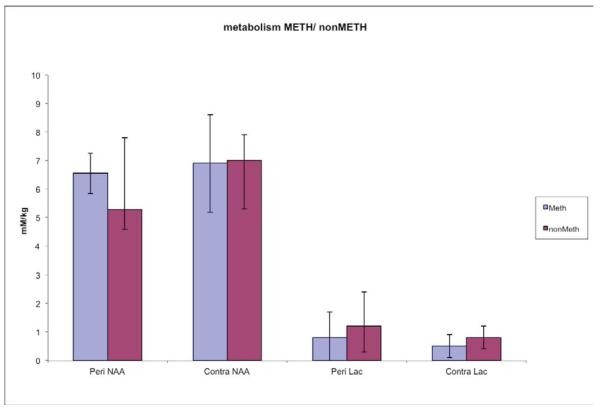
| [NAA] mM/kg | 0.03 | ||||
| Pericontusional | 5.81±2.0 | 6.55±0.7 | 5.29±2.5 | 0.21 | |
| Contralateral | 6.89±1.2 | 6.9±1.7 | 7.0±0.9 | 0.85 | |
| [Lactate]mM/kg | 0.17 | ||||
| Pericontusional | 1.02±1.1 | 0.8±0.9 | 1.2±1.2 | 0.47 | |
| Contralateral | 0.67±0.42 | 0.5±0.4 | 0.8±0.4 | 0.23 | |
| rCBF (cc/100g/min) | 0.72 | ||||
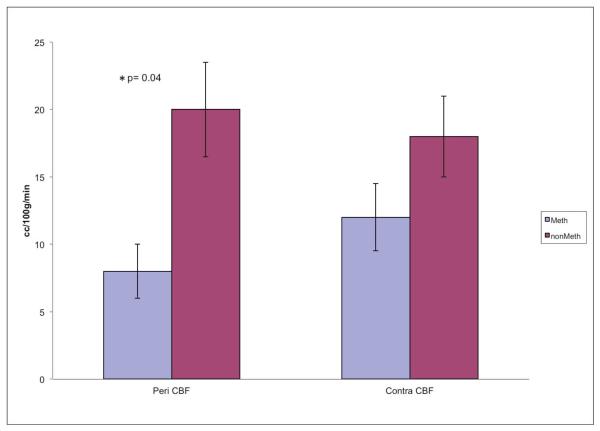
| Pericontusional | 15±12 | 8±8 | 20±12 | 0.04* | |
| Contralateral | 15±11 | 12±14 | 18±9 | 0.29 | |
NAA- N- acetyl-aspartate, CBF- cerebral blood flow, M:F- male: female METH positive- positive urine toxicology for methamphetamine on admission
statistical analysis performed on 13 subjects
Figure 3.
Figure 4.
Regional Cerebral Blood flow in Meth vs Non Meth
41% of the subjects tested positive for METH. The mean age, Glasgow Coma Scale and time to scan did not differ significantly between the subjects with or without METH use. The two subject groups also had similar regional NAA or lactate. Pericontusional blood flow was 60% lower in the METH users than the non-users, p=0.04(d = 1.18, large effect size); however, contralateral CBF did not differ between the groups(d = 0.57, moderate effect size).
Discussion
Brain metabolism and perfusion in TBI patients
Our findings validate prior reports that patients with TBI showed greater metabolic abnormalities in the pericontusional brain regions, but the perfusion changes were decreased in both the pericontusional and contralateral brain regions. In addition, the major findings of this study are that the subgroup of TBI patients who tested positive for METH displayed a different pattern of tissue metabolism and regional perfusion when compared with those who tested negative. This is potentially of great importance to regional tissue recovery after TBI through METH's effects on tissue perfusion and metabolism.
