Abstract
Auditory neuropathy/dys-synchrony is a form of hearing impairment in which cochlear outer hair cell function is spared but neural transmission in the auditory pathway is disordered. This condition, or group of conditions with a common physiologic profile, accounts for approximately 7% of permanent childhood hearing loss and a significant (but as yet undetermined) proportion of adult impairment. This paper presents an overview of the mechanisms underlying auditory neuropathy/dys-synchrony-type hearing loss and the clinical profile for affected patients. In particular it examines the perceptual consequences of auditory neuropathy/dys-synchrony, which are quite different from those associated with sensorineural hearing loss, and considers currently available, and future management options.
Introduction
The terms auditory neuropathy/dys-synchrony (AN) and auditory dys-synchrony (AD) have been used to describe a form of hearing impairment in which cochlear amplification (outer hair cell) function is normal but afferent neural conduction in the auditory pathway is disordered (Starr et al., 1996; Berlin et al., 2001). This paper provides an overview of the clinical features associated with this condition, the various mechanisms that may produce the AN/AD result profile, the unique perceptual disruptions that arise as a result, and the consequences for aural rehabilitation.
The Auditory Neuropathy/Dys-synchrony Result Pattern
The clinical findings that define auditory neuropathy/dys-synchrony are the demonstration of outer hair cell integrity in evoked otoacoustic emission and/or cochlear microphonic recordings, in conjunction with the inability to record evoked neural activity at the level of the VIII nerve (compound action potential) and brainstem (auditory brainstem response) (Figure 1). As such, the electrophysiologic result profile is classically “retrocochlear,” but the exact sites of origin and the pathologic mechanisms involved are yet to be determined. Other clinical features consistent with the AN/AD pattern include the presence of permanent or fluctuating hearing loss of varying degrees, normal radiologic findings, absence of middle-ear muscle reflexes, and speech perception deficits out of proportion with the behavioral audiogram.
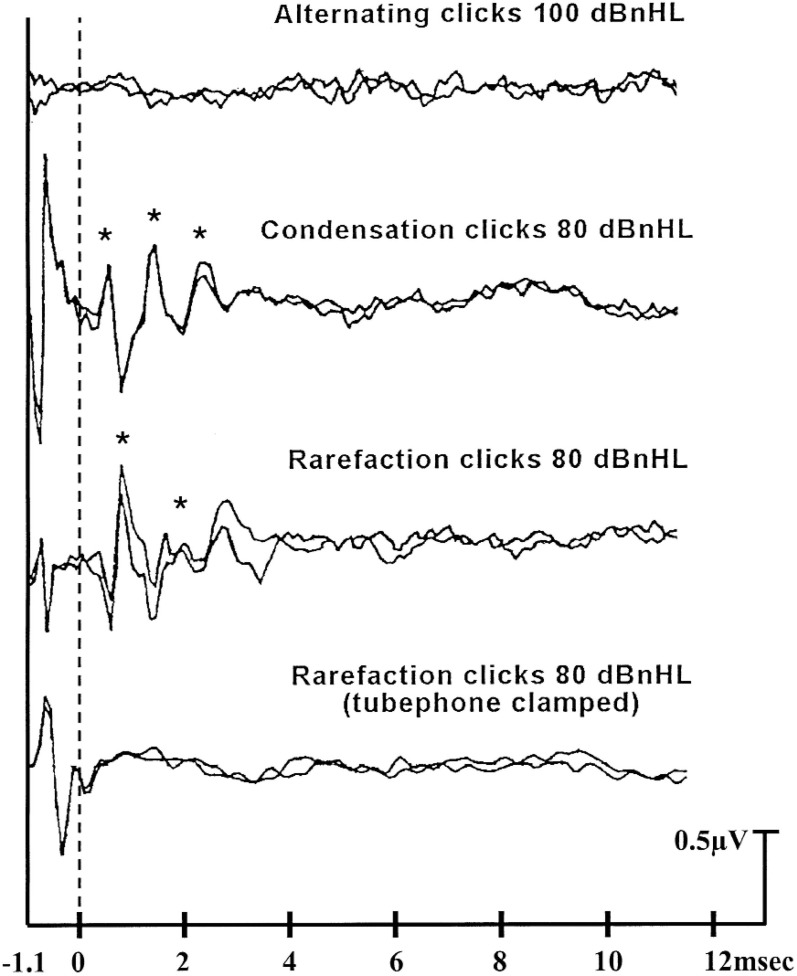
Figure 1.
ABR recordings for a 3-year-old child with AN/AD type hearing loss. The dotted line represents the point at which the stimulus reached the cochlea. The top tracings show no repeatable potentials to alternating clicks presented at 100 dBnHL. The middle tracing pairs show repeatable cochlear microphonic responses but absent brain stem response waveforms to unipolar stimuli at 80 dBnHL. The asterisks indicate the positive peaks in the cochlear microphonic waveform. The final tracings, in which only the stimulus artefact is evident, were obtained to rarefacting clicks presented with the tubephone clamped.
Decreased hearing sensitivity can result from dysfunction occurring at various sites in the peripheral and central auditory pathways. The most common form of permanent hearing loss is the result of an abnormality at the level of the cochlea and can be related to a loss or malfunction of the inner hair cells, loss or malfunction of the cochlear amplifier (which is thought to reside in the outer hair cells and provide an increase in hearing sensitivity of up to 30–40 dB) or a disruption of the driving force for the inner hair cell, known as the endocochlear potential (Ryan and Dallos, 1975). Cochlear level hearing deficit is variously referred to as sensory, inner ear, hair cell, cochlear, and sensorineural hearing loss. The last term has been used in recognition that some cochlear losses may also involve damage to neural elements that occur, for example, as a result sensory deprivation.
Hearing deficit can also be the result of abnormal transmission of neural signals through the auditory pathway or disordered processing of those signals in the auditory brainstem. Such losses, which can produce the auditory neuropathy/dys-synchrony result profile, have (until the advent of preneural assessment techniques) been indistinguishable from those centered at the cochlea. In recent times, however, the combination of preneural physiologic measures such as the cochlear microphonic and the otoacoustic emission, with neural responses such as the compound action potential and auditory brainstem response has made it possible to identify neural transmission disorders in subjects with cochlear (outer hair cell) function.
The Auditory Brainstem Response
The auditory brainstem response arises from activity occurring in the auditory pathway in the 10 to 15 ms immediately following the presentation of an abrupt auditory stimulus. The waveform complex consists of seven major peaks that are typically plotted with vertex positive waves pointing upwards and are labelled by Roman numerals. The neural generators responsible for the auditory brainstem response are yet to be clearly defined. The data suggest that both wave I and wave II are compound action potentials, with the former arising from the distal portion and the later from the proximal (brainstem) portion of the auditory nerve (Hashimoto et al., 1981; Møller and Jannetta 1981). The later waves are thought to have multiple generators, and are thought to have contributions from the superior olive and lemniscal pathways up to and including the inferior colliculus (Melcher et al., 1996a, 1996b, 1996c).
Auditory Brainstem Responses in Ears with Normal Hearing and Sensorineural Hearing Loss
Auditory brainstem response testing has been in widespread use as both a hearing screening and diagnostic measure for over 25 years. In subjects with normal hearing, repeatable auditory brainstem response waveforms can be reliably obtained to acoustic click and tone-burst stimuli presented at levels around 10–20 dBnHL (Hyde et al., 1990; Durieux-Smith et al., 1991; Stapells et al., 1994). In ears with significant hearing impairment, a reasonably close relationship between hearing level and auditory brainstem response threshold has been demonstrated (Gorga et al., 1985; Hyde et al., 1990; Picton et al., 1994; Stapells et al., 1995; Stapells and Oates, 1997). Mean auditory brainstem response/behavioral threshold difference levels of 10 dB or less have been obtained in these studies for both child and adult subjects.
This close correlation between auditory brainstem response thresholds and the behavioral audiogram in subjects with normal hearing or sensorineural loss allows a subject's audiogram to be predicted from evoked potential findings with reasonable confidence. Auditory brainstem response thresholds (when responses are obtained) typically overestimate the hearing levels slightly, and response absence at maximum presentation levels (about 100 dBnHL for acoustic clicks and about 100–110 dBnHL for tone bursts) is consistent with behavioral hearing levels in the severe-to-total hearing loss range (Brookhouser et al., 1990; Rance et al., 1998).
Auditory Brainstem Responses in Ears with Auditory Neuropathy/Dys-synchrony
In ears with auditory neuropathy/dys-synchrony, auditory brainstem responses are absent (or grossly abnormal) at maximum stimulus presentation levels regardless of behavioural hearing level (Starr et al., 1996; Rance et al., 1999; Sininger and Oba, 2001).1 In such cases, disruption of the auditory brainstem response is thought be the result of either a reduction in the number of neural elements available to contribute to the response, or a disruption in the temporal integrity of the neural signal.
The main positive peaks in the auditory brainstem response are separated by only about 1 ms. Thus, successful recording of the averaged response requires that the timing of discharges within the auditory brainstem be almost identical after each test stimulus. Various authors have suggested that a dys-synchrony in the neural firing of the order of fractions of a millisecond (Starr et al., 1991; Sininger et al., 1995; Kraus et al., 2000) is sufficient to disrupt the response and render the averaged potentials unrecognizable.
Cochlear Microphonics
The cochlear microphonic is a receptor potential produced by the polarization and depolarization of the cochlear hair cells. As such, the response is preneural and shows little or no latency delay from the onset of the stimulus. Starr et al. (2001a) for example, found that the initial peak in the cochlear microphonic waveform occurred in a group of normal subjects only 0.42 (0.2 ms after the stimulus reached the eardrum. The cochlear microphonic is recorded and extracted from the electroencephalograph in the same way as the auditory brainstem response, and appears as an alternating current potential that provides a bioelectric analog of the input (hence the term microphonic). As a result, this potential, unlike those produced by neural activity, shows a direct phase relationship with the stimulating waveform (Dallos and Cheatham, 1976).
In the past, cochlear microphonics have been difficult to distinguish from the electrical artifact that often accompanies the generation of a stimulus at the transducer (Eggermont, 1976). This difficulty occurs because of the temporal proximity of the cochlear microphonic response to the onset of the stimulus and because the cochlear microphonic response so closely resembles the stimulating waveform. The use of insert earphones in recent times has overcome this problem by removing the transducer from the recording site (i.e., reducing stimulus artifact) and by introducing a time delay as the stimulus passes down the earphone tube that separates the cochlear potentials from the artifact (Berlin et al., 1998).
The cochlear microphonic, when recorded from extra-tympanic sites such as the scalp or ear canal, is thought to be dominated by the activity of the outer hair cells (Dallos, 1973; Dallos and Cheatham, 1976; Norton et al., 1989). In the past, it was confused with the early components of the auditory brainstem response and was originally believed to be generated by the auditory nerve. However, the response does differ from neural potentials in a number of clinically obvious ways.
Most important, the cochlear microphonic is sensitive to the phase of the eliciting stimulus and can be identified by the 180° phase shift in the response that occurs when the stimulus phase changes (in the case of acoustic click stimuli) from rarefaction to condensation clicks (Sohmer and Pratt, 1976; Berlin et al., 1998) (see the middle tracings of Figure 1). In contrast, the polarity of neural responses is unaffected by the phase of the stimulus waveform, although variations in the latency of the compound action potential (Wave I in the auditory brainstem response) with the stimulus phase can give the appearance of response phase changes (Stockard et al., 1979).
The cochlear microphonic through its ability to reflect the integrity of cochlear hair cells can play a significant role in the identification of ears with auditory neuropathy/dys-synchrony. As discussed previously, an absence or severe abnormality of the auditory brainstem response at maximum presentation levels in ears with sensorineural hearing loss is consistent with significant cochlear damage. In such cases, the cochlear microphonic would also be expected to be absent. The presence of this response is indicative of at least some degree of outer hair cell function and is therefore suggestive of neural transmission abnormality in ears with absent or disrupted brainstem potentials (Chisin et al., 1979; Starr et al., 1991; Berlin et al., 1993; Starr et al., 1996; Berlin et al., 1998).
Otoacoustic Emissions
An otoacoustic emission is a release of sound energy in the cochlea that is recordable in the ear canal (Kemp, 1978). This sound appears to be a by-product of the active bioelectric process that exists within the normal cochlea. This active process, which is thought to enhance both the threshold sensitivity and frequency tuning of the inner ear transduction system, is considered to reside in the outer hair cells (Davis, 1983).
The relative ease with which otoacoustic emission testing can be performed, and the fact that emissions can be obtained in subjects of all ages, has led to the widespread investigation and use of this response as a hearing-screening tool. Although the data has, on the whole, suggested that the ability of otoacoustic emission-based procedures to predict audiometric threshold is limited, emission testing has proven to be useful as a screening measure capable of differentiating between ears with normal cochlear (outer hair cell) function and those with sensorineural hearing loss (Harris and Probst, 2002).
Approximately 99% of ears with audiometric thresholds in the normal range (<20 dBHL) have recordable emissions for both the transient (Kemp, 1978; Bonfils et al., 1988; Kapadia and Lutman, 1997) and distortion product (Lonsbury-Martin et al., 1990; Bonfils and Avan, 1992) test paradigms. In ears with cochlear hearing deficit however, the probability of eliciting an otoacoustic emission decreases as the degree of hearing loss increases such that the transiently evoked otoacoustic emission is absent in all cases with average hearing losses above 35 dBHL (Kemp, 1978; Collet et al., 1993), and the distortion product otoacoustic emission is absent for all ears with losses above 60 dBHL (Lonsbury-Martin et al., 1990; Bonfils and Avan, 1992; Gorga et al., 1997). As such, emission absence in an ear with normal middle ear function is indicative of significant cochlear hearing loss, whereas otoacoustic emission presence is indicative of normal peripheral (middle ear and cochlear outer hair cell) function.
The otoacoustic emission response, in providing an indirect measure of the function of the cochlear amplifier and outer hair cells, offers a means of differentiating between sensory and auditory neuropathy/dys-synchrony type hearing loss. Ears with absent auditory brainstem responses because of sensorineural hearing loss typically show audiometric thresholds in the severe to profound hearing loss range. Cochlear damage sufficient to cause a hearing loss of this degree typically disrupts the active cochlear mechanisms that generate the otoacoustic emission, resulting in response absence. Otoacoustic emission presence in ears with absent auditory brainstem responses is therefore suggestive of AN/AD rather than sensory type hearing loss.
Possible Mechanisms Producing the Auditory Neuropathy/Dys-synchrony Result Pattern
Patients with the physiologic characteristics that have been broadly categorized as auditory neuropathy/dys-synchrony can present with a range of clinical symptoms. The variability in the clinical features seen in this group may represent differing degrees of the same pathology or may be the result of a range of distinct auditory pathway disorders. Some possible sites of lesion include the cochlear inner hair cells, the synapse between the inner hair cells and type 1 auditory nerve fibers, and the auditory nerve itself (Starr et al., 1996; Rance et al., 1999; Amatuzzi et al., 2001).
Inner Hair Cell Loss
One mechanism that could produce the auditory neuropathy/dys-synchrony result pattern is pathology restricted to the inner hair cells. A peripheral site of a lesion such as this is consistent with the observation in AN/AD patients that even the earliest auditory brainstem response waves are absent, including wave I, which represents the first action potential in the auditory nerve. A specific inner hair cell abnormality could result in the decrement of the entire auditory brainstem response complex, with the preservation of outer hair cell responses.
At this stage, the integrity of inner hair cell function in living patients cannot be determined because suitable diagnostic tests are not available. There are, however, biologic precedents for selective inner hair cell loss in both the Bronx Waltzer mouse (Lenoir and Pujol, 1984; Schrott et al., 1989) and the Beethoven mouse models (Bussoli et al., 1997).
The auditory neuropathy/dys-synchrony physiologic profile has been chemically induced in chinchillas treated with antineoplastic agents (carboplatin) that produce selective inner hair cell lesion (Takeno et al., 1994, Wake et al., 1996; Liberman et al., 1997; Harrison, 1998; Salvi et al., 1999).2 Auditory brainstem response threshold disruption in these animals was considered to be due to a diminution in response amplitude that resulted from a reduction in the number of elements contributing to the volume conducted potential rather than from an increase in the firing threshold for the surviving elements because single-unit responses from inferior colliculus neurons showed normal response thresholds. As such, these findings suggest a mechanism whereby patients with auditory neuropathy/dys-synchrony-type hearing loss could demonstrate normal or near normal behavioral hearing thresholds (as has been reported in many human cases) in conjunction with severely disordered evoked potential findings. Behavioral hearing thresholds were however, not determined in the Harrison, (1998) study or in any of the mentioned investigations with experimental animals. Yet to be determined is whether normal sensitivity in a limited number of units in the central auditory system is sufficient for behavioral detection of low-level sounds.
Recent findings presented by Amatuzzi et al. (2001) have confirmed that selective inner hair cell loss can occur in humans. These authors carried out a detailed histologic evaluation of 15 nonsurvivors from a neonatal intensive care unit and identified 2 babies with loss of both inner and outer hair cells, 2 with loss of outer hair cells alone, and 3 babies with selective inner hair cell loss. Each of the cases with specific inner hair cell loss had an auditory brainstem response assessment before they died that showed no response at screening levels (40 dBnHL). None showed any evidence of cochlear neuron damage, suggesting that the mechanism for auditory brainstem response disruption was a paucity of contributing neural activity due to the reduced number of inner hair cells rather than an insult to the neural elements themselves.
The results presented by Amatuzzi et al. (2001) are inconsistent with the findings from a large body of adult human temporal bone work that has failed to show patterns of specific inner hair cell loss. The results for these oxygen-deprived youngsters do, however, fit with recent animal histologic evidence that suggests certain types of cochlear insult, notably those caused by to prolonged hypoxia, can have a greater effect on inner than outer hair cell survival (Bohne, 1976; Shirane and Harrison 1987a; Billet et al., 1989).
The Synapse Between the Inner Hair Cells and Auditory Nerve Terminals
A disorder at the synapse between the cochlear inner hair cells and type 1 auditory nerve fibers has also been proposed as a mechanism that could produce the auditory neuropathy/dys-synchrony result pattern (Starr et al., 1991). At the base of the inner hair cell are anatomic structures involved in the storage and release of neurotransmitters. Neurotransmitters act upon receptor sites in auditory nerve dendrites and initiate the generation of action potentials. Disorders at this site may be presynaptic (involving the release of transmitters) or postsynaptic (affecting the ability of the receptor sites on the auditory nerve dendrite to respond these substances) (Starr et al., 2000).
Mechanisms by which synaptic disruption might occur in the auditory pathway in human subjects are yet to be determined. Genetic dysfunction involving disruption of the otoferlin (OTOF) protein, which affects transmitter release and has been found in the inner hair cells has, however, been identified in subjects presenting with the auditory neuropathy/dys-synchrony result pattern (Varga et al., 2003).
Auditory Nerve Abnormality
As the term auditory neuropathy suggests, the affected site in many patients is thought to be the auditory nerve itself. Starr et al. (1996) coined the expression as 8 of the 10 subjects in their series had evidence of other peripheral nerve abnormality in addition to hearing loss.
The general (nonauditory) symptoms of peripheral neuropathy include weakness and muscle atrophy (if the motor nerves are involved) sensory loss, paresthesia (unusual sensations), and dysesthesia (discomfort). The commonly used diagnostic criteria include absent ankle jerks or reduction of vibration sense in the feet, abnormal results on nerve conduction studies, and abnormal sural nerve biopsy specimens.
Generalized neuropathic disorders have been indicated in 30% to 40% of reported auditory neuropathy/dys-synchrony cases overall and about 80% of patients with symptom onset occurring after age 15. The site of the disorder affecting the auditory nerve and auditory brainstem in these cases may be the myelin sheath or the neuron itself.
Myelin Disorder
Myelin serves in the central nervous system as an electrical insulator. It is manufactured and maintained by specialized cells known as oligodendroglia. The myelin sheath consists of a lamellar structure of lipids and proteins that wrap concentrically around the axon. Partial or complete loss of myelin can have profound effects on the generation and propagation of action potentials within auditory nerve fibers. Demyelination results in an increase in membrane capacitance and a decrease in membrane resistance, leading to a delayed excitation, a reduction in the velocity of action potential propagation, and an increase in conduction vulnerability (McDonald and Sears, 1970; Rasminsky and Sears, 1972; Pender and Sears, 1984). Fibers that are demyelinated to differing degrees conduct neural signals at different speeds, and the synchrony of discharges can be affected.
