Abstract
Background
The Trial to Assess Chelation Therapy (TACT) showed clinical benefit of an ethylene diamine tetraacetic acid (EDTA-based) infusion regimen in patients 50 years or older with prior myocardial infarction (MI). Diabetes prior to enrollment was a pre-specified subgroup.
Methods and Results
Patients received 40 infusions of EDTA chelation or placebo. 633 (37%) had diabetes (322 EDTA, 311 placebo). EDTA reduced the primary endpoint (death, reinfarction, stroke, coronary revascularization, or hospitalization for angina) [25% vs 38%, hazard ratio (HR) 0.59, 95% confidence interval (CI) (0.44, 0.79), p<0.001] over 5 years. The result remained significant after Bonferroni adjustment for multiple subgroups (99.4% CI (0.39, 0.88), adjusted p=0.002). All-cause mortality was reduced by EDTA chelation [10% vs 16%, HR 0.57, 95% CI (0.36, 0.88) p=0.011], as was the secondary endpoint (cardiovascular death, reinfarction, or stroke) [11% vs 17% HR 0.60, 95% CI (0.39, 0.91), p=0.017]. After adjusting for multiple subgroups, however, those results were no longer significant. The number needed to treat to reduce one primary endpoint was 6.5 over 5 years (95% CI (4.4, 12.7). There was no reduction in events in non-diabetics (n=1075, p=0.877), resulting in a treatment by diabetes interaction (p=0.004).
Conclusions
Post-MI diabetic patients age 50 or older demonstrated a marked reduction in cardiovascular events with EDTA chelation. These findings support efforts to replicate these findings and define the mechanisms of benefit. They do not, however, constitute sufficient evidence to indicate the routine use of chelation therapy for all post-MI diabetic patients.
Keywords: myocardial Infarction, diabetes mellitus, secondary prevention
For over 50 years, EDTA-based chelation therapy has been used by practitioners to treat complications of atherosclerosis, without a robust evidence base, and with increasing controversy.1,2,3 The Trial to Assess Chelation Therapy (TACT), developed in response to a Request for Proposals4 by the National Center for Complementary and Alternative Medicine and the National Heart, Lung, and Blood Institute, was designed as a pivotal trial of disodium EDTA chelation therapy for patients who had experienced a myocardial infarction (MI). EDTA chelation therapy was found to offer a modest, but significant, reduction in the primary composite cardiovascular endpoint.5 As part of the prospective analysis plan6, the presence of diabetes prior to enrollment was pre-specified for subgroup analysis.
Our initial report of TACT included the observation that there was an interaction between EDTA treatment and a self-reported history of diabetes5. EDTA is a potent metal chelator.7 Therefore, our preliminary observations were consistent with research supporting an important role for metal-catalyzed oxidation reactions in the development of advanced glycation end-products8, mediators of complications of diabetes. The present report provides greater detail on the effect of EDTA- based chelation therapy on diabetic patients who have had a prior MI.
Methods
The detailed methodology of TACT has been published.5 TACT was a double-blind 2X2 factorial trial in which patients (1708) were randomized to receive 40 infusions of disodium EDTA chelation or placebo and additionally to an oral high-dose vitamin and mineral regimen or oral placebo. This report describes the results of EDTA chelation versus placebo in a prespecified subgroup of patients with diabetes mellitus.
Study Population
Patients were at least 50 years old and had a history of MI at least 6 weeks prior to enrollment. Major exclusion criteria were: women of childbearing potential, a creatinine level greater than 176.8 μmol/L (2.0 mg/dL), platelet count less than 100,000/μL, abnormal liver function studies, blood pressure greater than 160/100 mm Hg, past intolerance to the chelation or vitamin components, chelation therapy within 5 years, or revascularization within 6 months. The study enrolled 1708 patients in 134 sites across the USA and Canada (Figure 1). The median duration of follow-up was 55 months. The institutional review board at each clinical site approved the study, and patients provided written informed consent. A Data and Safety Monitoring Board (DSMB) monitored the study.
Figure 1. Consort Diagram.
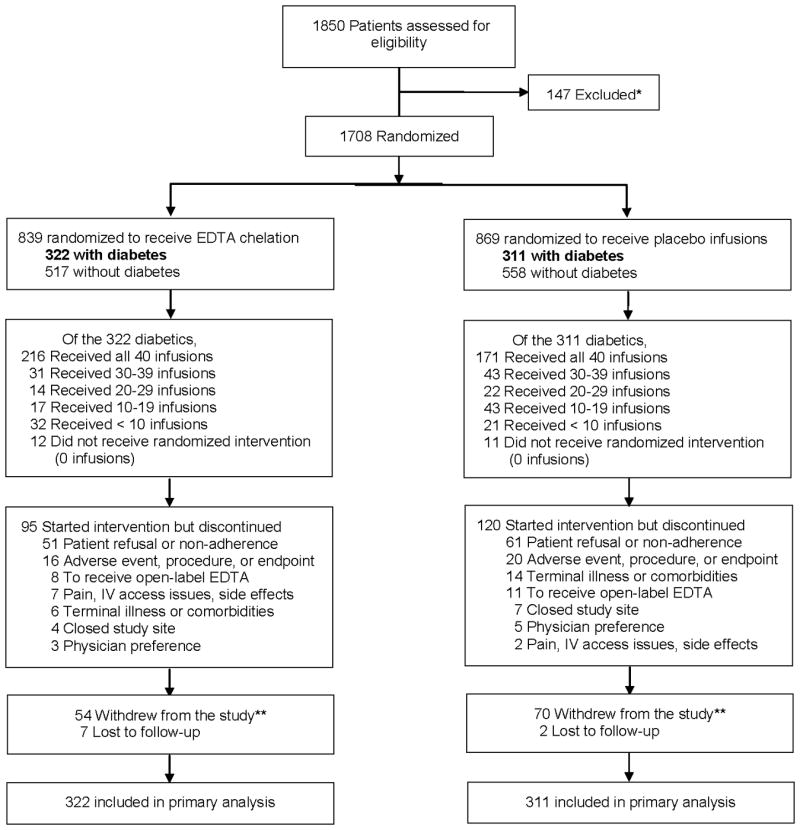
* Screened patients not randomized due to inclusion/exclusion criteria, unwillingness to participate, or other reasons ** Among patients who withdrew from the study or were lost to follow-up, 18 met the primary endpoint prior to withdrawal or becoming lost. Among the patients who had not experienced an event prior to withdrawal or becoming lost, 6 were found through search of death registries to have died. All of these events were included in the primary endpoint analysis
Diabetes Definition
Our prior report demonstrating a significant interaction (p=0.02) of EDTA therapy with the diagnosis of diabetes was based on patients' self-reported diagnosis of diabetes, present in 538 (31.5%) cases.5 The present analyses broadened the definition of diabetes to be more consistent with current guidelines9. Thus, patients included in the present diabetes subgroup had self-reported diabetes, or were taking oral or insulin treatment for diabetes, or had a fasting blood glucose of 6.99mmol/L (126 mg/dL) or greater at the time of enrollment in the study. This led to 633 (37.1%) patients with a diagnosis of diabetes eligible for analysis. The expansion of the diabetes definition was approved by the TACT Operations Group prior to performing the resulting analyses. Results are also provided, however, for the previously defined group of 538 patients (eTables 1-3).