Traumatic brain injury is associated with severe alterations in tissue metabolism and blood flow. These abnormalities occur in mild as well as severe TBI, which begin at the time of injury and continue through the subsequent hours and days. 28 Tissue near areas of injury (pericontusional) often behaves differently than tissue remote from focal injury. Cerebral microdialysis, positron emission tomography (PET), and 1H MRS16,29 have been used to study pericontusional tissue and changes in brain tissue metabolism. Cerebral microdialysis has demonstrated increased lactate-pyruvate ratios in pericontusional regions indicating greater cellular distress.30 Our 1H MRS findings demonstrate that as a group, our TBI subjects showed lower pericontusional NAA concentration relative to the contralateral NAA. The reduced NAA is consistent with the current literature indicating greater neuronal dysfunction in the pericontusional regions16,31, since declines in NAA levels are observed with cell death31, as well as mitochondrial dysfunction.32 The primary role of NAA is to serve as a molecular water pump for neurons.33 Water produced by glucose metabolism is then coupled to the movement of NAA along its intracellular-extracellular gradient. Thus NAA plays an important role in cellular metabolism. However, acutely decreased NAA level in the setting of TBI reflects only the severity of tissue injury, it cannot determine the ultimate tissue outcome, since some changes in NAA may reflect transient mitochondrial dysfunction.34
Cerebral lactate is also a useful marker of energy metabolism since elevated lactate levels indicate cerebral ischemia or mitochondrial failure.29 Our TBI subjects showed only a trend for higher lactate on 1H MRS in the pericontusional brain regions compare to the contralateral region. Since we do not have normal controls for comparison, we cannot determine whether the contralateral brain region also showed higher than normal lactate. Nevertheless, the tendency for higher lactate in the pericontusional region is consistent with a prior MRS study of TBI patients, and which suggests a failure of oxidative metabolism.16
The greater metabolic abnormalities in the pericontusional regions are also consistent with 15O-PET studies that found decreased pericontusional perfusion in patients after TBI without the expected compensatory increase or augmented cerebral perfusion pressure. The abnormal perfusion response suggests that pericontusional tissue is not able to compensate for fluctuations in perfusion and is more vulnerable to ischemic secondary injury.17,26 However, in our current study, both the pericontusional and contralateral regions of our entire cohort of TBI subjects showed decreased rCBF (~15 cc/100g/ min), which is significantly lower than the normal CBF is about 54 ±12 cc/100 gm/ min.27 These findings are consistent with prior reports that describe an acute decrease in global and regional CBF after TBI, with slow normalization of CBF over time (days to weeks).28,35 The degree of decrease in CBF after TBI is variable depending on the region sampled. PET imaging has demonstrated CBF as low as 11 cc/ 100g/ min in pericontusional areas and as high as 46 cc/100g/min in remote areas with healthy controls showing 49 cc/100g/min.36
Effects of METH on TBI brain metabolism and perfusion
In a non- trauma population, methamphetamine use has been associated with changes in brain perfusion and metabolism. Magnetic resonance spectroscopy studies in METH users indicate changes in glial markers (choline and myoinositol) as well as the neuronal marker NAA.
A study in abstinent METH users showed persistently lower than normal ratios of NAA to total creatine in the anterior cingulate cortex regardless of the time since last use.30 Perfusion MRI studies in abstinent METH users also showed altered regional CBF, with decreased relative blood flow in bilateral putamen and the right parietal region, but increased relative perfusion in temporoparietal white matter and left occipital cortex.9 A study using SPECT imaging in short term and long-term abstinent METH users also showed decreased regional CBF in the right anterior cingulate cortex.37
In the METH vs. non-METH analysis our findings demonstrate that the main difference between subjects who tested positive for METH and those who tested negative was lower pericontusional CBF in the METH-positive group. Likewise, the METH subjects showed a trend for larger regional differences in CBF with the pericontusional rCBF being the lowest. However, we did not observe an alteration in tissue metabolism between METH users and non-users, either in NAA or lactate.
This disconnection between tissue perfusion and metabolism after TBI has been previously described. Several authors described findings that suggest a non ischemic etiology for the metabolic markers of cellular distress after TBI. In subjects who underwent PET scanning and simultaneous microdialysis monitoring after TBI, Vespa et al demonstrated that the metabolic markers of cellular distress were much more frequently found than true ischemia seen on quantitative PET scanning.29 More recently, Soustiel et al performed ultrasound CBF studies and AVDO2 calculations to measure CMRO2 on patients after decompressive hemicraniectomy after TBI. They demonstrated that although the decompressive craniectomy only improved the rCBF but not the metabolic dysfunction of the tissue. Therefore, direct ischemia may not be responsible for the metabolic alterations seen after TBI.38 Similarly, we may not see the expected metabolic alterations in areas of low pericontusional rCBF because changes in rCBF may not be the predominant factor driving the metabolic dysfunction.