Although neurons that are not entirely myelinated are capable of conducting action potentials, they do so with prolonged refractory periods and an impaired ability to transmit high-frequency pulse trains (McDonald and Sears, 1970; Rasminsky and Sears, 1972; Pender and Sears, 1984). As a result, repetitive activation of demyelinated fibers results in a progressive increase in the conduction time of the action potential and may lead to an intermittent or total block in their propagation (conduction block) (Rasminsky and Sears, 1972).
The pathophysiologic changes in neural conduction properties associated with demyelination are likely to have profound effects on the auditory brainstem response which is reliant on the relatively precise synchronous response of a population of auditory nerve fibers to a transient acoustic stimulus. Reductions in the temporal synchrony of demyelinized VIII nerve fibers are likely to lead to a significant reduction in the amplitude of the averaged evoked response. Moreover, with more advanced lesions, the propagation of the action potential is likely to become increasingly vulnerable, and the risk of depolarization block is increased—especially for the relatively repetitious stimuli used to generate the auditory brainstem response.
Axonal Neuropathy
Axonal damage can occur in isolation as a result of specific disease processes or can occur in conjunction with or as a consequence of demyelinating conditions. As such, the functional distinction between myelin and axon related disorders can be blurred in some cases (Rapin and Gravel, 2003). Axonal neuropathies reduce the number of neural elements but do not directly affect conduction speed. The refractory periods of surviving elements also tend to be normal, allowing a reasonably unimpaired response to high-rate stimuli (Kuwabara et al., 1999). The classic signs of axonal neuropathy in the auditory pathway are, therefore, a reduction in the amplitude of the whole nerve action potential and auditory brainstem response rather than an increase in latency or a broadening of these potentials (as is the case for myelin related disorders). However, the absence of any evoked brainstem responses in most auditory neuropathy/dys-synchrony cases means that axonal and myelin related neuropathies are clinically indistinguishable.
Accurate differentiation between axonal and demyelinating neuropathies can only really be made from a histologic examination of the affected nerves. In the case of the auditory nerve, this can only be achieved on postmortem examination of the temporal bone or the brainstem at the point of entry of the auditory nerve.
Peripheral nerve studies can be done by taking a biopsy specimen of a small portion of another more accessible sensory nerve, and the results can be used to infer the function of the auditory nerve. Analyses of the sural nerve have, for example, been used in auditory neuropathy/dys-synchrony patients in this way (Butinar et al., 1999; Starr et al., 2001b).
In summary, neuropathic disorders of the peripheral nervous system, including the auditory nerve, can result in varying degrees of axon loss and myelin damage. Abnormal function in the auditory system resulting in the auditory neuropathy/dys-synchrony result pattern may therefore be related to disrupted neural synchrony resulting from myelin damage, a reduction in the number of functioning fibers caused by axonal loss, or in many cases, a combination of both.
Auditory Neuropathy or Auditory Dys-synchrony?
The previous sections have outlined a range of different pathologic mechanisms and sites of lesion that could produce the physiologic profile termed auditory neuropathy by Starr and colleagues in 1996. Some of these mechanisms, such as selective inner hair cell loss, may not directly affect the function of the auditory nerve, which has led some groups to suggest that the auditory neuropathy label is inappropriate at best, and at worst, is clinically misleading. Berlin et al. (2002) for example has suggested that implying the presence of an auditory nerve/brainstem abnormality may have serious clinical consequences, dissuading for example, clinicians from considering cochlear implantation in subjects who might be expected to benefit significantly from this procedure.
The term auditory dys-synchrony has been proposed as an alternative to auditory neuropathy (Berlin et al., 2001). As discussed previously, the absence of an auditory brainstem response in ears with measurable hearing levels is thought, in some cases at least, to be caused by a lack of temporal consistency in auditory brainstem response to series’ of audible stimuli. Myelin disorders can certainly affect the synchrony of neural discharges. However, some of the other mechanisms considered to result in a lack of measurable brainstem potentials may not involve dys-synchrony. Marsh (2002) for example argues that the temperature-dependant form of neuropathy is likely to reflect a conduction block rather than a disruption of the timing of neural signals. Auditory brainstem response absence in cases of axon-related neuropathies and inner hair cell lesions are also thought not to be primarily related to synchrony disruptions but to reduced numbers of neural elements contributing to the volume-conducted response.
Clearly, neither “auditory neuropathy” nor “auditory dys-synchrony” is adequate to describe the entire group of patients with absent auditory brainstem responses but present cochlear hair cell responses. The lack of an appropriate label is simply a reflection of our current inability to determine specific mechanisms in specific cases. For the purposes of this paper the term auditory neuropathy/dys-synchrony will be used.
Clinical Profile
Etiology
In most cases, auditory neuropathy/dys-synchrony type hearing loss presents in conjunction with specific medical risk factors. AN/AD can, however, occur in the absence of obvious medical problems or established hearing-related risk categories. For example, 3 of the 20 subjects presented in a survey of pediatric cases conducted in our laboratory (Rance et al., 1999) had no health concerns in their histories or evidence of permanent hearing loss of any kind in their immediate or extended families. The Sininger and Oba (2001) survey of adult and pediatric cases found that auditory neuropathy/dys-synchrony occurred without associated risk factors in 27% of patients.
A number of different etiologies have been associated with the auditory neuropathy/dys-synchrony result profile. These conditions can be broadly categorized as transient neonatal insults, infectious processes, and genetic or syndromal conditions.
Neonatal Insults
Thirteen of the 20 auditory neuropathy/dys-synchrony children described in the Rance et al. (1999) report presented with serious neonatal health concerns. This high proportion may have been associated with the manner in which the children were identified, with 12 of the subjects detected in an at-risk screening program. Subsequent findings presented by Sininger and Oba. (2001) have confirmed this result, however. Approximately 80% of the patients from their auditory neuropathy/dys-synchrony database with onset at less than 2 years of age (59 cases) presented with neonatal and/or familial risk factors. In fact, they found that almost half of their infant cases had both genetic and neonatal health factors and suggested that some children may be predisposed towards developing auditory neuropathy/dys-synchrony if they suffer some form of neonatal insult.
The most commonly reported neonatal conditions associated with auditory neuropathy/dys-synchrony are anoxia and hyperbilirubinemia (Stein et al., 1996; Berlin et al., 1997; Deltenre et al., 1999; Rance et al., 1999; Simmons and Beauchaine, 2000; Starr et al., 2000; Sininger and Oba, 2001; Franck et al., 2002; Madden et al., 2002; Dunkley et al., 2003). More than 50% of early onset AN/AD cases presented thus far have shown one or both of these conditions in their neonatal histories.
Excessive amounts of bilirubin (a byproduct of red-blood cell metabolism), which is often associated with liver immaturity in the newborn, can be toxic to the central nervous system and can result in significant neurologic insult known as kernicterus (Shapiro, 2003). Although many neonates (60%) experience some physiologic jaundice that is not toxic, unconjugated bilirubin (not bound to the albumin protein) can cross the blood-brain barrier and cause icteric staining of the central nervous system. Even short-term episodes of hyperbilirubinemia have been shown to result in both temporary and permanent evoked potential abnormalities, including elevated auditory brainstem response thresholds (Hung, 1989) and prolonged auditory brainstem response wave (I–V) latencies (Nakamura et al., 1985; Tan et al., 1992), suggesting that both the peripheral and central auditory systems are vulnerable to bilirubin insult.
Infectious Processes
Infection-related causes of auditory neuropathy/dys-synchrony have been suggested in a small but significant number of the cases reported recently. Starr et al. (2000) estimated that postviral infectious processes were involved in 10% of the 67 patients from their AN/AD database. Specific etiologic details were not presented, but other studies have reported that mumps (Prieve et al., 1991) and meningitis (Sininger et al., 1995; Rance et al., 1999) can be associated with the auditory neuropathy/dys-synchrony.
Genetic and Syndromal Factors
The auditory neuropathy/dys-synchrony result profile often occurs as a part of a generalized neuropathic disorder. Hereditary motor and sensory neuropathies such as Charcot-Marie-Tooth Syndrome (type I and II) make up a relatively high proportion of the adult AN/AD cases reported to date. Sininger and Oba, (2001) for example, report that 8 of their 13 patients with AN/AD symptom onset at age 10 years or older were confirmed hereditary motor and sensory neuropathy sufferers. Charcot-Marie-Tooth syndrome is a genetic disorder which involves the degeneration of the myelin sheaths and is thought to be related to an abnormality in the peripheral myelin protein 22 (PMP-22) on chromosome 17p 11.2 (Kovach et al., 1999) or a mutation of MPZ gene (Starr et al., 2003). Loss of axons of the distal portions of the peripheral nerves has also been reported with this condition (Chance and Fishbeck, 1994; Ouvrier, 1996).
Auditory brainstem responses have been reported to be absent or grossly abnormal in patients with Charcot-Marie-Tooth syndrome (Cassandro et al., 1986). Histopathologic results have shown evidence of cochlear hair cell survival in conjunction with loss of cochlear spiral ganglion cells and evidence of demyelinating processes in the VIII nerve (Nadol, 2001).
Hereditary motor and sensory neuropathies have also been linked to auditory neuropathy/dys-synchrony in recent studies involving Slovene, Italian, and Bulgarian Gypsy families (Butinar et al., 1999; Leonardis et al., 2000). The autosomal recessive condition, which in these cases produced both myelin and axonal damage, was mapped to the long arm of chromosome 8 (8q24). The disease process with this form of neuropathy tends to produce severe, progressive motor disabilities in early childhood and auditory pathway effects in adolescence.
Another inherited disease that is relatively commonly associated with auditory neuropathy/dys-synchrony is Friedreich's ataxia. Four cases of this autosomal recessive condition were described in the Sininger and Oba, (2001) series. Friedreich's ataxia is a neurodegenerative condition that is believed to be restricted to the brainstem and cerebellar parenchyma. Auditory brainstem response assessments in patients with Friedreich's ataxia have typically shown either complete response absence (Satya-Murti et al., 1980; Cassandro et al., 1986) or the presence of wave I and absent later responses (Jabbari et al., 1983). Histopathology (Spoendlin, 1974) has indicated that cochlear neurons and spiral ganglion cells are affected in Friedreich's ataxia, whereas cochlear structures (organ of Corti and hair cells) are unimpaired.
Isolated cases of auditory neuropathy/dys-synchrony have been reported with other genetic disorders. Some of these include Ehlers-Danlos syndrome (Sininger and Oba, 2001), an autosomal-dominant connective tissue condition related to serious vascular abnormalities, and Stevens-Johnson syndrome, a rare cutaneous disease typically triggered by drug therapy (Doyle et al., 1998). AN/AD has also been associated with syndromes affecting the immune system (Guillain-Barré syndrome) and mitochondrial enzymes (Deltenre et al., 1997; Corley and Crabbe, 1999).
Determination of genetic factors associated with AN/AD type hearing loss is currently an area of vigorous investigation. Recent reviews of the literature have been provided by Starr et al. (2003) and Rapin and Gravel (2003).
Age of Symptom Onset
The age of onset of auditory neuropathy/dys-synchrony type hearing loss has tended to fall into two distinct groups: those who present with symptoms in infancy, and those in whom the condition develops in adolescence or early adulthood. Only one in four auditory neuropathy/dys-synchrony cases are older than 10 years at symptom onset (Starr et al., 2000; Sininger and Oba, 2001). Starr et al. (2000) suggest that this comparatively low proportion may be because some affected patients lose their emissions over time, and as such, may not be recognizable as auditory neuropathy/dys-synchrony cases if otoacoustic emission response and not cochlear microphonics are the diagnostic criterion.
Another reason for the higher proportion of pediatric cases in the AN/AD spectrum could be because the physiologic test techniques required to identify the condition (auditory brainstem response/cochlear microphonics/otoacoustic emission) are more frequently used in screening and diagnostic programs in pediatric populations. Adult auditory neuropathy/dys-synchrony patients with symmetrical hearing thresholds and reasonable speech perception, for example, are unlikely to be considered for physiologic assessment.
The Prevalence of Auditory Neuropathy/Dys-synchrony
For the reasons outlined in the previous section, the prevalence of auditory neuropathy/dys-synchrony in adult populations is difficult to determine. At this stage, data are also insufficient to determine the condition's prevalence in the well-baby population, although the findings from universal screening programs should soon provide some insights in this regard.
Limited data do exist describing the proportion of affected children in at-risk infant populations (see Table 1 for details). Rance et al. (1999) presented results for 5,199 babies with specific risk factors for hearing loss. Twelve of these children showed evidence of auditory neuropathy/dys-synchrony presenting with absent auditory brainstem responses but present otoacoustic emissions and/or cochlear microphonic responses. This represents a reasonably high prevalence of 0.23% or 1 in every 433 of the subjects. Even higher AN/AD prevalence levels have been reported in other studies involving babies who have suffered severe neonatal health problems:
Table 1.
Prevalence of Auditory Neuropathy/Dys-synchrony in “At-Risk” Infant Populations
| Study | Population | No. of Subjects | No. of AN/AD Subjects | % of Total |
|---|---|---|---|---|
| Stein et al. (1996) | Special care nursery | 100 | 4 | 4.00 |
| Psarommatis et al. (1997) | Intensive care unit | 102 | 2 | 1.96 |
| Rance et al. (1999) | “At-risk” infants | 5199 | 12 | 0.23 |
Stein et al. (1996) identified 4 babies with the auditory neuropathy/dys-synchrony result pattern in a consecutive series of 100 children undergoing auditory brainstem response assessment in a special care nursery.
Psarommatis et al. (1997) found 2 cases in a study involving 102 neonatal intensive care unit graduates.
The higher incidences reported in these two studies (2%–4%) might be anomalies resulting from their small sample sizes. They do, however, demonstrate the significant risk of auditory pathway disorder that exists for children who have suffered a rocky neonatal course.
The proportion of permanent hearing loss related to auditory neuropathy/dys-synchrony in pediatric populations has been considered in a number of recent investigations (Table 2). Methodologic differences between studies—some, for example, have used cochlear microphonic testing whereas others have used otoacoustic emissions as their measures of preneural function—make direct comparison difficult. Overall however, the results are reasonably consistent and suggest that auditory neuropathy/dys-synchrony accounts for approximately 7% of permanent hearing loss in children.
Table 2.
Prevalence of Auditory Neuropathy/Dys-synchrony in Children with Permanent Hearing Loss
| Study | Population | No. of Cases Permanent Hearing Loss | No. of AN/AD Cases | % of Total |
|---|---|---|---|---|
| Kraus et al. (1984) | Hg. impaired children | 48 | 7 | 14.58 |
| Park Lee. (1998) | Hg. impaired children | 139 | 7 | 5.04 |
| Vohr et al. (1998) | Universal screening | 111 | 2 | 1.80 |
| Rance et al. (1999) | “At-risk” infants | 109 | 12 | 11.01 |
| Berlin et al. (2000) | Hg. impaired children | 1000 | 87 | 8.70 |
| Cone-Wesson et al. (2000) | Universal screening | 56 | 3 | 5.36 |
| Lee et al. (2001) | Hg. impaired children | 67 | 2 | 2.98 |
| Madden et al. (2002) | Hg. impaired children | 428 | 22 | 5.14 |
| Tang et al. (2004) | Hg. impaired children | 56 | 1 | 1.78 |
| Rance et al. (in press) | “At-risk” infants | 290 | 19 | 6.55 |
Measures of Outer Hair Cell Function
Cochlear microphonic and otoacoustic emissions tests have been used as indicators of cochlear (outer) hair cell function to aid in the identification of auditory neuropathy/dys-synchrony-type hearing loss. The results of these two techniques are not always consistent in affected ears, however. Such inconsistencies highlight the functional differences between the two responses and raise questions as to the best way to measure preneural function in the clinic.
The presence of cochlear microphonic responses was the primary identification method used in the study by Rance et al. (1999). In addition, transiently evoked otoacoustic emissions assessment was carried out in 33 of the affected ears. Robust otoacoustic emissions consistent with the presence of the cochlear “active process” and at least some degree of outer hair cell function were observed in 16 ears. However, 17 ears showed no emission response despite the presence of clear cochlear microphonic potentials.
Various explanations for this result mismatch were considered, including subtle middle ear pathology and the possibility that these ears had significant outer hair cell loss and that the cochlear microphonic response was actually produced by the inner hair cells. However, the most likely explanation seemed to be that the outer hair cells were present in these ears and were able to polarize and depolarize (producing the cochlear microphonic response), but that their function was impaired to the extent that they could not generate the mechanical cochlear processes reflected by the otoacoustic emissions.
Subsequent studies have also presented auditory neuropathy/dys-synchrony cases with absent emissions and normal cochlear microphonics (Starr et al., 2000; Trautwein et al., 2000; Sininger and Oba, 2001). Starr et al. (2000), in their survey of adults and children with auditory neuropathy, found that in 19 of 63 ears (30%) TEOAEs could not be detected. Interestingly, these authors found no relation between behavioral hearing level and otoacoustic emissions response/absence in their subjects, a result consistent with the findings from Rance et al. (1999).
Another notable finding from the Starr et al. (2000) study was that otoacoustic emission responses in some cases disappeared over time in the absence of confounding factors such as middle ear disease or the provision of amplification. In fact, 9 subjects in their sample who had originally shown clear responses later lost their transient evoked otoacoustic emissions. Deltenre et al. (1999) previously reported a similar result when they described the findings for 2 children who were identified with auditory neuropathy in infancy (showing present otoacoustic emissions/cochlear microphonic responses and absent auditory brainstem responses) but who subsequently lost their emissions. Cochlear microphonic responses in these children were relatively unchanged, with similar amplitudes obtained before and after emission loss and only a slight morphologic change reported in one case. Consistent with the findings of Rance et al. (1999) and Starr et al. (2000), behavioral hearing levels in the Deltenre et al. (1999) cases did not seem to be related to otoacoustic emission result. Behavioral audiograms obtained before and after the emission loss were unchanged in these children.
The mechanisms underlying the deterioration of otoacoustic emissions in subjects with auditory neuropathy are unclear at this stage. These processes may become more obvious as more cases are revealed and studied, but to date, no statistical relationship between otoacoustic emission loss and any particular pathology or disease process has been identified (Sininger and Oba, 2001). The time-course over which otoacoustic emission deterioration occurs is also uncertain and is clearly an issue that warrants further investigation. What is clear is that using otoacoustic emission testing as the sole diagnostic indicator of auditory neuropathy/dys-synchrony in subjects with absent or abnormal auditory brainstem response results will fail to identify a significant number of cases. A change in the operating definition of auditory neuropathy may therefore be warranted, making the presence of cochlear microphonic responses, which appear to be relatively unchanged in patients with deteriorating otoacoustic emissions, the primary measure of outer hair cell survival.
Behavioral Audiogram
Most reports on auditory neuropathy/dys-synchrony published before the mid-1990s described subjects with audiograms in the mild-to-moderate hearing loss range (Davis and Hirsh, 1979; Worthington and Peters, 1980; Lenhardt, 1981; Kraus et al., 1984). This bias towards losses of lesser degree may reflect that many of these early patients were only identified as a result of the inconsistency between behavioral and electrophysiologic findings. In clinics where tests of preneural function were not available, ears with absent auditory brainstem responses and hearing thresholds in the severe-to-profound range because of AN/AD would have been indistinguishable from their sensorineural counterparts.
Subsequent findings have shown behavioral thresholds that range from normal levels to total hearing loss. Rance et al. (1999), for example, found a reasonably even distribution of pure-tone average hearing levels across the audiometric range (Figure 2). Starr et al. (2000) and Sininger and Oba (2001) have subsequently reported a similar degree of audiometric variability in their surveys of clinical findings for affected children and adults. Starr et al. (2000) found average hearing levels in 31% of ears at less than 35 dBHL, 39% of ears between 35 and 70 dBHL, and 30% of ears at more than 70dBHL. Madden et al. (2002) also found an even spread of behavioral audiograms, with 6 (33%) in their group of 18 affected children presenting with audiograms in the normal-to-mild range, 6 in the moderate-to-severe range, and 6 in the profound hearing loss range.