Treatment
The 10 component 500 mL intravenous solution in TACT consisted of 3 g of disodium EDTA, adjusted downward based on estimated glomerular filtration rate; 7 g of ascorbic acid; 2 g of magnesium chloride; B-vitamins, and other components (eTable 4). The placebo solution consisted of 500 mL of normal saline and 1.2% dextrose (2.5 g total). The solution was infused over at least 3 hours through a peripheral intravenous line weekly for 30 weeks and then biweekly to bimonthly to complete 40 infusions.
All patients in the trial received a low-dose vitamin and mineral regimen daily while receiving infusions, in order to prevent depletion by the chelation regimen.9 Evidence-based post-MI therapy was encouraged and monitored by the Coordinating Centers.
Follow up
Patients were seen at the baseline visit and at each infusion visit. Once patients completed the infusion phase, they were followed via quarterly telephone calls, annual clinic visits, and a final visit at the 5-year follow-up or at the end of the study which ever came first. Laboratory evaluations included fasting blood glucose levels at baseline and throughout the infusion phase of the trial, and fasting lipids at baseline and prior to infusion #30.
End Points
The primary endpoint was a composite of death from any cause, reinfarction, stroke, coronary revascularization, or hospitalization for angina. The principal secondary endpoint consisted of a composite of cardiovascular death, reinfarction, or stroke. All end-point events were reviewed and adjudicated by a clinical events committee blinded to the randomized treatment assignment.
Statistical Analysis
Secure web-based permuted block randomization was stratified by clinical site (diabetes was not a stratification factor). Baseline characteristics of patients were descriptively summarized using the median and interquartile range for continuous variables and frequencies and percentages for categorical variables. The characteristics of patients with diabetes were compared to those of the patients without diabetes using the Wilcoxon rank-sum test for continuous variables and the conventional chi-square test for categorical variables. The Wilcoxon test was also used for comparing treatment groups with respect to the change in fasting blood glucose from baseline to the last infusion measurement. The log-rank test was used for comparing diabetics versus non-diabetics and the chelation versus placebo treatment arms with respect to the primary and secondary clinical outcomes. Although patients could experience more than 1 component of the composite primary and secondary end points, each patient was counted only once in treatment comparison of these endpoints using the time until the occurrence of their first event. All treatment comparisons were performed using two-sided significance tests, and included all patients in the treatment group to which they were randomized (intention to treat). Cumulative event rates were calculated according to the Kaplan-Meier method10. Relative risks were expressed as hazard ratios (HRs) with associated confidence intervals and were calculated using the Cox proportional hazards model11. The Cox model was also used for assessing a treatment by diabetes interaction. Although nominal p-values for treatment comparisons are reported, conservative Bonferroni12 adjusted confidence intervals and p-values, adjusted for 9 different subgroup factors, are also reported. Consistent with the overall study report 5, statistical significance for comparisons of the primary endpoint was defined as p<0.036. For other comparisons, significance was defined as p<0.05. Number needed to treat summaries with associated confidence intervals were calculated using the inverse of the absolute risk reduction in 5-year Kaplan-Meier event rates. Final statistical analyses were performed using SAS software, versions 8.2 and 9.2 (SAS Institute Inc).
Sensitivity analyses
To assess the robustness of study findings in the face of patients that withdrew consent or were lost to follow-up, post hoc sensitivity analyses were performed with imputation of missing outcome data, as previously published6. The event rates among patients that withdrew or were lost to follow-up in each treatment group were varied across a broad spectrum and included scenarios that were markedly unfavorable to chelation. These imputed event rates were combined with the observed event rates to assess the treatment effect and the robustness of the findings in the treatment group comparisons.
Results
A total of 1708 patients were enrolled in TACT, of which 633 (37.1%) had diabetes according to the expanded definition.