There are several important limitations to this study. First of all, the study is underpowered . Ideally we planned to enroll 30 subjects for 90% power to detect a 10% decrease in NAA and a 10% decrease in CBF. Unfortunately, we were unable to reach our enrollment target due to the challenges of performing acute MRI studies on severely injured subjects. Also, the heterogeneity of the clinical TBI group with varying severity of injury and regions of structural brain injury as well as the diverse injury mechanisms create variability in the data particularly in such a small sample. Additionally, the test used to demonstrate METH use was a urine test which was only reported as positive or negative and could not measure a specific concentration. It is not possible to determine when the subject last used the drug or how much they had used. Additionally, because the subjects were comatose and their families often did not know the previous drug use history it was impossible to separate those with a history of METH use from those who had used the drug only once. Therefore the study can only describe an effect of acute METH use coincident with a TBI. Although the average time to MRI scanning did not differ between the METH and non-METH groups, there was considerable variability in the time of MR scanning since the TBI (range 2–27 days). Changes in tissue chemistry after TBI are quite dynamic over time, and might have contributed to the variability of findings across subjects. For instance, rCBF can be low initially with gradual recovery, and transmembrane ion fluxes, excitotoxicity and electrical depolarizations can occur for several days post injury and are dynamic over time.35,28 Therefore, we would expect that the exact tissue response or recovery at the time of MR scanning was not equivalent across subjects, which might have influenced measurements of tissue perfusion and metabolism. However, we were successful in controlling variability due to sedation, pCO2 levels and blood pressure during MR scanning. There was also no difference in the range of time between TBI and MR scanning in the METH vs. nonMETH groups.
In conclusion, this small study demonstrates that tissue metabolism is regionally heterogeneous after TBI. The pericontusional NAA, which is a marker of neuronal injury, tended to be lower than the NAA in the contralateral hemisphere. Likewise, rCBF was reduced in both hemispheres in these TBI subjects. However, when the METH cohort was compared to the nonMETH cohort rCBF was significantly reduced in the pericontusional tissue. We did not observe any regional difference in tissue metabolism in the METH cohort, which may be due to the relatively small sample sizes of the subgroups.
Acknowledgement
This work was supported by NIH R03DA24199 (I START)
Footnotes
Author Disclosure Statement No competing financial interests.
References
- 1.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langlois J, Rutland-Brown W, Thomas K. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta (GA): 2004. [Google Scholar]
- 3.Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnaja E. Incidence and lifetime cost of injuries in the United States. Inj Prev. 2006 Aug;12(4):212–8. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andelic N, Jerstad T, Sigurdardottir S, Schanke A, Sandvik L, Roe C. Effects of acute substance use and pre-injury substance abuse on traumatic brain injury severity in adults admitted to a trauma centre. Journal of Trauma Management and Outcomes. 2012;4:6. doi: 10.1186/1752-2897-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolakowsky-Hayner SA, Gourley EV, 3rd, Kreutzer JS, Marwitz JH, Cifu DX, McKinley WO. Pre-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Inj. 1999 Aug;13(8):571–581. doi: 10.1080/026990599121313. [DOI] [PubMed] [Google Scholar]
- 6.Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995 Apr;76(4):302–309. doi: 10.1016/s0003-9993(95)80654-7. [DOI] [PubMed] [Google Scholar]
- 7.Parry-Jones BL, Vaughan FL, Miles CW. Traumatic brain injury and substance misuse: a systematic review of prevalence and outcomes research (1994–2004) Neuropsychological Rehabilitation. 2006;16:537–560. doi: 10.1080/09602010500231875. [DOI] [PubMed] [Google Scholar]
- 8.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004 Jun;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002 Jun;114(2):65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 10.Villemange V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally used by humans: evidence from [11C] WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neuroscience. 1998 Jan;18(1):419–27. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra loss. Brain Research. 2000;871:259–70. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- 12.Chan P, Chen JH, Lee MH, Deng JF. Fatal and non-fatal methamphetamine intoxication in the intensive care unit. JToxicology Clinical Toxicology. 1994;32(2):147–55. doi: 10.3109/15563659409000444. [DOI] [PubMed] [Google Scholar]