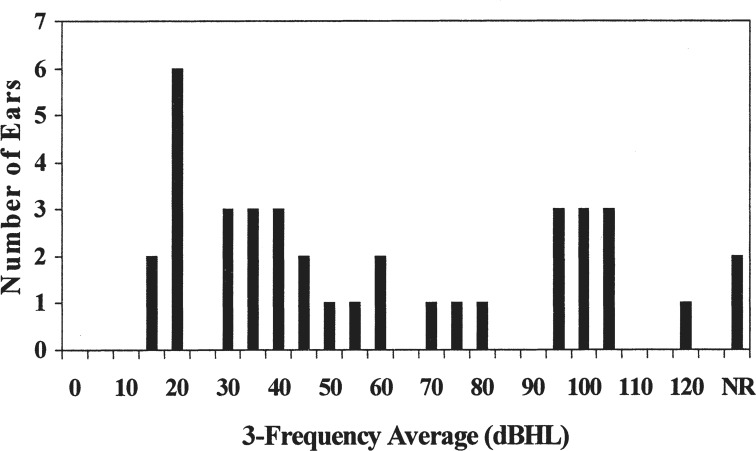
Figure 2.
The distribution of behavioral hearing thresholds (3-frequency average) for 38 ears with auditory neuropathy (Rance et al., 1999).
Threshold Stability
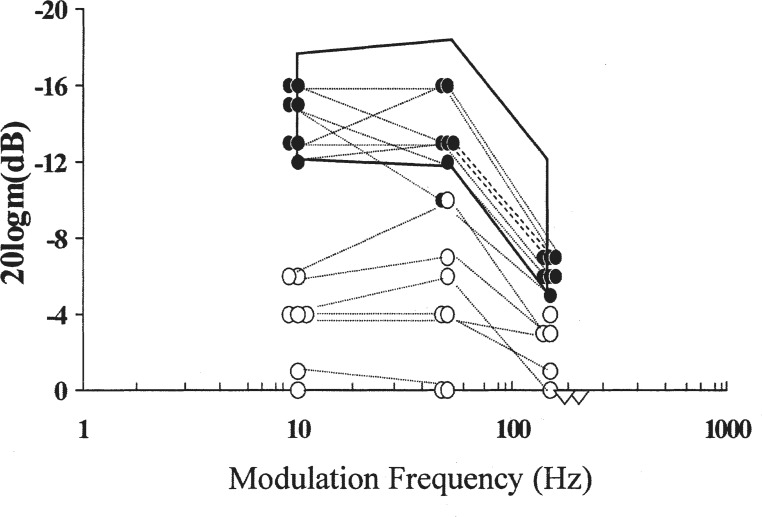
Fluctuation in both hearing level and perceptual ability is a reasonably common occurrence in patients with auditory neuropathy/dys-synchrony. Five of the 14 children presented by Rance et al. (1999), for whom repeated measures were available, showed significant hearing level fluctuations with threshold variances of approximately 20 dB. An example of the findings for one such child can be seen in Figure 3. These fluctuations, although not as dramatic as those reported by Gorga et al. (1995) and Starr et al. (1998) for their patients with temperature-sensitive neuropathy, were reported by parents and teachers to produce clear differences in functional hearing generally and speech understanding in particular. The Sininger and Oba (2001) and Starr et al. (2000) database findings have subsequently shown a similar proportion (29%) of ears with significant hearing level fluctuations.
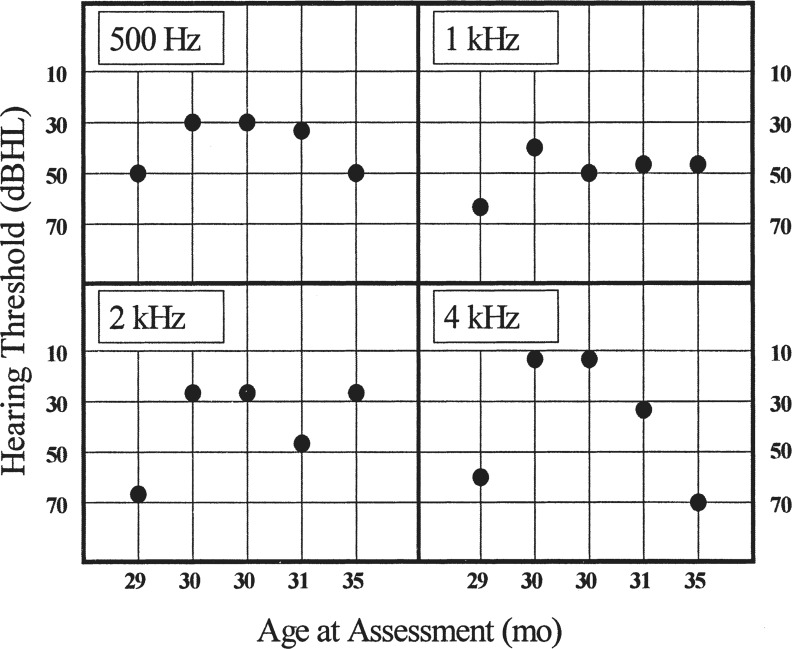
Figure 3.
Audiometric results for a 5-year-old child with auditory neuropathy/dys-synchrony type hearing loss. The five assessments were carried out over a 6-month period. Results obtained were considered to be an accurate reflection of the child's acuity for that day (Rance et al., 1999). Reproduced with permission of Lippincott, Williams Wilkins Publishing Group.
In addition to these ears with level fluctuation that show no overall directional trend, cases have been reported of long-term hearing deterioration and of long-term recovery with auditory neuropathy/dys-synchrony. Starr et al. (2000) and Sininger and Oba (2001) found that approximately 15% of the subjects in their database(s) showed deterioration of greater than 10 dB at three or more test frequencies over a series of hearing evaluations. In contrast, these authors found 1 patient who showed a 15 to 20 dB threshold improvement over time.
Other studies have reported dramatic hearing level improvements in affected children. Madden et al. (2002) presented evidence of spontaneous hearing recovery in 9 of the 22 auditory neuropathy/dys-synchrony children in their sample. In most, the behavioral audiogram improved from the profound to the moderate-to-severe range, but in 4 subjects, hearing thresholds reportedly improved to normal or near-normal levels. Hearing recovery was more likely in this group amongst the subjects who had suffered neonatal hyperbilirubinemia, and in all cases, had occurred before the age of 25 months.3 Other studies reporting improvements in hearing include Stockard et al. (1983), Kileny and Robertson (1985), Stein et al. (1996), and Berlin et al. (1997).
Hearing Loss Configuration
Audiograms with a low-frequency emphasis (reverse slope) are a reasonably common finding in both adults and children with auditory neuropathy/dys-synchrony. Eleven (28.9%) of the 38 ears presented in Rance et al. (1999) showed this configuration. The survey results presented by Sininger and Oba (2001) and Starr et al. (2000) showed similar findings, with rising audiograms reported in about 30% of ears in both studies. The high-frequency hearing loss configuration most commonly seen with sensorineural type hearing loss was only observed in approximately 10% of cases in these reports.
Acoustic Reflexes
Abnormal middle-ear muscle reflexes are a consistently reported finding for both adults and children with auditory neuropathy/dys-synchrony type hearing loss. Apart from isolated instances (3 of 44 subjects in Sininger and Oba, 2001; 1 child in Deltenre et al., 1997) acoustic reflexes have been absent to both ipsilateral and contralateral stimulation in almost all published cases, including those with normal or near-normal audiometric thresholds. The mechanism underlying this phenomenon has been a matter of some conjecture, but recent reports have shown that nonacoustic middle-ear muscle reflexes can be elicited in auditory neuropathy patients by tactile stimulation to the face, suggesting that the efferent components of the reflex arc (facial nerve and stapedius muscle) are intact (Gorga et al., 1995; Starr et al., 1998). Furthermore, Konradsson (1996), in a study involving 4 children with unilateral auditory neuropathy/dys-synchrony, found that an acoustic reflex in the AN/AD ear could be elicited by contralateral stimulation but that neither ipsilateral nor contralateral responses could be seen when the stimulus was directed to the affected side. As such, it is most likely that in patients with auditory neuropathy/dys-synchrony, the afferent pathway (auditory nerve) is not able to provide sufficiently high or sufficiently synchronized rates of discharge to activate the motor neurons of the stapedius muscle (Starr et al., 1998).
Evoked Potentials from the Central Auditory Pathways
As one of the signature features of the auditory neuropathy/dys-synchrony result profile is the absence or severe disruption of the auditory brainstem response, it might be expected that more central evoked responses such as the middle latency and cortical auditory evoked potential (CAEP) would be similarly affected. And yet, many of the reported cases have shown clearly identifiable responses with reasonably normal morphology and response latency (Gorga et al., 1995; Hood, 1999; Kraus et al., 2000; Rance et al., 2002; Zeng and Liu, in press). Figure 4 (from Rance et al., 2002) shows the similarity between averaged CAEP waveforms obtained for a group of AN/AD children with those from cohorts of age-matched children with normal hearing and sensorineural hearing loss.
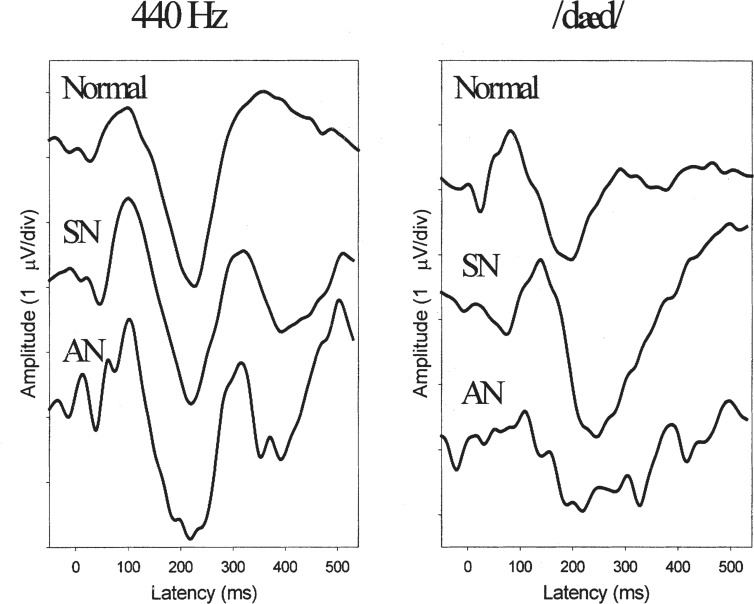
Figure 4.
Grand mean cortical event-related potential waveforms in response to tones (left panel) and to speech (right panel) for children with normal hearing (top traces), sensorineural (SN) hearing loss (440 Hz: N = 17; /dæd/: N = 15, middle traces), and auditory neuropathy (AN) (N = 11, bottom traces). Reproduced with permission of Lippincott, Williams Wilkins Publishing Group.
CAEPs may be recordable in some cases of auditory neuropathy/dys-synchrony because they are less dependent on synchronous neural firing than auditory brainstem responses. The peaks in the normal auditory brainstem response waveform are biphasic and are usually only separated by approximately 1 ms. Small variations in the timing of responses to individual stimuli can thus lead to cancellation in the averaged signal. In contrast, the component peaks in the CAEP waveform, which are much broader and are separated by 50 to 100 ms in adult subjects (and longer in children), are more resistant to subtle fluctuations in the timing of individual responses.
Evidence of the different tolerance of the auditory brainstem response and CAEPs to synchrony disruption has come from studies examining the timing of component responses. Starr et al. (1991) manipulated the synchrony of auditory brainstem responses by systematically varying the timing of each stimulus relative to the start of the averaging window. This study demonstrated that (for the cat auditory brainstem response at least), timing fluctuations of the order of tenths of a millisecond are sufficient to disrupt the averaged response. In contrast, studies considering the timing of responses from the auditory cortex have shown a much greater tolerance to temporal fluctuation. Michalewski et al. (1986), for example, determined the latency of various cortical event related potentials, including N1 and P2, in normal adult subjects for individual stimulus trials and showed peak latency standard deviations of about 17 ms for the N1 potential and 22 ms for the P2 potential. These individual trials, when subjected to conventional signal averaging procedures, produced robust waveforms.
The point at which synchrony disruptions associated with auditory neuropathy/dys-synchrony type loss might begin to affect averaged potentials from the auditory cortex is unclear at this stage. However, if the standard deviation of normal temporal fluctuation in these potentials is around 20 ms., then the level of dys-synchrony required to affect the CAEP waveform is likely to be of the order of tens of milliseconds. This level is significantly higher than that required to disrupt the auditory brainstem response and as such, the cortical event-related potentials can offer a gross measure of the effect of peripheral neural disruption on the signal reaching the auditory cortex. Furthermore, these responses may offer insights into the neural representation of speech in affected subjects (Rance et al., 2002).
Speech Perception in Adults with Auditory Neuropathy/Dys-synchrony
Speech perception difficulties are a consistently reported consequence of hearing impairment. In postlinguistically deafened adults with sensorineural loss, a reasonably strong relationship exists between the behavioral audiogram and open-set speech understanding. Not surprisingly, subjects with greater degrees of loss typically show poorer perception (Walden, 1984; Yellin et al., 1989). The exact cause(s) of the perceptual problems in these cases is still a matter of debate, but the general consensus is that speech understanding is limited by signal audibility for losses up to about 60 dBHL and by a combination of audibility and cochlear distortion effects for losses of greater degree (Glasberg and Moore, 1989; Moore, 1995).
In contrast, speech perception ability in adults diagnosed with auditory neuropathy/dys-synchrony-type hearing loss has shown no correlation with the pure-tone audiogram (Starr et al., 2000; Zeng et al., 2001b), and in most cases, has been significantly poorer than would have been expected for sensorineural losses of equivalent degree. Starr et al. (1996) presented open-set speech perception findings for 8 of the 10 subjects in their sample. Word recognition scores ranged from 0% to 92% and were significantly lower in 12 of the 16 ears than predicted from the norms generated by Yellin et al. (1989) for ears with sensorineural hearing loss. Similarly, Sininger and Oba (2001) reported speech discrimination scores (CID W-22 lists) for 36 of their (mostly adult) auditory neuropathy/dys-synchrony patients that showed 25 (69%) fell below the Yellin et al. (1989) normative range. Other examples of auditory neuropathy adults with extreme speech perception difficulties have been presented by Jerger et al., 1992; Berlin et al., 1993; Sininger et al., 1995; Widen et al., 1995; Berlin et al., 1996; Kaga et al., 1996; Starr et al., 2000; Zeng et al., 2001a; Mason et al., 2003; Starr et al., 2003; and Zeng and Liu, in press.
The data presented in these studies demonstrate that in many cases of adult auditory neuropathy/dys-synchrony, speech signal disruption can occur that is more extreme than that observed in sensorineural hearing loss. However, not all of the reported adult AN/AD cases have shown unusually poor speech understanding (at least in quiet listening conditions). For example, 25% of the ears presented by Starr et al. (1996) and 30% of the Sininger and Oba (2001) subjects showed speech perception scores within the normal range for sensorineural losses of equivalent degree. Most of the reported adult auditory neuropathy/dys-synchrony cases have suffered from progressive, generalized neuropathic conditions. It is therefore possible that in some of these patients with sensorineural-like speech perception ability, the disease process was less advanced than in their more affected peers, and hence their perception at the time of the assessments was less disrupted. Longitudinal monitoring of these cases will in time make this situation clearer. What the current results do show, however, is that good speech understanding is possible in ears with absent or grossly abnormal auditory brainstem responses.
In addition to the auditory neuropathy/dys-synchrony patients with “sensorineural-like” speech understanding, there have been cases of “normal” perception with AN/AD. Kraus et al. (2000) presented findings for a 24-year-old woman with an unremarkable medical history and normal hearing thresholds who had experienced difficulties in background noise throughout childhood. She obtained a perfect word recognition score on a CUNY-Sentence assessment for stimuli presented in quiet, demonstrating that open-set speech perception can be achieved despite measurable neural disruption in the auditory brainstem. Assessment in noise (in this case multi-talker babble) did show abnormally depressed results, however. On open-set word testing at a +3 dB signal-to-noise ratio for example, this subject scored only 10% correct where the mean score for a control group of normal subjects was 50%.
Shallop (2002) has also presented a case of a woman diagnosed with hearing thresholds in the mild-to-moderate range when in her late 20s, but who had reported difficulties in noise throughout childhood. Hearing in Noise Test (HINT) sentence testing in this case also showed 100% perception in quiet listening conditions but extreme difficulty in noise. Word identification for this subject fell to 25% at a +15 dB signal-to-noise ratio and to 0% at +12 dB. These cases illustrate the often-reported observation that adult auditory neuropathy/dys-synchrony sufferers have particular problems in background noise and suggest that although good speech understanding may be possible in ideal listening circumstances, even the least-impaired adult AN/AD subjects may struggle when redundancies in the speech signal are compromised.
Speech perception difficulties in background noise are not unique to auditory neuropathy/dys-synchrony-type hearing loss. Patients with sensorineural loss are also known to struggle with competing signals (Bilger et al., 1984). The effects of noise in AN/AD cases do, however, tend to be extreme. Zeng and Liu (in press), for example, recently studied in detail the perception of 14 (mostly adult) subjects and found consistent reductions in speech recognition ability, even at signal-to-noise ratios that show little or no effect on subjects with normal hearing (10 to 15 dB).
The mechanisms underlying these perceptual difficulties in noise are unclear. They are however consistent with the findings of recent psychophysical studies that have shown excessive masking of pure tones in auditory neuropathy/dys-synchrony subjects by simultaneous noise, as well as noise bursts presented before and after the test signal (Kraus et al., 2000; Zeng et al., 2001b; Zeng et al., in press).
In summary, most reported adult auditory neuropathy/dys-synchrony patients have shown severely disrupted speech perception. However, the proportion of AN/AD cases with particular speech perception problems has yet to be determined. Speech perception scores in 75% of the ears in the Starr et al. (1996) sample were poorer than expected from their behavioral audiogram, but in most instances, speech perception difficulty was the identifying characteristic in these patients. As mentioned, there are documented cases with perceptual abilities that fall within the expected performance range for sensorineural hearing loss, and there may be a population of adults who would fit the AN/AD physiologic profile but who are yet to be identified.
Speech Perception in Children with Auditory Neuropathy/Dys-synchrony
As with adult patients, disproportionate speech perception difficulties have been a consistently reported symptom in children with auditory neuropathy/dys-synchrony. Anecdotal evidence, beginning with the first auditory brainstem response papers to identify the condition in children (Davis and Hirsch, 1979; Worthington and Peters, 1980), has consistently suggested that young subjects with prelingual onset of AN/AD are at risk of significant perceptual problems and delays in speech and language development.
Despite the widely held concern regarding the integrity of the speech signal in pediatric auditory neuropathy/dys-synchrony cases, there has been a paucity of formal speech perception data presented in the literature. Amongst the papers that have presented formal data, it has been the opinion of the authors in almost all instances (Kraus et al., 1984; Starr et al., 1991; Gravel and Stapells, 1993; Gorga et al., 1995; Berlin et al., 1996; Konradsson, 1996; Doyle et al., 1998; Starr et al., 1998; Miyamoto et al., 1999; Rance et al., 1999; Simmons and Beauchaine, 2000; Lee et al., 2001) that perceptual abilities poorer than predicted by the behavioral audiogram were apparent in some or all of their patients.
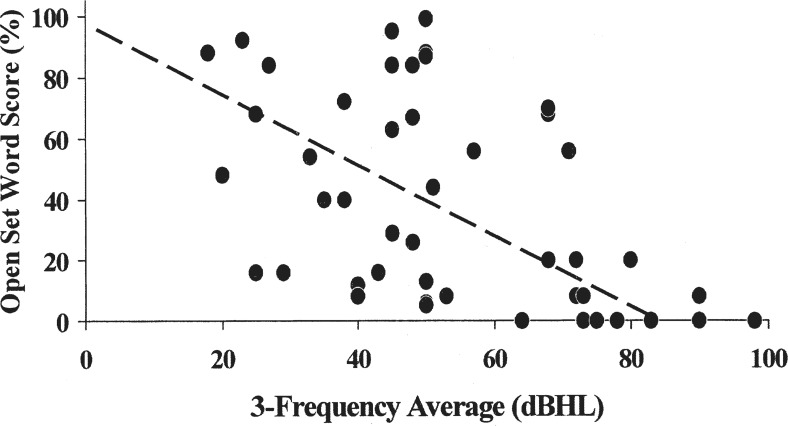
Comparisons between open-set word scores from subjects for whom 3-frequency average (1 kHz /2kHz /4 kHz) hearing levels were available, and the norms provided by Yellin et al. (1989) are shown in Figure 5. Overall, excluding the ears with pure-tone averages of 80 dBHL or more, for whom the minimum normal score in ears with sensorineural loss is zero, there are results for 41 individual ears showing the auditory neuropathy/dys-synchrony result pattern. Open-set word scores in 18 (44%) of these were within the expected range, and 23 (56%) of 41 ears were either borderline abnormal or significantly poorer than would have been expected for adults with equivalent degrees of sensorineural hearing loss.