Baseline characteristics of patients with and without diabetes
Compared with non-diabetic patients, fasting blood sugar and BMI were higher in patients with diabetes (Table 1). Diabetic patients also had a higher prevalence of congestive heart failure, stroke, hypertension and hypercholesterolemia than did non-diabetic patients. There was a particularly high prevalence of peripheral artery disease in diabetic compared with non-diabetic patients. The proportion of patients who had undergone a coronary revascularization procedure (either coronary artery bypass or percutaneous coronary intervention) procedure was over 80% and similar in the two groups. Diabetic patients were treated more aggressively with blockade of the renin-angiotensin system (73 vs 58%, p<0.001) and beta-blockers (75 vs 70%, p=0.012) than patients without diabetes. Diabetic patients had a lower fasting LDL-cholesterol than non-diabetic patients but lower HDL at study enrollment. (Table 1)
Table 1. Baseline Characteristics of Patients With or Without Diabetes.
| Diabetes (N=633) |
No Diabetes (N=1075) |
P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 65.4 (59.7, 71.3) | 65.2 (58.7, 72.5) | 0.784 |
| Female | 119 (19%) | 180 (17%) | 0.280 |
| Minority (Hispanic or non-Caucasian) | 68 (11%) | 88 (8%) | 0.077 |
| BMI (kg/m2) | 31.8 (28.0, 36.0) | 28.8 (25.9, 32.3) | <0.001 |
| History | |||
| Time from qualifying MI to randomization (years)* | 4.5 (1.5, 9.2) | 4.6 (1.8, 9.2) | 0.467 |
| Anterior MI | 239 (38%) | 435 (40%) | 0.269 |
| Congestive heart failure | 145 (23%) | 162 (15%) | < 0.001 |
| Valvular heart disease | 68 (11%) | 107 (10%) | 0.570 |
| Stroke | 51 (8%) | 60 (6%) | 0.045 |
| Peripheral vascular disease | 136 (22%) | 132 (12%) | < 0.001 |
| Hypertension | 494 (78%) | 675 (63%) | < 0.001 |
| Hypercholesterolemia | 528 (85%) | 842 (80%) | 0.013 |
| Atrial fibrillation | 85 (14%) | 110 (11%) | 0.041 |
| Former cigarette smoker | 354 (56%) | 601 (56%) | 0.994 |
| Coronary revascularization | |||
| CABG | 313 (49%) | 461 (43%) | 0.008 |
| PCI | 353 (56%) | 654 (61%) | 0.040 |
| Either CABG or PCI | 515 (81%) | 899 (84%) | 0.230 |
| Presenting Characteristics | |||
| Blood Pressure | |||
| Systolic | 130 (120, 140) | 130 (118, 140) | 0.094 |
| Diastolic | 74 (68, 80) | 77 (70, 81) | 0.001 |
| Concomitant Medications | |||
| Aspirin | 531 (84%) | 896 (83%) | 0.772 |
| Beta-blocker | 477 (75%) | 749 (70%) | 0.012 |
| Statin | 479 (76%) | 769 (72%) | 0.063 |
| ACE or ARB | 460 (73%) | 624 (58%) | < 0.001 |
| Clopidogrel | 161 (27%) | 264 (25%) | 0.642 |
| Warfarin | 65 (11%) | 83 (8%) | 0.070 |
| Aspirin, warfarin or clopidogrel | 582 (92%) | 970 (91%) | 0.202 |
| Diabetes medication | |||
| Insulin | 160 (26%) | 0 (0%) | < 0.001 |
| Oral hypoglycemic | 380 (61%) | 0 (0%) | < 0.001 |
| Multivitamin | 242 (40%) | 473 (45%) | 0.026 |
| Other vitamins/minerals | 292 (47%) | 560 (53%) | 0.020 |
| Herbal products | 190 (31%) | 370 (36%) | 0.088 |
| Laboratory Examinations | |||
| Fasting Glucose (mmol/L) | 7.3 (6, 9) | 5.4 (5, 5.8) | <0.001 |
| Creatinine (μmol/L) | 97.2 (79.6, 114.9) | 97.2 (79.6, 106.1) | 0.030 |
| Total cholesterol (mmol/L) | 4.2 (3.6, 5) | 4.3 (3.7, 5.1) | 0.047 |
| HDL (mmol/L) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.3) | <0.001 |
| LDL (mmol/L) | 2.1 (1.6, 2.9) | 2.3 (1.8, 3) | <0.001 |
| Triglycerides (mmol/L) | 1.7 (1.2, 2.6) | 1.5 (1, 2.1) | <0.001 |
Median, 25th and 75th percentiles are reported for all continuous variables.
BMI = Body Mass Index, MI = Myocardial Infarcction, CABG = Coronary Artery Bypass Graft, PCI = Percutaneous Coronary Intervention, ACEi = Angiotensin Converting Enzyme inhibitor, ARB = Angiotensin Receptor Blocker, HLD = High density Lipoprotein; LDL = Low density Lipoprotein.
Outcome events by diabetes status
When compared with non-diabetic patients, patients with diabetes were more likely to experience the primary endpoint [197 (31%) vs 286 (27%), log-rank p=0.009], the secondary endpoint [87 (14%) vs 122 (11%) p=0.057], and death from any cause [82 (13%) vs 98 (9%), log-rank p = 0.003].
Baseline characteristics of patients with diabetes by infusion arm
Among patients with diabetes, 322 were randomized to receive the EDTA chelation-based infusion regimen and 311 received placebo infusions. Baseline characteristics were similar between the treatment groups (Table 2).
Table 2. Baseline Characteristics of Patients With Diabetes by Infusion Arm.