- 13.Ashwal S, Holshouser BA, Shu SK, Simmons PL, Perkin RM, Tomasi LG, Knierim DS, Sheridan C, Craig K, Andrews GH, Hinshaw DB. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr Neurol. 2000 Aug;23(2):114–125. doi: 10.1016/s0887-8994(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 14.Marino S, Zei E, Battaglini M, Vittori C, Buscalferri A, Bramanti P, Federico A, De Stefano N. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 2006 May;78(5):501–7. doi: 10.1136/jnnp.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross BD, Ernst T, Kreis R, Haseler LJ, Bayer S, Danielsen E, Blüml S, Shonk T, Mandigo JC, Caton W, Clark C, Jensen SW, Lehman NL, Arcinue E, Pudenz R, Shelden CH. 1H MRS in acute traumatic brain injury. J Magn Reson Imaging. 1998;8(4):829–840. doi: 10.1002/jmri.1880080412. [DOI] [PubMed] [Google Scholar]
- 16.Schuhmann MU, Stiller D, Skardelly M, Bernarding J, Klinge PM, Samii A, Samii M, Brinker T. Metabolic changes in the vicinity of brain contusions: a proton magnetic resonance spectroscopy and histology study. J Neurotrauma. 2003 Aug;20(8):725–743. doi: 10.1089/089771503767869962. [DOI] [PubMed] [Google Scholar]
- 17.Hattori N, Huang SC, Wu HM, Liao W, Glenn TC, Vespa PM, Phelps ME, Hovda DA, Bergsneider M. PET investigation of post-traumatic cerebral blood volume and blood flow. Acta Neurochir Suppl. 2003;86:49–52. doi: 10.1007/978-3-7091-0651-8_11. [DOI] [PubMed] [Google Scholar]
- 18.Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci. 1996 Oct;801:187–98. doi: 10.1111/j.1749-6632.1996.tb17441.x. [DOI] [PubMed] [Google Scholar]
- 19.Krimer LS, Muly EC, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nature Neuroscience. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- 20.Brain Trauma Foundation & American Association of Neurological Surgeons. Joint Section on Neurotrauma and Critical Care . Management and Prognosis of Severe Traumatic Brain Injury (Part I and II) 2000. p. 286. [Google Scholar]
- 21.Provencher S. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001 Jun;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 22.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995 Sep;34(3):293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Gu H, Zhan W, Xu S, Silbersweig DA, Stern E. Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: characterization of functional contrast and susceptibility artifacts. Magn Reson Med. 2002;48:278–289. doi: 10.1002/mrm.10196. [DOI] [PubMed] [Google Scholar]
- 24.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998 Sep;40(3):383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 25.Rasband R, ImageJ WS. U.S. National Institutes of Hwealth. Bethesda, Maryland, USA: 1997–2011. http://imagej.nih.gov/ij/ [Google Scholar]
- 26.Steiner LA, Coles JP, Johnston AJ, Czosnyka M, Fryer TD, Smielewski P, Chatfield DA, Salvador R, Aigbirhio FI, Clark JC, Menon DK, Pickard JD. Responses of posttraumatic pericontusional cerebral blood flow and blood volume to an increase in cerebral perfusion pressure. J Cereb Blood Flow Metab. 2003 Nov;23(11):1371–1377. doi: 10.1097/01.WCB.0000090861.67713.10. [DOI] [PubMed] [Google Scholar]
- 27.Kety SS, Schmidt CF. The determination of cerebral blood flow in man by use of nitrous oxide in low concentrations. American J of Physiology. 1945;143:53–66. [Google Scholar]
- 28.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athletic training. 2001 Jul-Sep;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 29.Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005 Jun;25(6):763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005 Apr;62(4):444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 31.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993 Mar;13(3):981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dautry C, Vaufrey F, Brouillet E, Bizat N, Henry PG, Condé F, Bloch G, Hantraye P. Early N-acetylaspartate depletion is a marker of neuronal dysfunction in rats and primates chronically treated with the mitochondrial toxin 3-nitropropionic acid. J Cereb Blood Flow Metab. 2000 May;20(5):789–799. doi: 10.1097/00004647-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003 Jun;28(6):941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- 34.Xu S, Zhou J, Racz J, Shi D, Roys S, Fiskum G, Gullapalli R. Early Microstructural and Metabolic changes following Controlled Cortical Impact Injury in Rat: A Magnetic Resonance Imaging and Spectroscopy Study. Journal of Neurotrauma. 2011 Oct;28:2091–2102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997 Feb;86(2):241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 36.Hattori N, Huang SC, Wu HM, Liao W, Glenn TC, Vespa PM, Phelps ME, Hovda DA, Bergsneider M. Acute Changes in Regional Cerebral F-FDG kinetics in Patients with Traumatic Brain Injury. J of Nuclear Medicine. 2004 May;45:775–783. [PubMed] [Google Scholar]
- 37.Hwang J, Lyoo IK, Kim SJ, Sung YH, Bae S, Cho SN, Lee HY, Lee DS, Renshaw PF. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug Alcohol Depend. 2006 Apr;82(2):177–181. doi: 10.1016/j.drugalcdep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Soustiel JF, Sviri GE, Mahamid E, Shik V, Abeshaus S, Zaaroor M. Cerebral Blood Flow and Metabolism Following Decompressive Craniectomy for Control of Increased Intracranial Pressure. Neurosurgery. 2010 Jul;67(1):65–72. doi: 10.1227/01.NEU.0000370604.30037.F5. [DOI] [PubMed] [Google Scholar]