Figure 5.
Open-set word/average hearing level comparisons for 46 children with auditory neuropathy/dys-synchrony type hearing loss. The dashed line represents the minimum expected score for ears with sensorineural hearing loss (Yellin et al., 1989). Contributing studies are listed with the number of ears for each.
| Starr et al. (1991): | 4 |
| Sininger et al. (1995): | 2 |
| Berlin et al. (1996): | 2 |
| Konradsson. (1996): | 3 |
| Picton et al. (1998): | 2 |
| Starr et al. (1998): | 2 |
| Miyamoto et al. (1999): | 4 |
| Lee et al. (2001): | 4 |
| Rance et al. (2004): | 14 |
| Zeng et al. (in press): | 9 |
As with adult auditory neuropathy/dys-synchrony subjects, affected children are often reported to have extreme difficulty in background noise even if their speech perception is good in quiet listening conditions. For example, in their study involving 3 subjects with temperature-related AN/AD, Starr et al. (1998) found that 2 children who had 100% open set discrimination in quiet (when well), scored below the 10th percentile for age in background noise. Similarly, Gravel and Stapells (1993) found markedly abnormal results on the Pediatric Speech Intelligibility Test for a child when assessed in the presence of a competing signal. The use of personal frequency modulated (FM) systems to improve signal-to-noise ratios has thus been recommended by a number of authors (Berlin, 1999; Kraus et al., 2000).
While the poor speech perception ability reported for many children with auditory neuropathy/dys-synchrony-type hearing loss is likely to be the result of signal degradation in the auditory pathway, the test scores may in some instances have been influenced by nonauditory factors. Among adult subjects with late (postlinguistic) onset hearing loss, it is usual to assume that the knowledge of language structures and speech production abilities are uniform and are not likely to exert an influence over the speech perception test results. Performance variations are therefore considered to reflect differences in access to the sensory input. In young children, generally, and children with prelingual onset hearing loss, in particular, the assumption of uniformity cannot be made (Boothroyd, 1995). As such, speech perception findings in youngsters with early-onset auditory neuropathy/dys-synchrony may be limited by factors unrelated to the quality of the neural signal provided to the brain by the auditory pathway.
Some nonauditory factors that could influence speech perception test performance relate to the child's age and developmental level and include speech production skills, concentration span, and cognitive abilities (Tyler, 1993; Boothroyd, 1995). Consideration of these factors is particularly relevant to children with auditory neuropathy/dys-synchrony, as many affected subjects have had rocky neonatal periods and are at high risk of neurodevelopmental delay (Franck et al., 2002). Such delays could impact their ability to perform in the test session and their overall progress in areas such as speech and language development. Much of the literature regarding children with auditory neuropathy/dys-synchrony has been anecdotal, with presented cases offering at best patchy details about the general developmental progress of the subjects. One study involving subjects with early-onset AN/AD that did look in depth at general developmental level was reported by Franck et al. (2002). This study examined long-term outcomes in 9 AN/AD children (8 of whom had high-risk histories) and included neurologic and psychological evaluation of various aspects of development, including motor, cognitive, speech and language, and social and behavioral skills. The pattern of developmental deficits varied, but all 9 children showed some degree of global delay or neurologic abnormality. Other studies to report general developmental delays in children with auditory neuropathy/dys-synchrony include Worthington and Peters (1980), Gravel and Stapells (1993), Deltenre et al. (1997), and Corley and Crabbe (1999).
One set of results in which the effect of general developmental factors on speech perception testing can be excluded is that presented by Konradsson (1996). This study involved 3 children with unilateral auditory neuropathy/dys-synchrony who each showed perfect word discrimination scores for the better ear and disproportionately poor speech perception in the AN/AD affected ear. The poor speech perception result in these cases was likely to be caused by whatever mechanism disrupted the auditory brainstem response. However, sensory deprivation might also have played a role in the diminished auditory capacity of these subjects. The hearing losses in the 3 children were all of moderate or severe degree. If the losses were present from infancy at the levels obtained at the time of their speech assessments (6–11 years), then these ears are unlikely to have received any consistent auditory stimulation over an extended time period. This sensory deprivation could, in itself, cause alterations in the development and subsequent function of the auditory pathway, affecting the child's ability to make full use of their audition (Clopton and Silverman, 1978; Kitzes and Semple, 1985).
Long-term auditory deprivation may also have affected the speech perception abilities of other auditory neuropathy/dys-synchrony children reported in the literature. Most of them had not been provided with consistent amplification despite significantly elevated hearing levels.
The level of a child's speech and language development is another factor that can affect speech perception test performance (Boothroyd, 1995). Clearly this was not an issue in the unilateral cases presented by Konradsson (1996), but it may have affected the findings of some of the other studies involving children with significant bilateral hearing loses. The development of expressive speech and language skills in children with auditory neuropathy/dys-synchrony has not yet been addressed in detail, but it is clear from anecdotal reports that children with AN/AD often have significant speech production and language development problems (Davis and Hirsh, 1979; Worthington and Peters, 1980; Gravel and Stapells, 1993; Doyle et al., 1998). In some cases, these deficits may have affected the child's ability to score highly on both open- and closed-set speech perception assessments.
In summary, the speech perception findings for children with early-onset auditory neuropathy/dys-synchrony have resembled their adult counterparts, with many performing on formal assessments at levels poorer than would be expected for ears with sensorineural hearing losses of equivalent degree. However, it is unclear at this stage if the perceptual difficulties facing these children are qualitatively similar to those affecting adults with progressive neuropathic conditions. Furthermore, the effects of developmental factors associated with generalized neurologic abnormality and the lack of auditory stimulation during critical development periods (Deltenre et al., 1999) on speech perception test results have not yet been fully considered in these children.
Management of Auditory Neuropathy/Dys-synchrony
Amplification
The provision of hearing aids to patients (particularly children) with auditory neuropathy/dys-synchrony is currently a controversial issue. There are two main arguments against amplification for this population. The first relates to the issue of safety and the potential for damage to cochleae with outer hair cell function. The second concerns the inherent auditory pathway limitations in AN/AD subjects and the likelihood that conventional amplification will simply produce a louder but equally distorted signal.
Hearing aids can cause significant noise exposure that results in both temporary and permanent shifts in hearing threshold (Macrae, 1991, 1995). However, in children with sensorineural hearing loss in the mild-to-severe range, long-term amplification (5–9 years in the children studied by Macrae, 1995) at the real-ear insertion levels prescribed by the National Acoustics Laboratories (NAL) model appears to pose little or no risk of acoustic trauma, even with linear amplification techniques. High-gain amplification strategies necessary for adequate sound provision for children with profound loss (pure-tone average > 100 dBHL) have, however, produced significant threshold deterioration (up to 20 dB) in some cases (Macrae, 1995).
The potential for acoustic trauma through over-amplification is theoretically greater in ears with normal micromechanical cochlear processes (Starr et al., 1991). Permanent outer hair cell damage is a particular concern in ears with auditory neuropathy/dys-synchrony, as the efferent suppression and acoustic reflex mechanisms that are thought (amongst other things) to protect the cochlea from excessively loud sounds (Simmons, 1964; Borg et al., 1984) may be inactive (Berlin et al., 1993; Sininger et al., 1995; Hood et al., 1996; Starr et al., 1996).
Thus, it has been recommended that otoacoustic emissions be carefully monitored as a measure of outer hair cell health in auditory neuropathy/dys-synchrony ears that are being amplified (Hood, 1998) or that hearing aids not be considered unless emissions have already disappeared (Berlin, 1999). However, although otoacoustic emission amplitude reduction has been documented in children with high-powered amplification (Sininger and Oba, 2001; Trautwein et al., 2001), there have also been a number of reports of emission presence at normal amplitudes after long-term aid use (Katona et al., 1993; Doyle et al., 1998; Rance et al., 1999; Berlin et al., 2000; Starr et al., 2000; Lee et al., 2001; Sininger and Oba, 2001). Overall, no correlation has been established between hearing aid use and loss of otoacoustic emissions. Furthermore, a reasonably high proportion of subjects with AN/AD show otoacoustic emission amplitude reduction and subsequent loss in ears that have not been subjected to amplified sound at all (Deltenre et al., 1999; Starr et al., 2000).
The argument present by Hood (1998) and Berlin (1999) appears to be that hearing aid use should be limited to minimize damage to the outer hair cells and preserve the active cochlear mechanisms reflected by the otoacoustic emission. This contention is theoretically sound, but at this stage, there is no evidence that the processes generating the otoacoustic emission have any functional benefit in patients with auditory neuropathy/dys-synchrony. In fact, a number of authors (Deltenre et al., 1999; Rance et al., 1999; Starr et al., 2000) have presented results suggesting that the presence or absence of evoked otoacoustic emissions is unrelated to either hearing threshold sensitivity or speech perception ability in affected patients.
The second main argument against providing hearing aids to children and adults with auditory neuropathy/dys-synchrony rests on the assumption that increasing the amplitude of auditory signals will not overcome the pathologic mechanisms that have disrupted the auditory brainstem response and, in many cases, the unamplified speech signal. Berlin (1999), for example, advises against hearing aid fittings “not in an attempt to preserve (otoacoustic) emissions but simply because hearing aids are designed to compensate for missing outer hair cells.” The perceptual consequences of presenting high-level stimuli in ears with auditory neuropathy/dys-synchrony are yet to be fully investigated. As such, Cone-Wesson et al. (2001) have thus recommended that investigation of unaided speech perception performance-intensity functions be undertaken. Such investigations may be useful in improving our general understanding of perceptual deficits in AN/AD and may also provide helpful clinical insights when considering management options for individual subjects. A flat function, for example, may suggest that hearing aids will not substantially improve a particular subject's speech perception ability. Furthermore, speech performance rollover, such as seen with various types of retrocochlear abnormalities, may also argue against the usefulness of amplification (Cone-Wesson et al., 2001).
The potential for improvement in signal clarity with conventional amplification in ears with auditory neuropathy/dys-synchrony is unknown but is likely to be limited. While there is some evidence that the firing properties of afferent fibers in the auditory pathway of normally hearing subjects show increased phase locking and synchronous discharge as sensation levels increase (Javel, 1986; Phillips and Hall, 1990), similar improvements are yet to be demonstrated in subjects with auditory pathway abnormalities. What is clear in most patients with auditory neuropathy/dys-synchrony is that stimulus level increases fail to produce recordable auditory brainstem responses, even at levels well in excess of hearing threshold. This suggests no significant increase in either the amount (conduction block) or the synchrony of neural activity in the auditory brainstem.
One way in which amplification can improve speech perception ability in auditory neuropathy/dys-synchrony subjects with elevated hearing thresholds is by improving their access to speech sounds. A number of studies have now reported aided/unaided threshold improvements consistent with the level of gain provided by their hearing devices (Berlin et al., 1996; Deltenre et al., 1999; Trautwein et al., 2000; Cone-Wesson et al., 2001). Similar results were obtained for most of the children reported in Rance et al. (1999). Most of the subjects in this investigation showed aided thresholds that improved in accordance with NAL prescription targets to levels that afforded them complete access to the long-term 70-dBSPL speech spectrum.
Approaches to Fitting Hearing Aids in Subjects with Auditory Neuropathy/Dys-synchrony
The provision of hearing aids to subjects with auditory neuropathy/dys-synchrony has not been approached systematically. Many early-identified subjects, such as the adult presented by Prieve et al. (1991) who had been a consistent aid user for 28 years at the time of publication, were amplified as if they had sensorineural hearing losses because there was no evidence to suggest that they did not have a cochlear site of lesion. Management approaches for more recently identified cases of AN/AD have tended to be more varied, making interpretation of published results difficult.
Some authors have considered that amplification should not be used at all for children with auditory neuropathy/dys-synchrony (Berlin, 1999; Berlin et al., 2002), or that if hearing aids are trialed, they should only be fit monaurally and should be low-gain, wide-dynamic-range compression devices, even in subjects with severe-to-profound hearing loss (Hood, 1998). As a result, many clinics around the world have proceeded cautiously with aid fittings in newly diagnosed children, often under-amplifying them and potentially providing only limited access to the normal speech spectrum.
Hearing-Aid Performance in Subjects with Auditory Neuropathy/Dys-synchrony
Despite the considerable debate that currently exists about the potential risks and benefits of amplification in auditory neuropathy/dys-synchrony subjects, relatively few studies have presented evidence of aided function (speech perception results). Anecdotal reports of (on the whole) unsuccessful hearing aid fittings began to emerge about the time that the condition was first identified (Squires and Hecox, 1983; Kraus et al., 1984). Amongst adults with the late-onset form of AN/AD, acceptance of amplification has been almost universally poor, with reports ranging from little or no benefit (Berlin et al., 1993; Sininger et al., 1995; Widen et al., 1995; Berlin et al., 1996; Starr et al., 1996) to “detrimental effects” (Starr et al., 1996). Starr et al. (1996) did not elaborate on what these effects might be, but Sininger et al. (1995) reported that amplification in their 44-year-old subject with a moderate, predominantly low-frequency loss “interfered rather than helped with communication”.
Anecdotal reports of hearing aid outcome in children with auditory neuropathy/dys-synchrony have been more varied but in most cases have been poorer than would have been expected for ears with equivalent degrees of sensorineural hearing loss. Berlin and colleagues have suggested in a series of articles (Berlin et al., 1996; Berlin, 1999, Berlin et al., 2002; Berlin et al., 2003) that hearing aids produce no obvious benefits, although these papers have generally failed to cite specific examples or provide even basic statistics regarding the results obtained in their sample. In summarizing their findings for 193 auditory neuropathy/dys-synchrony patients seen over a 20-year period, these authors have concluded that “while hearing aids improved detection thresholds, the long-term value of hearing aids in understanding speech is far poorer than predicted based on the audiogram and/or articulation index alone” (Berlin et al., 2003). Berlin et al. (1998) did describe two pediatric cases for whom they concluded that there was “no compelling evidence” that amplification had helped. Both of these subjects had, however, only recently been fitted with low-gain hearing aids at the time of writing. Furthermore, audiometric details were not provided, making it difficult to interpret the outcome of these hearing aid trials.
Other authors have used conventional aiding strategies in fitting hearing devices to young children with auditory neuropathy/dys-synchrony and still found that amplification was of little benefit. Trautwein et al. (2000), for example, used the standard desired sensation level (DSL) amplification paradigm (Seewald et al., 1997) to fit an 18-month-old AN/AD child who later received a cochlear implant. Despite showing behavioral hearing levels in the profound range bilaterally, aided threshold testing in this child revealed good access to the normal 70-dBSPL speech spectrum, particularly in the low-to-mid frequencies, where detection thresholds of about 40 to 55 dBHL were obtained in both ears. Aided phoneme detection testing also revealed good speech sound awareness, with the child scoring 27/30 on the Ling Six Sounds Test. However, despite the good sound access provided by the amplification, speech discrimination was poor. Aided assessments at 3 years of age revealed “no closed- or open-set discrimination”.
A number of authors have described preoperative findings for young cochlear implant candidates with auditory neuropathy/dys-synchrony and severe-to-profound hearing loss. Miyamoto et al. (1999) presented a child with Friedreich's ataxia who had shown progressive hearing deterioration and assorted balance and neurologic problems. No formal results were presented in this study, but it was the opinion of the authors (after only 1 month of aid use), that the speech perception benefits were minimal. Similarly, Shallop et al. (2001) presented 5 children with auditory neuropathy/dys-synchrony and concluded that 4 “were indifferent to amplification”. The fifth child also showed no benefit from amplification but had limited experience because discomfort issues restricted device use to the assessment sessions.
The presence of profound hearing loss in the Miyamoto et al. (1999) and Shallop et al. (2001) cases may have affected speech perception performance by limiting access to the amplified speech spectrum. No such problems were encountered with the child reported by Simmons and Beauchaine (2000), who presented with hearing thresholds that were primarily in the mild-to-moderate hearing loss range. Aided benefit, as in the previously mentioned studies was, however, reported to be minimal, and amplification was removed after a “relatively short” time following a “lack of improvement in auditory behaviors”.
In contrast to the consistently poor findings with amplification in adult subjects with auditory neuropathy/dys-synchrony, a number of studies have provided anecdotal reports of positive outcomes in at least some children with AN/AD. Katona et al. (1993) presented preliminary findings for a profoundly deaf AN/AD child fitted with high-powered hearing aids (Phonak PP-C-LA) in infancy. These authors found no tolerance problems and reported good sound awareness and subjective performance at 8 months of age. Similarly, Franck et al. (2002) reported that a young child (2 years 10 months at publication) with severe-to-profound hearing loss was “showing some auditory benefit” with hearing aids that were initially set conservatively but were later configured in accordance with the DSL targets based on the behavioral audiogram. Madden et al (2002), in a larger scale investigation involving 16 children with auditory neuropathy/dys-synchrony type hearing loss, also found evidence of benefit with standard amplification and concluded that “patients in our study … have responded well to conventional rehabilitation and amplification.”
A survey of amplification outcomes in 29 auditory neuropathy/dys-synchrony children followed by the House Ear Institute was presented by Cone-Wesson et al. (2001). Fourteen (48%) had ceased to be consistent hearing aid-users, suggesting that they had received little or no benefit. Three children (10%), on the other hand, were considered to have shown “fair-to-good benefit,” as evidenced by improvement in aided thresholds and speech perception, and 5 children (17%) showed threshold improvements but no measurable perceptual benefits. Among the other cases, who were too young or too difficult to assess, or both, reports from parents and teachers indicated that “some” children had shown benefit from amplification.
Aided Open-Set Speech Perception Results in Children with Auditory Neuropathy/Dys-synchrony
To date only four publications have formally addressed the issue of amplification benefit using comparisons of open-set aided and unaided speech perception findings. The results of these studies are summarized in Figure 6.
Figure 6.
Open-set speech perception results (aided/unaided) for children with auditory neuropathy/dys-synchrony. Study A: Deltenre et al. (1999); Study B: Rance et al. (1999); Study C: Lee et al. (2001); Study D: Rance et al. (2002).
Deltenre et al. (1999) presented results for a young child who had suffered serious neonatal complications, including hyperbilirubinemia and respiratory distress syndrome, after a premature birth at 28 weeks. The auditory neuropathy/dys-synchrony result pattern was identified in infancy, and hearing thresholds in the moderate hearing loss range were subsequently established. Amplification was withheld until this child's otoacoustic emissions spontaneously disappeared at 4 years of age. The specifics of the aiding strategy were not defined, but the gain and maximum power output values were broadly consistent with the NAL/DSL prescriptions for sensorineural hearing loss of moderate degree. Improvements in this child's language skills were noted soon after fitting, and significant aided speech perception benefits were demonstrated on word identification score measurements at 6 and 7 years of age. Open-set word scores improved from 0% in the binaural unaided condition to 80% in the binaural aided condition at the first of these assessments and from 28% (binaural unaided condition) to 95% (binaural aided condition) at the second of these assessments.
In contrast, Lee et al. (2001) have presented speech perception data indicating no speech perception improvement with amplification in two auditory neuropathy/dys-synchrony children with bilateral moderate-to-severe hearing loss. Neither child presented with any risk factors when they were diagnosed and aided in infancy. No amplification details were provided, but the AN/AD diagnosis was not made until the children were assessed as part of a school-based otoacoustic emission survey program at 11 and 12 years of age, respectively. As such, it is reasonable to assume that they were aided according to the prescriptions for sensorineural hearing loss. In both children, similar (poor) open-set speech perception scores on the Cantonese Speech Discrimination Test were obtained in both the unaided and aided conditions. That these children were unable to effectively use their hearing for speech perception despite early diagnosis and fitting and approximately 10 years of listening experience suggests that the neural disruption, implied by the auditory neuropathy/dys-synchrony result pattern, was significantly affecting the integrity of the speech signal in these subjects.