| EDTA Chelation (N=322) |
Placebo (N=311) |
P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 65.1 (60.3, 71.1) | 66.2 (58.8, 71.5) | 0.843 |
| Female | 55 (17%) | 64 (21%) | 0.260 |
| Minority (Hispanic or non-Caucasian) | 31 (10%) | 37 (12%) | 0.357 |
| BMI (kg/m2) | 31.1 (27.9, 35.9) | 32.1 (28.4, 36.4) | 0.208 |
| History | |||
| Time from qualifying MI to randomization (years)* | 4.2 (1.6, 8.8) | 5.1 (1.4, 9.5) | 0.457 |
| Anterior MI | 128 (40%) | 111 (36%) | 0.292 |
| Congestive heart failure | 76 (24%) | 69 (22%) | 0.672 |
| Valvular heart disease | 34 11%) | 34 (11%) | 0.821 |
| Stroke | 26 (8%) | 25 (8%) | 0.987 |
| Peripheral vascular disease | 69 (22%) | 67 (22%) | 0.954 |
| Hypertension | 251 (78%) | 243 (78%) | 0.955 |
| Hypercholesterolemia | 273 (86%) | 255 (84%) | 0.436 |
| Atrial fibrillation | 36 (12%) | 49 (16%) | 0.086 |
| Former cigarette smoker | 181 (56%) | 173 (56%) | 0.882 |
| Coronary revascularization | |||
| CABG | 163 (51%) | 150 (48%) | 0.548 |
| PCI | 187 (58%) | 166 (53%) | 0.234 |
| Either CABG or PCI | 271 (84%) | 244 (78%) | 0.065 |
| Presenting Characteristics | |||
| Blood Pressure | |||
| Systolic | 130 (120, 140) | 130 (120, 140) | 0.681 |
| Diastolic | 74 (68, 80) | 74 (68, 80) | 0.937 |
| Concomitant Medications | |||
| Aspirin | 278 (86%) | 253 (81%) | 0.088 |
| Beta-blocker | 248 (77%) | 229 (74%) | 0.323 |
| Statin | 247 (77%) | 232 (75%) | 0.536 |
| ACEi or ARB | 234 (73%) | 226 (73%) | 1.000 |
| Clopidogrel | 86 (28%) | 75 (25%) | 0.550 |
| Warfarin | 34 (11%) | 31 (11%) | 0.845 |
| Aspirin, warfarin or clopidogrel | 296 (93%) | 286 (92%) | 0.909 |
| Diabetes medication | |||
| Insulin | 73 (23%) | 87 (29%) | 0.114 |
| Oral hypoglycemic | 191 (60%) | 189 (63%) | 0.585 |
| Multivitamin | 115 (37%) | 127 (43%) | 0.112 |
| Other vitamins/minerals | 142 (45%) | 150 (51%) | 0.147 |
| Herbal products | 94 (30%) | 96 (33%) | 0.419 |
| Laboratory Examinations | |||
| Fasting Glucose (mmol/L) | 7.1 (5.9, 8.9) | 7.4 (6, 9.1) | 0.167 |
| Creatinine (μmol/L) | 88.4 (79.6, 114.9) | 97.2 (79.6, 114.9) | 0.019 |
| Total cholesterol (mmol/L) | 4.1 (3.5, 4.9) | 4.3 (3.6, 5.2) | 0.037 |
| HDL (mmol/L) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) | 0.553 |
| LDL (mmol/L) | 2.1 (1.6, 2.7) | 2.2 (1.6, 3) | 0.111 |
| Triglycerides (mmol/L) | 1.7 (1.2, 2.5) | 1.8 (1.2, 2.7) | 0.299 |
Median, 25th and 75th percentiles are reported for all continuous variables.
BMI = Body Mass Index, MI = Myocardial Infarcction, CABG = Coronary Artery Bypass Graft, PCI = Percutaneous Coronary Intervention, ACEi = Angiotensin Converting Enzyme inhibitor, ARB = Angiotensin Receptor Blocker, HLD = High density Lipoprotein; LDL = Low density Lipoprotein.
Fasting glucose and diabetes medications during follow up
There was no EDTA-treatment-based difference in fasting blood glucose from baseline to last infusion requiring a blood draw (chelation glucose change from baseline to last followup: 1.0 mg/dL (-29, 24), placebo 1.5 mg/dL (-23, 25); p=0.64). Among patients with diabetes who completed 30 infusions and had paired medication data regarding insulin status at the pre-randomization visit and at the 30th infusion (n=429), 101 (23.5%) were receiving insulin for diabetes management at baseline, compared with 100(23.3%) at the 30th infusion. Among the 438 patients with paired data regarding oral hypoglycemic status, 282 (64.3%) were taking oral hypoglycemics at baseline, compared with 272 (62.1%) at the 30th infusion. These numbers, which reflect minimal changes in medications for diabetes, were consistent in the two treatment arms.
Outcome events in patients with diabetes by infusion group
The incidence of the primary endpoint over an extended follow-up of nearly 5 years was significantly lower in the EDTA chelation group, compared with placebo [HR 0.59, 95% CI (0.44,0.79), p<0.001] with a 15% absolute decrease in the 5-year Kaplan-Meier primary event rate (Figure 2a, Table 3) and a relative reduction of 41%. The result remained significant after Bonferroni adjustment for multiple subgroups (99.4% CI (0.39, 0.88), adjusted p=0.002). The number needed to treat to prevent a single event was 6.5 patients over 5 years (95% CI 4.4, 12.7). Rates of the secondary endpoint in diabetic patients were also lower for patients randomized to EDTA chelation, [HR 0.60 (0.39, 0.91), p=0.017], with a 5.1% absolute decrease in the 5-year Kaplan-Meier event rate and a relative reduction of 40% (Figure 3a). This result was not significant, however, after adjusting for multiple subgroups [99.4% CI (0.32, 1.09), adjusted p=0.153]. In contrast to the treatment effect observed in patients with diabetes, patients without diabetes (n=1075) did not have a treatment effect with regards to the primary endpoint (HR 1.02, 95% CI (0.81,1.28), p=0.877 or the secondary endpoint (HR 1.06, 95% CI (0.74, 1.50) (Figure 2b, Figure 3b, Table 4). There was a significant interaction between diagnosis of diabetes and EDTA treatment (p for interaction for the primary endpoint = 0.0037).
Figure 2a.
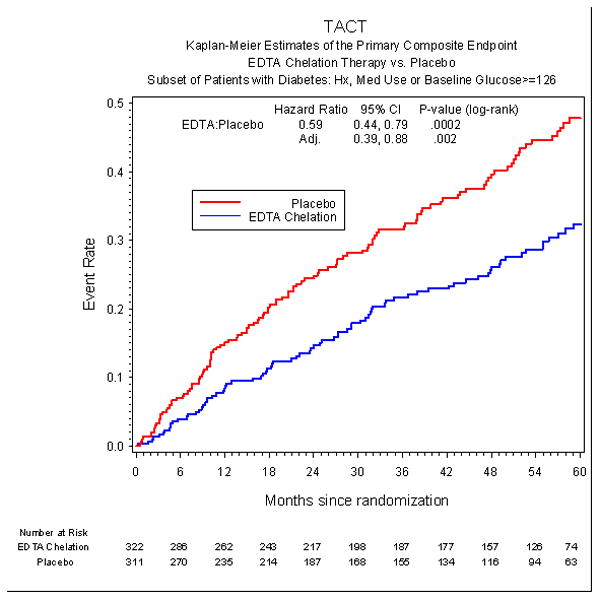
Primary endpoint in diabetic patients.
Table 3. Clinical End Points by Infusion Arms for Diabetic Patients.
| EDTA | Adjusted* | |||||
|---|---|---|---|---|---|---|
| Endpoint | Chelation (N=322) |
Placebo (N=311) |
Hazard Ratio (95% CI) |
P Value | CI | P-value |
| Primary Endpoint | 80 (25%) | 117 (38%) | 0.59 (0.44, 0.79) | <0.001 | (0.39, 0.88) | 0.002 |
| Death | 32 (10%) | 50 (16%) | 0.57 (0.36, 0.88) | 0.011 | (0.30, 1.06) | 0.099 |
| MI | 16 (5%) | 30 (10%) | 0.48 (0.26, 0.88) | 0.015 | (0.20, 1.13) | 0.135 |
| Stroke | 4 (1%) | 3 (1%) | 1.19 (0.27, 5.30) | 0.829 | (0.14, 9.88) | -- |
| Coronary revascularization | 48 (15%) | 62 (20%) | 0.68 (0.48, 0.99) | 0.042 | (0.40, 1.16) | 0.378 |
| Hospitalization for angina | 5 (2%) | 6 (2%) | 0.72 (0.22, 2.36) | 0.588 | (0.13, 3.87) | -- |
| Secondary Endpoint | 35 (11%) | 52 (17%) | 0.60 (0.39, 0.91) | 0.017 | (0.32, 1.09) | 0.153 |
| Cardiovascular Death | 19 (6%) | 27 (9%) | 0.63 (0.35, 1.13) | 0.118 | (0.27, 1.44) | -- |
Bonferroni adjustment for 9 subgroup factors. Confidence intervals are adjusted to a level of 99.4%, and p-value is 9 times the nominal p-value.
Figure 3a.
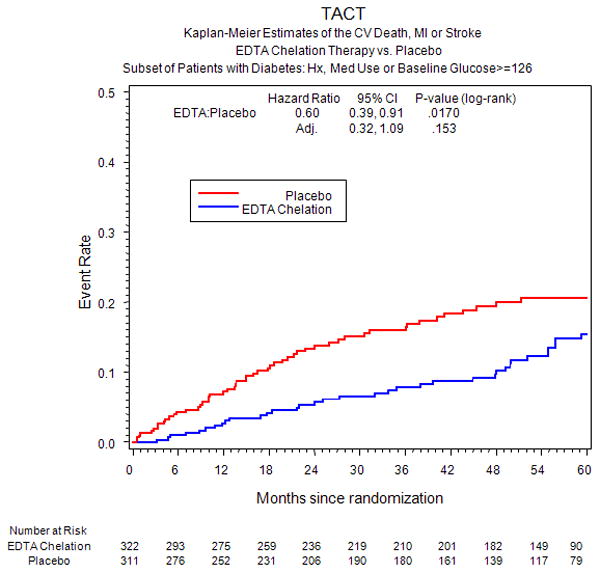
Secondary endpoint in diabetic patients.
Figure 2b.
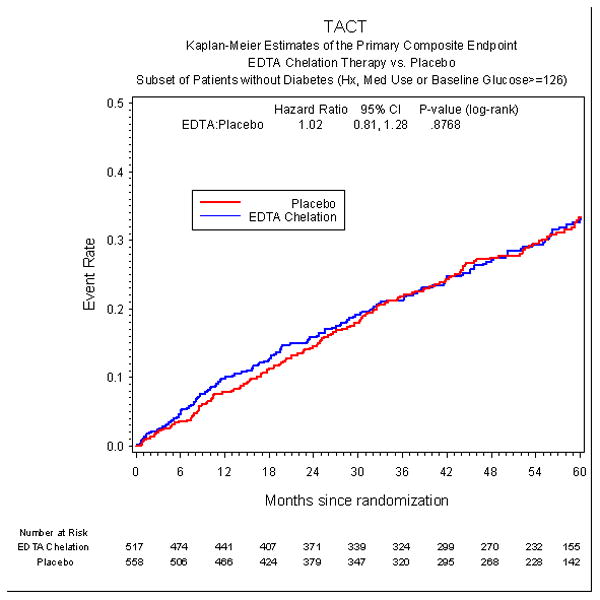
Primary endpoint in non-diabetic patients.
Figure 3b.
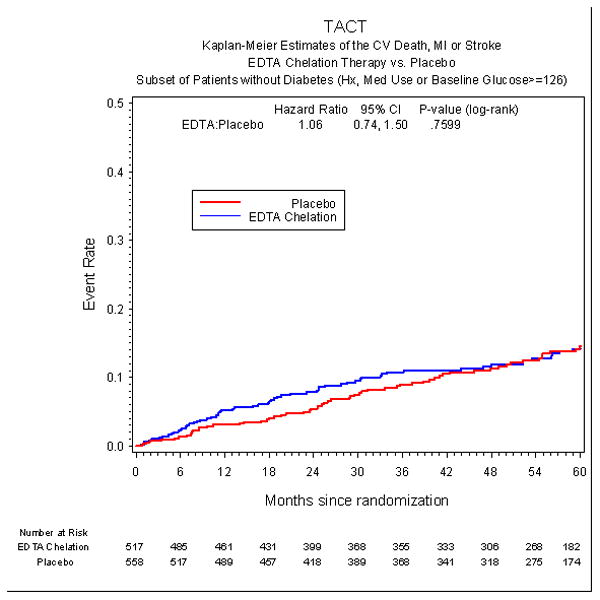
Secondary endpoint in non-diabetic patients.