Variable aided open-set speech perception results were obtained by Rance et al. (2002) in a study involving 15 children with the auditory neuropathy/dys-synchrony type hearing loss. The children had typically been diagnosed in infancy, and had all been fit with conventional amplification to the level of their hearing loss at least 12 months before speech perception was assessed. Each child scored at close to chance levels when evaluated in the unaided condition using the PBK Words Test presented live voice at conversational levels of about 70 dBSPL. In the aided condition, 7 subjects showed no significant improvement despite being afforded complete access to the aided speech spectrum by their hearing devices. However, the other 8 children did show a significant aided benefit, with a mean PBK-phoneme score of 67.2% and a mean difference score (aided-unaided) of 56.8%.
Amplification Outcomes and Degree of Hearing Loss
The relationship between sensorineural hearing loss and speech perception ability in postlinguistically deafened adults has been well documented (for reviews, see Walden, 1984 and Yellin et al., 1989). Not surprisingly, individuals with greater degrees of loss have consistently shown poorer discrimination ability. Similar results have been obtained in studies involving children with sensorineural hearing loss (Bamford et al., 1981; Boothroyd, 1997; MacArdle et al., 1999). Rance et al. (2002) sought to investigate the effect of degree of hearing loss on aided speech perception in children with auditory neuropathy/dys-synchrony type loss. This study compared the perceptual ability in a group of affected subjects with a cohort of age- and audiogram-matched children with sensorineural hearing loss.
Aided open-set speech perception ability in the children with sensorineural loss assessed in Rance et al. (2002) was correlated with the audiogram. Subjects with greater degrees of hearing loss tended to show poorer PBK scores than their counterparts with better audiometric thresholds. This was particularly the case for children with average hearing levels in excess of 60 dBHL. Interestingly, this result was not influenced by the audibility of the speech signal, as aided thresholds and articulation index scores were similar across children. These findings are consistent with the results of psychophysical studies in hearing impaired adults that suggest the limiting factors in speech understanding are cochlear distortion effects, which increase with greater degrees of sensorineural hearing loss and are perhaps related to the loss of the cochlear amplifier, rather than reduced audibility (Glasberg and Moore, 1989; Moore, 1995).
Overall, aided speech perception ability in the group of children with auditory neuropathy/dys-synchrony was not correlated with pure tone sensitivity. Some children with hearing levels in the severe-to-profound range showed reasonable speech perception, and yet others with only mild-to-moderate hearing loss had negligible perceptual ability (Figure 7). Aided PBK test performance among children with auditory neuropathy/dys-synchrony appeared to fall into two distinct categories. A child either scored at chance levels (<10%), suggesting no ability to make use of speech information, or performed at significant levels (>35%). Clearly, the subjects in the former group, all but one of whom had near complete access to the amplified speech spectrum with aided articulation index values of 0.75 or more, were suffering from significant distortion in the neural code.
Figure 7.
PBK phoneme scores in the aided test condition are shown as a function of 3-frequency average hearing level for children with sensorineural (open circles) and auditory neuropathy/dys-synchrony type hearing loss (filled circles).
In contrast, the other 8 auditory neuropathy/dys-synchrony subjects were able to make use of the access to the speech spectrum afforded by their hearing aids. In fact, the aided PBK scores obtained for this subset of the AN/AD group were indistinguishable from their sensorineural counterparts. That is, their speech perception scores were equivalent to the children with sensorineural hearing loss and were similarly correlated to the degree of hearing loss. Once again, it is unclear if these AN/AD children benefited from improvements in the clarity of the neural representation of speech or simply from being better able to hear speech with amplification. What is clear is that on speech perception testing (and anecdotal reports from teachers of the deaf and parents), the hearing aids afforded these children at least some degree of functional hearing.
Age at Intervention
Recent reports (Deltenre et al., 1999; Madden et al., 2002; Rance et al., 2002) describing amplification benefits in auditory neuropathy/dys-synchrony children do appear to be out of step with the weight of anecdotal evidence presented thus far. One possible explanation for the unusually positive findings in these papers may be related to the age at which the children received their hearing devices. The children in each of these studies were identified and amplified in infancy. The link between speech and language deficits in children with prelingual sensorineural hearing loss is well established. So too are the ameliorating effects of early diagnosis and intervention (Markides, 1986; Levitt and McGarr, 1988; Ramkalawan and Davis, 1992). Recent studies by Yoshinaga-Itano et al. (1998) and Møeller (2000) have shown that hearing-impaired children who are amplified and receive educational support in the first 6 months of life have significantly greater potential for speech and language development than do children who receive intervention at a later age. It thus seems logical that children with auditory neuropathy/dys-synchrony should show similar early intervention benefits (Deltenre et al., 1999). Concerns about the potential for benefit and for acoustic trauma have led to amplification delays of a number of years in many of the reported pediatric AN/AD cases. This sensory deprivation during critical developmental periods may in itself, have limited amplification outcomes in some cases.
Possible Differences Between Early-and Later-Onset Forms of Auditory Neuropathy/Dys-synchrony
The positive amplification results reported for some children with the auditory neuropathy/dys-synchrony result profile are inconsistent with the findings for affected adults. It is unclear at this stage whether the early-onset form of AN/AD (which is typically related to neonatal insult) results in perceptual disruptions that are qualitatively different from those observed in subjects with the late-onset form of the condition (typically associated with generalized neuropathy abnormality). However, if they do represent a similar level of neural disruption, one might expect the early-onset patients to cope better because they grow up with an impaired but reasonably consistent neural signal. The late-onset neuropathy cases, on the other hand, are faced with a different and deteriorating neural signal at a time (usually adolescence or adulthood) when their ability to cope with these changes may be reduced.
In summary, while amplification has shown little or no perceptual advantage in adult subjects with auditory neuropathy/dys-synchrony type hearing loss, some evidence suggests that a significant proportion of affected children can benefit from conventional hearing aids. That benefit has been documented with both anecdotal reports and speech perception tests. Prolonged exposure to high levels of sound can cause cochlear insult, but there is no compelling evidence at this stage that amplification has resulted in a permanent threshold shift or even permanent changes to outer hair cell function in AN/AD children. Since the loss of otoacoustic emissions can occur without amplification and does not appear to affect pure tone sensitivity or speech perception, monitoring the effects of noise exposure with otoacoustic emissions may be misleading.
Cochlear Implants
The first set of results for a confirmed auditory neuropathy/dys-synchrony subject who had undergone cochlear implantation were presented in Rance et al., 1999. Since then, the identification of greater numbers of AN/AD cases with severe-to-profound hearing loss, the often poor speech-perception performance of affected subjects, and limited success with conventional amplification has led many clinicians and patients to consider the cochlear implant management option. Despite the specific risks to cochlear structures during the procedure (O'Leary et al., 1991; Gstoettner et al., 1997) and outcome uncertainties related to cite of lesion variability, hundreds of children and adults around the world with AN/AD-type hearing loss have undergone the procedure.
Most implanted auditory neuropathy/dys-synchrony subjects have shown no particular device programming abnormalities and have received auditory sensations to electrical current at normal presentation levels. Furthermore, these patients, who typically presented with no little or no preoperative speech discrimination ability, have generally shown significant perceptual benefits and have performed on a range of speech discrimination tasks at levels consistent with their implanted sensorineural peers (Trautwein et al., 2000; Shallop et al., 2001; Trautwein et al., 2001; Madden et al., 2002; Shallop, 2002; Mason et al., 2003; Peterson et al., 2003; Zeng and Liu, in press). Postoperative speech production development has also been found to be equivalent in AN/AD cases to matched groups of sensorineural children (Buss et al., 2002).
The dramatic improvements afforded by the cochlear implant device raise questions about the particular advantages electrical stimulation strategies provide in these cases. One obvious benefit is that the implant device provides broad access to the speech spectrum. Preoperative hearing aid details have typically not been presented in the recent case reports, but as most implanted individuals have had profound hearing loss, it is likely that even with high-powered hearing aids they could not hear the entire range of speech sounds. Aided-audiograms obtained with the cochlear implant in auditory neuropathy/dys-synchrony cases have, in contrast, shown complete access to the normal speech spectrum (Trautwein et al., 2000; 2001; Shallop et al., 2001; Buss et al., 2002). Thus, the likely improved detection of speech sounds provided by a cochlear implant offers at least an opportunity for speech understanding in these cases.
In addition to improved sound detection, there is also some evidence that electrical signals produced by cochlear implants may stimulate the auditory pathway more efficiently in some auditory neuropathy/dys-synchrony subjects than is possible with acoustic stimulation. In most implanted AN/AD cases, electrically evoked physiologic responses such as the compound action potential (Trautwein et al., 2000; Shallop et al., 2001; Trautwein et al., 2001; Shallop, 2002) and auditory brainstem response (Shallop et al., 2001; Trautwein et al., 2001; Buss et al., 2002) have been recordable. In these subjects, for whom no repeatable brainstem responses could be observed to acoustic stimuli, this change represents either an improvement in the synchrony of neural firing or an increase in the number of contributing neural elements.
Considerable evidence from single unit recordings in animal models also indicates that electrical stimulation provides a higher degree of neural synchrony in the auditory pathway than acoustic stimuli (Weiss and Rose, 1988; Parkins, 1989; Dynes and Delgutte, 1992). Dynes and Delgutte (1992), for example, made discharge pattern recordings from auditory nerve fibers in anesthetized cats to bursts of sinusoidal current at a range of stimulus frequencies. These authors found that auditory nerve firing to electrical signals, particularly at high stimulus frequencies, showed a greater degree of neural synchrony than had been observed in equivalent studies using acoustic signals.
In addition to the general synchrony advantage that appears to exist for electrical stimulation, the manner in which modern cochlear implant systems present their stimuli may also be conducive to generating synchronised neural activity. Stimulation of the spiral ganglion is achieved not via the presentation of a continuous electrical analog of the acoustic waveform but by a series of biphasic current pulses. The discrete, pulsatile nature of these signals may in itself produce more synchronized patterns of neural activity (Berlin, 1999).
The presence of recordable electrically evoked potentials and improved speech perception observed in auditory neuropathy/dys-synchrony patients with cochlear implants may also be a reflection of the amount of neural activity elicited rather than a synchrony improvement. As discussed previously, scalp recorded brainstem responses may be unrecordable in some AN/AD cases as a result of depletion in the number of neural elements available to contribute to the volume-conducted evoked potential. This situation is thought to occur in cases of selective inner hair cell loss. Cochlear implants bypass this step in the auditory pathway, stimulating the neural elements directly (Javel and Shepherd, 2000).
The positive cochlear implant results and the often poor amplification outcomes reported for auditory neuropathy/dys-synchrony patients have recently led to a great deal of enthusiasm for the procedure as a management option. Some authors (Berlin et al., 2003) have even suggested that clinicians should “bypass the hearing aid trial before implantation.” Isolated cases of poor cochlear implant outcome in AN/AD patients have been reported in the literature, however. The case study presented in Rance et al. (1999) is a clear example of a child with no obvious impediments to perceptual performance (apart from the postoperatively diagnosed AN/AD) whose speech discrimination and even sound detection with the cochlear implant have been severely compromised. Results for this subject showed no open or even closed-set speech perception ability at 1year after implant and later findings (reported in Rance et al., 2002) after 3 years of consistent device use also showed no perceptual improvement.
Unlike most of his implanted auditory neuropathy/dys-synchrony counterparts, this subject showed no repeatable brainstem potentials to electrical stimulation. Current evidence is insufficient to draw firm conclusions, but it would appear that an auditory pathway that can produce recordable evoked potentials to electrical stimulation is more likely to be able to support useful levels of speech perception. Preoperative techniques that can test this ability, such as those that use a needle electrode placed on the cochlear round window to present an electrical stimulus, may have a significant role in the implant candidature process (WP Gibson, personal communication, 1999).
Miyamoto et al. (1999) have also presented a case in which presumed neuronal loss in the VIII nerve and spiral ganglion may have produced a poor implant result in a child with Friedreich's ataxia. This child, who initially presented at 4 years of age with only mild hearing loss and good speech perception (PBK phoneme score was 92% in the better ear), showed steady deterioration in his hearing, physical status, and vision.
When implanted at age 10.9 years, he had profound hearing loss bilaterally and was scoring at near chance levels on open-set word testing. This child had no reported cochlear implant programming problems, and free-field testing showed normal detection thresholds. However, despite being afforded good access to the speech spectrum, his speech perception ability was limited, perhaps affected by his generalized neurologic difficulties. His PBK phoneme score of 20% after 12 months of device use was similar to his last preoperative result and was significantly poorer than those of a control group of 7 children implanted after progressive sensorineural loss.
Cochlear Implant Candidature
Specific criteria for cochlear implant candidature vary from clinic to clinic, but in older children and adults, selection is typically based upon the pure-tone audiogram and a comparison of the candidate's aided speech perception ability with performance norms for cochlear implantees. While results presented to date in most implanted auditory neuropathy/dys-synchrony patients have been promising, there is no evidence that their speech perception performance is significantly better than that of their sensorineural peers. As such, it would seem reasonable at this stage to use the standard preoperative selection criteria when considering candidates with AN/AD.
Most of the auditory neuropathy/dys-synchrony cases implanted thus far have presented with audiograms in the severe-to-profound hearing loss range. As discussed previously, however, a significant number of cases have lesser degrees of hearing loss but little or no ability to use that hearing. This group of patients is now being also being considered for the procedure. Shallop (2002) has presented cochlear implant results for two auditory neuropathy/dys-synchrony patients who were within the mild-to-moderate hearing loss range and who showed severe speech perception deficits and little or no benefit from conventional amplification. One is an adult diagnosed with AN/AD in her late 20s but thought to have had a fluctuating mild-to-moderate hearing loss throughout her childhood. Open-set preoperative speech perception in quiet was 100% on HINT sentences, but she experienced extreme difficulty in speech discrimination tests involving background noise (0% discrimination score at a +12 dB signal-to-noise ratio) and considered her hearing loss to be a significant disability. At the time of writing, her postoperative perceptual ability had not yet been fully evaluated, but she had shown improved word recognition in noise and reported that her general ability to cope in challenging auditory environments had improved. Shallop (2002) also mentions (but provides no results) for a young auditory neuropathy/dys-synchrony child implanted despite the presence of hearing thresholds in the moderate (45–70 dBHL) range.
Cochlear implant candidacy in young (<3 years) children with sensorineural hearing loss is typically determined from audiometric findings. In particular, a child's access to the amplified speech spectrum is considered. The correlation that exists between hearing levels and speech perception in ears with this form of hearing loss allows perceptual ability to be predicted and compared with expected cochlear implant performance. Results obtained for auditory neuropathy/dys-synchrony subjects have, however, shown that for children with AN/AD-type hearing loss, such predictions cannot be made. As such, a conservative selection approach requires that AN/AD candidates not be considered until their aided speech perception ability can be accurately established. Unfortunately, such determinations are usually not possible until the child is at least 2 or 3 years old, and it is now well established that implantation before that age range is highly desirable in hearing impaired children (Dowell et al., 2002).4
It may however be reasonable to consider young auditory neuropathy/dys-synchrony candidates based on their audiometric results if they present with hearing thresholds in the severe-to-profound range, as “no” instances have been reported of AN/AD children showing speech perception abilities superior to those expected for sensorineural losses of equivalent degree. Some AN/AD subjects with lesser degrees of hearing loss do, however, show better aided speech perception than that of an average implanted child (Deltenre et al., 1999; Rance et al., 2002), indicating that cochlear implantation should not, as suggested by some authors, be considered the primary management option for all AN/AD cases.
In summary, cochlear implantation is currently the most successful remediation strategy for patients with poor sensitivity and speech understanding caused by auditory neuropathy/dys-synchrony. Most implantees with this form of hearing loss show good access to the normal speech spectrum and speech perception abilities comparable with their sensorineural counterparts. However, because isolated cases with poor results have been reported, preneural assessment techniques need to be incorporated into the preoperative test battery, and candidates identified with AN/AD condition need to be counseled accordingly.
Perceptual Disruption in Subjects with Auditory Neuropathy/Dys-synchrony
The fact that speech understanding is severely disrupted in many patients with AN/AD type hearing loss despite adequate sound detection suggests that distortion of suprathreshold cues is the limiting factor in perceptual performance. Understanding the ways in which basic perceptual features are affected by auditory neuropathy/dys-synchrony, and how this impacts upon speech discrimination has been the aim of a number of recent psychophysics-based investigations.
Speech Perception and Psychophysics
The essential features of complex sounds, including speech, are the relative intensity of different frequencies (the spectrum and its shape) and how these vary over time. A listener's ability to understand speech depends on how well they can perceive these features. Various perceptual abilities that underpin speech perception have been identified and investigated in subjects with normal hearing and hearing impairment. Some of these include frequency resolution, temporal resolution, and frequency discrimination.
Frequency Resolution
Frequency resolution, also referred to as frequency selectivity, is the ability of the auditory system to separate or resolve the components in a complex sound. Discrimination of vowel sounds in speech, for example, can only be achieved if the formant peaks can be spectrally separated (resolved) and coded independently in the auditory pathway (Moore, 1995). This spectral processing is thought to be achieved in the normal cochlea by means of basilar membrane mechanics and is aided by the active process that is mediated by the outer hair cells, which leads to amplification and sharpening of the peaks in basilar membrane movement (Yates et al., 1992).
Evidence for this outer hair cell contribution to resolution ability has been provided by lesion studies in various animal models that have selectively destroyed one type of hair cells (inner or outer) while keeping the other intact. When outer hair cells are damaged by aminoglycosides or acoustic over-stimulation, for example, the result is a significant broadening of both psychophysical and neural tuning curves (i.e., reduced frequency resolution ability) (Ryan and Dallos, 1975; Evans and Harrison, 1976; Liberman et al., 1986). In contrast, damage to the inner hair cells through carboplatin treatment results in significant loss of sensitivity but normal frequency resolution ability provided there is full outer hair cell retention and provided the active process mechanisms—typically inferred in animal studies from the presence of otoacoustic emissions—are functional (Wang et al., 1997; Salvi et al., 1999).
Sensorineural hearing loss has been shown in numerous studies to adversely affect frequency resolution ability (for a review see Moore, 1995). There appear to be two mechanisms by which this occurs. First, the need for higher signal levels in people with hearing loss leads, in itself, to a reduction in resolution because the basilar membrane travelling wave envelope is broader for high-level inputs (Moore and Glasberg, 1987; Glasberg and Moore, 1990). Second, in addition to this normal effect of level, subjects with cochlear hearing loss often show a further reduction in resolution ability that is thought to be the result of a loss of outer hair cell function, and hence, a disruption of the active process (Sellick et al., 1982).
The speech perception ability of adult listeners with sensorineural hearing loss has been positively correlated with frequency resolution ability in a number of studies (Dreschler and Plomp, 1980; Stelmachowitz et al., 1985; Moore, 1996). Furthermore, frequency resolution in cochlear implant patients (in this case the ability to distinguish different electrode positions) has also been related to speech perception (Henry et al., 2000).
If, in fact, frequency resolution is entirely determined by the active cochlear mechanisms, auditory neuropathy/dys-synchrony subjects with normal otoacoustic emissions would be expected to have normal resolution, at least at the basilar membrane level. This was the finding of Abdala et al. (2000) who, in a distortion product otoacoustic emission (DPOAE) suppression study, found evidence of normal cochlear tuning in both adult and pediatric subjects with AN/AD. These authors generated DPOAE suppression tuning curves by systematically varying the level and frequency of an ipsilaterally presented masking tone in 4 AN/AD subjects. The resulting suppression tuning curves in these cases were indistinguishable from those of 15 normal control subjects, suggesting normal cochlear level frequency selectivity.
Psychophysical investigation of frequency resolution in subjects with auditory neuropathy/dys-synchrony-type hearing loss has also, on the whole, shown normal results. Cacace et al. (1983) presented findings for 2 adult subjects with Friedreich's ataxia. Psychophysical tuning curves were plotted for stimulus frequencies of 500 Hz, 1 kHz, and 2 kHz in these subjects by using a simultaneous masking paradigm. Thresholds were obtained for tones at each of the test frequencies in the presence of masking tones of varying frequency. The resulting tuning curves were found to be sharply tuned and of normal morphology. Cacace et al. (1983) thus concluded that the structure and function of the outer hair cells in their patients was unaffected by the neural abnormality and that their frequency selectivity was normal.