Table 4. Clinical End Points by Infusion Arms for Non-Diabetic Patients.
| Endpoint | EDTA Chelation (N= 517) |
Placebo (N= 558) |
Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| Primary Endpoint | 142 (27%) | 144 (26%) | 1.02 (0.81, 1.28) | 0.877 |
| Death | 55 (11%) | 43 (8%) | 1.35 (0.90, 2.01) | 0.137 |
| MI | 36 (7%) | 37 (7%) | 1.03 (0.65, 1.64) | 0.872 |
| Stroke | 6 (1%) | 10 (2%) | 0.65 (0.24, 1.80) | 0.406 |
| Coronary revascularization | 82 (16%) | 95 (17%) | 0.90 (0.67, 1.21) | 0.474 |
| Hospitalization for angina | 8 (2%) | 12 (2%) | 0.71 (0.29, 1.74) | 0.440 |
| Secondary Endpoint | 61 (12%) | 61 (11%) | 1.06 (0.74, 1.50) | 0.760 |
| Cardiovascular Death | 31 (6%) | 24 (4%) | 1.37 (0.81, 2.34) | 0.239 |
Patients with diabetes randomized to EDTA chelation had a significant reduction in recurrent myocardial infarction (HR 0.48 (0.26, 0.88), p=0.015) (Figure 4a), in all-cause mortality (HR 0.57 (0.36, 0.88), p=0.011)(Figure 4b), and in coronary revascularizations (HR 0.68 (0.47, 0.99), p=0.042). However, after applying the Bonferroni adjustment to these results, they no longer met the criterion for significance. We also analyzed whether diabetic patients randomized in chelation sites were more likely to demonstrate a therapeutic benefit of EDTA chelation than patients randomized in conventional sites. The results show the opposite to be the case (eFigure 1).
Figure 4a.
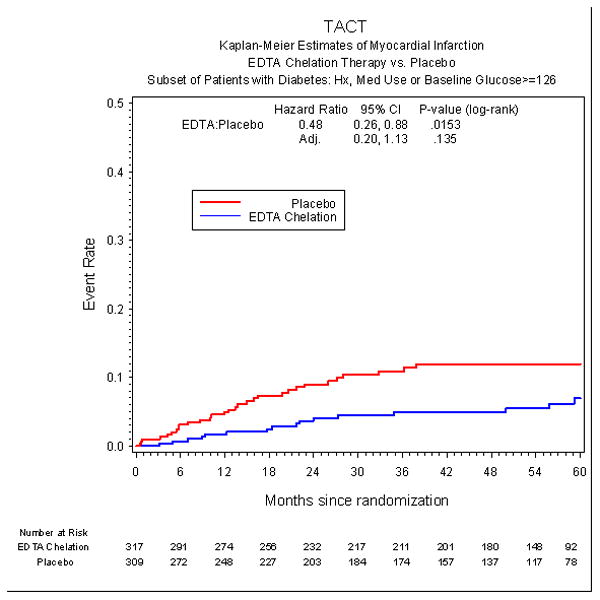
Myocardial Infarction in diabetic patients by infusion group.
Figure 4b.

Mortality in diabetic patients by infusion group.
Treatment adherence
Among the subgroup with diabetes, the median number of infusions received was 40 (25, 40); 73% completed 30 infusions, and 61% completed 40 infusions; 34% discontinued study infusions (n=120 (39%) in the placebo group, and n=95 (30%) in the chelation group).
Safety
There were 95 serious adverse events (non-endpoint events) in the diabetic population (56 placebo, 39 active). Adverse events attributable to the study medication led 5.7% to withdraw from the trial (20 placebo, 16 active).
Sensitivity analyses
As a sensitivity analysis, we assessed the baseline characteristics of the subgroup of patients who withdrew consent (eTable 5). We then assessed how the primary treatment comparison in the subgroup of patients with diabetes would be affected under a variety of assumptions regarding the occurrence of primary endpoint events among the patients who withdrew consent or were lost to follow-up and did not have an endpoint event prior to exiting the study (106 consent withdrawals, 9 lost to follow-up; eTable 6). To assess robustness of the results, these analyses focused on scenarios in which events among withdrawn or lost patients in the active arm were assumed to occur at a higher rate than withdrawn or lost patients in the placebo arm. For all realistic scenarios, the comparison of the two arms remained highly significant even if the relative increase of events among patients in the active arm who withdrew or were lost was as much as 100% higher than among withdrawn or lost patients in the placebo arm. The hazard ratio for all scenarios was in the range of 0.60 to 0.80, the p-values were very robust, and significance of the treatment effect was maintained, even for imputation scenarios that were very unfavorable to the EDTA chelation arm. Finally, we reported the small number of missing values for baseline characteristics in the overall (eTable 7) and the population with diabetes (eTable 8).
Discussion
The present study of EDTA-based chelation therapy in patients with diabetes and a prior myocardial infarction demonstrates a 41% (p<0.001) relative reduction in the risk of a combined cardiovascular endpoint; a reduction in risk of the composite of cardiovascular mortality, non-fatal stroke, or non-fatal myocardial infarction of 40% (p=0.017); a 52% reduction in recurrent myocardial infarction (p=0.015); and a reduction in death from any cause of 43% (p=0.011). These findings, if replicable, would have an impact on the health of patients with diabetes. We emphasize, however, that these results are based on a subgroup of the overall trial, albeit prespecified, and therefore must be interpreted with caution. Although there was a significant interaction of treatment with diabetes status, we have provided conservatively adjusted confidence intervals and p-values to account for the multiplicity of pre-specified subgroups. Even with adjustment, however, the effect of EDTA-chelation therapy in reducing the primary composite endpoint is highly significant. While the Bonferroni adjusted results for the components of the primary endpoint and for the secondary endpoint do not meet the nominal criterion for significance, the magnitude of the treatment effect for each major component, including mortality, and for the key secondary endpoint is remarkably consistent with the primary result.