Evidence of normal frequency resolution in subjects with auditory neuropathy/dys-synchrony type hearing loss was also obtained in a recent study in our laboratory (Rance et al., 2004). This study sought to characterize the perceptual abilities of 14 affected children, correlating their results on a range of psychophysical discrimination tasks with open-set speech perception performance. Data were also obtained from a cohort of subjects with sensorineural hearing loss matched for age and hearing level and from a group of children with normal hearing.
An estimate of auditory filter width was obtained in each case using a notched noise masking technique. In this procedure, a detection threshold for a 1-kHz tone was established in the presence of white noise and again in the presence of white noise with a 500-Hz notch centred at the stimulus frequency. The difference between thresholds obtained in the two conditions provides an estimate of the subject's ability to resolve the signal from the noise (a greater release from masking in the notched condition indicating better frequency resolution). Results obtained for the group of auditory neuropathy/dys-synchrony subjects in this study were similar to those of the normally hearing children and in fact, suggested auditory filters that were significantly narrower than those of their peers with sensorineural hearing loss (Figure 8).
Figure 8.
White/notched noise masker level differences for each subject. SN, sensorineural; AN/AD, auditory neuropathy/dys-synchrony (Rance et al., 2004).
In contrast, Kraus et al. (2000) have presented results suggesting abnormal frequency resolution in an adult AN/AD patient. This 24-year-old subject was assessed using a range of masking paradigms, including a simultaneous masking task that broadly resembled the test procedure used in Rance et al., 2004. A masking difference of only 3.5 dB was seen for this auditory neuropathy/dys-synchrony patient compared to a mean difference of 18 dB obtained from a cohort of normally hearing subjects, suggesting severely impaired frequency selectivity. Kraus et al. (2000) interpreted this finding as indicating a central coding deficit (assuming normal cochlear function from the presence of recordable otoacoustic emissions).
In summary, frequency resolution ability, which is thought to be primarily related to cochlear-level processing, appears to be normal in most auditory neuropathy/dys-synchrony patients reported thus far. This finding is consistent with the presence of otoacoustic emission responses in ears with AN/AD. These preneural responses are suggestive of normal cochlear outer hair cell function and “active process” mechanisms, and it is well established that the sharp frequency tuning of the basilar membrane is contingent upon outer hair cell integrity (Evans and Harrison, 1976; Yates et al., 1992; Moore, 1997).
Temporal Resolution
Temporal resolution is the ability to perceive changes in stimuli over time, for example, to detect a brief gap between two sounds or amplitude fluctuations in a continuous sound. The term refers to the detection of variations in the overall amplitude (the envelope) of the signal rather than rapid pressure changes associated with the fine structure of the sound (Viemeister and Plack, 1993). Results of studies examining the effects of sensorineural hearing loss on temporal resolution have suggested that once the effects of reduced sensation level or reduced audible bandwidth are accounted for, most subjects perform as well as normally hearing listeners (Moore, 1995; 1996).
In contrast, significant temporal resolution deficits have been demonstrated in auditory neuropathy/dys-synchrony subjects. Zeng et al. (1999) and Zeng et al. (in press), in related studies involving 21 adults and children with AN/AD-type hearing loss, have shown abnormal results on a range of temporal resolution measures, including gap detection (the identification of a silent period embedded within a burst of noise) and the temporal modulation transfer function (detection of sinusoidal amplitude fluctuations in the level of steady-state noise). Starr et al. (1991) have also presented findings showing profoundly impaired use of temporal cues (gap detection, monaural stimulus separation) in an 11-year-old subject with progressive AN/AD.
Furthermore, Zeng et al. (2001b), Zeng et al. (in press), and Kraus et al. (2000) have presented forward and backward masking data suggesting wider than normal temporal windows in adult auditory neuropathy/dys-synchrony subjects. These studies established detection thresholds for brief tonal stimuli presented at various timing intervals relative to the start and end of a longer duration masker. Performance on this task is thought to provide an estimate of how well a listener can separate sounds in time. Each of the subjects presented in these investigations showed abnormal backward and/or forward masking patterns, suggesting impaired temporal resolution ability.
In addition to the inability of auditory neuropathy/dys-synchrony subjects to perceive monaurally presented temporal cues (as in the previously mentioned studies), there is also evidence that affected subjects are impaired in their ability to integrate and make use of binaural temporal information. Abnormal masking level difference results, for example, have been a consistently reported finding in the AN/AD literature during the last decade (Starr et al., 1991; Berlin et al., 1993; Starr et al., 1996; Hood, 1999). This assessment rests on the principle that a signal embedded in noise is more easily detected if either the signal or the noise is out of phase relative to a competing signal in the contralateral ear. The ability to make use of these interaural phase differences is thought to be contingent upon an accurate neural representation at the level of the lower brainstem. Subjects with auditory neuropathy/dys-synchrony typically show no masking release with dichotic phase inversion, consistent with a degree of temporal disruption of the neural signal, whereas subjects with normal hearing usually show a masking level difference of approximately 10 dB (Licklider, 1948).
Localization ability based on interaural timing differences is another aspect of binaural auditory processing affected by auditory neuropathy/dys-synchrony-type hearing loss (Starr et al., 1991; Kaga et al., 1996; Zeng et al., in press). Subjects in the Zeng et al., study, for example were significantly impaired in their ability to make lateralization judgements from temporal cues. Interestingly, these listeners showed normal sound localization for discriminations based upon interaural intensity differences.
Severe speech perception difficulties have been consistently reported in AN/AD adult subjects with abnormal temporal resolution (Starr et al., 1991; Zeng et al., 1999; Zeng et al., 2001a; Zeng et al., in press). As such, these patients resemble other subject groups in whom temporal processing disorders have been correlated with speech perception deficits. Some of these include elderly listeners (Gordon-Salant and Fitzgibbons, 1993), patients with multiple sclerosis (Levine et al., 1993), and children with learning disabilities (Tallal, 1981; Kraus et al., 1996; Wright et al., 1997).
A correlation between temporal processing ability and speech perception has also been demonstrated for children with early-onset auditory neuropathy/dys-synchrony (Figure 9) (Rance et al., 2004). Amplitude modulation detection was abnormal in many of the subjects in this study, and the degree of the abnormality was strongly correlated with speech perception ability. (See Figure 10 for details). Seven of the 14 AN/AD children showed normal or only mildly impaired modulation detection ability, and all of these subjects demonstrated significant open-set speech discrimination (≥ 60%). In the other 7 subjects, however, the ability to perceive amplitude fluctuations even at relatively slow modulation rates was significantly depressed. C.N.C. phoneme scores in these cases indicated little or no open-set speech perception ability.
Figure 9.
Amplitude modulation detection thresholds in auditory neuropathy/dys-synchrony (AN/AD) subjects. Closed circles represent the findings for children in the AN/AD group with speech perception scores > 60%. Open circles represent the children in the AN/AD group with speech scores <30%. Open triangles show the findings for children in the AN/AD <30% group unable to detect a modulation depth of 0 dB (100%). The enclosed area shows the mean ±2 SD range for the normal group (Rance et al., 2004).
Figure 10.
Amplitude modulation detection threshold (10 Hz MF) plotted as a function of consonant-nucleus-consonant (CNC) phoneme score for auditory neuropathy/dys-synchrony subjects (Rance et al., 2004).
Because the auditory pathway abnormalities that produce the auditory neuropathy/dys-synchrony result profile are unclear, it is difficult to determine the exact mechanisms by which temporal cues are disrupted in affected subjects. Electrophysiologic results—in particular, the absence or distortion of averaged potentials in the auditory brainstem—do, however, point to disruptions in the synchrony of neural firing or some form of conduction block in the peripheral auditory system. Such disruptions could result in a time-smeared neural representation of acoustic stimuli with the degree of temporal distortion determined by the severity of the disruption (Starr et al., 1996; Zeng et al., 1999; Kraus et al., 2000).
The absence of auditory brainstem response in AN/AD subjects suggests a temporal disruption of at least 0.5 ms (Sininger et al., 1995; Kraus et al., 2000), and it may have been that the auditory neuropathy/dys-synchrony children described in Rance et al. (2004) with good speech perception and reasonably normal temporal modulation transfer function results had levels of brainstem asynchrony close to this limit. The impaired ability to accurately encode even low-rate (10 Hz) amplitude changes seen in some cases does, however, point to neural disruption of the order of tens of milliseconds and may suggest a different pathologic mechanism.
Frequency Discrimination
Frequency discrimination is the ability to perceive changes in frequency (or pitch) over time. For steady-state (pure tone) stimuli of 4 kHz and higher, frequency discrimination is thought to depend primarily on place mechanisms based on spatial changes in the basilar membrane excitation pattern (Moore, 1973b; Sek and Moore, 1995). In contrast, discrimination of stimuli of lower than 4 kHz is thought to be enhanced by the use of temporal information (Moore, 1973a; 1973b; Sek and Moore, 1995; Micheyl et al., 1998). It has been hypothesized that neural phase locking is important in the fine-tuning of discrimination abilities in this range (whereas for higher frequencies, limitations in neural refractory period prevent phase-related responses). Sek and Moore (1995), for example, showed that models of frequency discrimination based solely on excitation pattern information (and not taking into account phase locking) could not explain the variation of difference limens (DLF) across frequency. Specifically, these authors found that low-frequency DLFs are significantly smaller than predicted by place of excitation models.
Several studies have measured DLFs to fixed tonal stimuli in adults with cochlear hearing loss (Tyler et al., 1983; Freyman and Nelson, 1986; 1991; Moore and Peters, 1992). A high degree of intersubject variability has been reported, but overall, the findings indicate that discrimination ability is degraded by cochlear damage. Interestingly, DLFs are not strongly correlated with either the subjects’ hearing levels or frequency resolution ability (Tyler et al., 1983; Moore and Peters, 1992), suggesting that as with normally hearing subjects, temporal cues play an important role in the discrimination process.
Frequency discrimination abilities in subjects with auditory neuropathy/dys-synchrony are yet to be thoroughly investigated, but the data that has been presented thus far suggests extreme perceptual deficits in this regard. Starr et al. (1991) measured “just noticeable differences” for pairs of 500-ms tone-burst stimuli at octave frequencies from 250 Hz to 8 kHz in their 11-year-old AN/AD subject. Frequency discrimination results in this case were consistently depressed, showing just noticeable differences approximately 4.5 times higher than those obtained from 5 age-matched children across the test frequency range.
Zeng et al. (2001a; 2001b; in press) also found impaired frequency discrimination ability in 12 subjects with auditory neuropathy/dys-synchrony type hearing loss. In these studies, DLFs were obtained at octave frequencies (250 Hz-8 kHz) using a three alternative forced-choice adaptive procedure. Results for the AN/AD cases were considerably poorer than those obtained for a control group of normally hearing subjects. This was particularly the case in the low-to-mid frequency range (≤ 2 kHz), where DLFs were about one order of magnitude worse than those of the control group. A notable finding in the Zeng et al. subjects was that discrimination in the high-frequency range appeared to be less impaired, approaching the normal range at the 8-kHz test frequency. This result pattern may reflect a disruption of the low-frequency temporal discrimination processes in these AN/AD subjects, all of whom had shown abnormal results on a range of temporal discrimination tasks (Zeng et al., 2001a: 2001b; in press).
Frequency discrimination ability was similarly impaired in the auditory neuropathy/dys-synchrony children presented in Rance et al., 2004. In this study, the mean difference limen for 4-kHz pure tones was 4.5 times the normal value, whereas discrimination at 500 Hz averaged 11 times poorer than that of the normally hearing cohort. Furthermore, a comparison of DLF obtained to FM tones (which do not offer phase locking cues) and pure tones (which do) suggested that AN/AD children are less able to use phase-locking cues than subjects with normal hearing, or their counterparts with sensorineural hearing loss (Rance et al., 2004).
Frequency discrimination ability has also been correlated with speech understanding in subjects with auditory neuropathy/dys-synchrony. For example, in the children assessed by Rance et al. (2004), a strong relationship between open set-word score and DLF was obtained for all test conditions. As can be seen in Figure 11, the children the poorest frequency discrimination ability typically presented with the most impaired speech perception.
Figure 11.
Graph shows 500-Hz frequency discrimination limens for each subject plotted as a function of consonant-nucleus-consonant (CNC) phoneme score. SN, sensorineural; AN/AD, auditory neuropathy/dys-synchrony (Rance et al., 2004).
Disruption of Speech Perception in Auditory Neuropathy/Dys-synchrony Subjects
Strong correlations between fundamental processing deficits and speech perception difficulties have been found in many of the studies involving subjects with AN/AD type hearing loss. But what is it about specific temporal processing problems that can result in such extreme difficulties with speech understanding? One possibility is that the disorder affects an individual's ability to cope with the dynamic nature of speech signals. To accurately discriminate phonemes in running speech, or even in individual words, a listener must be able to perceive the characteristic spectral shapes of phonemes and, in addition, be able to rapidly update this perception to follow the flow of speech sounds. A listener must also be able to follow within-phoneme changes in spectral pattern that give cues to co-articulation. Auditory neuropathy/dys-synchrony subjects typically show normal spectral (frequency) resolution, and as such, there is reason to suppose that given a steady stimulus, they may be able to perceive the spectral shape adequately. However, their inability in many cases to perceive frequency and amplitude changes over time must lead to both a smearing of their spectral shape perception and a reduced ability to use amplitude envelope cues in speech.
Summary and Future Developments
Research in the field of auditory neuropathy/dys-synchrony type hearing loss has been dominated by discussion of the physiology underlying the condition. Questions such as: What are the mechanisms involved? Where is the site of lesion? and What should we call the disorder? have been addressed in detail. Defining the functional effects of this form of hearing loss and determining what can be done about them has received far less attention. Recent studies have, however, attempted to address this situation, providing insights into the particular ways in which perception is disrupted in affected patients and offering a base for the development of AN/AD-specific management strategies.
The perceptual profiles of subjects with auditory neuropathy/dys-synchrony are quite different from those with sensorineural hearing loss. Where subjects in the latter group typically present with impaired frequency resolution and normal temporal resolution, AN/AD patients usually show normal frequency resolution and varying degrees of temporal disruption. The severity of this timing abnormality, which affects both monaural and binaural temporal processing as well as the temporal aspects of frequency discrimination, appears to be strongly related to speech perception performance.
The particular perceptual deficits caused by auditory neuropathy/dys-synchrony require different management approaches. Cochlear implantation is currently the strategy of choice, with a high proportion of reported cases showing performance levels similar to their sensorineural peers. However, further research is warranted to determine optimal signal processing strategies for AN/AD subjects. For example, consideration of stimulus pulse rates may be important in some cases. The recent trend in cochlear implant signal processing has been towards providing higher rates of stimulation to improve perception generally and specifically to aid in the encoding of temporal cues (Zeng et al., in press). In some cases of auditory neuropathy/dys-synchrony, particularly those associated with axonal loss or demyelination, high presentation rates are more likely to produce adverse effects such as neural fatigue or conduction block (Stephanova and Daskalova, 2004). Such effects have been suggested for two cases reported in the literature thus far. The first was the case study presented in Rance et al. (1999). The second was a child described by Peterson et al. (2003) who initially performed poorly when programmed using the ACE strategy (at a rate of 900 Hz per channel) and subsequently improved when the presentation rate was reduced to 720 Hz per channel.
Despite concerns about the provision of hearing aids to patients with AN/AD-type hearing loss, there are a number of documented cases of significant benefit with conventional amplification strategies. Consideration of the basic perceptual problems associated with the condition may lead to refinements in aiding strategies and outcome improvements. For example, the psychophysical data indicates that detection of amplitude modulation is severely impaired in most cases. Conventional hearing aids that use linear amplification do not enhance the acoustic differences between sounds, and non-linear amplitude-compression circuits even reduce amplitude envelope fluctuations in most circumstances (Van Tasell, 1992). This has the overall effect of reducing the level difference between low-level and high-level speech inputs and is obviously not desirable for listeners who already have difficulty perceiving amplitude fluctuations.
For subjects who do not derive benefit from conventional hearing aids, the insights gained from psychophysical investigations into AN/AD type hearing loss may also provide a basis for the development of amplification devices that can be tailored to emphasise the perceptual cues most disrupted by the neural transmission disorder. Further research using digital speech processing systems for subjects with auditory neuropathy/dys-synchrony is required to determine how speech perception might be optimized.
The use of frequency-transposition amplification strategies is one option that has been proposed to minimize the frequency discrimination difficulties that affect many AN/AD subjects (Zeng et al., 2002; Zeng et al., in press). As discussed previously, various studies have shown that the discrimination of low-frequency signals in affected subjects is disrupted to a greater degree than was observed for high-frequency stimuli. These authors have suggested that either filtering out low-frequency sounds or transposing the acoustic speech signal into the high-frequency region may be beneficial in some cases. No formal results for this strategy have yet been published, but Zeng et al. (in press) indicate that they have successfully trialed a prototype device in “a small number of AN subjects”.
Zeng et al. (2001b) and Zeng et al. (in press) have also suggested that temporal processing difficulties in subjects with auditory neuropathy/dys-synchrony may be ameliorated by the use of “envelope expansion algorithms”. The aim of such speech processing strategies would be to improve speech perception in subjects with poor amplitude modulation detection ability by expanding the amplitude differences while maintaining the overall envelope shape and overall level in the speech signal, thereby making temporal envelope cues more salient. How successful such a strategy might be in an AN/AD subject with extreme amplitude modulation detection disruption is yet to be determined. As demonstrated in the Rance et al. (2004) and Zeng et al. (1999) findings, some affected individuals struggle to perceive even 100% amplitude fluctuations. In such cases, gating-type strategies that preserve the peaks in the speech amplitude envelope while removing all other components in the signal to produce amplitude fluctuations of effectively 100% could be useful. Such a processing strategy could also conceivably enhance the signal peaks by sharpening the transition from signal to no-signal periods.
Processing strategies that manipulate timing differences in the speech signal may also aid in the perception of temporal cues in some subjects with auditory neuropathy/dys-synchrony. Tallal et al. (1996) produced a processing algorithm that combined a peak enhancement strategy similar to that described in the previous paragraphs with a temporal expansion algorithm that prolonged the duration of the speech signal by 50%. The resulting fluent speech signal was considered to have maintained its spectral integrity and natural quality and when presented to a group of children with temporal-processing-related language learning disorders, was easier to understand than unprocessed speech. Interestingly, Tallal et al. (1996) found that intensive training with the acoustically modified signal subsequently improved the ability of their subjects to process natural speech, suggesting that habilitative programs with specialized materials can improve perceptual abilities in some subjects with temporal processing deficits. The applicability of such programs to subjects with auditory neuropathy/dys-synchrony type hearing loss is an area that needs to be investigated. To date no published studies describe the use of modified training materials with AN/AD subjects, although Zeng and Lui (in press) have used modified stimuli to test speech perception. In this case, they used clear speech sentence materials that use slower presentation rates and enhanced phonemic differences and were able to show a significant perceptual advantage over nonmodified sentence materials. Hopefully further refinement of the perceptual profiles for AN/AD patients will provide a framework for the development of individualized training programs.
In summary, the findings for patients with auditory neuropathy/dys-synchrony published during the last 20 years provide a useful reminder that whilst electroacoustic and evoked potential responses from the auditory pathways can offer powerful diagnostic insights, these responses are simply byproducts of complex physiologic processes and are not necessarily true indicators of perception. Attempts to characterize various aspects of the AN/AD perceptual profile have shown that results can be idiosyncratic despite a common pattern of physiologic results. Whether the perceptual variability seen in this population is due to different pathologic processes or different degrees of the same process is unclear. What is clear is that the management of affected patients and their families needs to be flexible and take into account individual differences.
Footnotes
An operational definition for grossly abnormal responses is yet to be determined but could include responses with latencies more than two standard deviations beyond the normal range, amplitudes significantly below normal and abnormal waveform morphology. Such definitions need to be applied with caution however, as severe sensory hearing loss can result in auditory brainstem responses that show prolonged latencies and poorly defined waveforms.
It should be noted that there is no evidence that carboplatin treatment results in specific inner hair cell loss in human subjects.
Madden et al. (2002) did note that maturational factors could have contributed to the thresholds improvements in their subjects (test age range, 6–25 months) but concluded that the observed changes were greater than would be predicted on the basis of development.
New techniques for the assessment of speech perception ability that are based upon visual reinforcement methods (such as the VRISD or habituation paradigms) are under development and may find clinical application in the near future (Houston et al., 2004).
References
- Abdala C, Sininger YS, Starr A. (2000). Distortion product otoacoustic emission suppression in subjects with auditory neuropathy. Ear Hear 21: 542–553 [DOI] [PubMed] [Google Scholar]
- Amatuzzi MG, Northrop C, Liberman C, et al. (2001). Selective inner hair cell loss in premature infants and cochlear pathological patterns from neonatal intensive care unit autopsies. Arch Otolaryngol Head Neck Surg 127: 629–636 [DOI] [PubMed] [Google Scholar]
- Bamford JM, Wilson IM, Atkinson D, Bench J. (1981). Pure tone audiograms from hearing-impaired children. Predicting speech-hearing from the audiogram. Brit J Audiol 15 (1): 3–10 [DOI] [PubMed] [Google Scholar]
- Berli CI. (1999). Auditory neuropathy: Using OAEs and ABRs from screening to management. Sem Hear 21: 307–315 [Google Scholar]
- Berlin CI, Bordelon J, Hurley A, et al. (1997). Autoimmune inner ear disease: Basic science and audiological issues. In Berlin CI. (ed): Neurotransmission and Hearing Loss: Basic Science, Diagnosis and Management. San Diego: Singular Publishing Group, 137–146 [Google Scholar]
- Berlin CI, Bordelon J, St. John P, et al. (1998). Reversing click polarity may uncover auditory neuropathy in infants. Ear Hear 19: 37–47 [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Cecola RP, et al. (1993). Does Type I afferent neuron dysfunction reveal itself through lack of efferent suppression? Hear Res 65: 40–50 [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Hurley MS, Wen H. (1996). Hearing aids: Only for hearing impaired patients with abnormal otoacoustic emissions. In Berlin CI. (ed): Hair Cells and Hearing Aids. San Diego: Singular Publishing Group, 99–111 [Google Scholar]
- Berlin CI, Hood LJ, Morlet T. (2000). The search for auditory neuropathy patients and connexin 26 patients in schools for the deaf. ARO Abstract 2000 23: 23–24 [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, et al. (2002). Auditory neuropathy/dys-synchrony: After diagnosis, then what? Sem Hear 23 (3): 209–214 [Google Scholar]
- Berlin CI, Hood LJ, Rose K. (2001). On renaming auditory neuropathy as auditory dys-synchrony. Audiol Today 13: 15–17 [Google Scholar]
- Berlin CI, Hood LJ, Rose K, et al. (2003). Auditory neuropathy/dys-synchrony (AN/AD): Management and results in 193 patients. Assoc Res Otol, Abstract #883.
- Bilger RC, Nuetzel JM, Raninowitz WM, et al. (1984). Standardization of a test of speech perception in noise. J Sp Hear Res 21: 32–48 [DOI] [PubMed] [Google Scholar]
- Billet TE, Thorne PR, Gavin JB. (1989). The nature and progression of injury in the organ of Corti during ischemia. Hear Res 41: 189–198 [DOI] [PubMed] [Google Scholar]
- Bohne BA. (1976). Mechanisms of noise damage in the inner ear. In Henderson D, Hamernick RP, Dosanjh DS, Mills JH. (eds): Effects of Noise on Hearing. New York: Raven, 41–68 [Google Scholar]
- Bonfils P, Avan P. (1992). Distortion-product otoacoustic emissions: Values for clinical use. Arch Otolaryngol Head Neck Surg 111: 1069–1076 [DOI] [PubMed] [Google Scholar]
- Bonfils P, Bertrand Y, Uziel A. (1988). Evoked otoacoustic emissions: Normative data and presbyacusis. Audiol 21: 27–35 [DOI] [PubMed] [Google Scholar]
- Boothroyd A. (1995). Speech perception tests and hearing impaired children. In Plant G, Spens KE. (eds): Profound deafness and speech communication. London: Tyne Wear, 345–371 [Google Scholar]
- Boothroyd A. (1997). Auditory capacity of hearing-impaired children using hearing aids and cochlear implants: Issues of efficacy and assessment. Scand Audiol Suppl 41: 17–25 [PubMed] [Google Scholar]
- Borg E, Counter SA, Rosler G. (1984). Theories of middle-ear muscle function. In Silman S. (ed.): The Acoustic Reflex: Basic Principles and Clinical Applications. Orlando: Academic Press, 63–99 [Google Scholar]
- Brookhouser PE, Gorga MP, Kelly WJ. (1990). Auditory brainstem response results as predictors of behavioral auditory thresholds in severe and profound hearing impairment. Laryngoscope 101: 803–810 [DOI] [PubMed] [Google Scholar]
- Buss E, Labadie RF, Brown CJ, et al. (2002). Outcome of cochlear implantation in pediatric auditory neuropathy. Otology Neurotol 21: 328–332 [DOI] [PubMed] [Google Scholar]
- Bussoli TJ, Kelly A, Steel P. (1997). Localization of the Bronx waltzer (bv) deafness gene to mouse chromosome 5. Mamm Genome 11: 714–717 [DOI] [PubMed] [Google Scholar]
- Butinar D, Zidar J, Leonardis L, et al. (1999). Hereditary auditory, vestibular and motor neuropathy in a Slovenian Roma (Gypsy) kindred. Ann Neurol 41: 36–44 [DOI] [PubMed] [Google Scholar]
- Cacace AT, Satya-Murti S, Gimes CT. (1983). Frequency selectivity and temporal processing in Friedreich's ataxia. Ann Otol Rhinol Laryngol 91: 276–280 [DOI] [PubMed] [Google Scholar]
- Cassandro E, Mosca F, Sequino L, et al. (1986). Otoneurological findings in Friedreich's ataxia and other inherited neuropathies. Audiol 21: 84–91 [DOI] [PubMed] [Google Scholar]
- Chance PF, Fischbeck KH. (1994). Molecular genetics of Charcot-Marie-Tooth disease and related neuropathies. Hum Mol Genet 1: 1503–1507 [DOI] [PubMed] [Google Scholar]
- Chisin R, Pearman M, Sohmer H. (1979). Cochlear and brain stem responses in hearing loss following neonatal hyperbilirubinemia. Ann Otol Rhinol Laryngol 81: 352–357 [DOI] [PubMed] [Google Scholar]
- Clopton BM, Silverman MS. (1978). Changes in latency and duration of neural responding following developmental auditory deprivation. Exp Brain Res 31: 39–47 [DOI] [PubMed] [Google Scholar]
- Collet L, Levy V, Veuillet E. (1993). Click-evoked otoacoustic emissions and hearing threshold in sensorineural hearing loss. Ear Hear 14 (2): 141–143 [DOI] [PubMed] [Google Scholar]
- Cone-Wesson B, Rance G, Sininger YS. (2001). Amplification and rehabilitation strategies for patients with auditory neuropathy. In Sininger YA, Starr A. (eds), Auditory Neuropathy. San Diego: Singular; Publishing, 233–250 [Google Scholar]
- Cone-Wessonn B, Vohr BR, Sininger YS, et al. (2000). Identification of neonatal hearing impairment: Infants with hearing loss. Ear Hear 21: 488–507 [DOI] [PubMed] [Google Scholar]
- Corley VM, Crabbe LS. (1999). Auditory neuropathy and a mitochondrial disorder in a child: Case study. J Am Acad Audiol 11: 484–488 [PubMed] [Google Scholar]
- Dallos P. (1973). The Auditory Periphery: Biophysics and Physiology. New York: Academic Press [Google Scholar]
- Dallos P, Cheatham MA. (1976). Production of cochlear potentials by inner and outer hair cells. J Acoust Soc Am 61: 510–512 [DOI] [PubMed] [Google Scholar]
- Davis H. (1983). An active process in cochlear mechanics. Hear Res 1: 79–90 [DOI] [PubMed] [Google Scholar]
- Davis H, Hirsh SK. (1979). The audiometric utility of the brain stem response to low frequency sounds. Audiol 11: 181–195 [DOI] [PubMed] [Google Scholar]
- Deltenre P, Mansbach AL, Bozet C, et al. (1999). Auditory neuropathy with preserved cochlear microphonics and secondary loss of otoacoustic emissions. Audiol 38 (4): 187–195 [DOI] [PubMed] [Google Scholar]
- Deltenre P, Mansbach AL, Bozet C, et al. (1997). Auditory neuropathy: A report on three cases with early onsets and major neonatal illnesses. Electroenceph Clin Neurophys 101: 17–22 [DOI] [PubMed] [Google Scholar]
- Dowell RC, Dettman SJ, Blamey PJ, et al. (2002). Speech perception in children using cochlear implants: Prediction of long-term outcomes. Cochlear Implant Int 3 (1): 1–18 [DOI] [PubMed] [Google Scholar]
- Doyle KJ, Sininger YS, Starr A. (1998). Auditory neuropathy in childhood. Laryngoscope 101: 1374–1377 [DOI] [PubMed] [Google Scholar]
- Dreschler WA, Plomp R. (1980). Relations between psychophysical data and speech perception for hearing-impaired subjects. I. J Acoust Soc Am 61: 1608–1615 [DOI] [PubMed] [Google Scholar]
- Dunkley C, Farnsworth A, Mason S, et al. (2003). Screening and follow up assessment in three cases of auditory neuropathy. Arch Dis Child 81: 25–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux-Smith A, Picton TW, Bernard P, et al. (1991). Prognostic validity of brainstem electric response audiometry in infants of a neonatal intensive care unit. Audiol 31: 249–265 [DOI] [PubMed] [Google Scholar]
- Dynes SBC, Delgutte B. (1992). Phase-locking of auditory-nerve discharges to sinusoidal electric stimulation of the cochlea. Hear Res 51: 79–90 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. (1976). Electrocochleography. In Keidel WD, Neff WD. (eds): Handbook of Sensory Physiology Vol. V/3 Auditory System. Berlin: Springer Verlag, 625–705 [Google Scholar]
- Evans E, Harrison R. (1976). Correlation between outer hair cell damage and deterioration of cochlear nerve tuning properties in the guinea pig. J Physiol 251: 43–44 [PubMed] [Google Scholar]
- Franck KH, Rainey DM, Montoya LA, et al. (2002). Developing a multidisciplinary clinical protocol to manage pediatric patients with auditory neuropathy. Sem Hear 23 (3): 225–237 [Google Scholar]
- Freyman RL, Nelson DA. (1986). Frequency discrimination as a function of tonal duration and excitation-pattern slopes in normal and hearing-impaired listeners. J Acoust Soc Am 71: 1034–1044 [DOI] [PubMed] [Google Scholar]
- Freyman RL, Nelson DA. (1991). Frequency discrimination as a function of signal frequency and level in normal-hearing and hearing-impaired listeners. J Speech Hear Res 31: 1371–1386 [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ. (1989). Psychoacoustic abilities of subjects with unilateral and bilateral cochlear impairments and their relationship to the ability to perceive speech. Scand Audiol Suppl 31: 1–25 [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ. (1990). Derivation of auditory filter shapes from notched-noise data. Hear Res 41: 103–138 [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. (1993). Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res 31: 1276–1285 [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B. (1997). From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear 11: 440–455 [DOI] [PubMed] [Google Scholar]
- Gorga MP, Stelmachowicz PG, Barlow SM, et al. (1995). Case of recurrent, sudden sensorineural hearing loss in a child. J Am Acad Audiol 1: 163–172 [PubMed] [Google Scholar]
- Gorga MP, Worthington DW, Reiland JK, et al. (1985). Some comparisons between auditory brainstem response threshold, latencies, and the pure-tone audiogram. Ear Hear 1: 105–112 [DOI] [PubMed] [Google Scholar]
- Gravel JS, Stapells DR. (1993). Behavioral, electrophysiologic, and otoacoustic measures from a child with auditory processing dysfunction: Case report. J Am Acad Audiol 1: 412–419 [PubMed] [Google Scholar]
- Gstoettner W, Plenk H, Jr., Franz P, et al. (1997). Cochlear implant deep insertion: Extent of insertion trauma. Acta Otolaryngol (Stockh) 111: 274–277 [DOI] [PubMed] [Google Scholar]
- Harris FP, Probst R. (2002). Exploring cochlear status with otoacoustic emissions. The potential for new clinical applications. In Ms robinette TJ. (eds.): Otoacoustic Emissions: Clinical Applications. New York: Thieme, 213–242 [Google Scholar]
- Harrison RV. (1998). An animal model of auditory neuropathy. Ear Hear 11: 355–361 [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Ishiyama Y, Yoshimoto T, et al. (1981). Brainstem auditory evoked potentials recorded directly from the human brainstem and thalamus. Brain 101: 841–859 [DOI] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, et al. (2000). The relationship between speech perception and electrode discrimination in cochlear implantees. J Acoust Soc Am 101: 1269–1280 [DOI] [PubMed] [Google Scholar]
- Hood LJ. (1998). Auditory neuropathy: What is it and what can we do about it? Hear Journal 51 (8): 10–18 [Google Scholar]
- Hood LJ. (1999). A review of objective methods of evaluating neural pathways. Laryngoscope 101: 1745–1748 [DOI] [PubMed] [Google Scholar]
- Hood LJ, Berlin C, Hurley A., Wen H. (1996). Suppression of otoacoustic emissions in normal hearing individuals. In Berlin C. (ed): Hair Cells and Hearing Aids. San Diego, Singular Publishing Group, 57–72 [Google Scholar]
- Houston DM, Ying EA, Pisoni DB, et al. (2004). Development of pre-word-learning skills in infants with cochlear implants. Volta Rev 103 (4): 303–326 [PMC free article] [PubMed] [Google Scholar]
- Hung KL. (1989). Auditory brainstem responses in patients with neonatal hyperbilirubinemia and bilirubin encephalopathy. Brain Dev 11: 297–301 [DOI] [PubMed] [Google Scholar]
- Hyde ML, Riko K, Malizia K. (1990). Audiometric accuracy of the click ABR in infants at risk for hearing loss. J Am Acad Audiol 1: 59–74 [PubMed] [Google Scholar]
- Jabbari B, Schwartz DM, MacNeil DM, et al. (1983). Early abnormalities of brainstem auditory evoked potentials in Friedreich's ataxia: Evidence of primary brainstem dysfunction. Neurology 31: 1071–1074 [DOI] [PubMed] [Google Scholar]
- Javel E. (1986). Basic response properties of auditory nerve fibers. In Altschuler RA, Hoffman RP. (eds.). Neurobiology of Hearing: The Cochlea. New York: Raven Press, 213–245 [Google Scholar]
- Javel E, Shepherd RK. (2000). Electrical stimulation of the auditory nerve. III. Response initiation sites and temporal fine structure Hear Res 140: 45–76 [DOI] [PubMed] [Google Scholar]
- Jerger JF, Ali A, Fong K, et al. (1992). Otoacoustic emissions, audiometric sensitivity loss, and speech understanding: A case study. J Am Acad Audiol 1: 283–286 [PubMed] [Google Scholar]
- Kaga K, Nakamura M, Shinogami M, et al. (1996). Auditory nerve disease of both ears revealed by auditory brainstem responses, electrocochleography and otoacoustic emissions. Scand Audiol 21: 233–238 [DOI] [PubMed] [Google Scholar]
- Kapadia S, Lutman M. (1997). Are normal hearing thresholds a sufficient condition for click-evoked otoacoustic emissions? J Acoust Soc Am 101: 3566–3576 [DOI] [PubMed] [Google Scholar]
- Katona G, Buki B, Farkas Z, et al. (1993). Transitory evoked otoacoustic emission. (TEOAE) in a child with profound hearing loss Int J Ped Otorhinolaryngol 21: 263–267 [DOI] [PubMed] [Google Scholar]
- Kemp DT. (1978). Stimulated acoustic emission from the human auditory system. J Acoust Soc Am 61: 1386–1391 [DOI] [PubMed] [Google Scholar]
- Kileny PR, Robertson CMT. (1985). Neurological aspects of infant hearing assessment. J Otolaryngol 11: 34–39 [PubMed] [Google Scholar]
- Kitzes LM, Semple MN. (1985). Effects of neonatal unilateral ablation: Single unit responses in the inferior colliculus. J Neurophysiol 51: 1483–1500 [DOI] [PubMed] [Google Scholar]
- Konradsson KS. (1996). Bilaterally presented otoacoustic emissions in four children with profound idiopathic unilateral hearing loss. Audiol 31: 217–227 [DOI] [PubMed] [Google Scholar]
- Kovach MJ, Lin JP, Boyajiev S. (1999). A unique point mutation in the PMP22 gene is associated with Charcot-Marie-Tooth disease and deafness. Am J Hum Genet 61: 1580–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham J, et al. (2000). Consequences of neural asynchrony: A case of auditory neuropathy. J Assoc Res in Otolaryngol 1 (1): 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, et al. (1996). Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science 271: 971–973 [DOI] [PubMed] [Google Scholar]
- Kraus NA, Ozdamar O, Stein L, et al. (1984). Absent auditory brainstem response: Peripheral hearing loss or brainstem dysfunction? Laryngoscope 91: 400–406 [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Nakajima Y, Hattori T, et al. (1999). Activity-dependent excitability changes in chronic inflammatory demyelinating polyneuropathy: A microneurographic study. Muscle Nerve 21: 899–904 [DOI] [PubMed] [Google Scholar]
- Lee JSM, McPherson B, Yuen KCP, et al. (2001). Screening for auditory neuropathy in a school for hearing impaired children. Int J Paed Otorhinolaryngol 61: 39–46 [DOI] [PubMed] [Google Scholar]
- Lenhardt ML. (1981). Childhood auditory processing disorder with brainstem evoked response verification. Arch Otolaryngol 101: 623–625 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Pujol R. (1984). Age-related structural investigation of the Bronx Waltzer mutant mouse cochlea: Scanning and transmission electron microscopy. Hear Res 11: 1123–1134 [DOI] [PubMed] [Google Scholar]
- Leonardis L, Popovic M, Timmerman V, et al. (2000). Hereditary motor and sensory neuropathy associated with auditory neuropathy in a Gypsy family. Eur J Physiol 431: 208–210 [PubMed] [Google Scholar]
- Levine RA, Gardner JC, Fullerton BC, et al. (1993). Effects of multiple sclerosis brainstem lesions on sound lateralization and brainstem auditory evoked potentials. Hear Res 61: 73–88 [DOI] [PubMed] [Google Scholar]
- Levitt H, McGarr N. (1988). Speech and language development in hearing impaired children. In Bess FH. (ed): Hearing Impairment in Children. Parkton, MD: York Press, 375–388 [Google Scholar]
- Liberman MC, Chesney CP, Kujawa SG. (1997). Effects of selective inner hair cell loss on DPOAE and CAP in carboplatin-treated chinchillas. Aud Neuro 1: 255–268 [Google Scholar]
- Liberman MC, Dodds LW, Learson DA. (1986). Structure-function correlation in noise damaged ears: A light and electron-microscopic study. In Salvi RJ, Henderson D, Hamernik RP, et al. (eds): Basic and Applied Aspects of Noise-Induced Hearing Loss. New York: Plenum, 163–176 [Google Scholar]
- Licklider JCR. (1948). The influence of interaural phase relation upon the masking of speech by white noise. J Acoust Soc Am 21: 150–159 [Google Scholar]
- Lonsbury-Martin BL, Harris FP, Stagner BB, et al. (1990). Distortion product emissions in humans: I. Basic properties in normally hearing subjects Ann Otol Rhino Laryngol 141: 3–14 [PubMed] [Google Scholar]
- MacArdle B, Hazan V, Prasher D. (1999). Speech pattern audiometry in hearing impaired children. Brit J Audiol 33 (6): 383–393 [DOI] [PubMed] [Google Scholar]
- Macrae JH. (1991). Permanent threshold shift associated with overamplification by hearing aids. J Sp Hear Res 31: 403–414 [DOI] [PubMed] [Google Scholar]
- Macrae JH. (1995). Temporary and permanent threshold shift caused by hearing aid use. J Sp Hear Res 31: 949–959 [DOI] [PubMed] [Google Scholar]
- Madden C, Rutter M, Hilbert L, et al. (2002). Clinical and audiological features in auditory neuropathy. Arch Otolaryngol Head Neck Surg 121: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Markides A. (1986). Age at fitting of hearing aids and speech intelligibility. Brit J Audiol 21: 165–167 [DOI] [PubMed] [Google Scholar]
- Marsh RR. (2002). Is it auditory dys-synchrony? Comment on “On renaming auditory neuropathy as auditory dys-synchrony”. Audiol Today 14 (3): 36–37 [Google Scholar]
- Mason JC, De Michele A, Stevens C, et al. (2003). Cochlear implantation in patients with auditory neuropathy of varied etiologies. Laryngoscope 113 (1): 45–49 [DOI] [PubMed] [Google Scholar]
- McDonald WI, Sears TA. (1970). The effects of experimental demyelination on conduction in the central nervous system. Brain 91: 583–598 [DOI] [PubMed] [Google Scholar]
- Melcher JR, Knudson IM, Fullerton BC, et al. (1996a). Generators of the brainstem auditory evoked potential in cat. I. An experimental approach to their identification. Hear Res 93: 1–27 [DOI] [PubMed] [Google Scholar]
- Melcher JR, Guinan JJ, Jr., Knudson IM, et al. (1996b). Generators of the brainstem auditory evoked potential in the cat. II. Correlating lesion sites with waveform changes Hear Res 91: 28–51 [DOI] [PubMed] [Google Scholar]
- Melcher JR, Kiang NY. (1996c). Generators of the brainstem auditory evoked potential in the cat. III: Identified cell populations. Hear Res 91: 52–71 [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Prasher DK, Starr A. (1986). Latency variability and temporal interrelationships of the auditory event-related potentials. (N1, P2, N2, and P3) in normal subjects Electroenceph Clin Neurophys 61: 59–71 [DOI] [PubMed] [Google Scholar]
- Micheyl C, Moore BCJ, Carlyon RP. (1998). The role of excitation-pattern cues and temporal cues in the frequency and modulation-rate discrimination of amplitude-modulated tones. J Acoust Soc Am 101: 1039–1050 [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Iler Kirk K, Renshaw J, et al. (1999). Cochlear implantation in auditory neuropathy. Laryngoscope 101: 181–185 [DOI] [PubMed] [Google Scholar]
- Møeller MP. (2000). Early intervention and language development in children who are deaf and hard of hearing. Pediatrics 106 (3): 1–21 [DOI] [PubMed] [Google Scholar]
- Møller AR, Janetta PJ. (1981). Compound action potentials recorded intercranially from the auditory nerve in man. J Exp Neurol 71: 862–874 [DOI] [PubMed] [Google Scholar]
- Moore BCJ. (1973a). Frequency difference limens for narrow bands of noise. J Acoust Soc Am 51: 888–896 [DOI] [PubMed] [Google Scholar]
- Moore BCJ. (1973b). Frequency difference limens for short-duration tones. J Acoust Soc Am 51: 610–619 [DOI] [PubMed] [Google Scholar]
- Moore BCJ. (1995). Speech perception in people with cochlear damage. Perceptual Consequences of Cochlear Damage. Oxford: Oxford University Press, pp 147–172 [Google Scholar]
- Moore BCJ. (1996). Perceptual consequences of cochlear hearing loss and their implications for the design of hearing aids. Ear Hear 11: 133–160 [DOI] [PubMed] [Google Scholar]
- Moore BCJ. (1997). Psychology of Hearing. Academic Press, San Diego [Google Scholar]
- Moore BCJ, Glasberg BR. (1987). Formulae describing frequency selectivity as a function of frequency and level and their use in calculating excitation patterns. Hear Res 21: 209–225 [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Peters RW. (1992). Pitch discrimination and phase sensitivity in young and elderly subjects and its relationship to frequency selectivity. J Acoust Soc Am 91: 2881–2893 [DOI] [PubMed] [Google Scholar]
- Nadol JB. (2001). Primary Cochlear Neuronal Degeneration. In Sininger YS, Starr A. (eds), Auditory Neuropathy. San Diego: Singular; Publishing, 99–140 [Google Scholar]
- Nakamura H, Takada S, Shimabuku R, et al. (1985). Auditory nerve and brainstem responses in new-born infants with hyperbilirubinemia. Ped 71: 703–708 [PubMed] [Google Scholar]
- Norton SJ, Ferguson R, Mascher K. (1989). Evoked otoacoustic emissions and extratympanic cochlear microphonics recorded from human ears. Abstracts of the Twelfth Midwinter Research Meeting of the Association for Research in Otolaryngology, 227 (A).