The US Centers for Disease Control and Prevention report that there are more than 24 million Americans with diabetes diagnosed, and an estimated 6 million more undiagnosed13. Minorities are disproportionately affected, adding to their burden of disease14. In a meta-analysis of almost a million patients, diabetes was associated with a two-fold increased risk of vascular death.15 Diabetes increases the risk of mortality and cardiovascular events in patients with established cardiovascular disease.16 This excess risk was demonstrated within our study as well, with a 27% relative increase in risk of the primary endpoint compared with the non-diabetic patients and a 56% relative increase in the risk of death. Moreover, patients with diabetes were more likely to be obese, and were more likely to have a history of congestive heart failure, stroke, peripheral artery disease, hypertension and hypercholesterolemia than those without diabetes. These differences in risk factors, of course, may explain some of the differences in clinical outcomes overall.
Analyses of prespecified subgroups in TACT suggested that patients with diabetes accrued particular benefit from EDTA-based infusions.6 The present work expands on those preliminary observations.
In this study, the EDTA-based chelation regimen markedly improved the clinical outcomes of patients with diabetes, with a number needed to treat to prevent 1 primary endpoint event of 6.5 over 5 years (95% confidence interval 4.4 to 12.7). Thus, the multicomponent EDTA-based chelation regimen demonstrated a robust reduction in events in this subgroup analysis. This has particular relevance when considering that patients were taking standard, evidence-based medications for post-MI patients, and patients with diabetes had a median LDL of 83 mg/dL. We found no improvement in glycemia in the diabetes subgroup. Other mechanisms must underlie these findings.
The benefits of the multicomponent EDTA-based infusions may be mediated through the chelation of metals, thereby reducing direct end-organ toxicity, as well as toxicity mediated through enhanced metal-catalyzed oxidation. Epidemiological studies support the concept that metals, including lead and cadmium, are linked to cardiovascular risk17,18,19,20 and EDTA chelates both21. Clinical trials of patients with advanced chronic kidney disease and chelatable lead, treated with EDTA infusions, have shown preservation of renal function.22,23 Yet these observations do not explain why there is a significant interaction of chelation treatment with diabetes status.
There are, however, hypotheses regarding specific effects of metals on patients with diabetes that have been proposed for over 20 years. Complications of diabetes are at least partially mediated through the accumulation of advanced glycation end products and activation of the receptor of advanced glycation end products (RAGE),24 with downstream inflammatory cascades.25,26 Glycation end-products are created by the non-enzymatic interaction of glucose with proteins, lipids, and nucleic acids27. Most AGEs require metal-catalyzed oxygen chemistry for their formation. Metals bind to glycation end-products and promote the formation of reactive oxygen species in an autocatalytic reaction. The resultant oxidized end-products accumulate in tissues and promote inflammation and oxidative stress, hallmarks of atherosclerosis. Thus, chelation of metal ions may have particular importance in patients with diabetes28,29. Interestingly, some medications commonly used in diabetes may also have chelating properties.30,31,32
The benefits reported here for EDTA chelation potentially support a mechanism linking metal ions to oxidative stress and vascular complications, particularly in diabetic patients and certainly merit further study. Of particular, albeit inferential importance, is the continued separation of event curves late in the trial, long after infusions have stopped, suggesting that removal of toxic xenobiotic metals may have long-term benefit in these patients.
There remain important limitations of these analyses. First and foremost, although this subgroup analysis was prespecified, subgroup findings, regardless of how robust they appear, must be considered hypothesis-generating, rather than conclusive or definitive and must be replicated. Likewise, p-values, although nominally significant, must also be interpreted cautiously, particularly as there were multiple subgroups analyzed. Adjusted p-values, using the conservative Bonferroni correction, have been displayed for comparison. An unexpectedly high number of patients withdrew consent, including a slightly higher percentage among diabetics compared to non-diabetics, somewhat limiting the events that could be accrued and attributed during follow-up. Given that more placebo patients than chelation patients withdrew consent, however, the bias is conservative. That is, the effect of active treatment is likely underestimated by the analyses presented. We performed sensitivity analyses of patients that withdrew consent, making adverse assumptions as to their outcomes in the active therapy arm, and found that the findings reported here remain robust. Finally, although there are plausible hypotheses regarding the effects of this therapy, we do not have measurements of the levels of metals, glycation end-products or oxidative stress to corroborate or refute our hypotheses. Therefore, future studies should be planned and include bioassay assessment of potential pathways to clarify the mechanisms of benefit.
Conclusions
Post-MI diabetic patients age 50 or older on evidence-based medications demonstrated a marked reduction in cardiovascular events, including total mortality in the unadjusted analyses, with EDTA-based chelation therapy. These findings support the initiation of clinical trials in patients with diabetes and vascular disease to replicate these findings, and define the mechanisms of benefit. They do not, however, constitute sufficient evidence to indicate the routine use of chelation therapy for all post-MI diabetic patients.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the scientific contributions and support of Mario Stylianou PhD, at the National Heart, Lung, and Blood Institute; the organizational skills of Ana Mon Project Leader at the Clinical Coordinating Center, Alyssa Cotler at the National Center for Complementary and Alternative Medicine, Susan Dambrauskas (formerly at the National Heart, Lung, and Blood Institute), and Vivian Thompson at the Duke Clinical Research Institute for their competent professional assistance.
Sources of Funding: The National Heart, Lung, and Blood Institute and the National Center for Complementary and Alternative Medicine provided funding and oversight, grant # U01AT001156 and U01HL092607.
Footnotes
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Center for Complementary and Alternative Medicine, or the National Institutes of Health.
Disclosures: Gervasio A. Lamas MD reports that from 2000 to 2003 he served as a consultant to OmniComm, the electronic data capture company used in the trial. No funds were received, and all ties were severed as of 09/10/2003. No other disclosures are reported from the authors.
Contributor Information
Esteban Escolar, The Columbia University Division of Cardiology at Mount Sinai Medical Center, Miami Beach FL.
Gervasio A. Lamas, The Columbia University Division of Cardiology at Mount Sinai Medical Center, Miami Beach FL.
Daniel B. Mark, Duke Clinical Research Institute, Durham NC.
Robin Boineau, National Heart, Lung, and Blood Institute, Bethesda MD.
Christine Goertz, Palmer Center for Chiropractic Research, Davenport IA.
Yves Rosenberg, National Heart, Lung, and Blood Institute, Bethesda MD.
Richard L. Nahin, National Center for Complementary and Alternative Medicine, Bethesda MD.
Pamela Ouyang, Johns Hopkins University, Baltimore, MD.
Theodore Rozema, Biogenesis Medical Center, Landrum SC.
Allan Magaziner, Magaziner Center for Wellness, Cherry Hill, NJ.
Richard Nahas, Seekers Centre for Integrative Medicine, Ottawa, ON.
Eldrin F. Lewis, Brigham and Women's Hospital and Harvard Medical School, Boston MA.
Lauren Lindblad, Duke Clinical Research Institute, Durham NC.
Kerry L. Lee, Duke Clinical Research Institute, Durham NC.
References
- 1.Clarke NE, Clarke CN, Mosher RE. The in vivo dissolution of metastatic calcium; an approach to atherosclerosis. Am J Med Sci. 1955;229:142–149. doi: 10.1097/00000441-195502000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetra acetic acid. Am J Med Sci. 1956;232:654–666. doi: 10.1097/00000441-195612000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Grier MT, Meyers DG. So much writing, so little science: a review of 37 years of literature on edetate sodium chelation therapy. Ann Pharmacother. 1993;27:1504–9. doi: 10.1177/106002809302701217. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Health. EDTA Chelation Therapy for Coronary Artery Disease, RFA-AT-01004. http://grants.nih.gov/grants/guide/rfa-files/RFA-AT-01-004.html.
- 5.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241–50. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Drisko JA, Lee KL. Design of the Trial to Assess Chelation Therapy (TACT) Am Heart J. 2012;163:7–12. doi: 10.1016/j.ahj.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cranton EM, Liu ZX, Smith IM. Textbook on EDTA Chelation Therapy. 2nd. Hampton Roads; Newburyport, Massachusetts: 2001. Urinary Trace and Toxic Elements and Minerals in Untimed Urine Specimens. [Google Scholar]
- 8.Monnier VM. Transition metals redox: reviving an old plot for diabetic vascular disease. J Clin Invest. 2001;107:799–801. doi: 10.1172/JCI12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36:S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 11.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 12.Miller RG. Simultaneous Statistical Inference. 2nd. New York: Springer-Verlag; 1981. [Google Scholar]
- 13.International Diabetes Federation Atlas. 2012 www.idf.org/diabetesatlas/5e/Update2012.
- 14.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221–32. doi: 10.7326/0003-4819-125-3-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sat- tar N, Selvin E, Hu FB, Danesh J. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) registry. Circulation. 2000;102:1014–1019. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 17.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 μmol/L (10 μg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 18.Nawrot TS, Staessen JA. Low-level environmental exposure to lead unmasked as silent killer. Circulation. 2006;114:1347–9. doi: 10.1161/CIRCULATIONAHA.106.650440. [DOI] [PubMed] [Google Scholar]
- 19.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, Cadmium, Smoking, and Increased Risk of Peripheral Arterial Disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 20.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas-Acien A. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters RS, Bryden NA, Patterson KY, Veillon C, Anderson RA. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol Trace Elem Res. 2001;83:207–21. doi: 10.1385/BTER:83:3:207. [DOI] [PubMed] [Google Scholar]
- 22.Lin JL, Lin-Tan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348:277–86. doi: 10.1056/NEJMoa021672. [DOI] [PubMed] [Google Scholar]
- 23.Chen KH, Lin JL, Lin-Tan DT, Hsu HH, Hsu CW, Hsu KH, Yen TH. Effect of chelation therapy on progressive diabetic nephropathy in patients with type 2 diabetes and high-normal body lead burdens. Am J Kidney Dis. 2012;60:530–8. doi: 10.1053/j.ajkd.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2013;pII:S1043–2760. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 26.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway: implications for diabetatherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2275–81. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 27.Goh SY, Cooper ME. The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 28.Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming 14 redox active “glycochelates”: implications for the pathogenesis of certain diabetic complications. Biochem Biophys Res Commun. 1998:250, 385–389. doi: 10.1006/bbrc.1998.9326. [DOI] [PubMed] [Google Scholar]
- 29.Frizzell N, Baynes JW. Chelation therapy: overlooked in the treatment and prevention of diabetes complications? Future Med Chem. 2013;5:1075–8. doi: 10.4155/fmc.13.73. [DOI] [PubMed] [Google Scholar]
- 30.Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: A fundamental mechanism of action of AGE Inhibitors, AGE Breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549–559. doi: 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, Ishikawa N, Nangaku M, Kurokawa K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol. 2002;13:2478–2487. doi: 10.1097/01.asn.0000032418.67267.f2. [DOI] [PubMed] [Google Scholar]
- 32.Forbes JM, Cooper ME, Thallas V, Burns WC, Thomas MC, Brammar GC, Lee F, Grant SL, Burrell LM, Jerums G, Osicka TM. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes. 2002;51:3274–3282. doi: 10.2337/diabetes.51.11.3274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