- O'Leary MJ, Fayad J, House WF, et al. (1991). Electrode insertion trauma in cochlear implantation. Ann Otol Rhinol Laryngol 100 (9): 695–699 [DOI] [PubMed] [Google Scholar]
- Ouvrier R. (1996). Correlation between the histopathologic, genotypic, and phenotypic features of hereditary peripheral neuropathies in childhood. J Child Neurol 11: 133–146 [DOI] [PubMed] [Google Scholar]
- Park MS, Lee JH. (1998). Diagnostic potential of distortion product otoacoustic emissions in severe or profound sensorineural hearing loss. Acta Otolaryngol. (Stockh) 111: 496–500 [DOI] [PubMed] [Google Scholar]
- Parkins CW. (1989). Temporal response patterns of auditory-nerve fibers to electrical stimulation in deafened squirrel monkeys. Hear Res 41: 137–168 [DOI] [PubMed] [Google Scholar]
- Pender MP, Sears TA. (1984) The pathophysiology of acute experimental allergic encephalomyelitis in the rabbit. Brain 101: 699–726 [DOI] [PubMed] [Google Scholar]
- Peterson A, Shallop J, Driscoll C, et al. (2003). Outcomes of cochlear implantation in children with auditory neuropathy. J Am Acad Audiol 14 (4): 188–201 [PubMed] [Google Scholar]
- Phillips D, Hall S. (1990). Response timing constraints on the cortical representation of sound time structure. J Acoust Soc Am 81: 1403–1411 [DOI] [PubMed] [Google Scholar]
- Picton TW, Durieux-Smith A, Champagne SC, et al. (1998). Objective evaluation of aided thresholds using auditory steady-state responses. J Am Acad Audiol 1: 315–331 [PubMed] [Google Scholar]
- Picton TW, Durieux-Smith A, Moran L. (1994). Recording auditory brainstem responses from infants. Int J Paed Otorhinolaryngol 21: 93–110 [DOI] [PubMed] [Google Scholar]
- Prieve BA, Gorga MP, Neely ST. (1991). Otoacoustic emissions in an adult with severe hearing loss. J Sp Hear Res 31: 379–385 [DOI] [PubMed] [Google Scholar]
- Psarommatis IM, Tsakanikos MD, Kontorgianni AD, et al. (1997). Profound hearing loss and presence of click-evoked otoacoustic emissions in the neonate: A report of two cases. Int J Ped Otorhinolaryngol 31: 237–243 [DOI] [PubMed] [Google Scholar]
- Ramkalawan TM, Davis AC. (1992). The effects of hearing loss and age of intervention on some language metrics in young hearing impaired children. Brit J Audiol 21: 97–107 [DOI] [PubMed] [Google Scholar]
- Rance G, Beer DE, Cone-Wesson B, et al. (1999). Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 21: 238–252 [DOI] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, et al. (2002). Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear 21: 239–253 [DOI] [PubMed] [Google Scholar]
- Rance G, Dowell RC, Rickards FW, et al. (1998). Steady-state evoked potential and behavioural hearing thresholds in a group of children with absent click-evoked auditory brainstem response. Ear Hear 11: 48–61 [DOI] [PubMed] [Google Scholar]
- Rance G, McKay C, Grayden D. (2004). Perceptual characterisation of children with auditory neuropathy. Ear Hear 21: 34–46 [DOI] [PubMed] [Google Scholar]
- Rance G, Roper R, Symonds L, et al. (in press). Hearing threshold estimation in infants using auditory steady state responses. J Am Acad Audiol. [DOI] [PubMed]
- Rapin I, Gavel J. (2003). “Auditory neuropathy”: Physiologic and pathologic evidence calls for more diagnostic specificity. Int J Ped Otorhinolaryngol 61: 707–728 [DOI] [PubMed] [Google Scholar]
- Rasminsky M, Sears TA. (1972). Internodal conduction in undissected demyelinated nerve fibers. J Physiol 221: 323–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Dallos P. (1975). Absence of cochlear outer hair cells: Effect on behavioural auditory thresholds. Nature 251: 44–46 [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D, et al. (1999). Auditory deprivation of the central auditory system resulting from selective inner hair cell loss: Animal model of auditory neuropathy. Scand Audiol Supp 51: 1–12 [PubMed] [Google Scholar]
- Satya-Murti S, Cacace AT, Hanson P. (1980). Auditory dysfunction in Friedreich's Ataxia: Result of spiral ganglion degeneration. Neurol 31: 1047–1053 [DOI] [PubMed] [Google Scholar]
- Schrott A, Stephan K, Spoendlin H. (1989). Hearing with selective inner hair cell loss. Hear Res 41: 213–220 [DOI] [PubMed] [Google Scholar]
- Seewald RC, Cornelisse LE, Ramji KV, et al. (1997). A Software Implementation of the Desired Sensation Level (DSL [i/o]) Method for Fitting Linear Gain and Wide-dynamic Range Compression Hearing Instruments. London, ON: Hearing Health Care Research Unit [Google Scholar]
- Sek A, Moore BCJ. (1995). Frequency discrimination as a function of frequency, measured in several ways. J Acoust Soc Am 91: 2479–2486 [DOI] [PubMed] [Google Scholar]
- Sellick PM, Patuzzi R, Johnstone BM. (1982). Measurement of basilar membrane motion in the guinea pig using the Mossbauer technique. J Acoust Soc Am 71: 131–141 [DOI] [PubMed] [Google Scholar]
- Shallop JK. (2002). Auditory neuropathy/dys-synchrony in adults and children. Sem Hear 23 (3): 215–223 [Google Scholar]
- Shallop JK, Peterson A, Facer GW, et al. (2001). Cochlear implants in five cases of auditory neuropathy: Postoperative findings and progress. Laryngoscope 111: 555–562 [DOI] [PubMed] [Google Scholar]
- Shapiro SM. (2003). Bilirubin toxicity in the developing nervous system. Ped Neurol 29 (5): 410–421 [DOI] [PubMed] [Google Scholar]
- Shirane M, Harrison RV. (1987). The effects of hypoxia on sensory cells of the cochlea. Scanning Microscopy 1: 1175–1183 [PubMed] [Google Scholar]
- Simmons JL, Beauchaine KL. (2000). Auditory neuropathy: Case study with hyperbilirubinemia. J Am Acad Audiol 11: 337–347 [PubMed] [Google Scholar]
- Simmons BF. (1964). Perceptual theories of middle ear muscle function. Ann Otol Rhino Laryngol 71: 724–740 [DOI] [PubMed] [Google Scholar]
- Sininger YS, Hood LJ, Starr A, et al. (1995). Hearing loss due to auditory neuropathy. Audiol Today 1: 10–13 [Google Scholar]
- Sininger YS, Oba S. (2001). Patients with auditory neuropathy: Who are they and what can they hear? In Sininger YS, Starr A. (eds): Auditory Neuropathy. San Diego: Singular; Publishing, 15–36 [Google Scholar]
- Sohmer H, Pratt H. (1976). Recording of the cochlear microphonic potential with surface electrodes. Electroencephalogr Clin Neurophysiol 41: 253–260 [DOI] [PubMed] [Google Scholar]
- Spoendlin H. (1974). Optic cochleovestibular degerations in heriditary ataxias. II. Temporal bone pathology in two cases of Friedreich's ataxia with vestibulocochlear disorders Brain 91: 41–48 [DOI] [PubMed] [Google Scholar]
- Squires KC, Hecox KE. (1983). Electrophysiological evaluation of higher level auditory processing. Sem Hear 4 (4): 415–432 [Google Scholar]
- Stapells DR, Gravel JS, Martin BA. (1995). Thresholds for auditory brainstem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear 11: 361–371 [DOI] [PubMed] [Google Scholar]
- Stapells DR, Oates P. (1997). Estimation of the pure-tone audiogram by the auditory brainstem response: A review. Audiol Neuro-Otol 2 (5): 257–280 [DOI] [PubMed] [Google Scholar]
- Stapells DR, Picton TW, Durieux-Smith A. (1994). Electrophysiologic measures of frequency specific auditory function. In Jacobsen JT. (ed), Principles and applications in auditory evoked potentials. Needham Heights, MA: Allyn Bacon, 251–283 [Google Scholar]
- Starr A, McPherson D, Patterson J, et al. (1991). Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain 111: 1157–1180 [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, et al. (2003). Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene. Brain 121: 1604–1619 [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Kim R. (2001b). Pathophysiology of auditory neuropathy. In Sininger YS, Starr A. (eds), Auditory Neuropathy. San Diego: Singular; Publishing, 67–82 [Google Scholar]
- Starr A, Picton TW, Sininger YS, et al. (1996). Auditory neuropathy. Brain 119 (3): 741–753 [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Nguyen T, et al. (2001a). Cochlear receptor. (microphonic, summating potentials, and otoacoustic emissions) and auditory pathway. (auditory brainstem potentials) activity in auditory neuropathy Ear Hear 21: 91–99 [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Pratt H. (2000). The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol 11 (3): 215–230 [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Winter M, et al. (1998). Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear 11: 169–179 [DOI] [PubMed] [Google Scholar]
- Stein L, Tremblay K, Pasternak J, et al. (1996). Brainstem abnormalities in neonates with normal otoacoustic emissions. Sem Hear 17 (2): 197–213 [Google Scholar]
- Stelmachowitz PG, Jesteadt W, Gorga MP, et al. (1985). Speech perception ability and psychophysical tuning curves in hearing-impaired listeners. J Acoust Soc Am 71: 621–627 [DOI] [PubMed] [Google Scholar]
- Stephanova DI, Daskalova M. (2004). Excitability properties of normal and demyelinated human motor nerve axons. Electromyogr Clin Neurophysiol 41: 147–152 [PubMed] [Google Scholar]
- Stockard JE, Stockard JJ, Coen RW. (1983). Auditory brainstem response variability in infants. Ear Hear 1: 11–23 [DOI] [PubMed] [Google Scholar]
- Stockard JE, Stockard JJ, Wesmoreland BF, et al. (1979). Brainstem auditory evoked responses: normal variation as a function of stimulus and subject characteristics. Arch Neurol 31: 823–831 [DOI] [PubMed] [Google Scholar]
- Tallal P. (1981). Language disabilities in children: Perceptual correlates. Int J Ped Otorhinolaryngol 1: 1–3 [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller SL, Bedi G, et al. (1996). Language comprehension in language learning impaired children improved with acoustically modified speech. Science 271: 81–84 [DOI] [PubMed] [Google Scholar]
- Tan KL, Skurr BA, Yip YY. (1992). Phototherapy and the brainstem auditory evoked response in neonatal hyperbilirubinemia. J Ped 121: 306–308 [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Ibrahim D, et al. (1994). Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res 71: 93–102 [DOI] [PubMed] [Google Scholar]
- Tang TPY, McPherson B, Yuen KCP, et al. (2004). Auditory neuropathy/auditory dys-synchrony in school children with hearing loss: Frequency of occurance. Int J Ped Otorhinolaryngol 61: 175–183 [DOI] [PubMed] [Google Scholar]
- Trautwein P, Shallop J, Fabry L, et al. (2001). Cochlear implantation of patients with auditory neuropathy. In Sininger YS, Starr A. (eds): Auditory Neuropathy. San Diego: Singular; Publishing, 203–232 [Google Scholar]
- Trautwein P, Sininger YS, Nelson R. (2000). Cochlear implantation of auditory neuropathy. J Am Acad Audiol 11: 309–315 [PubMed] [Google Scholar]
- Tyler RS. (1993). Speech perception by children. In: Tyler RS. (ed) Cochlear Implants: Audiological Foundations. San Diego: Singular, 191–256 [Google Scholar]
- Tyler RS, Wood EJ, Fernandes MA. (1983). Frequency resolution and discrimination of constant and dynamic tones in normal and hearing-impaired listeners. J Acoust Soc Am 71: 1190–1199 [DOI] [PubMed] [Google Scholar]
- Van Tasell DJ, Greenfield DG, Logemann JJ, et al. (1992). Temporal cues for consonant recognition: Training, talker generalization, and use in evaluation of cochlear implants. J Acoust Soc Am 91: 1247–1257 [DOI] [PubMed] [Google Scholar]
- Varga R, Kelley PM, Keats BJ, et al. (2003). Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin. (OTOF) gene J Med Genet 40: 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemeister NF, Plack CJ. (1993). Time analysis. In Yost WA, Popper N, Fay RR. (eds): Human Psychophysics. New-York: Springer-Verlag. [Google Scholar]
- Vohr BR, Carty LM, Moore PE, et al. (1998). The Rhode Island hearing assessment program: Experience with statewide hearing screening. (1993–1996). J Ped 133 (3): 353–357 [DOI] [PubMed] [Google Scholar]
- Wake M, Anderson J, Takeno S, et al. (1996). Otoacoustic emission amplification after inner hair cell damage. Acta Otolaryngol (Stockh) 111: 374–381 [DOI] [PubMed] [Google Scholar]
- Walden BE. (1984). Speech perception of the hearing impaired. In Jerger J. (ed): Hearing Disorders in Adults. San Diego: College-Hill Press, pp 263–309 [Google Scholar]
- Wang J, Powers NL, Hofstetter P, et al. (1997). Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res 101: 67–82 [DOI] [PubMed] [Google Scholar]
- Weiss TF, Rose C. (1988). Stages of degradation of timing information in the cochlea: A comparison of hair-cell and nerve-fiber responses in the alligator lizard. Hear Res 31: 167–174 [DOI] [PubMed] [Google Scholar]
- Widen JE, Ferraro JA, Trouba SE. (1995). Progressive neural impairment: Case report. J Am Acad Audiol 1: 217–224 [PubMed] [Google Scholar]
- Worthington DW, Peters J. (1980). Quantifiable hearing and no ABR: Paradox or error? Ear Hear 1: 281–285 [DOI] [PubMed] [Google Scholar]
- Wright BA, Lombardino LJ, King WM, et al. (1997). Deficits in auditory temporal and spectral resolution in language-impaired children. Nature 381: 176–178 [DOI] [PubMed] [Google Scholar]
- Yates GK, Johnstone BM, Patuzzi RB, et al. (1992). Mechanical preprocessing in the mammalian cochlea. Trends Neurosci 11: 57–61 [DOI] [PubMed] [Google Scholar]
- Yellin MW, Jerger J, Fifer RC. (1989). Norms for disproportionate loss in speech intelligibility. Ear Hear 10 (4): 231–234 [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedley AL, Coulter DK, et al. (1998). Language of early- and later-identified children with hearing loss. Ped 102 (5): 1161–1171 [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, et al. (in press). Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. [DOI] [PubMed]
- Zeng FG, Liu S. (in press). Clear speech perception in auditory neuropathy subjects. J Sp Hear Res. [DOI] [PubMed]
- Zeng FG, Oba S, Garde S, et al. (1999). Temporal and speech processing deficits in auditory neuropathy. Neuroreport 10 (16): 3429–3435 [DOI] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, et al. (2001a). Psychoacoustics and speech perception in auditory neuropathy. In Sininger YS, Starr A. (eds.): Auditory Neuropathy. San Diego: Singular; Publishing, 141–164 [Google Scholar]
- Zeng FG, Oba S, Starr A. (2001b). Supra threshold processing deficits due to desynchronous neural activities in auditory neuropathy. In Breebaart DJ, Houstma AJM, Kohlrausch A, et al. (eds): Physiological and Psychophysical Bases of Auditory Function. Maastricht, Netherlands: Shaker Publishing BV, 365–372 [Google Scholar]
- Zeng FG, Sheng L, Kong YY, et al. (2002). Treatment of auditory neuropathy: Cochlear implants or hearing aids. International Hearing Aid Research Conference. (IHCON) presentation. Lake Tahoe, August, 2002